#global defibrillator devices and equipment market
Text
Global Defibrillator Devices and Equipment Market Competitive Strategies and Forecasts to 2031

The Defibrillator Devices and Equipment Global Market Report 2021-31 by The Business Research Company describes and explains the global defibrillator devices and equipment market and covers 2016 to 2021, termed the historic period, and 2022 to 2026, termed the forecast period, along with further forecasts for the period 2026-2031. The report evaluates the market across each region and for the major economies within each region.
The Defibrillator Devices and Equipment Global Market Report 2022 covers defibrillator devices and equipment market drivers, defibrillator devices and equipment market trends, defibrillator devices and equipment market segments, defibrillator devices and equipment market growth rate, defibrillator devices and equipment market major players, and defibrillator devices and equipment market size.
View Complete Report:
The defibrillator devices and equipment market report provides in-depth analysis of the impact of COVID-19 on the global defibrillator devices and equipment industry along with revised market numbers due to the effects of the coronavirus and the expected defibrillator devices and equipment market growth numbers for 2022-2031.
The global defibrillator devices and equipment market size is expected to grow from $8.68 billion in 2021 to $9.73 billion in 2022 at a compound annual growth rate (CAGR) of 12.2%. The global defibrillator devices market share is expected to grow to $14.40 billion in 2026 at a CAGR of 10.3%.
Request Report Sample Now:
https://www.thebusinessresearchcompany.com/sample.aspx?id=2538&type=smp
Defibrillator Devices and Equipment Global Market Report 2022 is the most comprehensive report available on this market and will help gain a truly global perspective as it covers 60 geographies. The chapter on the impact of COVID-19 gives valuable insights on supply chain disruptions, logistical challenges, and other economic implications of the virus on the market. The chapter also covers markets which have been positively affected by the pandemic.
TBRC’s report covers the defibrillator devices and equipment market segments-
1) By Type: Implantable Defibrillators, External Defibrillators
2) By Implantable Defibrillator: Transvenous Implantable Cardioverter Defibrillator (T-ICDs), Subcutaneous Implantable Cardioverter Defibrillator (S-ICDs), Cardiac Resynchronization Therapy- Defibrillator (CRT-D), Single and Dual Chamber
3) By External Defibrillator: Manual External Defibrillator (MEDs), Automated External Defibrillator (AEDs), Wearable Cardioverter Defibrillator (WCDs)
4) By End-User: Hospitals, Pre-Hospitals, Public Access Market, Alternate Care Market, Home Healthcare
Table Of Contents
1. Executive Summary
2. Defibrillator Devices And Equipment Market Characteristics
3. Defibrillator Devices And Equipment Market Trends And Strategies
4. Impact Of COVID-19 On Defibrillator Devices And Equipment
5. Defibrillator Devices And Equipment Market Size And Growth
.
.
26. Africa Defibrillator Devices And Equipment Market
27. Defibrillator Devices And Equipment Market Competitive Landscape And Company Profiles
28. Key Mergers And Acquisitions In The Defibrillator Devices And Equipment Market
29. Defibrillator Devices And Equipment Market Future Outlook and Potential Analysis
30. Appendix
About The Business Research Company:
The Business Research Company is a market intelligence firm that excels in company, market, and consumer research. Located globally it has specialist consultants in a wide range of industries including manufacturing, healthcare, financial services, chemicals, and technology. It has offices in the UK, the US and India and a network of trained researchers in 20+ countries globally.
Contact Information:
The Business Research Company
Europe: +44 207 1930 708
Asia: +91 88972 63534
Americas: +1 315 623 0293
Email: [email protected]
Find us on
LinkedIn: https://in.linkedin.com/company/the-business-research-company
YouTube: https://www.youtube.com/channel/UC24_fI0rV8cR5DxlCpgmyFQ
Twitter: https://twitter.com/tbrc_info
Facebook: https://www.facebook.com/TheBusinessResearchCompany/
Blog: http://blog.tbrc.info/
#defibrillator devices and equipment market#global defibrillator devices and equipment market#defibrillator devices and equipment market industry#defibrillator devices and equipment market size#defibrillator devices and equipment market growth#defibrillator devices and equipment market scope#defibrillator devices and equipment market trends#defibrillator devices and equipment market analysis#defibrillator devices and equipment market share#defibrillator devices and equipment market report#defibrillator devices and equipment market research#defibrillator devices and equipment market segmentation
0 notes
Text
Good tourniquets save lives. Bad ones kill soldiers. The global market is awash with cheaply-made knock-offs: Handles that shear off under tension, rubber tubes that won’t tighten around a limb, devices that fail when they’re needed most. That’s why most armies buy in bulk from trusted suppliers. But Evgen Vorobiov prefers Amazon. Top of his Wish List at the moment are combat application tourniquets (CATs) from North American Rescue (five stars from 1,720 reviewers). Also on the list: burn dressings, compact chest seals, trauma shears and “The Original Rescue Essentials Brand QuikLitter”—a black canvas stretcher which promises low-cost casualty evacuation and patient transfer.
Before Russia launched its full-scale invasion in February 2022, Vorobiov, a lawyer, worked for the Ukrainian central bank and then on international projects trying to reform Ukraine’s financial system—“banking regulations, consumer protection, that kind of thing.” But, with Russian troops massing on Ukraine’s borders, he took some courses in tactical medicine, hoping to make himself useful if the worst happened. It did.
The Ukrainian army, dwarfed by its opponent, was supposed to collapse in days. But remarkably, it held the line, bolstered by a huge wave of volunteers and reservists. Trucks filled with Kalashnikov rifles drove into Kyiv’s neighborhoods and handed out weapons to anyone who wanted to join the fight. Engaged in constant combat for days on end, the armed forces quickly ran short of supplies. Vorobiov, with his basic knowledge of combat medicine, started reaching out to anyone he knew overseas who could help find CAT tourniquets, trauma bandages, chest seals and other lifesaving equipment. He and a couple of colleagues sourced gear from the UK, US, and the Netherlands and got it to Poland. Anyone they knew coming back to Ukraine via Poland was asked to bring bags of supplies, forming “a human chain” stretching from Europe to the frontline.
Eighteen months on, his operation has blossomed. Vorobiov’s intimate understanding of Ukrainian bureaucracy means he’s been particularly effective at getting sensitive shipments over the border, making him a focal point for other donors. He’s built a potent fundraising operation on social media, tapping into an international community of supporters to raise money and find supplies. And, by driving back and forth across Ukraine, delivering right into the hands of combat medics, he’s forged relationships with units who can tell him exactly what they need and when, creating a personalized military logistics operation from his living room in downtown Kyiv. In May, Vorobiov got a call from a medic working at a makeshift field hospital close to Bakhmut, the burned-out ruin of a town that was a bloody pivot point for the frontline in the first half of 2023. They were in desperate need of a portable ultrasound machine to scan casualties for internal injuries. Vorobiov tapped his network for money, and found a secondhand device in Poland for $3,400. When we meet, it’s sitting in his apartment waiting to go east, and he’s turned his attention to getting hold of a portable charging unit for a defibrillator. Soldiers ask for everything: Drones for artillery and reconnaissance units, portable generators, Starlink satellite internet terminals, 4x4s, the things they need to keep them online and alive, which are often the same thing in a war defined by the use of technology on the frontline.
For decades, Ukrainian civil society has been built horizontally. Rather than rely on government agencies for help, people have leant on personal connections—everyone knows someone who knows someone who can get what you need, help you out. This parallel state has been providing vital aid in eastern Ukraine since Russian proxies invaded in 2014. Since the full-scale invasion began it’s become super-charged, using social media and messaging platforms to go global. Vorobiov is just one link in a relay of money, supplies, innovations, and solidarity that is keeping Ukraine’s soldiers in the fight.
The Front Line Kitchen occupies a few cramped ground-floor rooms and a shed off a sloping street on the edge of Lviv’s picturesque old town. In the courtyard, volunteer cooks peel mountains of potatoes and beets among the organized chaos of plastic vegetable crates, cardboard boxes and IKEA bags overflowing with baked goods. Inside, fridge-sized dryers are filled with shredded vegetables, meat and mushrooms, waiting to go into vacuum-sealed ration packs.
The kitchen started years before the full-scale invasion, in the aftermath of the “Euromaidan” demonstrations and “Revolution of Dignity” in late 2013 and early 2014. Protests against the Kremlin-backed government of Viktor Yanukovich in Kyiv’s Independence Square—Maidan Nezalezhnosti—were met with a bloody crackdown by security forces. As the violence escalated, protesters formed self-defense forces and medical units, repelling assaults and even storming government buildings. In February 2014, Yanukovich fled Kyiv. Days later, Russia illegally annexed Crimea, and its proxies seized government buildings in Donetsk and Luhansk in the east of Ukraine, declaring themselves independent of Ukraine. They met little formal resistance: Under Yanukovich, Ukraine’s armed forces and intelligence agencies had been gutted.
That spring, Ukraine raised volunteer battalions, some directly linked to the self-defense units formed in Maidan. They were still ill-equipped, so they came to rely on other volunteers to supply them with basics—food, uniforms, medicines, vehicles—even weaponry. “The volunteers essentially replaced the function of the government for supplying the necessary resources,” says Roman Makukhin, a member of the National Interests Advocacy Network, a Kyiv-based NGO. “Protecting basically their neighbors, their friends, their brothers and sons.”
Oksana Mazar and Lyuda Kuvayskova, the Front Line Kitchen’s founders, met sewing camouflage nets and balaclavas for the volunteer detachments. Many of their friends, and Kuvayskova’s son, had been at Maidan. “The war had started, even if it wasn’t talked about like it’s a war,” Mazar says. “We just wanted to help, as the guys didn't have anything. No clothes, no shoes, and no food—because it was not [officially] a war.”
They started cooking meals for soldiers, experimenting with ways to turn home-made borscht and holubtsi (cabbage rolls) into ration packs that would survive the 1,000-kilometer journey to the Donbass, usually in the back of cars or trucks after being handed over to anyone heading that way. The cooks worked in small batches, drying food in friends’ kitchens, before they were gifted their current premises. They raised enough money to buy their own dryers, and gradually expanded. After the full-scale invasion began, the kitchen’s front yard was filled with volunteers and people bringing supplies. “They knew that we were doing food for the military, and they wanted to help,” Mazar says.
With 1 million Ukrainians mobilized to fight the Russians, the need has grown massively. The kitchen is now putting out 20,000 meals a day, sending truckloads of food east, and taking orders direct from the military. To scale up they’ve relied on donations, often sourced via the @frontlinekit Twitter account. The account is run by Richard Woodruff, who came to Ukraine from the UK early in the war, intending to join one of the international brigades in the Ukrainian army, despite having no military training. After seeing footage of the ferocious defense of Kyiv, “I kind of rethought my chances of survival,” he says. Instead, he arrived at Lviv train station a few weeks after the full scale invasion began, and soon found his way to the kitchen.
If the 1991 Gulf War was the first major conflict broadcast live on TV, the defense of Ukraine is the first full-scale interstate conflict to be shown in real time on Twitter. Ukrainians posted from the early hours of the invasion—air raid sirens sounding over a European capital in 2022; queues at the recruiting centers, calls for aid and statements of defiance. They recorded acts of insane valor, videoing themselves as they ambushed Russian columns with anti-tank missile launchers they’d barely been trained to use. Civilian drones pressed into service as surveillance tools provided a steady stream of high-definition footage made for phone screens, giving a gamer’s-eye view to the fighting. As Russian forces were pushed back, and the Ukrainian armed forces reclaimed land, the atrocities and scenes of destruction were shown live, along with poignant videos of liberating soldiers greeted by their ecstatic families. For those that wanted to see them, there were graphic videos: helmet cams showed firefights, drones dropping grenades on Russian soldiers and into the hatches of occupied vehicles.
Many of Ukraine’s new volunteers were “terminally online”—ordinary digital natives forced into a brutal conflict. Gen-Z recruits did dance videos for TikTok. Their meme game was wild. Woodruff’s Twitter bio reads “British Chef Fella”—a reference to the North Atlantic Fellas Organization, or NAFO—an online movement of Ukraine-supporting shitposters with shiba inu avatars who flood social media with memes mocking the “Vatniks” (Russian propagandists).
