#chorioretinitis
Text
Chorioretinal colobomatous scar
Chorioretinal colobomatous scars caused by presumed toxoplasmosis are characterized by sharply demarcated areas of retinal and choroidal atrophy. These lesions can be identified and monitored using fundus autofluorescence (FAF), which highlights areas of hypoautofluorescence due to RPE loss. Optical Coherence Tomography (OCT) provides detailed cross-sectional images, revealing structural…
0 notes
Text
Researchers create machine learning model predicting long-term vision in high myopia patients, a top cause of irreversible blindness
- By Nuadox Crew -
In a study conducted by Tokyo Medical and Dental University (TMDU) researchers, a machine-learning model has been devised to foresee the future visual outcomes of individuals grappling with severe shortsightedness, scientifically termed high myopia.
This model aims to predict whether these individuals will experience positive or negative vision changes down the line.
High myopia, a condition characterized by the inability to focus on distant objects while maintaining clarity in close-range vision, poses a significant risk of irreversible blindness, making it a pressing global health concern. The research, recently featured in JAMA Ophthalmology, sought to harness the power of machine learning to forecast the likelihood of visual impairment over extended periods.
By scrutinizing data collected from nearly a thousand patients at Tokyo Medical and Dental University's Advanced Clinical Center for Myopia, the team embarked on a comprehensive analysis. They meticulously evaluated 34 key variables routinely recorded during ophthalmic examinations, encompassing factors like age, current visual acuity, and corneal diameter. Employing various machine-learning algorithms, including random forests and support vector machines, the researchers discovered that a logistic regression-based model exhibited the highest efficacy in predicting visual impairment at 3- and 5-year intervals.
Yet, the study didn't stop at predictive accuracy. Recognizing the importance of translating complex data into practical clinical insights, the researchers ingeniously crafted a nomogram. This graphical representation of the model's outcomes serves as a user-friendly tool for both clinicians and patients. Each variable's significance in predicting future visual acuity is visually depicted as a line, convertible into points that collectively signify the risk of impending visual impairment.
The implications of this research extend beyond its technical achievements. For individuals facing the dire consequences of vision loss, both financially and physically, this advancement could offer a beacon of hope. The global economic impact of severe visual impairment was estimated at a staggering USD 94.5 billion in 2019.
While further validation on a larger scale remains imperative, this study underscores the potential of machine-learning models in combating the escalating public health challenge posed by high myopia-induced sight loss.
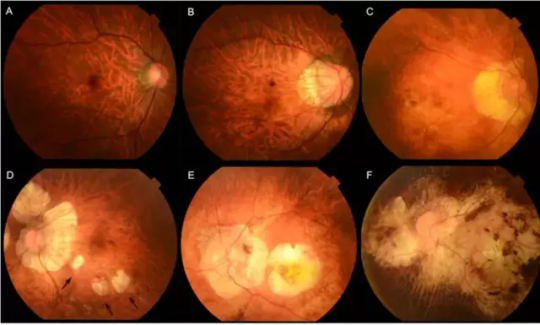
Image: Myopic maculopathy, also referred to as myopic macular degeneration, stands as a prominent characteristic of pathologic myopia. Within the META-PM classification system, myopic maculopathy lesions are grouped into five categories: from no myopic retinal lesions (category 0) to macular atrophy (category 4), including tessellated fundus (category 1, Figure 1A), diffuse chorioretinal atrophy (category 2, Figure 1B&C), patchy chorioretinal atrophy (category 3, Figure 1D arrows), and macular atrophy (category 4, Figure 1E&F). Credit: Tokyo Medical and Dental University.
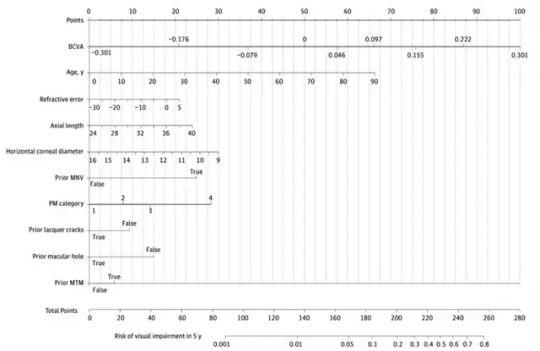
Image: A visual representation, known as a nomogram, was built to depict the model's insights. Longer lines indicate stronger variable impact on visual impairment (VI) after 5 years. Each variable corresponds to points, tallying up to calculate total points and the associated risk of VI. Credit: Tokyo Medical and Dental University.
--
Source: Tokyo Medical and Dental University (TMDU)
Full study: Wang Y, Du R, Xie S, et al. Machine Learning Models for Predicting Long-Term Visual Acuity in Highly Myopic Eyes. JAMA Ophthalmol. Published online October 26, 2023. doi:10.1001/jamaophthalmol.2023.4786
Read Also
New AI could predict whether or not those at high risk will develop glaucoma
#medtech#ophthalmology#eye#eyes#blindness#ai#artificial intelligence#diagnostics#health#medicine#health tech#machine learning
0 notes
Text
Lupine Publishers | Multiple Focal Choroidal Excavations in Association with Protein Rich Diet

Introduction
Choroidal excavation is a novel entity that is diagnosed with optical coherence tomography (OCT). In 1959, Klien, et al. [1] first described a concave-shaped chorioretinal abnormality with undifferentiated choriocapillaris and retinal pigment epithelium. Then in 2006, Jampol [2] defined similar changes with OCT and first used the term of focal choroidal excavation (FCE). In 2011, Margolis, et al. [3] described two FCE patterns, based on the shape of the lesions: nonconforming, if the photoreceptors are detached from retina pigment epithelium (RPE) and conforming, when the RPE follows the photoreceptor layer without any optically clear space between the outer retina and RPE. These two forms are also thought to convert to each other during the clinical process of the disease [4]. Although it is known to be mostly idiopathic, its relationship with central serous chorioretinopathy, choroidal neovascular membrane and inflammatory diseases such as multiple evanescent white dot syndrome and Vogt Koyanagi Harada disease has been reported [5]. In this report, we present a patient with multiple focal choroidal excavation following protein-rich diet.
