#Transdermal Drug Delivery Systems Market
Text
Transdermal Drug Delivery Systems Market Size, Share, Demand, Growth Drivers , Key Players and Forecast 2023-2028
IMARC Group has recently released a new research study titled “Transdermal Drug Delivery Systems Market: Global Industry Trends, Share, Size, Growth, Opportunity and Forecast 2023-2028”, offers a detailed analysis of the market drivers, segmentation, growth opportunities, trends and competitive landscape to understand the current and future market scenarios.How big is the transdermal drug…
View On WordPress
#Global Transdermal Drug Delivery Systems Market#Transdermal Drug Delivery Systems Market#Transdermal Drug Delivery Systems Market CAGr#Transdermal Drug Delivery Systems Market Outlook#Transdermal Drug Delivery Systems Market Report#Transdermal Drug Delivery Systems Market Scope#Transdermal Drug Delivery Systems Market Share#Transdermal Drug Delivery Systems Market Size
0 notes
Link
0 notes
Link
0 notes
Text
Transdermal Drug Delivery System Market Revenue Forecast 2030
Transdermal Drug Delivery System Market Growth & Trends
The global transdermal drug delivery system market is expected to reach USD 145.0 billion by 2030, registering a CAGR of 11.9% from 2023 to 2030, according to a new report by Grand View Research, Inc. Increasing adoption of transdermal patches, such as in the case of diabetes, which requires sustained and a daily dose of insulin is expected to provide the market with growth opportunities during the forecast period.
High demand for pain-free drug delivery is a major driving factor of the transdermal drug delivery systems market. In the case of diabetes, insulin has to be administered into the patient's body with the help of injections on a continuous basis. Continuous usage of injections to deliver insulin causes pain and increases the risk of transmission of chronic diseases, thus, making transdermal patches a better choice for delivering insulin in diabetic patients.
Request a free sample copy or view the report summary: https://www.grandviewresearch.com/industry-analysis/transdermal-drug-delivery-systems-industry
COVID-19 led to a significant fall in sales of transdermal drug delivery systems. This is because most of these drugs are prescribed drugs and the pandemic led to a sharp fall in patient visits to healthcare facilities. Factors such as lockdowns and travel restrictions led down by the government, to curb the spread of the virus, resulted in reduced patient visits. In addition, many individuals in order to avoid physical contact also avoided visiting healthcare facilities, as a precautionary measure.
However, with the world moving towards normalcy and various pharma companies focusing on developing new novel products, the market represents great opportunities for growth. Moreover, increasing investments by the government in R&D and healthcare are further accelerating the demand for transdermal drug delivery systems in the market.
Transdermal Drug Delivery System Market Report Highlights
The iontophoresis segment dominated the market in 2022 owing to its efficacy in delivering drugs. The mechanical arrays segment is expected to maximum growth due to the increasing number of product launches
The pain management segment accounted for the highest market share in 2022 due to increasing demand for transdermal patches used for pain management
The cardiovascular segment is anticipated to experience maximum growth during the forecast period due to the increasing utilization of these systems in the treatment of cardiovascular diseases
North America accounted for the highest market share in 2022 owing to a rise in investments by existing as well as new market players
Increasing healthcare expenditure and huge investments in research and development represent lucrative growth opportunities in the Asia Pacific region
Transdermal Drug Delivery System Market Segmentation
Grand View Research has segmented the global transdermal drug delivery system market based on technology, application, and region:
Transdermal Drug Delivery System Technology Outlook (Revenue in USD Million, 2018 - 2030)
Electroporation
Radio Frequency
Iontophoresis
Microporation
Thermal
Mechanical arrays
Ultrasound
Others
Transdermal Drug Delivery System Application Outlook (Revenue in USD Million, 2018 - 2030)
CNS
Pain Management
Cardiovascular
Hormone
Immunological
Metabolic
Gastrointestinal
Infection
Cancer
Others
Urological
Blood disorders
Respiratory
Musculoskeletal
Regional Insights
North America held a sizeable share of more than 58.2% in 2022. The growth in this region is majorly driven by patent expirations, resulting in more companies entering the market. In addition, repositioning of previously failed drugs, reformulation of existing drug compounds, and huge investments undertaken by the prominent as well as new market entrants are also presumed to be the key factors responsible for the substantial share captured by this region.
Factors such as favorable reimbursement scenarios and rising awareness by government organizations in Europe are contributing to the growth of the transdermal drug delivery system market in the region. Well-developed healthcare structure that covers the healthcare costs of the citizens, promotes more people opting for treatment in the region. This is expected to further boost the market growth during the forecast period.
The Asia Pacific market is anticipated to exhibit the fastest CAGR of above 13.4% during the forecast period, attributable to the growing healthcare spending and the rapidly evolving healthcare infrastructure. In addition, the growth is due to the high research and development intensity as well as the growing awareness in emerging economies, such as China and India.
List of Key Players in Transdermal Drug Delivery System Market
Novartis AG
Johnson & Johnson
Mylan Pharmaceuticals, Inc.
Boehringer Ingelheim GmbH
Biogel Technology, Inc.
Transdermal Technologies, Inc.
