#Amine Amino
Text
CAS NO.100-10-7 4-Dimethylaminobenzaldehyde Manufacturer/High quality/Best price/In stock /DA 90 DAYS
Quick Details
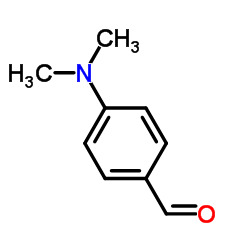
Product name:4-Dimethylaminobenzaldehyde
CAS: 100-10-7
Molecular formula:C9H11NO
Molecular weight:149.19
EINECS No.:202-819-0
Appearance:White to off white crystalline powder
Other names:HYDRAZINE METER SOLUTION;
DIMETHYLAMINOBENZALDEHYDE;
4-Dimethylaminobenzaldehyde;
p-Dimethylaminobenzaldehyde;
P-(Dimethylamino)benzaldehyde;
4-dimethyl amino benzaldehyde;
4-amino-2,3-dimethylbenzaldehyde;
N,N-dimethyl-4-amino benzaldehyde;
4-(N,N-Dimethyl)aminobenzaldehyde;
P-dimethylaminobenzaldehyde test solution(ChP);
4-(Dimethylamino)benzaldehyde test solution(ChP)
Density: 1.10g/mLat 20°C
Melting point: 72-75°C (lit.)
Boiling point: 176-177 °C (17 mmHg)
Flash point: 164°C
Water solubility: 0.3 g/L (20 ºC)
Solubility: alcohol: passes test (APHA ≤60)
Refractive index: n20/D 1.417
Acidity coefficient: pK1:1.647(+1) (25°C)
Port: any port in china
Packing: according to the clients requirement
Storage: Store in dry, dark and ventilated place.
Transportation: by sea or by air
payment methods: L/C, T/T, D/A, D/P, O/A, paypal, western union etc.accept all payment.
Application
1. Dye intermediates and analytical reagents. The product is used for the determination of indole, skatole, urea, tryptophan and ergot alkaloids, etc., and is also used to distinguish serum eruption and scarlet fever.
2. In terms of dyes, it can be used to synthesize pressure-sensitive dyes. Also used in the production of cationic brilliant red G (C.I.BasicRed52). It can also be used as a dye intermediate; as a reagent for the determination of urobilin, indole, alkaloids, etc., and as a chromatographic analysis reagent.
Superiority
1.supply sample
2.the packing can be according the customers` requirment
3.any inquiries will be replied within 24 hours
4.we provide commerical invoice, packing list, bill of loading, coa , health certificate and origin certificate. if your markets have any special requirements, let us know.
5.factory price.
6.prompt delivery. we have good cooperation with many professional forwarders, we can send the products to you once you confirm the order.
7.we can accept various payment methods, l/c, t/t, d/a, d/p, o/a, paypal, western union etc., and we cooperate with sinosure many years.
Anyway ,if you need any chemicals from China ,MIT -IVY INDUSTRY CO.,LTD can help you.
Company Information
MIT-IVY INDUSTRY CO.,LTD is a manufacturer and exporter of fine chemical dyes & pharmaceutical intermediates in China.
Mainly produce aniline series products ,chlorine series products,and epoxy curing agent
We are a company full of vitality. The company has a group of energetic, well-trained employees and strong technical research and development capabilities. We specialize in the production, development and sales of API intermediates, fine chemicals and plant extracts. Relying on advanced equipment and strict management, adhere to the business philosophy of "openness, tolerance, innovation, and sharing" to create a win-win cooperationplatform.Everything comes from innovation, it is our philosophy !
If you are interested in getting more quotations,
please add WHATSAPP:0086-13805212761 or E-MAIL:[email protected]
FAQ
Q1:Will you supply samples for testing?
A: For most of our products, samples are available, but please cover the shipping cost.
Q2:What's your MOQ?
A: For the high value product, our MOQ starts from 10g,100g and 1kg.
Q3:Which kind of payment terms do you accept?
A: Proforma invoice enclosed with our bank information will be sent after confirmation of order.
payment methods: L/C, T/T, D/A, D/P, O/A, paypal, western union etc.accept all payment.
