#agno3
Explore tagged Tumblr posts
Text
I can say from experience that chemists are a lot more insane than wizards
I was a wizard's apprentice, but we didn't get anything done because he kept putting more and more PPE on me while explaining the importance of safety. By the end I was wearing two hazmat suits, several pairs of gloves, three boots and a giant gasmask.
We were making a low-level healing potion.
#i will never forget how when i was at a national competition thar included a practical chemistry probe#we had to identify several substances one of which was AgNO3#needless to say 3/4 of the people there (myself included) had large black spots on their hands by the end of it#most did it on purpose
3K notes
·
View notes
Text



Chemical proof of past stages of existence AgNO3
#bloodborne#micolash host of the nightmare#laurence the first vicar#rom the vacuous spider#fromsoftware#my art#my doodles#babies who have not yet encountered the Horrors#click for better quality#i have the same resting bitch face as rom irl#Fatigue™ is gripping me very tightly and my social battery's dead. hope september treats my mind better#anyhow. photography exists in yharnam and i shall die on this hill
106 notes
·
View notes
Text
MCSR As Chemical Compounds
idk either man. expect very little actual explanation and a lot of chemical yapping from a very big nerd
Silverr as Silver Nitrate:
AgNO3
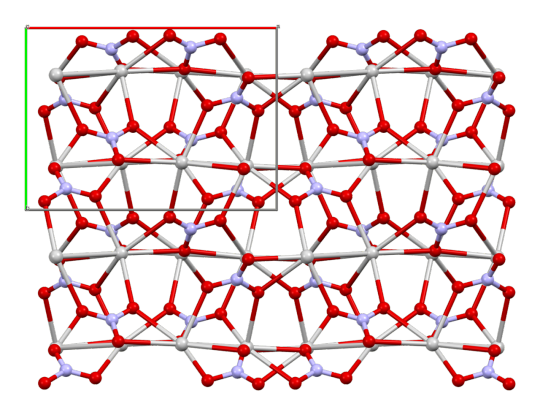
the above is the crystal structure
appearance is just a white crystal kinda like sugar
it took everything in me to not just make silverr plain Ag
silver nitrate is the most common precursor for all other important silver salts
also an extremely important compound in the development of photography! (and iirc silverr is a film major)
Feinberg as Ozone:
O3

produced during lightning strikes
pale blue at high ppm
only leaves gas state at cryogenic temperatures
naturally occurring in the stratosphere and absorbs UV rays from the sun
Fruit as Nickel(II) Chloride Hexahydrate:
NiCl2•6H2O

green
the non-hydrate form is a sort of olive-y yellow color
used to absorb ammonia in gas masks
Raddles as Potassium Permanganate:
KMnO4

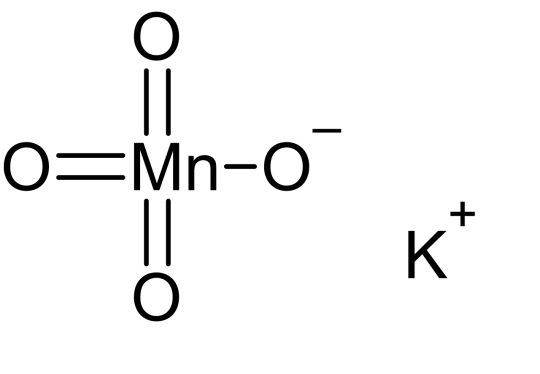
Sometimes referred to as Purple Potion Powder
goes CRAZY purple when dissolved and is lowkey my favorite chemical
very strong oxidizing agent
one time i stained my hand purple through my glove with this shit idk how it happened
if made in specific solvents can look extremely similar to dragon's breath in minecraft imo
K4 as Octathio[8]circulene:
C16S8
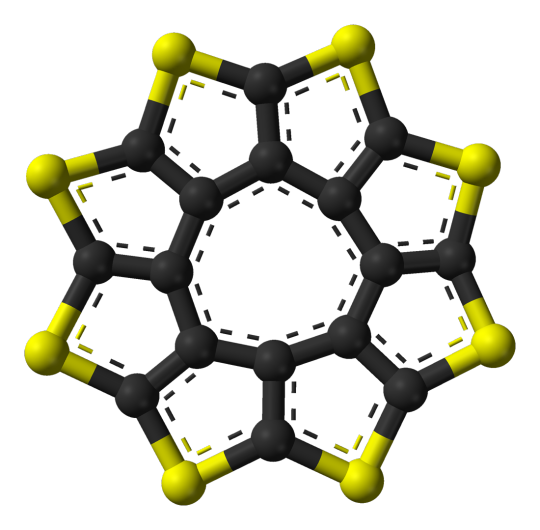
also referred to as Sulflower (like sulfur and sunflower haha get it)
planar which is fairly uncommon for molecules of this size
can be stacked together to make sheets of sulflowers
Cube as Cubane:
C8H8
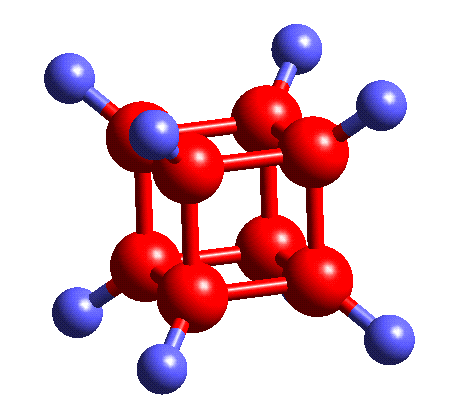
yeah this is self-explanatory
what is interesting though is that ring strain in 4 membered rings/squares is really high, so cubane existing is a bit of a chemical anomaly
i havent read into it enough to know for sure but i suspect that ring strain is why cubane is a precursor to a HELLA STRONG explosive compound
Reignex as PPTA:
Poly-p-paraphenylene terephthalamide
[-CO-C6H4-CO-NH-C6H4-NH-]n
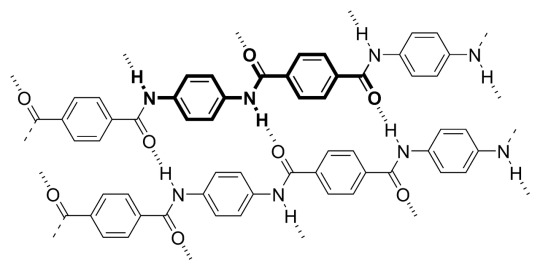
the name is complicated as shit but this is just kevlar!
aka bulletproof vest material
looks fluffy when not woven completely together
aligning of polymer chains w hydrogen bonds creates EXTREMELY high tensile strength
Mime as Phenylmagnesium Bromide:
C6H5MgBr

a common grignard reagent aka a compound that can be used in a grignard reaction, an extremely important reaction in organic synthesis as it creates new C-C bonds
another fun fact about grignard reagents is that if water is added to them- or even if they're handled in particularly moist air- they fucking explode
extremely strong nucleophile and base
Poundcake as Xenon Hexafluoride:
XeF6
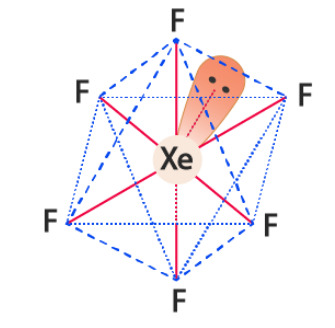
Noble gases don't react unless you REALLY make them
so a compound containing xenon is really interesting
colorless as a solid but sublimes (aka skips straight from solid to gas) into a bright yellow gas
fun fact a lot of instances where typical chemistry rules are broken (noble gases not reacting, octet rule in general, etc) involve fluorine to the point ive heard it referred to as a "batshit electron thief"
Fulham as Iron Hexacyanidoferrate:
C18Fe7N18


also known as prussian blue
extremely common pigment in paints and the first modern synthetic pigment
used extensively in The Great Wave
another one of my favorite molecules bc im biased and like inorganic chem aka things that contain metals
used as an antidote for heavy metal poisoning which is interesting bc it contains cyanide ligands!
Couriway as Bullvalene:
C10H10
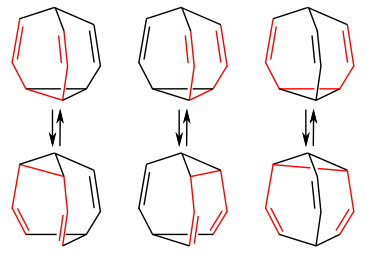
in a state of constant resonance
aka the double bonds are CONSTANTLY shifting and reforming bullvalene into... itself but moved around a little
the bonds fluctuate so rapidly that in nmr analysis each carbon and hydrogen in the entire molecule is read as equivalent (for my non-chem people that's very uncommon and very cool)
formed through photolysis (aka using light/photons to fuel a reaction)
#i made this for me and only me#chemistry is a disease and i will not be getting better anytime soon#90% of these picks are straight soul reads im gonna be so fr#mcsr#hbg#fruitberries#feinberg#couriway#fulham#president poundcake#raddles#silverrruns#reignex#talkingmime#cube1337x#k4yfour
90 notes
·
View notes
Text

