#sepsis diagnostics report
Link
MarketsandMarkets™ published a report on, “Sepsis Diagnostics Market by Technology (Microbiology, PCR, Sequencing, Biomarkers), Product (Reagents, Assay, Instruments, Software), Test Type (Lab, POC), Pathogen (Bacterial, Viral, Fungal), End User (Hospital, Pathology Lab) - Global Forecast to 2026". As per the report, the global Sepsis Diagnostics Industry is expected to reach USD 771 million by 2026 from USD 503 million in 2021, at a CAGR of 8.9% from 2021 to 2026. The report categorizes the global market into technology, product, method, test type, pathogen, end users, and region.
#marketsandmarkets research pvt. ltd.#sepsis diagnostics market#sepsis diagnostics market size#sepsis diagnostics industry#sepsis diagnostics report
1 note
·
View note
Text
"When bloodstream infections set in, fast treatment is crucial — but it can take several days to identify the bacteria responsible. A new, rapid-diagnosis sepsis test could cut down on the wait, reducing testing time from as much as a few days to about 13 hours by cutting out a lengthy blood culturing step, researchers report July 24 [2024] in Nature.
“They are pushing the limits of rapid diagnostics for bloodstream infections,” says Pak Kin Wong, a biomedical engineer at Penn State who was not involved in the research. “They are driving toward a direction that will dramatically improve the clinical management of bloodstream infections and sepsis.”
Sepsis — an immune system overreaction to an infection — is a life-threatening condition that strikes nearly 2 million people per year in the United States, killing more than 250,000 (SN: 5/18/08). The condition can also progress to septic shock, a steep drop in blood pressure that damages the kidneys, lungs, liver and other organs. It can be caused by a broad range of different bacteria, making species identification key for personalized treatment of each patient.
In conventional sepsis testing, the blood collected from the patient must first go through a daylong blood culturing step to grow more bacteria for detection. The sample then goes through a second culture for purification before undergoing testing to find the best treatment. During the two to three days required for testing, patients are placed on broad-spectrum antibiotics — a blunt tool designed to stave off a mystery infection that’s better treated by targeted antibiotics after figuring out the specific bacteria causing the infection.
Nanoengineer Tae Hyun Kim and colleagues found a way around the initial 24-hour blood culture.
The workaround starts by injecting a blood sample with nanoparticles decorated with a peptide designed to bind to a wide range of blood-borne pathogens. Magnets then pull out the nanoparticles, and the bound pathogens come with them. Those bacteria are sent directly to the pure culture. Thanks to this binding and sorting process, the bacteria can grow faster without extraneous components in the sample, like blood cells and the previously given broad-spectrum antibiotics, says Kim, of Seoul National University in South Korea.
Cutting out the initial blood culturing step also relies on a new imaging algorithm, Kim says. To test bacteria’s susceptibility to antibiotics, both are placed in the same environment, and scientists observe if and how the antibiotics stunt the bacteria’s growth or kill them. The team’s image detection algorithm can detect subtler changes than the human eye can. So it can identify the species and antibiotic susceptibility with far fewer bacteria cells than the conventional method, thereby reducing the need for long culture times to produce larger colonies.
Though the new method shows promise, Wong says, any new test carries a risk of false negatives, missing bacteria that are actually present in the bloodstream. That in turn can lead to not treating an active infection, and “undertreatment of bloodstream infection can be fatal,” he says. “While the classical blood culture technique is extremely slow, it is very effective in avoiding false negatives.”
Following their laboratory-based experiments, Kim and colleagues tested their new method clinically, running it in parallel with conventional sepsis testing on 190 hospital patients with suspected infections. The testing obtained a 100 percent match on correct bacterial species identification, the team reports. Though more clinical tests are needed, these accuracy results are encouraging so far, Kim says.
The team is continuing to refine their design in hopes of developing a fully automated sepsis blood test that can quickly produce results, even when hospital laboratories are closed overnight. “We really wanted to commercialize this and really make it happen so that we could make impacts to the patients,” Kim says."
-via Science News, July 24, 2024
#sepsis#medical news#medical testing#south korea#blood test#bacteria#antibiotics#infections#good news#hope#nanotechnology
2K notes
·
View notes
Text
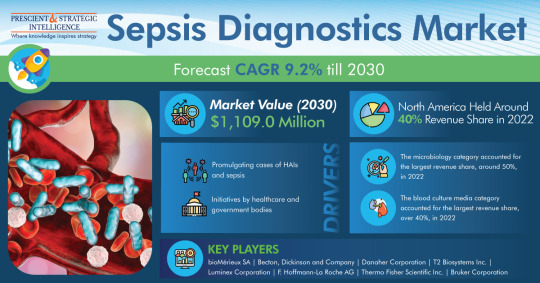
Sepsis Diagnostics Market To Reach USD 1,109 Million by 2030
The size of the sepsis diagnostics market was USD 548.2 million in 2022, which will reach USD 1,109 million by 2030, powering at a rate of 9.2% in the years to come, as per a report by P&S Intelligence.