The NAFO movement taunted Russia, at one stage managing to send the country’s ambassador in Vienna into a public meltdown. “Imagine, literally getting a world-class ambassador to speak with cartoon dogs on Twitter,” says Ivana Stradner, an adviser to the Foundation for Defense of Democracies think tank in Washington DC, an expert on misinformation and propaganda, and NAFO member. “This is the future of information warfare.”
NAFO does what state-backed information warriors, particularly those from democracies, can’t do. Its members make insane, often tasteless jokes, moving quickly to jump on trends. They’re good at memes, and flood the zone with infectious pro-Ukrainian vibes, humanizing, entertaining, and explaining to people far from the war why they should care. “I think NAFO, by boosting certain narratives, can actually also help people understand the severity of the situation and what's going on there,” Stradner says.
NAFO has helped raise millions of dollars through sales of merchandise (“I invaded Belgorod and all I got was this lousy T-shirt”) and crowdfunding campaigns. Now its avatars appear on the Twitter profiles of European politicians, on official Ukrainian defense channels, and on military equipment headed to the front. It has funded everything from food to medical supplies to a mobile artillery piece to the Georgian Legion, a unit of overseas volunteers that has been fighting since 2014. When the Frontline Kitchen’s vegetable shredder broke, Woodruff put out a call for funds to buy a new one. In the time it took him to drive to the supplier, the money had already been deposited in his account.
Social media works in tandem with the tight networks of Ukrainian society. This is a war being fought close to home—everyone knows someone at the front, and the soldiers are in constant contact. Link people like Vorobiov can connect those in the trenches with supporters in Kyiv or overseas. A unit under fire can ask for drones on Telegram, and within hours there’s a call for donations out on Twitter or Instagram. Vorobiov can deliver tourniquets to a combat medic near the front, and record a thank-you video to send directly to donors.
“I see a spike in donations when there is a story that I can tell of how donations help,” Vorobiov says. “Yesterday, I received a very long message from one of the medics, and she was telling me how medical supplies we brought to her helped her basically provide care to two servicemen. I posted that story on Twitter and folks started to donate.”
Sometimes, donors become more active participants. Last February, Polish filmmaker Maciej Zabojszcz was watching the conflict unfold over Twitter, and thinking about selling some of his military memorabilia to help raise money for a 4x4 for the Ukrainian army. But then, a graphic video emerged, apparently shot by Russian soldiers, of a Ukrainian prisoner of war being horrifically mutilated. “I felt like something changed,” he says. “I said, listen, let's not only buy one car.”
In the spring of 2022 he drove his first vehicle, a Nissan pickup, to Kyiv to deliver to the Georgian Legion. While there, he met Vorobiov, who was collecting some drones from Exen, another Polish volunteer. From then on, Zabojszcz was part of the network. Because they couldn’t order supplies online to be delivered to Ukraine, Vorobiov and others started putting Zabojszcz’s home as the delivery address. Each time he drives a car to Ukraine, he’s carrying helmets, body armor, drones, all kinds of medical supplies. When we met in March in Warsaw, he’d delivered seven 4x4s, and was fixing up an eighth.
Some Ukrainian units have a tradition of naming their vehicles, and the seventh car that Zabojszcz delivered, a Land Rover, was christened Mathilda. It was used to shuttle men from their barracks to the frontline through thick mud. “The whole unit was driving the car,” Zabojszcz says. “They were crazy about Mathilda.”
But after ten days of constant driving, Mathilda broke down. Another Polish volunteer found a local mechanic specialized in Land Rovers. They arranged an online consultation. The mechanic helped the soldiers figure out what was wrong and identify the part they needed to replace. The car broke on Monday. On Tuesday, a volunteer delivered the replacement part. “And on Thursday the car was fixed,” Zabojszcz says. “This is how this network works.”
Absorbing donations has required a degree of flexibility from the military establishment. Armies typically don’t like amateurs pitching in, turning up in warzones with stuff they’ve brought from home. Getting goods into Ukraine can be challenging—it’s understandably not legal for just anyone to move military equipment across borders—and even bringing in theoretically civilian items like cars, consumer drones, and generators requires customs forms and other paperwork. But volunteers say once they’ve got donations into the country, working with the military has been fairly easy. There’s still some admin, and donors have to have forms showing that the goods they’re delivering have been specifically asked for by a soldier, but mostly, they’ve integrated relatively seamlessly with the supply chains, with commanders on the ground sometimes turning a blind eye to help their soldiers get what they need.
This acceptance is driven partly by necessity—the military simply couldn’t supply its troops to the level it needed, and unlike its adversary, doesn’t want to send them into battle with tourniquets that snap under pressure and rations years past their expiration date. Volunteer networks can take orders, source, and deliver in a way that a centralized bureaucracy can’t. They’ve helped feed the battlefield innovations that have given outnumbered soldiers an edge, linking into the networks of workshops jury-rigging consumer drones; bringing 3D printers to the frontline to help turn hand grenades into air-dropped bombs.
“For the chaotic time after the invasion, these organizations created a stopgap solution for markets that the army could not operate,” says Simon Schlegel, senior Ukraine analyst at the Crisis Group think tank. “The army is good at buying in bulk, but these smaller operations are good at finding five pieces of Chinese-made drones in different countries and shipping them to Ukraine.”
President Volodymyr Zelenskyy understands this. He has, since the early days of the conflict, often made his social media addresses direct to citizens of other countries, not just to his fellow leaders. Volunteers—and the state’s own propagandists—have built a formidable ground game on social media, which has helped with donations, but also contributed to the ratcheting up of material being sent to the frontline by NATO partners. With public support for Ukraine high in their own countries, western leaders feel emboldened to hand over money and weapons. When those weapons deliver battlefield successes, the resulting content feeds back into the loop. “I think Ukraine is literally right now the superpower in this information war,” says Stradner.
The war, as seen through the filter of social media, has an oddly gamified quality. At times it seems it’s being won by jokes, by Ukrainian farmers pulling tanks behind tractors, by “Saint Javelin” (the “patron saint” of anti-tank missiles), and shiba inu soldiers. But it hasn’t been won yet, and many people at the far end of the volunteer supply chain have taken incredible risks, and exposed themselves to unspeakable horrors. In Lviv, I met Ernest Polanski, a Ukrainian volunteer taking a brief rest on his way back from delivering equipment to troops near Bakhmut.
What he saw there, he says, was “hell.” There was constant shelling, and the smell of corpses hung over the area. Whenever the bombardment stopped for longer than a few minutes, he wondered if something worse was about to come, “like a nuclear bomb,” he says. On the way back, he rescued three bedraggled kittens from the ruins.
Polanski has been driving back and forth from the frontlines since the early days of the war, and has lost count of the number of journeys he’s made, bringing generators, trench periscopes, medical gear and other supplies. Like other volunteers, he’s formed a special connection with a single unit, which he devotes most of his journeys to. He’s currently looking for €6,000 ($6,480) to buy new wheels for one of the unit’s 4x4s. “Not a lot of people want to go to this area,” he says. “But we have a special friendship with [this unit], and we want to help.”
The volunteer networks are made up of people from all over the world, but outside of Ukraine itself the cause has resonated more than anywhere in former Soviet nations, and in particular Baltic states like Lithuania, which see themselves as next in line if Ukraine falls. Traveling with Polanski on this journey to the front is one of his most committed supporters, the Lithuanian kickboxing champion Sergej Maslobojev. “Our country had the same problem years ago,” he says. “We feel their pain in our hearts.”
Maslobojev’s profile at home has meant he’s been able to fundraise for supplies, but, he says, it’s important for him to get out into the field to witness, and show the sacrifices still being made in the trenches of eastern and southern Ukraine. “When we listen to our news, usually we’re thinking that they're winning the war. Everything is going great. Why do we need to donate?” he says. “But when you go to the frontline and help those military guys, give them ammunition, extra food and the stuff that they really need. And they look at you with almost tears in their eyes and say, ‘nobody comes to us’. And then you understand why, in this moment.”
The day after Polanski and Maslobojev returned from Bakhmut, reports came through that the town had finally fallen. Individual defeats are hard to talk about in the context of fundraising campaigns and propaganda drives that are buoyed by a sense of inevitable victory. But they also underline the fragility of life close to the front. Almost all of the volunteers I spoke to in Ukraine had their own story of raising funds, or sourcing gear, only for the intended recipient to fall in battle before it could be delivered. All that does is make them more committed. Most say their supporters are also holding the line, a year and a half into the war.
“Sometimes it feels like this continuing western support is contingent on possible breakthroughs and huge victories. But I don't feel that, at least among my donors,” Vorobiov says. “You cannot afford hopelessness, because no one is going to support a lost cause. And we Ukrainians believe in winning this war. We have to infect others with that belief. But complacency is equally dangerous.”
5 notes
·
View notes
Text
Uninterruptible Power Supplies Market Scope & Growth Projection till 2032
Uninterruptible Power Supplies Market provides in-depth analysis of the market state of Uninterruptible Power Supplies manufacturers, including best facts and figures, overview, definition, SWOT analysis, expert opinions, and the most current global developments. The research also calculates market size, price, revenue, cost structure, gross margin, sales, and market share, as well as forecasts and growth rates. The report assists in determining the revenue earned by the selling of this report and technology across different application areas.
Geographically, this report is segmented into several key regions, with sales, revenue, market share and growth Rate of Uninterruptible Power Supplies in these regions till the forecast period
North America
Middle East and Africa
Asia-Pacific
South America
Europe
Key Attentions of Uninterruptible Power Supplies Market Report:
The report offers a comprehensive and broad perspective on the global Uninterruptible Power Supplies Market.
The market statistics represented in different Uninterruptible Power Supplies segments offers complete industry picture.
Market growth drivers, challenges affecting the development of Uninterruptible Power Supplies are analyzed in detail.
The report will help in the analysis of major competitive market scenario, market dynamics of Uninterruptible Power Supplies.
Major stakeholders, key companies Uninterruptible Power Supplies, investment feasibility and new market entrants study is offered.
Development scope of Uninterruptible Power Supplies in each market segment is covered in this report. The macro and micro-economic factors affecting the Uninterruptible Power Supplies Market
Advancement is elaborated in this report. The upstream and downstream components of Uninterruptible Power Supplies and a comprehensive value chain are explained.
Browse More Details On This Report at @https://www.globalgrowthinsights.com/market-reports/uninterruptible-power-supplies-market-101476
Global Growth Insights
Web: https://www.globalgrowthinsights.com
Our Other Reports:
Global Uninterruptible Power Supplies Market Growth
Global Drone Sensor Market Size
Global Rubber Track Pads for Excavators Market Share
Smart Grid Software Market Forecast
Defibrillator Market Size
Patient Throughput and Capacity Management Market Growth Rate
Composite Decking & Railing Market Analysis
Aircraft Engine, Parts and Equipment Market Share
Clinical Trial Patient Recruitment Services Market Growth
Carbon Credits Market
Global Touch Screen Controllers Market Size
Global Network Slicing Market Growth
Respiratory Drug Market Forecast
Global Sucrose Monolaurate (CAS 25339-99-5) Market Share
Retail Banking Market Growth Rate
Electric Vehicle (EV) Charging Infrastructure Market Size
Lithium-ion Battery Recycling Solution Market Share
1,8-Diaminonaphthalene Market Analysis
Polyurethane Dispersions (PUD) Market
Chromebook Market Growth
Global Solar Charger Market Growth
Global Semiconductor Wafer Fab Equipment (WFE) Market Size
Global Call Center Software Market Share
Unmanned Surface Vehicles Market Forecast
Zigbee and Thread Wireless Sensor Market Size
Mica Tape for Insulation Market Growth Rate
Electric Power Distribution Equipment Market Analysis
Thermally Fused Laminates (TFL) Market Share
Healthcare Nanotechnology Market Growth
Surface-Enhanced Raman Spectroscopy (SERS) Substrate Market
Global Smart Wireless Propane Tank Meter Market Size
Global DSL Modem Market Growth
Social Media Analytics Market Forecast
Global Lost and Found Software Market Share
Exome Sequencing Market Growth Rate
Polymer Based Thermal Interface Materials (TIM) Market Size
Luxury Crystal Ware Market Share
Accounts Payable Software Market Analysis
DNA-Encoded Library Market
Neuropathy Screening Devices Market Growth
#Uninterruptible Power Supplies Market Size#Uninterruptible Power Supplies Market Share#Uninterruptible Power Supplies Market Trends#Uninterruptible Power Supplies Market Industry#Uninterruptible Power Supplies Market Growth
0 notes
Text
The Expanding Market for Atrial Fibrillation Devices
Atrial fibrillation (AFib), a common type of arrhythmia, affects millions worldwide, posing significant health risks such as stroke, heart failure, and other cardiovascular complications. In response to this prevalent health issue, the market for atrial fibrillation devices has experienced substantial growth. This article delves into the dynamics of this burgeoning market, highlighting key drivers, technological advancements, and future prospects.