YEAH GO CRAZY
My piece for the @hermitzine!!!! This was inspired by MUZZ - Get Crazy (AgNO3 Remix)
I had so much fun working on this, and everyone was so nice! The zine is already out so go check it out here if you haven't already!!!
#theyre playing total chaos :)))))#hermitcraft#zombiecleo#impulsesv#docm77#cubfan135#ethoslab#falsesymmetry#geminitay#joe hills#xisumavoid#hermitcraft fanart#hermitcraft s9#hermitcraft total chaos#GO CHECK THE ZINE GOGOGOGOGO#mcyt#messiersart#I POSTED THIS SO LATE...... AUOGH..
195 notes
·
View notes
Text
Cl- + AgNO3 -> AgCl + NO3-
AgCl + 2 NH3 -> [Ag(NH3)2]+Cl-
[Ag(NH3)2] + Cl- + 2 H+ -> AgCl + 2 NH4+
ALT (Thiosulfate edition)
S2O32- + AgCl -> Ag2S203 + NO3-
Ag2S2O3 + H20 -> H2SO4 + Ag2S
ALT (Bromide edition)
Br- + AgNO3 -> AgBr + NO3-
AgBr + 2 NH3 -> [Ag(NH3)2]+Br-
[Ag(NH3)2]+Br- + 2 H+ -> AgBr + 2 NH4+
ALT (Iodide edition)
I- + AgNO3 -> AgI + NO3-
AgI + NH3 -> No Change
#feli speaks#uni posting#one proof down. many more to go. silvernitrate proof...... so common.... so fast....#though if u got more halogenides than just chloride you need to do some more shit of course#run the chloramin T proof to double check whether you have iodide or bromide#gotta undisturb the proof to check for halogenides if u got thiosulfate
3 notes
·
View notes
Text
If we mix aqueous solutions containing equal amounts of the soluble salts silver nitrate and sodium chloride (figure 10.16), we see immediate precipitation of the 'insoluble' salt AgCl, according to the equation:
AgNO3(aq) + NaCl(aq) → AgCl(s) + NaNO3(aq)

"Chemistry" 2e - Blackman, A., Bottle, S., Schmid, S., Mocerino, M., Wille, U.
5 notes
·
View notes
Text

