#Acute Leukemia Challenges US
Text
The Transformative Growth of the US Acute Myeloid Leukemia Market
Buy Now
What is the Size of US Acute Myeloid Leukemia Industry?
US Acute Myeloid Leukemia Market is expected to grow at a CAGR of ~ % in 2022 and is expected to reach ~USD Mn by 2028. The US Acute Myeloid Leukemia market is the rapid advancement in precision medicine and targeted therapies. The emergence of innovative treatments tailored to the genetic and molecular characteristics of individual AML patients has transformed the treatment landscape. Targeted therapies, such as FLT3 and IDH inhibitors, capitalize on specific genetic mutations driving AML, resulting in higher response rates and improved outcomes compared to traditional approaches.
Precision medicine's ability to identify the most effective treatment for each patient not only enhances therapeutic efficacy but also reduces unnecessary treatments, mitigating side effects and improving overall quality of life. This paradigm shift is bolstered by technological progress in molecular profiling techniques like next-generation sequencing, allowing for accurate genetic profiling and treatment customization.
Furthermore, regulatory support and accelerated approval pathways for targeted therapies have expedited their availability to patients. Pharmaceutical companies' increasing investment in research and development of novel agents, along with collaborations between academia and industry, leads to grow this market even more.

Click here to Download a Sample Report
US Acute Myeloid Leukemia Market by treatment type
The market is segmented by Treatment type into Chemotherapy, Targeted Therapy, Hormone Therapy, Immunotherapy and Others. Targeted Therapy is the dominant segment in the US Acute Myeloid Leukemia market in 2022. Specialty Centers, dedicated to the diagnosis, treatment, and management of specific medical conditions like AML, offer a concentrated and specialized approach to patient care. Their dominance in the AML market is driven by their unique capabilities to provide comprehensive and tailored treatments that address the complex needs of AML patients.
US Acute Myeloid Leukemia Market by end -user industry
The market is segmented by End-User into Hospitals, Homecare, Specialty Centers, Pharmacies and Others. Among these, Specialty Centers are the dominant End-User in the US Acute Myeloid Leukemia market in 2022. Specialty centers have a higher level of expertise and experience in treating specific types of cancer, including acute leukemia. They typically have specialized medical professionals, including hematologists and oncologists, who are well-versed in the latest treatment protocols and research. These centers are equipped with state-of-the-art diagnostic and treatment facilities that are specifically tailored to the needs of leukemia patients. This can include advanced imaging technologies, specialized laboratories, and access to cutting-edge therapies.
Click here to Download a Custom Report
US Acute Myeloid Leukemia companies market by Region
The US Acute Myeloid Leukemia market is segmented by Region into North, South, East, West. The dominance region is North in the US Acute Myeloid Leukemia Market in 2022. the North region often benefits from a higher level of funding for medical research, including AML. Federal funding agencies, private foundations, and pharmaceutical companies frequently channel resources into institutions within this region, allowing for greater research capabilities and clinical trials infrastructure.
Competition Scenario in US Acute Myeloid Leukemia Market
The US acute myeloid leukemia market was characterized by the presence of several prominent pharmaceutical companies and biotechnology firms striving to establish their presence and gain a competitive edge.
Larger corporations, smaller biotechnology companies making significant contributions to the AML landscape. Companies like Agios Pharmaceuticals and Daiichi Sankyo were gaining attention for their novel therapies targeting AML-associated mutations. These players are often at the forefront of introducing precision medicine approaches to AML treatment, taking advantage of advancements in genetic profiling and molecular diagnostics.
The competition is further intensified by research collaborations and partnerships between pharmaceutical companies, academic institutions, and research organizations. These collaborations aimed to combine resources, expertise, and insights to accelerate the development of effective therapies.
What is the Expected Future Outlook for the Overall US Acute Myeloid Leukemia market?
The US Acute Myeloid Leukemia market was valued at USD ~Million in 2022 and is anticipated to reach USD ~ Million by the end of 2028, witnessing a CAGR of ~% during the forecast period 2022- 2028. Pharmaceutical companies have been actively researching and developing novel therapies for Acute Myeloid Leukemia, with a focus on improving patient outcomes and reducing the side effects associated with traditional treatments like chemotherapy. The approval of new drugs, such as FLT3 and IDH inhibitors, has offered fresh therapeutic avenues for specific subsets of Acute Myeloid Leukemia patients with genetic mutations.
The future outlook for the US Acute Myeloid Leukemia market is anticipated to be influenced by a combination of factors including advancements in treatment options, evolving regulatory landscapes, and a growing understanding of the molecular basis of Acute Myeloid Leukemia. The market has been witnessing a shift towards personalized medicine, with increasing emphasis on targeted therapies and precision medicine approaches.
Furthermore, the integration of innovative technologies like next-generation sequencing (NGS) has enhanced our understanding of Acute Myeloid Leukemia’s molecular complexities, allowing for better patient stratification and treatment selection. This trend toward molecular profiling and personalized treatment regimens is likely to continue shaping the market landscape.
However, challenges persist. Despite progress, Acute Myeloid Leukemia remains a difficult-to-treat disease with a high relapse rate. Overcoming drug resistance and developing effective strategies for patients who do not respond well to existing therapies remain critical areas of focus.
Additionally, the cost of novel therapies and access to these treatments could also impact their adoption and availability. The regulatory environment, including expedited pathways for drug approvals, continue to influence market dynamics.
#US Acute Myeloid Leukemia Market Analysis#US Acute Myeloid Leukemia Industry#US Acute Myelogenous Market#US Bone Marrow Leukemia Industry#United States Acute Myeloid Leukemia Market#US Acute Myeloid Leukemia Market size#US Acute Myeloid Leukemia Market share#US Acute Myeloid Leukemia Market trends#US Acute Myeloid Leukemia Market growth#Chemotherapy in Acute Myeloid Leukemia Market US#Targeted Therapy in Acute Myeloid Leukemia Market US#Hormone Therapy in Acute Myeloid Leukemia Market US#End Users in US Acute Myeloid Leukemia Market#Specialty Centers in US Acute Myeloid Leukemia Market#Hospitals for Acute Myeloid Leukemia in US#Challenges in US Acute Myeloid Leukemia Market#Major Players in US Acute Myeloid Leukemia Market#Emerging Players in US Acute Myeloid Leukemia Market#US Acute Myeloid Leukemia Market Future Outlook#US Acute Myeloid Leukemia Market Growth Rate#leading companies US Acute Myeloid Leukemia Market#US Acute Myeloid Leukemia Market competitors#US Acute Myeloid Leukemia Market forecast#US Acute Myeloid Leukemia Market demand#US Acute Myeloid Market Challenges#Myelogenous Leukemia Opportunities US#Acute Leukemia Challenges US#Opportunities Myeloid Leukemia Market US#Leading Leukemia Pharmaceutical companies US#Major Competitors US Acute Leukemia Market
0 notes
Text
A Dive into CAR-T Cell Therapy
Imagine training your own soldiers to fight cancer. Not just any soldiers, but elite warriors genetically modified to recognize and demolish the enemy with laser-like precision. That's the essence of CAR-T cell therapy, a revolutionary approach turning heads in the medical world. In the fight against cancer, CAR-T cell therapy embodies this very concept, harnessing the body's own immune system to wage war against malignant cells.
T cells are the special forces of your immune system, constantly scanning for and eliminating threats. But sometimes, cancer cells outsmart them, hiding in plain sight. CAR-T cell therapy steps in, equipping these T-cells with a unique weapon: a Chimeric Antigen Receptor (CAR). Think of it as a GPS that locks onto a specific protein on cancer cells, guiding the T-cells straight to their target.
How does it work? Here's the simplified version:
Recruitment: First, T cells are extracted from your blood.
Modification Station: In the lab, scientists use a virus or other tools to insert the CAR gene into the T cells' DNA. This equips them with the cancer-targeting GPS.
Bootcamp Boost: The modified T cells are grown in a special environment, multiplying into a powerful army.
Redeployment: The CAR-T cell troops are infused back into your bloodstream, ready to seek and destroy.
Sounds amazing, right? But like any powerful technology, CAR-T comes with its own set of challenges. The treatment process is complex and expensive, and there can be serious side effects like cytokine release syndrome, where the immune system goes into overdrive. So, is CAR-T a miracle cure? Not yet. But for some patients with aggressive blood cancers like leukemia and lymphoma, it has shown remarkable results, offering hope where other treatments have failed. Researchers are constantly working to improve the safety and efficacy of CAR-T, making it a potential game-changer for even more cancers in the future.
CAR-T cell therapy has demonstrated remarkable efficacy in the treatment of certain types of hematologic malignancies, including acute lymphoblastic leukemia (ALL) and certain subtypes of non-Hodgkin lymphoma. Clinical trials have shown unprecedented response rates and durable remissions in patients who have exhausted all other treatment options. Furthermore, ongoing research is exploring the potential of CAR-T cell therapy in treating solid tumors, extending its therapeutic reach to a broader spectrum of cancers.
The future is bright for CAR-T. It's a testament to the power of human ingenuity and our ongoing quest to conquer one of humanity's greatest foes. While there's still a way to go, this groundbreaking therapy is a beacon of hope, reminding us that even the seemingly impossible can become reality.
3 notes
·
View notes
Text
Teen uses calculus learned through MITx to better understand his cancer treatment
High schooler Dustin Liang estimated his blood cell counts by applying knowledge from an MITx course and talking to doctors.
Sara Feijo | MIT Open Learning

