#edms - document management system
Explore tagged Tumblr posts
Text
0 notes
Text
2D Animation Services in London by Prabisha Consulting
Introduction In today's digital world, video content is one of the most effective ways to capture your audience’s attention. 2D animation is particularly powerful because of its ability to communicate complex ideas in an engaging and visually appealing way. Prabisha Consulting offers high-quality 2D animation services in London, tailored to meet your business needs and help you connect with your audience in a creative and memorable way.
The Power of 2D Animation 2D animation London is a versatile form of visual storytelling that can be used for various purposes, from promotional videos to explainer content. This form of animation is often more affordable and quicker to produce compared to other types, making it an ideal choice for businesses looking for high-quality, yet budget-friendly solutions. At Prabisha Consulting, we specialise in creating 2D animations that not only look great but also serve your specific marketing and communication goals.
Our 2D Animation Process At Prabisha Consulting, we follow a clear and efficient process to ensure that your 2D animation is created to the highest standards:
Initial Consultation: We begin by understanding your project requirements, business goals, and the message you want to convey through the animation.
Concept Development: Our team works with you to develop an animation concept that aligns with your brand’s identity and objectives.
Storyboarding: A storyboard is created to visualise the flow of the animation, giving you a clear idea of the final product before production begins.
Animation Production: We bring the concept to life with skilled animators, ensuring smooth transitions, vibrant visuals, and a high-quality end result.
Final Edits & Delivery: Once the animation is complete, we provide you with the final version in your preferred format, ready for distribution across your chosen platforms.
Why Choose Prabisha Consulting for 2D Animation?
Experienced Team: Our team of expert animators has years of experience in creating 2D animations for a wide range of industries.
Customised Approach: We offer tailored services to ensure that the animation aligns with your brand’s tone, style, and objectives.
High-Quality Results: We use the latest animation tools and techniques to produce visually stunning animations that capture attention and deliver results.
Affordable Services: We strive to provide high-quality animation services at competitive prices, ensuring that businesses of all sizes can benefit from our expertise.
Conclusion Prabisha Consulting offers professional 2D animation services in London, designed to help businesses create engaging, informative, and visually appealing content. Whether you're looking to explain a product, promote a service, or share your brand’s story, our 2D animation services are the perfect solution. Let us bring your ideas to life with animation that speaks to your audience and enhances your brand’s presence.
#prabishaconsulting#branding agency london#web development services#edms - document management system#artists on tumblr#2d animation london#video production london#prabisha consulting#2D animation London
0 notes
Text
Transform Business Efficiency with Comprehensive Content Management Services
In today’s fast-paced business landscape, managing a growing volume of content efficiently is paramount for companies to stay competitive. Whether it’s for internal processes, customer interactions, or compliance, content management is critical. Comprehensive content management services (CMS) helps streamline operations, improve collaboration, and ensure the security of data across all business functions. This article explores the importance of adopting an enterprise-level CMS and how it can transform business efficiency.
Understanding Content Management Services
Content management services (CMS) are essential for organizing, storing, and tracking business documents, multimedia content, and other digital assets. These systems not only allow businesses to store content but also manage workflows, monitor versioning, and enable easier access to key resources.
Effective CMS solutions offer businesses the ability to centralize all content in a single, easy-to-access location. The value lies not just in storing information but also in the automation, collaboration, and governance features that come with the system.
Key Benefits of Content Management Services
1. Streamlined Workflow Management
One of the most significant advantages of a comprehensive CMS is the improvement in workflow management. With an organized structure, businesses can automate repetitive tasks, such as document approvals, data entry, and content publication. This frees up employees to focus on more strategic tasks and reduces the chances of human error.
Moreover, automated workflows help ensure that the correct version of a document or content is always available, reducing delays caused by version control issues. Additionally, the ability to route documents through approval chains quickly enhances productivity and decision-making.
2. Improved Collaboration Across Teams
For businesses that rely on team collaboration, CMS tools provide a centralized platform where employees can work together more effectively. Teams can access, edit, and comment on documents in real time, no matter where they are located. This eliminates the need for back-and-forth emails and helps ensure everyone is working with the most up-to-date information.
In addition, content management services support role-based access control, allowing businesses to define who can access, edit, and distribute specific content. This provides an added layer of security, ensuring that sensitive information is only available to authorized personnel.
3. Enhanced Data Security and Compliance
As businesses handle sensitive and confidential data, security is a primary concern. A well-implemented CMS provides robust security features that help protect your content from unauthorized access, theft, and data breaches. CMS solutions often offer encrypted storage, user authentication, and audit trails, ensuring that every action taken within the system is logged and tracked.
For industries with strict compliance requirements, content management services make it easier to adhere to regulations by offering features like document retention policies, compliance tracking, and data integrity checks. These ensure that businesses can meet legal requirements and pass audits without disruption.
4. Increased Efficiency and Cost Savings
The ability to access and manage content easily reduces the time spent searching for files or manually sorting through documents. This improved efficiency can translate into direct cost savings as resources are optimized, and business processes are streamlined. By reducing the reliance on physical documents and implementing digital workflows, businesses can also cut costs related to printing, shipping, and storing paper records.
Furthermore, the automation of repetitive tasks reduces the need for manual input, saving both time and money. Employees can spend more time on high-impact activities, such as creative development or strategic decision-making, rather than administrative tasks.