Skyepharma, Watson Pharmaceuticals, Inc.
3M Company
Noven Pharmaceuticals, Inc.
4P Therapeutics, LLC
Transdermal Corporation
Echo Therapeutics, Inc.
Authoritative Research: https://www.grandviewresearch.com/industry-analysis/transdermal-drug-delivery-systems-industry
#Transdermal Drug Delivery System Market Size#Transdermal Drug Delivery System Market Share#Transdermal Drug Delivery System Market Growth#Transdermal Drug Delivery System Market Research
0 notes
Text
The U.S. transdermal drug delivery system market size was valued at USD 31.31 billion in 2022 and is expected to expand at a compound annual growth rate (CAGR) of 12.4% from 2023 to 2032.
0 notes
Text
Excellent growth of Transdermal Drug Delivery System Market

The transdermal drug delivery system market refers to the global market for drug delivery systems that use the skin as a route of administration. Transdermal drug delivery systems are used to deliver drugs through the skin and into the bloodstream, and are used in the treatment of a wide range of conditions, including pain management, hormone replacement therapy, nicotine addiction, and cardiovascular disease.
For Sample Report Click Here:-https://www.globmarketreports.com/request-sample/202344
The global transdermal drug delivery system market is expected to experience significant growth in the coming years, driven by factors such as the increasing prevalence of chronic diseases, the growing demand for non-invasive drug delivery systems, and the increasing adoption of transdermal drug delivery systems by pharmaceutical companies.
North America is expected to be the largest market for transdermal drug delivery systems, due to the high prevalence of chronic diseases, the presence of a well-established healthcare infrastructure, and the increasing adoption of advanced transdermal drug delivery systems. The Asia-Pacific region is also expected to experience significant growth in the coming years, driven by factors such as the increasing demand for non-invasive drug delivery systems and the growing focus of pharmaceutical companies on developing innovative transdermal drug delivery systems.
A transdermal drug delivery system (TDDS) is a medical technology that enables the transfer of drugs or other therapeutic agents through the skin and into the bloodstream. The system consists of a patch that is applied to the skin, which contains the drug in a specific formulation that allows it to penetrate the skin layers and reach the bloodstream.
TDDS has several advantages over traditional drug delivery methods, such as oral or injectable administration. It can provide a controlled and continuous release of the drug over a prolonged period of time, ensuring consistent blood levels of the drug. It also avoids the first-pass metabolism of the liver, which can reduce the effectiveness of the drug and cause unwanted side effects.
0 notes
Text
Transdermal Drug Delivery System Market Size, Share & Trends, Product, Region & Forecasts 2030
Transdermal Drug Delivery System Market Size, Share & Trends, Product, Region & Forecasts 2030
Description
The impact of a number of factors such as economic, legal, social, political, technological, and modern business developments on market dynamics is briefly examined in the Transdermal Drug Delivery System market analysis. The global Transdermal Drug Delivery System market analysis focuses on market share and competitiveness index, which helps evaluate the top player’s contributions to…
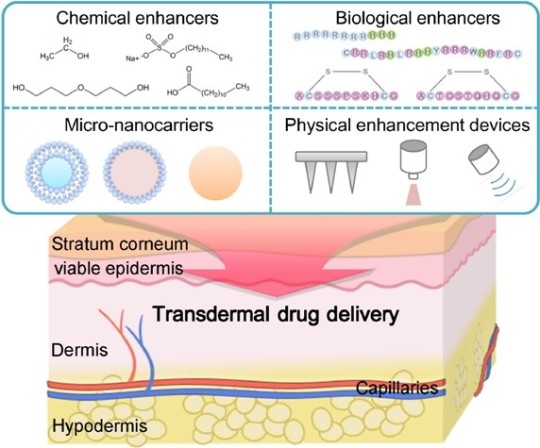
View On WordPress
0 notes
Note
What information do you have about Johnson & Johnson?
Alza filed an NDA for the transdermal opioid analgesic product in December 1987 for post-operative use and for the relief of chronic cancer pain. Developed by J&J subsidiary Janssen Pharmaceutica, fentanyl has been marketed since 1968 under the Sublimaze brand. The drug is currently approved for I.V. administration and used primarily as a short-acting analgesic during anaesthesia. "This new drug delivery system will make it possible for the first time to use fentanyl . . . outside the operating room to control moderate to severe pain." Source
US opioids: Johnson and Johnson and drug distributors offer $26bn to end thousands of lawsuits. Source
The drug company Johnson & Johnson (J&J) has expressed regret after court documents unsealed in talcum powder litigation showed that it funded a 1971 study in which Pennsylvania prison inmates, most of them black, were injected subcutaneously with asbestos. Source
Asbestos Prisoner Study May Spell More Problems for Johnson & Johnson
WASHINGTON - Global health care giant Johnson & Johnson (J&J) and its subsidiaries will pay more than $2.2 billion to resolve criminal and civil liability arising from allegations relating to the prescription drugs Risperdal, Invega and Natrecor, including promotion for uses not approved as safe and effective by the Food and Drug Administration (FDA) and payment of kickbacks to physicians and to the nation’s largest long-term care pharmacy provider. The global resolution is one of the largest health care fraud settlements in U.S. history, including criminal fines and forfeiture totaling $485 million and civil settlements with the federal government and states totaling $1.72 billion. Source
Johnson & Johnson paused all clinical trials of its experimental COVID-19 vaccine after a study participant became sick with an "unexplained illness."
