#US Acute Myeloid Leukemia Market
Text
Top 3 US Pharmaceutical Companies and Their Strategies in US Acute Myeloid Leukemia Market
Buy Now
US Acute Myeloid Leukemia Market is growing due to advancement in treatment approaches, increase healthcare costs and expenditure, growing investments in Research and Development, and a constant growth in population.
Story Outline
Pfizer Inc.- An American multinational company with the highest annual revenue of around 100 billion US$ in 2022 in the drug market. The company has made significant contributions to the US Acute Myeloid Leukemia Market through a sophisticated, robust, agile manufacturing infrastructure and investment in research and development.
Brystol Myers Squibb- One of the largest American pharmaceutical companies which consistently ranks on the Fortune 500 list of the largest US corporations. The company’s mission is to discover, develop, and deliver innovative medicines that help patients as well as prevail over serious diseases.
Novartis AG- The company with the fourth-largest revenue in the drug market which is focused to deliver high-value medicine that alleviate society’s greatest disease burdens through technology leadership in R&D and novel access approaches.
The US Acute Myeloid Leukemia Market is expected to grow at CAGR of 10.5% in the forecast period 2022-2028.
The Acute Myeloid Leukemia Market of US is driving growth at an amazing level. This surge is a result of advancement in treatment approaches, increases healthcare costs and expenditure, growing investments in Research and Development, and a constant growth in population.
Various pharmaceutical companies are actively shaping this growing market with their strategies and innovations.
This article provides an in-depth look at major pharmaceutical companies with their strategies and innovations.
1.Pfizer Inc.
Click here: To know more about Pfizer Inc.
Pfizer Inc. is an American multinational pharmaceutical company and headquartered at The Spiral in Manhattan, New York City. The company has made significant contributions to the US Acute Myeloid Leukemia Market through a sophisticated, robust, agile manufacturing infrastructure and investing in research and development.
The company employs more than 30,000 employees worldwide, have 35+ manufacturing sites, 300+ external suppliers, and have reached more than 180 countries. Pfizer tops the list of drug market by achieving a revenue of approx. 100 billion USD in 2022.
Pfizer has made significant contributions to the US acute Myeloid Leukemia Market. Some notable contributions are, in April 2017, the development and approval of a targeted therapy called “Rydapt”, which is an oral kinase inhibitor that targets multiple enzymes, including FLt3, which is often mutated in AML patients. The drug was approved by FDA in April 2017 for use in combination with Chemotherapy.
Furthermore, MYLOTARG is approved in combination with daunorubicin and cytarabine for the treatment of patients aged 15 and above with previously untreated, de novo, CD33-positive acute myeloid leukemia (AML), except Acute Promyelocytic Leukemia (APL).
The company’s purpose is “Breakthroughs that change patients' lives—fuels everything we do and reflects our passion for building on our legacy as one of the greatest contributors of good to the world.
The company says that its purpose ensures that its patients remain at the center of all that they do. They live with their purpose by sourcing the best science in the world; partnering with others in the healthcare system to improve access to their medicines.
Pfizer believes in growing partnerships with innovators to initiate forward great science and continually seek new partners that are actively researching bold scientific ideas. In December 2022, Pfizer announced its collaboration with Gero’s machine learning technology platform to discover potential therapeutic targets for fibrotic using large-scale human-based data.
Pfizer’s continuous clinical trials and collaboration with healthcare institutions and research organizations has majorly contributed in advancing Acute Myeloid Leukemia Market and the development of novel treatment strategies.
2.Bristol Myers Squibb

Click here: To Download a Sample Report
The Bristol Myers Squibb Company, is an American multinational pharmaceutical company headquartered in Princeton, New Jersey. BMS is one of the world’s largest pharmaceutical companies and consistently ranks on the Fortune 500 list of the largest US corporations.
The company employs more than 34,000 across more than 86 locations worldwide. The company’s revenue in 2022 was approximately 46 bn USD.
Their mission is to discover, develop, and deliver innovative medicines that help patients as well as prevail over serious diseases. Bristol believes in the power of science to address some most challenging diseases of today's world.
Bristol Myers is majorly known for its contributions to oncology, and immunology and its involvement in Acute Myeloid Leukemia Market with its broader focus on cancer treatments.
The significant development of BMS can be noted from June 2021, Bristol Myers Squibb received approval from European Commission for Onureg, a Frontline oral maintenance therapy for adult patients with acute Myeloid Leukemia who achieved their first complete remission (CR) or CR with incomplete blood count recovery following intensive induction chemotherapy. Onureg is expected to increase sales and product revenue, thereby increasing the US Acute Myeloid Leukemia Market growth.
Furthermore, the strategic cooperation between Evotec and Bristol Myers Squibb has grown in order to create a pipeline for programs addressing more neurological illnesses. In order to find altering therapies for a variety of neurodegenerative disorders, the firms started working together in 2016. The eight-year extension is intended to strengthen the strategic partnership even more.
3.Novartis AG

Click Here : To Download a Custom Report
Novartis AG is a healthcare company that majorly focuses on the discovery, development, manufacture and marketing of prescription and generic pharmaceutical and eye care products. Novartis offers generic medicines and biosimilars through Sandoz. The company conducts its research through The Novartis Institute for Biomedical Research (NIBR).
Novartis is one of the largest pharmaceutical companies in the world and the fourth largest by revenue in 2022, which was approx. 50.500 billion USD.
The company is functioning in more than 150 locations with around 1,10,000 employees working worldwide.
Novartis's strategy as a focused medicines company is to deliver high-value medicine that alleviates society’s greatest disease burdens through technology leadership in R&D an novel access approaches.
Novartis contribution to Acute Myeloid Leukemia Market includes, FDA approval of Novartis Scemblix (asciminib), with novel mechanism of action for the treatment of Leukemia in October,2021.
Furthermore, through their open approach Novartis is focusing on new technologies to develop next generation therapeutics. Currently, Novartis is working with Orionis Bioscience to find new targets at a genome-wide scale.
By combining development and drug discovery with innovation, they aim to achieve tenuous targets and to launch novel small molecule therapy for Acute Myeloid Leukemia patients more quickly. Thus driving a steered growth for the US Acute Myeloid Leukemia Market.
#US Acute Myeloid Leukemia Market#US Acute Myeloid Leukemia Market Revenue#US Acute Myeloid Leukemia Market Analysis#US Acute Myeloid Leukemia Industry#US Acute Myelogenous Market#US Bone Marrow Leukemia Industry#United States Acute Myeloid Leukemia Market#US Acute Myeloid Leukemia Market size#US Acute Myeloid Leukemia Market share#US Acute Myeloid Leukemia Market trends#US Acute Myeloid Leukemia Market growth#Chemotherapy in Acute Myeloid Leukemia Market US#Targeted Therapy in Acute Myeloid Leukemia Market US#Hormone Therapy in Acute Myeloid Leukemia Market US#End Users in US Acute Myeloid Leukemia Market#Specialty Centers in US Acute Myeloid Leukemia Market#Hospitals for Acute Myeloid Leukemia in US#Challenges in US Acute Myeloid Leukemia Market#Major Players in US Acute Myeloid Leukemia Market#Emerging Players in US Acute Myeloid Leukemia Market#US Acute Myeloid Leukemia Market Future Outlook#US Acute Myeloid Leukemia Market Growth Rate#leading companies US Acute Myeloid Leukemia Market#US Acute Myeloid Leukemia Market competitors#US Acute Myeloid Leukemia Market forecast#US Acute Myeloid Leukemia Market demand#US Acute Myeloid Market Challenges#Myelogenous Leukemia Opportunities US#Acute Leukemia Challenges US#Opportunities Myeloid Leukemia Market US
0 notes
Text
The Best News of Last Week - March 27, 2023
🐢 - Why did the 90-year-old tortoise become a father? Because he finally came out of his shell!
1. New Mexico governor signs bill ending juvenile life sentences without parole

New Mexico Governor Michelle Lujan Grisham has signed a bill into law that prevents juvenile offenders from receiving life sentences without eligibility for parole. The bill, known as the No Life Sentences for Juveniles Act, allows offenders who committed crimes under the age of 18 and received life sentences to be eligible for parole hearings 15 to 25 years into their sentences.
This legislation also applies to juveniles found guilty of first-degree murder, even if they were tried as adults. The move puts New Mexico in a group of at least 24 other states and Washington, DC, that have enacted similar measures following a 2021 Supreme Court ruling.
2. Promising pill completely eliminates cancer in 18 leukaemia patients

An experimental pill called revumenib has shown promise in curing terminal leukemia patients who were not responding to treatment in a long-awaited clinical trial in the United States. The drug works by inhibiting a specific protein called menin, which is involved in the machinery that gets hijacked by leukemia cells and causes normal blood cells to turn into cancerous ones.
The pill targets the most common mutation in acute myeloid leukemia, a gene called NPM1, and a less common fusion called KMT2A. The US Food and Drug Administration granted revumenib "breakthrough therapy designation" to fast-track its development and regulatory review based on the promising results of the trial.
3. Spain passes law against domestic animal abuse

Spain has passed a new law on animal welfare, accompanied by a reform of the penal code that increases prison sentences for those mistreating animals. The law will make compulsory training for dog owners, and will prohibit them from leaving their dogs alone for more than 24 hours.
It also mandates the sterilisation of cats, with exceptions for farms, and increases the penalties for mistreatment of animals to up to two years in prison, or three years in the event of aggravating circumstances.