Q4:How about your delivery time?
A: Generally, it will take 3 to 5 days after receiving your advance payment.
Q5:How do you treat quality complaint?
A:First of all, our quality control will reduce the quality problem near to zero.
If there is a quality problem caused by us, we will send you free goods for replacement or refund your loss.
Main products
Mit-Ivy is a well-known fine chemicals and pharmaceutical intermediates
manufacturer with strong R&D support in China.
Mainly involved Aniline, Chlorine products.
Payment:DA 60 DAYS
TEL:008619961957599 E-MAIL:[email protected]
产品
Product
CAS
N-甲基间甲苯胺
N-Methyl-M-Methylaniline
696-44-6
N-羟乙基苯胺
N-(2-hydroxyethyl)-Aniline
122-98-5
N-乙基对甲苯胺
N-ethyl-p-toluidine
622-57-1
N,N-二甲基邻甲苯胺
N,N-Dimethyl-o-toluidine
609-72-3
N-甲基邻甲苯胺
N-Methyl-o-methylaniline
611-21-2
N,N-二乙基对甲苯胺
N,N-Diethyl-p-toluidine
613-48-9
N,N-二乙基间甲苯胺
N,N-diethyl-m-toluidine
91-67-8
N-氰乙基-N-羟乙基间甲苯胺
N-cyanoethyl-n-hydroxyethyl-m-toluidine
119-95-9
N-乙基间甲苯胺
N-ethyl-m-toluidine
102-27-2
N-氰乙基-N-羟乙基苯胺
N-cyanoethyl-n-hydroxyethyl aniline
92-64-8
N-乙基邻甲苯胺
N-ethyl-o-toluidine
94-68-8
N,N-二羟乙基对甲苯胺
N,N-dihydroxyethyl-p-toluidine
.3077-12-1
N,N-二乙基苯胺
N,N-diethyl aniline
91-66-7
N-丁基-N-羟乙基苯胺
N-butyl-n-hydroxy aniline
3046-94-4
N-乙基-N-氰乙基间甲苯胺
N-ethyl-n-cyanoethyl-m-toluidine
148-69-6
N-丁基-N-氰乙基苯胺
N-butyl-n-cyano aniline
61852-40-2
N-甲基- N-羟乙基苯胺
N-methyl-n-hydroxyetjyl aniline
93-90-3
N,N-二丁基苯胺
N,N-dibutyl aniline
613-29-6
N-乙基-N-氰乙基苯胺
N-ethyl-n-cyanoethyl aniline
148-87-8
N-正丁基苯胺
N-Phenyl-N-butyl aniline
1126-78-9
N-乙基-N-羟乙基苯胺
N-ethyl-n-hydroxyethyl aniline
92-50-2
N-乙基-N-苄基间甲苯胺
N-ethyl-n-benzyl-m-toluidine
119-94-8
N-甲基-N-苄基苯胺
N-methyl-n-benzyl aniline
614-30-2
N-异丙基苯胺
N-isopropy aniline
768-52-5
N-乙基-N-苄基苯胺
N-ethyl-n-benzyl aniline
92-59-1
N-环已基苯胺
N-Cyclohexylaniline
1821-36-9
N,N-二甲基间甲苯胺
N,N,3-trimethyl- Dimethyl-m-toluidine
121-72-2
N-甲基甲酰苯胺
N-Methylformanilide
93-61-8
N-甲基-N-羟乙基对甲苯胺
N-(2-HYDROXYETHYL)-N-METHYL-4-TOLUIDINE
2842-44-6
N,N-二甲基对甲苯胺
N,N,4-trimethyl-;dimethyl-4-toluidine;
Dimethyl-p-toluidine
99-97-8
N-甲基对甲苯胺
N-Methyl-p-toluidine
623-08-5
N,N-二甲基苯胺
N,N-dimethyl aniline
121-69-7
N,N-二羟乙基苯胺
N,N-dihydroxyethyl aniline
120-07-0
N-乙基-N-羟乙基间甲苯胺
N-Ethyl-N-Hydroxyethyl-M-Toluidine
91-88-3
N,N-二羟乙基间甲苯胺
N,N-dihydroxyethyl-m-toluidine
91-99-6
N-乙基苯胺
N-ethyl aniline
103-69-5
N-甲基苯胺
N-methyl aniline
100-61-8
N-甲基对甲苯胺
4-Methyl-N-methylaniline
623-08-5
N-甲基-N-羟乙基苯胺
2-(N-Methylanilino)ethanol
93-90-3
N,N-二甲基对苯二胺