Johnson & Johnson has suspended international trials of a drug in the same class as an experimental drug made by Portuguese pharmaceutical company Bial, whose tests in France left one person brain dead and five others hospitalised. Source
1982 - McNeil
Product Recalled - Tylenol (acetaminophen) capsules
Reason for Recall - Medicine laced with potassium cyanide (poison) resulting in several patient deaths.
2009 to 2011 - McNeil
Product Recalled - Several OTC medicines including Tylenol, Motrin, Benadryl, St. Joseph aspirin, Sudafed, Pepcid, Mylanta, Rolaids, Zyrtec, Zyrtec Eye Drops (tens of millions of bottles)
Reason for Recall - Unpleasant smells causing nausea; tiny metal shards in liquid medicines; wrong ingredient levels
2010 - DePuy [Pinnacle Systems]
Product Recalled - ASR Hip Resurfacing System and ASR XL Acetabular System (metal-on-metal hip implants)
Reason for Recall - Metal poisoning (metallosis); loosening of the implant or joint dislocation; additional surgeries
2012 - Ethicon
Product Recalled - Gynecare Prolift Kit, Gynecare Prolift+M Kit, Gynecare TVT Secure and Gynecare Prosima Pelvic Floor Repair System Kit (transvaginal mesh implants)
Reason for Recall - Perforation of organs; vaginal bleeding and scarring; mesh erosion; severe pain
2014 - Ethicon
Product Recalled - Power Morcellators
Reason for Recall - Spread of uterine cancer; rapid progression of the disease; death
2019 – Johnson & Johnson
Product Recalled – 33,000 bottles of Johnson’s Baby Powder
Reason for Recall – The FDA found a small amount of asbestos — a known carcinogen — in a sample
Xarelto
Number of Lawsuits - 13,511
Injuries - severe, sometimes deadly bleeding events, blood clots, wound leaks, infection
J&J was involved in seven of 2017’s top ten health-care-related verdicts.
The company was also involved in the third-largest pharmaceutical settlement with the U.S. Department of Justice. In 2013, J&J paid the Justice Department more than $2.2 billion. The settlement resolved civil and criminal allegations involving Risperdal, Invega and Natrecor.
May 2017
J&J paid $33 million to most U.S. states and the District of Columbia. The states charged J&J with misrepresenting the manufacturing practices behind certain drugs. This included its Motrin products. These products were later recalled.
Oz
4 notes
·
View notes
Text
Understanding the Growth Dynamics of the Oral Transmucosal Drugs Market
The Oral Transmucosal Drugs Market is projected to be valued at USD 16.57 billion in 2024 and is anticipated to grow to USD 22.97 billion by 2029, with a compound annual growth rate (CAGR) of 6.75% over the forecast period (2024-2029).
The oral transmucosal drugs market has been gaining traction due to its unique drug delivery system that allows medications to be absorbed directly into the bloodstream through the oral mucosa, bypassing the digestive system. This method provides a faster onset of action and is beneficial for patients who struggle with oral intake or those requiring rapid relief. In this blog, we will explore the current trends, key drivers, and challenges shaping the market landscape, based on insights from the market research industry.
1. Market Overview: A Shift Towards Patient-Centric Drug Delivery
The global oral transmucosal drug market is experiencing notable growth due to its convenience and improved patient compliance. This mode of administration is particularly useful in treating conditions like breakthrough cancer pain, migraines, and anxiety, where rapid drug action is critical. The ability to deliver precise dosages through buccal, sublingual, or nasal routes offers a viable alternative to traditional oral or intravenous methods.
Market research points to a growing interest in transmucosal drug delivery systems as pharmaceutical companies look for ways to improve drug efficacy and enhance patient experiences.
2. Key Drivers of Market Growth
a. Advances in Drug Formulations
Continuous advancements in drug formulations are driving the oral transmucosal drugs market forward. Innovations in bioavailability and the stability of drugs administered through the mucosal lining are improving therapeutic outcomes. Pharmaceutical firms are investing in research to create formulations that ensure quicker absorption and minimal side effects.
b. Rising Demand for Pain Management Solutions
Chronic pain management remains one of the top application areas for oral transmucosal drugs. Cancer patients, especially those experiencing breakthrough pain, benefit significantly from this delivery system. Furthermore, the rise in demand for pain management due to the aging population is expected to fuel the market's expansion.
c. Patient Preference for Non-invasive Drug Delivery
Patients are increasingly favoring non-invasive drug delivery methods over traditional injections or tablets. Oral transmucosal administration provides a less invasive approach, making it suitable for individuals with swallowing difficulties, such as pediatric or elderly patients, or those who require rapid symptom control.
