Don't wanna be here? Send us removal request.
Text
Ineffective task monitoring
Once a task has been assigned, ignoring it until it is done can lead to unexpected results. What’s in your head is not what’s in the employee’s head.

#qms#QMS documentation#eqms#qmsWrapper#qms planning#monitoring#comic#compliance#documentation#cup of joe#cupofjoe#ISO#risk#risk management#Iso 9001#ISO9001#ISO 13485#iso 13485 documents#ISO 13485:2016#management#requirements#project management#medical manufacturers#medical#task#task management#standard#quality management system#Systems Integration#artists on tumblr
1 note
·
View note
Text
Vague wording
Vague wording used in requirement documents
Whether you are freshly minted into the QMS position or you are a founder of a Startup or a product manager with a new project… your strategy requires QMS oversight.
QMS Joe is here to guide you through the plethora of information and support you with handy tips & tricks to streamline your way to compliance.
Use precise requirements as much as possible
If the requirement is not clear enough the employees or the sub-contractor could misunderstand or make incorrect decisions related to the given task that might end in an unplanned surprise, and could easily lead to an unsatisfied customer.
Customer satisfaction can be improved by using precise requirements as much as possible in QMS.

#QMS#eqms#QMS documentation#qmsWrapper#qms planning#standard#software#cloud based software#requirements#quality management system#systems integration#quality#quality manual#quality requirements#ISO#risk#risk management#Iso 9001#iso 13485 documents#ISO 13485#ISO 13485:2016#customer#customer experience#customerreview#customer service#customer satisfaction#planning#resource#tips#tricks
0 notes
Text
Identifying potential hazards
Identifying potential hazards at the beginning of project planning
Assemble a cross-functional team, e.g., design engineer, product development engineer, member of manufacturing team, someone from packaging, someone from marketing, medical consultant, medical writers and quality engineers, to brainstorm potential hazard scenarios. The increased number of perspectives will increase your chances of uncovering more hazard.
It is easier and cheaper to fix risks at the beginning of planning, rather than during production or worse, once the product is in use.

#QMS#eqms#QMS documentation#qmsWrapper#project management#processes#project#iso 13485 documents#documentation#ISO#Iso 9001#ISO9001#ISO 13485#Iso 14971#fda#fda 510k#risk#risk management#management#quality#quality management system#quality manual#medical manufacturers#medical#audit#project planning#infographic#software#compliance#mtq
0 notes
Text
Incorrect Resource Management
Companies are often failing when it comes to properly organize their resources, whether it is material, equipment, people, knowledge, time, financial, etc. Resource management means not just monitoring and following the amount of existing resources, but also balancing between the planned activities and the changing resource requirements. Good resource management is vital to project success, and yet one of the most difficult things to achieve, maintain, and control.

In developing resource plans, there is little chance that the project manager will have all of the necessary resources assigned to the project at its start. Changing resource requirements and needs have to be properly monitored, preferably foreseen and implemented in QMS processes. Since projects are unique resource management and planning should continue throughout the project life cycle.
#eqms#QMS#QMS documentation#qmsWrapper#iso 13485 documents#documentation#project management#processes#project#fda 510k#fda#cup of joe#cupofjoe#comics#comic#compliance#team collaboration#team#quality#quality management system#quality manual#management#medical manufacturers#medical#risk
1 note
·
View note
Text
Quality Planning - Learning to Think Ahead
To turn your ideas into success you must cultivate the skill of looking one move ahead…whether you are good in playing chess or not. Every project should have a quality plan. In reality, very few do.
Why is quality planning important?
Not planning the work means you are constantly retracing your steps instead of moving from one area to the next, you waste time trying to remember what to do and trying to make decisions on the fly. Without a strong quality planning, a project carries an increased risk that you won't be satisfied with the results. Things go much more smoothly if you take some time to consider what you need to do and plan the path for doing it.
Is producing a quality plan a complex job?
Producing a quality plan is not complex. The development of a Project Quality Plan should be a team process, that depends as much on communicating information as it does on planning. Once everyone understands the ultimate reason for what they are doing, people are on the same page and working toward the same goals.
The Quality plan is not just a specific and detailed listing of all quality requirements and standards, use Quality Planning to develop awareness of potential quality issues. Based on this awareness it’s much easier to prepare plans and actions to counter any weaknesses or deficiencies in the project execution…thus ensuring that all quality standards are met effectively.
What are the main elements of a good Quality Plan?
A good Project Quality Plan is defined as a set of activities planned at the beginning of the project that helps achieve Quality in the project being executed.
It includes main points like:
The project’s activities and the relations between them
Development strategy
Milestones clearly identified and dated
Concrete and measurable criteria for project activities and milestones completion Project schedule
Responsible for each activity
Resources for each project activity – human, equipment, material, financial
Quality plan should be regularly updated as project activities proceed
Quality plan should be available for relevant persons
Quality plan and every update should be approved and re-approved
Learning to Think Ahead
Having uncovered the quality issues, be sure you have a mechanism in place to fix the problems. There must be some follow up process to allocate fixes to particular people and ensure they actually make the changes. This implies that time must be built into the schedule for rework following quality events.
There is an overhead in undertaking quality checks, but this is offset by not having to fix things further down the line. Inevitably, the later you find a problem, the longer it takes to fix.
A plan puts the structure in place that allows the Quality Management efforts to gain traction and produce real results.
Now consider that the effectiveness of a QMS software impacts how well the whole organization meets customer expectations. qmsWrapper offers a full circle of QMS, Quality managers with little experience or knowledge of the supported Standards, can easily step into the role and effectively start. That’s the true benefit of a quality management system.