Case
21-year-old male patient applied with blurred vision in his right eye since one week. He was on a protein rich diet since he was doing bodybuilding and had been using approximately 2g/kg/day protein powder since 3 months. His best corrected visual acuity was 0,6 in his right eye (RE) and 0,9 in his left eye (LE). There was no refractive disorder. Slit lamp examination and intraocular pressure were normal. Fundus examination revealed cavitary lesions in the macula of the RE (Figure 1a) and pigmentary changes above the macula of the LE (Figure 1b). OCT showed multiple conforming (Figure 2a) and non-conforming (Figure 2b) type of focal choroidal excavations with small retinal pigment epithelium detachments in the right eye and a small retinal pigment epithelium detachment in the left eye (Figure 2c). When evaluated with fundus autofluorescence (FAF), there have been hypo and hyperautofluorescent areas in the macula of the right eye in accordance with the pigmentary changes (Figure 3a) and hypoautofluorescent area above the macula of the left eye (Figure 3b). Fundus fluorescein angiography showed multiple hyperfluorescent window defects in the macula of the RE and hyperfluorecence consistent with window defect above macula of the LE. There was no ischemia, leakage, macular edema and all vascular structures were normal . Protein powder stopped and the patient was examined 6 months after the diagnosis. No changes in the findings were detected.
Read more about this article : https://lupinepublishers.com/ophthalmology-journal/fulltext/multiple-focal-choroidal-excavations-in-association-with-protein-rich-diet.ID.000158.php
Read More About Lupine Google Scholar: https://scholar.google.com/citations?view_op=view_citation&hl=en&user=T4c9RDkAAAAJ&citation_for_view=T4c9RDkAAAAJ:blknAaTinKkC
0 notes
Text
Charge syndrome

#Charge syndrome skin#
#Charge syndrome skin#
As well as, rhombencephalic abnormalities, hypothalamo-hypophyseal dysfunction, external/middle ear malformations, mediastinal visceral malformations, and mental retardation.ĭental malocclusion, Deep palmar crease, Delayed puberty, Malar flattening, Delayed eruption of teeth, Brachydactyly, Clinodactyly of the 5th finger, Broad columella, Chorioretinal coloboma, Anophthalmia, Cleft upper lip, Retinal detachment, Epicanthus, Split hand, Esophageal atresia, Cupped ear, Dandy-Walker malformation, Cryptorchidism, Dysphagia, Duodenal atresia, Downslanted palpebral fissures, Double outlet right ventricle, Facial palsy, Facial asymmetry, Feeding difficulties in infancy, External ear malformation, Posterior choanal atresia, Overfolded helix, Hand polydactyly, Nystagmus, Obsessive-compulsive behavior, Optic atrophy, Renal hypoplasia, Omphalocele, Preaxial hand polydactyly, Autosomal dominant inheritance, Parathyroid hypoplasia, Preauricular skin tag, Otitis media, Patent ductus arteriosus, Sinusitis, Retinal coloboma, Retinopathy, Renal agenesis, Webbed neck, Vesicoureteral reflux, Aplasia/Hypoplasia of the earlobes, Aplasia/Hypoplasia of the corpus callosum, Aplasia/Hypoplasia of the cerĬHARGE syndrome is a complex genetic disorder caused by heterozygous pathogenic CHD7 variants (most often private, truncating), which are usually de novo but can be inherited in an autosomal dominant fashion. Other symptoms may include a cleft palate, issues with balance, kidney health concerns and intellectual disability. Physical features of the syndrome include asymmetrical facial palsy, short and wide ears with little or no earlobe, small thumbs and fingers, and upper body hypotonia. The main symptoms of the syndrome, and from which the syndrome derives its name, are:Ĭoloboma: a slit in the iris or retina that may lead to vision lossĬranial nerve abnormalities: issues with the connection between the nose and throatĬhoanal Atresia: a blockage in the back of an individual’s nose that can make breathing difficult. What are the main symptoms of CHARGE syndrome?

0 notes
Text
Iris publishers-Open access journal of Rheumatology & Arthritis Research| Behind the Scenes Of Cns Vasculitis: A Rare Case of Cns Vasculitis with Two Primary Malignancies

Abstract
To highlight the importance of a broad differential diagnosis, including malignancy, in patients with central nervous system vasculitis (CNSV) presenting as multiple, recurrent cryptogenic strokes.
Recent Findings
We describe a rare entity, CNSV in the setting of two underlying neoplastic disorders and a chorioretinal inflammatory disease. The clinical presentation, imaging study results, histopathologic features, and response to treatment are reported.
Summary
Cancer-associated CNSV is a rare cause of recurrent cryptogenic strokes. This case highlights the importance of clinical suspicion of and comprehensive work-up for neoplasms in a patient presenting with brain lesions consistent with CNSV.
Keywords: Vasculitis; Infarction; Visual loss; Retina; Paraneoplastic syndrome
Abbreviations:
APMPPE- Acute posterior multifocal placoid pigment epitheliopathy
CNSV- Central Nervous system Vasculitis
MRI- magnetic resonance imaging
MRA- magnetic resonance angiography
ANA- anti-nuclear antibody
CSF- Cerebrospinal fluid
PET- positron emission tomography
CT - computerized tomography
CYC- cyclophosphamide
Introduction
Acute posterior multifocal placoid pigment epitheliopathy (APMPPE) is a rare, self-limiting chorioretinal inflammatory disorder that is often characterized by a flu-like prodrome and usually presents in young adults [1]. Neurological complications are rare but potentially fatal manifestations of APMPPE that have been previously reported in 56 cases, with the most common being CNSV (50% of the cases) [2]. CNSV is a diagnostically challenging disease and has been rarely associated with malignancies, especially with Hodgkin lymphoma [3].
We report one patient with APMPPE and neurological symptoms consistent with CNSV, who was diagnosed with two primary malignancies during the medical work-up for CNSV.