5. Better Content Quality and Consistency
A CMS ensures that content is standardized across an organization. Whether it's marketing materials, internal documents, or customer-facing content, consistency is key to maintaining a professional image. By centralizing all content, businesses can create templates, apply uniform formatting, and ensure that branding is adhered to at every touchpoint.
Furthermore, version control ensures that content is always up to date, minimizing the risk of outdated or conflicting information being used. This helps build trust with customers and clients, who rely on accurate and consistent communication.
Types of Content Management Services
When selecting a content management service, businesses have several options based on their specific needs. Below are the primary types of CMS solutions:
1. Document Management Systems (DMS)
DMS are designed to store and track business documents. These systems typically include features such as document storage, version control, document search capabilities, and access controls. DMS solutions are ideal for businesses that focus on managing a high volume of written documents, such as contracts, legal papers, and financial reports.
2. Enterprise Content Management (ECM) Systems
ECM systems are more comprehensive and are used to manage the entire lifecycle of business content, from creation to archiving. ECM systems are typically integrated with other enterprise applications like Customer Relationship Management (CRM) or Enterprise Resource Planning (ERP) systems. They provide businesses with a robust solution for managing documents, records, multimedia content, and workflows across the organization.
3. Web Content Management (WCM)
WCM solutions are specifically focused on managing digital content on websites. These tools allow businesses to create, manage, and optimize content for the web, including images, videos, articles, and blogs. WCM systems are crucial for businesses that prioritize content marketing, customer engagement, and SEO optimization.
4. Cloud-Based CMS
Cloud-based CMS solutions offer the flexibility of storing content remotely on secure cloud servers. These systems are ideal for businesses that need to provide remote access to content for teams across multiple locations. With cloud CMS, businesses can scale storage and functionality as needed, without the need for on-site infrastructure.
How to Implement Content Management Services
Implementing content management services within a business requires careful planning and execution. Below are the key steps to ensure successful CMS integration:
1. Assess Business Needs
Before selecting a CMS, businesses should evaluate their content management needs. This includes understanding the type and volume of content they manage, the required workflows, and security needs. By assessing these factors, businesses can select a CMS that best aligns with their operational goals.
2. Select the Right CMS
There are numerous CMS platforms available, each offering different features and capabilities. It’s crucial to choose a system that can meet both the current and future needs of the organization. Consider factors such as scalability, ease of use, and integration capabilities when selecting a CMS.
3. Train Employees
Proper training is essential for ensuring that employees can effectively use the new system. Providing training on how to navigate the CMS, manage content, and leverage key features will help businesses realize the full benefits of the system.
4. Monitor and Optimize
After implementing a CMS, businesses should regularly monitor its performance and make adjustments as needed. This includes evaluating system efficiency, gathering feedback from employees, and optimizing workflows to improve productivity.
Conclusion
Comprehensive content management services are not just a luxury but a necessity for businesses looking to streamline their operations, enhance collaboration, and maintain data security. By investing in an effective CMS, businesses can transform their content management process, improve workflow efficiency, and reduce operational costs. The ability to automate processes, maintain consistency, and ensure regulatory compliance positions businesses for long-term success in today’s competitive environment.
Adopting the right CMS solution will allow companies to stay agile, adapt to changing business needs, and ultimately, drive growth and profitability. A well-managed content strategy is a powerful tool in enhancing overall business efficiency and delivering value to both internal teams and customers.
#dms system#electronic document management system#cloud based document management#document management companies#electronic document management solutions#enterprise document management#electronic data management system#dms document management system#dms services#document management system india#dms software india#best document management software in india#document management system dms#document management company#dms software company#edms#online document management system#document management software india#document management system companies in india#document management system in india#document storage systems#Document Storage#physical records management#physical document management#physical document management system#records management system software#record management system#Record management software#Record storage software#records management solutions
0 notes
Text
#Electronic Document Management Systems (Edms) Market#Electronic Document Management Systems (Edms) Market Trends#Electronic Document Management Systems (Edms) Market Growth#Electronic Document Management Systems (Edms) Market Industry#Electronic Document Management Systems (Edms) Market Research#Electronic Document Management Systems (Edms) Market Report
0 notes
Text
First day at work ✨



It's funny that this is not my first work experience, and still... I feel like a kid on the first day of school. Will be working in Project Management for a Software company that makes EDMS (electronic document management systems) I'M SO HYPED ! Discovering KM (knowledge management) a couple years ago was life changing and getting to work for a company that produces systems like the ones I programmed, designed and used for years is so exciting.
To-do list :
▢ check transportation
▢ pick my work computer
▢ check if the company has it's own note-taking/km system or make one
▢ put down list of tasks & prep timetable
✨it's going to be great✨
#workblr#note taking#stemblr#mnemochat#first days are always a vibe#trying to be positive#knowledge management#project management
10 notes
·
View notes
Text
0 notes
Text
Boost Your Career with a Document Controller Course in Abu Dhabi
In today’s fast-paced professional landscape, accurate documentation is at the heart of every successful project and organization. Whether it's engineering, construction, oil & gas, or government sectors, skilled document controllers are in high demand. If you're in Abu Dhabi and looking to enhance your career in this field, enrolling in a Document Controller Course in Abu Dhabi is the perfect step forward.
At EduBrand, we understand the importance of strong document management skills in ensuring the smooth operation of complex projects. Our Document Controller Course in Abu Dhabi is carefully designed to equip students with practical, job-ready knowledge that aligns with international standards.

Why Become a Document Controller?
