🧪 26. She/her. Bi. 🌈Mentally ill medical lab science student with an affinity for Chappell Roan, pottery, animals, and nature. Feminist af. 🍉 Free Palestine 🍉Eat the rich.
Don't wanna be here? Send us removal request.
Text








@tolerateit EDITING PROMPT OF THE MONTH: Pride
Favorite LGBTQ Artists: Chappell Roan
6K notes
·
View notes
Text

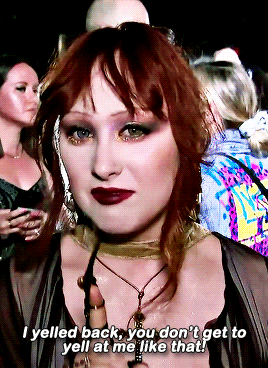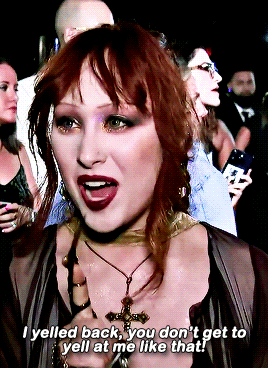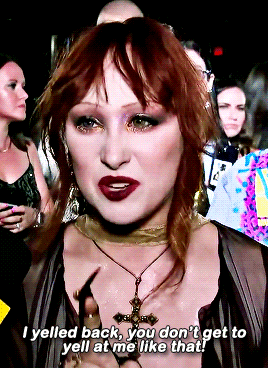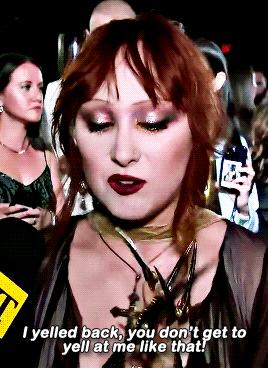







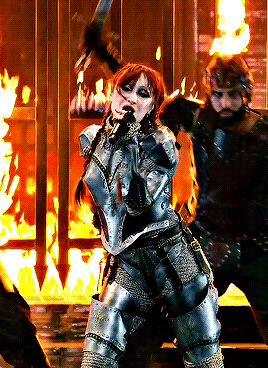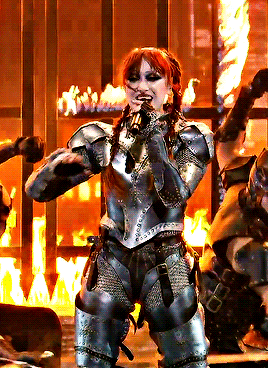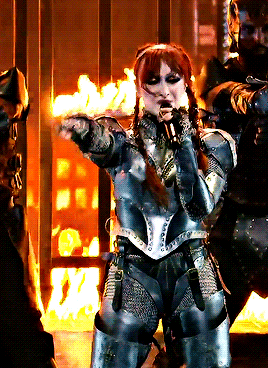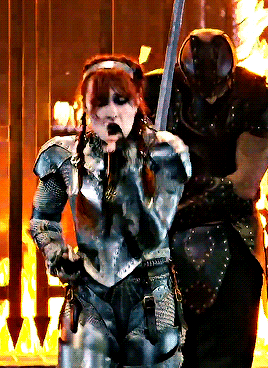
CHAPPELL ROAN Video Music Awards 2024
19K notes
·
View notes
Text

October 16th & 17th, 2023
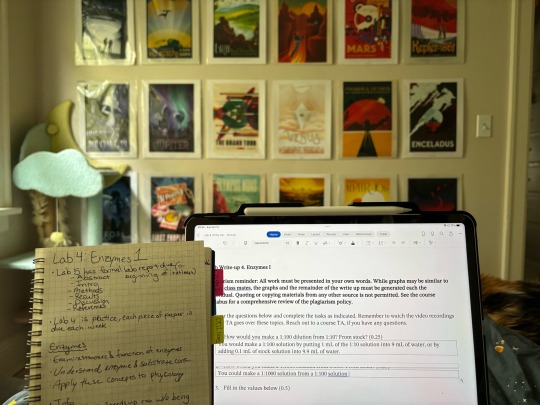
The last week has been rough with pcos symptoms, and the pain has been making it difficult to study. Spent one day studying in bed but dragged myself to class and the library yesterday to finish up more class work. Feeling like I've taken on too much but hoping I just need to readjust to the full schedule. <3
Hope everyone is having a great beginning to the school year!
#studyblr#study motivation#study aesthetic#chemistry#science#studying#studyspo#fraisespersonal#biology#pchem
42 notes
·
View notes
Text
what if i stopped stressin and trusted everything will be okay and felt deep peace inside
13K notes
·
View notes
Text


October 5, 2023
Been fueled by only coffee this week, bio notes are extensive and made a 130,000 cell excel sheet for my numerical data analysis class to show the absorptions at different rotational states of carbon monoxide 👌🏻 Feeling those 400 level chemistry vibes 🧪
#studyblr#study motivation#study aesthetic#chemistry#science#studying#ochem#studyspo#fraisespersonal#biology#data analysis#excel#excel spreadsheet
25 notes
·
View notes
Text


October 2, 2023
Been working lots and lots on keeping up with my intro to bio class, very very grateful I took ochem before this. 🧪🧫
#studyblr#study motivation#study aesthetic#chemistry#science#studying#ochem#studyspo#fraisespersonal#biology
16 notes
·
View notes
Text


july 19th 2023
well, hello there! today the plan is:
• grammar 5 & 6
• continue studying some vocab & start using vocabulary in use: advanced
• write! my! to-do lists! on my planner! (really need to use my planner)
I’m really lucky to have the free time to self-study for my cae exam so I’m really trying to do my best. also, i had to add a picture of my cat bc as you may know she’s my study partner hehe <3
806 notes
·
View notes
Text
When sodium hypochlorite (bleach) solution is added to luminol, a chemical reaction occurs that releases energy in the form of light. This is called chemiluminescence. The bleach solution acts as an oxidizing agent, which means it takes electrons away from the luminol molecule. This causes the luminol molecule to become excited, and it releases the energy as light.
🎥 Courtesy: Kendra Frederick
The luminol molecule is made up of two amino groups, a carbonyl group, and an azo group. The amino groups are electron-rich, while the carbonyl group is electron-poor. The azo group is a conjugated system, which means that the electrons in the double bonds can move freely from one atom to another.
When sodium hypochlorite (bleach) solution is added to luminol, the bleach molecules react with the amino groups of the luminol molecule. This reaction takes electrons away from the luminol molecule, which causes the luminol molecule to become oxidized. The oxidized luminol molecule is in an excited state, which means that it has more energy than it normally does.
The excited luminol molecule then releases the extra energy as light. This light is called chemiluminescence. The light emitted by the chemiluminescence reaction is blue because the luminol molecule has a blue fluorescence.
The chemiluminescence reaction between luminol and sodium hypochlorite is catalyzed by the presence of a metal ion, such as iron or copper. The metal ion helps to stabilize the excited state of the luminol molecule, which makes it more likely to release the extra energy as light.
The chemiluminescence reaction is very sensitive to impurities, so it is important to use pure chemicals. The reaction can also be affected by the pH of the solution. The optimal pH for the reaction is around 9.
The chemiluminescence reaction between luminol and sodium hypochlorite can be used to detect blood, as the iron in hemoglobin can catalyze the reaction. The reaction is also used in some commercial products, such as glow sticks and emergency lights.
I hope you enjoyed learning about this. ❤️🙏
5K notes
·
View notes
Text


The key is to go to this dude early so you can get around.
2K notes
·
View notes

