3. Challenges and Restraints
a. Regulatory and Approval Complexities
The complexity of obtaining regulatory approvals for transmucosal drugs can be a hurdle for market players. The stringent evaluation of safety, efficacy, and potential risks associated with absorption variability can delay the introduction of new drugs into the market. Pharmaceutical companies need to navigate these regulatory landscapes carefully.
b. Competition from Other Drug Delivery Systems
While oral transmucosal delivery offers distinct advantages, it faces competition from other emerging drug delivery technologies such as transdermal patches, inhalation systems, and implantable devices. Each method comes with its own set of benefits and limitations, leading to a competitive landscape where companies need to differentiate their offerings.
4. Innovations and Opportunities in the Market
a. Breakthroughs in Bioadhesive Technologies
New developments in bioadhesive technologies are enhancing the effectiveness of oral transmucosal drugs. These innovations improve the adhesion of drugs to the mucosal surfaces, ensuring prolonged contact and better absorption rates. The integration of nanoparticles and microencapsulation techniques is also opening new avenues for controlled drug release.
b. Personalized Medicine and Custom Drug Formulations
With the rise of personalized medicine, there is increasing demand for customizable drug formulations in the oral transmucosal segment. Tailoring drug delivery to individual patient needs based on genetic, metabolic, and lifestyle factors offers new possibilities for the industry. This personalized approach aligns with broader trends toward precision medicine, which seeks to optimize treatment outcomes.
5. Future Outlook: Market Expansion and Growth Potential
According to recent market research, the oral transmucosal drugs market is projected to expand significantly over the next decade. This growth will be driven by continued advancements in drug delivery technologies, a rising prevalence of chronic diseases requiring quick therapeutic responses, and increased investments in R&D by pharmaceutical companies. Moreover, the shift towards more patient-centric healthcare solutions will continue to push this market forward.
Final Thoughts
The oral transmucosal drugs market is positioned for substantial growth due to its unique benefits and applications in various therapeutic areas. As pharmaceutical companies focus on enhancing drug delivery methods, investing in innovative formulations, and navigating regulatory challenges, this market will continue to evolve. Understanding the market trends, drivers, and opportunities can help stakeholders in the healthcare sector make informed decisions to capitalize on this burgeoning industry.Call to Action: Interested in exploring more insights on the oral transmucosal drug market? Stay ahead of the curve with comprehensive market research reports that delve deeper into emerging trends and forecast analyses for the next decade.
#Oral Transmucosal Drugs Market trends#Oral Transmucosal Drugs Market size#Oral Transmucosal Drugs Market share#Oral Transmucosal Drugs Market analysis#Oral Transmucosal Drugs Market forecast#Oral Transmucosal Drugs Market demand
0 notes
Text
The Non-Viral Drug Delivery Systems Market To Grow Owing To Increasing Advantages Over Viral Delivery Methods

Non-viral drug delivery systems have gained immense popularity in recent years owing to their advantages over viral delivery methods including low immunogenicity, larger transgene capacity and ease of production. Non-viral techniques involve encapsulating drugs into nanoparticles, liposomes or conjugating them to targeting moieties and are generally considered safer than viral vectors.
The Non-Viral Drug Delivery Systems Market is estimated to be valued at US$ 8.1 Bn in 2024 and is expected to exhibit a CAGR of 13% over the forecast period 2024-2031.
Key Takeaways
Key players operating in the non-viral drug delivery systems market are Arcturus Therapeutics, Bio-Path Holdings, CureVac, Entos Pharmaceuticals, eTheRNA Immunotherapies. The companies are investing heavily in R&D to develop novel non-viral vectors with higher efficiency and safety. The growing demand for targeted drug delivery systems is a major factor driving the non-viral drug delivery systems market. Non-invasive therapeutic delivery through oral, transdermal and inhalation routes has gained prominence. Technological advancements like lipid nanoparticles, polymeric nanoparticles and conjugation with cell-penetrating peptides have increased the delivery of macromolecules.
Market Trends
One of the major trends in the non-viral drug delivery systems market is the rising focus on gene therapy. Non-viral gene delivery methods offer less immunogenic and inflammatory responses making them safer for repeated administration. mRNA-based therapies and applications in cancer immunotherapy are emerging as lucrative opportunities. Another key trend is the development of stimuli-responsive delivery systems that are designed to release drug payloads in response to specific biochemical cues like pH, redox potential or enzymatic activity at the site of action.
Market Opportunities
Targeted delivery to tumors using actively/passively targeted nanoparticles presents a huge opportunity. The application of nanotechnology has allowed efficient delivery of anti-cancer drugs, imaging agents and nucleic acids selectively to tumor tissues. Oligonucleotide therapeutics also offer lucrative opportunities given the advances in mRNA vaccines. Non-invasive delivery through pulmonary route for treatment of lung cancers and respiratory diseases is an emerging area of focus.
Impact Of COVID-19 On Non-Viral Drug Delivery Systems Market Growth
The COVID-19 pandemic has significantly impacted the non-viral drug delivery systems market. During the initial phases of the pandemic, most non-essential research was put on hold or delayed, impacting the development of new drug delivery technologies. Companies focused their efforts on developing COVID-19 vaccines and therapeutics to address the urgent medical need. This diverted resources away from other drug delivery applications.
