#reactive oxygen species (ROS)
Explore tagged Tumblr posts
Text
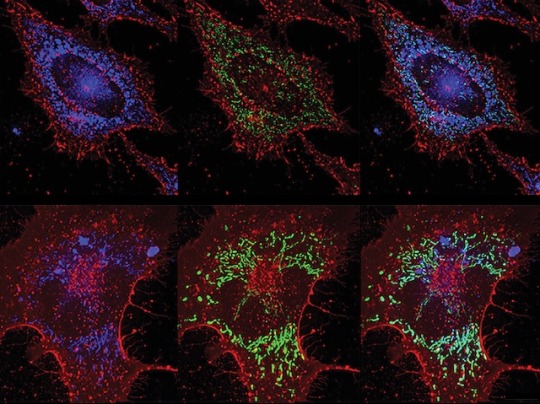
ROS vs Bacteria
Inducing lung lining cells to produce bacteria-killing reactive oxygen species (highly reactive chemicals that can cause oxidative damage) protects against pneumonia without reliance on antibiotics
Read the published research paper here
Image from work by Yongxing Wang and colleagues
Department of Pulmonary Medicine, University of Texas MD Anderson Cancer Center, Houston, TX, USA
Image originally published with a Creative Commons Attribution 4.0 International (CC BY 4.0)
Published in PLOS Pathogens, September 2023
You can also follow BPoD on Instagram, Twitter and Facebook
#science#biomedicine#immunofluorescence#biology#reactive oxygen species#ROS#sci art#pulmonary#lungs#pneumonia#antibiotics#antibiotic resistance
10 notes
·
View notes
Text
Mitochondrial Dysfunction in Beckers Muscular Dystrophy
Introduction
Beckers Muscular Dystrophy (BMD) is a genetic neuromuscular disorder caused by mutations in the DMD gene, leading to defective dystrophin production. While dystrophin primarily serves as a structural protein, emerging evidence indicates its role in mitochondrial function and cellular metabolism. This article explores mitochondrial dysfunction in BMD, focusing on bioenergetics, oxidative stress, mitochondrial dynamics, and metabolic consequences.
Bioenergetic Impairment
Mitochondria are the primary energy-producing organelles, generating adenosine triphosphate (ATP) through oxidative phosphorylation (OXPHOS). In BMD, mitochondrial bioenergetics are disrupted due to reduced dystrophin-associated glycoprotein complex (DGC) stability, affecting intracellular signaling and energy metabolism. Studies show that muscle fibers from BMD patients exhibit reduced ATP production, mitochondrial membrane potential (ΔΨm) depolarization, and decreased respiratory chain efficiency. Impaired complex I and complex IV activities have been reported, contributing to decreased oxidative phosphorylation and subsequent muscle weakness.
Oxidative Stress and ROS Accumulation
Mitochondria are a significant source of reactive oxygen species (ROS), which play dual roles as signaling molecules and contributors to oxidative damage. In BMD, excessive ROS production due to dysfunctional electron transport chain (ETC) exacerbates oxidative stress. Studies have demonstrated elevated lipid peroxidation, increased protein carbonylation, and mitochondrial DNA (mtDNA) damage in BMD-affected muscles. Reduced expression of key antioxidant enzymes, such as superoxide dismutase (SOD) and glutathione peroxidase (GPx), further impairs the ability to counteract oxidative damage. The resulting oxidative burden contributes to muscle fiber degeneration, chronic inflammation, and apoptosis.
Mitochondrial Dynamics: Fission and Fusion Imbalance
Mitochondria continuously undergo fission and fusion processes to maintain cellular homeostasis. These dynamics are critical for mitochondrial quality control, ensuring the removal of damaged mitochondria via mitophagy. In BMD, an imbalance between fission and fusion leads to mitochondrial fragmentation and defective turnover. Key regulators such as dynamin-related protein 1 (DRP1) and mitofusin-2 (MFN2) exhibit altered expression, resulting in increased mitochondrial fission and reduced fusion. This dysregulation impairs mitochondrial network integrity, contributing to decreased ATP production and enhanced susceptibility to apoptosis.
Calcium Homeostasis and Mitochondrial Dysfunction
Dystrophin deficiency in BMD disrupts sarcolemmal stability, leading to aberrant calcium (Ca²⁺) handling. Elevated intracellular Ca²⁺ levels induce mitochondrial Ca²⁺ overload, impairing bioenergetic function and promoting mitochondrial permeability transition pore (mPTP) opening. mPTP dysregulation results in mitochondrial swelling, cytochrome c release, and apoptotic cascade activation. Additionally, excessive mitochondrial Ca²⁺ uptake alters ATP synthesis efficiency, exacerbating muscle fiber necrosis and degeneration.