Document controllers play a vital role in managing and securing company documents, controlling access, ensuring version control, and maintaining compliance with regulations. With the growth of industries like construction, oil & gas, and IT in Abu Dhabi, the demand for certified professionals is on the rise.
Our course gives you a competitive edge by training you in the latest document control software, archiving procedures, and workflow systems. Whether you're a fresher or an experienced admin professional looking to specialize, this course opens doors to new career opportunities.
What You'll Learn
The Document Controller Course in Abu Dhabi offered by EduBrand covers all essential areas of document management, including:
Introduction to document control systems
Document numbering and version control
Filing systems and electronic document management systems (EDMS)
Document review and approval processes
ISO standards related to documentation
Security and confidentiality protocols
Hands-on training with software tools used in the UAE market
We provide a blend of theoretical knowledge and real-world application, so you're not just learning the concepts—you’re learning how to apply them on the job.
Who Should Enroll?
This course is ideal for:
Fresh graduates seeking admin-related roles
Admin professionals aiming to upskill
Engineers and project support staff looking to specialize
Professionals transitioning into the construction or oil & gas sectors
No prior experience in document control is required, making it accessible for beginners as well.
Why EduBrand?
EduBrand is a trusted name in Abu Dhabi for professional and career-focused training. Our trainers are experienced industry professionals who bring real-world insight into the classroom. With flexible schedules, weekend classes, and certification support, we make it easy for working professionals to upgrade their skills without disrupting their current job.
Our certification is recognized across various industries in the UAE, helping you stand out in a competitive job market.
Enroll Today
The Document Controller Course in Abu Dhabi is more than just a qualification—it’s your pathway to career stability and growth in some of the region's most promising industries. Don’t miss the chance to upgrade your skills with EduBrand.
👉 Visit https://edubrand.ae/document-controller/ to learn more and register today.
0 notes
Text
The Critical Role of Engineering Data Management in Electronics Control Panel Manufacturing
The electronics control panel manufacturing industry is a highly specialized sector where precision, efficiency, and compliance are non-negotiable. Control panels are the nerve centers of industrial automation, power distribution, and machinery operations, meaning any error in design, procurement, or assembly can lead to costly failures, safety hazards, and project delays.

To mitigate these risks, manufacturers must adopt Engineering Data Management (EDM)—a systematic approach to organizing, storing, and controlling engineering data throughout the product lifecycle. EDM ensures that all stakeholders—from design engineers to procurement teams and production managers—work with accurate, up-to-date information.
In this blog, we will explore why EDM is indispensable for control panel manufacturers, the challenges it addresses, and best practices for implementation.
Why Engineering Data Management is Essential in Control Panel Manufacturing
1. Ensuring Design Accuracy & Version Control
Control panel designs involve complex schematics, wiring diagrams, and component specifications. Without a structured EDM system:
Engineers may work on outdated drawings, leading to mismatched components.
Multiple file versions can cause confusion, resulting in incorrect assemblies.
Manual updates increase the risk of human error.
How EDM Helps:
Maintains a single source of truth for all design documents.
Tracks revisions with audit trails, ensuring only the latest versions are used.
Supports CAD/EDA (Computer-Aided Design/Electronic Design Automation) integrations for seamless updates.
2. Facilitating Cross-Department Collaboration
A control panel’s journey from concept to delivery involves multiple teams:
Design Engineers create schematics and layouts.
Procurement sources components based on the Bill of Materials (BOM).
Production assembles the panels according to approved designs.
Quality Assurance verifies compliance with industry standards.
Without EDM:
Emailing files back and forth leads to version mismatches.
Delays occur when teams wait for manual approvals.
Miscommunication results in incorrect part orders or assembly errors.
With EDM:
Cloud-based platforms enable real-time collaboration.
Role-based access ensures stakeholders see only relevant data.
Automated workflows speed up approvals and reduce bottlenecks.
3. Compliance with Industry Standards & Regulations
Control panels must comply with stringent standards such as:
IEC 61439 (Low-voltage switchgear and controlgear assemblies)
UL 508A (Industrial control panels)
NEC (NFPA 70) (National Electrical Code)
Challenges Without EDM:
Manual tracking of certifications is time-consuming.
Missing documentation can lead to failed inspections.
Non-compliance risks legal penalties and reputational damage.
How EDM Ensures Compliance:
Centralizes certification documents, test reports, and compliance records.
Automates alerts for expiring certifications.
Links design files to regulatory requirements, ensuring adherence at every stage.
4. Accelerating Time-to-Market
In a competitive industry, faster project delivery is a key differentiator. Delays in design finalization, procurement, or production can cost customers time and money.
How EDM Speeds Up Processes:
Automated BOM Generation: Reduces manual entry errors and speeds up procurement.
Digital Twin Simulations: Allows virtual testing before physical production.
Integration with ERP/MRP Systems: Ensures seamless data flow from design to manufacturing.
5. Reducing Costs & Minimizing Waste
Mistakes in control panel manufacturing can be expensive—wrong components, rework, and scrap materials eat into profits.
EDM’s Cost-Saving Benefits:
Eliminates Rework: Accurate data prevents assembly errors.
Optimizes Inventory: Syncs BOMs with procurement to avoid overstocking or shortages.
Identifies Cost-Effective Alternatives: Tracks component performance and supplier pricing for better sourcing decisions.