N,N-DIMETHYL-P-PHENYLENEDIAMINE
99-98-9
3-(甲氨基)甲苯
3-(Methylamino)toluene
696-44-6
N,N-二异丙醇对甲苯胺
DIPROPOXY-P-TOLUIDINE
38668-48-3
N,N-二乙基邻甲苯胺
N,N-DIETHYL-O-TOLUIDINE
606-46-2
N-甲基对硝基苯胺
N-Methyl-4-nitroaniline
100-15-2
N,N-二苄基苯胺
N,N-DIBENZYLANILINE
91-73-6
N-苯基乙醇胺
2-Anilinoethanol
122-98-5
N-苄基苯胺
N-Phenylbenzylamine
103-32-2
N-羟乙基间甲苯胺
N-2-HYDROXYETHYL-M-TOLUIDINE
102-41-0
N-乙基N氯乙基间甲苯胺
N-ETHYL-N-CHLOROETHYL-M-TOLUIDINE
22564-43-8
N,N-二乙基-4-氨基-2-甲基苯甲醛
4-Diethylamino-2-methylbenzaldehyde
92-14-8
间甲苯胺
M-Toluidine MT
108-44-1
1,4-二溴-2,5-二碘苯
1,4-DIBROMO-2,5-DIIODOBENZENE
63262-06-6
N,N-二羟乙基对苯二胺硫酸盐
N,N-Bis(2-hydroxyethyl)-p-phenylenediamine sulphate
54381-16-7
N-乙基-N-苄基-4-氨基苯甲醛
4-(N-Ethyl-N-benzyl)amino-benzoaldehyde
67676-47-5
N,N-二乙基-4-氨基苯甲醛
4-Diethylaminobenzaldehyde
120-21-8
对二甲胺基苯甲醛
p-Dimethylaminobenzaldehyde
100-10-7
2-氨基噻唑
2-Aminothiazole
96-50-4
对甲苯胺
P-Toluidine PT
106-49-0
N,N-双(2-羟基丙基)苯胺
N,N-BIS(2-HYDROXYPROPYL)ANILINE
3077-13-2
N-乙基-N-氰乙基苯胺
3-Ethylanilinopropiononitrile
148-87-8
N-乙基-N-(3'-磺酸苄基)苯胺
N-Ethyl-N-benzylaniline-3'-sulfonic acid
101-11-1
邻苯甲酰苯甲酸甲酯
Methyl 2-benzoylbenzoate
606-28-0
对羟基苯甲酸甲酯
Methylparaben
99-76-3
十四酸异丙酯
Isopropyl myristate
110-27-0
棕榈酸异丙酯
Isopropyl palmitate
142-91-6
邻甲苯胺
O-Toluidine OT
95-53-4
4-甲基-N-苯基苯胺
N-PHENYL-P-TOLUIDINE
620-84-8
N,N-二甲基苄胺
N,N-Dimethylbenzylamine BDMA
103-83-3
N,N-二甲基甲酰胺
N,N-Dimethylformamide DMF
.68-12-2
N-甲基甲酰胺
N,N-Dimethylformamidedimethyl acetal (DMF-DMA)
4637-24-5
N,N-二甲基乙酰胺
N,N-Dimethylacetamide DMAC
127-19-5
N,N-二乙基间甲苯甲酰胺
避蚊胺
N,N-diethyl-m-toluamide DEET
134-62-3
N,N-二乙基羟胺
N,N-Diethylhydroxylamine DEHA
3710-84-7
N,N-二甲基-间甲基苯胺
N,N-DIMETHYL-M-TOLUIDINE
121-72-2
N-甲基二苯胺
N-Methyldiphenylamine
552-82-9
N,N-二氰乙基苯胺
N,N-Dicyanoethylaniline
1555-66-4
N-乙基-2-硝基苯胺
N-Ethyl-2-Nitro-Benzenamine
10112-15-9
N-(2-羟乙基)乙二胺
AEEA
111-41-1
二乙烯三胺(DETA)
Diethylenetriamine DETA
111-40-0
三乙烯二胺
Triethylenediamine
280-57-9
三乙烯四胺
TriethylenetetramineTETA
112-24-3
四乙烯五胺
TEPA
112-57-2
间二氯苯
1,3-Dichlorobenzene MDCB
541-73-1
间二三氟甲苯
1,3-Bis(trifluoromethyl)-benzene
402-31-3
粉末丁腈橡胶
MITIVY33-1(POLYMER/ADHESIVE COMPOUNDING)
9003-18-3
十六烷基氯化吡啶
Cetylpyridinium chloride monohydrate
6004-24-6
对氯甲苯
4-Chlorotoluene
106-43-4
无水硫酸钠
SODIUM SULFATE
7757-82-6 /15124-09-1
碱性嫩黄
Auramine O
2465-27-2
偶氮二异丁腈
2,2'-Azobis(2-methylpropionitrile)
78-67-1
Read the full article