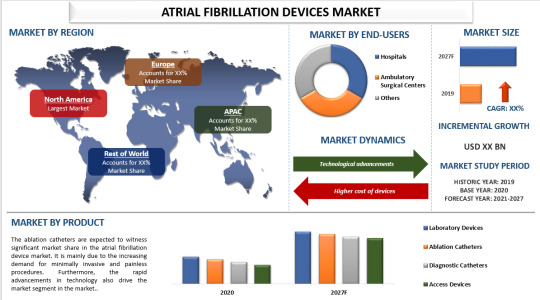
Market Overview
The global atrial fibrillation devices market has been expanding rapidly, driven by an increasing prevalence of AFib and a growing elderly population. According to recent studies, the AFib prevalence is projected to rise significantly over the next decade. The market encompasses a variety of devices, including diagnostic tools, ablation catheters, and implantable devices such as pacemakers and defibrillators, each playing a crucial role in managing and treating AFib.
Key Market Drivers
1. Aging Population: The global increase in the elderly population is a primary driver of the AFib devices market. Older adults are more susceptible to atrial fibrillation, necessitating effective diagnostic and treatment solutions.
2. Technological Advancements: Innovations in medical technology have led to the development of more efficient and accurate AFib management devices. These advancements include improved imaging techniques, advanced mapping systems, and sophisticated ablation technologies, all of which enhance the efficacy of AFib treatments.
3. Rising Awareness and Diagnosis Rates: Increased awareness about the symptoms and risks associated with AFib has led to higher diagnosis rates. Early diagnosis is crucial for effective management, thereby driving the demand for diagnostic devices and monitoring systems.
4. Minimally Invasive Procedures: The shift towards minimally invasive surgical procedures has significantly impacted the market. Catheter ablation, a minimally invasive procedure used to treat AFib, has gained popularity due to its effectiveness and shorter recovery times compared to traditional surgical methods.
For a comprehensive analysis of the market drivers, visit https://univdatos.com/report/atrial-fibrillation-devices-market/
Technological Innovations
Technological innovation is at the heart of the atrial fibrillation devices market's growth. Several groundbreaking advancements have revolutionized the way AFib is diagnosed and treated:
- 3D Mapping Systems: These systems provide detailed, real-time images of the heart’s electrical activity, allowing for precise identification and targeting of abnormal signals during ablation procedures. This technology improves the success rates of ablation therapies and reduces the risk of complications.
- Cryoablation Technology: Unlike traditional radiofrequency ablation, cryoablation uses extreme cold to destroy abnormal heart tissue. This method has shown promising results, offering a safer and more effective alternative for certain patients.
- Wearable Devices: The integration of wearable technology in healthcare has introduced new possibilities for continuous AFib monitoring. Devices such as smartwatches equipped with ECG capabilities enable real-time tracking of heart rhythms, facilitating early detection and timely medical intervention.
- Implantable Loop Recorders (ILRs): ILRs are small devices implanted under the skin to continuously monitor heart activity over long periods. These devices are particularly useful for patients with intermittent AFib, providing valuable data that can inform treatment decisions.
Market Challenges
Despite the promising growth, the atrial fibrillation devices market faces several challenges:
- High Costs: The cost of advanced AFib devices and procedures can be prohibitive for many patients, limiting access to cutting-edge treatments. Efforts to reduce costs and improve insurance coverage are essential to broaden the market.
- Regulatory Hurdles: Stringent regulatory requirements for medical devices can slow the approval process, delaying the availability of new technologies. Navigating these regulations requires significant time and resources.
- Technical Limitations: While advancements are being made, some devices still face technical limitations, such as difficulty in accurately targeting ablation sites or issues with long-term device performance.
For a sample report, visit https://univdatos.com/get-a-free-sample-form-php/?product_id=22580
Future Prospects
The future of the atrial fibrillation devices market looks promising, with continuous advancements in technology and a growing emphasis on early diagnosis and intervention. Personalized medicine, driven by genetic and biomarker research, is expected to play a significant role in the development of tailored AFib treatments. Additionally, the integration of artificial intelligence and machine learning in diagnostic and monitoring devices will further enhance the precision and efficacy of AFib management.
In conclusion, the atrial fibrillation devices market is on a robust growth trajectory, fueled by demographic trends, technological innovation, and increased awareness. Addressing the existing challenges and leveraging emerging opportunities will be crucial in shaping the future landscape of AFib management, ultimately improving patient outcomes and quality of life.
Contact Us:
UnivDatos Market Insights
Email - [email protected]
Contact Number - +1 9782263411x
Website -www.univdatos.com
#Atrial Fibrillation Devices Market#Atrial Fibrillation Devices Market Size#Atrial Fibrillation Devices Market Growth#Atrial Fibrillation Devices Market Forecast
0 notes
Text
10 Essential FAQs About the Medical Devices Market: A Must-Read for Investors and Innovators
Q1. What is the size of the global medical devices market?
Ans. The global medical devices market is estimated to be worth over USD 568 billion in 2023 and is expected to reach USD 772.3 billion by 2028, growing at a CAGR of around 5.2%.
Q2. What are the major product segments in the medical devices market?
Ans. The largest segments include:
Diagnostics: Imaging equipment (MRI, CT scanners, etc.), laboratory instruments, and in vitro diagnostics (IVDs).
Therapeutics: Cardiac devices (pacemakers, defibrillators), orthopedic implants (joints, spinal devices), and surgical instruments.
Other: Dental equipment, ophthalmic devices, rehabilitation equipment, and home healthcare devices.

Q3. Which regions are leading the medical devices market?
Ans. North America: Holds the largest share, driven by advanced healthcare infrastructure and high disposable incomes.
Europe: Strong market with well-established regulatory frameworks and aging population.
Asia Pacific: Fastest-growing region due to population growth, rising healthcare spending, and improving medical facilities.
Q4. What are the driving factors for medical devices market growth?
Ans. Aging population: Increased demand for healthcare services and devices for chronic conditions.
Technological advancements: New generations of devices with improved functionality and minimally invasive procedures.
Rising healthcare spending: Increasing affordability and government initiatives in some regions.
Q5. What are the major medical devices market challenges?
Ans. Regulatory hurdles: Stringent regulatory requirements and lengthy approval processes for new devices.
Cybersecurity threats: Increasing vulnerabilities of connected medical devices to hacking and data breaches.
Cost containment pressures: Healthcare systems striving for cost-efficiency, impacting device manufacturers.
Q6. What are the latest trends in the medical devices market?
Ans. Artificial intelligence (AI): Integrating AI into diagnostics, surgical robots, and personalized medicine.
Telemedicine: Remote monitoring and healthcare delivery through connected devices.
3D printing: Personalization of medical devices and development of complex implants.
Wearable devices: Growing adoption for fitness tracking, chronic disease management, and remote monitoring.
Q7. Who are the major players in the medical devices market?
Ans. Here are some Medical Device Market Players
Johnson & Johnson
Medtronic
Siemens Healthineers
Abbott Laboratories
Stryker
Q8. How is the COVID-19 pandemic impacting the medical devices market?
Ans. Increased demand for ventilators and other critical care devices.
Disruptions in supply chains and manufacturing due to lockdowns and travel restrictions.
Shift towards telemedicine and virtual consultations.
Q9. What are the ethical considerations in the medical devices market?
Ans. Access to devices for patients in developing countries.
Data privacy and security concerns with connected devices.
Ensuring equitable distribution of new technologies and treatments.
Q10. What does the future hold for the medical devices market?
Ans. The market is expected to continue growing with advancements in technology, personalized medicine, and increasing demand in emerging economies. However, navigating regulatory challenges, cost pressures, and ethical considerations will be crucial for sustainable growth.
0 notes
Text
Global Emergency Medical Equipment Market Is Estimated To Witness High Growth Owing To Advancements in Technology

The global Emergency Medical Equipment market is estimated to be valued at US$ 23.82 billion in 2022 and is expected to exhibit a CAGR of 6.3% over the forecast period 2022-2030, as highlighted in a new report published by Coherent Market Insights.
Market Overview:
Emergency medical equipment includes devices and tools used by healthcare professionals to provide immediate medical assistance during emergencies. These equipment are crucial in saving lives and treating patients in critical conditions. The market for emergency medical equipment is driven by factors such as the increasing incidence of accidents and injuries, coupled with the need for immediate medical care. Moreover, the advancements in technology have led to the development of innovative and efficient equipment that are improving patient outcomes.
Market Key Trends:
One key trend in the global Emergency Medical Equipment market is the adoption of advanced technologies in medical devices. Healthcare providers are increasingly investing in innovative equipment that can provide accurate and timely diagnosis and treatment. For instance, the use of portable ultrasound devices has become common in emergency situations as they enable quick and accurate assessment of internal injuries. Similarly, the integration of artificial intelligence (AI) algorithms in emergency equipment is improving decision-making and enhancing patient care.
PEST Analysis:
- Political: The government plays a crucial role in regulating the production, distribution, and usage of emergency medical equipment. It sets standards and guidelines to ensure the safety and efficacy of these devices.
- Economic: The emergency medical equipment market is influenced by economic factors such as healthcare expenditure, insurance coverage, and reimbursement policies. The affordability and accessibility of these equipment are important considerations for market growth.
- Social: The increasing awareness about the importance of emergency medical care and the rising demand for prompt medical assistance are major social factors driving market growth.
- Technological: Advances in technology have revolutionized emergency medical equipment. Devices such as automated defibrillators, portable ultrasound, and telemedicine solutions are improving the delivery of emergency medical care.
Key Takeaways:
1: The Global Emergency Medical Equipment Market Size is expected to witness high growth, exhibiting a CAGR of 6.3% over the forecast period. This growth can be attributed to increasing accidents and injuries, which highlight the need for immediate medical care. The advancements in technology have led to the development of efficient and innovative equipment that are enhancing patient outcomes.
2: The Asia Pacific region is anticipated to be the fastest-growing and dominating region in the global Emergency Medical Equipment market. The growing population, increasing healthcare expenditure, and rising awareness about the importance of emergency medical care are driving market growth in this region.
3: Key players operating in the global Emergency Medical Equipment market include 3M, Abbott, Asahi Kasei Corporation, B. Braun Medical, BD, Cardinal Health, GE Healthcare, Henry Schein, Johnson & Johnson, Philips Healthcare, Smith & Nephew, and Stryker Corporation.
In conclusion, the global Emergency Medical Equipment market is on a growth trajectory fueled by advancements in technology and the increasing demand for immediate medical care. The adoption of advanced technologies and the integration of AI algorithms in emergency equipment are key trends shaping the market. However, government regulations and economic factors play a crucial role in shaping the market landscape. Market players should focus on innovation and ensuring affordability and accessibility of emergency medical equipment to capitalize on the growing market opportunities.
#Emergency Medical Equipment Market#Emergency Medical Equipment Market Growth#Emergency Medical Equipment Market forecast#Emergency Medical Equipment Market analysis#Emergency Medical Equipment Market values#Emergency Medical Equipment#Medical Equipment#Coherent Market Insights
0 notes
Text
Medical Batteries Market Forecast 2024 to 2032
Medical batteries are specialized batteries used in various medical devices and equipment to provide power for their operation. These batteries are designed to meet the specific requirements of medical applications, which often involve high reliability, long life, and consistent performance.