Implementing Engineering Data Management in Control Panel Manufacturing
1. Choose the Right EDM/PLM Software
Not all data management systems are suited for electrical engineering. Look for solutions that:
Support electrical CAD (eCAD) integrations (e.g., EPLAN, AutoCAD Electrical).
Offer version control, change management, and approval workflows.
Provide cloud-based access for remote teams.
2. Integrate with ERP & Quotation Systems
To maximize efficiency:
Link EDM with Enterprise Resource Planning (ERP) for real-time inventory updates.
Connect to quotation software to ensure accurate cost estimations based on the latest BOMs.
3. Standardize Documentation Processes
Use templates for schematics, wiring diagrams, and test reports.
Automate compliance documentation to reduce manual effort.
4. Train Teams on EDM Best Practices
Conduct workshops on data entry protocols, revision control, and system navigation.
Assign data stewards to oversee accuracy and updates.
5. Leverage Analytics for Continuous Improvement
Track design changes, production errors, and component failures to identify trends.
Use insights to refine processes and reduce recurring issues.
Conclusion
Engineering Data Management is no longer optional for electronics control panel manufacturers—it’s a strategic necessity. By implementing a robust EDM system, manufacturers can: ✔ Eliminate costly errors caused by outdated or incorrect data. ✔ Speed up project timelines with seamless collaboration. ✔ Ensure compliance with industry regulations. ✔ Reduce waste and optimize costs through accurate BOM management. ✔ Gain a competitive edge by delivering high-quality panels faster.
In an industry where precision and efficiency define success, investing in EDM is a game-changer. Manufacturers that embrace digital transformation today will lead the market tomorrow.
Is your control panel manufacturing process optimized with EDM? If not, now is the time to take the first step toward smarter, more efficient operations.
#project management software#stock management software#inventory management software#product management system#quotation management software#product management software
0 notes
Text
🚀 Become a Certified Document Control Specialist! 🚀
Master document control & electronic records management with this comprehensive 5-day course!
✅ Implement Electronic Document Management Systems (EDMS) efficiently ✅ Learn document security, compliance & ISO 9000 standards ✅ Automate workflows & enhance document retrieval speed ✅ Gain a professional certification & boost your career in document management!
🔗 Enroll Now
#DocumentControl #EDMS #RecordsManagement #ISO9000 #Compliance #CareerGrowth #Training
#document control#recordsmanagement#RecordsManagement#ISO9000#Compliance#management#business strategy#companies
0 notes
Text
New Product Development in Pharma: Strategies for Faster Market Entry

Bringing a new pharmaceutical product to market is a time-intensive and highly regulated process. Traditional New Product Development (NPD) in the pharmaceutical industry takes an average of 10-15 years, from discovery to commercialization. However, in a competitive landscape, companies must adopt innovative strategies to accelerate drug development, streamline approvals, and reduce time-to-market while maintaining compliance and quality.
Key Challenges in Pharma NPD
1. Lengthy Regulatory Approvals
Pharmaceutical companies must comply with FDA (USA), EMA (Europe), MHRA (UK), and other global agencies, each having distinct approval processes. Regulatory bottlenecks often lead to delays in clinical trials, submissions, and market entry.
2. Complex Clinical Trials
Clinical trials, the most time-consuming phase of drug development, face challenges such as patient recruitment, ethical approvals, and protocol compliance. Delays in trial execution directly impact market timelines.
3. High R&D Costs and Uncertain ROI
Developing a new drug requires billions in investment, with no guarantee of success. Pharma companies must manage budget constraints, risk assessment, and resource allocation efficiently to ensure commercial viability.
4. Supply Chain and Manufacturing Bottlenecks
Scaling up drug production while ensuring quality, compliance, and global distribution readiness remains a significant challenge. Manufacturing delays can stall product launches, affecting revenue generation.
Strategies for Faster Market Entry in Pharma NPD
1. Implement AI and Digital Technologies in Drug Discovery
AI and machine learning can significantly accelerate drug discovery by analyzing vast datasets to identify potential drug candidates faster. AI-driven platforms help predict molecule behavior, optimize formulations, and reduce preclinical testing time.
2. Optimize Regulatory Submissions with Automation
Using Regulatory Information Management Systems (RIMS) and Electronic Document Management Systems (EDMS) can help automate regulatory submissions, reducing errors and approval delays. Proactive communication with regulatory agencies through early-stage consultations can also streamline the approval process.
3. Leverage Adaptive and Decentralized Clinical Trials
Traditional clinical trials are time-consuming and expensive. Implementing adaptive trial designs allows real-time modifications based on interim results, leading to faster decision-making. Decentralized clinical trials (DCTs) using remote patient monitoring and digital platforms improve recruitment speed and engagement, reducing trial durations.
4. Strengthen Cross-Functional Collaboration
Breaking down silos between R&D, regulatory, manufacturing, and commercial teams ensures a seamless transition from drug discovery to market launch. A centralized project management approach enhances communication and decision-making efficiency.
5. Utilize Fast-Track Regulatory Pathways
Many regulatory agencies offer accelerated approval pathways for breakthrough drugs. Pharma companies should explore:
Fast Track (FDA) – Speeds up drug reviews for serious conditions.
Breakthrough Therapy Designation (FDA) – Provides early-stage guidance for promising treatments.
Priority Review (FDA, EMA) – Shortens review times for high-impact drugs.
Adaptive Licensing (EMA) – Allows phased market access while additional data is gathered.
6. Optimize Supply Chain and Manufacturing Scalability
Ensuring an agile supply chain with on-demand manufacturing, predictive analytics, and AI-driven inventory management can reduce delays in production and distribution, supporting faster market entry.