Metabolic Alterations and Energetic Deficits
Skeletal muscle metabolism in BMD is characterized by a shift from oxidative to glycolytic energy production. Defective mitochondrial respiration forces muscle fibers to rely on glycolysis for ATP generation, leading to increased lactate accumulation and metabolic acidosis. This metabolic shift results in early fatigue, reduced endurance, and inefficient energy utilization. Transcriptomic analyses have identified downregulation of genes involved in fatty acid oxidation and tricarboxylic acid (TCA) cycle activity, further confirming the metabolic shift towards glycolysis. Such metabolic alterations compromise muscle function and regeneration capacity, contributing to disease progression.
Mitochondrial Quality Control and Mitophagy Defects
Mitophagy, a selective form of autophagy responsible for degrading damaged mitochondria, is impaired in BMD. The PINK1/Parkin pathway, essential for mitochondrial quality control, is downregulated in dystrophic muscle, leading to the accumulation of dysfunctional mitochondria. Defective mitophagy contributes to mitochondrial swelling, increased oxidative stress, and cellular energy deficits. Additionally, impaired mitophagy reduces the capacity for mitochondrial biogenesis, further exacerbating mitochondrial dysfunction and muscle pathology.
Conclusion
Mitochondrial dysfunction in BMD arises from bioenergetic impairments, oxidative stress, disrupted mitochondrial dynamics, altered Ca²⁺ homeostasis, metabolic deficits, and defective mitophagy. These abnormalities collectively contribute to muscle degeneration and disease progression. Understanding these mitochondrial defects provides valuable insights into the pathophysiology of BMD, emphasizing the need for targeted researc
h to mitigate mitochondrial dysfunction and improve muscle health in affected individuals.
#Beckers Muscular Dystrophy#Mitochondrial dysfunction in BMD#Oxidative stress in muscular dystrophy#Mitochondrial bioenergetics in BMD#ATP production in muscle disease#Reactive oxygen species (ROS) in BMD#Electron transport chain dysfunction#Mitochondrial DNA damage in BMD#Calcium homeostasis in muscular dystrophy#Mitochondrial fission and fusion imbalance#Mitophagy defects in BMD#Muscle fiber degeneration in BMD#Glycolytic metabolism in muscular dystrophy#Mitochondrial membrane potential disruption#Sarcolemmal instability in BMD#Superoxide dismutase (SOD) in muscle health#Mitochondrial permeability transition pore (mPTP)#Fatty acid oxidation in muscle disease#Dystrophin-associated glycoprotein complex (DGC)#Metabolic deficits in Beckers muscular dystrophy
0 notes
Text
youtube
#WO₃₋ₓ@Ferrocene-Folic Acid#photothermal therapy#chemodynamic therapy#reactive oxygen species#Fenton reaction#cancer nanomedicine#targeted drug delivery#tumor microenvironment#immunogenic cell death#nanoplatform#folate receptor targeting#oxidative stress#tumor ablation#ferrocenyl compounds#nanoparticle-mediated therapy#oxygen-deficient tungsten oxide#cancer immunotherapy#ROS generation#synergistic therapy#advanced oncology treatments.#Youtube
1 note
·
View note
Text
The role of antioxidants in periodontal health | ICPA Health Products Ltd.

Periodontal disease is a persistent inflammatory condition that impacts the tissues encircling our teeth. This condition arises from an imbalance between oral biofilms and the body’s immune response, potentially leading to the loss of tooth-supporting tissues. When this inflammation is confined to the protective periodontium, it’s termed gingivitis. However, when it extends to the periodontal supporting structures, it’s recognized as periodontitis. This prevalent oral disease, affecting 10%-15% of adults, is primarily driven by bacterial plaque microorganisms. Additionally, factors such as systemic health, oral hygiene, age, gender, and smoking play significant roles in its development.
The double-edged sword of Reactive Oxygen Species (ROS)
Reactive oxygen species (ROS) serve as crucial players in our body’s defense against invading pathogens. These molecules have antimicrobial properties that help combat infections in the oral cavity. However, ROS can be a “double-edged sword” since an excessive presence of these molecules can become cytotoxic to our own cells. ROS plays pivotal roles in cell signaling, gene regulation, and antimicrobial defense. An overabundance of ROS, coupled with inadequate antioxidant capacity, can result in oxidative stress within the affected periodontal tissues. This, in turn, leads to pathological changes and the destruction of host tissues, ultimately culminating in the loss of teeth as their supporting structures degrade. Inside cells, ROS can inflict damage on biomolecules and cell membranes, further exacerbating the situation.