The Medical Batteries Market was valued at USD 1,632.27 Million in 2022 and is expected to register a CAGR of 2.63% by 2032.
The continuous advancement of medical technology has led to the development of sophisticated and portable medical devices that require reliable power sources. As medical devices become more advanced and portable, the demand for compact and high-performance batteries to power these devices increases. This key factor is expected to drive global market growth during the forecast period.
Get a Free Sample Copy
Market Segmentation (by Type)
•Li-ion Battery
•Ni-Cd Battery
•Nimh Battery
•Alkaline-manganese Battery
Market Segmentation (by Application)
•Pacemakers
•Infusion Pumps
•Implantable Cardioverter defibrillators
Key Company
•Siemens Ag
•GE Healthcare
•Maxim Integrated
•Panasonic Corp
•Texas Instruments
•Quallion LLC
•Stmicroelectronics N.V
•Ultralife Corp
•Electrochem Solutions
•EaglePicher Technologies
Read more
0 notes
Text
Medical Device Software Development: From Software Design to Launch
Picture this: the global population is aging, quicker than you can say ‘baby boomers’, and their demand for medical products is shooting up like fireworks on New Year’s Eve.
According to the United Nations, by the time 2050 rolls around, we’ll have 1.5 billion people aged 65 and older, a giant leap from 793 million in 2019. Now, translate that into potential users, potential customers, who will be clamoring for the latest in medical instrument technology. We’re talking about a market on the cusp of exploding, like a star going supernova!
You want figures? We’ve got them. Statista foresees that the medical device software market will surge from its current worth of $570 billion to a whopping $719 billion by 2028. It’s like witnessing a small town growing into a megacity overnight.
From diagnostic tools to therapeutic devices, the future of healthtech is practically being written in code.
We’re helped to build and test software for dozens of digital health projects. Now, we offer you to delve into the intricacies of medtech software development, navigating the maze of regulatory landscape and many other challenges that developers face in this brave new world.
So, ready to decode the future of safe and effective medical devices? Let’s dive in!
Custom Medical Device Software: Types and Application
What’s the secret ingredient that brings a medical gadget to life? It’s the medical device software (MDS) – an essential component that orchestrates the functionality of these gadgets whether they’re seamlessly integrated or operating independently.
Take, for instance, a wearable fitness tracker on your wrist, quietly monitoring your health metrics (like heart rate) and counting steps as you go about your day. This everyday device is empowered by complex software designed to deliver critical data right at your fingertips.
On a larger scale, there’s radiology imaging software, enhancing X-Ray machines’ ability to produce detailed images, or ventilator software, adjusting the air flow delivered to patient rooms in hospitals. The breadth and depth of applications are truly extraordinary.
Let’s turn our attention to exploring the diverse types of clinical device software.
Key MDS Types In the Current Market
In a nutshell, there are two primary categories:
1. Embedded Medical Systems and Software
This software is integrated directly into the med. device and controls its core functionalities. Examples of devices that use embedded software include pulse oximeters, electronic defibrillators, and various types of medical imaging equipment.
2. Software as a Medical Device (SaMD)
Unlike embedded software, SaMD operates independently of any physical device. It is used for medical data visualization, processing, management, and specific technical diagnostics. One advantage of SaMD is that it can be more easily updated compared to traditional medical devices.
Grown Factors Every Medical Device Software Development Company Should Consider
The expansion in this sector hinges primarily on two driving forces: our escalating reliance on software within healthcare devices and the burgeoning rise of telemedicine and remote patient monitoring. These dynamics pave the way for a broad spectrum of stakeholders, ranging from manufacturers of medical tools to hospitals, NGOs, and healthtech companies, enabling them to enhance care delivery and pioneer innovative solutions.
On top of that, new technologies like AI, ML, and IoT are creating fresh opportunities. AI and ML are paving the way for smarter patient monitoring, while IoT is connecting medical instruments like never before. To stay ahead, companies need to keep up with trends, regulatory changes, and tech advances.
Healthtech device software is changing the game for doctors and patients alike. While the growing market value and potential revenue are a big draw, they’re not the only reasons companies are investing in custom medical instrument software development.
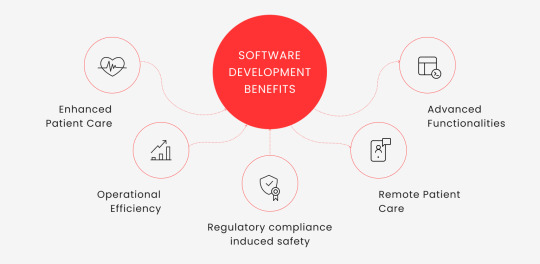
Top-5 Reasons Why Companies Need Software for Medical Devices
Putting it simply, medtech software development is vital for healthcare organizations, offering numerous benefits:
Enhanced Patient Care. Custom healthtech device software enables real-time patient monitoring, accurate diagnosis, and effective treatment plans, improving patient outcomes.
Operational Efficiency. Medical soft automates administrative tasks, reduces paperwork, and streamlines workflows, freeing up staff time for patient care.
Regulatory compliance induced safety. Software for a medical product must comply with stringent regulations. Failing to do so comes with massive fines. Custom software can be designed to meet these specific requirements, ensuring devices are safe for patient use and don’t cause financial trouble.
Remote Patient Care. Medical soft facilitates remote patient care, enabling data-driven decision-making and providing a platform for telemedicine services.
Advanced Functionalities. With technologies like AI and ML, healthtech software can support functionalities like predictive analysis and personalized patient care. This is particularly important considering the existing drag of the legacy business.
Medical app and desktop software development is a necessity for healthcare organizations, not a luxury. It significantly enhances patient care and operational efficiency, making it a worthwhile investment.
However, developing medtech software requires specialized skills and knowledge of healthcare regulations. Many companies partner with experienced software development services to leverage their expertise and ensure project success. However, before going into detail about compliance, let’s look at the type of medical gadgets one can upgrade with medical gadget software development services.
How Software Solutions Make Medical Devices Better
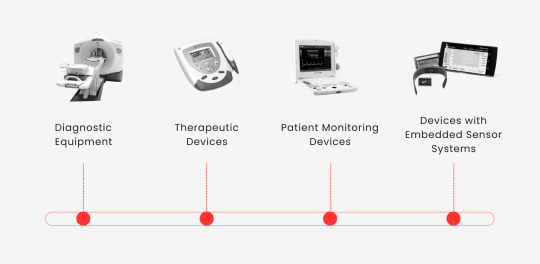
In the rapidly evolving healthcare landscape, software solutions play a crucial role in augmenting the capabilities of medical devices. This might be as simple as improving the readability of data from a heart monitor or as complex as enabling remote diagnostics. Here’s how these advancements contribute to enhancing medical devices:
Diagnostic Equipment. MRI, CT scanners, and X-ray machines rely on software for data processing, image creation, abnormality identification, and diagnostic reporting.
Therapeutic Devices. Software in devices like insulin pumps and pacemakers ensures accurate medication or electrical stimulation delivery.
Patient Monitoring Devices. Heart rate, blood pressure, and glucose monitor software collects and analyzes patient data, providing real-time feedback and alerting healthcare providers to significant changes.
Devices with Embedded Sensor Systems. Wearable fitness trackers and smartwatches use software to analyze real-time health data, provide insights, and offer personalized health and fitness recommendations.
Hospitals and clinics use these medical tools for diagnosis, treatment, patient monitoring, baby care devices management and personal health tracking. In such a context, developing software for these devices is a chance to kill two birds with one store — make healthcare services more effective, and build new revenue streams. Nevertheless, only a few companies dare to enter the realm of healthcare software development in general. The reason for that is two-fold—compliance and regulation.
Regulatory Requirements for SAMD Development and Beyond
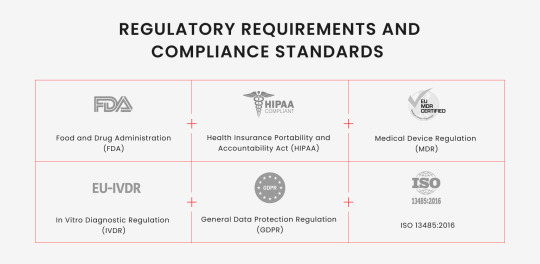
Navigating the regulatory landscape is a critical aspect of healthtech device software development. With various international and regional standards in place, understanding these regulations is essential to ensure medical tools’’ safety, effectiveness, and quality. The regulatory environment for the phenomenon is intricate and governed by various international and regional standards.
In the United States, the Food and Drug Administration (FDA) oversees the development and distribution of medical instruments, including software. The FDA’s regulations aim to ensure the safety and effectiveness of these devices, focusing on risk management and quality assurance. In addition, there is the Health Insurance Portability and Accountability Act (HIPAA). HIPAA is a crucial aspect of medtech software development in the United States, as it sets the standard for protecting sensitive patient data. Any healthcare-related software must ensure that all necessary physical, network, and process security measures are followed.
The Medical Device Regulation (MDR) and In Vitro Diagnostic Regulation (IVDR) regulate medical gadget software in the European Union. These regulations, effective in May 2021, emphasize clinical evaluation and post-market surveillance, requiring manufacturers to monitor and report their devices’ performance continuously. Regarding data protection, adhering to General Data Protection Regulation (GDPR) is vital.
In the global landscape, ISO 13485:2016 is a globally recognized standard for quality management systems in the medical instrument industry. It supports healthtech device software creators in designing quality management systems that establish and maintain their processes’ effectiveness. This standard ensures the consistent design, development, production, installation, and delivery of healthtech devices that are safe for their intended purpose.
Regulations and standards environment for new software in the healthcare industry is complex but necessary to ensure patient safety and device effectiveness. Adherence to these regulations, from the FDA guidelines to the ISO standards, is a legal obligation and a commitment to quality and safety in healthcare. As we move forward to the dev process, keeping compliance in mind is a must.
Understanding the Impact of IEC 62304 on Healthtech Software Development
In the realm of digital health software development, one international standard has taken precedence as a guiding force: the International Electrotechnical Commission’s IEC 62304. This standard lays out the lifecycle requirements for medtech device software, providing a comprehensive framework that ensures safety and performance.
Key facets of IEC 62304 include:
Risk Management. The standard insists on the adoption of risk management process compliant with ISO 14971, which ensures all possible risks are identified, evaluated, and mitigated effectively.
Software Development Lifecycle (SDLC). IEC 62304 mandates a well-structured SDLC, which includes planning, development, testing, maintenance, and eventual retirement of the software.
Software Classification. It introduces a classification scheme (Classes A, B, C) based on the potential risk to patients — the higher the risk, the stricter the development requirements.
Configuration Management. A robust configuration management system is necessary, allowing for the traceability of all software versions and modifications.
Problem Resolution. The standard requires a systematic process for identifying, documenting, and resolving all software-related problems.
Software Maintenance: It prescribes the need for ongoing maintenance to address software issues, continuously improve functionality, and manage updates.
IEC 62304, by covering every aspect of medtech software development, from inception to sunset, offers a clear and solid foundation for developers. It ensures patient safety and product efficacy while providing a roadmap for consistent, high-quality software development.
In conclusion, compliance with IEC 62304 is not just a regulatory requirement, but a blueprint for success. It streamlines processes, mitigates risks, and instills confidence among users, ultimately elevating the software reliability and credibility in a dynamic and demanding healthcare landscape.
The Ins and Out of the Medical Device Software Development Process
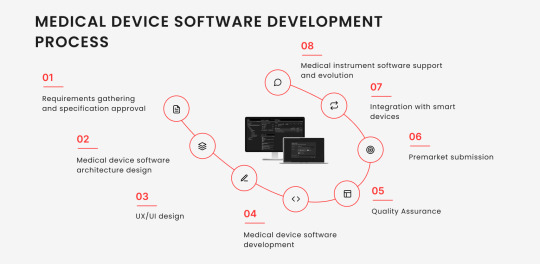
Developing software for medical instruments is a meticulous process involving several critical steps. From identifying a market need to ensuring the software’s safety and effectiveness, each stage of the software development life cycle (SDLC) plays a crucial role in successfully deploying healthtech device software. At this point, let’s take a closer look at these steps.