Conclusion
Pharma companies must adopt a tech-driven, regulatory-smart, and collaborative approach to reduce time-to-market. By integrating AI in drug discovery, automating regulatory processes, optimizing clinical trials, and leveraging fast-track approvals, organizations can achieve faster commercialization without compromising safety or compliance. In today’s fast-paced industry, speed is a competitive advantage—companies that embrace digital transformation and strategic execution will lead the future of pharmaceutical innovation.
0 notes
Text
Document Controller
Document ControllerJob Location : QatarExperience : Proven work experience as a Document Controller or in a similar role. Experience with Electronic Document Management Systems (EDMS) and Data Management. Qualification : BSc degree in Project Management or relevant field. Knowledge of Microsoft Office (especially MS Excel) and Oracle systems. Excellent communication skills in both written and…
0 notes
Text
Video Production Services in London – Prabisha Consulting
Introduction In today's digital world, high-quality video content is essential for businesses to engage their audience effectively. If you're looking for video production services in London, Prabisha Consulting is the ideal partner to bring your vision to life. Our professional team is skilled in creating impactful videos tailored to your brand's goals.
Why Choose Prabisha Consulting for Video Production?
Expert Team: Our experienced video production team ensures every project is handled with the utmost professionalism.
Tailored Solutions: We understand that each client has unique needs. We provide customised video production that aligns with your business objectives.
Creative Approach: We combine technical expertise with creative flair to deliver videos that captivate and inspire.
Our Services We offer a range of Video production London that can benefit various industries:
Corporate Videos: Enhance your brand with corporate videos that communicate your values, services, and products clearly.
Promotional Videos: We help you create videos that promote your services or products to attract and engage potential customers.
Event Coverage: From conferences to live events, we provide full-service event video production.
Training and Educational Videos: Whether you're training employees or educating your audience, we create clear and effective instructional videos.
Benefits of Video Production Services
Enhanced Branding: Professional video production enhances your brand's image and credibility.
Increased Engagement: Videos are more likely to be shared and viewed, increasing your audience reach.
High-Quality Content: We use state-of-the-art equipment and editing techniques to ensure the highest production quality.
Conclusion At Prabisha Consulting, we specialise in delivering exceptional video production services in London. Our tailored, creative approach ensures that your video content stands out, enhances your brand, and effectively engages your target audience. Whether you need corporate, promotional, or event videos, we’ve got you covered. Contact us today to elevate your video content to new heights!
#prabishaconsulting#2d animation london#edms - document management system#web development services#across the spiderverse
0 notes
Text
Electronic Document Management Software
In today's digital era, businesses are increasingly relying on technology to streamline their operations, improve productivity, and ensure the smooth handling of critical information. One of the most essential tools that have become a cornerstone in achieving these objectives is Electronic Document Management Software (EDMS). As businesses continue to generate and store vast amounts of data, the need for an efficient system to manage, access, and secure these documents has never been more critical. This is where PDMPL (Pridex Data Management India Pvt. Ltd.) steps in, offering cutting-edge solutions through their advanced Electronic Document Management Software.
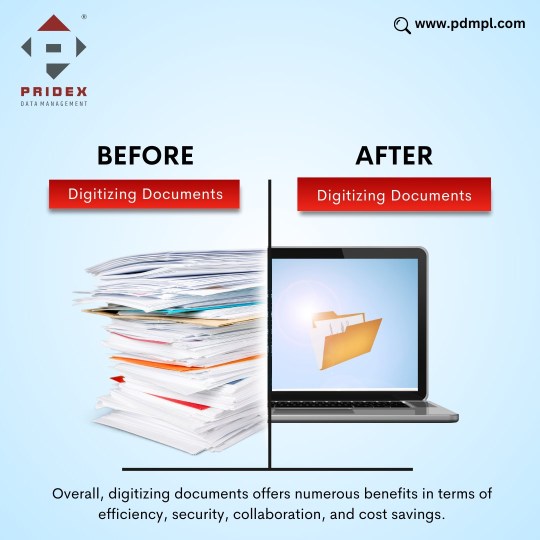
Understanding Electronic Document Management Software (EDMS)
Electronic Document Management Software is a system that helps businesses digitize, store, manage, and retrieve documents in a secure and organized manner. With the advent of the digital age, physical document storage is becoming obsolete, and businesses need a modern solution that offers greater efficiency and accessibility. EDMS enables organizations to scan and convert paper documents into digital formats, making it easier to manage and share them across departments.
The key features of an EDMS include document scanning, storage, retrieval, version control, security, and sharing. These features not only improve the efficiency of day-to-day operations but also enhance compliance with industry regulations, reduce operational costs, and ensure data security.
How PDMPL’s Electronic Document Management Software is Revolutionizing the Industry
PDMPL, a leading provider of data management solutions, has developed a robust and scalable Electronic Document Management Software that caters to the needs of modern businesses. Their EDMS integrates seamlessly with existing business workflows, offering a complete solution for managing both physical and digital documents. Let’s take a look at the benefits and features that make PDMPL’s EDMS stand out in the market.
1. Efficient Document Storage and Retrieval
One of the primary benefits of PDMPL’s EDMS is the ability to store documents electronically, making it easier for businesses to organize and retrieve them quickly. With traditional document management systems, searching for physical files can be time-consuming and prone to errors. However, with PDMPL’s EDMS, documents are stored in an organized, searchable, and easily accessible format. The software uses advanced indexing and metadata tagging, allowing users to search for documents by keywords, dates, and other criteria.