My copic marker drawing of Cyrene Gyllenkrantz, my original character
#aesthetic#art#artists on tumblr#copic art#copic drawing#copic markers#dark academia#gothic#my art#oc art#vamptember#vampyr#vampire#vampcore#fantasy#scifi#scifiart#cyborg#cyberpunk#dark aesthetic#dark acamedia#dark acadamia aesthetic#concept art#original character#original art#armour#SoundCloud
3 notes
·
View notes
Text
Chemistry Sem 4 End - Mod 2
Precipitation and neutralization of liq. ammonia
Liquid NH3 contains a strongly electronegative element, nitrogen, which causes hydrogen bonding. NH3 has sizable dielectric constant and has a high ionizing capacity.
Precipitation Reaction:
The solubility of different substances in liq. NH3 and H2O are different.
A number of reaction which are not possible in water can occur in liq. ammonia.
Silver nitrate gives AgCl ppt. in water
KCl + AgNO3 <-H2O-> KNO3 + AgCl ppted
While on the other hand, KNO3 and AgCl react in liq. ammonia to give KCL ppt.
AgCL + KNO3 <-Liq. NH3-> KCl ppted + AgNO3
-
Neutralization Reaction:
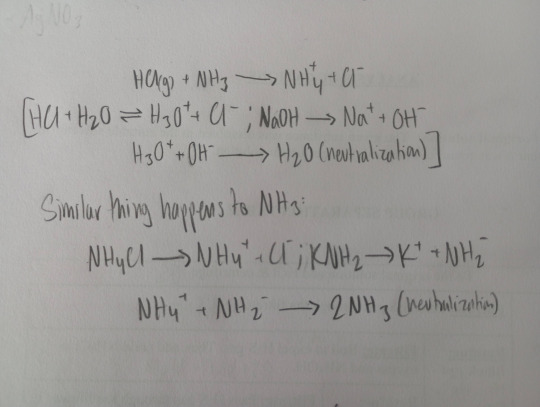
The role of HCl in water is the same as NH4Cl in ammonia.
The role of KOH in aqueous solution is the same as KHN2 in NH3.
NH3Cl is a strong acid and KNH2 is a strong base in ammonia solution.
--- toxicity of As, Hg, Pb
Arsenic Poisoning
Arsenic is an essential ultra-trace element present in red algae, chicks, humans, and some other mammals.
In chicks, deficiency of arsenic results in depressed growth.
But when present in more than ultra trace quantities, it is moderately toxic to plants and highly toxic to mammals.
Lewisite is a deadly poisonous gaseous arsenic compound: - Cl2-AsCH=CHCl It binds to enzymes in living species and weakens its activity.
Arsenic contaminated drinking water can cause severe damage to our skin, liver, and kidneys.
Arsenic compounds are used as insecticides by farmers. Such pesticides, mining operations, and the burning of coal are the chief sources of arsenic pollution in the air and water.
-
Mercury Poisoning
Mercury has no biological function.
Hg is highly toxic to fungi, plants, and animals. It is a contaminated poison for mammals.
The intake of Hg-contaminated fish can cause serious issues.
If Hg is dissolved into the blood and carried to the brain, it can cause irreversible damage to the central nervous system.
Monomethyl and dimethyl mercury ([CH3Hg] and [(CH3)2Hg)] cause nervous disorders in marine life.
Contact with mercury causes nervousness, fear, inability to make decisions, heaviness, irritability, headaches, pessimism, fatigue, sleeplessness, trembling of limbs, falling teeth, and diarrhea. Also genetic changes.
Mercury is primarily from two sources: natural volcanic eruption or weathering of mercury-containing rocks, and as a waste product in industrial processes.
Animal charcoal and penicillamine both act as antidotes for Hg poisoning.
-
Lead Poisoning
Lead has no biological function.
Pb is a cumulative poison as it continuously accumulates in the tissues of organisms.
Lead in very low concentrations such as 0.2ppm in the human body can cause metabolic disturbances.
Lead can bind to cellular enzymes, this disrupts the functioning of cells and organs of the muscular, circulatory and nervous systems.
Lead damaged organs such as the liver, kidneys, and intestine.
Lead develops issues and abnormalities in fertility and pregnancy.
Lead causes coagulation of proteins.
Lead can be absorbed by skin, resulting in many skin diseases.
The deposition of lead in bones and teeth causes tiredness, headache, loss of appetite, anemia, muscular weakness, etc.
Excessive intake of lead causes disruption of hemoglobin synthesis, loss of appetite, anemia, kidney disfunction, nervous disorders, and brain damage.
Air gets polluted by PbBr2 and PbCl2 through the combustion of petrol.
Lead pollution is also caused by industries involved in lead mining, extraction, purification, and the manufacture of alloys and paints.
2PbCO3.Pb(OH)2, a lead based pigment causes serious health hazards.
Even prolonged use of lead utensils causes 'lead sickness.'
In the case of lead poisoning, intravenous injection of CaNa2.EDTA can treat the patient.
--- Hemoglobin structure, how does it carry oxygen
Hemoglobin or Hb is the red pigment which is present in red blood cells in the human body.
For each 100ml of blood in a normal human male there is around 15g of Hb.
About 65% of iron in the human body is present as Hb.
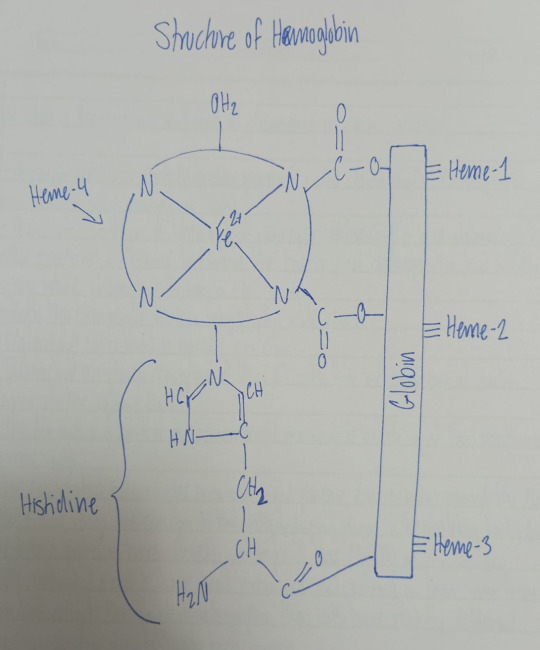
Hb is Fe(II) porphyrin, it contains four identical units arranged roughly tetrahedrally.
Each histidine unit has one heme group attached to it.
The molar mass of Hb is about 64500. Each Hb molecule has 4 heme groups: heme-1, heme-2, heme-3, heme-4, they are bound to globin on its surface.
Therefore Hb is a heme containing protein
The 4 heme groups present in the structure of Hb are the subunits of Hb.
Hb is an octahedral complex of Fe(II). Fe(II) occupies the central position and the four corners of the square base are occupied by the four N-atoms of heme group. One axial position is occupied by N-atom of histidine and the other axial position is occupied by H2O molecule.
The 4 subunits are linked together through salt bridged present between the four polypeptide chains. it is now believed that the salt bridges present in between the polypeptide chains of Hb introduces stain in the molecule of Hb.
Hb which has not been oxidized is called deoxy-hemoglobin or just hemoglobin while Hb which has been oxidized is called oxygenated-hemoglobin or oxyhemoglobin.
Fe(II) present in Hb can be oxidized to Fe(III) to form Fe(III) protein called met-hemoglobin. Fe(III) protein is responsible for the brown color of old meat and dried blood.
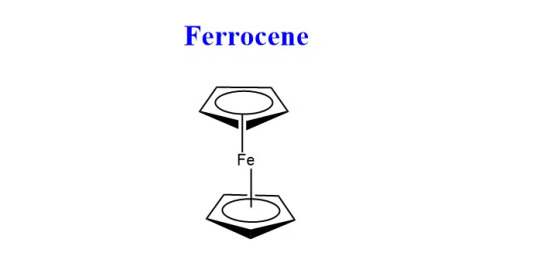
--- Synthesis of Ferrocene + properties
Aka dicyclopentadienyl iron, has the chemical formula (C5H5)2Fe
Ferrocene is the first discovered metallocene sandwich compound.
Metallocene is derived from “Metallo”- transition metal and “Cene” - benzene.
The cyclopentadienyl ligands C5H5 bond in such a way that iron is present in the center of the complex resulting in a sandwich structure
Primarily ferrocene is used as a catalyst in the industrial world
-
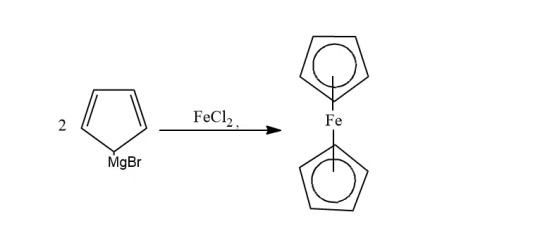
Synthesis
Can be prepared: - From Grignard's reagent - From sodium cyclopentadienide - From cyclopentadiene
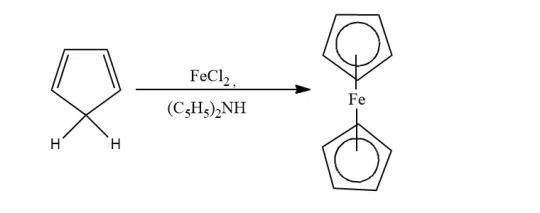
Grignard's: When the Grignard reagent is treated with iron chloride(II), ferrocene is formed
Sodium Cyclopentadienide: Cyclopentadiene is treated with sodium metal to form cyclopentadienide, which on reacting with FeCl2 forms ferrocene.
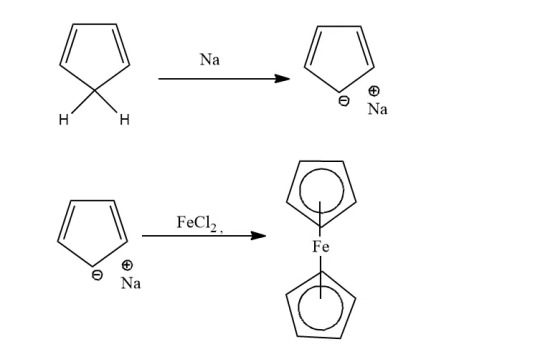
Cyclopentadiene: When cyclopentadiene is treated with iron chloride in the presence of a strong base, ferrocene is obtained. Lab method.
-
Physical Properties
Orange crystalline solid at room temp w a camphor like odor.
Stable organometallic compound w melting point 173-174°C and boiling point 249°C.
less soluble in water but soluble in organic solvents like acetone.
-
Chemical Properties
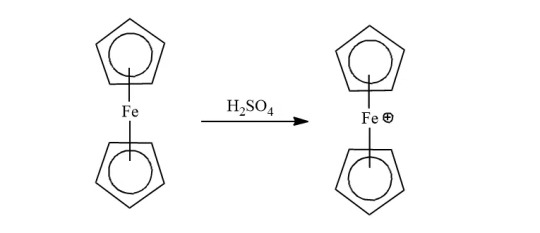