4. Bravery medals for women who raced into 'rough, crazy' surf to save drowning girls
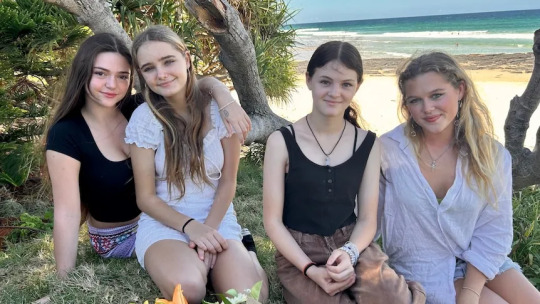
Elyse Partridge (far left) and Bella Broadley (far right) raced into dangerous surf to save Chloe and Violet from drowning.(ABC North Coast: Hannah Ross)
Bella Broadley and Elyse Partridge saved two 11-year-old girls from drowning at Angels Beach near Ballina, an unpatrolled beach in Australia. The younger girls, Chloe and Violet, became trapped in a rip and overwhelmed by waves and the current. Bella and Elyse jumped into action, using an esky lid as a flotation device to help them swim to the girls. Elyse helped Chloe back to shore while Bella swam further out to help Violet.
Elyse and Bella were on Wednesday named on the Governor General's Australian Bravery Decorations Honours List, which recognised 66 Australians for acts of bravery.
5. Almost every cat featured in viral Tik Tok posted by Kansas City animal shelter adopted
Let's find homes for the rest
youtube
6. A 90-year-old tortoise named Mr. Pickles just became a father of 3. It's a big 'dill'

These critically endangered tortoises are native to Madagascar and have seen their numbers decline due to over-collection for illegal sales on the black market. Captive breeding programs have helped produce new radiated tortoises, but the species still faces extinction in the wild.
That's why the arrival of these hatchlings, born to 90-year-old Mr. Pickles and his 53-year-old partner Mrs. Pickles, is such great news. Mr. Pickles is considered the most genetically valuable radiated tortoise in the Association of Zoos and Aquariums' Species Survival Plan, and the births represent a significant contribution to the survival of the species.
7. EU strikes ‘ground-breaking’ deal to cut maritime emissions

The European Parliament and EU ministers have agreed on a new law to cut emissions in the maritime sector. The law aims to reduce ship emissions by 2% as of 2025 and 80% as of 2050, covering greenhouse gas, methane, and nitrous oxide emissions.
The European Commission will review the law in 2028 and will decide whether to place carbon-cutting requirements on smaller ships. The agreement will also require containerships and passenger ships docking at major EU ports to plug into the on-shore power supply as of 2030. Penalties collected from those that fail to meet the targets will be allocated to projects focused on decarbonising the maritime sector.
- - - -
That's it for this week :)
This newsletter will always be free. If you liked this post you can support me with a small kofi donation:
Buy me a coffee ❤️
Also don’t forget to share this post with your friends.
468 notes
·
View notes
Quote
For the past decade, the Dutch immunologist Jacques (Sjaak) Neefjes has been on a mission to bring back a cancer drug that hasn’t been available in Europe since 2004. “I’m still flabbergasted that a compound that could have helped thousands of people was taken off the market,” says Neefjes. Why it was removed seems something of a mystery, but as far as he can tell, it was simply a lack of demand.
His latest research shows that this drug, aclarubicin, can improve the survival of people with acute myeloid leukemia (AML) much better than other forms of chemotherapy. If it had been available in Europe for the past 20 years, Neefjes estimates that it could have helped 100,000 people.
But while the maths sounds simple, the path of Neefjes’s research has been far from smooth. In his attempts to revive aclarubicin, he has smuggled small amounts from China, found old samples in a Finnish freezer, and funded part of his work using an inheritance from a Dutch MP who was murdered 10 years ago.
“It has been a tough battle,” says Neefjes. And it’s nowhere near over. Next, he has to produce enough of the drug to run clinical trials for AML patients in Europe.
One man’s mission to revive a forgotten, life-saving cancer drug | Cancer research | The Guardian
3 notes
·
View notes
Text
Advances in Leukemia Therapeutics: Innovations and Emerging Trends
The Evolving Landscape of Leukemia Treatment

Leukemia, a group of malignancies that affect the blood and bone marrow, continues to challenge the medical community with its complexity and variability. Recent years have seen significant strides in leukemia therapeutics, driven by advancements in drug development, personalized medicine, and novel therapeutic strategies. These innovations are transforming treatment paradigms, offering new hope to patients and paving the way for more effective and targeted therapies.
Breakthroughs in Drug Development
One of the most significant advancements in leukemia therapeutics is the development of targeted therapies. Unlike traditional chemotherapy, which indiscriminately kills rapidly dividing cells, targeted therapies are designed to attack specific molecules involved in cancer progression. For instance, tyrosine kinase inhibitors (TKIs) like imatinib, dasatinib, and nilotinib have revolutionized the treatment of chronic myeloid leukemia (CML). These drugs target the BCR-ABL protein, a key driver of CML, leading to improved outcomes and reduced side effects compared to conventional treatments.
In addition to TKIs, the approval of novel agents such as venetoclax has marked a significant leap forward. Venetoclax targets the BCL-2 protein, which is often overexpressed in leukemia cells, thereby promoting their survival. By inhibiting BCL-2, venetoclax enhances the effectiveness of chemotherapy and has shown promise in treating acute myeloid leukemia (AML) and chronic lymphocytic leukemia (CLL).
Advances in Immunotherapy
Immunotherapy is another area where substantial progress has been made. Chimeric antigen receptor (CAR) T-cell therapy represents a groundbreaking approach, especially for acute lymphoblastic leukemia (ALL) and some cases of CLL. This therapy involves modifying a patient's T-cells to express a CAR that targets leukemia-specific antigens. The modified T-cells are then reintroduced into the patient’s body, where they identify and destroy leukemia cells.
Recent studies have demonstrated the efficacy of CAR T-cell therapies, leading to impressive remission rates in patients with refractory or relapsed leukemia. However, this approach is not without challenges. The risk of cytokine release syndrome (CRS) and neurotoxicity remains a concern, and ongoing research aims to enhance the safety and effectiveness of CAR T-cell therapies.
The Leukemia Therapeutics Market size was USD 16 Billion in 2023 and is expected to Reach USD 27.28 Billion by 2031 and grow at a CAGR of 6.9% over the forecast period of 2024-2031.
Precision Medicine and Genetic Insights
The era of precision medicine has ushered in a new approach to leukemia treatment, with a focus on tailoring therapies based on individual genetic profiles. Advances in genomic sequencing have identified specific mutations and genetic alterations associated with different types of leukemia. For example, mutations in the FLT3 gene are common in AML, and targeted inhibitors like midostaurin are being used to address these mutations.
Similarly, research into the genetic underpinnings of CLL has led to the development of drugs that target specific mutations or pathways involved in the disease. By understanding the unique genetic landscape of each patient’s leukemia, clinicians can select the most effective therapies and potentially improve treatment outcomes.
Future Directions and Challenges
While the progress in leukemia therapeutics is promising, several challenges remain. The high cost of novel therapies, including CAR T-cell treatments, poses a significant barrier to access for many patients. Additionally, the development of resistance to targeted therapies and immunotherapies requires ongoing research to develop new and effective treatment options.
Looking ahead, researchers are exploring combination therapies, novel drug targets, and personalized approaches to overcome these challenges. The integration of artificial intelligence and machine learning in drug discovery and treatment planning holds the potential to further accelerate progress in leukemia therapeutics.
Conclusion
The field of leukemia therapeutics is undergoing a transformative phase, with innovations in drug development, immunotherapy, and precision medicine leading the way. These advancements are not only enhancing treatment outcomes but also offering new hope to patients. As research continues to evolve, the focus will remain on overcoming current challenges and pushing the boundaries of what is possible in the fight against leukemia.
0 notes
Text
Olutasidenib Market Research Trends Analysis by 2024-2034

Olutasidenib Market Introduction:
Olutasidenib is emerging as a pivotal drug in the oncology field, targeting cancers with specific genetic mutations. Developed to inhibit the mutant form of the isocitrate dehydrogenase 1 (IDH1) enzyme, olutasidenib offers a targeted approach for treating cancers driven by IDH1 mutations, particularly acute myeloid leukemia (AML).
Unlock your exclusive sample PDF now:
Market Trends
Increasing Adoption in Clinical Practice: Olutasidenib's clinical trials have shown promising results, particularly in treating acute myeloid leukemia (AML) and other hematologic malignancies. The drug's effectiveness in targeting IDH1 mutations has garnered interest from oncologists, leading to its gradual adoption in clinical settings. As more data becomes available, the use of olutasidenib is expected to become more widespread, further driving market growth.
Growing Pipeline of IDH1-Mutant Cancers: The olutasidenib market is benefiting from an expanding pipeline of IDH1-mutant cancers. Research is ongoing to evaluate the drug's efficacy in various cancer types beyond AML, including solid tumors. This broadening scope is likely to enhance market potential and create new opportunities for pharmaceutical companies.
Increasing Investment and Partnerships: Pharmaceutical companies are investing heavily in the development and commercialization of targeted therapies like olutasidenib. Strategic partnerships and collaborations are becoming more common as companies seek to leverage each other’s strengths in research, development, and market access. These alliances are crucial for accelerating the drug’s availability and expanding its reach.
Opportunities in the Olutasidenib Market
Expanding Indications: While olutasidenib is currently approved for specific indications, there is significant potential for expanding its use. Ongoing research is exploring its effectiveness in additional cancer types and stages. Successfully gaining approval for these new indications could substantially increase the drug's market share.
Global Market Expansion: As the drug continues to demonstrate its efficacy, there is a growing opportunity for global market expansion. Different regions may have varying rates of IDH1-mutant cancers, which could influence the drug's market dynamics. Companies that successfully navigate regulatory pathways in diverse markets will have a competitive advantage.
Personalized Medicine: The rise of personalized medicine is a key factor driving the olutasidenib market. With a focus on precision oncology, treatments are increasingly tailored to the genetic profiles of individual patients. Olutasidenib’s targeted approach aligns well with this trend, offering the potential for more effective and personalized cancer treatment.
Olutasidenib Market Segments
by Type
Generic Olutasidenib
Branded Olutasidenib
by Distribution Channel
Hospital Pharmacies
Retail Pharmacies
Online Pharmacies
Olutasidenib Market Key Market Players
AbbVie Inc.