2. Enhanced Security and Compliance
Security is a major concern for businesses dealing with sensitive information. PDMPL’s Electronic Document Management Software ensures that all documents are protected by advanced encryption protocols, ensuring confidentiality and data integrity. The software also offers role-based access control, allowing businesses to restrict access to specific users based on their roles within the organization. Additionally, PDMPL’s EDMS helps businesses comply with industry regulations such as HIPAA, GDPR, and other compliance standards by ensuring that documents are securely stored and handled.
3. Collaboration and Sharing Capabilities
PDMPL’s EDMS enables seamless collaboration among team members, regardless of their location. With cloud-based storage, employees can access documents from any device with internet access, making it easier to work remotely or collaborate with external stakeholders. The software supports real-time document sharing and editing, allowing users to work together on the same document without the risk of data loss or version control issues.
4. Automated Workflow Management
Another standout feature of PDMPL’s EDMS is its ability to automate document-centric workflows. Businesses can define specific workflows for various tasks such as document approvals, reviews, and sign-offs. This automation helps eliminate bottlenecks, reduces human error, and speeds up the overall process. With automated workflows, businesses can ensure that tasks are completed on time and that no steps are missed, ultimately increasing productivity and efficiency.
5. Cost Reduction and Space Saving
Traditional document management methods involve the use of physical storage space, such as filing cabinets and storage rooms, which can be costly and inefficient. PDMPL’s Electronic Document Management Software eliminates the need for physical storage, significantly reducing overhead costs associated with printing, filing, and storing paper documents. Furthermore, the digitization of documents allows businesses to save physical space, creating a more organized and efficient work environment.
6. Scalability and Customization
PDMPL’s EDMS is designed to grow with your business. Whether you're a small startup or a large enterprise, the software can scale to meet your specific needs. The system is highly customizable, allowing businesses to tailor it to their unique workflows and requirements. Whether you need custom document templates, integrations with other software, or specific user permissions, PDMPL’s EDMS can be adapted to suit your needs.
7. Disaster Recovery and Backup
Data loss can be catastrophic for any business. PDMPL’s EDMS ensures that all documents are backed up securely, both on-premises and in the cloud. This redundancy guarantees that documents can be restored in the event of system failure, natural disaster, or cyberattack. With the right backup strategy in place, businesses can avoid the risks associated with data loss and ensure business continuity.
Why Choose PDMPL’s Electronic Document Management Software?
PDMPL’s Electronic Document Management Software offers a comprehensive and reliable solution for businesses seeking to optimize their document management processes. With a focus on efficiency, security, and compliance, PDMPL provides a solution that empowers businesses to manage their documents with ease.
Their software offers unparalleled customer support and technical expertise, ensuring that businesses receive the guidance they need to implement and maintain an effective document management system. Whether you're looking to digitize paper-based files or streamline your existing digital document management process, PDMPL is the trusted partner you need.
Conclusion
In a world where data is king, the need for an efficient, secure, and scalable document management system has never been more critical. PDMPL’s Electronic Document Management Software provides businesses with a comprehensive solution that ensures smooth, secure, and efficient document handling. From improved collaboration to enhanced security, PDMPL’s EDMS is a must-have tool for businesses looking to stay ahead of the curve in today’s competitive landscape. If you are looking for a reliable and cutting-edge solution to manage your documents, look no further than PDMPL.
0 notes
Text
Quality Control Review of Regulatory Clinical Documents: The Tools of Trade and Best Practices

Quality control (QC) in the pharmaceutical industry is crucial in reviewing regulatory clinical documents to ensure the accuracy, consistency, and compliance of documents submitted to regulatory authorities for approval.
What is a QC review?
In medical writing, QC means ensuring the document’s content, style and format are of high quality. This does not just ‘happen’ but because of systematic quality control review following client-specified checklists and standard operating procedures (SOPs) to ensure a thorough and consistent review. Even well-written documents may have “Errors”, but the QC experts can spot them with a “fresh set” of eyes that a writer might overlook. Hence, a quality control check is essential before submission, because a good quality review takes time but is well worth the effort.
Who should perform the QC review?
Ideally, the QC review should be performed by somebody other than the author or medical writer of the document can be called a QC reviewer/editor, who may have less, equal, or greater experience than the author. The type of documents they might receive include:
Investigator’s brochures (IBs)
Briefing books (BBs)
Clinical study reports (CSRs)/addendums
Protocol and protocol amendments
Module 2 documents/summaries
Safety narratives
Regulatory authority responses and
Paediatric study plans etc.
The “Tools of the Trade”: The Best practices to perform the QC
Many pharmaceutical companies or CROs have their own set of standards for producing their documents. They may be described in company-specified templates, document-specified guidelines (e.g., QC Process guideline for Clinical submission documents: Protocol, IB’s and CSR, etc), style guides, SOPs, etc. Apart from that, one must have a QC checklist to ensure that a review's quality and level of detail are consistent. A detailed QC checklist is an important tool to help the QC reviewer cover all the aspects of a review which will keep them on track of what they have checked and what’s left to do. Also, it will help them manage time efficiently. The entire QC review/editing process will follow the below sequencing steps:
1. Receiving the workflow notification from the Medical Writer:
The QC reviewer/editor will receive the request from the author (Medical Writer) with the scope of the review mentioned and the link (to navigate the working document) through the workflow notification generated from the respective electronic data management system’s (EDMS) eg, MEDIVA, PleaseReview or InteliNotion, etc. along with the source documents/links and authoring instructions (if any).