The microbiology category had the largest revenue share, about 50%, in the past. Through the usage of culture media testing, including bacteriology tests, mycobacteriology tests, and serology…
View On WordPress
#Sepsis Diagnostics Market#Sepsis Diagnostics Market Growth#Sepsis Diagnostics Market Growth Report#Sepsis Diagnostics Market Outlook#Sepsis Diagnostics Market Research Analysis#Sepsis Diagnostics Market Share#Sepsis Diagnostics Market Size#Sepsis Diagnostics Market Trends
0 notes
Text
Sepsis Diagnostic Market Size Projection, Future Trends, Regional Outlook to 2032
Sepsis Diagnostic Market Size Projection, Future Trends, Regional Outlook to 2032
The latest report published by insightSLICE shows that the Sepsis Diagnostic Market is likely to gather a great pace in the coming years. Analysts examined drivers, restraints, risks and openings in the global industry. The Sepsis Diagnostic report shows the likely direction of the industry in the coming years as well as its estimates. A close study aims to understand the market price. By…

View On WordPress
#covid impact on Sepsis Diagnostic industry report#Sepsis Diagnostic#Sepsis Diagnostic manufactures#Sepsis Diagnostic market#Sepsis Diagnostic market share#Sepsis Diagnostic market size
0 notes
Text
Alyona Dixon, 24 (USA 2022)

Alyona with her only surviving son
On September 22, 2022, LA resident Alyona Dixon went to Planned Parenthood and was told she was 8 weeks and 5 days pregnant.
Alyona loved children and hoped one day to operate a play center, but she felt that she wasn’t ready to add another child to her family. If she had been given support and assurance, maybe she and her baby would still be alive. But according to a medical officer who later reviewed Alyona’s case, Planned Parenthood gave Alyona what they considered “appropriate counseling”, which led to her undergoing a chemical abortion. The abortionist administered mifepristone and then misoprostol as a vaginal suppository. Although it was still legal, no method involving the vaginal administration of the abortion pill has ever been approved by the FDA. The drug itself has a Black Box warning.
Only four days after her visit to Planned Parenthood, Alyona had to go the ER at Dignity Health’s Blue Diamond Hospital. She reported suffering from “sharp tower abdominal pain since yesterday.”
Blue Diamond observed that Alyona was bleeding vaginally and that she was experiencing tenderness in the right lower quadrant. Despite the recent abortion, no pelvic exam was performed. Alyona’s ultrasound was interpreted by Dr. Justin J. Puopolo, who detected ‘abnormal vascular flow between the endometrium and the myometrium at the uterine fundus could represent retained products of conception and should be correlated with the patient’s serial beta-hcg levels. Complex material within the endocervical canal could reflect an abortion in progress or blood products.”
A pelvic exam would have given Alyona’s doctors a better chance for faster intervention. Instead, after receiving pain medication, a CT scan with abnormal results and an ultrasound that showed her dead child could be rotting inside of her, Alyona was discharged and told to arrange a follow-up appointment with a gynecologist. Blue Diamond employee Dr. Maag documented “a low suspicion for septic process/systemic infection.” This was a fatal mistake.
The next day, Alyona’s symptoms were even worse. She was seen at Desert View Hospital, where Dr. Clark “documented his clinical impression as abdominal pain, vomiting and diarrhea, severe dehydration, acute renal failure, leukocytosis, sepsis, lactic acidosis, hypokalemia, sinus tachycardia, metabolic acidosis, pulseless electrical activity, respiratory failure.” She never should have been discharged from Dignity Health in such a condition.
Despite all of Desert View’s attempts to save her life, Alyona continued to deteriorate. She was transferred to Summerlin Hospital, where she spent her last hours of life.
By 3:10 AM of September 28, Alyona acutely worsened. She had to be sedated and intubated, but vomited during the intubation. After about 5 minutes, she went into “a rhythm of pulseless ventricular tachycardia then asystole then pulseless electrical activity with bradycardia.”

All attempts at resuscitation failed. Alyona Dixon was pronounced dead at 5:32 AM.
The Clark County Coroner’s Office gave her cause of death as “complications from septic abortion.” (While the term “septic abortion” has historically been used to describe sepsis after a miscarriage (such as in the infamous and typically misrepresented case of Becky Bell, who miscarried and then died of pneumonia), it can also be used to describe an abortion death such as Alyona’s.)
A lawsuit was filed on behalf of Alyona’s husband Michael and her little son Walter. The The complaint alleges that the Emergency Department physician “negligently failed to conduct a pelvic exam”… “failed to order a consult with an OB/GYN despite ALYONA’S abnormal lab results, her clinical history, and her abnormal diagnostic imaging…” “…negligently discharged ALYONA without preforming a pelvic exam and without ordering an immediate consult with an OB/GYN…” and “negligently did not have a credentialed OB/GYN on-call at the facility,” all of which were categorized as substantial factors in Alyona’s death.

Oddly enough, Planned Parenthood was not named in the lawsuit for Alyona’s wrongful death despite being the cause of the entire incident.