Olutasidenib Industry: Regional Analysis
North America Market Forecast
With a Olutasidenib Market share of more than 47%, North America leads the global olutasidenib industry in terms of revenue generation. Because of its sophisticated healthcare system and large R&D expenditures, North America is one of the top markets for olutasidenib. Novel treatments are highly sought for due to the high incidence of acute myeloid leukemia (AML) and the large number of clinical studies carried out in the United States and Canada. Market potential is further enhanced by this region's established healthcare infrastructure and emphasis on innovative treatments.
European Market Data
Europe's market for olutasidenib is bolstered by its extensive clinical research endeavors and strong healthcare infrastructure. Demand is increased by the high cancer incidence rates and favorable regulatory regimes found in nations like Germany, France, and the UK. The potential for Olutasidenib's expansion in Europe is enhanced by the region's investment in cancer research and development, as well as the enhancement of patient access to innovative medicines.
Challenges and Considerations
High Development Costs: Developing and bringing a novel drug to market involves substantial investment. The costs associated with clinical trials, regulatory approvals, and market access can be significant. Companies must carefully manage these expenses to ensure a favorable return on investment.
Competition and Market Saturation: The oncology market is highly competitive, with numerous therapies targeting similar pathways. As more drugs enter the market, olutasidenib will need to demonstrate clear advantages in efficacy and safety to maintain its competitive edge.
Regulatory Hurdles: Navigating the regulatory landscape can be complex and time-consuming. Companies must adhere to rigorous standards to gain approval for new indications and international markets. Successfully overcoming these regulatory hurdles is essential for maximizing market potential.
Olutasidenib and Clinical Trials
Before its approval, Olutasidenib Market underwent rigorous clinical trials to evaluate its safety and efficacy. These studies demonstrated promising results in terms of:
Response Rate: A significant number of patients experienced complete or partial remission of their AML.
Survival: Patients treated with olutasidenib showed improved overall survival compared to standard treatment options.
Key benefits of olutasidenib include:
Targeted therapy: Specifically addresses the underlying genetic cause of AML in patients with the IDH1 mutation.
Oral administration: Convenient and patient-friendly compared to traditional intravenous chemotherapy.
Improved outcomes: Demonstrated effectiveness in clinical trials, leading to increased response rates and improved survival.
Future Outlook
The future of the olutasidenib market appears promising, with ongoing research and development efforts poised to enhance its therapeutic potential. As the drug continues to show efficacy in clinical trials and expands its indications, it is likely to become a cornerstone of treatment for IDH1-mutant cancers. Additionally, advancements in personalized medicine and global market expansion will further drive growth.
Conclusion,
Olutasidenib represents a significant advancement in targeted cancer therapy. Its market trajectory will be shaped by continued research, strategic partnerships, and the evolving landscape of oncology. For investors, healthcare professionals, and patients alike, the Olutasidenib Market offers a glimpse into the future of personalized and effective cancer treatment.
0 notes
Text
Tibsovo Available in Afghanistan | Bangladesh | Bhutan| India| Maldives| Nepal| Pakistan and Sri Lanka|
Brand Name – Tibsovo
Active Ingredient – Ivosidenib
Strength – 250 mg
Originator Name/Marketing-authorisation Holder – Servier Pharmaceuticals LLC
What is Tibsovo?
TIBSOVO (ivosidenib) is an isocitrate dehydrogenase 1 (IDH1) enzyme inhibitor. The drug Ivosidenib is a small molecule inhibitor that targets the mutant isocitrate dehydrogenase 1 (IDH1) enzyme.
What is the use of Tibsovo?
The medicine ivosidenib is used to treat acute myeloid leukemia (AML), relapsed or refractory acute myeloid leukemia and locally advanced or metastatic cholangiocarcinoma.
Dosage and Administration of Tibsovo
Recommended Dosage
Newly diagnosed AML
The recommended dosage of ivosidenib is 500 mg taken orally once a day until disease progression or unacceptable toxicity.
One should start ivosidenib administration on Cycle 1 Day 1 in combination with azacitidine 75 mg/m2 subcutaneously or intravenously once a day on Days 1-7 of each 28-day cycle.

Relapsed or refractory AML
The recommended dosage of ivosidenib 500 mg taken orally once a day until disease progression or unacceptable toxicity.
Cholangiocarcinoma
The recommended dosage of ivosidenib is 500 mg taken orally once a day until disease progression or unacceptable toxicity.
Instruction for Administration
Administer ivosidenib with or without food. Do not administer TIBSOVO tablets with a high-fat meal because of increased ivosidenib concentration. Do not split, crush, or chew TIBSOVO tablets. Administer Ivosidenib tablets orally at about the same time each day. If a dose of TIBSOVO is missed or not taken at the usual time, one should administer the dose as soon as possible and a minimum 12 hours before the next scheduled dose. Return to the normal schedule the next day. One should not administer 2 doses within 12 hours.
Side Effects of Tibsovo
In the case of AML
changes in certain blood cell counts
diarrhea
increased blood sugar
changes in certain liver function tests
inflammation in arms or legs
fatigue
levels of electrolytes in the blood
nausea
vomiting
reduced appetite
joint pain
Precautions & Warnings
Differentiation Syndrome in AML
Patients with newly diagnosed AML and patients with relapsed or refractory AML treated with ivosidenib experienced differentiation syndrome. Differentiation syndrome is associated with the rapid spreading and differentiation of myeloid cells and may be fatal if not treated.
If severe signs and symptoms persist for more than 48 hours after initiating corticosteroids, interrupt ivosidenib until signs and symptoms are no longer fatal.
QTc Interval Prolongation
Patients treated with Ivosidenib tablets can develop QT (QTc) prolongation [see Clinical Pharmacology and ventricular arrhythmias. One should frequently monitor may be necessary for patients with congenital long QTc syndrome, congestive heart failure, electrolyte abnormalities, or those taking medications that prolong the QTc interval. One should permanently discontinue Ivosidenib in patients who develop QTc interval prolongation with symptoms of life-threatening arrhythmia.
Guillain-Barré syndrome
Patients who are treated with Ivosidenib could develop Guillain-Barré syndrome. Monitor patients taking TIBSOVO for the onset of new signs or symptoms of motor and sensory neuropathy, such as unilateral or bilateral weakness, difficulty breathing or sensory alterations, paresthesias. One should permanently discontinue Ivosidenib in patients who are diagnosed with GuillainBarré syndrome.
Handling and Storage of Tibsovo
Store ivosidenib at room temperature between 68°F to 77°F (20°C -25°C).
Keep ivosidenib and all medicines out of the reach of children.
Frequently Asked Questions (FAQ)
What is Tibsovo approved for?
The medicine Tibsovo is approved for the treatment of Acute Myeloid Leukemia and Locally Advanced or Metastatic Cholangiocarcinoma.
Is Tibsovo FDA approved for AML?
Yes, Tibsovo was approved by the FDA in 2018 for Acute Myeloid Leukemia (AML) and Locally Advanced or Metastatic Cholangiocarcinoma.
What is the mechanism of Tibsovo?
The drug, Ivosidenib is a small molecule inhibitor that targets the mutant isocitrate dehydrogenase 1 (IDH1) enzyme.
How to import a Tibsovo 250 mg tablet in India?
For medicine, Tibsovo Servier is the innovator, and Ikris Pharma Network is an authorized partner of it, who can help access this medicine in India for the patient through legal procedures.
Where can I buy Tibsovo medicine?
In India, you can buy Tibsovo with the help of Ikris Pharma Network, an authorized partner of “Servier” for this medicine.
Tibsovo Introduction
Tibsovo (Ivosidenib) was approved by the FDA in 2018 and Ivosidenib is an isocitrate dehydrogenase-1 (IDH1) inhibitor indicated for the treatment of adult patients of Acute Myeloid Leukemia (AML) and Locally Advanced or Metastatic Cholangiocarcinoma. Ivosidenib is a small molecule inhibitor that targets the mutant isocitrate dehydrogenase 1 (IDH1) enzyme.
Tibsovo at a glance
Tibsovo Tablets approved in multiple countries..
Tibsovo was first approved in the United States.
Commercialized by Servier Pharmaceuticals LLC.
Approved for Acute Myeloid Leukemia and Cholangiocarcinoma.
Servier Pharmaceuticals is providing innovative therapeutics to patients with serious unmet medical requriements. They have approved products for Acute Myeloid Leukemia (AML) and Cholangiocarcinoma.
Ikris Pharma Network is authorized by Servier Pharmaceuticals to provide access to their drugs to Indian patients through Named-patient Import.
#Tibsovo available in Afghanistan#tibsovo price in india#tibsovo 250 mg#buy tibsovo tablets from india#Tibsovo in Saudi Arabia and the United Arab Emirates#tibsovo in india#tibsovo supplier in delhi#Tibsovo in nepal#Tibsovo price in india
0 notes
Text
Leukemia Therapeutics Market Size: Competitive Landscape Analysis

The Leukemia Therapeutics Market size was USD 16 Billion in 2023 and is expected to Reach USD 27.28 Billion by 2031 and grow at a CAGR of 6.9% over the forecast period of 2024-2031.The leukemia therapeutics market is experiencing rapid growth, driven by advancements in targeted therapies and personalized medicine. With an increasing understanding of the genetic and molecular mechanisms underlying various leukemia subtypes, pharmaceutical companies are developing innovative treatments that offer improved efficacy and reduced side effects. The market is also benefiting from a surge in research and development activities, coupled with supportive regulatory frameworks that expedite the approval process for novel drugs. Furthermore, the rise in leukemia prevalence, along with heightened awareness and early diagnosis, is expanding the patient pool eligible for these cutting-edge therapies. This dynamic landscape presents significant opportunities for stakeholders, ranging from biotech startups to established pharmaceutical giants, to make impactful contributions to leukemia care and patient outcomes.
Get Sample of This Report @ https://www.snsinsider.com/sample-request/3135
Market Scope & Overview
The market report gives thorough information to help readers comprehend the current situation of the industry. The report relied on primary and secondary research, as well as private databases and a paid data source. The market research report sheds light on the history and future of the Leukemia Therapeutics Market business. The report analyses the market size and includes information on market drivers, limitations, and opportunities.