Step #1. Requirements gathering and specification approval
The initial phase of the process involves:
Identifying the software concept.
Prioritizing software requirements.
Creating a detailed specification of the medical instrument software.
This phase also includes risk analysis and creating a risk management plan, following the ISO 14971 standard in medical tool manufacturing.
Any oversights or missteps at this juncture can lead to significant issues further down the road. Moreover, this stage serves as the platform to identify potential software-related hazards, assess the associated risks, and evaluate their potential impact.
Step #2. Medical device software architecture design
Next, software developers design a reliable and scalable healthcare device software architecture. This architecture allows for adding new modules or device types with minimal rework. It ensures system configurability, clear module interfaces, and good encapsulation of every module.
The architecture design phase is pivotal in shaping the software’s performance, scalability, and ease of maintenance and updating. This stage also serves as a platform where engineers of clinical device software examine its interoperability with other systems and devices, aiming for a seamless and secure exchange of data.
Step #3. UX/UI design
Designing software for medical devices is an intricate task that hinges greatly on user experience. Our aim is to create a user interface that is not only sleek but also accessible for everyone, no matter their age or level of tech expertise.
Consider, for example, a heart rate monitor app on a smartwatch. The interface needs to be easy for a tech-savvy young adult to navigate, but also simple enough for an elderly person who may not be as comfortable with technology. This delicate balance is one of the world’s most vital challenges in medical device software design.
The interaction between the user and the software significantly impacts user satisfaction. So, we don’t just build software; we design software that helps people. It has to be intuitive and user-friendly, serving healthcare professionals and patients alike.
In short, the focus is on creating a software design for medical devices that enhances healthcare delivery and user satisfaction, while being easily navigable by all users. It’s all about making sure technology is an aid, not a hindrance, to care.
In one of my healthcare projects, we worked on a medical device that pricked your arm and drew blood. We didn’t want our users feeling uneasy or scared about it. So, we prioritized their experience and expectations from designing the app’s interface stage to drafting the packaging. We wanted them to trust us and our device. And that kind of trust only comes when you genuinely empathize with your end users. By empathizing, you create devices and interfaces that cover practical functionality and a human touch.
Yehor Sokhan, Head of Design at QArea
Step #4. Medical device software development
The software is developed with cross-platform compatibility during this phase to cater to multiple operating systems. It is integrated seamlessly with healthcare software (EHR, ADT) via HL7 v.3 or FHIR. The development methodology can be either Waterfall (full-fledged version delivered in one iteration) or Iterative (Agile, Scrum) with MVP delivery and updates every 2-4 weeks.
This step is all about coding the software according to the specifications and design documents created in the previous steps. It’s also the moment where the software’s ability to interact with wearable and non-wearable smart devices is considered, ensuring regular communication with the IoMT system for patient monitoring.
Step #5. Quality Assurance
During software testing and quality assurance, OWASP’s Secure Software Development Life Cycle (S-SDLC) practices are applied. These involve comprehensive multi-level QA. In addition, the step includes continuous testing, software validation/verification, and regular code reviews.
Testing strategies validate that the software meets its intended use purpose and can operate safely under normal and abnormal conditions. This step is crucial to ensure the software is defect-free and performs as expected. It’s also the stage where the software’s cybersecurity measures are considered, including data encryption, secure user authentication, and regular security audits.
Step #6. Premarket submission
To meet the requirements of the FDA and the Council of the European Union and ensure software safety, development services are provided according to ISO 13485, IEC 62304, and IEC 82304-1. During this stage, experts prepare detailed documentation for FDA 510 (k) premarket notification, CE marking, HIPAA compliance audits, etc. This step is critical to ensure the software meets all regulatory requirements and is safe for use.
Step #7. Integration with smart devices
At this stage, the software for the medical instrument is enabled to ensure smooth interaction with the devices. Stable communication with the IoMT system is ensured for remote patient monitoring, and comprehensive analytics of patient-generated health data collected by devices is provided. This step ensures the software can effectively communicate with other devices and systems.
Step #8. Medical instrument software support and evolution
Finally, if required, support is provided for the healthtech device software. It manages the software’s performance and security. Routine software administration tasks are mainly performed, and the software is helped to evolve further. This step is crucial to ensure the software is always up-to-date.
The stages above give you an idea of custom medical tool software development. The rule of thumb dictates—the better you handle each step, the more cost-efficient the process will be. Yet, even for clinical device software developers with years of experience, some crucial challenges remain to consider during the entire dev and design process.
Medical device software engineering challenges: From regulation to device application
The development of healthtech device software involves navigating a complex landscape of challenges. Developers must address many factors, from regulatory compliance to cybersecurity, to ensure successful software deployment and operation.
Challenge 1: Regulatory compliance
Again and again, regulatory compliance is crucial to remember within the dev and design process. The price of non-compliance can go as high as $1.5 million. Medical device software must adhere to FDA guidelines, HIPAA, and EU MDR regulations.
Developers must stay updated with these regulations and design the software to meet these standards. Outsourcing to experienced partners can be a viable strategy to navigate these complex regulatory waters.
Challenge 2: Interoperability
Medtech devices frequently have to communicate and exchange data with a variety of other systems and devices. This interaction is vital for the consolidation and comprehension of the data received from multiple sources, aiding in accurate diagnosis and effective treatment plans.
Let’s take the example of a heart rate monitor and a glucose level tracker in a hospital setting. These devices need to share information with the hospital’s main system so doctors can get a complete view of a patient’s health status. To enable this seamless exchange of information, developers adopt standard communication protocols and data formats, such as HL7 or FHIR.
In addition, the integration of these protocols is not a one-time event but must be maintained throughout the software lifecycle. As technology evolves and upgrades, these standards may also be updated. Your development team needs to ensure that the software remains compatible with these changes, maintaining the smooth flow of data exchange.
Challenge 3: Cybersecurity
Healthtech devices handle sensitive data, making them attractive targets for cybercriminals. A recent report by IBM indicates the average cost of a healthcare data breach surpasses $10 million. To avoid data breaches, MDS developers must implement robust security measures, including Advanced Encryption Standard (AES), SSH File Transfer Protocols (SFTP), encryption tools like BitLocker/FileVault, two-factor (2FA) or multi-factor (MFA) user authentication, single sign-on (SSO), and regular security audits including vulnerability assessment, penetration testing, and code reviews.
Challenge 4: Usability
The usability of SAMD and software for medical procedures and monitoring plays a crucial role in their effectiveness. It’s essential that these tools are user-friendly for everyone involved — from healthcare professionals to patients.
Let’s think about a future medical app designed to help patients track their symptoms. If the app is too complex, it may discourage users or lead to inaccurate data input. That’s why developers need to adopt user-centered design principles.
When we design and build healthtech soft, it’s not just about technical functionality. The software must be intuitive, ensuring users can operate it efficiently and effectively without added stress.
Challenge 5: Software validation
Rigorous testing and validation processes should be in place to verify the software’s functionality and performance. This includes coordinating, designing, and conducting trials and research studies for product validation.
To develop and deploy medical device software successfully, several hurdles must be overcome. For those developing this type of software, smooth sailing is possible through continuous learning about new regulatory changes, making certain that the software can interact seamlessly with other systems, implementing strong cybersecurity defenses, prioritizing user-friendly design, and thoroughly validating the software. It’s essentially about remaining adaptable, secure, user-oriented, and thorough in all stages of the software creation process.
The future of medical device app development
The medical device software development services now have a potential to thrive is an ever-evolving landscape. Technological advancements and the need for improved healthcare solutions also drive it. The following points highlight some of the key trends shaping this field:
Artificial Intelligence (AI) and Machine Learning (ML). These technologies are revolutionizing healthtech device software capabilities by enhancing diagnostics, predictive analytics, and personalized medicine. They analyze vast data sets to identify patterns and make predictions, improving patient outcomes. For example, AI can analyze imaging data for early disease detection.
Internet of Medical Things (IoMT). The IoMT, a connected infrastructure of medical tools, software applications, and health systems, allows devices to communicate and exchange data, improving efficiency and patient care. The trend of increasing device connectivity is expected to continue.
Telemedicine and Remote Patient Monitoring. The COVID-19 pandemic has accelerated the need for distance patient monitoring and telemedicine. Medical device software is being developed to facilitate remote consultations, monitor patient health data, and provide real-time feedback to healthcare providers.
Personalized Medicine. Leveraging AI and data analytics, healthtech device software is being developed to provide personalized treatment plans based on a patient’s unique characteristics. This approach can improve treatment outcomes and patient satisfaction.
Blockchain Technology. Blockchain can provide a secure and transparent platform for patient data exchange. It can maintain patient privacy, ensure data integrity, and facilitate interoperability between healthcare systems.
These trends highlight the dynamic nature of medical device software development. Outsourcing to experienced medical software development services can be a strategic move to leverage the opportunities presented by these trends.
Wrapping up
Faced with rigid regulations and constantly shifting trends, the challenges of development of medical device software are hefty but, with the right approach, entirely surmountable.
Agile development plays a significant role in tackling these challenges, allowing for flexibility and quick adaptation to changing circumstances or requirements. Mobile development, too, is an exciting frontier that brings healthcare tools right into the hands of those who need them, making care more accessible and efficient.
It’s in this environment that the innovative medical software truly shines — helping healthcare providers deliver superior patient care, improving clinical outcomes, and streamlining workflows.
The importance of high-quality medical tool software continues to grow exponentially. This makes it an ideal time to explore and leverage the immense potential that software design for healthtech devices holds.
However, navigating these waters and turning challenges into opportunities requires not just the right tools and strategies, but also the right team. A professional development partner, experienced in the nuances of healthcare software, can guide you through the intricate labyrinth of regulations, lifecycle management, and more, ensuring your product is not just compliant, but a step ahead.
The journey may be intricate, but the results — improved healthcare outcomes through powerful, intuitive software — are undoubtedly worth it.
0 notes
Text
North American Arrhythmia Management System (AMS) Market Analysis Demand, Statistics, Top Manufacturers, Revenue by Reports and Insights 2030
The latest market report published by Credence Research, Inc. “Global North American Arrhythmia Management System (AMS) Market: Growth, Future Prospects, and Competitive Analysis, 2016 – 2028. The North American Arrhythmia Management System (AMS) market has been gradually growing in recent years and is expected to grow at a 6.60% CAGR between 2023 and 2030. The market was valued at USD 3.7 billion in 2022 and is expected to expand to USD 5.78 billion by 2030.
North American Arrhythmia Management System (AMS) market. Our in-depth analysis focuses on key trends, market segmentation, major players, growth drivers, challenges, and future opportunities. As leaders in the field of arrhythmia management, we aim to provide valuable insights for industry stakeholders, healthcare professionals, and investors looking to understand and capitalize on this flourishing market.
North American Arrhythmia Management System (AMS) Market Top Report Findings shed light on the current state of arrhythmia management in this region. This comprehensive study provides an extensive analysis of key factors affecting market growth, including technological advancements, regulatory frameworks, and competitive landscape. The report highlights that the North American AMS market is witnessing a steady expansion due to increasing prevalence of cardiac disorders and rising awareness regarding early diagnosis and treatment options. It also reveals that advanced technologies such as implantable cardioverter-defibrillators (ICDs) are gaining significant traction among healthcare professionals for their effectiveness in managing arrhythmias.
Key Segments of the North American AMS Market
Test Equipment Segment
The AMS market is segmented by test equipment, with the Electrocardiogram (ECG) leading the pack. ECG remains a critical tool for diagnosing arrhythmias, providing valuable insights into the patient's heart rhythm and guiding appropriate treatment plans.
Site of Origin Atrial Segment
When it comes to the site of origin atrial, Sinus Bradycardia emerges as the leading segment. This condition is characterized by a slower-than-normal heart rate and requires effective monitoring and management.
Type Segment
Among different types of arrhythmias, Supraventricular Tachycardias show the highest Compound Annual Growth Rate (CAGR) during the forecast period. Supraventricular tachycardias refer to a group of arrhythmias originating above the ventricles, demanding accurate detection and timely interventions.