The link between oxidative stress and periodontal disease
Emerging research has elucidated the connection between oxidative stress and periodontal disease. In the early stages of periodontal disease, especially in the case of periodontitis, a prominent oxidative process unfolds, characterized by elevated levels of reactive oxygen and nitrogen species (ROS and RNS). This oxidative onslaught can upset the balance of the body’s response, triggering changes in biomolecules, especially lipids, proteins, and nucleic acids, ultimately leading to damage to periodontal tissues.
The role of antioxidants
To counteract the deleterious effects of excessive free radicals generated during oxidative stress, our bodies possess an antioxidant defense system. Antioxidants can inhibit and reduce the damage caused by these harmful molecules. These natural antioxidants are found in various sources, including foods, teas, vitamins, minerals, and more. They are also used in auxiliary treatments for conditions such as cardiovascular diseases, pulmonary diseases, aging, and atherosclerosis, all of which share physiological links with periodontal diseases. This suggests that the application of antioxidants might also yield benefits in managing periodontal health.
Promising results
Recent scientific inquiry supports the idea that antioxidants can play a pivotal role in the treatment of periodontitis. A meta-analysis comprising fifteen clinical trials demonstrated uniformly positive outcomes associated with antioxidant supplementation during periodontitis treatment. These findings offer hope and promise for those seeking alternative therapies to complement traditional periodontal treatments.
Conclusion
As we delve deeper into the intricacies of periodontal disease, the role of oxidative stress and antioxidants emerges as a significant area of interest. Oxidative stress appears to be a key contributor to the development and progression of periodontal
diseases. Antioxidants, with their ability to neutralize harmful free radicals, hold promise as adjunct therapies in managing and mitigating the effects of periodontal disease. With ongoing research in this field, we are one step closer to a more comprehensive understanding of periodontal health and the potential for novel treatments that can preserve our smiles for years to come.
#Periodontal disease#Reactive oxygen species (ROS)#periodontal tissues#oral disease#oxidative stress and periodontal disease.#What is oxidative stress?#dental care#application of antioxidants#treatment of periodontitis#periodontal health
0 notes
Text

Horses are among the world’s most elite athletes: When galloping, they can consume twice as much oxygen per kilogram as the fittest humans. All that oxygen supercharges horses’ cells’ energy-producing compartments as they crank out ATP, the chemical needed to power their impressive muscles. But making so much cellular fuel so quickly comes with a catch: the manufacture of pernicious byproduct molecules called reactive oxygen species (ROS), which can wreak havoc in cells.
How horses dealt with this biological trade-off and evolved into premier endurance athletes has long intrigued biologists. Researchers report today in Science that they have uncovered a big part of it, identifying a key mutation that lets horses safely produce so much ATP. The trait helped pave the way for horses to go from dog-size critters millions of years ago to the high-endurance athletes we know today.
The study’s detailed molecular work makes it “exceptional,” says José Calbet, an expert on the cellular responses to exercise at the University of Las Palmas de Gran Canaria who wasn’t involved with the study.
The mutation in question occurs in the gene that encodes a protein called KEAP1, which acts as a biochemical bouncer, binding to a different protein called NRF2 to prevent it from entering the cell’s nucleus, where it would otherwise activate stress-response genes that help blunt cell damage.
But ROS can help NRF2 sneak in by causing KEAP1 to release its bind on the protein, allowing it to enter the nucleus and trigger the cell’s stress-response genes.
Johns Hopkins University ophthalmologist and clinician scientist Elia Duh, a senior author of the new study, didn’t set out to study horses. Initially, Duh was interested in the KEAP1-NRF2 system because its role in activating stress-response genes makes it a tempting target for treating inflammation—and aging-related conditions, such as blinding retinal diseases, irritable bowel syndrome, and neurodegeneration.
Duh wondered whether any insights could be gleaned from studying the evolution of these proteins in different animals. So, he teamed up with Gianni Castiglione, an evolutionary biologist and biochemist at Vanderbilt University. Together, they scanned hundreds of vertebrate genomes looking for notable mutations to the gene for KEAP1.
The team’s genomic work revealed birds had almost completely lost the gene, presumably an adaptation to the extreme demands of flight. When they looked in horses, researchers noticed what initially appeared to be a DNA sequence that encoded an unusually short—and therefore presumably nonfunctional—version of the KEAP1 protein. But when Duh’s and Castiglione’s team grew horse cells in culture, it discovered the protein was very much there and working. “Naturally, I was worried I was doing something wrong,” Castiglione says. “Then one day, a light bulb went off.”