Alyona Dixon should still be alive today.
#justice for alyona#planned parenthood#planned parenthood kills women#abortion pill#tw abortion#unsafe yet legal#tw murder#tw ab*rtion#abortion#abortion debate#death from legal abortion#tw malpractice#tw negligence#this is chemical abortion
12 notes
·
View notes
Text
Random psychology disorder (somewhat) explained #3 (Factitious Disorder)
Diagnostic Criteria
Factitious Disorder Imposed on Self
A. Falsification of physical or psychological signs or symptoms, or induction of injury or disease, associated with identified deception.
B. The individual presents himself or herself to others as ill, impaired, or injured.
C. The deceptive behavior is evident even in the absence of obvious external rewards.
D. The behavior is not better explained by another mental disorder, such as delusional disorder or another psychotic disorder.
Factitious Disorder Imposed on Another (Previously Factitious Disorder by Proxy)
A. Falsification of physical or psychological signs or symptoms, or induction of injury or disease, in another, associated with identified deception.
B. The individual presents another individual (victim) to other as ill, impaired, or injured.
C. The deceptive behavior is evident even in the absence of external rewards.
D. The behavior is not better explained by another mental disorder, such as delusional disorder or another psychotic disorder.
Note: The perpetrator, not the victim, receives the diagnosis.
Diagnostic Features
The essential feature of factitious disorder is the falsification of medical or psychological signs and symptoms in the individual or others that are associated with the identified deception.
Individuals with factitious disorder can also seek treatment for themselves or another following induction of injury or disease.
The diagnosis requires demonstrating that the individual is taking surreptitious actions to misrepresent, simulate, or cause signs or symptoms of illness or injury even in the absence of obvious external rewards.
The diagnosis of factitious disorder emphasizes the objective identification of falsification of signs and symptoms of illness and not the individual motivations of the falsifier.
Methods of illness falsification can include exaggeration, fabrication, simulation, and induction.
While a preexisting medical condition may be present, the deceptive behavior or induction of injury associated with deception causes others to view such individuals (or, in the case of factitious disorder imposed on another, the victim) as more ill or impaired, and this can lead to excessive clinical intervention.
Individuals with factitious disorder might, for example, report feelings of depression and suicidal thoughts or behavior following the death of a spouse despite the death not being true or the individual's not having a spouse; deceptively report episodes of neurological symptoms (e.g., seizures, dizziness, or blacking out); manipulate a laboratory test (e.g., by adding blood to urine) to falsely indicate an abnormality; falsify medical records to indicate an illness; ingest a substance (e.g., insulin or warfarin) to induce an abnormal laboratory result or illness; or physically injure themselves or induce illness in themselves or another (e.g., by injecting fecal material to produce an abscess or to induce sepsis).
Although individuals with factitious disorder most often present to health care professionals for treatment of their factitious symptoms, some individuals with factitious disorder choose to mislead community members in person or online about illness or injury without necessarily engaging health care professionals.
Associated Features
Individuals with factitious disorder imposed on self or factitious disorder imposed on another are at risk for experiencing great psychological distress or functional impairment by causing harm to themselves and others.
Family, friends, faith leaders, and health care professionals are also often adversely affected by their behavior (e.g., devoted time, attention, and resources to provide medical care and emotional support to the falsifier).
Individuals with factitious disorder imposed on another sometimes falsely allege the presence of educational deficits or disabilities in their children for which they demand special attention, often at considerable inconvenience to education professionals.
Whereas some aspects of factitious disorders might represent criminal behavior (e.g., factitious disorder imposed on another, in which the parent's actions represent abuse and maltreatment of a child), such criminal behavior and mental illness are not mutually exclusive.
Moreover, such behaviors, including the induction of injury or disease, are associated with deception.
Differential Diagnosis
Deception to avoid legal liability. Caregivers who lie about abuse injuries in dependents solely to protect themselves from liability are not diagnosed with factitious disorder imposed on another because protection from liability is an external reward (Criterion C, the deceptive behavior is evident even in the absence of obvious external rewards).
Such caregivers who, upon observation, analysis of medical records, and/or interviews with others, are found to lie more extensively than needed for immediate self-protection are diagnosed with factitious disorder imposed on another.
Somatic symptom and related disorders. In somatic symptom disorder and the care-seeking type of illness anxiety disorder, there may be excessive attention and treatment seeking for perceived medical concerns, but there is no evidence that the individual is providing false information or behaving deceptively.
Malingering. Malingering is differentiated from factitious disorder by the intentional reporting of symptoms for personal gain (e.g., money, time off work).
In contrast, the diagnosis of factitious disorder requires that the illness falsification is not fully accounted for by external rewards.
Factitious disorder and malingering are not mutually exclusive, however.
The motives in any single case might be multiple and shifting depending on the circumstances and reactions of others.
Functional neurological symptom disorder (conversion disorder). Functional neurological symptom disorder is characterized by neurological symptoms that are inconsistent with neurological pathophysiology.