2. Confirming the required/source documents:
Get confirm the required/source documents with the Medical Writer that are available through the links shared through annotations in the document that may include:
Document current template
Source documents (Data Tables such as TLFs, Protocol and its amendments, CSR and SAP results), etc depending on the document to be reviewed.
Style guide (covering text styles, punctuation, abbreviations, capitalization, number and date formats, preferred word choice, etc).
Authoring instructions if any (covering use of company-specific authoring toolbars, cross-referencing and in-text referencing etc).
SOPs
QC checklists and attestation forms
3. Start editing the document:
Start editing the documents by “TRACK changes” ON with the below points in mind as main points of “standard QC checks” with the examples:
i. Formatting:
Page layout
Pagination
Headers and footers
ii. Consistency:
Synopsis/summary/conclusion versus the main body of the document: eg, Protocols
Text: study endpoints, and results eg, CSRs
Tabular data quoted in text: especially for protocols, the schedule of assessments, and study schema versus the text
iii. Tables/Figures:
Formatting and layout
Consistency in the style of tables throughout the document (eg, Heading and Caption styles)
Clarity of presentation/legends
Ensuring the sources in the footnotes (correctly cited) and their designators have corresponding footnotes etc.
iv. References:
Correct citations
Format of in-text citations (insert EndNote to the Reference list)
Confirming the reference list completeness
v. Internal/External Links:
Internal links-confirms the internal links for the sections, In-text tables, and or figures.
External links are referenced accurately and consistently throughout the document and the blue text has been applied to the source sections/citations to external documents where a link will be created during the publishing phase of the document.
vi. General:
Data accuracy check versus source documents/and their content
Spell check and grammar
Sentence construction
Abbreviations (client-specific if any)
Check for TOC, List of Tables/Figures and Appendices, etc. for completeness
Template verification etc.
vii. Flagging to the Medical Writer:
Flagging (comment alerts) issues to Medical Writer is a crucial step for maintaining document standards: Here are some of the best tips to do it effectively:
Tag Online to Medical Writer: Tag all comments to the Medical Writer online, which will enable them to receive mail alerts/notifications, and if you are working in a document editor like MS Word, use the track changes features or comments to highlight issues directly in the text.
Be Specific (easy navigation): Identify the issue. Instead of saying “There’s a mistake”, specify what the mistake is and where it’s the location (Include the section number in the source document, eg: SAP section 7.1.1).
Provide Context: Explain why it’s an issue. For example, “This section contradicts the data presented in the synopsis/or per the source documents like protocol”.
Provide Evidence-based comments: backed by relevant data, studies, or guidelines and a style guide to address the comments effectively.
Suggest Solutions: If possible, offer a way to fix the problem. This can help the Medical Writer understand the issue better and correct it more efficiently.
Be Professional and Polite: Use a respectful tone. For example, “I noticed a potential inconsistency in the text/Tables against its source documents on Page 56. Could you please review it?”
Prioritization of Comments: Address the critical comments first that can affect the data consistency (major), followed by less urgent ones, to manage time efficiently.
Summarize major issues: If there are multiple issues, provide a summary at the end of your feedback to ensure the Medical Writer understands the key areas that need further attention.
Compliance Tips/Comment resolution: Here are some tips for resolving the comments during the QC check:
Clarification Requests/Seek for additional information: The Medical Writer can approach the QC reviewer to seek additional information or clarification on the comment to ensure resolution.
Consistency Checks: Ensure that the resolution aligns with the overall document’s tone, style, and content.
Documentation of Changes: Document the changes made in response to comments for transparency and reference.
Provide Feedback loop: Implement a feedback loop where the QC reviewer/editor reviews the resolution to “confirm” satisfaction.
Complete the comment resolution during the QC period: Follow the client specification rules to ensure the comment resolution happens during the QC timeline provided.
Use automated tools: Utilize automated tools or software to track and manage comments and their resolutions for future tracking.
Checklist filling and Uploading:
Ensure to fill the QC checklist (specific to each type of document) to confirm its contents like text, tables/figures appendices, etc. by ticking the boxes where the QC reviewer/editor can check off that each item in the list has been checked (Yes, No or NA).
Also, include a comment box where the QC reviewer/editor can explain why an item is not checked and in which specific findings can be described.
In addition, the checklist also has a space box that includes the version number of the document and the date of the reviewed document together with its title, project code/EDMS link, and a space to sign (Electronic Signature) and date the form to record that the QC has been completed.
6. Checklist Sign through DocuSign/ Adobe Acrobat Signature:
Once the checklist is filled, the QC reviewer will use the tools (DocuSign/Adobe Acrobat Signature) to sign the documents electronically. It helps eliminate the need for printing, signing, and scanning physical copies. Also, sending, and managing the documents digitally.
7. Check the documents back to EDMS:
After, the QC review/editing of the document is completed, “Save” the document with all the comments and changes Check it back into the respective EDMS and attach/upload the signed QC checklist (PDF) along with it.