As it turns out, the computer algorithm scientists had used to scan the horse genome had made a mistake. The algorithm had spotted a specific kind of mutation in the part of the KEAP1 gene that changed the messenger RNA from CGA—which codes for the amino acid arginine—to UGA, which is what’s known as a “stop codon.”
Normally, the cellular machinery interprets UGA as a sign to stop translating the RNA into a protein. But instead, the horses’ genetic machinery recodes the stop codon into a different amino acid, cysteine, causing it to ignore that order. This phenomenon, known as a stop codon read-through, is common among viruses but rare in multicellular organisms.
“The identification of this evolutionarily significant UGA recoding event represents a potentially seminal finding, offering a model for uncovering other yet-unidentified cases of stop codon read-through,” says Hozumi Motohashi, a biologist at Tohoku University who has studied KEAP1 and NRF2.
That the replacement is a cysteine is particularly notable, Castiglione says. KEAP1 senses cellular stress through its cysteines, which contain sulfur atoms whose reactions with ROS, induce the chemical changes that cause KEAP1 to let go of NRF2. The mutation the researchers had identified adds another place on KEAP1 for ROS to interact, which makes the protein more sensitive to stress—and lets horse cells respond much faster to the cellular stress of intense exercise. “It does make complete sense [that] by introducing another cysteine, another sulfur, you would have heightened sensitivity,” Castiglione says.
What’s more, this tweaking of KEAP1 is a “[key] genetic component to the puzzle of the evolution of horses,” Duh says. “Once they figured out how to run, they could occupy all kinds of ecological niches,” Castiglione adds.
The finding could also point the way toward new kinds of drugs to treat diseases by targeting the specific parts of the KEAP1 protein that help horses hoof it. “By looking at what evolution has figured out, we know this is a viable strategy,” Castiglione says.
Source
811 notes
·
View notes
Text
ROS detoxification enzymes and antioxidants function in cells as a network supported by various antioxidant recycling systems that replenish the level of reduced antioxidants (Figure 24.20).

"Plant Physiology and Development" int'l 6e - Taiz, L., Zeiger, E., Møller, I.M., Murphy, A.
#book quotes#plant physiology and development#nonfiction#textbook#reactive oxygen species#ros#detoxification#enzymes#plant cells#network#recycling#biosynthetic pathways
0 notes
Text
Also preserved in our archive (Daily updates!)
Published Sept 3, 2024
By Chuck Dinerstein, MD, MBA
New research reveals that fibrin, a key component of blood clots, may be the secret culprit behind the devastating neurological and inflammatory aftermath of the virus, including long COVID. From dense, stubborn clots to brain fog, the interaction of COVID’s spike protein with fibrin could be the missing link — and a potential target for life-saving therapies.
Coagulopathy, the formation of small blood clots that go on to wreck respiratory and neurologic havoc, has long been a clinical hallmark of COVID, and now it's oft-ignored Long COVID. A new study suggests that fibrin, a key component of blood clots, plays a role.
Fibrin provides structure to a blood clot and is derived from fibrinogen, a soluble blood protein when the coagulation cascade is activated. If you think of a blood clot as nature’s way of plugging a leak, fibrin deposition is frequently found where there is damage to the walls of blood vessels and the vessels making up the blood-brain barrier. Fibrin serves as a plug and a signal for a greater inflammatory and immune response.
Given the unique clinical presentation of clotting in COVID compared to other respiratory viruses, the researchers hypothesized that COVID directly binds to fibrinogen, promoting blood clot formation and altering clot structure and function. They found that the spike protein of the virus binds to fibrinogen and fibrin at specific binding sites, suggesting that the virus might contribute to abnormal clotting by interacting with fibrinogen.
They found that the spike protein altered the structure of clots, making them denser and more resistant to the body’s natural means of removing clots, a process called fibrinolysis. Additionally, the spike protein enhanced the inflammatory signals from fibrin, increasing oxidative forces (reactive oxygen species or ROS) released from macrophages, a first responder of the immune system.
In converting fibrinogen to fibrin, the spike's binding site (epitope) is exposed. Therapeutically, having identified binding regions, the research found that antibodies could disrupt and reduce these pro-inflammatory effects implicated in acute and long COVID. Among the inflammatory effects reduced by blocking the actions of fibrinogen was the deposition of collagen in the lungs, which creates a barrier to oxygen passage and helps to explain the refractory response to supplemental oxygen we have seen in patients.
Fibrin also suppresses natural killer (NK) cells, which are called "natural killers" because they can recognize and kill stressed cells without prior exposure to a particular pathogen, making them critical first responders. The suppression of NK cell activity results in enhancing viral persistence and lung inflammation.