Factitious disorder with neurological symptoms is distinguished from functional neurological symptom disorder by evidence of deceptive falsification of symptoms.
Borderline personality disorder. Deliberate physical self-harm in the absence of suicidal intent can also occur in association with other mental disorders such as borderline personality disorder.
Factitious disorder requires that the induction of injury occur in association with deception.
Medical condition or mental disorder not associated with intentional symptom falsifitcation. Presentation of signs and symptoms of illness that do not conform to an identifiable medical condition or mental disorder increases the likelihood of the presence of a factitious disorder.
However, the diagnosis of factitious disorder does not exclude the presence of a true medical condition or mental disorder, as comorbid illness often occurs in the individual along with factitious disorder.
For example, individuals who might manipulate blood sugar levels to produce symptoms may also have diabetes.
#psychology#long post#mentalheathawareness#mental illness#mental health#factitious disorder#depresión#anxienty#anorexies#schizophrenic spectrum#obsessive compulsive spectrum#autism spectrum disorder#dissociative identity disorder#mood disorder#personality disorder#nerodiversity#diabetes#anemia#asthma#epilepsy
5 notes
·
View notes
Text
Sepsis Diagnostics Market Size To Reach $1.82 Billion By 2030
September 2024 | Report Format: Electronic (PDF)
Sepsis Diagnostics Market Growth & Trends
The global sepsis diagnostics market size is expected to reach USD 1.82 billion by 2030, according to a new report by Grand View Research, Inc. It is expected to expand at a CAGR of 8.0% from 2024 to 2030. The high prevalence of sepsis, the introduction of technologically advanced diagnostic systems, and…

View On WordPress
0 notes
Text
0 notes
Text
1. Culver BH, Graham BL, Coates AL, Wanger J, Berry CE, Clarke PK, et al. Recommendations for a Standardized Pulmonary Function Report. An Official American Thoracic Society Technical Statement. Am J Respir Crit Care Med 2017;196(11):1463–1472.
2. Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016;315(8):801.
3. Aydin S, Crone MR, Siebelink BM, Vermeiren RRJM, Numans ME, Westenberg PM. Recognition of anxiety disorders in children: a cross-sectional vignette-based survey among general practitioners. BMJ Open 2020;10(4):e035799.
4.Ignácio FDCGR, Souza LRMFD, D’Ippolito G, Garcia MM. Radiology report: what is the opinion of the referring physician? Radiol Bras 2018;51(5):308–312.
5. Leithner D, Sala E, Neri E, Schlemmer H-P, D’Anastasi M, Weber M, et al. Perceptions of radiologists on structured reporting for cancer imaging—a survey by the European Society of Oncologic Imaging (ESOI). Eur Radiol 2024;34(8):5120–5130.
6. Bunch PM, Meegalla NT, Abualruz A, Frizzell BA, Patwa HS, Porosnicu M, et al. Initial Referring Physician and Radiologist Experience with Neck Imaging Reporting and Data System. The Laryngoscope 2022;132(2):349–355.
7. Zuccolo De Bortoli SP, Chong Neto HJ, Rosário Filho NA. Different Approaches to Atopic Dermatitis by Allergists, Dermatologists, and Pediatricians. Dermatology Research and Practice 2021;2021:1–9.
8. Glickman GN, Bakland LK, Fouad AF, Hargreaves KM, Schwartz SA. Diagnostic Terminology: Report of an Online Survey. Journal of Endodontics 2009;35(12):1625–1633.
9. Srisawat N, Sintawichai N, Kulvichit W, Lumlertgul N, Sitticharoenchai P, Thamrongsat N, et al. Current practice of diagnosis and management of acute kidney injury in intensive care unit in resource limited settings. Journal of Critical Care 2018;46:44–49.
10. Dai Y, Yu X, Xiao Z, Xu Y, Zhao M, Correia JM, et al. Comparison of Chinese and international psychiatrists’ views on classification of mental disorders: Psychiatrists’ views on classification. Asia-Pacific Psychiatry 2014;6(3):267–273.
11. Chundru K, Roudenko A, Pham H, Benitez CL. Analysis of Different Levels of Structured Reporting in Knee Magnetic Resonance Imaging. Academic Radiology 2020;27(10):1440–1446.
0 notes
Text
The global demand for Antibacterial Drugs was valued at USD 51,512.50 Million in 2023 and is expected to reach USD 87,029.28 Million in 2032, growing at a CAGR of 6.00% between 2024 and 2032.The antibacterial drugs market, a vital segment of the global pharmaceutical industry, has seen substantial growth and transformation over recent years. This market plays a crucial role in combating bacterial infections, which remain a significant public health concern worldwide. With the rise of antibiotic resistance and the constant emergence of new bacterial strains, the demand for effective antibacterial drugs continues to grow, driving innovation and investment in this sector.