When Dustin Liang was diagnosed with T-cell acute lymphoblastic leukemia in June, the cancer consumed his life. But despite a monthlong hospital stay, aggressive chemotherapy treatments, and ongoing headaches, fatigue, loss of appetite, and nausea, the 17-year-old high school senior enrolled in MITx’s class 18.01.1x (Calculus 1A: Differentiation).
MITx, part of MIT Open Learning, offers hundreds of high-quality massive open online courses adapted from the MIT classroom for learners worldwide. The Calculus 1A: Differentiation course was designed and created by the Department of Mathematics and offered through the MITx program. Liang took the free course this summer in between treatment sessions and medical tests so that he could meet the four-year math requirement to graduate from a Massachusetts high school — an arrangement he made with his school.
In class, Liang learned how to differentiate functions and how to make linear and quadratic approximations. He then applied this knowledge to estimate his blood cell counts. “I was in a hospital bed when I saw the doctor draw a graph of my neutrophils on a whiteboard, and I thought you could apply a quadratic approximation to it to estimate my blood cell counts at a certain time in the future,” Liang recalls. “I talked to the doctors about it, and they said it was a good idea but that they currently didn’t have the technology to do that.”

In Calculus 1A, Liang was learning how to predict the near future value of a function using linear or quadratic approximation methods. After seeing a doctor’s chart of his neutrophils, Liang hypothesized that he could use quadratic approximation to predict his neutrophil count.
“Given a series of points of the blood cell counts, a function can be modeled,” Liang explains. “So, predicting a future point not far away is mathematically feasible.”
Determined to test his idea, Liang called his mentor, Jiawen Sun, who works in a London security exchange firm as a trading analyst simulating and modeling stock market behavior. Sun helped Liang create a graph to estimate Liang’s neutrophil count at a certain time. When Liang compared the graph to his blood test results, he found that the math worked.
“I was able to predict the blood cell counts. It was a little off, but close enough,” Liang says. “There are some challenges in simulating the function of blood cells. However, the human blood cell counts turned out to be converging easier than the stock market to simulate.”
Keep reading.
Make sure to follow us on Tumblr!
3 notes
·
View notes
Text
Engineered T Cells Market — Forecast(2024–2030).

Engineered T Cells Market Overview
Engineered T cells market represents a revolutionary advancement in cellular therapy, leveraging cutting-edge biotechnology to treat various diseases, primarily cancer. Engineered T cells involve modifying a patient’s T cells to enhance their ability to recognize and attack diseased cells, offering new hope for conditions that were previously difficult to treat. This comprehensive market overview provides insights into the current landscape, key players, applications, challenges, and future trends shaping this rapidly evolving sector.
Request Sample
Market Dynamics
1. Market Growth and Drivers
The engineered T cells market is experiencing substantial growth, driven by several factors:
Increasing Cancer Incidence: The global rise in cancer cases is a major driver, as engineered T cell therapies, such as CAR-T (Chimeric Antigen Receptor T-cell) therapy, offer novel treatments for various cancers.
Advancements in Technology: Innovations in genetic engineering, such as CRISPR and advanced gene-editing techniques, are enhancing the efficacy and safety of engineered T cell therapies.
Growing Investment: Significant investments from both public and private sectors in research and development are fueling advancements and commercialization in this field.
2. Technology and Innovation
Two primary technologies dominate the engineered T cells market:
CAR-T Therapy: This involves modifying T cells to express chimeric antigen receptors (CARs) that target specific proteins on cancer cells. Approved CAR-T therapies, such as Kymriah (Novartis) and Yescarta (Kite Pharma), have shown remarkable success in treating hematologic cancers, including leukemia and lymphoma.
TCR Therapy: T-cell receptor (TCR) therapies focus on enhancing T cells to recognize specific cancer antigens presented by MHC (Major Histocompatibility Complex) molecules. TCR therapies are designed to target a broader range of cancers and are currently in various stages of clinical development.
Inquiry Before Buying
Key Players
Several companies are leading the engineered T cells market, each contributing to the development and commercialization of these therapies:
Novartis: A pioneer in CAR-T therapy, Novartis’ Kymriah was one of the first CAR-T therapies to receive FDA approval. The company continues to advance its pipeline of cell therapies and explore new indications.
Gilead Sciences: Through its subsidiary Kite Pharma, Gilead Sciences has developed Yescarta, another leading CAR-T therapy. Gilead is actively involved in expanding its cell therapy portfolio and researching new treatment options.
Bristol-Myers Squibb: With its acquisition of Celgene, Bristol-Myers Squibb has gained access to innovative CAR-T therapies like Breyanzi. The company is also exploring other cell and gene therapies.
Bluebird Bio: Known for its focus on gene therapies, Bluebird Bio is developing both CAR-T and TCR therapies. The company is advancing its investigational therapies through various stages of clinical trials.
Adaptimmune: Specializing in TCR therapies, Adaptimmune is developing innovative treatments targeting specific cancer antigens. The company is actively working on expanding its clinical trials and therapeutic indications.
Schedule a Call
Applications
1. Oncology
The primary application of engineered T cells is in oncology. CAR-T therapies have shown significant efficacy in treating:
Hematologic Cancers: CAR-T therapies like Kymriah and Yescarta have been particularly effective in treating blood cancers, including acute lymphoblastic leukemia (ALL) and diffuse large B-cell lymphoma (DLBCL).
Solid Tumors: Research is ongoing to extend the use of CAR-T therapies to solid tumors, such as breast, lung, and pancreatic cancers. Challenges include identifying suitable target antigens and overcoming the tumor microenvironment’s immunosuppressive effects.
2. Other Diseases
Beyond oncology, engineered T cells are being explored for:
Autoimmune Diseases: There is potential for engineered T cells to target autoreactive T cells involved in autoimmune conditions such as type 1 diabetes and multiple sclerosis.
Infectious Diseases: Research is investigating the use of engineered T cells to target chronic viral infections, including HIV and hepatitis B.
Buy Now
Regulatory Landscape
1. Approval Process
Engineered T cell therapies must undergo rigorous regulatory scrutiny to ensure their safety and efficacy. The approval process typically involves:
Preclinical Studies: Initial research to evaluate the safety and effectiveness of the therapy in animal models.
Clinical Trials: Phases I through III trials to assess safety, efficacy, and optimal dosing in humans. Successful trials are crucial for obtaining regulatory approval.
Regulatory Review: Submission of clinical trial data to regulatory agencies such as the FDA (U.S. Food and Drug Administration) and EMA (European Medicines Agency) for review and approval.
2. Challenges
Cost: Engineered T cell therapies are expensive to develop and administer, posing challenges for widespread adoption and accessibility.
Manufacturing Complexity: The process of modifying and expanding T cells is complex and requires specialized facilities and expertise.
Side Effects: Potential side effects, such as cytokine release syndrome (CRS) and neurotoxicity, need to be carefully managed and mitigated.
Future Trends
1. Innovations in Technology
Future developments in the engineered T cells market are expected to include:
Next-Generation CAR-T Therapies: Improvements in CAR design, such as dual-targeting CARs and armored CARs, aim to enhance efficacy and reduce side effects.
Combination Therapies: Combining engineered T cells with other modalities, such as immune checkpoint inhibitors, may improve treatment outcomes and address limitations.
2. Personalized Medicine
The shift towards personalized medicine will likely drive market growth. Tailoring therapies to individual patients’ genetic and tumor profiles can enhance treatment efficacy and minimize adverse effects.
3. Global Expansion
As research advances and manufacturing capabilities improve, engineered T cell therapies are expected to become more widely available across different regions, including emerging markets. Collaborative efforts between pharmaceutical companies and research institutions will play a key role in expanding access to these therapies.
The global market size for engineered T cells was $20.21 billion in 2022, and is expected to grow to $348.9 billion by 2032.
For More Information click Here
1 note
·
View note
Text
Advances in Leukemia Therapeutics: Innovations and Emerging Trends
The Evolving Landscape of Leukemia Treatment