In additional studies in mice, the researchers found that this fibrin-dependent inflammatory response occurs independently of the active virus, suggesting a potential mechanism for persistent symptoms in Long COVID. [1] Therapeutically, in their mouse model, the use of a monoclonal antibody targeting the fibrin epitope, in addition to reducing the lung’s inflammatory response, reduced neuroinflammation (associated with long COVID’s brain fog). There were reductions in fibrin deposition and microglial reactivity “leading to improved neuronal survival and reduced white-matter injury.” Microglia are the primary immune cells of the central nervous system.
To summarize:
Coagulopathy in COVID-19 is a primary driver of thrombo-inflammation and neuropathology rather than a consequence of systemic inflammation. Fibrin plays a causal immunomodulatory role in promoting hyperinflammation, neuropathological alterations, and increased viral load in COVID-19 by modulating NK cells, macrophages, and microglia. Elevated fibrinogen levels and BBB permeability in COVID-19 contribute to neuropathology, and targeting fibrin may offer a dual mechanism of action by inhibiting fibrin-spike interactions and exerting anti-inflammatory effects. A fibrin-targeting antibody effectively blocks many pathological effects of fibrin, providing neuroprotection and reducing thrombo-inflammation. Their findings have limitations, including how they measured changes in brain tissue, the use of mouse models, and the fact that our inflammatory response may have more than one pathway that results in COVID-19’s deleterious effects. For Long COVID, the fibrin-targeted antibody does not interfere with normal clotting, acting solely on fibrin's inflammatory responses, making it a candidate to protect against pulmonary and cognitive impairment; that will, of course, require clinical trials.
And there you have it—the silent saboteur behind the lingering specter of Long COVID. Fibrin is not just a bystander in the aftermath of COVID-19; it's a key player driving the chronic symptoms that continue to baffle patients and clinicians alike. The discovery that the virus’s spike protein meddles with fibrin, transforming it into a resilient, inflammatory force, opens a new frontier in the fight against the pandemic’s long tail. The research, though groundbreaking, is still in its early days, confined to animal models, and the complexities of human biology could introduce new challenges.
But if the science holds, targeting fibrin could offer a two-for-one punch against the clotting and inflammation that underpin much of the damage COVID-19 leaves in its wake. For the millions grappling with the enduring effects of Long COVID, this could be a glimmer of hope—a chance to reclaim their lives.
[1] The inquisitive with a conspiratorial bent might link these inflammatory responses in the absence of infection to deaths felt to be due to the COVID vaccines, which employ the spike as antigenic stimulus. The researchers note that most hematologic changes are triggered by the vaccine vector (an adenovirus) and that “COVID-19 RNA vaccines lead to small amounts of spike protein accumulating locally and within draining lymph nodes where the immune response is initiated, and the protein is eliminated.”
Source: Fibrin drives thrombo-inflammation and neuropathology in COVID-19 Nature DOI: 10.1038/s41586-024-07873-4 www.nature.com/articles/s41586-024-07873-4
#mask up#wear a mask#public health#pandemic#covid#covid 19#wear a respirator#coronavirus#still coviding#sars cov 2#fibrin#long covid#brain fog#chronic illness
77 notes
·
View notes
Text
Now this takes me back to a 20 year old hyperfixation on herbs and plants with medicinal benefits. I did not expect to find this in my search.
70 notes
·
View notes
Text
you know taking a radiation biology class has made me like my url a lot more. niche reference to ro's old band name now it's like haha i am killing you with low let particles that penetrate your entire body. i am generating reactive oxygen species and damaging your dna. you will develop cancer in about 30 years
#unless it's like an acute high dose i guess. then you'll get radiation sickness. and die#but slowly developing cancer is funnier to me#don't take that out of context btw
14 notes
·
View notes
Text
"We don't have special rights for anyone. We have equal rights for everybody." -Tom Allen
The Left Raged Against Government Waste Until Musk Arrived
by William Haupt III, The Center Square
"We don't have special rights for anyone. We have equal rights for everybody." -Tom Allen Margaret Thatcher once reminded us, "You'd be surprised at what a few dedicated patriots will do to make their country a better place to live in. In fact, they are the only people who try...
‘Transphobic’ Toddler Suspended From Nursery, Proving the Woke Mind Virus Has Taken Over the UK
by JD Rucker
A child who is either 3- or 4-years-old has been suspended from a nursery for being reported as "transphobic."
You can't make this up. According to Telegraph: A toddler was suspended from nursery after being accused of being transphobic or homophobic, The Telegraph can reveal. Department for Education (DfE)...