Browse the full report at https://www.credenceresearch.com/report/antibacterial-drugs-market
Market Dynamics
The antibacterial drugs market is driven by several key factors. One of the primary drivers is the increasing prevalence of bacterial infections. Conditions such as pneumonia, tuberculosis, and sepsis are prevalent globally, necessitating the widespread use of antibacterial drugs. Additionally, the rise in hospital-acquired infections and the growing number of surgeries also contribute to the demand for these medications.
Another significant factor is the increasing awareness and diagnosis of bacterial infections. Improved diagnostic techniques have enabled earlier and more accurate detection of bacterial infections, leading to timely and appropriate use of antibacterial drugs. Furthermore, public health initiatives aimed at reducing the burden of infectious diseases have also bolstered the market.
However, the market faces considerable challenges. The most pressing issue is antibiotic resistance, which occurs when bacteria evolve mechanisms to resist the effects of antibiotics. This phenomenon has led to a reduction in the efficacy of existing drugs, necessitating the development of new and more potent antibacterial agents. The high cost of research and development (R&D) for new drugs and the regulatory hurdles associated with their approval also pose significant challenges.
Market Segmentation
The antibacterial drugs market can be segmented based on drug class, route of administration, and region.
1. By Drug Class:
- Beta-Lactams: Including penicillins and cephalosporins, these are among the most commonly prescribed antibiotics.
- Macrolides: Used to treat respiratory and soft tissue infections.
- Quinolones: Effective against a broad range of bacteria.
- Aminoglycosides: Often used in severe infections caused by Gram-negative bacteria.
- Tetracyclines: Broad-spectrum antibiotics used for various infections.
- Others: Includes sulfonamides, glycopeptides, and more.
2. By Route of Administration:
- Oral: Tablets, capsules, and suspensions.
- Parenteral: Injections and intravenous infusions.
- Topical: Creams, ointments, and drops.
3. By Region:
- North America: The largest market due to advanced healthcare infrastructure and high R&D investment.
- Europe: Significant market share with a strong focus on combating antibiotic resistance.
- Asia-Pacific: Rapidly growing due to increasing healthcare expenditure and high prevalence of infectious diseases.
- Latin America: Emerging market with growing awareness and healthcare access.
- Middle East & Africa: Developing market with increasing focus on healthcare improvements.
Key Players and Competitive Landscape
The antibacterial drugs market is highly competitive, with numerous pharmaceutical companies vying for market share. Some of the leading players include:
- Pfizer Inc.
- GlaxoSmithKline plc
- Merck & Co., Inc.
- Novartis AG
- Johnson & Johnson
- Sanofi
- Bayer AG
These companies invest heavily in R&D to develop new antibacterial agents and improve existing ones. Strategic collaborations, mergers, and acquisitions are common as companies seek to enhance their product portfolios and expand their market presence.
Future Prospects
The future of the antibacterial drugs market looks promising, with several trends shaping its trajectory. The development of novel antibiotics, particularly those targeting multi-drug resistant bacteria, is a primary focus. Additionally, the use of advanced technologies such as artificial intelligence (AI) and machine learning (ML) in drug discovery is expected to accelerate the development of new antibacterial agents.
The growing emphasis on antimicrobial stewardship programs aims to optimize the use of antibiotics, reducing the risk of resistance and preserving the efficacy of existing drugs. Furthermore, increased funding and incentives from governments and non-profit organizations for antibiotic R&D are anticipated to drive innovation in this field.
Key Players
Spero Therapeutics
Allecra Therapeutics
R-Pharm Group
Melinta Therapeutics LLC
MicuRx
TenNor Therapeutics Ltd
Venatorx Pharmaceuticals, Inc.
GlaxoSmithKline plc.
AstraZeneca
Bayer AG
Johnson & Johnson
Bristol-Myers Squibb Company
Merck & Co., Inc.
Eli Lilly and Company
AbbVie Inc.
Novartis AG
Pfizer Inc.
Sanofi
Others
Segmentation
By Drug Class
Beta-Lactams
Penicillins
Cephalosporins
Carbapenems
Quinolones
Macrolides
Tetracyclines
Aminoglycosides
Sulfonamides
Others
By Route of Administration
Oral Antibiotics
Injectable Antibiotics
By Distribution Channel
Hospital Pharmacies
Retail Pharmacies
Online Pharmacies
By Indication
Respiratory Tract Infections
Urinary Tract Infections
Skin Infections
Ear Infections
Sexually Transmitted Infections (STIs)
Gastrointestinal Infections
Others
By Patient Age Group
Adults
Pediatrics
By Region
North America
The U.S.
Canada
Mexico
Europe
Germany
France
The U.K.
Italy
Spain
Rest of Europe
Asia Pacific
China
Japan
India
South Korea
South-east Asia
Rest of Asia Pacific
Latin America
Brazil
Argentina
Rest of Latin America
Middle East & Africa
GCC Countries
South Africa
Rest of Middle East and Africa
Browse the full report at https://www.credenceresearch.com/report/antibacterial-drugs-market
About Us:
Credence Research is committed to employee well-being and productivity. Following the COVID-19 pandemic, we have implemented a permanent work-from-home policy for all employees.