The market research report examines the primary strategies used by top industry players to advance their operations in the worldwide market while maintaining a competitive advantage over their competitors. The Leukemia Therapeutics Market study also depicts the competitive landscape of the industry's major competitors, as well as the top organizations’ percentage market share.
Market Segmentation Analysis
By Type
Chronic Lymphocytic Leukemia
Acute Lymphocytic Leukemia
Chronic Myeloid Leukemia
Acute Myeloid Leukemia
Others
By Drug Class
Targeted Therapy
Chemotherapy and Immunotherapy
By Distribution channel
Hospital Pharmacies
Online Providers
Drug Store
Retail Pharmacies
COVID-19 Pandemic Impact Analysis
In this Leukemia Therapeutics Market analysis, the impact of COVID-19 on the market is analyzed at both the global and country levels. According to this analysis, the COVID-19 pandemic and the post-pandemic phase had a significant impact on both the supply and demand sides of the market.
Regional Outlook
The report's accurate data will help market participants make informed decisions about regional expansions and investments. The report looks at the dynamics of the Leukemia Therapeutics Market in relation to worldwide market conditions.
Competitive Analysis
The data in this section will assist readers in understanding the primary strategies used by leading market players to control the global Leukemia Therapeutics Market . The research report includes a PORTER, SVOR, and PESTEL analysis, as well as an examination of the potential impact of microeconomic market variables. External and internal elements that are projected to have an impact on the company have been studied, presenting decision-makers with a clear picture of the industry's future.
Key Questions Answered in the Leukemia Therapeutics Market Report
What is the forecasted growth rate, market share, and m market size?
Who are the market's dominant players, and how have they gained a competitive advantage over their competitors?
Conclusion
The Leukemia Therapeutics Market research report's estimations and estimates examine the impact of different political, social, and economic factors, as well as current market conditions, on market growth. All of this important information will assist the reader in better understanding the market.
About Us
SNS Insider is a market research and insights firm that has won several awards and earned a solid reputation for service and strategy. We are a strategic partner who can assist you in reframing issues and generating answers to the trickiest business difficulties. For greater consumer insight and client experiences, we leverage the power of experience and people.
When you employ our services, you will collaborate with qualified and experienced staff. We believe it is crucial to collaborate with our clients to ensure that each project is customized to meet their demands. Nobody knows your customers or community better than you do. Therefore, our team needs to ask the correct questions that appeal to your audience in order to collect the best information.
Related Reports
Protein Engineering Market Share
AI in Cancer Diagnosis Market Share
Blood Group Typing Market Share
Immunomodulators Market Share
Lymphoma Treatment Market Share
0 notes
Text
Global Leukemia Therapeutics Market, Forecast 2027
Leukemia Therapeutics Market
Leukemia is a type of blood cancer caused by gene mutation leading to abnormal development of WBCs in the bone marrow and causes leukemia. Leukemia is also caused due to risk factors such as genetic disorders such as Down syndrome, previous cancer treatments, exposure to chemicals, smoking, and family history of leukemia or other blood disorders. The major types of leukemia are Acute Myeloid Leukemia (AML), Chronic Myelogenous Leukemia (CML), and Chronic Lymphocytic Leukemia (CLL), Acute Lymphocytic Leukemia (ALL). The majority of the AML, CML & CLL cases could be observed in adult populations, and ALL could be seen in all age groups. Leukemia treatment is based on its type, age, and ability to cope-up with the treatments.
Market Size & Growth Rate:
The leukemia therapeutics market was valued at USD 13.5 billion in 2021 and is projected to reach USD 17.1 billion by 2027, with a CAGR of 7.2% over the forecast period 2022-2027. The growth could be attributed to the return of demand to pre-pandemic levels, increasing authorization of novel & innovative medicines, extensive research, disease prevalence, and immunotherapies.
Click here for full report:
https://www.pharmanucleus.com/reports/leukemia-therapeutics-market
Market Dynamics:
On account of the growing number of people suffering from leukemia, the leukemia therapeutics treatment market has been driven by technological research & developments, the merging of healthcare organizations and new approvals in the field of blood cancer diagnostics across the globe.
The Leukemia & Lymphoma Society Therapy Acceleration Program (LLS TAP)-April 2021, announced five new investments in increasing the development of new and improved immunotherapies in Abintus Bio, Nexlmmune, Caribou Biosciences and Immune-Onc Therapeutics for the treatment of leukemia cancer.
Moreover, raising public awareness about the benefits of preventive healthcare is likely to move the demand for leukemia therapeutics treatment forward throughout the forecast period. Additionally, the Government initiatives and programs at increasing cancer awareness are expected to drive leukemia therapeutics treatment market expansion until 2027.
Market Drivers:
The growing research and technological development activities.
Increasing product approval and product launch by the market players are expected to propel the leukemia therapeutics market, and also technological developments are likely to create various new opportunities that will impact this leukemia therapeutics market growth in the forecast period. For instance,
In May 2022, Ivosidenib (Tibsovo, Servier Pharmaceuticals LLC) received FDA approval for combination with azacitidine in newly diagnosed AML with a susceptible IDH1 mutation in adults 75 years and older.
On June 2021, Jazz Pharmaceuticals received FDA approval for a new form of Rylaze (asparaginase erwinia chrysanthemi recombinant rywn) to be developed as part of a treatment for children and adults with ALL
In October 2021, Kite Pharma received FDA approval for use of the CAR T-cell therapy brexucabtagene autoleucel developed by Tecartus (the first CAR T-cell therapy approved) in adults with B-cell precursor ALL
In November 2018, Pfizer Inc. received U.S. FDA approval for Glasdegib (DAURISMO), the Hedgehog pathway inhibitor for the treatment of adults suffering from AML
Increasing incidence of leukemia to boost leukemia therapeutics market growth.
One of the foremost driving factors influencing the global market is the increasing prevalence of leukemia across the globe, according to the National Cancer Institute (NCI), approximately 1.5% of people were diagnosed with leukemia during their lifetime. Additionally, in the report-2022, the estimated number of living people with new leukemia cases will be 60,650, and the number of death cases will be 24,000 in the United States.
According to the American Cancer Society-2022, the CLL segment is anticipated 20,160 diagnosis cases including both children & adults in US leukemia therapeutic market, as CLL is the first line of treatment for it followed by AML and CML.
Click here for full report:
Get more details on this report - Request Free Sample
Substantial progress has been made against cancer in recent decades. As of 2019, the rate has declined by 32%, mostly because of advances in early detection and treatment for some cancers including leukemia. It reflects largely driven by progress against leukemia cancer.
Challenges:
The high cost of treatment and stringent regulatory scenario restrain the market growth.
High cost of treatment and stringent regulatory scenario restraint the market growth over the forecast period. For instance, the NCI-2022 report estimates that cancer-related direct medical costs in the US were USD 183 billion in 2015 and are projected to increase to USD 246 billion by 2030, a 34% increase based only on population growth and aging.
The low diagnosis rate of leukemia & lack of proper healthcare facilities in developing countries
Adverse reactions of leukemia therapeutics
Competitive landscape:
Novartis International AG, Bristol Myers Squibb, Sanofi S.A, Pfizer Inc., Amgen Inc., Takeda Pharmaceutical, Celgene Corporation, GlaxoSmithKline plc, Abintus Bio, Nexlmmune, Exelixis Inc., MorphoSys AG, Otsuka Holdings Company Ltd, AbbVie, Ambit Biosciences corporation, Ariad Pharmaceuticals, Gennzme Corporation, Rpche, and Others.
Key Developments:
Get more details on this report - Request Free Sample
Regional Analysis:
North America was the largest region in the leukemia therapeutics market in recent years and it is anticipated to capture the highest share of this market over the forecast period 2022-2027, with the U.S. accounting for the maximum contribution and it has well-developed structural healthcare systems. In addition, the high prevalence of leukemia and the rising geriatric population, based on the NCI-2022 report, leukemia represents 3.2% of all new cancer cases in the U.S., and the incidence rate increased in children and adolescents by about 1% per year and was stable in adults ages 20 and older. The presence significant of key players, some of the best research universities, and encouraging new product developments & launches for research lead to more significant assets in the pipeline. For instance,
In October 2022, The American Cancer Society (ACS) approved funding for 89 new Extramural Discovery Science (EDS) research grants totaling USD 54.3 million. It will fund investigators at 65 institutions across the United States from January 1, 2023.?
In November 2021, Pfizer Company acquired Trillium Therapeutics for approximately USD 2.22 billion, to enhance its oncology portfolio with the addition of next-generation immune therapies.
#Leukemia Therapeutics Market#leukemia care#global therapeutics market#innovative treatments#groundbreaking developments
0 notes
Text
Acute Myeloid Leukemia Market
#Acute Myeloid Leukemia Marketscope#Acute Myeloid Leukemia Market report#Acute Myeloid Leukemia Market share
0 notes
Text
Hematopoietic Stem Cells Transplantation Market 2023-2035 to Grow at a CAGR of ~15% Driving Factors, Size, Revenue, Segments, Expansion, Demand
Global Hematopoietic Stem Cells Transplantation Market Key Insights
During the forecast period of 2023-2035, the global hematopoietic stem cells transplantation market is expected to reach an estimated value of ~USD 6,000 billion by 2035, by expanding at a CAGR of ~15%. The market further generated a revenue of ~USD 2,500 million in the year 2022. Major key factors propelling the growth of hematopoietic stem cells transplantation market worldwide are the increasing prevalence of cancer and the rising prevalence of hematopoietic stem cell transplantation.
Market Definition of Hematopoietic Stem Cells Transplantation
Hematopoietic stem cell transplantation is a process of sourcing healthy multipotent stem cells for replacing the old degenerative cells of the body. After the transplantation of healthy hematopoietic stem cells in the patient’s body, they produce red blood cells, white blood cells, and platelets to repair and replace the patient’s hematopoietic system. The procedure is done to cure a disease, such as cancer, or bone marrow failure, and even in some cases to change the gene pattern. These cells are sourced from the umbilical cord of a baby, peripheral blood cells, and matching bone marrow.