Case Presentation
A 59-year-old female presented to the hospital with bilateral blurry vision, floaters and light flashes that persisted over a twoweek period. These symptoms were preceded by holocephalic aching headaches associated with temporal tenderness two weeks prior to the vision symptoms. The patient has a medical history of recurrent herpes zoster, hyperlipidemia, and type 2 diabetes mellitus. Review of systems was notable for fatigue, fever, chills, night sweats, dry mouth, and weight loss of 64 pounds in the past year. Physical exam revealed normal vital signs, a palpable left submandibular lymph node, impaired visual acuity to counting fingers at 3 feet in the left eye and 20/200 in the right eye, and fundus exam revealed yellow-white placoid lesions with areas of chorioretinal atrophy (Figures 1-4) The remainder of the eye exam was unremarkable. Neurological examination revealed impaired range of central visual field and decreased sensation to pinprick in the right cranial nerve V3 (mandibular) distribution and right upper extremity. Brain magnetic resonance imaging (MRI) showed a subacute lacunar infarct in the dorsal left putamen/capsular junction and minimal white matter chronic microvascular ischemic disease (Figure 5). Cranial magnetic resonance angiography (MRA) demonstrated no evidence of flow-limiting stenosis or occlusion. The patient was treated with atorvastatin 40 mg and aspirin 325 mg for secondary stroke prevention.





Ophthalmologic findings triggered a concern for cerebral vasculitis, thus immunologic workup was done which was unremarkable except for a weakly positive anti-nuclear antibody (ANA) (titer of 1:160). The results of the tests for anti-dsDNA and anti-extractable nuclear antigen antibodies, viral hepatitis B and C, and HIV screening were all negative.
Laboratory studies at presentation demonstrated mildly elevated inflammatory markers (Table 1). Cerebrospinal fluid (CSF) demonstrated lymphocytic pleocytosis and elevated protein levels (Table 2). CSF studies for varicella zoster virus PCR, IgG, and IgM were negative. Transthoracic echocardiogram and cardiac MRI did not demonstrate any findings to suggest acute or chronic cardiopulmonary abnormality. The patient was started on methylprednisolone 1000 mg intravenously daily for 3 days followed by prednisone 60 mg by mouth daily with a slow taper to manage her suspected CNSV.
Table 1:Relevant lab studies.

Table 2:CSF Findings.

Catheter-based angiogram of the brain was negative for vasculitic changes. Given the lack of definite diagnosis, a brain biopsy was ordered. However, a pre-operative head CT scan showed multiple interval lacunar infarcts within the right periventricular corona radiata, right cerebellum, mesial left temporal lobe, and left occipital lobe, and an evolving subacute infarct within the left basal ganglia. Additionally, whole body PET CT imaging showed a region with increased uptake in the right breast with associated hypermetabolic right axillary lymphadenopathy and left submandibular lymph node, but no evidence of large-vessel vasculitis (Figure 6). At that time the brain biopsy was aborted pending work up for these lesions, prednisone was titrated to 100 mg by mouth daily, and the patient was discharged to facilitate necessary outpatient oncologic workup, including breast biopsy.
Four days later, immediately following her breast biopsy, the patient presented again with an episode of numbness and paresthesia on the left side of her body, speech difficulty, and facial droop. A repeat MRI confirmed the presence of multiple new acute infarcts in the superior frontal gyrus and bilaterally in the occipital lobe (Figure 7).
The breast biopsy revealed invasive ductal carcinoma. A needle core biopsy of the patient’s submandibular lymph node was consistent with follicular lymphoma; however the sample was too scant for grading. An open brain biopsy (right occipital craniotomy and biopsy of dura and brain with neuro navigation) was performed, which showed no evidence of neoplastic process or definite features of vasculitis. Repeat catheter angiography of the brain demonstrated multifocal regions of vessel wall narrowing leading to occlusion, most pronounced in distal bilateral middle cerebral arteries and distal left posterior cerebral artery territories concerning for vasculitis or other cause of vasospasm. No verapamil was administered due to recent stroke and risk of reperfusion hemorrhage.
Based on discussion with oncology, both breast malignancy and follicular lymphoma were thought to be indolent and thus a decision was made to start induction immunosuppression for CNSV.
The patient was started on cyclophosphamide (CYC) 600 mg/ m2 IV every 4 weeks along with tapering the prednisone, which was well-tolerated for 3 doses of CYC. However, one week after her third dose she had an episode of right leg weakness and numbness and presented to the emergency room, where brain MRI showed new subacute/acute infarcts in both cerebral hemispheres and the left genu of the corpus callosum. CT angiogram showed new multifocal areas of moderate to severe narrowing of the bilateral pericallosal arteries and posterior cerebral arteries. Transthoracic echocardiogram was again negative for acute or chronic abnormalities and repeat CSF studies showed normal leukocyte count and protein level.
At this point, the decision was made to add rituximab 375 mg/ m2 IV weekly to her CYC therapy and increase the prednisone dose to 60 mg. At the time of submission of this case report, the patient had received a total of 5 doses of CYC and 6 doses of rituximab. Clinically, the patient did well with no recurrence of neurologic deficits.
Discussion
CNSV was found in some studies to be associated with APMPPE. The mechanism that leads to this association is unknown but is often attributed to an underlying vasculitis process [2]. Our experience here demonstrates the difficulty of diagnosing CNSV, a rare disease that is sometimes associated with other pathologies. Ambiguous clinical presentation and radiographic imaging can often mimic other disease processes and can present diagnostic and therapeutic challenges. Angiography has a sensitivity for diagnosing CNSV between 50% and 90% while some report the sensitivity of brain biopsy to be less than 50%, although the latter might be helpful in excluding the presence of other syndromes [4]. In our case, the diagnostic interventions were further complicated by the discovery of two primary malignancies as associated and potentially causative pathologies.
To our knowledge there is only one other report in the literature of a case of APMPPE associated with a malignancy. However, in that case the patient presented differently. He was diagnosed with and treated for metastatic clear cell renal carcinoma before the APMPPE was diagnosed. The development of APMPPE was proposed to be a result of the spreading of tumor-originated circulating immune complexes [5]. CNSV has been rarely associated with malignancy. CNSV has been previously reported in a patient who presented with intractable seizures and was later diagnosed with invasive lobular carcinoma of the breast [6]. Two additional patients presenting with CNSV after receiving treatment for malignant vaginal squamous cell carcinoma and invasive breast ductal carcinoma responded to methylprednisolone/plasmapheresis and to prednisone, respectively [7].