Leukemia, a group of malignancies that affect the blood and bone marrow, continues to challenge the medical community with its complexity and variability. Recent years have seen significant strides in leukemia therapeutics, driven by advancements in drug development, personalized medicine, and novel therapeutic strategies. These innovations are transforming treatment paradigms, offering new hope to patients and paving the way for more effective and targeted therapies.
Breakthroughs in Drug Development
One of the most significant advancements in leukemia therapeutics is the development of targeted therapies. Unlike traditional chemotherapy, which indiscriminately kills rapidly dividing cells, targeted therapies are designed to attack specific molecules involved in cancer progression. For instance, tyrosine kinase inhibitors (TKIs) like imatinib, dasatinib, and nilotinib have revolutionized the treatment of chronic myeloid leukemia (CML). These drugs target the BCR-ABL protein, a key driver of CML, leading to improved outcomes and reduced side effects compared to conventional treatments.
In addition to TKIs, the approval of novel agents such as venetoclax has marked a significant leap forward. Venetoclax targets the BCL-2 protein, which is often overexpressed in leukemia cells, thereby promoting their survival. By inhibiting BCL-2, venetoclax enhances the effectiveness of chemotherapy and has shown promise in treating acute myeloid leukemia (AML) and chronic lymphocytic leukemia (CLL).
Advances in Immunotherapy
Immunotherapy is another area where substantial progress has been made. Chimeric antigen receptor (CAR) T-cell therapy represents a groundbreaking approach, especially for acute lymphoblastic leukemia (ALL) and some cases of CLL. This therapy involves modifying a patient's T-cells to express a CAR that targets leukemia-specific antigens. The modified T-cells are then reintroduced into the patient’s body, where they identify and destroy leukemia cells.
Recent studies have demonstrated the efficacy of CAR T-cell therapies, leading to impressive remission rates in patients with refractory or relapsed leukemia. However, this approach is not without challenges. The risk of cytokine release syndrome (CRS) and neurotoxicity remains a concern, and ongoing research aims to enhance the safety and effectiveness of CAR T-cell therapies.
The Leukemia Therapeutics Market size was USD 16 Billion in 2023 and is expected to Reach USD 27.28 Billion by 2031 and grow at a CAGR of 6.9% over the forecast period of 2024-2031.
Precision Medicine and Genetic Insights
The era of precision medicine has ushered in a new approach to leukemia treatment, with a focus on tailoring therapies based on individual genetic profiles. Advances in genomic sequencing have identified specific mutations and genetic alterations associated with different types of leukemia. For example, mutations in the FLT3 gene are common in AML, and targeted inhibitors like midostaurin are being used to address these mutations.
Similarly, research into the genetic underpinnings of CLL has led to the development of drugs that target specific mutations or pathways involved in the disease. By understanding the unique genetic landscape of each patient’s leukemia, clinicians can select the most effective therapies and potentially improve treatment outcomes.
Future Directions and Challenges
While the progress in leukemia therapeutics is promising, several challenges remain. The high cost of novel therapies, including CAR T-cell treatments, poses a significant barrier to access for many patients. Additionally, the development of resistance to targeted therapies and immunotherapies requires ongoing research to develop new and effective treatment options.
Looking ahead, researchers are exploring combination therapies, novel drug targets, and personalized approaches to overcome these challenges. The integration of artificial intelligence and machine learning in drug discovery and treatment planning holds the potential to further accelerate progress in leukemia therapeutics.
Conclusion
The field of leukemia therapeutics is undergoing a transformative phase, with innovations in drug development, immunotherapy, and precision medicine leading the way. These advancements are not only enhancing treatment outcomes but also offering new hope to patients. As research continues to evolve, the focus will remain on overcoming current challenges and pushing the boundaries of what is possible in the fight against leukemia.
0 notes
Text
A Visit with Our 2024 Pedal Partner
If you are in Massachusetts or nearby, you may have seen more cyclists on the road than usual. Many of us are taking our last long training rides before the Pan-Mass Challenge.
I went on a 60 mile solo ride on Saturday followed up by a 30 mile ride on Sunday with the team to visit with our 2024 Pedal Partner, Teagan.
As some of you may know from previous emails, she completed just over 2 years of treatment for B-cell Acute Lymphoblastic Leukemia.

It was good to see her in good spirits and living her best life :-)
Donations like yours help finance the studies that make studies, trials, and protocols for treating cancer like her's possible.
100% of every donation goes directly to the Dana-Farber Cancer Institute in Boston and supports cancer research & patient care.
Click here to make $25 donation
Click here to make a $50 donation
Click here to make a $100 donation
Click here to make a $250 donation
Click here to make a $500 donation
Click here to make a $1,000 donation
Click here to make a donation of any other amount
Click here to donate using your Fidelity Donor Advised Fund
Thank you for your support!
Marty Middelmann
My 2024 Fundraising Goal: $12,000
Raised for 2024: $6,847
Number of Donors: 69
Corporate Matches: $450 (Including $200 Pending)
My Progress Towards that Goal: 57.1%
0 notes
Text
Dr. Purvi Kadakia Kutty: Leading the Fight Against Cancer as Kharghar's Top Oncologist
Pediatric Oncology is a specialized branch of medicine that focuses on the diagnosis, treatment, and management of cancer in children and adolescents. It deals specifically with Child Oncologist in navi mumbai, which can vary significantly from cancers aggressiveness, and treatment approaches. Pediatric oncologists are medical experts who provide comprehensive care to young patients with cancer, working closely with a multidisciplinary team to ensure the best possible outcomes and quality of life for these young patients and their families.

Conditions Treated in Pediatric Oncology:
Pediatric oncologists manage various types of childhood cancers, which can affect different parts of the body. Some common childhood cancers treated in Pediatric Oncology include:
Leukemias: Cancers that affect the bone marrow and blood, including Acute Lymphoblastic Leukemia (ALL) and Acute Myeloid Leukemia (AML).
Lymphomas: Cancers that arise from the lymphatic system, such as Hodgkin's lymphoma and Non-Hodgkin lymphoma.
Brain Tumors: Tumors that develop in the brain or spinal cord, including astrocytomas, medulloblastomas, and ependymomas.
Germ Cell Tumors: Tumors that arise from germ cells, commonly found in the ovaries or testes.
Dr. Purvi Kadakia Kutty, M.D. Pediatrics, FNB Pediatric Hematology & Oncology is a Consultant Pediatric Hematology & Oncology at Apollo Hospitals, Navi Mumbai.She is an Honorary-visiting consultant in the Division of Pediatric Hematology Oncology at L.T.M.G.H., Sion Hospital. She has experience of over 10 years in the field of Pediatric Hematology, Immunology & Oncology. She is also experienced in Pediatric Bone marrow transplantsfor benign and malignant hematological conditions.Kharghar, Navi Mumbai
Diagnostic Tests in Pediatric Oncology:
To diagnose Child Oncologist, pediatric oncologists may utilize a range of diagnostic tests, including:
Biopsy: A procedure to obtain a tissue sample for examination under a microscope to determine if cancer cells are present.
Imaging Studies: Techniques like X-rays, CT scans, MRI, and PET scans are used to visualize tumors and evaluate the extent of cancer.
Bone Marrow Aspiration and Biopsy: These tests help assess if cancer has spread to the bone marrow.
Lumbar Puncture (Spinal Tap): A procedure to collect cerebrospinal fluid for evaluation if cancer involves the central nervous system.
Dr. Purvi Kadakia Kutty's dedication to oncology and her unwavering commitment to her patients make her one of the best oncologists in Kharghar. Her extensive training, compassionate care, and holistic approach to treatment ensure that her patients receive the best possible care during their most challenging times. Dr. Kutty stands out as a beacon of hope and excellence in the field of oncology.
Visit: https://childoncologist.com/
Contact: +91 77381 62020
#ChildOncologist#ChildOncologistinKharghar#ChildOncologistinnavimumbai#Bestoncologistinnavimumbai#bestoncologistinKharghar
0 notes
Text
Know about Blincyto Injection in India
Blincyto is used for the treatment of a certain type of cancer (Acute Lymphocytic Leukemia). Indian Pharma network legally offers the best Blincyto injection price in India and other countries where the drug is not available. The prices of this medication can vary over time and may be influenced by factors such as location, required dosage, and any applicable discounts. To obtain current and accurate information on the price of Blincyto 35 mcg vials in india.