Request Free Sample Copy of this Report @ https://www.researchnester.com/sample-request-3937
Global Hematopoietic Stem Cells Transplantation Market: Growth Drivers
The growth of the global hematopoietic stem cells transplantation market can majorly be attributed to the higher number of people suffering from cancer and the deaths attributed to cancer, followed by the growing adoption of hematopoietic stem cells transplantation. According to the World Health Organization, in 2020, around 10 million deaths were caused by cancer and it was known as the leading cause of death in the world. Moreover, every year, around 400,000 children develop cancer in the world. Furthermore, as of 2020, in the world, around 2 million hematopoietic cell transplants have been carried out in the world and there are around 1,500 transplantation centers are set up in the world. On the other hand, the market growth can also be attributed to the rising need for kidney transplantation. In order to stay alive, around 2 million people in the world need dialysis and kidney transplantation.
The global hematopoietic stem cells transplantation market is also estimated to grow majorly on account of the following:
Rising Cases of Cancer
Number of People Suffering from Organ Failure
Rise in Research and Development on Stem Cells
Increasing Number of Hematopoietic Stem Cells Transplant
Development of Customized Drugs
Request for customization @ https://www.researchnester.com/customized-reports-3937
Global Hematopoietic Stem Cells Transplantation Market: Restraining Factor
Hematopoietic stem cells transplantation is also known to be associated with side effects to the heart, lungs, mouth, and others. Besides this, the cost of the treatment is very high and, in many places, there is a shortage of skilled experts who can perform the transplantation. Hence, these factors are expected to be the major hindrance to the growth of the global hematopoietic stem cells transplantation market during the forecast period.
Global Hematopoietic Stem Cells Transplantation Market Segmentation
By Transplant Type (Allogenic, and Autologous)
By Disease Type (Acute Myeloid Leukemia, Acute Lymphoblastic Leukemia, Hodgkin Lymphoma, Multiple Myeloma, and Others)
The multiple myeloma segment, amongst all the other segments, is anticipated to garner the largest revenue by the end of 2035. The growth of the segment can be attributed to the growing cases of multiple myeloma in the world and the surge in use of stem cell transplantation to treat multiple myeloma. In 2020, there were 131,518 cases of multiple myeloma for every 100,000 people, according to the IARC database. Furthermore, in the United States, around 34,000 adults are suffering from multiple myeloma. Moreover, nearly 13,000 people die of the disease in the nation, and approximately 120,000 deaths were reported worldwide in 2020.
By Application (Bone Marrow Transplant (BMT), Peripheral Blood Stem Cells Transplant (PBSCT), Cord Blood Transplant (CBT), and Others)
By Region
The North America hematopoietic stem cells transplantation market is anticipated to hold the largest market share by the end of 2035 among the market in all the other regions. The market size of North America is expected to grow on the account of rising cases of cancer and a higher number of transplants carried out in the region. The number of organ transplants carried out in the United States increased to 41,354 in 2021. It is approximately 6% higher than the previous higher. Furthermore, in 2022, around 61,000 new cases of leukemia were reported in the United States, and nearly 24,000 deaths were attributed to it.
Know More About the Complete Study @ https://www.researchnester.com/reports/hematopoietic-stem-cells-transplantation-market/3937
The market research report on global hematopoietic stem cells transplantation also includes the market size, market revenue, Y-o-Y growth, and key player analysis applicable for the market in North America (U.S., and Canada), Latin America (Brazil, Mexico, Argentina, Rest of Latin America), Asia-Pacific (China, India, Japan, South Korea, Singapore, Indonesia, Malaysia, Australia, New Zealand, Rest of Asia-Pacific), Europe (U.K., Germany, France, Italy, Spain, Hungary, Belgium, Netherlands & Luxembourg, NORDIC (Finland, Sweden, Norway, Denmark), Ireland, Switzerland, Austria, Poland, Turkey, Russia, Rest of Europe), and Middle East and Africa (Israel, GCC (Saudi Arabia, UAE, Bahrain, Kuwait, Qatar, Oman), North Africa, South Africa, Rest of Middle East and Africa).
Key Market Players Featured in the Global Hematopoietic Stem Cells Transplantation Market
Some of the key players of the global hematopoietic stem cells transplantation market are Escape Therapeutics, Inc., Regen BioPharma, Inc., ThermoGenesis Holdings, Inc., Lonza Group Ltd., CSG-BIO Company, Inc., CBR Systems, Inc., Pluristem Inc., Global Cord Blood Corporation, ViaCord, LLC, Cynata Therapeutics Limited, and others.
About Research Nester
Research Nester, which is a leading service provider for strategic market research and consulting services, aims to provide unbiased, unparalleled market insights and industry analysis. These analyses help conglomerates, executives, and industries to take wise decisions for their businesses as well as for their future marketing strategy, expansion and investment among others. We believe that our expertise in the field of market research can help businesses to expand to its new horizon. Our team of research analysts can provide businesses a right guidance at the right time, while our out of box thinking helps our clients to take wise decision in order to avoid future uncertainties.
Contact for more Info:
AJ Daniel
Email: [email protected]
U.S. Phone: +1 646 586 9123
U.K. Phone: +44 203 608 5919
#Hematopoietic Stem Cells Transplantation Market#Hematopoietic Stem Cells Transplantation#Hematopoietic Stem Cells Transplantation Market share
0 notes
Text
Global Dasatinib Drugs Market Is Estimated To Witness High Growth Owing To Increasing Demand for Targeted Cancer Therapies
The global Dasatinib Drugs Market is estimated to be valued at US$ 4.35 billion in 2023 and is expected to exhibit a CAGR of 6.0% over the forecast period (2023-2030), as highlighted in a new report published by Coherent Market Insights.
Market Overview:
Dasatinib is an oral medication used for the treatment of chronic myeloid leukemia (CML) and Philadelphia chromosome-positive acute lymphoblastic leukemia (Ph+ ALL). It is classified as a tyrosine kinase inhibitor and works by blocking the action of abnormal proteins that signal the growth of cancer cells. The advantages of dasatinib include its high efficacy in treating CML, ability to target specific cancer cells, and reduced side effects compared to traditional chemotherapy. The increasing incidence of CML and the demand for targeted cancer therapies are driving the growth of the dasatinib drugs market.
Market Key Trends:
One key trend in the dasatinib drugs market is the growing focus on precision medicine. Precision medicine aims to tailor treatment strategies based on a patient's genetic makeup, lifestyle, and environmental factors. Dasatinib, being a targeted therapy, aligns with the principles of precision medicine by selectively inhibiting specific cancer cells, thereby reducing the likelihood of adverse effects. For example, research studies have identified specific mutations in the BCR-ABL gene, which can affect the response to dasatinib treatment. This trend emphasizes the importance of personalized therapies and molecular testing in optimizing patient outcomes.
PEST Analysis:
- Political: Government regulations and policies related to drug approvals, intellectual property rights, and reimbursement can significantly impact the availability and affordability of dasatinib drugs.
- Economic: The cost-effectiveness of dasatinib drugs compared to other treatment options, as well as healthcare expenditure and insurance coverage, can influence market growth.
- Social: The increasing prevalence of cancer, especially CML, and the awareness and acceptance of targeted therapies among patients and healthcare professionals are driving market demand.
- Technological: Advances in genetic testing technologies and biomarker identification are enabling the identification of patients who are likely to benefit the most from dasatinib treatment.
Key Takeaways:
1: The Global Dasatinib Drugs Market Demand is expected to witness high growth, exhibiting a CAGR of 6.0% over the forecast period, due to increasing demand for targeted cancer therapies. Dasatinib's efficacy in treating CML and its ability to selectively target specific cancer cells position it as a preferred treatment option.
2: In terms of regional analysis, North America is expected to dominate the dasatinib drugs market due to the high prevalence of CML in the region and the well-established healthcare infrastructure. Asia Pacific is projected to be the fastest-growing region, driven by the increasing incidence of CML and the rising adoption of targeted therapies.
3: Key players operating in the global dasatinib drugs market include Bristol-Myers Squibb Company, Novartis International AG, Teva Pharmaceutical Industries Ltd., Sun Pharmaceutical Industries Ltd., Pfizer Inc., Cipla Ltd., Hetero Drugs Limited, Natco Pharma Limited, Dr. Reddy's Laboratories Ltd., and Aurobindo Pharma Ltd. These companies focus on research and development activities to develop innovative therapies and expand their product portfolios.
#Dasatinib Drugs Market#Dasatinib Drugs Market Outlook#Dasatinib Drugs Market Insights#Dasatinib Drugs Market Forecast#lymphoblastic leukemia#chronic myeloid leukemia#cancer cells#Dasatinib#chemotherapy drug#coherent market insights
0 notes
Text
The Transformative Growth of the US Acute Myeloid Leukemia Market
Buy Now
What is the Size of US Acute Myeloid Leukemia Industry?
US Acute Myeloid Leukemia Market is expected to grow at a CAGR of ~ % in 2022 and is expected to reach ~USD Mn by 2028. The US Acute Myeloid Leukemia market is the rapid advancement in precision medicine and targeted therapies. The emergence of innovative treatments tailored to the genetic and molecular characteristics of individual AML patients has transformed the treatment landscape. Targeted therapies, such as FLT3 and IDH inhibitors, capitalize on specific genetic mutations driving AML, resulting in higher response rates and improved outcomes compared to traditional approaches.
Precision medicine's ability to identify the most effective treatment for each patient not only enhances therapeutic efficacy but also reduces unnecessary treatments, mitigating side effects and improving overall quality of life. This paradigm shift is bolstered by technological progress in molecular profiling techniques like next-generation sequencing, allowing for accurate genetic profiling and treatment customization.
Furthermore, regulatory support and accelerated approval pathways for targeted therapies have expedited their availability to patients. Pharmaceutical companies' increasing investment in research and development of novel agents, along with collaborations between academia and industry, leads to grow this market even more.