However, as the pandemic progressed, companies recognized the long-term market potential for non-viral delivery platforms to address future pandemics and other diseases. Nanoparticle-based delivery systems can effectively transport mRNA, DNA and protein therapeutics into cells, making them well-suited for developing new classes of antiviral drugs and vaccines. Several companies utilized their non-viral platforms to design COVID-19 vaccines and therapies during clinical trials.
Going forward, governments and healthcare agencies are expected to prioritize research into development capabilities for rapid responses to health emergencies. Non-viral delivery technologies can play a major role here through their ability to package different types of biologics and enable faster discovery processes compared to viral vectors. Companies are also advancing formulations tuned for stability at varying temperatures and extended shelf-life to address logistical challenges in vaccine distribution globally.
North America Dominates Non-Viral Drug Delivery Systems Market
The North America region currently dominates the Non-Viral Drug Delivery Systems Market in terms of value. This is due to presence of established pharmaceutical and biotechnology companies engaged in development and commercialization of delivery platforms for various biologics. Large companies have made significant investments setting up research centers focused on non-viral technologies.
Government funding for innovation is also strong through the National Institute of Health and Department of Defense programs. Academic research is rapidly advancing new formulations and delivery routes. The U.S. and Canada also have a well-developed regulatory system to approve new drug-device combination products incorporating non-viral carriers. High healthcare spends per capita contribute to faster market uptake of advanced therapeutics enabled by these platforms.
Asia Pacific Emerging As Fastest Growing Region
Going forward, the Asia Pacific region is expected to offer the fastest market growth opportunities for non-viral drug delivery systems. This is attributable to rising chronic disease prevalence in highly populated countries like China and India. Governments are investing significantly to build local R&D capabilities through initiatives such as the Made in China 2025 policy.
Countries like South Korea and Japan also have large biotechnology industries focusing on formulations. At the same time, reduced manufacturing and labor costs are attracting global pharmaceutical companies to outsource production to Asia Pacific contract development and manufacturing organizations. This will help expand regional production capacities for various non-viral technologies.
Get more insights on this topic: https://www.ukwebwire.com/non-viral-drug-delivery-systems-market-are-estimated-to-witness-high-growth-owing-to-advancements-in-nanotechnology/
About Author:
Priya Pandey is a dynamic and passionate editor with over three years of expertise in content editing and proofreading. Holding a bachelor's degree in biotechnology, Priya has a knack for making the content engaging. Her diverse portfolio includes editing documents across different industries, including food and beverages, information and technology, healthcare, chemical and materials, etc. Priya's meticulous attention to detail and commitment to excellence make her an invaluable asset in the world of content creation and refinement. (LinkedIn - https://www.linkedin.com/in/priya-pandey-8417a8173/)
What Are The Key Data Covered In This Non-Viral Drug Delivery Systems Market Report?
:- Market CAGR throughout the predicted period
:- Comprehensive information on the aspects that will drive the Non-Viral Drug Delivery Systems Market's growth between 2024 and 2031.
:- Accurate calculation of the size of the Non-Viral Drug Delivery Systems Market and its contribution to the market, with emphasis on the parent market
:- Realistic forecasts of future trends and changes in consumer behaviour
:- Non-Viral Drug Delivery Systems Market Industry Growth in North America, APAC, Europe, South America, the Middle East, and Africa
:- A complete examination of the market's competitive landscape, as well as extensive information on vendors
:- Detailed examination of the factors that will impede the expansion of Non-Viral Drug Delivery Systems Market vendors
FAQ’s
Q.1 What are the main factors influencing the Non-Viral Drug Delivery Systems Market?
Q.2 Which companies are the major sources in this industry?
Q.3 What are the market’s opportunities, risks, and general structure?
Q.4 Which of the top Non-Viral Drug Delivery Systems Market companies compare in terms of sales, revenue, and prices?
Q.5 Which businesses serve as the Non-Viral Drug Delivery Systems Market’s distributors, traders, and dealers?
Q.6 How are market types and applications and deals, revenue, and value explored?
Q.7 What does a business area’s assessment of agreements, income, and value implicate?
*Note:
1. Source: Coherent Market Insights, Public sources, Desk research
2. We have leveraged AI tools to mine information and compile it
#Non-Viral Drug Delivery Systems Market Trend#Non-Viral Drug Delivery Systems Market Size#Non-Viral Drug Delivery Systems Market Information#Non-Viral Drug Delivery Systems Market Analysis#Non-Viral Drug Delivery Systems Market Demand
0 notes
Link
North America accounted for the largest share of the global transdermal drug delivery system market in 2020 and is expected to retain its position through...
0 notes
Link
0 notes
Text
Cyproterone Acetate Market Poised to Grow at a Robust Pace due to Rising Demand for Anti-Androgens

The cyproterone acetate market has witnessed rising demand over the years owing to increasing applications of cyproterone acetate as a powerful anti-androgen. Cyproterone acetate is an anti-androgen steroidal drug that is used for the treatment of hypersexuality or hypersexual disorder, enlarged prostate or benign prostatic hypertrophy, and hirsutism or excessive hair growth in women. It acts by competitively inhibiting androgens like testosterone and dihydrotestosterone from binding to androgen receptors in target tissues.