#qms#eqms#QMS documentation#qmsWrapper#qms planning#planning#fda#fda 510k#Iso 9001#ISO9001#ISO 13485#ISO#cup of joe#cupofjoe#iso 13485 documents#compliance#comic#artists on tumblr#art#software#cloud based software#checklist#project management#processes#project#programming#progress#monitoring#quality manual#management
0 notes
Text
Monitor who does what with what efficiency
Productivity is all about efficiency -- doing more, faster and with less. A good quality management software can help you to get the most out of your day.
qmsWrapper gives a real-time visual overview of all user tasks - percentage done, reports and assignments. Monitor who does what with what efficiency. Instantly identify any overlaps in the work and easily reassign tasks as needed, follow the over-all productivity and keep track of milestones, QMS issues, deadlines, upcoming events, to-dos and due dates at a glance.

There are multiple views to give you different perspectives on the projects. View work by week either through a Gantt view or Roadmap, view the status of each member on task completion charts, or on the team dashboard. In Gantt view you have the opportunity to see all tasks in a 6-month period, whether in the future or in the past, so you can do an analytic overview of completed tasks or plan for the next period.
With qmsWrapper you can coordinate valuable team resources across the company, make longer period plans and anticipate potential bottlenecks way in advance. Overlapping tasks can be seen, so you can act before the problem of overtasking occurs and avoid project delays.
With qmsWrapper you can maintain Strategic Focus and agile response to the goals and objectives to be achieved.
#cup of joe#project management#team collaboration#qms#QMS documentation#qmsWrapper#eQMS#joe#iso#ISO 13485#Iso 14971#iso 13485 documents#ISO 13485:2016#Quality management system#quality manual#Medical manufacturers#management#iso9001#iso 9001#risk#risk management#strategic focus#medical#audit#fda 510k#FDA#project#approved#risk assessment#capa
0 notes
Text
Documenting Compliance
Whether you are freshly minted into the QMS position or you are a founder of a Startup, or a product manager with a new project… your strategy requires QMS oversight.
qmsJoe is here to guide you through the plethora of information and support you with handy tips & tricks to streamline your way to compliance.
Simply having a signed document is no longer the standard of proof
For the FDA, the law is clear in 21 CFR 820.100 which states that “all activities required … and their results, shall be documented”. This is not a “should be” but a “shall be” requirement. FYI, it’s not optional. It is not enough to complete a signed QMS form saying the action was completed, any lack of documentation of QSR related activities or remedial actions, means that they never happened, signed form or not.
There are many examples where the FDA looked past the form to the work that should have been the end result of the produced work. If the tasks or work that should have resulted in the completion of the form is lacking, then FDA can simply conclude that there is inadequate proof as the activities and their results were not documents. It does not mean you didn’t perform the QSR activities themselves, it means you didn’t document them, and as such to the FDA it is like these activities never happened.

In qmsWrapper any and all tasks, issues, documents can be tagged as a QMS event, that gets automatically reported to the QMS system, where not just project managers but any QMS manager can oversee the progress; CAPA can be followed back to its origin. Resolution with a signed document, electronic or otherwise, now has a date stamped electronic trail, that, if required, can be printed.
Management through quality is smart management that effectively avoids adding another layer of management by encouraging team based collaboration compliance.
#ISO 13485#iso 13485 documents#ISO 13485:2016#Iso 14971#fda 510k#FDA#CAPA#eQMS#QMS#quality#quality manual#Quality management system#cup of joe#cupofjoe#Medical manufacturers#compliance#audit#qmsWrapper#QMS documentation#comic#document#document management#management#risk#risk assessment#approved files#procedures#small companies#startups#developers & startups
0 notes
Text
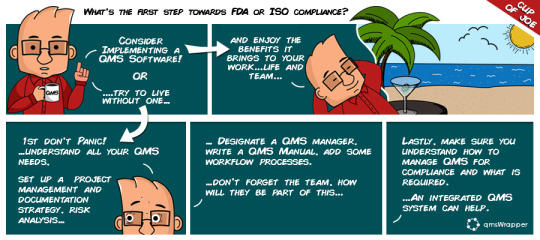
What's the first step towards FDA or ISO compliance ?
Whether you are freshly minted into the QMS position or you are a founder of a Startup, or a product manager with a new project… your strategy requires QMS oversight and you have several questions like “where to start” and “what to do”.
There is a lot of information on the Web but it is not all very helpful. The danger resides in fluff pieces, written for promotional purposes.
qmsJoe is here to guide you through the plethora of information and support you with handy tips & tricks to streamline your way to compliance.
#qms#qms documentation#iso 13485:2016#iso 13485#iso 13485 documents#cup of joe#cupofjoe#software#cloud based software#qmswrapper#eqms#capa#fda 510k#iso 14971#processes#quality#quality manual
2 notes
·
View notes