Click here to Download a Sample Report
US Acute Myeloid Leukemia Market by treatment type
The market is segmented by Treatment type into Chemotherapy, Targeted Therapy, Hormone Therapy, Immunotherapy and Others. Targeted Therapy is the dominant segment in the US Acute Myeloid Leukemia market in 2022. Specialty Centers, dedicated to the diagnosis, treatment, and management of specific medical conditions like AML, offer a concentrated and specialized approach to patient care. Their dominance in the AML market is driven by their unique capabilities to provide comprehensive and tailored treatments that address the complex needs of AML patients.
US Acute Myeloid Leukemia Market by end -user industry
The market is segmented by End-User into Hospitals, Homecare, Specialty Centers, Pharmacies and Others. Among these, Specialty Centers are the dominant End-User in the US Acute Myeloid Leukemia market in 2022. Specialty centers have a higher level of expertise and experience in treating specific types of cancer, including acute leukemia. They typically have specialized medical professionals, including hematologists and oncologists, who are well-versed in the latest treatment protocols and research. These centers are equipped with state-of-the-art diagnostic and treatment facilities that are specifically tailored to the needs of leukemia patients. This can include advanced imaging technologies, specialized laboratories, and access to cutting-edge therapies.
Click here to Download a Custom Report
US Acute Myeloid Leukemia companies market by Region
The US Acute Myeloid Leukemia market is segmented by Region into North, South, East, West. The dominance region is North in the US Acute Myeloid Leukemia Market in 2022. the North region often benefits from a higher level of funding for medical research, including AML. Federal funding agencies, private foundations, and pharmaceutical companies frequently channel resources into institutions within this region, allowing for greater research capabilities and clinical trials infrastructure.
Competition Scenario in US Acute Myeloid Leukemia Market
The US acute myeloid leukemia market was characterized by the presence of several prominent pharmaceutical companies and biotechnology firms striving to establish their presence and gain a competitive edge.
Larger corporations, smaller biotechnology companies making significant contributions to the AML landscape. Companies like Agios Pharmaceuticals and Daiichi Sankyo were gaining attention for their novel therapies targeting AML-associated mutations. These players are often at the forefront of introducing precision medicine approaches to AML treatment, taking advantage of advancements in genetic profiling and molecular diagnostics.
The competition is further intensified by research collaborations and partnerships between pharmaceutical companies, academic institutions, and research organizations. These collaborations aimed to combine resources, expertise, and insights to accelerate the development of effective therapies.
What is the Expected Future Outlook for the Overall US Acute Myeloid Leukemia market?
The US Acute Myeloid Leukemia market was valued at USD ~Million in 2022 and is anticipated to reach USD ~ Million by the end of 2028, witnessing a CAGR of ~% during the forecast period 2022- 2028. Pharmaceutical companies have been actively researching and developing novel therapies for Acute Myeloid Leukemia, with a focus on improving patient outcomes and reducing the side effects associated with traditional treatments like chemotherapy. The approval of new drugs, such as FLT3 and IDH inhibitors, has offered fresh therapeutic avenues for specific subsets of Acute Myeloid Leukemia patients with genetic mutations.
The future outlook for the US Acute Myeloid Leukemia market is anticipated to be influenced by a combination of factors including advancements in treatment options, evolving regulatory landscapes, and a growing understanding of the molecular basis of Acute Myeloid Leukemia. The market has been witnessing a shift towards personalized medicine, with increasing emphasis on targeted therapies and precision medicine approaches.
Furthermore, the integration of innovative technologies like next-generation sequencing (NGS) has enhanced our understanding of Acute Myeloid Leukemia’s molecular complexities, allowing for better patient stratification and treatment selection. This trend toward molecular profiling and personalized treatment regimens is likely to continue shaping the market landscape.
However, challenges persist. Despite progress, Acute Myeloid Leukemia remains a difficult-to-treat disease with a high relapse rate. Overcoming drug resistance and developing effective strategies for patients who do not respond well to existing therapies remain critical areas of focus.
Additionally, the cost of novel therapies and access to these treatments could also impact their adoption and availability. The regulatory environment, including expedited pathways for drug approvals, continue to influence market dynamics.
#US Acute Myeloid Leukemia Market Analysis#US Acute Myeloid Leukemia Industry#US Acute Myelogenous Market#US Bone Marrow Leukemia Industry#United States Acute Myeloid Leukemia Market#US Acute Myeloid Leukemia Market size#US Acute Myeloid Leukemia Market share#US Acute Myeloid Leukemia Market trends#US Acute Myeloid Leukemia Market growth#Chemotherapy in Acute Myeloid Leukemia Market US#Targeted Therapy in Acute Myeloid Leukemia Market US#Hormone Therapy in Acute Myeloid Leukemia Market US#End Users in US Acute Myeloid Leukemia Market#Specialty Centers in US Acute Myeloid Leukemia Market#Hospitals for Acute Myeloid Leukemia in US#Challenges in US Acute Myeloid Leukemia Market#Major Players in US Acute Myeloid Leukemia Market#Emerging Players in US Acute Myeloid Leukemia Market#US Acute Myeloid Leukemia Market Future Outlook#US Acute Myeloid Leukemia Market Growth Rate#leading companies US Acute Myeloid Leukemia Market#US Acute Myeloid Leukemia Market competitors#US Acute Myeloid Leukemia Market forecast#US Acute Myeloid Leukemia Market demand#US Acute Myeloid Market Challenges#Myelogenous Leukemia Opportunities US#Acute Leukemia Challenges US#Opportunities Myeloid Leukemia Market US#Leading Leukemia Pharmaceutical companies US#Major Competitors US Acute Leukemia Market
0 notes
Text
Isocitrate Dehydrogenase (IDH) Inhibitors Market Growth, Trends Analysis Report 2033
The market for isocitrate dehydrogenase (IDH) inhibitors worldwide has a market value of US$ 1.7 billion in 2022 and is anticipated to grow at a CAGR of 33% from 2023 to 2033 to reach US$ 39.16 billion.
Additionally, the development of more potent and selective IDH inhibitors, as well as the expansion of their use into other cancer types, is expected to further contribute to the growth of the market. Bayer and Agios Pharmaceuticals are among the key players in the IDH inhibitors market, with their respective drugs BAY 1436032 and ivosidenib being approved by the US FDA for the treatment of IDH-mutated cancers. These drugs have shown promising results in clinical trials, including high response rates and extended survival in patients with AML.
The IDH inhibitors market is a rapidly growing field with significant potential for the treatment of several types of cancer. Continued research and development efforts, as well as the development of new drugs and treatment strategies, are expected to contribute to the growth of the market.
Get a PDF Sample with Latest Market Insights @
https://www.futuremarketinsights.com/reports/sample/rep-gb-16847
Key Takeaways from the Market Study
The global Isocitrate dehydrogenase (IDH) inhibitors market is expected to grow with a 33% CAGR during 2023 to 2033.
Oral route of administration is expected to hold 49% of the market share in 2023 for Isocitrate dehydrogenase (IDH) inhibitors market.
North America is expected to possess 46% market share for Isocitrate dehydrogenase (IDH) inhibitors market in 2023.
Europe Isocitrate dehydrogenase (IDH) inhibitors market size is expected to possess 43% market share in 2023.
“The development of combination therapies and alternative treatment strategies is being explored to further optimize the clinical benefit of IDH inhibitors. This, in turn, is fueling the growth of IDH inhibitors market.” states an FMI analyst
Competitive Landscape
Key players in the isocitrate dehydrogenase (IDH) inhibitors market are Bayer, Agios Pharma, Daiichi Sankyo, Ohm Oncology, Celgene, Philogen S.p.A., Tragara, Aslan Pharmaceuticals, Pfizer, Inc. and Sun Pharmaceutical Industries Ltd.
Bayer is collaborating with other pharmaceutical companies, such as Agios Pharmaceuticals, in the development of IDH inhibitors. The two companies have a joint development and commercialization agreement for ivosidenib (AG-120), an oral small molecule inhibitor of the mutated IDH1 enzyme, which has been approved by the US FDA for the treatment of relapsed or refractory acute myeloid leukemia (AML) with an IDH1 mutation.
Agios’ Pharma lead IDH inhibitor candidate is ivosidenib (AG-120), an oral small molecule inhibitor of the mutated IDH1 enzyme. Ivosidenib works by inhibiting the production of the oncometabolite 2-hydroxyglutarate (2-HG) and inducing differentiation of leukemia cells. Ivosidenib has been approved by the US FDA for the treatment of relapsed or refractory acute myeloid leukemia (AML) with an IDH1 mutation.
For more Report Customization, connect with us at @
https://www.futuremarketinsights.com/customization-available/rep-gb-16847
More Valuable Insights
Future Market Insights, in its new offering, presents an unbiased analysis of the global Isocitrate dehydrogenase (IDH) inhibitors market, presenting historical analysis from 2018 to 2022 and forecast statistics for the period of 2023 to 2033.
The study reveals essential insights on the basis of Type (IDH1 Mutant Medullary Malignant Tumor, IDH2 Mutant Medullary Malignant Tumor, Peptides, Small Molecule, Others) Molecule Types (Monoclonal Antibody, Peptides, Small Molecules, Others) Route of Administration (Oral, Parenteral, Subcutaneous) Region (North America, Latin America, Europe, South Asia, East Asia, Oceania, Middle East & Africa)
About the Healthcare at Future Market Insights
The healthcare domain team at Future Market Insights offers expert analysis, time-efficient research, and strategic recommendations with an objective to provide authentic insights and accurate results to help clients worldwide. With a repertoire of over 100+ reports and 1 Billion+ data points, the team has been analyzing the industry lucidly in 50+ countries for over a decade. The team provides a brief analysis of key trends including competitive landscape, profit margin, and research development efforts.
Last few days to get reports at discounted prices, offer expires soon!