Ferrocene can undergo numerous electrophilic reactions faster than benzene. But, it can not undergo an electrophilic reactions with electrophiles which are strong oxidants like H2SO4 or HNO3.
It can also undergo formylation and carboxylation reactions to give mono-functionalized derivatives, since the functional group extensively deactivates the ferrocenyl group.
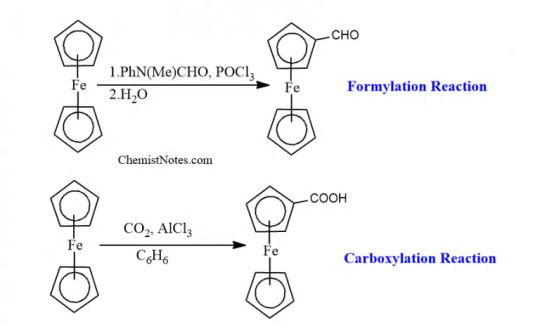
Friedel craft acylation reaction can be given by Ferrocene.
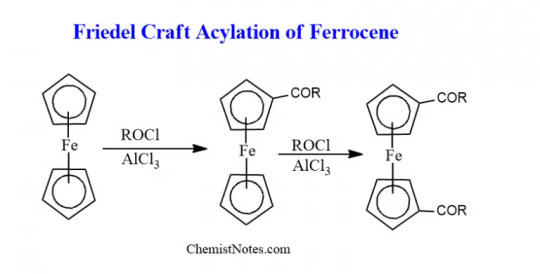
--- Solubility Product + Common ion effect
Solubility Product
When a saturated solution of a salt, for example AB, is in contact with the solid phase, the following equilibrium forms:
AgCl (undissolved solid) <--> AgCl (dissolved but unionized) <--> Ag⁺ + Cl⁻ (ions)
AgCl is sparingly soluble in water
The solid is in equilibrium with the ions of solution and this applying the law of mass action, in general: [Ag⁺][Cl⁻]/[AgCl] = K
Now since the solution is saturated and is in contact with the solid, the concentration of the unionized salt molecules should be constant at constant temp.
Hence: [Ag⁺][Cl⁻]/Constant = K where [Ag⁺][Cl⁻] = new constant, [Ag⁺][Cl⁻] = Ksp
Thus, the product of the ionic concentration in a saturated solution of a soluble electrolyte at a constant temp in a constant quantity called solubility product (Ksp)
Ex: using excess reagent, since BaSO4 is insoluble in H2O a small amount would remain in solution if equal amounts of two reagents, like BaCl2 + H2SO4 are taken.
Therefore when carrying out complete precipitation, the precipitant must always be added in a little excess such that the ionic products of compound to be precipitated far exceeds its solubility product.
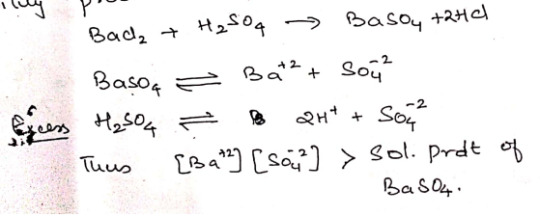
-
Common Ion Effect
Consider dissociation of a weak electrolyte in water, there exists an equilibrium between ions and unionized molecules to which the law of mass action can be applied.
ex: AB <--> A⁺ + B⁻, [A⁺][B⁻]/[AB] = K (ionization constant)
If the above electrolyte solution added another strong electrolyte with a common ion, either A⁺ or B⁻, the cation or anion will increase.
In order to keep 'K' constant, [AB] should increase in concentration.
This suppression in dissociation is due to a common ion, hence the phenomenon is called common ion effect.
Thus, the suspension of the dissociation of a weak electrolyte in presence of a strong electrolyte which have a common ion with each other is called Common Ion Effect.
-
In group II, cations are ppted as sulphides in acidic medium while in group IV, the cations are ppted as sulphines in basic medium.
The weak electrolyte H2S ionizes in aqueous solution to give sulphide ions in sufficient amounts to exclude the solubility product of group II and the group IV radicals.
But when the solution is made acidic with the addition of dil. HCl, H⁺ ions from the HCl suppresses the ionization of H2S due to the common ion effect
This causes sulphide ion concentration to decrease, thus only the sulphides of group II with a much lower solubility product than the group IV are ppted.
In the presence of NH4OH, the OH- ions combine with the H+ of H2S, forming unionized water, resulting in greater ionization of H2S molecules to give H+ and S-.
This increases the concentration of S- ions, it becomes so high that the solubility product of group IV sulphides is exceeded and this ppted out.
H2S <--> 2H⁺ + S⁻2 is feebly ionized
HCl <--> H⁺ + Cl⁻ is highly ionized
---
Flame test + brown ring test
Flame Test
Flame test is a quick test used in semi-micro analysis to identify the presence of a relatively small number of metal ions in a compound
Flame test is effective in indicating the presence of group I and group V cations
- Principle:
If you excite an atom or an ion by strongly heating, electrons can be promoted from their normal unexcited state into higher orbitals.
As they fall back down to lower levels, energy is released as light.
Each jump involves a specific amount of energy being released as light, and each corresponds to a particular wavelength.
As a result of all these jumps, a spectrum of lines will be produced, some of which will be in the visible part of the spectrum.
The visible color is a combination of all those individual colors.
- Procedure:
To the salt mixture taken in a watch glass, add 2 drops of conc. HCl. Mix with a glass rod to make a paste, expose the end of the rod to the flame of the bunsen burner.
If an apple green color is observed, barium cation from group V may be present.
If a brick red color is observed, calcium cation may be present.
If a crimson red color is observed, strontium cation may be present
-
Brown Ring Test
Brown ring tests is a common nitrate test which determines the presence of nitrate anion in any solution.
- Principle
The nitrate ion functions as an oxidizing agent.
Iron (II) reduces the nitrate ion in the reaction mixture, and iron (II) is oxidized to iron (III).
When nitric oxide is reduced to NO-, ferrous nitroso sulphate complex is formed, which creates a brown ring at the intersection of two layers
- Procedure
A small portion of Na2CO3 extract is taken in a semi-micro test tube
It is acidified by adding a few drops of dil. H2SO4 till no more effervescence is evolved, then followed by a dilute solution of FeSO4.
By holding the test tube at an inclined position, conc. H2SO4 is slowly added along the inner wall of the test tube.
If a brown ring is formed at the junction of the two layers, the presence of nitrate ion is confirmed.
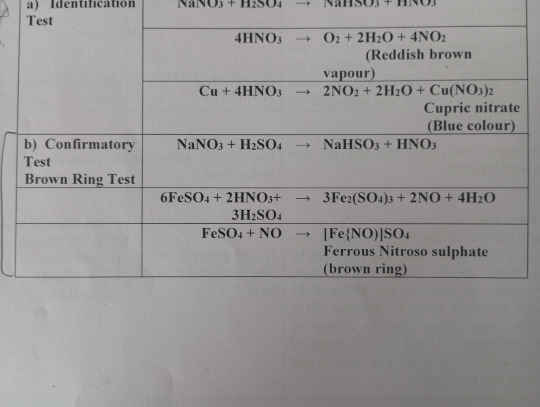
---
Classify organometallic compounds
Organometallic compounds, or organometallics, are compounds where the central metal atom is linked directly with the C atom of the organic ligand.
These compounds contain 1 or more M-C bonds.
The metal atom may be a transition metal, lanthanide, actinide, or a main group element.
Although B, Si, Ge, As, etc. are all non-metallic elements, they are also classified as organometallic compounds.
Cyanides and carbides have M-C bonds, but these compounds are not organometallic compounds because metal is not attached to the carbon of alkyl group.
Classification on the bases of nature of M-C bond: - Ionic bonded - σ-bonded - π-bonded - Bridge bonded
Ionic bonded - The organometallic compounds of highly electropositive metals are usually ionic in nature - In these compounds, the hydrocarbon residue exists as a carbanion with a negative charge, it is attracted to the metal atom by non-directional electrostatic forces. - Colorless, non-volatile, very reactive solids, insoluble in organic solvents. - ex: OMCs with alkali (no lithium), alkaline earth metals, etc. - R2M (M=Ca or Ba), R⁻Na⁺
σ-bonded - C atom of organic ligand bonds to the metal by a 2e⁻, 2 centered covalent bond. - Metals with low electropostive nature form sigma-bonded covalent bonds. - Formed by most elements with values of electropositivity > 1, such as non-metals and weakly electropositive metals. - M and C share a pair of electrons, forming a covalent bond. - ex: (CH3)2Zn
π-bonded - Specific to transition metals - First π-bonded OMC was prepared by ferrocene: (C5H5)2Fe - Ferrocene has a sandwich structure in which the iron atom lies between 2 planar C5H5 rings:

- Bonding involves overlap of π-electrons of the cyclopentadienyl rings with unfilled d-orbitals of the metal.
Bridge-bonded - The compounds in which a loosely bonded electron deficient species exists with the coordination of metals like Li, Be, Al, etc. - This group includes the organometallic compounds with bridging alkyl groups. - Ex: Al2Me6
--- Reactions in HF
Anhydrous HF is a strong acidic solvent
It has high dielectric constant = 84
High dipole moment = 1.9D
Boiling point = 19.4°C and Freezing point = -83°C
It acts as a better solvent than water.
-
Auto Ionization:
Aka self ionization, refers to the process in which a molecules can donate a proton to another molecule of the same species, resulting in the formation of ions.
HF + HF -> H2F⁺ + F⁻
Only a few substances can donate H⁺ to HF:

Precipitation:
Perchlorates, sulphates of non-alkali metals get precipitated when their fluorides dissolved in liq. HF are treated with solution of alkali metal sulphate, perchlorate, etc. NaClO4 + TiF -HF-> TiClO4 ppted + NaF Na2SO4 + NiF2 -HF-> NiSo4 ppted + 2NaF
Acid-Base Reactions:
3HF <--> H2F⁺ (acid) + HF2⁻ (base)
All substances which can form H2F⁺ ion will behave as an acid and substances which yield HF2⁻ ion behave as a base. HNO3(base) + HF -> H2NO3+ + F- BF3(acid) + 2HF -> BF4- + H2F+ HClO4(acid) + HF -> ClO4- + H2F+ HClO4(base) + HF -> H2ClO4+ + F-
Protonation:
Organic compounds like ethanol, benzene, etc. get protonated when dissolved in HF. C2H5OH + HF -> C2H5OH2+ + F- C6H6 + HF -> C6H7+ + H-
---
(5M) Solvolysis reaction of liq. ammonia w ex
Solvolysis is a type of nucleophilic substitution or elimination reaction where the nucleophile is a solvent molecule.
Ammonolysis or ammolysis is solvolysis by ammonia
The attachment of one or more solvent molecules to a solute species through any chemical linkage.
Its products are called solvates H2O -> Hydration -> Hydrates liq. NH3 -> Ammonation -> Ammonites BF3 + NH3 -> BF3.NH3 SiF4 + 2NH3 -> SiF4.2NH3
--- (5M) Bio significance of Na, K, Mg, Cl
Sodium:
Na⁺ cation is a major ion present in the extracellular fluids of organisms, it can be found in large quantities in bones as phosphate. May also be present in chloride and bicarbonate.
Na⁺ ion activates some enzymes in animals.
Imp source for sodium is common table salt (NaCl), which is used in all cooking.
Excessive intake of Na⁺ ions can cause hypertension, for example when fish take in too much saline water where NaCl is in large amounts.
In biological systems, Na⁺ ions regulate acid-base equilibrium.
Sodium is important for the formation of HCl acid in the stomach., the conduction of nerve impulses, and muscle contraction
Since herbivorous animals fail to receive enough Na⁺ ions from their diets, they look out for 'salt-lick' to bridge the gap in their Na⁺ requirements.
Sodium also helps in maintaining osmotic pressure of the body fluid, protecting the organism from fluid loss.
Sodium helps in the prevention of normal irritability of muscle and permeability of cells
Injectable medicines are dissolved in NaCl before they can be administered to humans
Na⁺ cations are essential for both nerve action and heart function.
Na⁺ cation is also responsible for the transport of glucose and amino aids into the cell.
-
Potassium:
K⁺ cation is primarily in intra-cellular fluid as well as extra-cellular fluid.
Imp sources is almost all foods like coffee, tea, cocao, dried beans, molasses, leafy green veg, milk, fish, chicken, pork, dried apricots and peach, banana, orange juice, etc.
K⁺ ion is essential for nerve impulse and muscle contraction.
K⁺ ions are important for all organisms with the potential exception of blue green algae.
When injected intravenously potassium is moderately toxic to mammals.
Much like sodium, potassium also influences acid-base equilibrium in extracellular fluid.
Potassium controls osmotic pressure and water retention
Potassium is essential for metabolic functions such as protein biosynthesis by ribosomes.
Enzymes such as glycolytic enzyme pyruvate kinase requires K⁺ for optimization.
K⁺ ions are required in cells for glucose metabolism, protein synthesis, and activation of some enzymes.
-
Magnesium
Mg is an important non-transition element to all organism. It is present in great concentration in red blood cells.
Imp sources are almonds, cereals, beans, green veg, potatoes, and cheeses.
Mg(II) specifically plays an essential role in many metallo-enzymes
Mg2⁺ cation is present in chlorophyll of plants, enabling the process of photosynthesis.
Constipation, obesity, liver, and gall bladder disorders can all be treated with Mg(II) salts.
Mg forms a vital complex with ATP which is required for most enzymatic reaction involving ATP within the cell.
Magnesium ions are present in important enzymes such as phosphate and aminopeptidase.
Magnesium deficiency is induced by tetanus like cramps and abnormal swelling of the atrial walls in plants. Mg deficiency destroyed the green color of leaves.
-
Chlorine
Cl⁻ ion is an essential electrolyte, commonly found in the body's fluids and blood.
An imp source for sodium is common table salt (NaCl), which is used in all cooking.
Cl⁻ may be present as chlorides (HCl, NaCl, KCl, etc.)
Chlorine is primarily absorbed from the gastrointestinal tract.
Cl⁻ ion helps the body to maintain normal hydration and osmotic pressure, prevents excessive fluid loss.
Cl salts aid in the maintenance of normal acid-base equilibrium.
Chloride ions play an important role in the gaseous transport of CO2.
NaCl and KCl maintain the correct viscosity of blood.
Gastric HCl is an important digestive fluid.
Chlorine ions are mostly excreted by the kidneys in urine.
Deficiency of chlorine in the body leads to hypochloremia: poor appetite, increased urination, irritability, muscle twitching
Excess of chlorine in the body leads to hyperchloremia: diarrhea, vomiting, fatigue, dehydration
--- (5M) Reactions for identification of CO3(2-) and NH4+ ions
Analysis of NH4⁺ cation:
Ammonium salts when titrated with NaOH solution produces ammonia gas with a pungent odor.
NH4Cl + NaOH -> NaCl + NH3 + H2O
The evolution of NH3 gas allows us to confirm that NH4⁺ is present.
To confirm the evolved gas in NH3:
i) Expose the gas to a filter paper paper dipped in CuSO4 solution - If the paper turns blue, the gas in NH3 and HN4⁺ is initially confirmed. - CuSO4 + 4NH3 -> [Cu(NH3)4]SO4 - [Cu(HN3)4]SO4 is tetramine copper complex, it is a deep blue color.
ii) Expose the gas to a filter paper dipped in Nessler's reagent. - If the paper turns brown, NH4⁺ is confirmed. - Ammonium chloride reacts with Nessler's reagent to form a brown ppt called iodide of Millon's base.

-
Analysis of CO3(2-) anion
Carbonate salts when treated with dilute HCl acid produces carbon dioxide gas along with brisk effervescence.
Na2CO3 + 2HCl -> 2NaCl + CO2 +H2O
Confirming that the gas emitted is CO2 will confirm the presence of CO3(2-).
If we pass the gas through lime water and the lime water turns milky, the gas if confirmed to be CO2 and CO3(2-) is confirmed.
CO2 + Ca(OH)2 (lime water) -> CaCO3 + H2O
---
Chlorophyll Structure

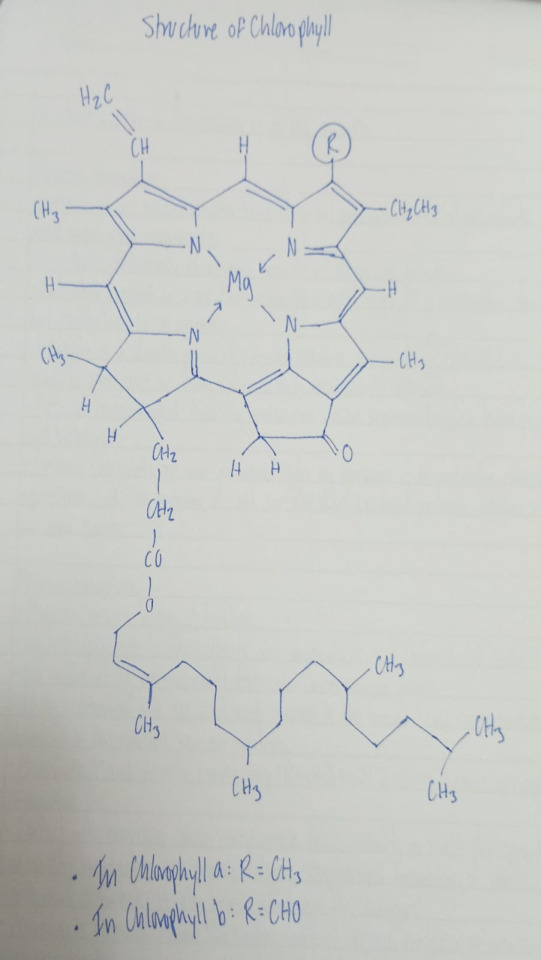
---
Grignard's Reagent


0 notes
Text
What Are Laboratory Chemicals And Why Are They Essential?

Lab chemicals are important for many scientific experiments, research, and industrial processes. These substances help to carry out accurate tests that form the basics of chemistry, biology, physics, and environmental science. In this blog, we will discuss different types of laboratory chemicals, their uses in scientific work as well as leading laboratory chemicals manufacturers and suppliers in India.
What are Laboratory Chemicals?
Laboratory chemicals are substances used in laboratories for research or experimental purposes such as analysis, synthesis, or for experiment. They can be elements, compounds, or mixtures that serve various roles like reagents for reactions; solvents; indicators, etc.
Common Laboratories Chemicals And Their Uses
Below are some common laboratory chemicals, detailing their specific uses and significance:
Acetic Acid Glacial (1%)
It is a highly concentrated form of acetic acid commonly used as a reagent in chemical reactions at 1% concentration; solvent for different organic syntheses and for pH adjustment of solutions.
Acetocarmine Sulphate (Nucleic Acid)
Acetocarmine Sulphate is primarily used as a stain in microscopy for observing nucleic acids, especially chromosomes during cell division. It Helps enhance the contrast of the structures, making them more visible under microscopes.
Barium Sulphate
Widely employed as a radiopaque agent during medical imaging procedures because it makes certain areas more visible under x-rays than others; also acts as filter material in the plastics industry alongside rubber production and other uses – such filters may remove contaminants from liquids or gasses passing through them based on their pore sizes which allow only desired molecules to pass through while blocking unwanted ones, Analytical chemists often use it when looking at ways of determining sulfate ions within samples.
Carbon Disulphide
Carbon disulfide is a volatile solvent used in chemical synthesis and industrial applications. It is often used for the extraction of oils and fats and in the manufacture of rayon and cellophane
Copper Sulphate
Copper sulfate is widely used in laboratories for various purposes, including as a reagent in Fehling’s solution for testing reducing sugars, as a fungicide, and in the preparation of Bordeaux mixture for agricultural use.
Potassium And Sodium Tartrates
These potassium and sodium tartrates are used in laboratories as reagents in Fehling’s solution and in the food industry as emulsifiers and acidity regulators. They are also involved in the manufacture of some medicinal products.