Buying injection Blincyto 38.5 mcg from Indian Pharma networks offers a reliable and convenient solution for those in critical need of Blincyto 38.5 MCG. This is an immunotherapy used for treating certain types of leukemia legally provides access to high-quality medications, ensuring patients receive authentic and promising treatments. Our streamlined ordering procedure and knowledgeable staff make obtaining this medicine easier, mainly for those facing health challenges. TIP's dedication to patient well-being and easy accessibility make TIP a reliable source for Blincyto injections in India
Blinatumomab, a breakthrough immunotherapy to treat certain types of leukemia, remains a relatively high-cost medicine globally. The medication Blinatumomab price can vary because it may not be easily accessible or affordable in India. Kindly dial our TOLL-FREE Number: 1800-889-1064 or Call/WhatsApp: +91 9310090915 to contact us. Access to such specialty therapies may depend on factors like hospital availability, and local regulations. For the most relevant and latest information on blinatumomab's price and availability at The Indian Pharma (TIP), one can directly contact TIP or consult with doctors in India, as the pharmaceutical landscape is dynamic and subject to change.
Blincyto, a groundbreaking drug for a rare type of leukemia, is available at a high price or cost. In India, the medicine Blincyto price is not fixed by the local pharmacies. The affordability of Blincyto vials can be a valid concern for patients across the world. However, Indian pharmaceutical importers may potentially import Blincyto injection to make it accessible for India-based patients at a more reasonable price. This involves navigating tough regulations. While the importation of Blincyto can boost access to cutting-edge therapeutic options. Blincyto, a breakthrough therapeutic option for Acute Lymphoblastic Leukemia (ALL), can be imported by Noida-based pharmaceutical importer, The Indian Pharma (TIP). Kindly Call/WhatsApp: +91 9310090915 for Blincyto price in India. This specialty medicinal product harnesses the potency of bispecific antibodies to target unhealthy cells, offering new hope to patients. As for Blincyto price, it's necessary to note that the prices of medications can vary because of factors like dosage and market fluctuations. TIP strives to provide reasonable pricing and availability to life-saving medicines like Blincyto, ensuring that patients in India have legal access to cutting-edge therapies. Contact TIP for specific pricing information as well as availability.
Blincyto (blinatumomab) is a specialty drug used in the treatment of certain types of acute lymphoblastic leukemia(ALL). The Indian Pharma (TIP) is offering Blincyto at the most reasonable price range. The overall cost of Blincyto therapy can vary widely on behalf of the patient's body weight, the time span of therapy, and the specific treatment plan recommended by the doctor. It's important to note that drug prices can fluctuate because of factors like currency exchange rates and pharmaceutical market dynamics,
Blincyto (blinatumomab) is a specialized immunotherapy drug used for treating certain sorts of leukemia. The cost of Blincyto in India can vary significantly on behalf of factors such as the dosage recommended, length of treatment, and the hospital or healthcare facility where the drug is administered. Generally, it is considered an expensive drug because of its specialized nature.
Buying Blincyto (blinatumomab) online can be risky. Blincyto is a prescription medication used to treat certain types of leukemia, and it should only be obtained through a licensed importer like THE INDIAN PHARMA (TIP). Kindly Call/WhatsApp: +91 9310090915 to buy Blincyto online through legal channels. Buying medications online poses significant dangers, including the risk of receiving counterfeit or fake products, which can be hazardous or ineffective. But TIP is known for offering the quality and genuine medicines at the most competitive price. Always prioritize your health as well as safety by obtaining Blincyto through TIP, which is a legitimate and authorized channel.
#Blincyto price in india#Blincyto in India#Blincyto injection price#Blincyto 35 mcg#buy Blincyto 38.5 Single-Dose Vial
0 notes
Text
Oncology Drugs Market Growth, Trends, Size, Share, Demand And Top Growing Companies 2031

In a landscape where the battle against cancer rages on, advancements in healthcare systems, public health measures, and novel pharmaceutical therapies have ushered in a new era of hope. According to the National Cancer Institute, the United States saw an estimated 1,806,590 new cancer cases and approximately 606,520 deaths due to the disease in 2020. However, over the past five decades, cancer survival rates have soared from 50% in 1970 to an impressive 70%, thanks to a trifecta of progress.
For more information: https://www.fairfieldmarketresearch.com/report/oncology-drugs-market
Unprecedented Growth Trajectory:
The global oncology therapy sales are forecasted to surpass US$ 300 billion by 2026, with oncology contributing 21.7% to total pharmaceutical sales. Fueling this growth are the top 10 pharmaceutical companies, which have declared oncology as their key focus area, driving multibillion-dollar M&A deals and strategic collaborations. Pfizer's acquisition of Array BioPharma for US$11 billion in 2019 and AbbVie's strategic partnership with Genmab for a bispecific antibody development deal worth US$3 billion are testament to this focus.
Diverse Indications Drive Demand:
While oncology represents over 20 different indications, a significant portion of revenue stems from just five of them: breast cancer, multiple myeloma, non-small-cell lung carcinoma (NSCLC), prostate cancer, and non-Hodgkin's lymphoma (NHL), which collectively accounted for approximately 65% of the market in 2020. Moreover, with breast, lung, and colorectal cancers expected to collectively account for ~50% of all new cancer diagnoses by 2026, the demand for innovative therapies continues to surge.
Disruptive Trends Reshape Landscape:
Innovation in oncology is accelerating, with disruptive technologies such as cell therapy, RNA therapy, viral vectors, and stem cell therapy gaining traction. Recent approvals of CAR-T cell therapies like Kymriah and Yescarta for acute lymphocytic leukemia (ALL) and diffuse large B-cell lymphoma (DLBCL) respectively signal a new frontier in cancer treatment. Precision medicine is also driving progress, with over 160 oncology biomarkers approved by 2019, paving the way for more targeted and effective therapies.
Impact of COVID-19:
Despite remarkable progress, oncology has been among the worst-hit therapeutic areas amid the COVID-19 pandemic. Decreased demand for physician-administered products, disruptions in cancer screenings, and a decline in new clinical trials have posed significant challenges. However, the industry remains resilient, adapting to the evolving landscape and ensuring continued innovation.
Immuno-Oncology Leads the Way:
Immuno-oncology sales are expected to soar to ~US$ 95 billion by 2026, with agents and protein kinase inhibitors comprising ~65% of sales. With over 550 active cell- and gene-therapy agents under clinical development, the future of cancer treatment looks promising. Investments in combination studies and the exploration of new mechanisms underscore the industry's commitment to advancing immuno-oncology therapies.Roche and Keytruda: Leading the Charge:
In a highly concentrated market where the top 10 companies capture over 75% of the market value, F. Hoffmann-La Roche AG (Roche) and Merck & Co. stand out as leaders. While Roche maintains its global leadership position, Merck's Keytruda is poised to become the world's top-selling oncology
0 notes
Text
Informative Report on MRD Testing Market | BIS Research