A study looking at patients diagnosed with any type of vasculitis and lymphoma over a 32-year period found the incidence of lymphoma presenting together with CNSV to be 10/168, or 5.9%. All of the patients in that study were treated with prednisone and two patients also received cyclophosphamide. Overall, 6/10 patients responded to therapy and showed almost no residual deficits [3]. Significant in our case is the simultaneous presence of two primary malignancies in addition to APMPPE and a CNSV process. Cyclophosphamide may be an appropriate firstline treatment in a patient with CNSV. However, in the setting of underlying malignancy, our patient did not respond well to cyclophosphamide despite adequate dosing for 12 weeks. The addition of rituximab to cyclophosphamide was possibly beneficial due to the underlying follicular lymphoma, pointing to the possibility of a paraneoplastic etiology of the CNSV. The relationship between systemic malignancies and CNSV continues to be difficult to assess. A cell-mediated immune process triggered by abnormal serum proteins or tumor antigens has been suggested as a possible etiopathogenetic mechanism [6,7]. Treatment recommendations and patient outcomes vary in the literature. Additionally, these clinical entities are often difficult to diagnose which leads to further problems in determining the optimal treatment plan. Our case demonstrates the importance of considering a broad differential in a patient presenting with APMPPE and atypical persistent neurologic symptoms. Further studies are needed to help guide treatment for CNSV when associated with malignancy Appendix 1.
Conflict of Interest
The authors report no financial disclosures
Acknowledgement
None
Read more: Full Text
For more articles in Archives of Rheumatology & Arthritis Research please click on:
https://irispublishers.com/arar/archive.php
For more open access journals in Iris Publishers
#Iris Publishers#Iris Publishers LLC#cerebrospinal fluid#CNS Vasculitis#chorioretinal inflammatory disorder
1 note
·
View note
Text
The classical triad of congenital toxoplasmosis is: hydrocephalus, intracranial calcification, and chorioretinitis. Congenital chorioretinitis is associated with viral infections such as CMV or toxoplasmosis. In the case of CMV, the disease progressively improves with time and throughout infancy. On the other hand, babies with congenital toxoplasmosis may be asymptomatic at the time of birth, but their condition progressively worsens over a long span of time and becomes clinically significant during old age.
3 notes
·
View notes
Text
Ocular toxoplasmosis
Ocular toxoplasmosis is characterized by a pathognomonic lesion, typically presenting as a focal, necrotizing retinochoroiditis. This lesion is often accompanied by adjacent old chorioretinal scars and active inflammation. Fundus imaging reveals a white or yellowish focus of retinal necrosis with surrounding inflammation. Fluorescein angiography (FA) and Optical Coherence Tomography (OCT) can be…
0 notes
Text
chorioretinitis Übersetzung chorioretinitis Deutsch
chorioretinitis auf Deutsch übersetzen, Bedeutung für chorioretinitis, Was ist chorioretinitis.
chorioretinitis Deutsch übersetzen #chorioretinitis
0 notes
Text
Penyakit Sifilis Sembuh
Penyakit sifilis sembuh lebih mudah jika pengobatannya dilakukan lebih awal, yakni pada tahap awal infeksinya. Penyakit sifilis umumnya menyebar dan berkembang dalam tubuh melalui empat tahap. Pada tiap tahap, sifilis akan memperlihatakan tanda yang berbeda. tanda-tanda tersebut menjadi penting untuk mengidentifikasi tingkat keparahan dan menentukan metode pengobatan. penyembuhan agar penyakit sifilis sembuh harus dilakukan oleh dokter. Pengobatan secara sembarangan justru akan memperburuk keadaan terutama jika obat yang digunakan tidak tepat sesuai kondisi penyakit.

Sifilis primer
Setelah masa inkubasi 3–4 minggu (kisaran 1–13 minggu), lesi utama (solid chancre) berkembang di tempat di mana infeksi terjadi. Lesi dapat muncul di mana saja, tetapi paling umum di tempat area seperti batang penis, dubur (pria dan wanita), vulva, serviks, rektum dan perineum. Lesi ini biasanya sembuh dalam 3–12 minggu. Setelah itu, orang dengan sifilis tampak benar-benar sehat.
Sifilis sekunder
Spirochete Treponema pallidum penyebab sifilis akan menyebar dalam aliran darah dan menghasilkan lesi mukokutan yang luas (ruam), edema kelenjar getah bening dan, beberapa yang jarang di organ lain. Gejala ini biasanya mulai muncul 6–12 minggu setelah lesi muncul. Gejala yang mengikutinya antara lain, demam, kehilangan nafsu makan, mual dan kelelahan. Selain itu, sakit kepala (karena meningitis), gangguan pendengaran (karena otitis media), masalah keseimbangan (karena labirinitis), gangguan penglihatan (karena retinitis atau uveitis), dan nyeri tulang (karena periostitis) juga dapat terjadi.
Periode laten
Tahap sifilis laten terjadi sebelum 1 tahun setelah infeksi atau terlambat hingga 30 tahun setelah infeksi terjadi. Gejala dan tanda tidak ada pada tahap ini dan biasanya diketahui melalui tes darah.
Sifilis terlambat atau tersier
Sekitar sepertiga dari orang yang tidak diobati mengembangkan sifilis terlambat, dan biasanya kondisi akan menjadi tanda-tanda sifilis tersier seperti sifilis tersier jinak, sifilis kardiovaskular dan neurosifilis.
Sifilis tersier jinak biasanya berkembang dalam 3–10 tahun infeksi dan dapat mempengaruhi kulit, tulang, dan organ dalam. Gumma — massa lunak, destruktif, meradang, yang biasanya terbatas, tetapi dapat menembus ke dalam organ atau jaringan secara difus. Jika menyerang tulang, sifilis akan menyebabkan nyeri yang dalam dan tumpul, yang biasanya memburuk di malam hari.
Sifilis kardiovaskular biasanya muncul 10–25 tahun setelah infeksi awal dengan gejala-gejala seperti ekspansi aneurisma aorta ascenden, ketidakcukupan katup aorta, penyempitan pembuluh darah koroner dan sebagainya.
Sementara dalam masalah neurosifilis, ada beberapa bentuk yaitu neurosifilis asimptomatik, neurosifilis meningovaskular dan neurosifilis parenkim.