Potassium Bromide
Infrared spectroscopy samples are commonly prepared using Potassium Bromide. As a reagent in analytical chemistry with applications within the pharmaceutical industry there were also other uses.
Potassium Chloride
It is used For the different chemical reactions at laboratories, it is used as a reagent together with being used to prepare buffer solutions. Also, it serves as an electrolyte replenisher during medical treatments.
The Function Of Chemicals And Laboratory Reagents
To create reactions and study the outcomes, substances are used in the lab commonly known as laboratory reagents or chemicals. They are necessary for qualitative as well as quantitative analysis; this helps scientists understand the composition, properties and behavior of different materials. Each may have its own purpose in an experiment so there can be many types of them.
Common Laboratory Reagents
Laboratory Reagents are compounds or mixtures introduced into systems to elicit a chemical reaction or test if one does occur. Some examples include:
Hydrochloric Acid (HCL)
Sodium Hydroxide (NaOH)
Ethanol (C2H5OH)
Sulphuric Acid (H2SO4)
Silver Nitrate (AgNO3)
These are some of the most commonly used reagents in labs, they could be used for different analytical methods like titrations, precipitations and pH adjustments.
A Chemical reagent is a substance or compound that is added to a reaction to determine whether it occurs or not. For example Fehling’s solution which can detect presence-reducing sugar by heating after mixing with a sample gives positive results indicated by brick red precipitate showing these sugars were present.
Why Choose Science Lab Export?
Lab chemicals and reagents form the backbone for scientific research and industrial processes worldwide. This is because they enable accurate experimentation; new product development as well as understanding nature better. These products therefore cannot be overlooked be it medical imaging, agricultural testing or even synthesis of chemicals during various experiments we do need them always.
It is important to comprehend different types of Lab chemical, if one wants to succeed academically or professionally in this field. If you require high-quality lab chemicals, consider Science Lab export. As a leading manufacturer and supplier of lab chemicals, we provide a wide-range of products to meet our scientific needs.
#laboratory chemicals manufacturers and suppliers in India#laboratory chemicals manufacturers and suppliers#laboratory chemicals manufacturer
0 notes
Text
0 notes
Text
What are the Benefits and Limitations of Tollens Test?
Tollens test, also known as the silver mirror test, is a chemical test used to distinguish between aldehydes and ketones. This test is based on the oxidation-reduction reaction between aldehydes and reagent, which results in the formation of a silver mirror on the inner surface of the reaction vessel.
Principle of Tollens Test
The principle behind the Tollens test lies in the fact that aldehydes are readily oxidized to carboxylic acids, while Tollen’s reagent, which is an alkaline solution of silver nitrate, acts as an oxidizing agent. When an aldehyde is present in the solution, it reduces Tollen’s reagent, causing elemental silver to precipitate and form a mirror-like coating.
What is Tollens Reagent?
Tollens reagent, also known as silver mirror reagent, is a solution that contains silver ions in an alkaline medium. It is prepared by adding silver nitrate to a solution of sodium hydroxide until a slight precipitate of silver oxide is formed. The precipitate is then dissolved by adding ammonia solution drop by drop until the solution becomes colorless.
Tollens Reagent Preparation
To prepare Tollens reagent, follow these steps:
Dissolve 5 grams of silver nitrate (AgNO3) in 50 mL of distilled water.
In a separate container, dissolve 5 grams of sodium hydroxide (NaOH) in 100 mL of distilled water.
Slowly pour the sodium hydroxide solution into the silver nitrate solution while stirring.
A brown precipitate of silver oxide (Ag2O) will form.
Add dilute ammonia solution drop by drop to the brown precipitate until it dissolves completely and the solution turns colorless.
Test the resulting solution with litmus paper to ensure it is slightly alkaline. If necessary, adjust the pH by adding more sodium hydroxide or dilute ammonia solution.
Tollens Test Procedure
The Tollens test procedure is as follows:
Take a small quantity of the unknown compound and dissolve it in water or ethanol, depending on its solubility.
Transfer this solution into a clean test tube.
Add a few drops of Tollens reagent to the test tube.
Gently heat the mixture by placing the test tube in a water bath or by using a Bunsen burner.
Observe the reaction mixture to form a silver mirror on the test tube’s inner surface.
The appearance of a silver mirror indicates a positive Tollens test, confirming the presence of an aldehyde or alpha hydroxy ketone.
Benefits of Tollens Test
The Tollens test offers several benefits in organic chemistry:
It provides a simple and reliable method for detecting the presence of aldehydes and alpha hydroxy ketones.
The formation of a silver mirror is a visual confirmation, making the test easy to interpret.
It does not require expensive equipment and can be performed using basic laboratory apparatus.
The Tollens test can be used qualitatively as well as quantitatively for determining the concentration of aldehydes in a given sample.
Limitations of Tollens Test
While the Tollens test is a valuable tool, it does have some limitations:
It only detects aldehydes and alpha hydroxy ketones, not other functional groups.
The reaction requires the presence of an acidic hydrogen atom adjacent to the carbonyl group.
It may not work efficiently for highly reactive aldehydes or alpha hydroxy ketones.
The test can yield false-positive results if reducing agents other than aldehydes or alpha hydroxy ketones are present in the solution.
In conclusion, the Tollens test is a useful chemical test that provides a simple and reliable method for detecting the presence of aldehydes and alpha hydroxy ketones. By observing the formation of a silver mirror, chemists can confirm these compounds in a sample. The Tollens test has its limitations, but when used appropriately, it can provide valuable information in organic chemistry analysis.
Keen on effortlessly mastering concepts, as explained above? Dive into our Tutoroot Blog section for simplified learning. Enhance your understanding of subjects and have your questions clarified through Tutoroot online tuition. Immerse yourself in the world of Tutoroot’s online home tuitions by scheduling a FREE DEMO session today.
0 notes
Text
Navigating Regulatory Frameworks in the Silver Nitrate Market