MRD is a sophisticated diagnostic technique used primarily in oncology to detect and quantify residual cancer cells that may remain in the body during or after treatment.
The global MRD Testing market is a rapidly growing segment in the healthcare industry, driven by the increasing demand for accurate and sensitive methods to monitor and manage cancer patients.
The MRD Testing market was valued at $1.67 billion in 2023 and is expected to reach $6.67 billion by 2033, growing at a CAGR of 14.81% between 2023 and 2033.
MRD Overview
MRD assessment is a supplementary approach to detect extremely low levels of blood cancer cells and solid tumors following the treatment of conditions such as acute and chronic leukemia, lymphoma, or multiple myeloma. MRD specifically pertains to the minute population of cancer cells that persist in the body despite achieving complete remission (CR) through chemotherapy or stem cell transplantation.
MRD Testing market is clinically important as it helps to monitor treatment response, predict relapse risk, guide personalized therapies, and serve as an endpoint in clinical trials. By identifying and addressing MRD, healthcare providers can strive for better treatment outcomes and improved patient survival rates.
MRD , however, utilizes advanced molecular and genetic technologies to detect and quantify these residual cancer cells with unparalleled sensitivity.
Have a look at our sample page here !
Market Segmentation
By Technology
By Target Detection
By End Users
By Region
China dominated the Asia-Pacific Minimal Residual Testing market in 2022 with a share of 36.08%. Although the market is expected to remain in a strong growth phase due to the massively growing number of cancer cases and the rising health-related awareness among people in Asia-Pacific, a significant barrier to the increasing adoption is an uneven economic balance among countries within the region.
Key Factors
The MRD Testing market has experienced significant growth in recent years, driven by several key factors like
advancements in technology
rising cancer burden,
clinical evidence supporting MRD monitoring
Key Players In the MRD Testing Market includes
QIAGEN N.V.
Thermo Fisher Scientific Inc.
Sysmex Corporation
Mission Bio
OPKO Health
Bio-Rad Laboratories, Inc
ICON plc
Hoffmann-La Roche
and many others
MRD Testing Market
MRD Testing is a crucial component of cancer management aimed at detecting and monitoring residual cancer cells in patients who have undergone treatment. The primary goal of MRD Testing is to identify and quantify the small number of cancer cells that may remain in the body after treatment, even when the patient shows no signs of disease.
MRD Testing represents a paradigm shift in cancer care, moving towards a more personalized and proactive approach by enabling clinicians to monitor disease dynamics with greater precision, ultimately leading to improved patient outcomes and enhanced quality of life.
Applications for MRD Market
Treatment Response Monitoring
Relapse Prediction
Treatment Decision-making
Prognostic Assessment
Clinical Trials and drug development
MRD Testing Market Dynamics
Market Drivers
Advent of Minimal Residual Testing and its Awareness among Consumers
Increasing Incidence of Cancer Cases Demanding Minimal Residual Testing
Rise in administration of solid tumors
Expanding Medicare Coverage for Minimal Residual Testing
Market Challenges
Early Risk of Progression and relapse due to MRD negative result
Substantially high costs of Minimal Residual Testing
Market Opportunities
Advancements in the Development Of Companion Diagnostics
Rising Number of Clinical Trials in Prolonged MRD-Negative Treatment Decisions
Recent Developments in the Minimal Residual Testing Market
•Quest Diagnostics acquired Haystack Oncology, expanding its oncology portfolio with the inclusion of advanced liquid biopsy technology. This addition aimed to enhance personalized cancer care by offering highly sensitive diagnostic capabilities.
Integrated DNA Technologies launched the Archer FUSIONPlex Core Solid Tumor Panel, a pioneering cancer research testing solution that has been enhanced and fine-tuned to include a broader range of single nucleotide variant (SNV) and indel coverage.
Visit our precision medicine page here!
Key Questions Answered
Q What is Minimal Residual Testing ?
Minimal residual disease (MRD) testing is a supplementary approach to detect extremely low levels of blood cancer cells and solid tumors following the treatment of conditions such as acute and chronic leukemia, lymphoma, or multiple myeloma. MRD specifically pertains to the minute population of cancer cells that persist in the body despite achieving complete remission (CR) through chemotherapy or stem cell transplantation.
Q What kinds of New Strategies are adopted by the existing market players to strengthen their positions in the Industry ?
The global Minimal Residual Testing market is currently witnessing several developments, primarily aimed at introducing new products and services. Major manufacturers of Minimal Residual Testing products, along with the service providers, are actively undertaking significant business strategies to translate success in research and development into the commercial clinical setting.
Conclusion
MRD Testing Market represents a dynamic and evolving landscape, characterized by innovation, collaboration, and a shared commitment to revolutionizing cancer management. With its profound impact on clinical decision-making and patient care, Minimal Residual Testing stands as a beacon of hope in the fight against cancer, heralding a new era of precision oncology.
0 notes
Text
Top 3 US Pharmaceutical Companies and Their Strategies in US Acute Myeloid Leukemia Market
Buy Now
US Acute Myeloid Leukemia Market is growing due to advancement in treatment approaches, increase healthcare costs and expenditure, growing investments in Research and Development, and a constant growth in population.
Story Outline
Pfizer Inc.- An American multinational company with the highest annual revenue of around 100 billion US$ in 2022 in the drug market. The company has made significant contributions to the US Acute Myeloid Leukemia Market through a sophisticated, robust, agile manufacturing infrastructure and investment in research and development.
Brystol Myers Squibb- One of the largest American pharmaceutical companies which consistently ranks on the Fortune 500 list of the largest US corporations. The company’s mission is to discover, develop, and deliver innovative medicines that help patients as well as prevail over serious diseases.
Novartis AG- The company with the fourth-largest revenue in the drug market which is focused to deliver high-value medicine that alleviate society’s greatest disease burdens through technology leadership in R&D and novel access approaches.
The US Acute Myeloid Leukemia Market is expected to grow at CAGR of 10.5% in the forecast period 2022-2028.
The Acute Myeloid Leukemia Market of US is driving growth at an amazing level. This surge is a result of advancement in treatment approaches, increases healthcare costs and expenditure, growing investments in Research and Development, and a constant growth in population.
Various pharmaceutical companies are actively shaping this growing market with their strategies and innovations.
This article provides an in-depth look at major pharmaceutical companies with their strategies and innovations.
1.Pfizer Inc.
Click here: To know more about Pfizer Inc.
Pfizer Inc. is an American multinational pharmaceutical company and headquartered at The Spiral in Manhattan, New York City. The company has made significant contributions to the US Acute Myeloid Leukemia Market through a sophisticated, robust, agile manufacturing infrastructure and investing in research and development.
The company employs more than 30,000 employees worldwide, have 35+ manufacturing sites, 300+ external suppliers, and have reached more than 180 countries. Pfizer tops the list of drug market by achieving a revenue of approx. 100 billion USD in 2022.
Pfizer has made significant contributions to the US acute Myeloid Leukemia Market. Some notable contributions are, in April 2017, the development and approval of a targeted therapy called “Rydapt”, which is an oral kinase inhibitor that targets multiple enzymes, including FLt3, which is often mutated in AML patients. The drug was approved by FDA in April 2017 for use in combination with Chemotherapy.
Furthermore, MYLOTARG is approved in combination with daunorubicin and cytarabine for the treatment of patients aged 15 and above with previously untreated, de novo, CD33-positive acute myeloid leukemia (AML), except Acute Promyelocytic Leukemia (APL).
The company’s purpose is “Breakthroughs that change patients' lives—fuels everything we do and reflects our passion for building on our legacy as one of the greatest contributors of good to the world.
The company says that its purpose ensures that its patients remain at the center of all that they do. They live with their purpose by sourcing the best science in the world; partnering with others in the healthcare system to improve access to their medicines.
Pfizer believes in growing partnerships with innovators to initiate forward great science and continually seek new partners that are actively researching bold scientific ideas. In December 2022, Pfizer announced its collaboration with Gero’s machine learning technology platform to discover potential therapeutic targets for fibrotic using large-scale human-based data.
Pfizer’s continuous clinical trials and collaboration with healthcare institutions and research organizations has majorly contributed in advancing Acute Myeloid Leukemia Market and the development of novel treatment strategies.
2.Bristol Myers Squibb

Click here: To Download a Sample Report
The Bristol Myers Squibb Company, is an American multinational pharmaceutical company headquartered in Princeton, New Jersey. BMS is one of the world’s largest pharmaceutical companies and consistently ranks on the Fortune 500 list of the largest US corporations.
The company employs more than 34,000 across more than 86 locations worldwide. The company’s revenue in 2022 was approximately 46 bn USD.
Their mission is to discover, develop, and deliver innovative medicines that help patients as well as prevail over serious diseases. Bristol believes in the power of science to address some most challenging diseases of today's world.
Bristol Myers is majorly known for its contributions to oncology, and immunology and its involvement in Acute Myeloid Leukemia Market with its broader focus on cancer treatments.
The significant development of BMS can be noted from June 2021, Bristol Myers Squibb received approval from European Commission for Onureg, a Frontline oral maintenance therapy for adult patients with acute Myeloid Leukemia who achieved their first complete remission (CR) or CR with incomplete blood count recovery following intensive induction chemotherapy. Onureg is expected to increase sales and product revenue, thereby increasing the US Acute Myeloid Leukemia Market growth.
Furthermore, the strategic cooperation between Evotec and Bristol Myers Squibb has grown in order to create a pipeline for programs addressing more neurological illnesses. In order to find altering therapies for a variety of neurodegenerative disorders, the firms started working together in 2016. The eight-year extension is intended to strengthen the strategic partnership even more.
3.Novartis AG