Request Discount@ https://www.futuremarketinsights.com/request-discount/rep-gb-16847
Key Segments Profiled in the Isocitrate Dehydrogenase (IDH) Inhibitors Industry Survey
Type:
IDH1 Mutant Medullary Malignant Tumor
IDH2 Mutant Medullary Malignant Tumor
Peptides
Small Molecule
Others
Molecule Types:
Monoclonal Antibody
Peptides
Small Molecules
Others
Route of Administration:
Oral
Parenteral
Subcutaneous
0 notes
Text
Tibsovo for acute myeloid leukemia-aml
Indication and Usage
Tibsovo is an isocitrate dehydrogenase-1 (IDH1) inhibitor licenced by the FDA for the treatment of patients who have a susceptible IDH1 mutation as identified by an FDA-approved test with:
Acute Myeloid Leukaemia (AML) is a type of blood cancer.
Newly diagnosed AML patients above the age of 75 or who have comorbidities that prohibit the administration of aggressive induction chemotherapy AML that has relapsed or is refractory
Cholangiocarcinoma, Locally Advanced or Metastatic
Patients with previously treated locally advanced or metastatic cholangiocarcinoma
This efficient medication is offered on the market and can be obtained from any recognised vendor. Additionally, the Tibsovo price in India is modest and highly affordable, making it simple to include it in the course of therapy.
Dosage and Administration
It is available as Tibsovo 250 mg Tablets. TIBSOVO is intended to be taken orally once daily until disease progression or intolerable toxicity occurs.
One can take Tibsovo with or without food. Because of an increase in Tibsovo concentration, do not take Tibsovo with a high-fat meal [see Warnings and Precautions (5.2) and Clinical Pharmacology. Tibsovo pills should not be divided or crushed. Take Tibsovo pills orally at the same time every day. If a Tibsovo dose is vomited, do not give a replacement dose; instead, wait until the next planned dose is due. If a Tibsovo dose is missed or not taken on time, provide it as soon as feasible and at least 12 hours before the next planned dose. The following day, resume your regular schedule. Do not take two doses within 12 hours.
Warnings and Precautions
Monitor electrocardiograms and electrolytes for QTc interval prolongation. If QTc interval prolongation occurs, reduce or suspend the dose, then resume the dose or permanently cease Tibsovo.
Guillain-Barré Syndrome: Keep an eye on patients for signs and symptoms of new motor and/or sensory abnormalities. In individuals with Guillain-Barré syndrome, quitTibsovo for good.
This potent medicine is available on the market and can be acquired from any reputable dealer. Furthermore, the Tibsovo cost in India is tiny and incredibly reasonable, making it simple to incorporate into therapy. You can reach us at: For more information, contact Ikris Pharma Network at 1800 889 1064.
Drug Interaction
Strong or moderate CYP3A4 Inhibitors: With strong CYP3A4 inhibitors, reduce TIBSOVO dosage. Patients should be monitored for an increased risk of QTc interval prolongation.
CYP3A4 Inducers: Avoid using TIBSOVO (7.1, 12.3) concurrently.
Avoid simultaneous usage with TIBSOVO (7.2, 12.3) if you have sensitive CYP3A4 substrates.
QTc Prolonging Drugs: Avoid using in conjunction with TIBSOVO. If coadministration is inevitable, patients should be monitored for an increased risk of QTc interval prolongation.
You can easily buy Tibsovo tablets from India at a lower cost from Indian Pharma Network at a more reasonable and pocket-friendly price. We are the most trustworthy and reliable generic medicine supplier in India.
Adverse Reactions
Although the price of Tibsovo is affordable, it has some following drug interactions which need to be known before using the drug. For more detail, ask your doctor.
Fatigue, arthralgia, leukocytosis, diarrhea, edema, nausea, dyspnea, mucositis, and ECG were the most prevalent side effects (20%) in AML patients. QT prolongation, rash, cough, decreased appetite, myalgia, constipation, and pyrexia are all symptoms of QT prolongation.
Hemoglobin decreased, calcium decreased, sodium decreased, magnesium decreased, uric acid increased, potassium decreased, alkaline phosphatase increased, aspartate aminotransferase increased, phosphate decreased, and creatinine increased were the most common laboratory abnormalities (20%) in AML patients.
Fatigue, nausea, stomach discomfort, diarrhea, cough, decreased appetite, ascites, vomiting, anemia, and rash were the most prevalent adverse effects (15%) in patients with cholangiocarcinoma.
The most prevalent laboratory abnormalities (10%) in individuals with cholangiocarcinoma were decreased hemoglobin, increased aspartate aminotransferase, and elevated bilirubin.
Tibsovo: Mechanism of Action
Ivosidenib is a small molecule inhibitor that works by inhibiting the mutant isocitrate dehydrogenase 1 (IDH1) enzyme. Susceptible IDH1 mutations in AML patients are those that result in increased levels of 2-hydroxyglutarate (2-HG) in leukemia cells and where efficacy is predicted by 1) clinically meaningful remissions with the recommended dose of ivosidenib and/or 2) inhibition of mutant IDH1 enzymatic activity at ivosidenib concentrations sustainable at the recommended dosage according to validated methods. The most common of these mutations in AML patients are R132H and R132C substitutions.
In vitro, ivosidenib inhibited selected IDH1 R132 mutants at substantially lower doses than wild-type IDH1. Ivosidenib inhibited the mutant IDH1 enzyme, which lowered 2HG levels and increased myeloid development in vitro and in vivo in mouse xenograft models of AML with an IDH1 mutation. Ivosidenib lowered ex-vivo 2-HG levels, blast numbers, and the percentage of mature myeloid cells in blood samples from patients with AML with mutant IDH1.
Description about the drug
C28H22ClF3N6O3 is the chemical formula, and the molecular weight is 583.0 g/mol. Ivosidenib is essentially insoluble in aqueous solutions with pH values ranging from 1.2 to 7.4.
The film-coated 250 mg tablet of Tibsovo (ivosidenib) is available for oral use. Inactive components in each tablet include colloidal silicon dioxide, croscarmellose sodium, hypromellose acetate succinate, magnesium stearate, microcrystalline cellulose, and sodium lauryl sulfate. FD&C blue #2, hypromellose, lactose monohydrate, titanium dioxide, and triacetin are all included in the tablet coating.
The drug can be purchased from licensed pharmaceutical suppliers who have the authorization to do that under a valid and recognised prescription following necessary protocols. Indian Pharma Network is among the most trusted and reliable dealers of generic medicines, and you can buy tibsovo online from us at very affordable rates.

1 note
·
View note
Text
Global Hemato Oncology Testing Market worth USD 4.0 billion by 2024 : Increasing Incidence of Hematologic Cancer
The study involved four major activities in estimating the current size of the hemato oncology testing market. Exhaustive secondary research was done to collect information on the market and its different subsegments. The next step was to validate these findings, assumptions, and market sizing with industry experts across the value chain through primary research.
In the secondary research process, various secondary sources such as annual reports, press releases & investor presentations of companies, white papers, certified publications, articles by recognized authors, gold-standard & silver-standard websites, regulatory bodies, and databases (such as D&B Hoovers, Bloomberg Business, and Factiva) were referred to in order to identify and collect information for this study.
According to the new market research report “Hemato Oncology Testing Market by Product & Services (Assay Kits, Services), Cancer (Leukemia (Acute Lymphoblastic, Acute Myeloid), Non-Hodgkin’s Lymphoma), Technology (PCR, NGS, IHC), End-User (Clinical Laboratories, Hospitals) – Global Forecast to 2024″, published by MarketsandMarkets™, the Hemato Oncology Testing Market is projected to reach USD 4.0 billion by 2024 from USD 2.0 billion in 2019, at a CAGR of 14.8%.
Browse in-depth TOC on “Hemato Oncology Testing Market“
77 – Tables
29 – Figures
116 – Pages
Download PDF Brochure: https://www.marketsandmarkets.com/pdfdownloadNew.asp?id=262472877
Growth in this market is driven mainly by the rising prevalence of lymphoma & myeloma cancers, growing focus on personalized medicine, and increasing collaborations for developing assays.
The services segment accounted for the largest share of the product & services segment in the Hemato Oncology Testing Market in 2018.
Based on product & services, the market is segmented into assay kits and services. In 2018, the services segment accounted for the largest share of the product & services segment in the Hemato Oncology Testing Market. The large share of this segment can be attributed to the rising prevalence of hematologic cancers and increasing aging population.
Availability of robust infrastructure and high-end equipment for conducting hemato oncology tests are the supporting factors for clinical laboratories segment
Based on end-user, the Hemato Oncology Testing Market has been segmented into four types, i.e., clinical laboratories, hospitals, academic & research institutes, and other end-users. Other end-users include CROs and pharmaceutical & biotechnological companies. Clinical laboratories accounted for the largest share of the market in 2018. This segment is expected to grow at the highest rate in the coming years. This is mainly due to the presence of advanced diagnostic equipment such as analyzers and the presence of skilled professionals to perform these tests.
North America accounted for the largest share of the Hemato Oncology Testing Market in 2018
North America accounted for the largest share of the Hemato Oncology Testing Market in 2018. The large share of this segment can primarily be attributed to the high incidences of hematologic cancer, aging population, awareness regarding advanced treatment methods, and the strong presence of industry players in the region. These trends are likely to drive market growth during the forecast period.
Request Sample Pages:https://www.marketsandmarkets.com/requestsampleNew.asp?id=262472877
Some of the leading players in the Hemato Oncology Testing Market include F. Hoffmann-La Roche Ltd. (Switzerland), Abbott Laboratories (US), Thermo Fisher Scientific, Inc. (US), QIAGEN N.V. (Germany), Bio-Rad Laboratories, Inc. (US), Illumina, Inc. (US), MolecularMD (Ireland), Invivoscribe, Inc. (US), Asuragen, Inc. (US), Adaptive Biotechnologies (US), ArcherDx, Inc. (US), and ARUP Laboratories Inc. (US).