Silver Nitrate: An Essential Chemical Compound for Businesses History and Discovery of Silver Nitrate Silver nitrate is an inorganic compound with chemical formula AgNO3. It was discovered in the late 16th century through the reaction of silver metals with dilute nitric acid. Since then, its wide applications have been found and it became commercially important. Key historical milestones include early uses as cauterizing and disinfecting agents in medicine. Purification and Manufacturing Silver nitrate is commercially produced through the dissolution of silver metal in hot, concentrated nitric acid. This yields a crude impure salt containing residues and byproducts. Further purification is done by recrystallization to produce a white, crystalline powder or colorless crystalline chunks. Strict quality control is required as even small impurities can negatively impact end uses. Medical Applications Silver nitrate finds enduring usefulness as a powerful antiseptic and escharotic agent in medicine. It is commonly used to treat a variety of external wounds and infections by cauterization. This involves applying the compound to affected areas, where it destroys pathogens and seals lesions. Internally, it can help stop minor bleeding when swabbed onto irritated tissues. Silver nitrate solutions also assist in various dermatological procedures like treating keratoses or destroying tissue samples for examination. The astringent and disinfectant properties contribute to its ongoing role in surgical and clinical settings. Electronics and Battery Functions Within electronics manufacturing, silver nitrate finds an assortment of specialized purposes. It provides the necessary silver component for decades-established processes like toner fusing in photocopiers. Silver nitrate also assists with silver plating of electrical contacts and circuit boards for dependability. Looking ahead, R&D explores its potential uses in organic electronics, printed flexible circuits, and other emerging applications. Laboratory and Analytical Reagent Due to its distinctive properties, silver nitrate maintains a ubiquitous role across scientific labs globally. It acts as a qualitative reagent for identifying ions like halides or ammonia. For instance, NaCl solution yields a white AgCl precipitate with silver nitrate. Analytical chemists further leverage its solubility behavior in qualitative and quantitative analyses. Silver nitrate also enables titration experiments for determining chlorine or nitrate content. Safety Considerations and Regulations While silver nitrate offers invaluable industrial and medical applications, it requires responsible usage due to certain hazards. Primary concerns relate to its caustic nature, potential for skin/eye irritation, and toxicity if ingested. Proper protective equipment like gloves and goggles are necessary during handling. Workplaces must implement safety protocols for silver nitrate storage, spill response procedures, and disposal compliance. Regulators globally issue guidelines to minimize risks. For example, many jurisdictions classify it as hazardous and restrict quantities for commercial/individual use. International standards organizations provide best practices to govern transportation and facility safety when dealing with this essential inorganic salt. In summary, silver nitrate's long history of diverse commercial use stems from its unique chemical properties and reactivity profile. While industrialization introduced mass production capabilities, each application domain still depends on a consistent supply of high-purity silver nitrate. Continued innovation also explores new functional roles, such as in emerging technologies.
0 notes
Text
قارن بين كل من نسيج عضو
قارن بين كل من نسيج عضو
قارن بين كل من میتوکندریا بلاستيدات خضراء
توقع هل لنباتات أخرى أنسجة تسهم في انتقال الماء
وضح ماذا تعمل لتحسين هذه التجربة
ماذا يحدث عند خلط محلول (AgClO 3(aq بمحلول NaNO3
استنتج لماذا تكون بعض أنسجة النبات حمراء اللون؟
إذا خلط محلول مائي من كبريتات النيكل ll بمحلول مائي من هيدروكسيد الصوديوم فهل يحدث تفاعل مرئي
فسر ما إذا كانت النتائج لهذه التجربة تؤكد فرضيتك.
ميز بين المخلوط والمحلول والمركب
ما ضوابط التجربة ؟ وما متغيرات التجربة
قارن بين لون ساق نبات الكرفس قبل التجربة، وفي أثنائها، وبعدها
بالاعتماد على قراءتك السابقة حول الطريقة ��لتي يؤدي بها النبات وظائفه، اكتب فرضية حول أين ينتقل الماء في النبات
ارسم خريطة مفاهيم على شكل سلسلة تبين فيها مستويات التنظيم الخلوية من الخلية إلى الجهاز، وأعط أمثلة على مستويات التنظيم.
لماذا يجب على الخلايا المتخصصه ان تعمل كمجموعة
وضح العلاقه بين الخلايا والنسيج وبين النسيج والعضو
وضعت قطعة من فلز الألومنيوم في محلول KCI المائي، ووضعت قطعة أخرى من الألومنيوم في محلول AgNO3 المائي. هل يحدث تفاعل في كلتا الحالتين؟ لماذ
قارن بين الخلايا الموجوده في جذور النبات وفي اوراقه وفي سيقانه
صف ثلاثة من انواع الخلايا في جسم الانسان
ميز بين مركب أيوني ومركب تساهمي مذابين في الماء. وهل تتأين المواد التساهمية جميعها عند إذابتها في الماء
ما المصطلح الذي يطلق على نسيجين او اكثر يعملان معا
0 notes
Text
Silver Nitrate Market Trends: From Automotive Applications to Electronics
Historical Significance:
Silver nitrate has a rich history dating back to the 13th century, where it was discovered by Albertus Magnus. Over the centuries, its applications evolved, and by the 19th century, it became a key component in the field of photography. Today, it continues to find applications in diverse industries.
The Silver Nitrate Market stands as a critical player in the global chemical industry, holding significance across various sectors such as healthcare, photography, and electronics. As a versatile compound, silver nitrate (AgNO3) plays a pivotal role in several applications, contributing to its consistent demand and market growth
Healthcare Sector: One of the primary applications of silver nitrate lies in the healthcare industry. It is extensively used as a cauterizing agent to remove unwanted tissues and as a topical antimicrobial agent in wound care. The compound's antimicrobial properties make it valuable in preventing infections and promoting healing.
Photography and Imaging:
In the realm of photography, silver nitrate plays a crucial role in the production of light-sensitive materials. Its light-sensitive nature makes it an essential component in traditional photographic films and papers. Although digital photography has become dominant, silver nitrate still finds applications in niche areas and artistic photography.
Electronics and Mirrors: The electronics industry relies on silver nitrate for various processes, including the manufacturing of conductive inks and the coating of mirrors. The compound's ability to conduct electricity makes it valuable in the production of electronic components, while its reflective properties contribute to high-quality mirrors.
Emerging Applications:
As technology advances, new applications for silver nitrate continue to emerge. The compound is gaining attention for its potential role in antibacterial coatings, water purification, and even in certain medical diagnostic procedures. Researchers are exploring innovative ways to harness its properties for sustainable and eco-friendly solutions.