Christian Actor Neal McDonough Says “Beacon of Light” Gold Company Can Be Trusted in These Crazy Times
by Sponsored Post
Bible-believing Christians and those who are concerned about the economic future of America have the consensus view that strange times are ahead. Even with fiscal spirits raised by the election of President Donald J. Trump, geopolitical turmoil continues to threaten the markets. Physical precious metals like gold and silver continue to draw Americans to them even as price records are being broken every week. ...
Over 50% of Parents Supporting Adult Children, Two-Thirds Plan to Cut Them off in Next 3-4 Years
by Tyler Durden, Zero Hedge
(Zero Hedge)—More than 50% of parents with a child older than 18 are providing them with at least some financial support, according to a recent report by savings.com. Key findings from the report: Half of parents with adult children provide regular financial assistance to their grown offspring. The average support per adult child...
Here’s What’s At Stake In Wisconsin’s Supreme Court Election
by Daily Wire
Wisconsin voters will head to the polls on Tuesday to decide the ideological slant of the State Supreme Court, bringing to a close the most expensive state judicial race in history. The race — between conservative candidate Brad Schimel and liberal Judge Susan Crawford — will have nationwide consequences, as...
Trump Has Begun to Clean House at the Department of Justice
by Townhall
President Donald J. Trump is taking a chainsaw to the Department of Justice. The unsavory elements are being cut away, and, again, this shouldn’t be a shock to anyone paying attention to the 2024 campaign. The cancerous elements that infected this institution under Joe Biden, which were weaponized to go...
Mitochondrial Dysfunction in Neurodegenerative Disorders
by Dr. Joseph Mercola
Mitochondrial dysfunction is a key driver of neurodegeneration, with research showing that a single resting cortical neuron requires 4.7 billion ATP molecules every second for energy When mitochondria lose their efficient shape, electrons escape and form reactive oxygen species (ROS), triggering cellular damage and stress that particularly affects brain cells...
8 notes
·
View notes
Text
Mitochondrial Dysfunction in SLC6A1: A Molecular and Cellular Perspective
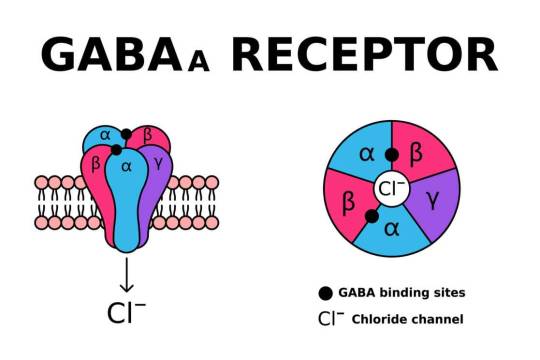
SLC6A1 encodes the gamma-aminobutyric acid (GABA) transporter type 1 (GAT1), a crucial component of inhibitory neurotransmission. Pathogenic variants in SLC6A1 lead to neurological disorders, primarily epilepsy, developmental delay, and neuropsychiatric conditions. While its role in GABAergic signaling is well established, emerging evidence suggests an intersection with mitochondrial dysfunction, which exacerbates disease pathology. This article explores the molecular and cellular mechanisms linking SLC6A1 mutations to mitochondrial impairment, highlighting alterations in energy metabolism, oxidative stress, and mitochondrial dynamics.
1. Introduction The SLC6A1 gene encodes the GAT1 transporter, responsible for reuptaking GABA from the synaptic cleft into presynaptic neurons and astrocytes. Disruptions in SLC6A1 impair inhibitory neurotransmission, contributing to hyperexcitability in neuronal circuits. Recent studies indicate a link between SLC6A1 dysfunction and mitochondrial abnormalities, underscoring a metabolic component to disease pathogenesis. The mitochondrial connection is crucial as these organelles regulate neuronal energy homeostasis and apoptosis. Understanding these mechanisms is essential for dissecting the full scope of SLC6A1-related disorders.
2. Role of SLC6A1 in Cellular and Mitochondrial Function Neurons exhibit high metabolic demand, relying heavily on mitochondria for adenosine triphosphate (ATP) production. GABA metabolism interfaces with mitochondrial pathways, influencing oxidative phosphorylation (OXPHOS) and redox balance. SLC6A1 mutations impair GABA uptake, potentially disrupting mitochondrial function through dysregulated Krebs cycle activity, altered ATP synthesis, and excessive reactive oxygen species (ROS) generation. Additionally, GABAergic dysfunction affects calcium signaling, further impacting mitochondrial integrity.