Country Segment
The United States is the driving force behind the growth of the North American Arrhythmia Management System (AMS) industry. Canada is the second largest country in the market, while Mexico is expected to be the fastest-growing country in this sector
Browse 220 pages report North American Arrhythmia Management System (AMS) Market By Test Equipment (Electrocardiogram (ECG), Holter monitor) By Site of Origin Atrial (Sinus bradycardia, Premature atrial contractions (PACs), Wandering atrial pacemaker, Atrial tachycardia, Multifocal atrial tachycardia, Supraventricular tachycardia (SVT), Atrial flutter, Atrial fibrillation)- Growth, Future Prospects & Competitive Analysis, 2016 – 2030)- https://www.credenceresearch.com/report/north-american-arrhythmia-management-system-ams-market
Scarcity of Qualified Healthcare Workers
Effectively utilizing AMS technology requires skilled healthcare professionals, including cardiologists, electrophysiologists, and technicians. However, certain areas face a shortage of skilled professionals, hindering the uptake and implementation of AMS technology. Limited training opportunities and the specialized nature of arrhythmia therapy can exacerbate this challenge.
Limited Reimbursement Coverage
Although reimbursement policies may incentivize AMS adoption, limited coverage for specific devices and services can hinder market growth. Insurance companies and government healthcare initiatives may not fully cover the cost of all AMS devices and procedures. This limited reimbursement coverage may create cost constraints for patients and healthcare providers, influencing the adoption of advanced AMS systems.
Focus on Patient Education and Engagement
Educating patients about arrhythmias, self-monitoring techniques, and the importance of adhering to treatment plans can improve patient engagement and self-care. AMS providers can develop instructional tools, mobile applications, or interactive platforms to educate patients and promote active involvement in managing their condition.
Advancements in Wearable Devices and Sensors
Wearable devices such as smartwatches, ECG monitors, or patches enable continuous monitoring of heart rhythms throughout the day, facilitating real-time surveillance and analysis. This constant monitoring aids in the early detection of anomalies and arrhythmias, providing valuable insights for healthcare practitioners. Long-term data collection through wearable devices allows for identifying patterns, triggers, and evaluating the effectiveness of treatment strategies, enhancing arrhythmia management practices.
Competitive Landscape
The North American Arrhythmia Management System (AMS) market is highly competitive, with several leading players vying for market share. Some notable competitors in the market include:
Applied Cardiac Systems
AliveCor
Biotronik
Biotricity
GE Healthcare
iRhythm Technologies
Koninklijke Philips N.V.
Medtronic plc.
Nihon Kohden Corporation
St. Jude Medical (Abbott Laboratories)
Spacelabs Healthcare (OSI Systems Inc.)
Welch Allyn (Hillrom Services Inc.)
These key players focus on product innovation, expanding their market reach, and maintaining competitive pricing to stay ahead of the competition.
Future Outlook
The North American Arrhythmia Management System (AMS) market holds immense promise, driven by the rising demand for remote patient monitoring and telemedicine solutions. Key growth factors, such as the increasing prevalence of arrhythmia and the growing senior population, continue to propel the market forward. To remain competitive and successful, key businesses in the sector must prioritize product innovation, expand market reach, and maintain a customer-centric approach.
Why to Buy This Report-
The report provides a qualitative as well as quantitative analysis of the global North American Arrhythmia Management System (AMS) Market by segments, current trends, drivers, restraints, opportunities, challenges, and market dynamics with the historical period from 2016-2020, the base year- 2021, and the projection period 2022-2028.
The report includes information on the competitive landscape, such as how the market's top competitors operate at the global, regional, and country levels.
Major nations in each region with their import/export statistics
The global North American Arrhythmia Management System (AMS) Market report also includes the analysis of the market at a global, regional, and country-level along with key market trends, major players analysis, market growth strategies, and key application areas.
Browse Full Report: https://www.credenceresearch.com/report/north-american-arrhythmia-management-system-ams-market
Visit: https://www.credenceresearch.com/
Related Report: https://www.credenceresearch.com/report/medical-animation-market
Related Report: https://www.credenceresearch.com/report/ecoa-esource-and-clinical-trials-market
Browse Our Blog: https://www.linkedin.com/pulse/north-american-arrhythmia-management-system-ams-market-singh
Browse Our Blog: https://tealfeed.com/north-american-arrhythmia-management-system-ams-scifx
About Us -
Credence Research is a viable intelligence and market research platform that provides quantitative B2B research to more than 10,000 clients worldwide and is built on the Give principle. The company is a market research and consulting firm serving governments, non-legislative associations, non-profit organizations, and various organizations worldwide. We help our clients improve their execution in a lasting way and understand their most imperative objectives. For nearly a century, we’ve built a company well-prepared for this task.
Contact Us:
Office No 3 Second Floor, Abhilasha Bhawan, Pinto Park, Gwalior [M.P] 474005 India
0 notes
Text
Cardiovascular Biomaterial Market Research Report, Growth, Analysis and Forecast 2028
Global Cardiovascular Biomaterial Market, By Type (Natural, Ceramic, Metallic, Polymer), Product (Catheters, Stents, Implantable Cardiac Defibrillators, Pacemakers, Sensors, Heart Valves, Vascular Grafts, Guidewires, Ventricular Assist Devices), Country (U.S., Canada, Mexico, Germany, Italy, U.K., France, Spain, Netherland, Belgium, Switzerland, Turkey, Russia, Rest of Europe, Japan, China, India, South Korea, Australia, Singapore, Malaysia, Thailand, Indonesia, Philippines, Rest of Asia- Pacific, Brazil, Argentina, Rest of South America, South Africa, Saudi Arabia, UAE, Egypt, Israel, Rest of Middle East and Africa) Industry Trends and Forecast to 2028.
An expert team performs systematic, object-oriented and complete market research study to provide the facts associated with any subject in the field of marketing via Cardiovascular Biomaterial marketing report. The report has a lot to offer to both established and new players in the Cardiovascular Biomaterial industry with which they can completely understand the market. SWOT analysis and Porter’s Five Forces analysis methods are used wherever applicable, while generating this report. One of the most important parts of an international Cardiovascular Biomaterial market report is competitor analysis with which businesses can estimate or analyse the strengths and weaknesses of the competitors.
Key Players
The major players covered in the cardiovascular biomaterial market report are DSM, Wright Medical Group N.V, Zimmer Biomet, Bayer AG, BASF SE, CRS Holdings Inc., Invibio Ltd., Foster Corporation, CVD Equipment Corporation, Abbott, Baxter, Medtronic, Johnson & Johnson Private Limited , Boston Scientific Corporation , Edwards Lifesciences Corporation, BD, Molnlycke Health Care AB, Smith+Nephew, Integra LifeSciences, Messe-Düsseldorf GmbH, AnteoTech, and ANYGEN among other domestic and global players. Market data is available for global, North America, South America, Europe, Asia-Pacific (APAC) and Middle East and Africa (MEA) separately. DBMR analysts understand competitive strengths and provide competitive analysis for each competitor separately.
Browse More Info @ https://www.databridgemarketresearch.com/reports/global-cardiovascular-biomaterial-market
With the help of credible Cardiovascular Biomaterial market analysis report, businesses can make out the reaction of the consumers to an already existing product in the market. The report includes estimations of recent state of the market, CAGR values, market size and market share, revenue generation, and necessary changes required in the future products. A wide-ranging competitor analysis helps build superior strategies of production, improvement in certain product, its advertising or marketing and promotion for the business. Exhaustive and comprehensive market study performed in the wide ranging Cardiovascular Biomaterial market report offers current and forthcoming opportunities that put light on the future market investment.
Key questions answered in the report:
Which product segment will grab a lion’s share?
Which regional market will emerge as a frontrunner in coming years?
Which application segment will grow at a robust rate?
Report provides insights on the following pointers:
Market Penetration: Comprehensive information on the product portfolios of the top players in the Cardiovascular Biomaterial Market.
Product Development/Innovation: Detailed insights on the upcoming technologies, R&D activities, and product launches in the market.
Competitive Assessment: In-depth assessment of the market strategies, geographic and business segments of the leading players in the market.
Table Of Content
Part 01: Executive Summary
Part 02: Scope Of The Report
Part 03: Global Market
Part 04: Global Market Size
Part 05: Global Market Segmentation By Product
Part 06: Five Forces Analysis
More Reports:
Diuretic Drugs Market
Patient Engagement Technology Market
Healthcare Business Intelligence Market
Chinese Hamster Ovary cells (CHO) Market
Anti-cancer Drug Market
About Us:
Data Bridge Market Research set forth itself as an unconventional and neoteric Market research and consulting firm with unparalleled level of resilience and integrated approaches. We are determined to unearth the best market opportunities and foster efficient information for your business to thrive in the market
Contact:
Data Bridge Market Research
Tel: +1-888-387-2818
Email: [email protected]
0 notes
Text
Primary Lithium Batteries for Medical Market Outlook on Key Growth Trends, Factors and Forecast 2032

Primary Lithium Batteries for Medical Market Overview:
The primary lithium batteries for medical market refers to the sector that provides non-rechargeable lithium batteries specifically designed for medical devices and applications. These batteries offer reliable and long-lasting power to various medical devices, ensuring uninterrupted operation in critical healthcare settings. The Global Primary Lithium Battery Market Size Reached USD 3035.2 Million in 2022. It is Expected to Grow at a CAGR of 4.3% 2023-2030. Here is an overview of the primary lithium batteries for medical market, including key factors that drive its growth:
Key Factors:
Medical Device Applications: Primary lithium batteries are widely used in a range of medical devices, including implantable medical devices, patient monitoring systems, infusion pumps, defibrillators, hearing aids, insulin pumps, neurostimulators, and diagnostic equipment. These batteries provide a safe and dependable power source for essential medical functions.
Reliability and Longevity: Medical devices require a consistent and reliable power supply to ensure accurate measurements, continuous monitoring, and proper functioning. Primary lithium batteries are known for their stable voltage output and extended shelf life, ensuring the reliability and longevity of medical devices during critical operations.
Compact and Lightweight: Primary lithium batteries offer a high energy density, meaning they can store a significant amount of energy in a compact size. This characteristic is crucial for medical devices, which often require small and lightweight power sources to be portable and non-intrusive.
Safety and Regulatory Compliance: Primary lithium batteries for medical applications are designed and manufactured to meet strict safety standards and regulatory requirements, including those specified by organizations such as the International Electrotechnical Commission (IEC) and the Food and Drug Administration (FDA). These batteries undergo rigorous testing and validation to ensure their safety in medical environments.
Shelf Life and Storage Stability: Primary lithium batteries have a long shelf life and can retain their charge for extended periods. This is important for medical devices that may have intermittent usage or require stockpiling for emergencies. The ability to store these batteries for an extended time without significant loss of capacity or performance is a key advantage in the medical field.
Durability and Performance in Extreme Conditions: Medical devices may operate in various environments, including high or low temperatures, humidity, and mechanical stress. Primary lithium batteries are designed to withstand these challenging conditions, offering excellent performance and durability to ensure uninterrupted operation in critical healthcare settings.
Increasing Medical Device Adoption: The growing adoption of medical devices and technological advancements in healthcare drive the demand for primary lithium batteries. The rising prevalence of chronic diseases, an aging population, and the need for advanced diagnostic and treatment methods contribute to the increasing use of medical devices that rely on reliable power sources.
Technological Advancements: Ongoing advancements in primary lithium battery technology, including improvements in energy density, safety features, and integration with medical devices, further propel the growth of the market. These advancements enhance the performance, efficiency, and usability of medical devices, driving the demand for primary lithium batteries.
Emphasis on Portable and Wearable Devices: The trend towards portable and wearable medical devices, such as remote patient monitoring systems and wearable biosensors, requires compact and long-lasting power solutions. Primary lithium batteries provide the necessary energy supply for these devices, supporting the shift towards personalized and patient-centric healthcare.