Kerusakan lain akibat sifilis
Selain bahaya sifilis tersebut, manifestasi sifilis berupa kerusakan mata dan telinga dapat terjadi pada semua tahap penyakit.
Sindrom mata dapat memengaruhi hampir semua bagian mata; mereka termasuk keratitis menengah, uveitis, chorioretinitis, retinitis, vaskulitis retina, dan saraf kranial dan neuropati penglihatan.
Otosifilis dapat memengaruhi koklea (menyebabkan gangguan pendengaran dan dering di telinga) atau sistem vestibular (menyebabkan pusing dan nistagmus).
Lesi trofik sekunder akibat hipestesia kulit atau di sekitar jaringan artikular dapat terjadi pada tahap selanjutnya. Ulkus trofik dapat berkembang di telapak kaki dan menembus jauh ke tulang di bawahnya.
Arthropathy neurogenik (sendi Charcot), penghancuran sendi tanpa rasa sakit dengan pembengkakan tulang dan rentang gerakan abnormal, adalah manifestasi klasik neuropati.
Pengobatan agar penyakit sifilis sembuh lebih cepat adalah saat sifilis masih dalam tahap primer. Jika dibiarkan, sifilis akan menyebar dan menyebabkan berbagai macam komplikasi tersebut. Pengobatan yang tepat bisa Anda dapatkan di rumah sakit atau klinik-klinik penyakit kelamin agar mendapatkan perawatan yang memadai.
1 note
·
View note
Link
The journal of Ocular Infection and Inflammation is a peer reviewed, Open Access journal that aims to publish research dealing with ophthalmology and the various ocular diseases like blepharitis, keratitis, chorioretinitis, ocular histoplasmosis syndrome etc. The journal focuses on the various aspects of ocular research like ocular infection, ocular tumors, ophthalmic inflammation, immuno ophthalmology etc.
1 note
·
View note
Text
Age Related Macular Degeneration Treatment In Ghatkopar, Mumbai by Dr. jatin ashar
Age Related Macular Degeneration
Human eye has various important parts like Cornea, Pupil, Iris, Lens and Retina. The macula is located in the center of the retina, the light-sensitive tissue at the back of the eye. The retina instantly converts light, or an image, into electrical impulses. The retina then sends these impulses, or nerve signals, to the brain. When the cells of the macula deteriorate, images are not received correctly. In early stages, macular degeneration does not affect vision. Later, if the disease progresses, people experience wavy or blurred vision, and, if the condition continues to worsen, central vision may be completely lost. People with very advanced macular degeneration are considered legally blind. Macular Degeneration is the leading cause of vision loss, more than cataracts and glaucoma combined.
Macular degeneration is classified as:
Dry Age related Macular Degeneration
Wet Age related Macular Degeneration.
Pathophysiology
The dry form is more common than the wet form, with about 85 to 90 percent of AMD patients diagnosed with dry AMD. The less common wet AMD usually leads to more serious vision loss.
Dry AMD causes changes of the retinal pigment epithelium, typically visible as dark pinpoint areas. The retinal pigment epithelium plays a critical role in keeping the cones and rods healthy and functioning well. Accumulation of waste products from the rods and cones can result in drusen, which appear as yellow spots. Areas of chorioretinal atrophy (referred to as geographic atrophy) occur in more advanced cases of dry AMD. There is no elevated macular scar (disciform scar), edema, hemorrhage, or exudation.
Dry AMD has three stages, all of which may occur in one or both eyes:
Early AMD - People with early AMD have either several small drusen or a few medium-sized drusen. At this stage, there are no symptoms and no vision loss.
Intermediate AMD - People with intermediate AMD have either many medium-sized drusen or one or more large drusen. Some people see a blurred spot in the center of their vision. More light may be needed for reading and other tasks.
Advanced AMD - In addition to drusen, people with advanced dry AMD have a breakdown of light-sensitive cells and supporting tissue in the central retinal area. This breakdown can cause a blurred spot in the center of your vision. Over time, the blurred spot may get bigger and darker, taking more of your central vision. You may have difficulty reading or recognizing faces until they are very close to you.
Wet AMD occurs when new abnormal blood vessels develop under the retina in a process called choroidal neovascularization (abnormal new vessel formation). Localized macular edema or hemorrhage may elevate an area of the macula or cause a localized retinal pigment epithelial detachment. Eventually, untreated neovascularization causes a disciform scar under the macula.
Symptoms
Dry macular degeneration symptoms usually develop gradually and without pain. They may include:
Visual distortions, such as straight lines seeming bent
Reduced central vision in one or both eyes
The need for brighter light when reading or doing close work
Increased difficulty adapting to low light levels, such as when entering a dimly lit restaurant
Increased blurriness of printed words
Decreased intensity or brightness of colors
Difficulty recognizing faces
What causes macular degeneration?
Though macular degeneration is associated with aging, there is genetic component to the disease. A strong association between development of AMD and presence of a variant of a gene known as complement factor H (CFH) is observed. This gene deficiency is associated with almost half of all potentially blinding cases of macular degeneration.
Other investigators have found that variants of another gene, complement factor B, may be involved in development of AMD.
Specific variants of one or both of these genes, which play a role in the body's immune responses, have been found in 74 percent of AMD patients who were studied. Other complement factors also may be associated with an increased risk of macular degeneration.
Oxygen-deprived cells in the retina produce a type of protein called vascular endothelial growth factor (VEGF), which triggers the growth of new blood vessels in the retina.
The normal function of VEGF is to create new blood vessels during embryonic development, after an injury or to bypass blocked blood vessels. But too much VEGF in the eye causes the development of unwanted blood vessels in the retina that easily break open and bleed, damaging the macula and surrounding retina.
Risk Factors
The biggest risk factor for Macular Degeneration is age. Your risk increases as you age, and the disease is most likely to occur in those 55 and older.
Other risk factors include:
Genetics – People with a family history of AMD are at a higher risk.
Race – Caucasians are more likely to develop the disease than African-Americans or Hispanics/Latinos.
Smoking – Smoking doubles the risk of AMD.
Diagnosis
AMD is detected during a comprehensive eye exam that includes:
Visual acuity test - This eye chart test measures how well you see at various distances.