Click Here : To Download a Custom Report
Novartis AG is a healthcare company that majorly focuses on the discovery, development, manufacture and marketing of prescription and generic pharmaceutical and eye care products. Novartis offers generic medicines and biosimilars through Sandoz. The company conducts its research through The Novartis Institute for Biomedical Research (NIBR).
Novartis is one of the largest pharmaceutical companies in the world and the fourth largest by revenue in 2022, which was approx. 50.500 billion USD.
The company is functioning in more than 150 locations with around 1,10,000 employees working worldwide.
Novartis's strategy as a focused medicines company is to deliver high-value medicine that alleviates society’s greatest disease burdens through technology leadership in R&D an novel access approaches.
Novartis contribution to Acute Myeloid Leukemia Market includes, FDA approval of Novartis Scemblix (asciminib), with novel mechanism of action for the treatment of Leukemia in October,2021.
Furthermore, through their open approach Novartis is focusing on new technologies to develop next generation therapeutics. Currently, Novartis is working with Orionis Bioscience to find new targets at a genome-wide scale.
By combining development and drug discovery with innovation, they aim to achieve tenuous targets and to launch novel small molecule therapy for Acute Myeloid Leukemia patients more quickly. Thus driving a steered growth for the US Acute Myeloid Leukemia Market.
#US Acute Myeloid Leukemia Market#US Acute Myeloid Leukemia Market Revenue#US Acute Myeloid Leukemia Market Analysis#US Acute Myeloid Leukemia Industry#US Acute Myelogenous Market#US Bone Marrow Leukemia Industry#United States Acute Myeloid Leukemia Market#US Acute Myeloid Leukemia Market size#US Acute Myeloid Leukemia Market share#US Acute Myeloid Leukemia Market trends#US Acute Myeloid Leukemia Market growth#Chemotherapy in Acute Myeloid Leukemia Market US#Targeted Therapy in Acute Myeloid Leukemia Market US#Hormone Therapy in Acute Myeloid Leukemia Market US#End Users in US Acute Myeloid Leukemia Market#Specialty Centers in US Acute Myeloid Leukemia Market#Hospitals for Acute Myeloid Leukemia in US#Challenges in US Acute Myeloid Leukemia Market#Major Players in US Acute Myeloid Leukemia Market#Emerging Players in US Acute Myeloid Leukemia Market#US Acute Myeloid Leukemia Market Future Outlook#US Acute Myeloid Leukemia Market Growth Rate#leading companies US Acute Myeloid Leukemia Market#US Acute Myeloid Leukemia Market competitors#US Acute Myeloid Leukemia Market forecast#US Acute Myeloid Leukemia Market demand#US Acute Myeloid Market Challenges#Myelogenous Leukemia Opportunities US#Acute Leukemia Challenges US#Opportunities Myeloid Leukemia Market US
0 notes
Text
CAR-T Therapy Market Share, Growth, Sales, Trends, Supply, Forecast 2030
The “CAR-T Therapy Market Share, Size, and Trends | 2030” is market research by The Insight Partners. The CAR-T Therapy market has perceived tides of change in the recent past. This study offers precise projections after detailed scrutiny of a range of factors impacting the business. Considering the present market scenario, this report brings forward correct predictions on revenue, market size, and CAGR of the CAR-T Therapy market. The novel market research which is based on a fact-based foundation is now accessible for purchase. This report can make a variance in wide decision-making and drive business forward in the right direction.
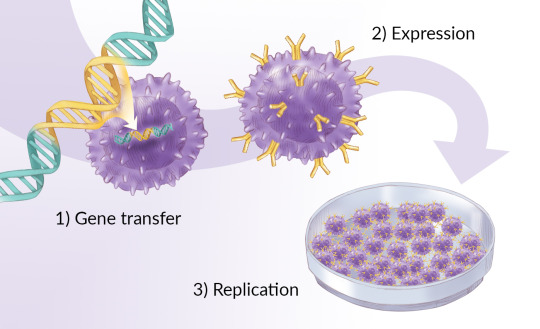
Business is no longer a game of instincts when it comes to capitalizing on new production lines. In a highly competitive CAR-T Therapy market, companies may face several challenges. Having trusted market research is always endorsed for both veteran and new entrants. CAR-T Therapy Market report presents a thorough analysis of local, regional, and global market scenarios through the following details.
Report Attributes
Details
Segmental Coverage
By Targeted Antigen
CD 19
BCMA
HER2
GD2
CD 20
CD22
CD30
CD33
HER1
Therapeutic Application
Acute Lymphocytic Leukemia
Chronic Lymphocytic Leukemia
Diffuse Large B-Cell Lymphoma
Follicular Lymphoma
Geography
North America
Europe
Asia Pacific
and South and Central America
Regional and Country Coverage
North America (US, Canada, Mexico)
Europe (UK, Germany, France, Russia, Italy, Rest of Europe)
Asia Pacific (China, India, Japan, Australia, Rest of APAC)
South / South & Central America (Brazil, Argentina, Rest of South/South & Central America)
Middle East & Africa (South Africa, Saudi Arabia, UAE, Rest of MEA)
Market Leaders and Key Company Profiles
Novartis International AG
Kite Pharma, Inc. (Gilead Sciences, Inc.)
Juno Therapeutics (Celgene Corporation)
Bluebird Bio, Inc. (Celgene Corporation)
Sorrento Therapeutics Inc.
Mustang Bio, Inc
Aurora Biopharma Inc.
Legend Biotech (Genscript Biotech Corporation)
Pfizer, Inc.
CARsgen Therapeutics, Ltd.
Other key companies
Competitive Landscape
Knowing the state of rivals is a strategically right move to outperform them. This report is the right place to explore key strategies, developments, and recent launches by key CAR-T Therapy market players. This report emphasizes an analysis of business strategies and expected growth opportunities for brands.
Key Coverings:
Current and Future Market Estimates- CAR-T Therapy Market Share, CAGR, and Forecast | 2030
Market Dynamics – Drivers, Challenges, Regional Trends, and Market Opportunities
Market Segmentation – Product, Application, End-use Industries, and Regional Growth Prospects.
Competition Matrix – Key Market Players and Strategies
Recent Developments and Innovation Contributing Market Growth
Need a Customized Market Research Report?
You can always share any specific requirements that you have, and our team will adjust the scope of research offerings as per your needs.
The following are some customizations our clients frequently ask for:
The CAR-T Therapy market report can be customized based on specific regions/countries as per the intention of the business
The report production was facilitated as per the need and following the expected time frame
Insights and chapters tailored as per your requirements.
Depending on the preferences we may also accommodate changes in the current scope.
Key Questions Addressed in the CAR-T Therapy Market Research Include:
What are present CAR-T Therapy market values, and what can be expected in the upcoming decade?
What are the key segments in the CAR-T Therapy market?
What is the regional distribution of the CAR-T Therapy market report?
What are the key players and their recent strategies?
What are the key factors driving CAR-T Therapy market growth?
What are regulatory concerns and requirements businesses have to compel?
About Us:
The Insight Partners is a one-stop industry research provider of actionable intelligence. We help our clients in getting solutions to their research requirements through our syndicated and consulting research services. We specialize in industries such as Semiconductor and Electronics, Aerospace and Defense, Automotive and Transportation, Biotechnology, Healthcare IT, Manufacturing and Construction, Medical Devices, Technology, Media and Telecommunications, Chemicals and Materials.
Contact Us: www.theinsightpartners.com
0 notes
Text
What is Acute Biphenotypic Leukemia and how can cord blood banking help since approved by the FDA?

I am ready to enroll in cord blood banking NOW and get my special discount!

By clicking on either buttons, you are agreeing to our TOS and disclaimers and will be redirected to an affiliate cord blood banking provider. We might get financial compensation if you sign up with them through our affiliate links. Unlock your special discounts by adding your promo code.CORD300 in the coupon field to get $300 OFF cord blood and tissue banking. OR cord200 to get $200 OFF if you are getting cord blood banking only.
I want more information on cord blood banking