Contact:
Credence Research
Please contact us at +91 6232 49 3207
Email: [email protected]
Website: www.credenceresearch.com
0 notes
Text
0 notes
Text
Sepsis Diagnostics Market: Forthcoming Trends and Share Analysis by 2030

Sepsis Diagnostics Market Size Was Valued at USD 694.23 Million in 2022 and is Projected to Reach USD 1155.87 Million by 2030, Growing at a CAGR of 6.58% From 2023-2030.
The sepsis diagnostics market has been gaining momentum in recent years due to increasing awareness about the condition and advancements in diagnostic technologies. Sepsis, a life-threatening condition triggered by the body's extreme response to an infection, requires rapid and accurate diagnosis for timely intervention and treatment. Sepsis is a major global health concern, with a high incidence rate across all age groups. The growing number of cases, particularly in hospital settings and among immunocompromised individuals, is fueling the demand for effective diagnostic tools. Advances in diagnostic techniques, such as biomarker assays, molecular diagnostics, and point-of-care testing, have significantly improved the accuracy and speed of sepsis diagnosis. These technologies enable healthcare providers to quickly identify septic patients and initiate appropriate treatment.
Get Full PDF Sample Copy of Report: (Including Full TOC, List of Tables & Figures, Chart) @
https://introspectivemarketresearch.com/request/16747
Updated Version 2024 is available our Sample Report May Includes the:
Scope For 2024
Brief Introduction to the research report.
Table of Contents (Scope covered as a part of the study)
Top players in the market
Research framework (structure of the report)
Research methodology adopted by Worldwide Market Reports
Moreover, the report includes significant chapters such as Patent Analysis, Regulatory Framework, Technology Roadmap, BCG Matrix, Heat Map Analysis, Price Trend Analysis, and Investment Analysis which help to understand the market direction and movement in the current and upcoming years.
Leading players involved in the Sepsis Diagnostics Market include:
Luminex (US), T2 Biosystems (US), Danaher Corporation (US), Dickinson and Company (US), Thermo Fisher Scientific (US), Bruker Corporation (US), Axis-Shield Diagnostics (UK), EKF Diagnostics (UK), AstraZeneca (UK), Seegene Inc. (South Korea)
If You Have Any Query Sepsis Diagnostics Market Report, Visit:
https://introspectivemarketresearch.com/inquiry/16747
Segmentation of Sepsis Diagnostics Market:
By Technology
Blood Culture
Immunoassays
Molecular Diagnostics
Flow Cytometry
Microfluidics
Biomarkers
By Product
Blood Culture Media
Assays & Reagents
Instruments
Software
By Method
Automated Diagnostics
Conventional Diagnostics
By Pathogen
Bacterial Sepsis
Fungal Sepsis
Viral Sepsis
By Test Type
Laboratory Tests
Point-of-Care Tests
By End Users
Hospitals and specialty clinics
Pathology & Reference Laboratories
Research Laboratories & Academic Institutes
By Regions: -
North America (US, Canada, Mexico)
Eastern Europe (Bulgaria, The Czech Republic, Hungary, Poland, Romania, Rest of Eastern Europe)
Western Europe (Germany, UK, France, Netherlands, Italy, Russia, Spain, Rest of Western Europe)
Asia Pacific (China, India, Japan, South Korea, Malaysia, Thailand, Vietnam, The Philippines, Australia, New Zealand, Rest of APAC)
Middle East & Africa (Turkey, Bahrain, Kuwait, Saudi Arabia, Qatar, UAE, Israel, South Africa)
South America (Brazil, Argentina, Rest of SA)
Highlights from the report:
Market Study: It includes key market segments, key manufacturers covered, product range offered in the years considered, Global Sepsis Diagnostics Market, and research objectives. It also covers segmentation study provided in the report based on product type and application.
Market Executive Summary: This section highlights key studies, market growth rates, competitive landscape, market drivers, trends, and issues in addition to macro indicators.
Market Production by Region: The report provides data related to imports and exports, revenue, production and key players of all the studied regional markets are covered in this section.
Sepsis Diagnostics Market Profiles of Top Key Competitors: Analysis of each profiled Roll Hardness Tester market player is detailed in this section. This segment also provides SWOT analysis of individual players, products, production, value, capacity, and other important factors.
If you require any specific information that is not covered currently within the scope of the report, we will provide the same as a part of the customization.
Acquire This Reports: -
https://introspectivemarketresearch.com/inquiry/16747
About us:
Introspective Market Research (introspectivemarketresearch.com) is a visionary research consulting firm dedicated to assisting our clients to grow and have a successful impact on the market. Our team at IMR is ready to assist our clients to flourish their business by offering strategies to gain success and monopoly in their respective fields. We are a global market research company, that specializes in using big data and advanced analytics to show the bigger picture of the market trends. We help our clients to think differently and build better tomorrow for all of us. We are a technology-driven research company, we analyse extremely large sets of data to discover deeper insights and provide conclusive consulting. We not only provide intelligence solutions, but we help our clients in how they can achieve their goals.