The Global cyproterone acetate market is estimated to be valued at US$ 327.1 Mn in 2024 and is expected to exhibit a CAGR of 4.5% over the forecast period 2024 to 2031.
Key Takeaways
Key players operating in the cyproterone acetate market are Curia, Axplora, LGM Pharma, Hubei Gedian Humanwell Pharmaceutical, Cipla, NEWCHEM SPA, Swati Spentose, Teva API, Teva Pharmaceutical Industries, Unipex, Zhejiang Xianju Pharmaceutical Co. Ltd, KRKA, Cambrex, Sicor De México, Zhejiang Xianju Xianle, Shaoxing Hantai Pharma, and Farmabios. The key players are engaged in developing improved formulations and delivery methods of cyproterone acetate to increase market share.
The rising prevalence of hormonal disorders like hypersexuality, hirsutism and benign prostatic hyperplasia has opened up opportunities for pharmaceutical manufacturers in the Cyproterone Acetate Market Size Furthermore, increasing awareness about treatment options and availability of generic drugs are fueling the demand.
Technological advancements in drug delivery systems like transdermal patches, gels, and nano-formulations are allowing for better management of hormonal disorders and can drive the adoption of cyproterone acetate.
Market Drivers
A key driver for the cyproterone acetate market is the rising geriatric population who are more prone to benign prostatic hyperplasia. Furthermore, changing lifestyles and increasing stress levels have contributed to the rise in disorders like hypersexuality which is also propelling the demand. Favorable government policies for generic drugs and availability of generic versions of cyproterone acetate at lower costs are facilitating greater market penetration.
Challenges in Cyproterone Acetate Market:
Patent Expiry of Acetate Products. Cyproterone Acetate Market Size And Trends drugs are widely used in the treatment of sex hormone-dependent disorders like precocious puberty, hirsutism and acne, however many patented drugs containing cyproterone acetate are going off patent in the coming years leading to increased competition in the generic market.
Stringent Regulatory Guidelines: Approval processes for new drugs and formulations containing cyproterone acetate are long and stringent. Regulatory authorities like USFDA and EMA impose rigorous evaluation procedures to ensure safety and efficacy. This increases clinical trial timelines and costs.
Potential Side Effects: Cyproterone acetate has few potential side effects if taken for a long duration. Side effects reported include nausea, vomiting, headache, breast tenderness or swelling. Rare side effects include hepatitis, changes in liver function values and blood clotting problems. Potential risks require monitoring and further research on safety.
SWOT Analysis:
Strengths: High growth opportunities in generic drugs market. Well established uses in treatment of hormonal disorders.
Weaknesses: Patents expiry of branded drugs. Potential side effects require further safety evaluation.
Opportunities: Developing novel drug delivery systems to reduce side effects. Approval of new treatment indications will boost growth.
Threats: Stringent regulations delay market approvals. Intense competition from existing and emerging players.
Geographical Regions:
Currently North America accounts for the largest share in cyproterone acetate market, mainly driven by high treatment rates for hormonal disorders. Emergence of new generics and strong reimbursement structure also supports growth. Europe follows North America and holds significant revenue share due to rising healthcare expenditures.
Fastest Growing Region:
Asia Pacific region is poised to be the fastest growing market for cyproterone acetate. Increasing patient pool suffering from hormonal imbalance, rising medical tourism, growing healthcare infrastructure and entry of low-cost generics will drive robust growth in Asia Pacific during forecast period. India and China will be the key revenue generators in this region.
Current challenges in Cyproterone Acetate Industry:
The cyproterone acetate industry faces challenges from generic competition as major patents expire. Several drugs containing cyproterone acetate as active ingredient lost exclusivity in last few years leading to availability of low-cost generics. This has significantly reduced prices of branded formulations impacting revenues of innovator companies. Another challenge is the stringent regulatory norms for approval of new drugs. The regulatory processes have become complex over time warranting extensive clinical data and trials increasing costs and timelines for industry players. Potential side effects also require further evaluation on long term safety profile through ongoing clinical research. High development costs and regulatory hurdles restrict entry of new market entrants. Overall, generic competition and regulatory challenges are major pain points for cyproterone acetate industry currently.
Get More Insights On, Cyproterone Acetate Market
For More Insights Discover the Report In language that Resonates with you
French
German
Italian
Russian
Japanese
Chinese
Korean
Portuguese
About Author:
Ravina Pandya, Content Writer, has a strong foothold in the market research industry. She specializes in writing well-researched articles from different industries, including food and beverages, information and technology, healthcare, chemical and materials, etc. (https://www.linkedin.com/in/ravina-pandya-1a3984191
#Cyproterone Acetate Market Share#Cyproterone Acetate Market Size#Cyproterone Acetate Market Trends#Cyproterone Acetate#Cyproterone Acetate Market
0 notes
Text
0 notes
Text
Softgel Capsules Market Detailed Analysis And Forecast 2024-2033 | Global Insight Services
Softgel capsules are a type of dietary supplement that is encased in a soft, gel-like shell. These capsules are designed to be easy to swallow and to dissolve quickly in the stomach.