Dilated eye exam - Drops are placed in your eyes to widen the pupils. Your eye care professional uses a special magnifying lens to examine your retina and optic nerve for signs of AMD and other eye problems. After the exam, your close-up vision may remain blurred for several hours.
Tonometry - An instrument measures the pressure inside the eye. Numbing drops may be applied to your eye for this test.
Both forms of age - related macular degeneration (AMD) are diagnosed by funduscopic examination. Visual changes can often be detected with an Amsler grid.
Color photography and fluorescein angiography are done when findings suggest wet AMD. Angiography shows and characterizes subretinal choroidal neovascular membranes and can delineate areas of geographic atrophy. Optical coherence tomography (OCT) aids in identifying intraretinal and subretinal fluid and can help assess response to treatment.
What Treatments Are Available for Macular Degeneration?
There’s no cure for macular degeneration. Treatment may slow it down or keep you from losing too much of your vision. Your options might include:
Lifestyle changes - like dieting, exercise, avoiding smoking, and protecting your eyes from ultraviolet light.
Anti-angiogenesis drugs - These medications – aflibercept (Eylea), bevacizumab (Avastin), pegaptanib (Macugen), and ranibizumab (Lucentis) -- block the creation of blood vessels and leaking from the vessels in your eye that cause wet macular degeneration. Many people who’ve taken these drugs got back vision that was lost. You might need to have this treatment multiple times.
Laser therapy - High-energy laser light can destroy abnormal blood vessels growing in your eye.
Photodynamic laser therapy - Your doctor injects a light-sensitive drug verteporfin (Visudyne) into your bloodstream, and it’s absorbed by the abnormal blood vessels. Your doctor then shines a laser into your eye to trigger the medication to damage those blood vessels.
Low vision aids - These are the devices that have special lenses or electronic systems to create larger images of nearby things. They help people who have vision loss from macular degeneration make the most of their remaining vision.
Submacular surgery - This removes abnormal blood vessels or blood.
Retinal translocation - A procedure to destroy abnormal blood vessels under the center of your macula, where your doctor can’t use a laser beam safely. In this procedure, your doctor rotates the center of your macula away from the abnormal blood vessels to a healthy area of your retina. This keeps you from having scar tissue and more damage to your retina. Then, your doctor uses a laser to treat the abnormal blood vessels.
Important Reminder: This information is only intended to provide guidance, not a definitive medical advice. Please consult eye doctor about your specific condition. Only a trained, experienced board certified eye doctor can determine an accurate diagnosis and proper treatment.
To schedule an appointment with our experts for Age Related Macular Degeneration Management please call us at +91 8451045935, +91-8451045934 or visit our clinic at Address.
Tags= eye specialist in ghatkopar, eye clinic in ghatkopar west
0 notes
Text
Age Related Macular Degeneration Treatment In Ghatkopar, Mumbai
Age Related Macular Degeneration
Human eye has various important parts like Cornea, Pupil, Iris, Lens and Retina. The macula is located in the center of the retina, the light-sensitive tissue at the back of the eye. The retina instantly converts light, or an image, into electrical impulses. The retina then sends these impulses, or nerve signals, to the brain. When the cells of the macula deteriorate, images are not received correctly. In early stages, macular degeneration does not affect vision. Later, if the disease progresses, people experience wavy or blurred vision, and, if the condition continues to worsen, central vision may be completely lost. People with very advanced macular degeneration are considered legally blind. Macular Degeneration is the leading cause of vision loss, more than cataracts and glaucoma combined.
Macular degeneration is classified as:
Dry Age related Macular Degeneration
Wet Age related Macular Degeneration.
Pathophysiology
The dry form is more common than the wet form, with about 85 to 90 percent of AMD patients diagnosed with dry AMD. The less common wet AMD usually leads to more serious vision loss.
Dry AMD causes changes of the retinal pigment epithelium, typically visible as dark pinpoint areas. The retinal pigment epithelium plays a critical role in keeping the cones and rods healthy and functioning well. Accumulation of waste products from the rods and cones can result in drusen, which appear as yellow spots. Areas of chorioretinal atrophy (referred to as geographic atrophy) occur in more advanced cases of dry AMD. There is no elevated macular scar (disciform scar), edema, hemorrhage, or exudation.
Dry AMD has three stages, all of which may occur in one or both eyes:
Early AMD - People with early AMD have either several small drusen or a few medium-sized drusen. At this stage, there are no symptoms and no vision loss.
Intermediate AMD - People with intermediate AMD have either many medium-sized drusen or one or more large drusen. Some people see a blurred spot in the center of their vision. More light may be needed for reading and other tasks.
Advanced AMD - In addition to drusen, people with advanced dry AMD have a breakdown of light-sensitive cells and supporting tissue in the central retinal area. This breakdown can cause a blurred spot in the center of your vision. Over time, the blurred spot may get bigger and darker, taking more of your central vision. You may have difficulty reading or recognizing faces until they are very close to you.
Wet AMD occurs when new abnormal blood vessels develop under the retina in a process called choroidal neovascularization (abnormal new vessel formation). Localized macular edema or hemorrhage may elevate an area of the macula or cause a localized retinal pigment epithelial detachment. Eventually, untreated neovascularization causes a disciform scar under the macula.
Symptoms
Dry macular degeneration symptoms usually develop gradually and without pain. They may include:
Visual distortions, such as straight lines seeming bent
Reduced central vision in one or both eyes
The need for brighter light when reading or doing close work
Increased difficulty adapting to low light levels, such as when entering a dimly lit restaurant
Increased blurriness of printed words
Decreased intensity or brightness of colors
Difficulty recognizing faces
What causes macular degeneration?
Though macular degeneration is associated with aging, there is genetic component to the disease. A strong association between development of AMD and presence of a variant of a gene known as complement factor H (CFH) is observed. This gene deficiency is associated with almost half of all potentially blinding cases of macular degeneration.
Other investigators have found that variants of another gene, complement factor B, may be involved in development of AMD.
Specific variants of one or both of these genes, which play a role in the body's immune responses, have been found in 74 percent of AMD patients who were studied. Other complement factors also may be associated with an increased risk of macular degeneration.