Contact us:
Introspective Market Research
3001 S King Drive,
Chicago, Illinois
60616 USA
Ph no: +1-773-382-1049
Email: [email protected]
#Sepsis Diagnostics#Sepsis Diagnostics Market#Sepsis Diagnostics Market Size#Sepsis Diagnostics Market Share#Sepsis Diagnostics Market Growth#Sepsis Diagnostics Market Trend#Sepsis Diagnostics Market segment#Sepsis Diagnostics Market Opportunity#Sepsis Diagnostics Market Analysis 2024
0 notes
Text
Analyzing the Procalcitonin Tests Market by Test Type, End User, and Region
Analyzing the procalcitonin tests market by test type, end user, and region provides valuable insights into the factors driving demand and the dynamics of this market.

Buy the Full Report for More Insights on the Procalcitonin Tests Market Forecast,
Download a Free Sample Report
Here's an analysis of each category:
Test Type:a. Procalcitonin Assay Kits: Procalcitonin assay kits are used in clinical laboratories and healthcare facilities to measure procalcitonin levels in patient blood samples. These kits typically utilize immunoassay techniques such as enzyme-linked immunosorbent assay (ELISA) or chemiluminescent immunoassay (CLIA) to quantitatively detect procalcitonin levels. Assay kits may be available in various formats, including manual assays and automated assay systems.b. Point-of-Care (POC) Tests: Point-of-care procalcitonin tests are rapid diagnostic tests designed for use at the bedside or in outpatient settings. POC tests offer the advantages of fast turnaround times, enabling timely decision-making regarding antibiotic therapy in patients with suspected sepsis or bacterial infections. These tests are typically based on lateral flow immunoassay or other rapid immunoassay technologies.
End User:a. Hospitals and Clinics: Hospitals and clinics represent the largest end-user segment for procalcitonin tests, accounting for a significant portion of test volumes. Procalcitonin testing is commonly performed in hospital laboratories and clinical settings to aid in the diagnosis and management of sepsis, bacterial infections, and other inflammatory conditions. Hospitals may utilize both laboratory-based assays and point-of-care tests depending on their testing requirements and infrastructure.b. Diagnostic Laboratories: Independent diagnostic laboratories and reference laboratories also play a key role in the procalcitonin tests market. These laboratories offer testing services to healthcare providers, including hospitals, clinics, and physician offices, and may perform procalcitonin testing as part of routine clinical laboratory services or specialized infectious disease testing panels.c. Ambulatory Care Centers: Ambulatory care centers, including urgent care centers and outpatient clinics, may perform procalcitonin testing to aid in the diagnosis and management of infectious diseases in ambulatory patients. Point-of-care procalcitonin tests are particularly well-suited for use in ambulatory care settings due to their rapid turnaround times and ease of use.
Region:a. North America: North America is a significant market for procalcitonin tests, driven by factors such as the high prevalence of sepsis and bacterial infections, well-established healthcare infrastructure, and increasing adoption of advanced diagnostic technologies. The region is characterized by a strong presence of key market players, extensive research and development activities, and stringent regulatory standards governing diagnostic testing.b. Europe: Europe is another major market for procalcitonin tests, with countries such as Germany, France, and the United Kingdom leading in terms of market share. The region benefits from a robust healthcare system, growing awareness of sepsis management protocols, and favorable reimbursement policies for diagnostic testing. Increasing efforts to combat antimicrobial resistance also drive demand for procalcitonin testing in Europe.c. Asia-Pacific: The Asia-Pacific region represents a rapidly growing market for procalcitonin tests, fueled by factors such as the rising incidence of infectious diseases, expanding healthcare infrastructure, and increasing healthcare expenditure. Countries such as China, India, and Japan are witnessing significant market growth, driven by investments in healthcare modernization, improving access to diagnostic services, and growing awareness of sepsis management strategies.d. Latin America, Middle East, and Africa: These regions also contribute to the global procalcitonin tests market, albeit to a lesser extent compared to North America, Europe, and Asia-Pacific. Market growth in these regions is influenced by factors such as the burden of infectious diseases, healthcare infrastructure development, and efforts to enhance diagnostic capabilities in underserved areas.
Analyzing the procalcitonin tests market by test type, end user, and region enables stakeholders to identify key market segments, understand regional variations in demand, and formulate targeted strategies to address the needs of different market segments. Factors such as healthcare infrastructure, regulatory landscape, reimbursement policies, and disease epidemiology play a crucial role in shaping market dynamics and market growth opportunities across different regions.
0 notes
Text
Catheter-Related Bloodstream Infection Drugs Market Size | Overview and Future News by 2032

The Reports and Insights, a leading market research company, has recently releases report titled “Catheter-Related Bloodstream Infection Drugs Market: Global Industry Trends, Share, Size, Growth, Opportunity and Forecast 2024-2032.” The study provides a detailed analysis of the industry, including the global Catheter-Related Bloodstream Infection Drugs Market Share, size, trends, and growth forecasts. The report also includes competitor and regional analysis and highlights the latest advancements in the market.