Softgel capsules are often used to deliver vitamins, minerals, and other nutrients to the body. They may also be used to deliver medications, such as those used to treat pain or to promote weight loss.
Softgel capsules are generally considered safe for most people. However, as with any dietary supplement, it is important to speak with a healthcare provider before taking them.
Key Trends
The softgel encapsulation market is growing at a rapid pace due to the many advantages that softgels offer over other drug delivery systems. Some of the key trends in softgel capsules technology include:
Increased use of softgels for oral delivery of drugs: Softgels offer many advantages over tablets and capsules for oral drug delivery, including improved bioavailability, better patient compliance, and more. As a result, more and more drugs are being formulated as softgels.
Increased use of softgels for other delivery methods: In addition to oral delivery, softgels can also be used for topical, transdermal, and injectable drug delivery. This versatility makes them an attractive option for a variety of different drugs.
Improved manufacturing processes: Softgel manufacturing technology has come a long way in recent years, and there are now a variety of different ways to produce high-quality softgels. This has led to increased efficiency and lower costs, making softgels more affordable for both manufacturers and consumers.
New and innovative uses for softgels: Softgels are being used for a variety of new and innovative applications, such as delivery of nutraceuticals, cosmetics, and even vaccines. This is opening up new markets for softgel manufacturers and providing more options for consumers.
Key Drivers
The key drivers of the softgel capsules market are the rising demand for dietary supplements and the growing pharmaceutical industry. The increasing health consciousness among people and the rising incidence of chronic diseases are also driving the growth of the market. Moreover, the easy availability of softgel capsules and the increasing number of manufacturing companies are providing a boost to the market growth. However, the high cost of softgel capsules and the stringent regulations imposed by the government are restraining the market growth.
Unlock Growth Potential in Your Industry – Get Your Sample Report Now@ https://www.globalinsightservices.com/request-sample/GIS22197
Research Objectives
Estimates and forecast the overall market size for the total market, across product, service type, type, end-user, and region
Detailed information and key takeaways on qualitative and quantitative trends, dynamics, business framework, competitive landscape, and company profiling
Identify factors influencing market growth and challenges, opportunities, drivers and restraints
Identify factors that could limit company participation in identified international markets to help properly calibrate market share expectations and growth rates
Trace and evaluate key development strategies like acquisitions, product launches, mergers, collaborations, business expansions, agreements, partnerships, and R&D activities
Thoroughly analyze smaller market segments strategically, focusing on their potential, individual patterns of growth, and impact on the overall market
To thoroughly outline the competitive landscape within the market, including an assessment of business and corporate strategies, aimed at monitoring and dissecting competitive advancements.
Identify the primary market participants, based on their business objectives, regional footprint, product offerings, and strategic initiatives
Request Customization@ https://www.globalinsightservices.com/request-customization/GIS22197
Market Segments
The Softgel Capsules Market is segmented based on application, sales channel, and region. Based on application, the market is bifurcated as health supplements and pharmaceuticals. According to the sales channel, it is categorized into supermarket & hypermarket, pharmacy & drug store, and online provider. Region-wise, the market is segmented into North America, Europe, Asia-Pacific, and the Rest of the World.
Key Players
The Softgel Capsules Market report includes players such as BASF SE, Catalent, Inc., Colorcon Inc., Fuji Capsules Co., Ltd., InovoBiologic Inc., NOW Foods Inc., Sirio Pharma Co., Ltd., Super Spectrim, Thermo Fisher Scientific, Inc. (Patheon), and Trigen Laboratories, Inc.
Drive Your Growth Strategy: Purchase the Report for Key Insights@ https://www.globalinsightservices.com/checkout/single_user/GIS22197
Research Scope
Scope – Highlights, Trends, Insights. Attractiveness, Forecast
Market Sizing – Product Type, End User, Offering Type, Technology, Region, Country, Others
Market Dynamics – Market Segmentation, Demand and Supply, Bargaining Power of Buyers and Sellers, Drivers, Restraints, Opportunities, Threat Analysis, Impact Analysis, Porters 5 Forces, Ansoff Analysis, Supply Chain
Business Framework – Case Studies, Regulatory Landscape, Pricing, Policies and Regulations, New Product Launches. M&As, Recent Developments
Competitive Landscape – Market Share Analysis, Market Leaders, Emerging Players, Vendor Benchmarking, Developmental Strategy Benchmarking, PESTLE Analysis, Value Chain Analysis
Company Profiles – Overview, Business Segments, Business Performance, Product Offering, Key Developmental Strategies, SWOT Analysis.
With Global Insight Services, you receive:
10-year forecast to help you make strategic decisions
In-depth segmentation which can be customized as per your requirements
Free consultation with lead analyst of the report
Infographic excel data pack, easy to analyze big data
Robust and transparent research methodology
Unmatched data quality and after sales service
Contact Us:
Global Insight Services LLC
16192, Coastal Highway, Lewes DE 19958
E-mail: [email protected]
Phone: +1-833-761-1700
Website: https://www.globalinsightservices.com/
About Global Insight Services:
Global Insight Services (GIS) is a leading multi-industry market research firm headquartered in Delaware, US. We are committed to providing our clients with highest quality data, analysis, and tools to meet all their market research needs. With GIS, you can be assured of the quality of the deliverables, robust & transparent research methodology, and superior service.