#100-10-7#4-(Dimethylamino)benzaldehydetestsolution(ChP)#4-(NN-Dimethylamino)benzaldehyde#4-amino-23-dimethylbenzaldehyde#4-dimethylaminobenzaldehyde#4-Dimethylaminobenzaldehyde#4-Dimethylaminobenzaldehyde100-10-7#4-DimethylaminobenzaldehydeSupplierinChina#Amines#API#APIintermediates#APIs#casno.100-10-7#chemicalrawmaterials#Chemicals#DIMETHYLAMINOBENZALDEHYDE#Dyeintermediates#FactorySupplyp-Dimethylaminobenzaldehyde#Finechemicals#HYDRAZINEMETERSOLUTION#inorganicrawmaterials#intermediate#intermediates#medicalrawmaterials#NN-dimethyl-4-aminobenzaldehyde#organicchemicalrawmaterials#organicintermediates#Organicrawmaterials#p-Dimethylaminobenzaldehyde#P-dimethylaminobenzaldehydetestsolution(ChP)
1 note
·
View note
Text




cas 1193389-70-6 (4-Fluoro-phenyl)-piperidin-4-yl-amine dihydrochloride, 501673-99-0 1-BOC-4-[(4-METHYLPHENYL)AMINO]-PIPERIDINE
#cas 1193389-70-6 (4-Fluoro-phenyl)-piperidin-4-yl-amine dihydrochloride#501673-99-0 1-BOC-4-[(4-METHYLPHENYL)AMINO]-PIPERIDINE
0 notes
Text
Different Amino Acid-Based Surfactants in Use Today
Surfactants are famous for their ability to form fine lather because of their wonderful detergency and emulsifying properties. The amino acid-based surfactants have high biodegradability, excellent surface-active properties, and low toxicity. They tolerate hard water well and are mild on the eyes and skin. Being environmentally friendly, they don't pose any threat to the ecosystem. These are milder than the corresponding carboxylates and sulfates.
Evolution of the Cleaning Products
Soaps are salts of fatty acids and came into use in the personal cleansing segment in the early 1900s. In the 1950s, a syndet surfactant got introduced as personal cleansing bars. During the 1990s, liquid cleansing technology came into the limelight. These body washes and shower gels use a combination of sodium lauryl ether sulfate (SLES), an amphoteric surfactant such as Cocamidopropyl betaine, and an anionic surfactant. In amine oxide based surfactants, amino acids form the natural building block for the surfactants.
Using Facial Cleansers and Toothpastes
Ajinomoto pioneered the synthesis of amino acid-based surfactants in the 1970s. Acyl sarcosinate was the first such surfactant used in toothpaste. After this, acyl glutamates became popular for their application in transparent soap bars and facial cleaning. Acyl glycinate found favor with the manufacturers for their lather properties and mildness. Other than glutamates, sarcosinates, and glycinates, we have other amino acid head groups such as taurates and alaninates.