Acute Biphenotypic Leukemia (ABL) is a rare and aggressive form of leukemia that affects approximately 1 to 2% of all adult leukemias. It is characterized by the presence of both myeloid and lymphoid markers on the cancerous cells, making it difficult to diagnose and treat. Due to its rarity and complexity, ABL poses significant challenges for doctors and researchers, and has a lower survival rate compared to other types of leukemia. However, recent advancements in medical technology have shed new light on potential treatments for ABL, including cord blood banking. In 2019, the U.S. Food and Drug Administration (FDA) approved the use of cord blood stem cells for the treatment of ABL, providing a promising new option for patients and their families. In this article, we will delve into the specifics of acute biphenotypic leukemia, its symptoms and treatment options, and how cord blood banking can play a crucial role in improving the chances of survival for those diagnosed with this challenging disease.
Understanding Acute Biphenotypic Leukemia: Symptoms and Diagnosis
Acute Biphenotypic Leukemia (ABL) is a rare and aggressive form of leukemia characterized by the presence of both myeloid and lymphoid features in the leukemic cells. The symptoms of ABL are similar to those of other types of leukemia and may include fatigue, weakness, recurrent infections, easy bruising or bleeding, bone pain, and enlarged lymph nodes. However, due to the biphenotypic nature of the disease, ABL can present with a wide range of symptoms, making diagnosis challenging. To accurately diagnose ABL, a thorough evaluation of the patient's medical history, physical examination, blood tests, bone marrow biopsy, and other diagnostic tests may be necessary. Early and accurate diagnosis is crucial for determining the most appropriate treatment approach and improving the prognosis for individuals affected by ABL.
Importance of Early Detection for Treatment Success
Timely detection of Acute Biphenotypic Leukemia (ABL) plays a pivotal role in the successful management and treatment of this aggressive disease. Early diagnosis allows for prompt initiation of treatment strategies, increasing the chances of a favorable outcome. With ABL, like many other types of leukemia, delayed diagnosis can result in disease progression and complications, making it more challenging to achieve remission. The timely identification of ABL enables healthcare professionals to tailor treatment plans that target the specific characteristics of the disease, optimizing the effectiveness of therapies and minimizing potential adverse effects. Moreover, early detection allows patients to access appropriate support services and resources to cope with the physical and emotional challenges of ABL, contributing to improved overall well-being and quality of life. Therefore, recognizing the importance of early detection for treatment success is fundamental in providing the best possible care for individuals affected by ABL.
Role of Cord Blood Banking in ABL Treatment
Cord blood banking has emerged as a valuable resource in the treatment of Acute Biphenotypic Leukemia (ABL). Cord blood contains a rich source of hematopoietic stem cells, which have the potential to differentiate into various blood cell types. These stem cells can be utilized in the transplantation process to replace and replenish the diseased bone marrow with healthy cells. The unique advantage of cord blood banking is that it provides a readily accessible and compatible source of stem cells, which eliminates the need for finding a matched donor. This is particularly beneficial in cases where a suitable bone marrow donor cannot be identified. The use of cord blood stem cells in ABL treatment has shown promising results, with studies demonstrating successful engraftment and improved overall survival rates. The approval of cord blood banking by the FDA further validates its role as a viable treatment option for ABL, offering hope and potential for improved outcomes in patients facing this challenging disease.
FDA Approval of Cord Blood Banking for ABL
The recent approval by the FDA for cord blood banking in the treatment of Acute Biphenotypic Leukemia (ABL) marks a significant milestone in the field of regenerative medicine. This regulatory approval reaffirms the safety and efficacy of utilizing cord blood stem cells for ABL treatment. With this approval, healthcare providers and patients can now have confidence in the utilization of cord blood banking as a viable treatment option. The FDA's thorough evaluation process ensures that the collected cord blood units meet stringent quality and safety standards, providing reassurance to both patients and healthcare professionals. This approval opens up new avenues of hope for individuals diagnosed with ABL, as cord blood banking offers a readily available source of hematopoietic stem cells that can be used in transplantation procedures. The FDA's endorsement solidifies the role of cord blood banking in the fight against ABL, highlighting its potential to improve patient outcomes and enhance the quality of life for those affected by this devastating disease.
How Cord Blood Banking Works: Explained
Cord blood banking is a process that involves collecting and storing the blood found in the umbilical cord and placenta after childbirth. This blood is a rich source of hematopoietic stem cells, which have the ability to develop into different types of blood cells. The collection process is simple and painless, typically performed shortly after the baby's birth. Once collected, the cord blood is processed and cryogenically preserved in specialized laboratories. These stored cord blood units can then be used in the future for various medical purposes, including the treatment of certain diseases and conditions, such as Acute Biphenotypic Leukemia (ABL). When a patient requires cord blood transplantation, the stored cord blood unit is matched to the patient's HLA (Human Leukocyte Antigen) type. The stem cells from the cord blood unit can then be infused into the patient's bloodstream, where they have the potential to replace damaged or diseased cells and contribute to the patient's recovery. Cord blood banking provides a valuable resource for patients with ABL and other conditions, offering a potentially life-saving option that has been approved by the FDA.
Benefits of Using Cord Blood for ABL Treatment
The use of cord blood for the treatment of Acute Biphenotypic Leukemia (ABL) offers several significant benefits. Firstly, cord blood contains a rich source of hematopoietic stem cells, which have the potential to develop into various types of blood cells. These stem cells can help replenish and rebuild the patient's damaged or diseased blood cells, promoting the recovery and restoration of a healthy immune system. Additionally, cord blood transplantation has shown lower rates of immune rejection compared to other sources, increasing the likelihood of a successful engraftment and reducing the risk of complications. Furthermore, cord blood units are readily available and can be accessed quickly, making it an efficient and convenient option for patients in need of urgent treatment. The FDA approval of cord blood banking further validates its safety and effectiveness, ensuring that patients and healthcare providers can confidently rely on this innovative therapy for ABL treatment.
Comparing Cord Blood Banking Options
When considering cord blood banking options, it is essential to thoroughly compare and evaluate the various services available. One crucial factor to consider is the accreditation and certifications held by the cord blood banks. Look for banks that are accredited by reputable organizations such as the American Association of Blood Banks (AABB) or the Foundation for the Accreditation of Cellular Therapy (FACT). These accreditations indicate that the bank adheres to strict quality standards and follows proper procedures for collection, processing, and storage of cord blood units. Additionally, it is important to assess the storage facilities and practices of the banks. Ensure that the bank has state-of-the-art cryogenic storage systems and backup power sources to guarantee the long-term preservation of the cord blood units. Moreover, consider the transparency and accessibility of the bank's online portal or customer service. It should provide detailed information about the status and availability of the stored cord blood unit, as well as the option for easy retrieval when needed. By carefully comparing these crucial factors, you can make an informed decision and choose the most suitable cord blood banking option for your specific needs.
Financial Considerations for Cord Blood Banking
When it comes to cord blood banking, it is crucial to carefully consider the financial aspects involved. The cost of cord blood banking can vary significantly between different providers, so it is essential to compare the pricing structures and services offered. Some banks charge an upfront fee for collection and processing, while others may have annual storage fees. It is important to assess these fees and determine if they fit within your budget. Additionally, inquire about any additional costs that may arise in the future, such as retrieval or shipping fees if the cord blood unit is needed for transplantation. Moreover, it is advisable to check if the cord blood bank offers any financial assistance or payment plans to make the process more manageable. Understanding the financial considerations associated with cord blood banking will help you make an informed decision and ensure that you are prepared for the costs involved in preserving this valuable resource.
Success Stories of ABL Patients
ABL, or Acute Biphenotypic Leukemia, is a rare and aggressive form of leukemia that presents challenges in treatment and prognosis. However, there have been inspiring success stories of ABL patients who have overcome the odds and achieved remission or even complete recovery. These success stories not only highlight the resilience and strength of the individuals, but also the advancements in medical research and treatment options. Through a combination of chemotherapy, targeted therapies, and sometimes stem cell transplantation, ABL patients have been able to achieve positive outcomes and regain their health. These success stories serve as a beacon of hope for patients diagnosed with ABL, showing that with early detection, appropriate treatment, and ongoing support, a favorable outcome is possible.
Future of Cord Blood Banking in ABL Treatment.
Cord blood banking has emerged as a promising avenue in the treatment of Acute Biphenotypic Leukemia (ABL). The utilization of cord blood stem cells, derived from the umbilical cord and placenta after childbirth, has shown potential for treating various hematological disorders, including ABL. The FDA's approval of cord blood banking for use in transplantation has further bolstered the credibility and potential of this approach. The unique properties of cord blood stem cells, such as their abundance, immunological naivety, and potential for reduced graft-versus-host disease, make them a valuable resource in ABL treatment. Ongoing research and clinical trials are exploring ways to optimize the use of cord blood stem cells in the context of ABL, including strategies to enhance engraftment, minimize relapse rates, and improve overall patient outcomes. With advancements in technology and a growing understanding of the biology behind ABL, the future of cord blood banking in ABL treatment holds great promise, offering new possibilities for patients and their families in the fight against this challenging disease.In conclusion, Acute Biphenotypic Leukemia is a rare and aggressive form of cancer that requires immediate and effective treatment. The recent approval of cord blood banking by the FDA offers a promising solution for those diagnosed with this disease. By preserving cord blood at birth, families can have access to a potentially life-saving treatment option in the future. This is a significant advancement in the fight against Acute Biphenotypic Leukemia, giving hope to patients and their loved ones. It is crucial to continue raising awareness and supporting research in this area to improve treatment outcomes and ultimately save lives.
FAQ
What is Acute Biphenotypic Leukemia and how does it differ from other types of leukemia?Acute biphenotypic leukemia is a rare type of leukemia where the cancerous cells exhibit characteristics of both myeloid and lymphoid cells. This makes it unique from other types of leukemia, which typically involve either myeloid or lymphoid cells exclusively. The diagnosis and treatment of acute biphenotypic leukemia can be challenging due to its mixed cell lineage, requiring a tailored approach that incorporates treatments for both myeloid and lymphoid leukemias.How can cord blood banking help in the treatment of Acute Biphenotypic Leukemia, now that it has been approved by the FDA?Cord blood banking can aid in the treatment of Acute Biphenotypic Leukemia by providing a source of stem cells for transplant. These stem cells can help rebuild the patient's immune system after high-dose chemotherapy. The FDA approval of cord blood banking ensures that these stem cells are safe and effective for use in treating various diseases, including Acute Biphenotypic Leukemia. This advancement offers new treatment options and hope for patients battling this aggressive form of leukemia.What are the potential benefits of using cord blood stem cells in the treatment of Acute Biphenotypic Leukemia?Cord blood stem cells offer benefits in the treatment of Acute Biphenotypic Leukemia due to their ability to regenerate healthy blood cells and immune system components after intense chemotherapy. They also provide a lower risk of graft-versus-host disease compared to bone marrow transplants and can be more readily available for patients in need. Additionally, cord blood stem cells have shown promising results in reducing relapse rates and improving overall survival outcomes in patients with Acute Biphenotypic Leukemia.Are there any limitations or risks associated with using cord blood stem cells for treating Acute Biphenotypic Leukemia?While cord blood stem cells can be used to treat Acute Biphenotypic Leukemia, there are limitations such as the potential for graft-versus-host disease and the risk of relapse due to incomplete eradication of leukemia cells. Additionally, finding a suitable match and the cost of the procedure can also be limiting factors. However, research is ongoing to improve outcomes and reduce risks associated with cord blood stem cell transplantation for Acute Biphenotypic Leukemia.How does the FDA approval of cord blood banking for the treatment of Acute Biphenotypic Leukemia impact patients and healthcare providers in terms of treatment options and outcomes?The FDA approval of cord blood banking for the treatment of Acute Biphenotypic Leukemia expands treatment options for patients and healthcare providers, offering a potentially life-saving alternative for those who may not find a suitable bone marrow donor. This approval can improve outcomes by increasing access to stem cell transplants and reducing the risks associated with finding a matched donor. Additionally, it may lead to advancements in personalized medicine and regenerative therapies, ultimately benefiting both patients and healthcare providers through improved treatment efficacy and patient outcomes.
Read the full article
0 notes
Text
Pediatric Oncology: Common Cancer In Children’s Care And Treatment