Oxygen-deprived cells in the retina produce a type of protein called vascular endothelial growth factor (VEGF), which triggers the growth of new blood vessels in the retina.
The normal function of VEGF is to create new blood vessels during embryonic development, after an injury or to bypass blocked blood vessels. But too much VEGF in the eye causes the development of unwanted blood vessels in the retina that easily break open and bleed, damaging the macula and surrounding retina.
Risk Factors
The biggest risk factor for Macular Degeneration is age. Your risk increases as you age, and the disease is most likely to occur in those 55 and older.
Other risk factors include:
Genetics – People with a family history of AMD are at a higher risk.
Race – Caucasians are more likely to develop the disease than African-Americans or Hispanics/Latinos.
Smoking – Smoking doubles the risk of AMD.
Diagnosis
AMD is detected during a comprehensive eye exam that includes:
Visual acuity test - This eye chart test measures how well you see at various distances.
Dilated eye exam - Drops are placed in your eyes to widen the pupils. Your eye care professional uses a special magnifying lens to examine your retina and optic nerve for signs of AMD and other eye problems. After the exam, your close-up vision may remain blurred for several hours.
Tonometry - An instrument measures the pressure inside the eye. Numbing drops may be applied to your eye for this test.
Both forms of age - related macular degeneration (AMD) are diagnosed by funduscopic examination. Visual changes can often be detected with an Amsler grid.
Color photography and fluorescein angiography are done when findings suggest wet AMD. Angiography shows and characterizes subretinal choroidal neovascular membranes and can delineate areas of geographic atrophy. Optical coherence tomography (OCT) aids in identifying intraretinal and subretinal fluid and can help assess response to treatment.
What Treatments Are Available for Macular Degeneration?
There’s no cure for macular degeneration. Treatment may slow it down or keep you from losing too much of your vision. Your options might include:
Lifestyle changes - like dieting, exercise, avoiding smoking, and protecting your eyes from ultraviolet light.
Anti-angiogenesis drugs - These medications – aflibercept (Eylea), bevacizumab (Avastin), pegaptanib (Macugen), and ranibizumab (Lucentis) -- block the creation of blood vessels and leaking from the vessels in your eye that cause wet macular degeneration. Many people who’ve taken these drugs got back vision that was lost. You might need to have this treatment multiple times.
Laser therapy - High-energy laser light can destroy abnormal blood vessels growing in your eye.
Photodynamic laser therapy - Your doctor injects a light-sensitive drug verteporfin (Visudyne) into your bloodstream, and it’s absorbed by the abnormal blood vessels. Your doctor then shines a laser into your eye to trigger the medication to damage those blood vessels.
Low vision aids - These are the devices that have special lenses or electronic systems to create larger images of nearby things. They help people who have vision loss from macular degeneration make the most of their remaining vision.
Submacular surgery - This removes abnormal blood vessels or blood.
Retinal translocation - A procedure to destroy abnormal blood vessels under the center of your macula, where your doctor can’t use a laser beam safely. In this procedure, your doctor rotates the center of your macula away from the abnormal blood vessels to a healthy area of your retina. This keeps you from having scar tissue and more damage to your retina. Then, your doctor uses a laser to treat the abnormal blood vessels.
Important Reminder: This information is only intended to provide guidance, not a definitive medical advice. Please consult eye doctor about your specific condition. Only a trained, experienced board certified eye doctor can determine an accurate diagnosis and proper treatment.
To schedule an appointment with our experts for Age Related Macular Degeneration Management please call us at +91 8451045935, +91-8451045934 or visit our clinic at Address.
Tag = eye specialist in ghatkopar east, eye clinic in ghatkopar east, eye specialist in ghatkopar, eye clinic in ghatkopar west
For more information = https://www.mumbaieyecare.com/
some more blogs = https://eye-specialist-in-ghatkopar.blogspot.com/2022/06/corneal-arcus-or-arcus-senilis-causes.html
#eye specialist in ghatkopar east#eye clinic in ghatkopar east#eye specialist in ghatkopar#eye clinic in ghatkopar west
0 notes
Text
Age Related Macular Degeneration Treatment In Ghatkopar, Mumbai by Dr. Jatin Ashar
Age Related Macular Degeneration
Human eye has various important parts like Cornea, Pupil, Iris, Lens and Retina. The macula is located in the center of the retina, the light-sensitive tissue at the back of the eye. The retina instantly converts light, or an image, into electrical impulses. The retina then sends these impulses, or nerve signals, to the brain. When the cells of the macula deteriorate, images are not received correctly. In early stages, macular degeneration does not affect vision. Later, if the disease progresses, people experience wavy or blurred vision, and, if the condition continues to worsen, central vision may be completely lost. People with very advanced macular degeneration are considered legally blind. Macular Degeneration is the leading cause of vision loss, more than cataracts and glaucoma combined.
Macular degeneration is classified as:
Dry Age related Macular Degeneration
Wet Age related Macular Degeneration.
Pathophysiology
The dry form is more common than the wet form, with about 85 to 90 percent of AMD patients diagnosed with dry AMD. The less common wet AMD usually leads to more serious vision loss.
Dry AMD causes changes of the retinal pigment epithelium, typically visible as dark pinpoint areas. The retinal pigment epithelium plays a critical role in keeping the cones and rods healthy and functioning well. Accumulation of waste products from the rods and cones can result in drusen, which appear as yellow spots. Areas of chorioretinal atrophy (referred to as geographic atrophy) occur in more advanced cases of dry AMD. There is no elevated macular scar (disciform scar), edema, hemorrhage, or exudation.
Dry AMD has three stages, all of which may occur in one or both eyes:
Early AMD - People with early AMD have either several small drusen or a few medium-sized drusen. At this stage, there are no symptoms and no vision loss.
Intermediate AMD - People with intermediate AMD have either many medium-sized drusen or one or more large drusen. Some people see a blurred spot in the center of their vision. More light may be needed for reading and other tasks.
Advanced AMD - In addition to drusen, people with advanced dry AMD have a breakdown of light-sensitive cells and supporting tissue in the central retinal area. This breakdown can cause a blurred spot in the center of your vision. Over time, the blurred spot may get bigger and darker, taking more of your central vision. You may have difficulty reading or recognizing faces until they are very close to you.