Improvement in the Quality Levels
You can contact your surfactant chemical supplier to get more information on this matter. Technically, taurates do not belong to the amino acid group. But because they have similar properties and taurine is an amino-sulfonic acid, they get grouped in the same category.
Customer-centric solutions from prominent manufacturers have enhanced the quality of the cleaning products in use today. We can use the surfactants as emulsifiers and as preservatives. Your surfactant chemical supplier will tell you how to do this. Biosurfactants in use today are naturally occurring substances.
0 notes
Text
The glutamate:glyoxylate aminotransferase catalyzes the transamination of glyoxylate with glutamate, yielding the amino acid glycine (see Figure 8.8 and Table 8.2, reaction 5).
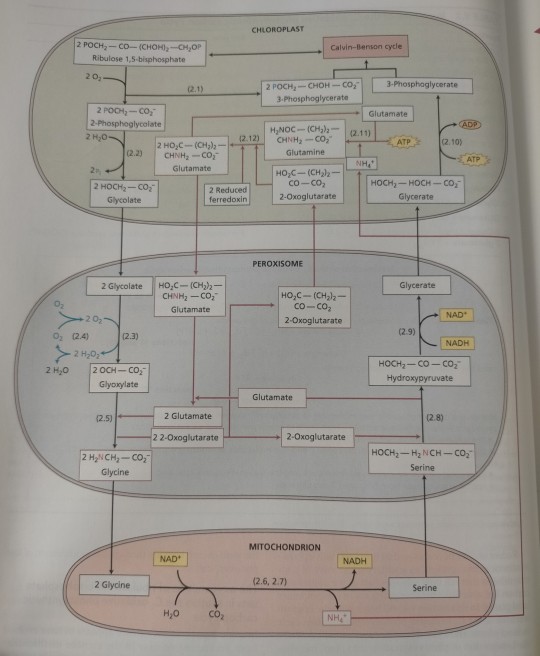


"Plant Physiology and Development" int'l 6e - Taiz, L., Zeiger, E., Møller, I.M., Murphy, A.
#book quote#plant physiology and development#nonfiction#textbook#carbon cycle#glutamate#catalyze#amino acid#glycine#photosynthesis#photochemistry#photorespiration#amine
0 notes
Link
0 notes
Video
এই জুম্মা মিস করিও না, আজ বছরের শেষ জুম্মা। Don't miss this Jumma, today is the last Jumma of the yearyear. #friends #JummahDay #Alhamdulillah #Allah #hadiya #amino #amin https://www.instagram.com/p/CmyHLhSoss_/?igshid=NGJjMDIxMWI=
0 notes
Text
NameReaction, The Kabachnik–Fields reaction

The Kabachnik–Fields reaction is a three-component organic reaction forming α-aminomethyl phosphonates from an amine, a carbonyl compound, and a dialkyl phosphonate, (RO)2PH. Aminophosphonates are synthetic targets of some importance as phosphorus analogs of α-amino acids.

#chemical reactions#chemistry class#chemistry#science#kingdraw#chemblr#online chemist#chem#dailychem#uni studyblr
4 notes
·
View notes
Note
would mer ezra smell fishy
Not at all! He smells like the sea, clean and salty, just like all living fish. Only dead fish have that unpleasant “fishy” odor. Here’s why:
In order to maintain fluid balance, ocean creatures must fill their cells with amino acids and amines to counter the saltiness of seawater. Ocean fish tend to rely on trimethylamine oxide (TMAO) for this purpose.