3. Energy Metabolism and ATP Production Mitochondria generate ATP primarily through OXPHOS. Deficient GABA uptake alters cellular excitability, increasing ATP demand while simultaneously impairing ATP synthesis. Studies show that neurons with SLC6A1 mutations exhibit reduced mitochondrial membrane potential (∆ψm), leading to inefficient ATP generation. Moreover, compensatory glycolysis often fails to meet neuronal energy demands, resulting in cellular stress and neuronal dysfunction.
4. Oxidative Stress and ROS Dysregulation Mitochondria are primary sites of ROS production, which serve as signaling molecules in normal physiology but become deleterious when unregulated. SLC6A1 mutations contribute to ROS imbalance, leading to oxidative stress and lipid peroxidation. Elevated ROS levels have been reported in neurons with impaired GABAergic signaling, suggesting that SLC6A1 mutations exacerbate mitochondrial oxidative damage. This process triggers mitochondrial DNA (mtDNA) mutations, protein oxidation, and lipid peroxidation, further compromising mitochondrial integrity.
5. Calcium Homeostasis and Mitochondrial Dysfunction Neuronal activity depends on tightly regulated calcium homeostasis. Mitochondria buffer intracellular calcium, maintaining synaptic function and preventing excitotoxicity. SLC6A1 dysfunction alters calcium flux due to disrupted GABAergic inhibition, leading to excessive mitochondrial calcium uptake. This triggers the mitochondrial permeability transition pore (mPTP), resulting in bioenergetic failure and apoptotic signaling cascades. Elevated cytosolic calcium further dysregulates mitochondrial enzyme activity, exacerbating metabolic dysfunction.
6. Mitochondrial Dynamics and Biogenesis Mitochondria undergo continuous fission and fusion to adapt to cellular demands. Impaired mitochondrial dynamics are observed in neurons harboring SLC6A1 mutations, leading to fragmented and dysfunctional mitochondria. The fusion-fission imbalance results in defective mitochondrial quality control, accumulation of damaged organelles, and impaired biogenesis. Downregulation of mitophagy-related proteins such as PINK1 and Parkin has been documented in models of SLC6A1 dysfunction, suggesting defective clearance of impaired mitochondria.
7. Synaptic Dysfunction and Mitochondrial Interactions Neurotransmission relies on synaptic mitochondria to meet localized energy demands. GABAergic synapses, in particular, require significant mitochondrial support due to their reliance on ATP-dependent vesicular transport and receptor function. SLC6A1 mutations disrupt synaptic mitochondrial positioning, reducing ATP availability at synapses. This impairment contributes to synaptic dysfunction, decreased inhibitory tone, and aberrant excitatory-inhibitory balance, which are hallmarks of SLC6A1-related neurological disorders.
8. Neuroinflammation and Mitochondrial Dysfunction Mitochondria modulate immune responses through ROS production and inflammatory cytokine signaling. Neurons with SLC6A1 mutations exhibit increased inflammatory markers, such as interleukin-6 (IL-6) and tumor necrosis factor-alpha (TNF-α), indicative of neuroinflammation. Mitochondrial dysfunction exacerbates this process by activating microglia and astrocytes, leading to chronic neuroinflammatory states. This further damages neuronal mitochondria, perpetuating a vicious cycle of dysfunction and degeneration.
9. Genetic and Epigenetic Influences on Mitochondrial Dysfunction Mutations in SLC6A1 not only affect protein function but also influence mitochondrial gene expression and epigenetics. Studies indicate altered expression of nuclear-encoded mitochondrial genes, including those involved in OXPHOS. Additionally, epigenetic modifications such as DNA methylation and histone acetylation impact mitochondrial biogenesis and function in SLC6A1-related disorders. Dysregulated mitochondrial gene transcription exacerbates bioenergetic failure, compounding neurological deficits.
10. Conclusion Mitochondrial dysfunction is an emerging pathological mechanism in SLC6A1-related disorders, contributing to energy deficits, oxidative stress, impaired calcium homeostasis, defective mitochondrial dynamics, and synaptic dysfunction. Understanding the interplay between SLC6A1 mutations and mitochondrial abnormalities provides insights into disease pathogenesis, paving the way for targeted metabolic and neuroprotective interventions. Future research should focus on elucidating the precise molecular pathways linking SLC6A1 dysfunction to mitochondrial pathology, ultimately aiding in the development of novel therapeutic strategies.