0 notes
Text
نیترات نقره
نیترات نقره
در حال حاضر موسسه ی مبتکران شیمی به عنوان یکی از تامین کنندگان محصولات شیمیایی کشور, طیف گسترده ای از مواد شیمیایی آزمایشگاهی و صنعتی را از برندهای معتبر در اختیار تولید کنندگان قرار می دهد. برای کسب اطلاعات بیشتر می توانید با همکاران ما در ارتباط باشید.
خرید نیترات نقره
جهت اطلاع از نحوه ی فروش این محصول و ثبت سفارش شما می توانید با همکاران ما در واحد فروش موسسه مبتکران شیمی در ارتباط باشید.
اطلاعات محصول
کشور تولید کننده: چین
بسته بندی: بطری های شیشه ای تیره 1 کیلویی
درصد خلوص : 99.9%
CAS NO:7761-88-8
آنالیز محصول
نیترات نقره چیست؟
نیترات نقره (Silver nitrate) با فرمول شیمیایی AgNO3 یک جامد شفاف، کریستالی و بی رنگ است. نیترات نقره در حضور مواد آلی و نور خورشید به رنگ خاکستری یا سیاه تغییر رنگ می دهد. این ترکیب شیمیایی به صورت مایع بی رنگ، برای سنتز بسیاری از نمک های مربوطه از جمله کلوئید و هالید های نقره که به ترتیب در پزشکی و عکاسی کاربرد دارد.
همچنین این ماده در شیمی تجزیه، نوعی معرف مهم به حساب می آید.این ماده دارای فرمول شیمیایی AgNO3 و ساختار مولکولی زیر می باشد.
ساختار مولکولی نیترات نقره
این ماده به دو صورت مایع و جامد وجود دارد.
AgNO3 چه به صوت مایع و چه به صورت جامد در آب و گیلیسیرین به راحتی حل می گردد ولی با اغلب الکل ها به خوبی واکنش نمی دهد و برای انحلال پذیری به حرارت نیاز دارد.
.تولید نیترات نقره
برای سنتز این ترکیب از واکنش ورقه های نقره با اسید نیتریک استفاده می شود. در نتیجه این واکنش، نیترات نقره، آب و اکسدهای نیتروژن حاصل می شود. میزان محصولات جانبی در این واکنش به غلظت اسید نیتریک استفاده شده بستگی دارد. معادله واکنش انجام شده به صورت زیر می باشد. در معادله 1 وقتی از نیتریک اسید سرد و رقیق استفاده می شود نیتروژن اکسید به عنوان محصول جانبی تولید می گردد. در معادله دوم از اسید نیتریک گرم و غلیظ استفاده می شود و در نتیجه محصول جانبی تولید شده دی اکسید نیتروژن است.
3Ag + 4HNO3 → 3AgNO3 + 2H2O + NO
Ag + 2HNO3 → AgNO3 + H2O + NO2
خواص فیزیکی
کریستال های شفاف AgNO3 در دمای 212 درجه ی سانتی گراد ذوب می شوند. در صورت حرارت دیدن این ماده تا دمای 320 درجه ی سانتی گراد AgNO3 اکسیژن را از دست می دهد و نیتریت نقره را تشکیل می دهد. در دماهای بسیار بالا AgNO3 طبق واکنش زیر تجزیه می گردد و نقره تشکیل می شود.
واکنش تجزیه ی نقره نیترات
. کاربرد های نیترات نقره
AgNO3 در سنتز سایر مواد شیمیایی نقشی کلیدی ایفا میکند. در زیر به چند تا از جالب ترین واکنش های این ترکیب شیمیایی می پردازیم:
واکنش نقره نیترات با آمونیاک
ساخت آیینه با تهیه ی آزمایشی ساده با کمک AgNO3 امکان پذیر خواهد بود. با قرار دادن شیشه ای در محلول حاوی نیترات نقره و آمونیاک در محیطی که به وسیله ی سدیم هیدروکساید بازی شده است و افزودن مقداری شکر و حرارت دادن مخلوط حاصله می توان شیشه را تبدیل به آیینه نمود.
واکنش آمونیاک با نقره نیترات
واکنش نیترات نقره با اسید کلریدریک
از واکنش این ترکیب با هیدروکلریک اسید، محلولی از اسید نیتریک و همچنین رسوب نقره کلرید تشکیل می شود. معادله واکنش آن به صورت زیر است:
AgNO3 + HCl → AgCl + HNO3
همچنین از واکنش این ترکیب با سدیم کلرید هم می توان نقره کلرید به دست آورد. AgCl در صنایع گوناگون کاربرد فراوانی دارد.
واکنش تولید نقره کلرید
واکنش نقره نیترات با سدیم کربنات
با واکنش دادن این دو ماده با هم شاهد ایجاد رسوبی در انتهای ظرف آزمایش خواهیم بود. طبق واکنش زیر از واکنش بی کربنات سدیم و این ترکیب شیمیایی می توان نقره کربنات به دست آورد.
واکنش تولید نقره کربنات
واکنش مس با نقره نیترات
با قرار دادن سیم مسی در محلول Silver nitrate شاهد جابه جایی یون مس با Ag هستیم.همانطور که در شکل نیز مشهود است رنگ محلول به آبی تغییر پیدا خواهد کرد و شاهد نشستن یونAg بر روی سیم هستیم.
نیترات نقره و مس
کاربرد نیترات نقره در پزشکی
به طور کلی ترکیبات حاوی نقره دارای خاص ضد باکتریایی و ضد قارچ هستند. نقره نیترات نیز به دلیل داشتن نقره در ترکیب خود، دارای این خاصیت بوده و از آن در درمان زخم ها و همچنین به منظور جلوگیری از عفونت در زخم استفاده می کنند. به طور معمول از آن به صورت موضعی و در کرم ها و پماد ها استفاده می گردد. از دیگر کاربردهای این ماده استفاده از لوسیون 50% آن برای رفع زگیل های پوستی می باشد. همچنین تحقیقاتی در زمینه کاربرد نیترات نقره برای ضدعفونی در مواد ضدعفونی کننده و تاثیرات آن در از بین بردن مبکروب ها نیز انجام شده که نشان از تاثیرگذاری این ماده دارد.
تزیینات و آبکاری اشیا
آبکاری نقره: قبل از استفاده از این ماده در آبکاری، نکات ایمنی را رعایت کنید و حتما از دستکش استفاده نمایید. برای این کار نصف ��یمانه از Silver nitrate را در ظرفی ریخته و آنقدر آب به آن اضافه می نماییم تا پودر نیترات نقره به طور کامل حل گردد(حدود 1 لیتر). سپس در ظرفی جداگانه نصف پیمانه از سیانید پتاسیم را با حدود یک لیتر آب حل می نماییم. و در نهایت دو محلول ساخته شده را با هم مخلوط می نماییم.رنگ به دست آمده توسط این آبکاری نقره ای می باشد. برای آبکاری نقره نیاز به منبع تغذیه ی 9 الی 12 ولت می باشد.آبکاری نیازمند تهیه ی آند و کاتد است.
آند:
سیمی را به قطب مثبت منبع (+) که معمولا قرمز رنگ است متصل کنید.
سپس سر دیگر سیم را به یک قطعه فلز نقره متصل نمایید. این قطعه نقش تامین فلز نقره را برای آبکاری ایفا خواهد کرد.Ag به عنوان آند در این فرایند نقش خواهد داشت.
کاتد:
سیمی دیگر را به قطب منفی (-) منبع که معمولا سیاه رنگ است متصل کنید. سر دیگر سیم را به چیزی که می خواهیم با Ag آن را آبکاری نماییم اتصال داده و کاتد و آند تهیه شده را به آرامی در محلول ساخته شده فرو می بریم. و اختلاف پتانسیل را به کمک منبع تغذیه اعمال میکنیم تا آبکاری آغاز گردد.
با گذشت زمان شاهد نشستن یون های Ag بر روی شی مورد آبکاری خواهیم بود.
آبکاری نقره
فرایند اتمام آبکاری ممکن است طولانی مدت شود و از یک روز تا یک هفته به طول انجامد. مدت زمانی که طول می کشد تا آبکاری به اتمام برسد به اندازه ی شی ای که می خواهیم آبکاری کنیم و قدرت منبع تغذیه بستگی دارد. لازم است شی مورد آبکاری (کاتد) را هر روز بررسی کنید و در صورتی که احساس کردید آبکاری به کندی انجام می گیرد و بسیار طولانی گشته است از یک منبع تغذیه ی دیگر استفاده نمایید.
عکاسی
چاپ عکس به کمک نمک ها در دهه ی اول سال 1830 توسط ویلیام هنری فاکس تالبوت برای چاپ نگاتیو کشف شد. تالبوت می دانست که AgCl برای چاپ عکس بسیار مناسب است اما نمی توانست آن را بر روی کاغذ پوشش دهد.بنابراین به فکر راه بهتری بود. تالبوت ابتدا محلول آب نمک را ساخت و به آن این ماده را افزود.طی همین فرایند توانست AgCl را به آرامی بر روی کاغذ پوشش دهد و در فرایند چاپ از آن استفاده نماید. یکی از اولین تصاویر ظاهر شده به کمک این ماده را در زیر می توانید مشاهده نمایید:
تصویر چاپ شده به کمک نیترات نقره
امروزه در هنگام چاپ عکس های نگاتیوی از سدیم تیو سولفات به عنوان تثبیت کننده و مقداری اسید استیک در محلولی که تالوت کشف کرد استفاده می نمایند.
storage شرایط نگهداری نقره نیترات
به دلیل تجزیه شدن محلول این ماده توسط نور، باید از ظروف شیشه ای تیره و یا ظروف شیشه ای که با استفاده از فویل های آلومینیومی پوشانده شده اند، برای ذخیره سازی آن استفاده نمود.
از نگهداری آن در کنار مواد آتش زا اکیدا خودداری شود.
msds Msds نقره نیترات
msds نقره نیترات
AgNO3 بسیار خورنده می باشد و خسارات زیادی می تواند به پوست وارد کند.
هنگام استفاده از این ماده پوشیدن دستکش امری ضروری است.
این ماده جز مواد مخرب محیط زیست دسته بندی می شود و لازم است از ورود آن به محیط زیست به صورت جد خودداری گردد.
AgNO3 یک اکسید کننده به شمار می رود و می تواند سبب تشدید آتش سوزی شود.
بنابراین از نگهداری آن در کنار مواد اشتعال زا و یا حرارت خودداری نمایید.
هنگام کار با این محصول لازم است که از مصرف غذا،نوشیدنی و یا کشیدن سیگار دوری کنید.
بلعیدن این ماده خطرناک است و موجب اسهال، استفراغ و درد خفیف شکم می شود . در صورت خوردن آن برای درمان لازم است به صورت خوراکی آب نمک، شیر یا سفیده ی تخم مرغ همراه با آب را مصرف نمایید و سپس به یک مرکز درمانی مراجعه کنید. مصرف مواد ذکر شده از غشاهای داخلی مری و معده در برابر یون های سمی آزاد شده ی Ag محافظت به عمل می آورد و نمی گذارد رسوب کلرید نقره در بدن تشکیل شود.
قبل از استفاده از این محصول msds آن را به طور دقیق مطالعه نمایید.
.برای دیدن سایت اصلی کلیک کنید
0 notes
Text
Tính chất hóa học của muối lớp 9 – Các dạng bài hay gặp
Trong đời sống, khi nói đến muối thì đó là một loại gia vị có vị mặn. Công thức của muối ăn là NaCl (Natri Clorua). Tuy nhiên, đối với hóa học, muối có nhiều loại khác nhau, cơ chế tạo ra muối trong hóa học là từ một hay nhiều nguyên tử kim loại hoặc cation NH4+ và liên kết với một hay nhiều gốc axit khác nhau. Vậy tính chất hóa học của muối là gì
Tính chất hóa học của muối lớp 9 như thế nào?
Muối có thể làm đổi màu chất chỉ thị màu
Quỳ tím chuyển sang màu đỏ khi:
Muối có tính axit;
Khi kim loại yếu kết hợp với gốc axit mạnh.
Quỳ tím chuyển sang màu xanh khi:
Muối có tính bazo mạnh hơn;
Khi kim loại mạnh kết hợp với gốc axit yếu.
Quỳ tím không chuyển màu khi:
Muối trung tính;
Khi kim loại mạnh kết hợp với gốc axit mạnh hoặc tính chất của cả 2 ngang bằng nhau.
>> Tham khảo: Ứng dụng học trực tuyến hàng đầu Việt Nam – Toppy
Khi muối tác dụng với kim loại
Muối tác dụng với kim loại sẽ tạo nên muối mới và kim loại mới.
Ví dụ minh họa:
Fe + CuSO4 → FeSO4 + Cu↓
Cu + 2AgNO3 → Cu(NO3)2 + 2Ag↓
Khi muối tác dụng với axit
Muối khi tác dụng với axit sẽ tạo thành axit mới và muối mới. Tuy nhiên, nếu thành phẩm của phản ứng khi tạo ra là axit yếu thì không thể tồn tại được mà sẽ tự chuyển hóa thành chất khác bền hơn.
Ví dụ minh họa:
AgNO3 + HCl → AgCl ↓ + HNO3
CaCO3 + 2HCl → CaCl2 + CO2↑ + H2O
Khi muối tác dụng với muối
Khi 2 muối tác dụng với nhau sẽ tạo nên 2 loại muối mới.
Ví dụ minh họa: AgNO3 + NaCl → NaNO3 + AgCl↓. Phản ứng này làm xuất hiện kết tủa trắng do AgCl sinh ra.
Đọc bài viết đầy đủ tại :https://toppy.vn/blog/tinh-chat-hoa-hoc-cua-muoi/
Tham khảo thêm các bài viết tương tự : https://toppy.vn/blog/
0 notes