The problem, or stink, arises when fish are killed and bacteria and fish enzymes convert TMAO into trimethylamine (TMA), which gives off the characteristic fishy odor. [Source]

#softpad answers#merboy ezra#hjbenderart#ezra bridger#star wars rebels#au#merfolk au#merpeople#mermeta#when i was a kid i wanted to be a marine biologist#i guess i'm making up for that
24 notes
·
View notes
Photo
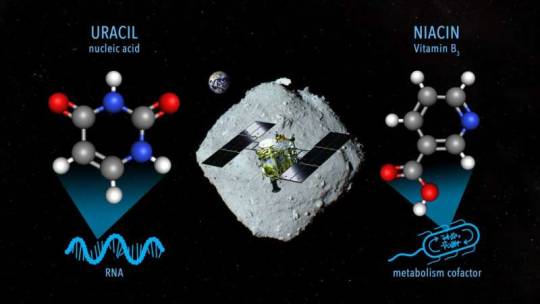
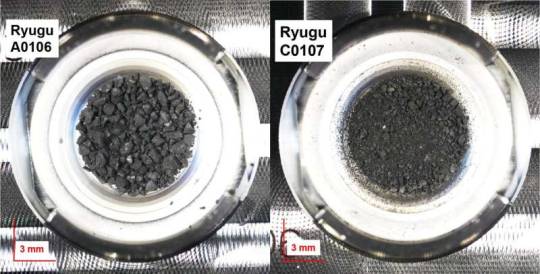
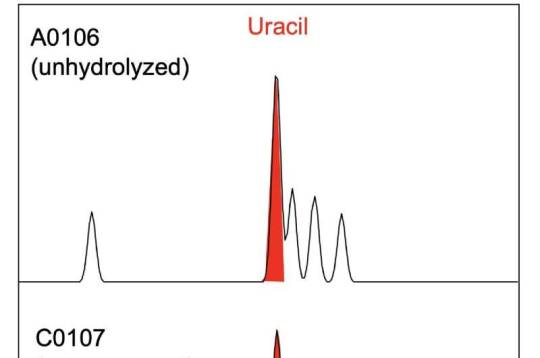
RNA molecule uracil found in asteroid Ryugu samples Researchers have analyzed samples of the asteroid Ryugu collected by the Japanese Space Agency's Hayabusa2 spacecraft and found uracil, one of the informational units that make up RNA, the molecules that contain the instructions for how to build and operate living organisms. Nicotinic acid, also known as Vitamin B3 or niacin, which is an important cofactor for metabolism in living organisms, was also detected in the same samples. This discovery by an international team, led by Associate Professor Yasuhiro Oba at Hokkaido University, adds to the evidence that important building blocks for life are created in space and could have been delivered to Earth by meteorites. The findings were published in the journal Nature Communications. "Scientists have previously found nucleobases and vitamins in certain carbon-rich meteorites, but there was always the question of contamination by exposure to the Earth's environment," Oba explained. "Since the Hayabusa2 spacecraft collected two samples directly from asteroid Ryugu and delivered them to Earth in sealed capsules, contamination can be ruled out." The researchers extracted these molecules by soaking the Ryugu particles in hot water, followed by analyses using liquid chromatography coupled with high-resolution mass spectrometry. This revealed the presence of uracil and nicotinic acid, as well as other nitrogen-containing organic compounds. "We found uracil in the samples in small amounts, in the range of 6–32 parts per billion (ppb), while vitamin B3 was more abundant, in the range of 49–99 ppb," Oba elaborated. "Other biological molecules were found in the sample as well, including a selection of amino acids, amines and carboxylic acids, which are found in proteins and metabolism, respectively." The compounds detected are similar but not identical to those previously discovered in carbon-rich meteorites. The team hypothesizes that the difference in concentrations in the two samples, collected from different locations on Ryugu, is likely due to the exposure to the extreme environments