Wet AMD occurs when new abnormal blood vessels develop under the retina in a process called choroidal neovascularization (abnormal new vessel formation). Localized macular edema or hemorrhage may elevate an area of the macula or cause a localized retinal pigment epithelial detachment. Eventually, untreated neovascularization causes a disciform scar under the macula.
Symptoms
Dry macular degeneration symptoms usually develop gradually and without pain. They may include:
Visual distortions, such as straight lines seeming bent
Reduced central vision in one or both eyes
The need for brighter light when reading or doing close work
Increased difficulty adapting to low light levels, such as when entering a dimly lit restaurant
Increased blurriness of printed words
Decreased intensity or brightness of colors
Difficulty recognizing faces
What causes macular degeneration?
Though macular degeneration is associated with aging, there is genetic component to the disease. A strong association between development of AMD and presence of a variant of a gene known as complement factor H (CFH) is observed. This gene deficiency is associated with almost half of all potentially blinding cases of macular degeneration.
Other investigators have found that variants of another gene, complement factor B, may be involved in development of AMD.
Specific variants of one or both of these genes, which play a role in the body's immune responses, have been found in 74 percent of AMD patients who were studied. Other complement factors also may be associated with an increased risk of macular degeneration.
Oxygen-deprived cells in the retina produce a type of protein called vascular endothelial growth factor (VEGF), which triggers the growth of new blood vessels in the retina.
The normal function of VEGF is to create new blood vessels during embryonic development, after an injury or to bypass blocked blood vessels. But too much VEGF in the eye causes the development of unwanted blood vessels in the retina that easily break open and bleed, damaging the macula and surrounding retina.
Risk Factors
The biggest risk factor for Macular Degeneration is age. Your risk increases as you age, and the disease is most likely to occur in those 55 and older.
Other risk factors include:
Genetics – People with a family history of AMD are at a higher risk.
Race – Caucasians are more likely to develop the disease than African-Americans or Hispanics/Latinos.
Smoking – Smoking doubles the risk of AMD.
Diagnosis
AMD is detected during a comprehensive eye exam that includes:
Visual acuity test - This eye chart test measures how well you see at various distances.
Dilated eye exam - Drops are placed in your eyes to widen the pupils. Your eye care professional uses a special magnifying lens to examine your retina and optic nerve for signs of AMD and other eye problems. After the exam, your close-up vision may remain blurred for several hours.
Tonometry - An instrument measures the pressure inside the eye. Numbing drops may be applied to your eye for this test.
Both forms of age - related macular degeneration (AMD) are diagnosed by funduscopic examination. Visual changes can often be detected with an Amsler grid.
Color photography and fluorescein angiography are done when findings suggest wet AMD. Angiography shows and characterizes subretinal choroidal neovascular membranes and can delineate areas of geographic atrophy. Optical coherence tomography (OCT) aids in identifying intraretinal and subretinal fluid and can help assess response to treatment.
What Treatments Are Available for Macular Degeneration?
There’s no cure for macular degeneration. Treatment may slow it down or keep you from losing too much of your vision. Your options might include:
Lifestyle changes - like dieting, exercise, avoiding smoking, and protecting your eyes from ultraviolet light.
Anti-angiogenesis drugs - These medications – aflibercept (Eylea), bevacizumab (Avastin), pegaptanib (Macugen), and ranibizumab (Lucentis) -- block the creation of blood vessels and leaking from the vessels in your eye that cause wet macular degeneration. Many people who’ve taken these drugs got back vision that was lost. You might need to have this treatment multiple times.
Laser therapy - High-energy laser light can destroy abnormal blood vessels growing in your eye.
Photodynamic laser therapy - Your doctor injects a light-sensitive drug verteporfin (Visudyne) into your bloodstream, and it’s absorbed by the abnormal blood vessels. Your doctor then shines a laser into your eye to trigger the medication to damage those blood vessels.
Low vision aids - These are the devices that have special lenses or electronic systems to create larger images of nearby things. They help people who have vision loss from macular degeneration make the most of their remaining vision.
Submacular surgery - This removes abnormal blood vessels or blood.
Retinal translocation - A procedure to destroy abnormal blood vessels under the center of your macula, where your doctor can’t use a laser beam safely. In this procedure, your doctor rotates the center of your macula away from the abnormal blood vessels to a healthy area of your retina. This keeps you from having scar tissue and more damage to your retina. Then, your doctor uses a laser to treat the abnormal blood vessels
Tags - eye specialist in ghatkopar, eye clinic in ghatkopar, Age Related Macular Degeneration Treatment In Ghatkopar
For more information link - www.mumbaieyecare.com
#eye specialist in ghatkopar#eye clinic in ghatkopar#health#Age Related Macular Degeneration Treatment In Ghatkopar
0 notes
Text
Widefield Imaging Systems Market By Treatment Type, Device, End User, and Geography Forecast till 2030

Widefield imaging systems are being used more frequently in the global healthcare sector as a result of this factor. In the years ahead, this scenario is likely to create a plethora of expansion opportunities for vendors in the global widefield imaging systems market. Many important parameters, such as component, indication, modality, end-user, and region, are used to segment the global Widefield Imaging Systems Market.
The Widefield Imaging Systems Market is divided into two categories based on modality: standalone and portable. Diabetic retinopathy, retinopathy of prematurity, paediatric retinal diseases, retinal vein occlusion, ocular oncology, uveitis, chorioretinal disease, and glaucoma have all seen significant increases in the past few years.
Exceptional growth in diabetic retinopathy cases is expected to fuel expansion opportunities in the global Widefield Imaging Systems Market from 2020 to 2030. Wide-field fluorescence microscopy is a technique for imaging. Widefield imaging systems are in high demand among hospitals, specialty clinics, and ambulatory surgical centres, among other end-users.
Read more @ https://creativeedge16.blogspot.com/2022/04/widefield-imaging-systems-market.html
#Widefield Imaging Systems#Widefield Imaging Systems Market#medical imaging#Coherent Market Insights
0 notes
Text
Chorioretinitis (retinal lesions) + hydrocephalus + jaundice + HSM = congenital toxoplasmosis; comes from oocytes in cat feces or undercooked meat.
2 notes
·
View notes