Browse Adjacent Markets: Medical Devices Market Research Reports & Consulting
Browse Related Reports:
Molecular Diagnostics Market by Application (Infectious Disease (Hepatitis, HIV), Oncology, Genetic Testing), Technology (PCR, DNA Sequencing & NGS), End User (Hospital/Academic Laborato
https://www.marketsandmarkets.com/Market-Reports/molecular-diagnostic-market-833.html
Companion Diagnostics Market by Indication (Breast cancer, NSCLC, Colorectal cancer, Neurological disorders, Infectious Diseases), Technology (PCR, IHC, NGS, ISH), End User (Pharmaceutical & Biopharmaceutical
Companies, Reference Lab) – Global Forecast to 2022
https://www.marketsandmarkets.com/Market-Reports/companion-diagnostics-market-155571681.html
About MarketsandMarkets™
MarketsandMarkets™ provides quantified B2B research on 30,000 high growth niche opportunities/threats which will impact 70% to 80% of worldwide companies’ revenues. Currently servicing 7500 customers worldwide including 80% of global Fortune 1000 companies as clients. Almost 75,000 top officers across eight industries worldwide approach MarketsandMarkets™ for their painpoints around revenues decisions.
Our 850 fulltime analyst and SMEs at MarketsandMarkets™ are tracking global high growth markets following the “Growth Engagement Model – GEM”. The GEM aims at proactive collaboration with the clients to identify new opportunities, identify most important customers, write “Attack, avoid and defend” strategies, identify sources of incremental revenues for both the company and its competitors. MarketsandMarkets™ now coming up with 1,500 MicroQuadrants (Positioning top players across leaders, emerging companies, innovators, strategic players) annually in high growth emerging segments. MarketsandMarkets™ is determined to benefit more than 10,000 companies this year for their revenue planning and help them take their innovations/disruptions early to the market by providing them research ahead of the curve.
MarketsandMarkets’s flagship competitive intelligence and market research platform, “Knowledge Store” connects over 200,000 markets and entire value chains for deeper understanding of the unmet insights along with market sizing and forecasts of niche markets.
Contact:
Mr. Shelly Singh
MarketsandMarkets™ INC.
630 Dundee Road
Suite 430
Northbrook, IL 60062
USA: +1-888-600-6441
Email: [email protected]
Research Insight: https://www.marketsandmarkets.com/ResearchInsight/hemato-oncology-testing-market.asp
Visit Our Website: https://www.marketsandmarkets.com
Content Source: https://www.marketsandmarkets.com/PressReleases/hemato-oncology-testing.asp
0 notes
Text
Acute Myeloid Leukemia Therapeutics Market Size Forecast to Reach $18.3 Billion by 2026
Acute Myeloid Leukemia Therapeutics market size is forecast to reach $18.3 billion by 2026, growing at a CAGR of 7.2% during the forecast period 2021-2026. Leukemia affects the white blood cells and bone marrow. It is characterized by the rapid increase of abnormal blood cells growth that reduces the numbers of fully modified blood cells which leads to the typical symptoms of anemia, bleeding, and high risk of infection. Leukemia can grow along the lymphoid stem cell lines or myeloid depending upon the epigenetic and genetic mutations of the pluripotent stem cells. The leukemia that occurs along the myeloid are known as acute myeloid leukemia and it is a serious condition that is suffered by adult people. Various types of treatments that includes cytarabine, mitoxantrone, tyrosine kinase inhibitors, and imatinib among others are used for acute myeloid leukemia therapeutics. Increasing prevalence of acute myeloid leukemia and increased drug approval rate for acute myeloid leukemia are the major factors driving the growth of the market. Large number of treatment options available for treatment of acute myeloid leukemia and increasing funding for cancer research & development of new therapies is set to further enhance the overall market development of the Acute Myeloid Leukemia Therapeutics Market for the period 2021-2026.
Acute Myeloid Leukemia Therapeutics Market Segment Analysis – By Treatment Type
Cytarabine held the largest share in the Acute Myeloid Leukemia Therapeutics Market in 2020 and is estimated to grow at a CAGR 7.7% during the forecast period 2021-2026. Cytarabine is used for the treatment of different types of leukemia such as acute and chronic myeloid leukemia among others. Owing to its effectiveness in the treatment of acute myeloid leukemia, it is being widely used during chemotherapy process. Macmillan Cancer Support, an organization that is working for cancer have stated that cytarabine is more effective for the treatment of acute myeloid treatment. Chemotherapy kills the cancer cells that metastasized across the different parts of the body. Cytarabine is estimated to register the highest CAGR over the period 2021-2026.
Request for Sample Report @ https://www.industryarc.com/pdfdownload.php?id=507054
Report Price: $ 5900 (Single User License)
Acute Myeloid Leukemia Therapeutics Market Segment Analysis – By End User
Hospitals held the largest share in the Acute Myeloid Leukemia Therapeutics Market in 2020 and is estimated to grow at a CAGR 7.4% during the forecast period 2021-2026. Chemotherapy is majorly used for consolidation therapy of acute myeloid leukemia. In hospitals, younger adults are given mainly four rounds of high or immediate dose of cytarabine at monthly intervals and several different regimes are used for older patients. Chemotherapy is usually given in the hospital and recovery time can be spent at home. Hospitals are estimated to register the highest CAGR over the forecast period 2021-2026.
Acute Myeloid Leukemia Therapeutics Market Segment Analysis – By Geography
North America dominated the Acute Myeloid Leukemia Therapeutics Market with a major share of 38.3% in 2020. This is attributed to the increasing research in the field of regenerative medicine for treatment of acute myeloid leukemia. Growing awareness on personalized medicine, high incidences of leukemia, and huge investment in the development of pipeline drug is also increasing the growth of the market in this region.
However, Asia Pacific is estimated to grow at a higher CAGR during the forecast period 2021-2026 owing to the rising investment and increasing cancer awareness. Improving healthcare infrastructure and increasing inclination towards targeted therapies is also increasing the growth of the market in this region.
Acute Myeloid Leukemia Therapeutics Market Drivers
Increasing prevalence of acute myeloid leukemia
Increasing prevalence of acute myeloid leukemia is increasing the growth of the Acute Myeloid Leukemia Therapeutics Market. Acute Myeloid Leukemia begins in the bone marrow and quickly moves into the blood. It can also spread to other parts of the body such as liver, spleen, testicles, and central nervous system. It develops from those cells that turns into white blood cells. Radiation therapy or surgery in cancer patients removes the cancer cells in a specific area but chemotherapy works through the body and gives a better result. According to the estimation done by The American Cancer Society, around 61,780 new cases and 22,840 deaths from leukemia has been reported in 2019.Thus, increasing the growth of the Acute Myeloid Leukemia Therapeutics Market during the forecast period 2021-2026.
Increasing Cancer Awareness
Increasing cancer awareness is increasing the growth of the Acute Myeloid Leukemia Therapeutics Market. This is attributed to the fact that according to the World Health Organization, one third of all cancers are preventable and this can be done by taking a free cancer risk test that identifies the behaviors that can lead to cancer and ways to reduce the risk. As of 2019, 16.9 million cancer survivors are there in U.S. and number of cancer survivors are set to increase to 22.2 million by 2030.Thus, increasing the growth of the Acute Myeloid Leukemia Therapeutics Market during the forecast period 2021-2026.
Download Sample Report @ https://www.industryarc.com/pdfdownload.php?id=507054
Acute Myeloid Leukemia Therapeutics Market Challenges
High Cost of the Therapeutics Process and its Safety Concerns
Some of the factors that are set to impede the growth of the Acute Myeloid Leukemia Therapeutics Market are high cost of the therapeutics process and its safety concerns. Lack of targeted therapies in current acute myeloid leukemia therapeutics and difficult approval process for drugs is also set to hinder the growth of the market during the forecast period 2021-2026.
Acute Myeloid Leukemia Therapeutics Market Landscape
Product launches, mergers and acquisitions, joint ventures, and R&D activities are key strategies adopted by players in the Acute Myeloid Leukemia Therapeutics Market. In 2020, the Acute Myeloid Leukemia Therapeutics Market share is consolidated by the top ten players present in the market. Acute Myeloid Leukemia Therapeutics Market, top 10 companies are Pfizer Inc., Eisai Inc., Cephalon Inc., Celegene Corporation, Bristol,, Novartis AG, Genzyme Corporation, Eli Lily & Company, Amgen, and Gilead Sciences among others.
Key Takeaways
North America dominated the Acute Myeloid Leukemia Therapeutics Market in 2020 owing to the increasing research in field of regenerative medicine for treatment of acute myeloid leukemia and growing awareness on personalised medicines. The Acute Myeloid Leukemia Therapeutics Market scope for different regions will be provided in the final report.
Increasing advantages of biopharmaceuticals over conventional medicines and strong biopharmaceuticals pipeline are likely to aid the market growth of the Acute Myeloid Leukemia Therapeutics Market report.
Detailed analysis of the Strength, Weakness, and Opportunities of the prominent players operating in the market will be provided in the Acute Myeloid Leukemia Therapeutics Market report.
High cost of the therapeutics process and its safety concerns is poised to create hurdles for the Acute Myeloid Leukemia Therapeutics Market.
Related Reports:
A. Acute Lymphoblastic Leukemia Therapeutics Market
https://www.industryarc.com/Research/Acute-Lymphoblastic-Leukemia-Therapeutics-Market-Research-509670
B. B-cell Chronic Lymphocytic Leukemia Market
https://www.industryarc.com/Research/B-cell-Chronic-Lymphocytic-Leukemia-Market-Research-509962
For more Lifesciences and Healthcare Market reports, please click here
About IndustryARC: IndustryARC primarily focuses on Cutting Edge Technologies and Newer Applications market research. Our Custom Research Services are designed to provide insights on the constant flux in the global supply-demand gap of markets. Our strong team of analysts enables us to meet the client research needs at a rapid speed, with a variety of options for your business. Any other custom requirements can be discussed with our team, drop an e-mail to [email protected] to discuss more about our consulting services.
#AcuteMyeloidLeukemiaTherapeuticsMarket#AcuteMyeloidLeukemiaTherapeuticsMarketshare#AcuteMyeloidLeukemiaTherapeuticsMarketsize
0 notes