of space. They also hypothesized that the nitrogen-containing compounds were, at least in part, formed from the simpler molecules such as ammonia, formaldehyde and hydrogen cyanide. While these were not detected in the Ryugu samples, they are known to be present in cometary ice—and Ryugu could have originated as a comet or another parent body that had been present in low temperature environments. "The discovery of uracil in the samples from Ryugu lends strength to current theories regarding the source of nucleobases in the early Earth," Oba concludes. "The OSIRIS-REx mission by NASA will be returning samples from asteroid Bennu this year, and a comparative study of the composition of these asteroids will provide further data to build on these theories." TOP IMAGE....A conceptual image for sampling materials on the asteroid Ryugu containing uracil and niacin by the Hayabusa2 spacecraft (NASA Goddard/JAXA/Dan Gallagher). Credit: NASA Goddard/JAXA/Dan Gallagher CENTRE IMAGE....Photographs of samples A0106 and C0107 collected from the asteroid Ryugu, during the 1st touchdown sampling and 2nd touchdown sampling, respectively. Credit: Yasuhiro Oba et al, Nature Communications, March 21, 2023 LOWER IMAGE....Mass chromatograms from the first (top) and second (center) samples from asteroid Ryugu, showing the presence of uracil (red peak). They were compared to a sample of pure uracil (bottom). Credit: Yasuhiro Oba et al, Nature Communications, March 21, 2023
2 notes
·
View notes
Text
A Level Organic Chemistry - Types of Cpds
Alkanes
Alkenes
Arenes - benzene, methylbenzene (aka toluene)
Halogen derivatives - halogenoalkane (aka alkyl halide), halogenoarenes (aka aryl halide)
Alcohols - primary, secondary and tertiary alcohols + phenols
Carbonyls - aldehyde, ketone, benzaldehyde
Carboxylic acids and their derivatives - Carboxylic acids, esters, acyl chlorides and amides
Nitrogen compounds - aliphatic amines, phenylamines, amino acids
#study#knowledge#learning#studyblr#studyhaus#science#chemistry#organicchem#organic chemistry#alevel#stem#studynotes
3 notes
·
View notes
Text




cas 1193389-70-6 (4-Fluoro-phenyl)-piperidin-4-yl-amine dihydrochloride, 501673-99-0 1-BOC-4-[(4-METHYLPHENYL)AMINO]-PIPERIDINE
#cas 1193389-70-6 (4-Fluoro-phenyl)-piperidin-4-yl-amine dihydrochloride#501673-99-0 1-BOC-4-[(4-METHYLPHENYL)AMINO]-PIPERIDINE
0 notes
Text
The properties of amino acids arise from a combination of the chemistry of amines (chapter 19) and that of carboxylic acids (chapter 23).
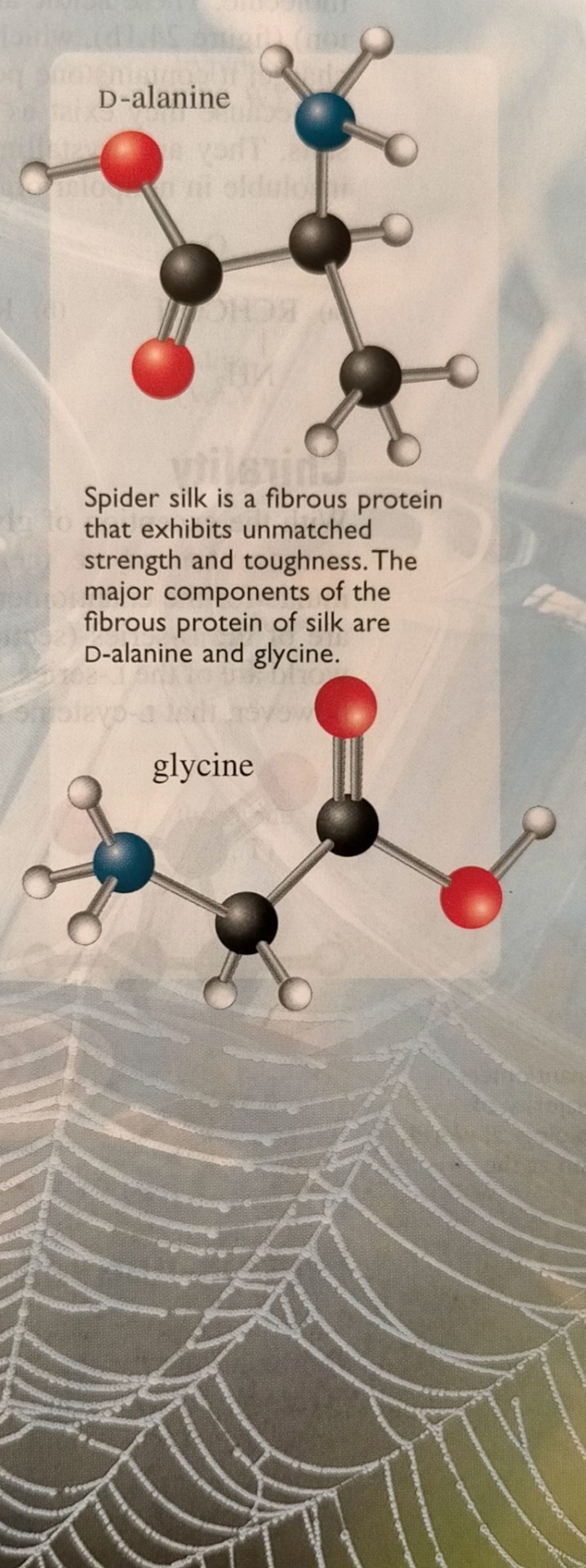
"Chemistry" 2e - Blackman, A., Bottle, S., Schmid, S., Mocerino, M., Wille, U.