8. In Closing:
In conclusion, when we return the reviewed copy of the document and checklist to the Medical Writer, ensure that our comments/edits are clear and easy to understand. More than just a quick read-through, QC reviews/edits are comprehensive and detailed exercises that can also be a learning experience for both the reviewer and the Medical Writer. So, overall, the regulatory agency/authority as a reader, will expect high-quality documents, but this requires an organized review process. As well as QC guidance documents, a good QC checklist will also contribute towards a review that is performed well and will improve the standard of the reviewed documents.
#medical device regulatory consulting#regulatoryaffairs#medical devices#regulatoryapproval#regulatoryrequirements
0 notes
Text
Cut costs and boost productivity with electronic document management
In today’s corporate operations, document management is undeniably critical. As digital transformation advances, traditional paper-based document management has revealed numerous shortcomings, such as limited storage capacity, low retrieval efficiency, and poor document security. To address these challenges, an increasing number of businesses are adopting Electronic Document Management Systems (EDMS). These systems not only enhance file management efficiency but also significantly reduce operational costs and boost productivity. This article explores how to achieve these benefits, particularly through the adoption of efficient systems like 8Manage EDMS.

1.Overview of Electronic Document Management Systems
An Electronic Document Management System (EDMS) is a specialized software platform designed to store, manage, retrieve, and track electronic documents. Compared to traditional paper-based document management, EDMS offers higher efficiency and lower costs. It allows businesses to categorize, archive, share, and retrieve documents quickly and accurately.
EDMS enables organizations to store all critical documents and data in a centralized database, ensuring secure, convenient access and compliance. With advancements in technology, many EDMS platforms now incorporate features like Optical Character Recognition (OCR), artificial intelligence, and automated workflows, further improving document management efficiency and intelligence.
2.How EDMS Reduces Operational Costs
1. Lower Physical Storage Costs
Traditional document management relies heavily on paper files, requiring extensive physical storage space, especially as businesses expand and paper documents proliferate. By digitizing documents and storing them electronically, EDMS drastically reduces the need for physical storage space and associated costs.
For example, 8Manage EDMS can swiftly scan paper documents into electronic formats and store them in the cloud or on servers, eliminating the issue of accumulated archives and significantly cutting physical space and storage costs.
2. Enhanced Document Management Efficiency
Manual document handling is time-consuming and prone to inefficiencies. EDMS allows for organized categorization and keyword-based searches, saving substantial time and labor. Automated classification and tagging further enhance the intelligence and efficiency of document management.
3. Minimized Errors and Redundancy
Manual document management often leads to errors, such as misplaced files, archiving mistakes, or version inconsistencies, which impact operational efficiency and pose compliance risks. EDMS automates processes to ensure accuracy and standardization. With systems like 8Manage EDMS, each document update is automatically version-controlled, minimizing human error and redundant tasks.
4. Improved Remote Work and Collaboration
In modern enterprises, team members often work from various locations. EDMS facilitates seamless information sharing and collaboration, regardless of geography. With cloud-based access and real-time collaboration features in 8Manage EDMS, team members can share and edit documents anytime, anywhere, greatly enhancing efficiency.
3. How EDMS Boosts Productivity
1. Streamlined Document Review and Approval
Traditional document approval processes are cumbersome, often involving physical signing and passing documents back and forth. EDMS automates approval workflows, ensuring faster and more transparent processes. With 8Manage EDMS, automated approval flows notify relevant personnel, record feedback, and eliminate delays associated with manual handling.
2. Intelligent Data Analysis and Reporting
EDMS can analyze and summarize vast amounts of document data, providing valuable insights for decision-making. Businesses can track document access, version history, and usage frequency, identifying opportunities for better document management or archiving. This supports data-driven, strategic decisions.
3. Improved Employee Efficiency
A unified document management platform reduces the time employees spend searching, organizing, and managing files. With smart search and one-click archiving features in 8Manage EDMS, employees can quickly locate and process needed documents, focusing more on core tasks and improving overall efficiency.
4. Ensured Document Security and Compliance
EDMS platforms typically include robust access controls to restrict document access based on roles, preventing sensitive information from leaking. For instance, 8Manage EDMS provides granular permissions, enabling tailored access according to employee roles and responsibilities, ensuring document security. Moreover, operation logs are automatically recorded, ensuring compliance with regulatory standards.
4. The Future of EDMS
With continuous advancements in artificial intelligence, big data, and cloud computing, EDMS will become even more intelligent and automated. Future systems will not only enhance document management efficiency but also provide stronger decision support through deep learning and data analysis. Additionally, with the widespread adoption of 5G technology, real-time document management and collaboration capabilities will further improve.

FAQs
1.What are the main features of an EDMS?
Key features include document storage, categorization, retrieval, sharing, version control, access management, approval workflows, and data analysis. EDMS helps businesses efficiently manage vast amounts of documents while ensuring security and compliance.
2.How to choose the right EDMS for your business?
Consider factors like functionality, security, compliance support, cloud storage options, intelligent automation features, user-friendliness, and customer support. For example, 8Manage EDMS provides comprehensive solutions to meet diverse business needs.
3.Can EDMS improve collaboration efficiency?
Yes, EDMS enhances collaboration by enabling real-time sharing, online editing, and automated approvals, allowing team members to work efficiently across locations and reducing delays caused by manual processes.
Conclusion
In summary, EDMS offers significant potential to reduce operational costs and boost productivity. With intelligent and automated features, systems like 8Manage EDMS empower businesses to manage documents more effectively, enhancing overall operational efficiency and employee productivity. For businesses seeking to maintain a competitive edge, adopting an efficient EDMS is undoubtedly a wise choice.
0 notes