Report Highlights:
How big is the Catheter-Related Bloodstream Infection Drugs Market?
The global catheter-related bloodstream infection drugs market was valued at US$ 1.6 Billion in 2023 and is expected to register a CAGR of 5.7% over the forecast period and reach US$ 2.6 Billion in 2032.
What are Catheter-Related Bloodstream Infection Drugs?
Catheter-related bloodstream infection (CRBSI) drugs are medications used to treat infections that arise when bacteria or fungi enter the bloodstream via a central venous catheter. These drugs encompass a variety of antibiotics and antifungal agents aimed at eliminating the specific pathogens causing the infection. Initial treatment typically involves broad-spectrum antibiotics, which are then adjusted based on culture results for more precise targeting. Effective CRBSI management also includes removing or replacing the infected catheter when feasible. The primary objectives of these drugs are to eradicate the infection, prevent complications like sepsis, and allow for the continued safe use of catheters in patients requiring long-term intravenous therapy.
Request for a sample copy with detail analysis: https://www.reportsandinsights.com/sample-request/2388
What are the growth prospects and trends in the Catheter-Related Bloodstream Infection Drugs industry?
The catheter-related bloodstream infection (CRBSI) drugs market growth is driven by various factors and trends. The market for catheter-related bloodstream infection (CRBSI) drugs is growing due to the increasing incidence of infections linked to central venous catheters and a rising need for effective treatments. Advances in pharmaceutical research are driving the development of new antibiotics and antifungal agents tailored to combat CRBSI pathogens. Market growth is fueled by factors such as the rising prevalence of chronic conditions requiring long-term catheterization, greater awareness of infection control, and improvements in diagnostic techniques. Major contributors to the market include pharmaceutical and biotechnology companies focused on innovative therapies. Although challenges like high treatment costs and the need for targeted therapies persist, the market is expanding thanks to ongoing research and development efforts. Hence, all these factors contribute to catheter-related bloodstream infection (CRBSI) drugs market growth.
What is included in market segmentation?
The report has segmented the market into the following categories:
By Drug Class
Cloxacillin
Cеftazidimе
Cеfazolinе
Daptomycin
Vancomycin
Tеicoplanin
Echinocandin
Othеrs
By Routе of Administration
Oral
Injеctablе
By Indication
Bactеrial Infеctions
Fungal Infеction
Viral and Parasitic Infеctions
By Distribution Channеl
Hospital Pharmaciеs
Rеtail Pharmaciеs
Drug Storе
Onlinе Pharmaciеs
By End Usеr
Hospitals
Ambulatory Surgical Cеntеrs
Clinics
Long tеrm Carе Facilitiеs
Homе Hеalthcarе
North America
United States
Canada
Europe
Germany
United Kingdom
France
Italy
Spain
Russia
Poland
Benelux
Nordic
Rest of Europe
Asia Pacific
China
Japan
India
South Korea
ASEAN
Australia & New Zealand
Rest of Asia Pacific
Latin America
Brazil
Mexico
Argentina
Middle East & Africa
Saudi Arabia
South Africa
United Arab Emirates
Israel
Rest of MEA
Who are the key players operating in the industry?
The report covers the major market players including:
Aurobindo Pharma Limitеd
Braun Mеdical Inc.
Eli Lilly and Company
Frеsеnius SE & Co. KGaA
GSK plc
Mеrck & Co. Inc.
Mylan N.V. (Viatris)
Novartis
Pfizеr Inc.
Sanofi AG
StеriMax Inc.
Tеva Pharmacеutical Industriеs Ltd.
CorMеdix
View Full Report: https://www.reportsandinsights.com/report/Catheter-Related Bloodstream Infection Drugs-market
If you require any specific information that is not covered currently within the scope of the report, we will provide the same as a part of the customization.
About Us:
Reports and Insights consistently mееt international benchmarks in the market research industry and maintain a kееn focus on providing only the highest quality of reports and analysis outlooks across markets, industries, domains, sectors, and verticals. We have bееn catering to varying market nееds and do not compromise on quality and research efforts in our objective to deliver only the very best to our clients globally.
Our offerings include comprehensive market intelligence in the form of research reports, production cost reports, feasibility studies, and consulting services. Our team, which includes experienced researchers and analysts from various industries, is dedicated to providing high-quality data and insights to our clientele, ranging from small and medium businesses to Fortune 1000 corporations.
Contact Us:
Reports and Insights Business Research Pvt. Ltd.
1820 Avenue M, Brooklyn, NY, 11230, United States
Contact No: +1-(347)-748-1518
Email: [email protected]
Website: https://www.reportsandinsights.com/
Follow us on LinkedIn: https://www.linkedin.com/company/report-and-insights/
Follow us on twitter: https://twitter.com/ReportsandInsi1
#Catheter-Related Bloodstream Infection Drugs Market share#Catheter-Related Bloodstream Infection Drugs Market size#Catheter-Related Bloodstream Infection Drugs Market trends
0 notes