0 notes
Text
Understanding the Growth of the Dinoprostone Market
Dinoprostone, also known as prostaglandin E2 (PGE2), is a naturally occurring prostaglandin used in various medical applications, primarily for labor induction and cervical ripening. The Dinoprostone Market has witnessed considerable growth due to its effectiveness in obstetric and gynecological applications. This article explores the market size, share, industry trends, and forecasts for the Dinoprostone Market through 2032.
Market Overview
The Dinoprostone Market is growing steadily, driven by increasing demand for obstetric interventions and advancements in gynecological treatments. Dinoprostone is widely used in labor induction, cervical ripening, and as an abortifacient. Its efficacy and safety profile have made it a preferred choice in medical practice.
Dinoprostone Market Size and Share
Dinoprostone Market Size was estimated at 13.22 (USD Billion) in 2023. The Dinoprostone Market Industry is expected to grow from 13.85(USD Billion) in 2024 to 20.0 (USD Billion) by 2032. The dinoprostone Market CAGR (growth rate) is expected to be around 4.71% during the forecast period (2024 - 2032).Several factors contribute to this robust growth:
Rising Birth Rates: Increasing birth rates in developing regions are driving the demand for labor induction agents, including dinoprostone.
Advancements in Obstetric Care: Technological advancements and improved obstetric care practices are boosting the adoption of dinoprostone for safe and effective labor induction.
Increasing Awareness: Growing awareness about the benefits of medically assisted labor induction is contributing to market growth.
Regulatory Approvals: Favorable regulatory approvals and guidelines supporting the use of dinoprostone in obstetric care are enhancing its market penetration.
Key Trends in the Dinoprostone Market
Technological Innovations: The development of novel drug delivery systems, such as controlled-release formulations and transdermal patches, is improving the efficacy and convenience of dinoprostone administration.
Rising Demand for Minimally Invasive Procedures: The trend towards minimally invasive gynecological procedures is driving the adoption of dinoprostone for cervical ripening and labor induction.
Growing Focus on Maternal Health: Increased focus on maternal health and initiatives aimed at reducing maternal mortality rates are promoting the use of dinoprostone in obstetric care.
Emerging Markets: Rapid economic growth and improving healthcare infrastructure in emerging markets are creating new opportunities for market expansion.
Collaborations and Partnerships: Key market players are engaging in collaborations and partnerships to enhance their product portfolios and expand their market presence.
Regional Analysis
The Dinoprostone Market exhibits regional variations in terms of market size and growth.
North America: Dominates the market, accounting for the largest share, primarily due to the high adoption rate of advanced obstetric practices and the presence of major pharmaceutical companies.
Europe: Follows closely, driven by well-established healthcare systems, high awareness levels, and favorable regulatory frameworks.
Asia-Pacific: Expected to witness the highest growth rate during the forecast period, fueled by increasing birth rates, improving healthcare access, and rising awareness about maternal health.
Latin America and Middle East & Africa: These regions are also experiencing steady growth, supported by improving healthcare facilities and increasing government initiatives to address maternal and child health issues.
Competitive Landscape
The Dinoprostone Market is highly competitive, with several key players striving to expand their market share through strategic initiatives such as mergers and acquisitions, partnerships, and new product launches. Major companies in the market include: Esaote SpA ,Koninklijke Philips ,Hitachi ,Mindray Medical International ,General Electric ,Siemens Healthineers ,Samsung Electronics ,Canon Medical Systems ,Neusoft Corporation ,United Imaging Healthcare ,Hologic ,Carestream Health ,Fujifilm Holdings ,Planmed ,Ziehm Imaging.
Future Outlook
The future of the Dinoprostone Market looks promising, with continuous advancements in obstetric and gynecological care and a growing focus on maternal health. Key factors that will shape the market include:
Innovative Drug Formulations: Ongoing research and development efforts to create more effective and convenient dinoprostone formulations will drive market growth.
Increased Focus on Patient Safety: Enhancing patient safety and reducing adverse effects through improved drug delivery systems and formulations will be crucial for market expansion.
Global Collaborations: International collaborations between pharmaceutical companies, research institutions, and healthcare providers will accelerate the development and commercialization of new dinoprostone products.
Regulatory Support: Favorable regulatory frameworks and expedited approval processes for innovative obstetric drugs will encourage market growth.
Rising Healthcare Expenditure: Increased healthcare spending, particularly in emerging economies, will improve access to obstetric care and boost market growth.
Conclusion
The Dinoprostone Market is assured of significant growth, driven by rising birth rates, advancements in obstetric care, and a growing focus on maternal health. The market's future will be shaped by technological innovations, patient-centric approaches, and early diagnosis efforts. As key players continue to invest in research and development and strategic initiatives, the Dinoprostone Market will witness robust growth, offering improved treatment options and better quality of life for patients worldwide.
0 notes