In summary, the primary lithium batteries for medical market is driven by the wide range of medical device applications, reliability, longevity, compactness, safety compliance, shelf life, durability, technological advancements, increasing medical device adoption, and the emphasis on portable and wearable devices. As the healthcare industry continues to evolve and demand reliable power solutions, the market for primary lithium batteries for medical applications is expected to grow.
Demand:
Primary lithium batteries are widely used in medical devices due to their long shelf life, high energy density, and reliable performance. These batteries provide a stable power source for various medical applications, including implantable devices, diagnostic equipment, monitoring devices, and portable medical devices.
Implantable medical devices such as pacemakers, defibrillators, neurostimulators, and drug delivery systems often rely on primary lithium batteries. These batteries are preferred in implantable devices because they provide a long operational life, minimizing the need for battery replacement surgeries.
Diagnostic equipment such as glucose meters, blood pressure monitors, and various medical sensors also utilize primary lithium batteries for their compact size and high energy capacity. These batteries offer reliable power for accurate readings and prolonged use.
Portable medical devices, such as infusion pumps, portable oxygen concentrators, and handheld monitors, often utilize primary lithium batteries as well. These batteries provide the necessary energy for these devices to be portable and convenient for patients.
The demand for primary lithium batteries in the medical market is influenced by factors such as technological advancements in medical devices, an aging population, increasing prevalence of chronic diseases, and the overall growth of the healthcare industry. To obtain the most up-to-date information on the specific demand for primary lithium batteries in the medical market, it would be best to consult industry reports, market research, and battery manufacturers who specialize in medical applications.
We recommend referring our Stringent datalytics firm, industry publications, and websites that specialize in providing market reports. These sources often offer comprehensive analysis, market trends, growth forecasts, competitive landscape, and other valuable insights into this market.
By visiting our website or contacting us directly, you can explore the availability of specific reports related to this market. These reports often require a purchase or subscription, but we provide comprehensive and in-depth information that can be valuable for businesses, investors, and individuals interested in this market.
“Remember to look for recent reports to ensure you have the most current and relevant information.”
Click Here, To Get Free Sample Report: https://stringentdatalytics.com/sample-request/primary-lithium-batteries-for-medical-market/6710/
Market Segmentations:
Global Primary Lithium Batteries for Medical Market: By Company
• EVE Energy
• SAFT
• Hitachi Maxell
• GP Batteries International
• Energizer
• Duracell
• Varta
• Changzhou Jintan Chaochuang Battery
• Vitzrocell
• FDK
• Panasonic
• Murata
• Wuhan Lixing (Torch) Power Sources
• Newsun
• Renata SA
• Chung Pak
• Ultralife
• Power Glory Battery Tech
• HCB Battery
• EEMB Battery
Global Primary Lithium Batteries for Medical Market: By Type
• Li/SOCL2
• Li/MnO2
• Li-SO2
• Others
Global Primary Lithium Batteries for Medical Market: By Application
• Portable Medical Electronics
• Insulin Pumps
• Portable Ultrasound Equipment
• Others
Global Primary Lithium Batteries for Medical Market: Regional Analysis
All the regional segmentation has been studied based on recent and future trends, and the market is forecasted throughout the prediction period. The countries covered in the regional analysis of the Global Primary Lithium Batteries for Medical market report are U.S., Canada, and Mexico in North America, Germany, France, U.K., Russia, Italy, Spain, Turkey, Netherlands, Switzerland, Belgium, and Rest of Europe in Europe, Singapore, Malaysia, Australia, Thailand, Indonesia, Philippines, China, Japan, India, South Korea, Rest of Asia-Pacific (APAC) in the Asia-Pacific (APAC), Saudi Arabia, U.A.E, South Africa, Egypt, Israel, Rest of Middle East and Africa (MEA) as a part of Middle East and Africa (MEA), and Argentina, Brazil, and Rest of South America as part of South America.
Visit Report Page for More Details: https://stringentdatalytics.com/reports/primary-lithium-batteries-for-medical-market/6710/
Reasons to Purchase Primary Lithium Batteries for Medical Market Report:
• To gain insights into market trends and dynamics: this reports provide valuable insights into industry trends and dynamics, including market size, growth rates, and key drivers and challenges.
• To identify key players and competitors: this research reports can help businesses identify key players and competitors in their industry, including their market share, strategies, and strengths and weaknesses.
• To make informed business decisions: this research reports provide businesses with data-driven insights that can help them make informed business decisions, including strategic planning, product development, and marketing and advertising strategies.
About US:
Stringent Datalytics offers both custom and syndicated market research reports. Custom market research reports are tailored to a specific client's needs and requirements. These reports provide unique insights into a particular industry or market segment and can help businesses make informed decisions about their strategies and operations.
Syndicated market research reports, on the other hand, are pre-existing reports that are available for purchase by multiple clients. These reports are often produced on a regular basis, such as annually or quarterly, and cover a broad range of industries and market segments. Syndicated reports provide clients with insights into industry trends, market sizes, and competitive landscapes. By offering both custom and syndicated reports, Stringent Datalytics can provide clients with a range of market research solutions that can be customized to their specific needs
Contact US:
Stringent Datalytics
Contact No - +1 346 666 6655
Email Id - [email protected]
Web - https://stringentdatalytics.com/
#Non-rechargeable Batteries#Long Life Batteries#High Energy Density Batteries#Battery Power#Medical Sensors#Medical Diagnostics#Cardiac Devices#Neurostimulators#Hearing Aids#Infusion Pumps#Portable Medical Devices#Remote Monitoring#Medical Wearables.
1 note
·
View note
Text
Home Healthcare Market to be Worth $514.5 Billion by 2030 - Exclusive Report by Meticulous Research®
According to a new market research report titled, ‘Home Healthcare Market by Offering (Fertility, Pregnancy, Cholesterol Tests, Oxygen Delivery, Dialysis, IV, Patient Support Equipment), Services (Rehabilitation, Telehealth, Nursing, Hospice), Application (Cardiovascular, Diabetes)- Global Forecast to 2030,’ published by Meticulous Research®, the home healthcare market is projected to reach $514.5 billion by 2030, at a CAGR of 7.9% from 2023 to 2030.
Population aging is the dominant demographic trend of the twenty-first century—a reflection of increasing longevity, declining fertility, and the progression of a large number of individuals to older ages. The OECD forecasts that, globally, the population aged 60 and over is expected to reach 2.1 billion by 2050. As per the World Health Organization (WHO) statistics, the global population in the age group of 60 years and above was expected to increase from 1 billion in 2020 to 1.4 billion in 2022. The growth in the aging population has resulted in the increased adoption of home healthcare services due to the high costs of hospital healthcare services.
Download Free Report Sample Now @ https://www.meticulousresearch.com/download-sample-report/cp_id=1280
The geriatric population is highly susceptible to diseases such as high blood pressure, diabetes, arthritis, and cardiovascular diseases. Therefore, the growing global geriatric population, coupled with the fact that approximately 70% of home health patients belong to the age group of 65 years and above, is expected to drive the demand for home healthcare products and services during the forecast period.
Impact of COVID-19 on the Home Healthcare Market
The COVID-19 pandemic positively impacted the home healthcare market. The pandemic has increased the preference for home healthcare due to the risks of acquiring infections during hospital and clinic visits for treatment and related purposes. The pandemic has also increased the adoption of telehealth, making it a primary source of care for many patients. According to the 2021 World Economic Forum’s 5G Outlook Series report, there has been an increase of 490% in telemedicine visits for urgent cases. Furthermore, the outbreak of the COVID-19 pandemic increased the demand for home healthcare infrastructure and increased investments from governments in promoting homecare services. For instance, in August 2022, Fresenius Medical Care North America, the North American division of Fresenius Medical Care AG & Co. KGaA (Germany), acquired Interwell Health U.S. This acquisition was aimed at bringing Fresenius Health Partners’ expertise in kidney care and value-based performance and contracting.
The home healthcare market is segmented based on Offering (Home Tests and Patient Monitoring Equipment [Fertility Tests & Aids, Pregnancy Tests, Gender, DNA, and Parental Tests, Cholesterol Tests, HIV Tests, Holter Monitors, Blood Pressure Monitors, Oximeters, Heart Rate Monitors,
Thermometers, Stethoscopes, Defibrillators, Pedometers, Scales & Body Fat Monitors, Peak Flow Meters, Apnea Monitors, Baby Monitors, Coagulation Monitors, Diabetes Management]), Home Therapeutic Equipment ({Home Respiratory Therapy Equipment, [Continuous Positive Airway Pressure Equipment (CPAP Machines, CPAP Accessories & Consumables)], Oxygen Delivery Equipment, Nebulizers & Accessories, Ventilators & Accessories}, Home Dialysis Equipment, {Home Peritoneal Dialysis Products, Home Hemodialysis Products}, Home IV Equipment {IV Pumps, Other Home IV Equipment}, Other Equipment), Patient Support Equipment, {Mobility Assist Equipment, [Wheelchairs], Walking Assist Devices {Walking Assist Devices, [Walkers and Rollators, Canes & Walking Sticks, Crutches], Mobility Scooters, Medical Furniture and Accessories, Bathroom Safety Equipment), Services (Rehabilitation Services, Infusion Services, Skilled Care/Nursing, Unskilled Care Services, Telehealth Services, Hospice Care Services, Respiratory Therapy Services, Other Services), Application (Cardiovascular Diseases & Hypertension, Diabetes, Respiratory Diseases, Pregnancy, Mobility Disorders, Cancer, Wound Care, and Other Applications) and Geography. The study also evaluates industry competitors and analyzes their market shares at the country and regional levels.
Speak to our Analysts to Understand the Impact of COVID-19 on Your Business : https://www.meticulousresearch.com/speak-to-analyst/cp_id=1280
Based on offering, in 2023, the home therapeutic equipment segment is expected to account for the largest share of the home healthcare market. The growth of this segment is attributed to increasing cases of chronic kidney and respiratory diseases, the high costs of therapeutic services in hospitals, and the availability of rental products, including ventilators, nebulizers, and oxygen delivery equipment.
Based on services, the skilled care/nursing segment is projected to register the highest CAGR during the forecast period. The growth of this segment is attributed to the wide range of daily tasks performed by nurses to provide adequate care for patients receiving homecare, the rising adoption of home-based treatments that require technical knowledge of operating healthcare products which includes home dialysis equipment, and home IV equipment, home respiratory therapy equipment, and the increasing demand for home care for the elderly population.
Based on application, in 2023, the diabetes segment is expected to account for the largest share of the home healthcare market. The growth of this segment is attributed to the rising number of diabetic patients globally who are more prone to diseases such as cardiovascular diseases and Alzheimer's disease. Furthermore, the growing need to monitor the heart rate, hemoglobin, cholesterol, and blood pressure levels of diabetic patients contributes to the growth of this market segment.
“Exciting news! We are thrilled to announce an incredible opportunity for you!
For a limited time only, we are delighted to offer an exclusive flat 30% discount on all our remarkable research reports. It's our way of showing appreciation for your continuous support and commitment to staying informed. This remarkable discount allows you to unlock a wealth of valuable insights and expert analysis at an unbeatable price. Whether you're a business professional, an academic researcher, or an avid learner, this offer is tailor-made to enhance your knowledge and empower your decision-making.”
Quick Buy – Home Healthcare Market - Global Opportunity Analysis And Industry Forecast (2023-2030), Research Report: https://www.meticulousresearch.com/Checkout/85264114
Based on geography, in 2023, North America is expected to account for the largest share of the home healthcare market, followed by Europe and Asia-Pacific. Furthermore, in 2023, the U.S. is expected to be the largest shareholding market in North America. The market growth in the U.S. is attributed to the high disposable incomes of the population, the rising number of patients with chronic diseases, and government investments in promoting home healthcare. However, Asia-Pacific is expected to witness a rapid growth during forecast period. The growth of this regional market is attributed to the increasing geriatric population, the rising prevalence of chronic diseases in developing nations, the increasing investments in home healthcare in developing countries such as China and India, and the higher preference for home-based treatments due to the high costs of hospital services. Furthermore, government initiatives promoting homecare and the launch of advanced homecare products are driving the growth of the home healthcare market in Asia-Pacific.