Understanding, Common Childhood Cancers and Treatments
Pediatric oncology, including pediatric leukemia, pediatric brain tumors, and other common cancers in children requires specialized care and treatment. It is a challenging road for children and their families.
In this blog, explore and learn about pediatric cancer, understand the different forms in which pediatric oncology manifests, know how we diagnose it and learn about the treatments we offer and the remarkable success stories we’ve witnessed at VCC.
What is Pediatric Cancer?
Pediatric cancer, also known as childhood cancer, is a term that no parent ever wants to hear. It’s when a child’s body begins to grow abnormal cells that can turn into cancer. These cancers can affect various parts of the body, and they are indeed challenging to deal with.
Different Types of Pediatric Cancers
Pediatric cancers come in various forms, including acute leukemias, lymphomas, kidney cancers, and more. Two of the most common ones are pediatric leukemia and pediatric brain tumors. These are formidable opponents that need our full attention.
Acute leukemias are a group of cancers that can grow rapidly in the blood and bone marrow. Within this group, there’s pediatric acute lymphoblastic leukemia, where abnormal white blood cells multiply quickly. It’s like a fierce battle, but it’s one we can win.
Lymphomas affect the lymphatic system, and they can be Hodgkin lymphoma or non-Hodgkin lymphoma. Yes, they can even affect young warriors.
Kidney cancers, although less common in children, can still happen. It’s like an unexpected twist in the story, but it’s a story we can rewrite.
Understanding these possible pediatric cancers is crucial because it helps us identify them early and take action. Vydehi Cancer Center specializes in diagnosing and treating all these types, and we’ve seen countless brave children fight and win these battles.
How We Figure It Out at VCC
At Vydehi Cancer Center, we know that every dhildhood cancer situation is unique. That’s why we use a variety of methods to find out what’s happening. We start with some general tests, like checking the blood to see if there’s anything unusual.
In case there is any suspicion or no clarity, we need dig deeper, and use special tests like bone marrow aspirations, flow cytometry, and cytogenetics. This is to provide the best possible diagnostic which is really helpful not only in early stage cancer detection but also for proper and complete treatment.
How We Fight It at VCC
Now, let’s talk about the part that matters most – fighting back. At Vydehi Cancer Center, we offer different ways to battle pediatric cancers. Our main weapons are chemotherapy and radiotherapy, which we use depending on the type of cancer and how far it’s spread.
In some cases, when it’s like a mighty dragon that needs to be vanquished, we might recommend a bone marrow transplant. But don’t worry, we’re here every step of the way, supporting you and your child.
Our Success Stories at VCC
At Vydehi Cancer Center, we are incredibly proud of our success rates. We’ve seen our young warriors win battles with pediatric cancer, achieving an impressive overall success rate of 70-80 percent. And when it comes to pediatric leukemias, our success rate soars to an incredible 90 percent. These are real stories of triumph, and we’re honored to be part of them.
In conclusion, pediatric cancer is a challenging journey, but it’s one that can lead to victory. Vydehi Cancer Center, with its focus on pediatric oncology and remarkable success stories, stands as a source of hope for children and families on this path. If your child is facing pediatric cancer, remember, that you’re not alone, and Vydehi Cancer Center is here to walk this journey with you.
#vydehicancercenter#cancertreatment#childhoodcancer#childhoodcancerawareness#pediatriccancer#leukemia#childhoodcancersucks#childhoodcanceradvocate
0 notes
Text
Acute Myeloid Leukemia Treatment in India
Acute Myeloid Leukemia (AML) is a type of cancer that affects the blood and bone marrow, leading to the rapid growth of abnormal white blood cells. In India, like many other countries, the diagnosis and treatment of AML have evolved significantly over the years. The healthcare system in India has made notable strides in providing advanced and comprehensive care for leukemia patients, including various treatment modalities and support services.
The first step in managing AML is an accurate diagnosis, often achieved through bone marrow aspiration and biopsy, blood tests, and imaging studies. Once diagnosed, the treatment plan is tailored to the specific characteristics of the leukemia cells and the overall health of the patient. In India, major healthcare centers and specialized oncology hospitals are equipped with state-of-the-art diagnostic facilities and experienced hematologists and oncologists who play a crucial role in determining the most effective treatment approach.
The primary treatment options for AML in India include chemotherapy, targeted therapy, stem cell transplantation, and supportive care. Chemotherapy, the most common initial treatment, involves the use of powerful drugs to destroy cancer cells. Specialized oncology centers in India administer chemotherapy based on well-established protocols, often following international guidelines. This helps ensure that patients receive standardized, evidence-based care.
Targeted therapy is another approach gaining prominence in the treatment of AML. This method involves drugs that specifically target certain molecules or pathways involved in the growth of cancer cells, minimizing damage to healthy cells. The availability and use of targeted therapies in India have increased, providing more options for personalized and effective treatment.
Stem cell transplantation, particularly allogeneic transplantation, is considered in cases where the AML is high-risk or has relapsed. This procedure involves replacing the patient's diseased bone marrow with healthy stem cells from a compatible donor. Specialized transplant centers in India have experienced teams of transplant physicians, hematologists, and supportive care staff to manage the complex process of transplantation.
Supportive care is an integral component of AML treatment, addressing the side effects of both the disease and its treatment. This includes managing infections, providing blood transfusions, and supporting patients through the emotional and physical challenges of cancer therapy. Palliative care is also increasingly recognized as an essential aspect of leukemia treatment, focusing on improving the quality of life for patients and their families.
Access to advanced medical technologies, ongoing research, and international collaborations contribute to the continuous improvement of AML treatment in India. Clinical trials evaluating new therapies and treatment approaches are conducted at leading cancer centers, providing patients with opportunities to participate in cutting-edge research.
While the availability of sophisticated treatments is crucial, it is also noteworthy that the cost of leukemia treatment can be a significant concern for many patients. In India, efforts are being made to enhance affordability through government initiatives, insurance coverage, and collaborations with pharmaceutical companies to provide access to essential drugs at lower costs.
In conclusion, the landscape of Acute Myeloid Leukemia treatment in India has evolved, with a focus on comprehensive, personalized, and evidence-based care. The integration of advanced therapies, supportive care, and ongoing research contributes to improved outcomes for AML patients, making strides toward a future where leukemia is not only treatable but also more manageable for individuals and their families.
Al Afiya Medi Tour is a leading medical tourism company. We are offer medical tourism services in India foreign patients. Some of the main countries are Bangladesh, south Africa, Uganda, Zambia, Namibia, Iraq, Kenya, Nigeria and so on. We provide free assistance for TURP surgery cost in India, lung cancer treatment, stomach cancer treatment in India, liver transplant costa, best hospital for heart valve replacement, bone marrow transplant cost , arthroscopic surgery, brain tumor surgery cost in India, kidney transplant, liver cancer treatment, best bone marrow hospital etc. If you are searching for free medical and healthcare consulting to find the best hospitals and top doctors and surgeons in India for any treatment then contact us- Alafiyameditour.com.
Source: https://alafiyameditour1.blogspot.com/2023/11/acute-myeloid-leukemia-treatment-in.html
0 notes
Text
PMC at Fenway, Our Pedal Partner, and a Big Milestone!
Living Proof Celebration at Fenway Park
This past Friday I took my family to PMC Night at Fenway Park where the Boston Red Sox hosted the Pan-Mass Challenge and honored Living Proof Riders, riders who have survived cancer, by allowing them to parade around the field on their bikes.
Among them was a PHAT Tuesday Teammate, Sharon Foster. This year she will be riding in her 7th Pan-Mass Challenge. Sharon is active, healthy and thriving today due to the amazing treatment from her doctor at the Dana-Farber Cancer Institute.

Teagan's Last Treatment...
This Tuesday, our 2023/2024 PMC Pedal Partner, Teagan, is scheduled for what is supposed to be her LAST treatment. They also are waiting to hear about port removal. Teagan has been battling B-cell Acute Lymphoblastic Leukemia since being diagnosed in May of 2022.

It has been a tough journey for Teagan. She has been hospitalized a number of times with bacterial infections, side effects from her Chemotherapy treatments, and even severe acute necrotizing pancreatitis with hypovolemic shock. In addition to this Teagan has had additional complications from pancreatitis and other reactions to various medications.
Teagan is fortunate that her oncologist is the doctor who wrote the protocol for treatment of her specific type of leukemia. Hopefully this last scheduled treatment today is the beginning of the end of her 2+ year cancer journey.
Prior to receiving her diagnosis, Teagan was a dedicated, competitive dancer. With hard work and a little bit of physical therapy, she has returned to dance on a small scale. She's your typical high school girl; she loves her friends, her phone, music, clothes and shopping.
In Other News...
As of last Friday, the Pan-Mass Challenge surpassed $28 million raised so far this year, bringing the fundraising total since 1980 to $1 billion for Dana-Farber Cancer Institute.
With just over 2 weeks to go I am just over halfway to my $12,000 goal. I am hoping to at least hit $10,000 by the end of July. Please help me reach my goals by making a donation.
100% of every donation goes directly to the Dana-Farber Cancer Institute in Boston and supports cancer research & patient care.
Click here to make $25 donation
Click here to make a $50 donation
Click here to make a $100 donation
Click here to make a $250 donation
Click here to make a $500 donation
Click here to make a $1,000 donation
Click here to make a donation of any other amount
Click here to donate using your Fidelity Donor Advised Fund
Thank you for your support!
Marty Middelmann
My 2024 Fundraising Goal: $12,000
Raised for 2024: $6,447
Number of Donors: 64
Corporate Matches: $450 (Including $200 Pending)
My Progress Towards that Goal: 53.7%
0 notes