#SLC6A1 gene#Mitochondrial dysfunction#GABA transporter (GAT1)#Neurological disorders#Oxidative stress#Mitochondrial energy metabolism#ATP production#Reactive oxygen species (ROS)#Mitochondrial membrane potential#Calcium homeostasis#Neuronal excitability#Mitochondrial biogenesis#Mitochondrial dynamics#Synaptic dysfunction#Neuroinflammation#Mitochondrial quality control#Mitochondrial permeability transition pore (mPTP)#Neurodegeneration#Epigenetic modifications in mitochondria#Mitochondrial oxidative phosphorylation (OXPHOS)
0 notes
Text
youtube
#Photodynamic therapy#aggregation-induced emission#AIE-PSs#photosensitizers#reactive oxygen species#tumor microenvironment#cancer therapy#ROS generation#non-invasive treatment#tumor targeting#cancer research#precision medicine#light activation#photostability#intracellular aggregation#aggregation-enhanced photosensitizers#oncology advancements#tumor selectivity#biophotonics#therapeutic precision.#Youtube
0 notes
Text
A team of scientists has developed a new solution for the treatment of rheumatoid arthritis (RA). The work has been published in Nature Nanotechnology. RA is a chronic disease that, unfortunately, has no cure. The disease triggers a mix of troublesome symptoms like inflamed joints, harmful cytokines, and immune system imbalances, which work together to create a relentless cycle of worsening symptoms. While targeting some of these factors can provide short-term relief, others remain unresolved, leading to a frustrating cycle of remission and flare-ups. One of the major hurdles in RA treatment is the inability to restore the immune system to its healthy state. This leaves the body unable to control the continuous production of harmful substances like reactive oxygen species (ROS) and inflammatory cytokines, leading to persistent inflammation and discomfort. In essence, the ideal treatment for RA should not only provide immediate relief from inflammation and symptoms but also address the root cause by restoring the immune system to its normal, balanced state.
Continue Reading
142 notes
·
View notes
Text
Alcohol consumption is a significant risk factor for various cancers, including those of the mouth, throat, esophagus, liver, colon, rectum, and breast. When alcohol is metabolized in the body, it converts to acetaldehyde, a toxic compound that can damage DNA and hinder its repair mechanisms. This DNA damage can lead to uncontrolled cell growth, a hallmark of cancer development.

Additionally, alcohol can generate reactive oxygen species (ROS), leading to oxidative stress and further DNA damage. It also impairs the body's ability to absorb essential nutrients like vitamins A, C, D, E, and folate, which play protective roles against cancer. Moreover, alcohol increases estrogen levels, potentially elevating the risk of breast cancer. Given these mechanisms, it's crucial to be aware of the cancer risks associated with alcohol consumption and consider moderating intake to reduce potential harm.
6 notes
·
View notes
Text

Garlic - helps with blood sugar regulation, antioxidant with anti-inflamatory properties, and assists in lowering blood pressure.
Honey - antioxidant flavonoids and phenolic acids help neutralize reactive oxygen species (ROS) in your body which can build up in cells and cause damage, reduces inflammation, improves blood sugar regulation, improves cholesterol and triglyceride levels, and prevent heart disease.
Chicken - zinc, iron, B vitamins, protein, reduces blood pressure, lowers risk of heart disease
2 notes
·
View notes
Text
Rapid SSA responses to different abiotic stress conditions, including heat, cold, salinity, and high light intensity, have been demonstrated to be mediated by a self-propagating wave of ROS production, which travels at a rate of approximately 8.4 cm min^-1 and is dependent on the presence of a specific NADPH oxidase, respiratory burst oxidase homolog D (RBOHD), which is located on the plasma membrane (Figure 24.14).
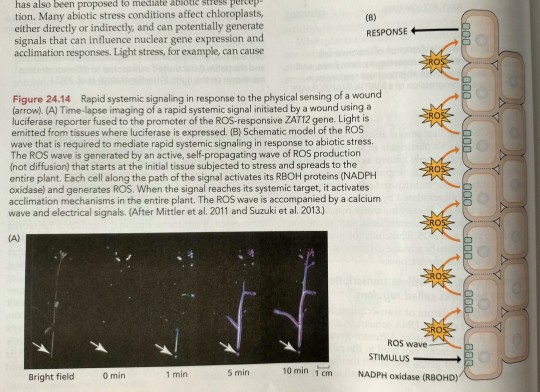
"Plant Physiology and Development" int'l 6e - Taiz, L., Zeiger, E., Møller, I.M., Murphy, A.
#book quotes#plant physiology and development#nonfiction#textbook#systemic acquired acclimation#saa#abiotic stress#heat#cold#salinity#high light#light intensity#reactive oxygen species#ros#nadph oxidase#plasma membrane#plant cells
0 notes