The report also includes an extensive assessment of the key growth strategies adopted by leading market players in the past three to four years. In the last couple of years, the home healthcare market has witnessed various developments.
Some of the key players operating in the home healthcare market are Abbott Laboratories (U.S.), Amedisys, Inc (U.S.), Owens & Minor (U.S.), B. Braun SE (Germany), Baxter International, Inc. (U.S.), BAYADA Home Health Care (U.S.), Convatec (U.K.), Halyard Health, Inc. (U.S.), Covidien (Ireland), Fisher & Paykel (New Zealand), Fresenius Medical (Germany), GE Healthcare (U.S.), Johnson & Johnson (U.S.), and F. Hoffmann-La Roche AG (Switzerland).
To gain more insights into the market with a detailed table of content and figures, click here: https://www.meticulousresearch.com/product/home-healthcare-market-1280
Scope of the Report:
Home Healthcare Market, by Offering
Home Tests and Patient Monitoring Equipment
Fertility Tests & Aids
Pregnancy Tests
Gender, DNA, and Parental Tests
Cholesterol Tests
HIV Tests
Holter Monitors
Blood Pressure Monitors
Oximeters
Heart Rate Monitors
Thermometers
Stethoscopes
Defibrillators
Pedometers
Scales & Body Fat Monitors
Peak Flow Meters
Apnea Monitors
Baby Monitors
Coagulation Monitors
Diabetes Management
Home Therapeutic Equipment
Home Respiratory Therapy Equipment
Continuous Positive Airway Pressure Equipment
CPAP Machines
CPAP Accessories & Consumables
Oxygen Delivery Equipment
Nebulizers & Accessories
Ventilators & Accessories
Home Dialysis Equipment
Home Peritoneal Dialysis Products
Home Hemodialysis Products
Home IV Equipment
IV Pumps
Other Home IV Equipment
Other Equipment
Patient Support Equipment
Mobility Assist Equipment
Wheelchairs
Walking Assist Devices
Walkers and Rollators
Canes & Walking Sticks
Crutches
Mobility Scooters
Medical Furniture and Accessories
Bathroom Safety Equipment
Home Healthcare Market, by Services
Rehabilitation Services
Infusion Services
Skilled Care / Nursing
Unskilled Care Services
Telehealth Services
Hospice Care Services
Respiratory Therapy Services
Other Services
Home Healthcare Market, by Application
Cardiovascular Diseases & Hypertension
Diabetes
Respiratory Diseases
Pregnancy
Mobility Disorders
Cancer
Wound Care
Other Applications
Home Healthcare Market, by Geography
North America
U.S.
Canada
Europe
Germany
France
Italy
U.K.
Spain
Rest of Europe (RoE)
Asia-Pacific
China
Japan
India
Rest of Asia-Pacific
Latin America
Brazil
Mexico
Rest of Latin America
Middle East & Africa
Download Free Report Sample Now @ https://www.meticulousresearch.com/download-sample-report/cp_id=1280
1 note
·
View note
Text
Emergency Room Equipment Industry Current Trends and Challenges Analysis by 2023-2030

The emergency room equipment market refers to the global industry involved in the manufacturing and distribution of medical devices and equipment specifically designed for use in emergency departments or emergency rooms (ERs) of hospitals and healthcare facilities.
For Sample Report Click Here:- https://www.marketinforeports.com/Market-Reports/Request-Sample/485270
Emergency rooms are critical areas where patients with acute illnesses, injuries, or life-threatening conditions receive immediate medical attention. To effectively manage these situations, ERs require specialized equipment and devices to provide rapid assessment, diagnosis, and treatment. The emergency room equipment market encompasses a wide range of products that are essential for the functioning of an emergency department.
Some common types of emergency room equipment include:
Diagnostic equipment: This includes devices such as X-ray machines, CT scanners, ultrasound systems, electrocardiograms (ECGs), and vital signs monitors. These tools help healthcare professionals quickly evaluate patients and diagnose their conditions.
Life support equipment: Emergency rooms are equipped with various life support devices, including ventilators, defibrillators, cardiac monitors, and infusion pumps. These devices are crucial for stabilizing patients in critical condition or cardiac arrest.
Surgical instruments: Emergency room equipment also includes a variety of surgical instruments and supplies for performing emergency procedures. These may include surgical trays, sterile gloves, sutures, forceps, and scalpels.
Trauma care equipment: ERs are often equipped with specialized equipment for managing trauma cases, such as trauma beds, immobilization devices, cervical collars, traction devices, and splints.
Resuscitation equipment: Automated external defibrillators (AEDs), airway management devices (such as endotracheal tubes and laryngoscopes), and other resuscitation equipment are essential for responding to cardiac arrests and maintaining airway patency.
Patient monitoring systems: These systems provide continuous monitoring of patients’ vital signs, such as heart rate, blood pressure, oxygen saturation, and temperature. This allows healthcare providers to track patients’ conditions and respond promptly to any changes.
The emergency room equipment market is driven by factors such as the increasing number of emergency visits, rising demand for advanced diagnostic tools, and technological advancements in medical devices. The market includes several key players, including medical device manufacturers, distributors, and service providers. These companies strive to develop innovative and efficient equipment to enhance patient care in emergency settings.
0 notes
Text
Advanced ceramics market growth & demand analysis till 2031
The global advanced ceramics market. According to the study, the market reached a valuation of around US$ 60 Billion in 2020, which amounts to around 20% share of the overall ceramics market.
Sales of advanced ceramics are slated to rise at a CAGR of 7% to top US$ 120 Billion by 2031. Demand for alumina ceramics is set to increase at a CAGR of 6% across the forecast period of 2021 to 2031.
Download Sample Copy of This Report:
https://www.factmr.com/connectus/sample?flag=S&rep_id=2993?VM
The readability score of the Advanced Ceramics Market Demand report is good as it offers chapter-wise layout with each section divided into a smaller sections.
The report encompasses graphs and tables to show the entire assembling. Pictorial demonstration of the definite and estimated values of key segments is visually appealing to readers.
This Advanced Ceramics Market outlook report explicates on vital dynamics such as the drivers, restraints and opportunities for key players and competitive analysis of Advanced Ceramics Market along with key stakeholders as well as emerging players associated with the manufacturing of product.
The Key trends Analysis of Extended Oral Antibiotics Market also provides dynamics that are responsible for influencing the future Sales and Demand of over the forecast period.
What differences can the Advanced Ceramics Market report make on the revenue impacts and strategies of businesses?
Fact.MR strives to provide comprehensive assessments of opportunities in various regions and technology segments. The study also offers an uncluttered data-driven insights into the growth avenues of the Advanced Ceramics Market and all its segments. Some of the ways the study can make a discernible impact are by offering evidence-based perspectives on:
Attractiveness quotient of emerging product/technology types in various products in the Automated External Defibrillators Market.
Micro-economics factors that may hamper the prospects of some of the key segments
Recent spate of research and development (R&D) funding on key Advanced Ceramics Market
New business models paving way for disruptions in demand dynamic of key segments
Regional markets that will be future engine of growth and the industry trends that will support these markets
Challenges overcoming which may offer industry players competitive edge
Competitive Landscape
Leading companies in the market for advanced ceramics are putting a lot of effort into creating innovative technologies for unique applications.
Competition in the market has been observed to intensify in recent years. Major players in the market for advanced ceramics are investing in improving their product offerings as well as expanding their production capacities to cater to rising demand.
Key Segments Covered in Advanced Ceramics Industry Research
· Material
Alumina Ceramics
Ceramic-based Components
Titanate Ceramics
Zirconia Ceramics
Silicon Carbide Ceramics
Ceramic Filters
Others
Silicon Nitride Ceramics
Magnesium Silicate Ceramics
Pyrolytic Boron Nitride Ceramics
Aluminium Nitride Ceramics
Electroceramics
Structural Ceramics
Technical Ceramics
High-tech Ceramics
Ferrite Ceramics
Transparent Ceramics
· Class
Ceramic Matrix Composites
Ceramic Coatings
Monolithic Ceramics
· Application
Electrical Equipment
Catalyst Support
Electronic Devices
Wear Parts
Engine Parts
Filters
Bioceramics
· End User
Electrical & Electronics Sector
Transportation Sector
Medical Sector
Defense & Security Sector
Environmental Sector
Chemical Sector
Get Full Access of Complete Report:
https://www.factmr.com/checkout/2993
Questionnaire answered in the Market outlook Report of advanced ceramics include:
What is the key strategy deployed by large players to maximize Automated External Defibrillators Market growth?
What are the main challenges faced by players in the Advanced Ceramics Market Demand?
With the advent of technological advancement, how will the Advanced Ceramics Market landscape change over the forecast period?
What does player bring to the table which is unique as a strategy, and is easy to emulate for new investors in the Advanced Ceramics Market size?
How will be insights and market estimations provided in the Fact.MR report on the Demand of advanced ceramics make a difference?
The study takes a closer look at the major economic turmoil, with a focus on the recent COVID-19 pandemic disruptions
The assessment of key growth dynamics highlights the attractiveness of new automation technologies and offers readers insight on the prospect of these during the forecast period
The study tries to offer a balance perspective of the opportunities in mature and the most lackluster markets
Provides scrutiny of the industry trends that have shaped recent government policies
Provides an account of major breakthroughs in all segments that might change the course of the market considerably
Provides an incisive analysis of socio-political milieu in which the key markets operate, and how will that influence the lucrativeness of the overall Automated External Defibrillators Market
Analyzes how collaborations and partnerships among players from different industries shape the key growth dynamics in the near future
Evaluates the role of various stages of funding on new growth avenues in key regional markets
Contact:
US Sales Office : 11140 Rockville Pike
Suite 400 Rockville, MD 20852
United States
Tel: +1 (628) 251-1583
E-Mail: [email protected]
0 notes
Text
Did you Know the Future of Medical Kits
Medical kits refer to the segment of the healthcare industry that produces and sells pre-packaged kits containing various medical supplies and equipment. These kits are designed to be used in a variety of medical settings, including hospitals, clinics, ambulances, and in-home care.
Medical kits can be specialized for a particular medical condition, such as diabetes or asthma, or they can be more general and contain a variety of basic medical supplies, such as bandages, gauze, and antiseptics. Some medical kits may also contain more advanced equipment, such as defibrillators, airway management devices, and respiratory therapy devices.
Upcoming Trends: Medical Kit Industry
The global medical kits market is expected to grow in the coming years, driven by factors such as the increasing prevalence of chronic diseases, the growing demand for home healthcare services, and the need for emergency medical services.

0 notes
Text
What products can be used instead of RRC2020?
Currently, there is a global shortage of ventilators, so even companies in other industries are looking to provide manufacturing to meet the necessary demand. To drive new product development in the shortest possible time, RRC Power Solutions now offers a ready-to-use solution for the necessary portable power supply: the Ventilator Power Pack.
This package offers several benefits: As the included RRC standard batteries are globally recognized, they can be used anywhere in the world. Many well-known medical device manufacturers already rely on RRC standard battery packs.
Typically, it takes several years to develop a medical device and find a suitable portable power solution, and RRC standard batteries can be used without additional development time and cost. Furthermore, the batteries are developed and tested according to the highest standards (ISO13485 + FDA QS.Reg 820) and offer high performance.
What should I do if I don’t want to use the RRC2020 battery? Is there a replacement for this battery in China? There is one in China, that is TEFOO ENERGY smart lithium battery pack.
Features of TEFOO ENERGY smart lithium battery pack:
Comply with regulatory requirements in multiple regions around the world
Standard SMBus v1.1 interface
Integrated LED state of charge (SOC) indicator
Use Panasonic batteries, which have the highest energy density and stability in the market and meet JEITA charging optimization rules
Comprehensive safety protection functions, such as overcharge, overdischarge, overcurrent, overtemperature, short circuit, etc.
Equipped with equalization function to effectively improve battery life
TI precise power calculation
Standard lithium battery packs are widely used in medical equipment outdoor rescue defibrillation monitors, blood pressure monitors, oxygen concentration ECG monitors, handheld ECG monitors, single-lead ECG instruments, etc.

E-Mail:[email protected]
Official Website:www.tefoo-energy.com
0 notes