#book quotes#chemistry#nonfiction#textbook#chemical properties#amino acid#carboxylic acid#alanine#glycine#spider silk#protein#spider web
0 notes
Text
Ceftiofur CAS#: 80370-57-6

IdentificationPhysical DataSpectraRoute of Synthesis (ROS)Safety and HazardsOther Data
Identification
Product NameCeftiofurIUPAC Name(6R,7R)-7-amino]-3-(furan-2-carbonylsulfanylmethyl)-8-oxo-5-thia-1-azabicyclooct-2-ene-2-carboxylic acidMolecular StructureCAS Registry Number 80370-57-6EINECS Number1308068-626-2MDL NumberMFCD00864953SynonymsCeftiofur80370-57-6CeftiofurumNaxcelExcenelUNII-83JL932I1CCeftiofur crystalline free acidCeftiofurum HSDB 744583JL932I1CDTXCID5026702DTXSID7046702Ceftiofur (INN)Ceftiofurum (Latin)(6R,7R)-7-(2-(2-Amino-4-thiazolyl)glyoxylamido)-3-(mercaptomethyl)-8-oxo-5-thia-1-azabicyclo(4.2.0)oct-2-ene-2-carboxylic acid, 7(sup 2)-(Z)-(O-methyloxime), 2-furoate (ester)CEFTIOFUR CEFTIOFUR (MART.)CEFTIOFUR Naxcel (veterinary)Molecular FormulaC19H17N5O7S3Molecular Weight523.6InChIInChI=1S/C19H17N5O7S3/c1-30-23-11(9-7-34-19(20)21-9)14(25)22-12-15(26)24-13(17(27)28)8(5-32-16(12)24)6-33-18(29)10-3-2-4-31-10/h2-4,7,12,16H,5-6H2,1H3,(H2,20,21)(H,22,25)(H,27,28)/b23-11-/t12-,16-/m1/s1InChI KeyZBHXIWJRIFEVQY-IHMPYVIRSA-NIsomeric SMILESCO/N=C(/C1=CSC(=N1)N)C(=O)N23N(C2=O)C(=C(CS3)CSC(=O)C4=CC=CO4)C(=O)O
Patent InformationPatent IDTitlePublication DateWO2008/47376AN IMPROVED PROCESS FOR THE PREPARATION OF CEPHALOSPORIN ANTIBIOTIC2008
Physical Data
AppearanceOff-white or light yellowish powder
Description (Association (MCS))Solvent (Association (MCS))Temperature (Association (MCS)), °CPartner (Association (MCS))Adsorptionaq. phosphate buffer37pyrographite
Spectra
Description (IR Spectroscopy)Bands, Spectrum
Description (UV/VIS Spectroscopy)Spectrum
Route of Synthesis (ROS)
Route of Synthesis (ROS) of Ceftiofur CAS# 80370-57-6
ConditionsYieldStage #1: ceftiofur With triethylamine In tetrahydrofuran; water at -5 - 0℃; for 2h;Stage #2: With sodium 2-ethylhexanoic acid In tetrahydrofuran; water at 8 - 10℃; for 1h;Experimental Procedure4; 5 EXAMPLE-4 Preparation of ceftiofur sodium (Ib) from 7- 3-(mercaptomethyl)-8-oxo-5-thia-1-azabicyclo-oct-2-ene-2-carboxylic acid (ceftiofur) (Ia)140 ml of tetrahydrofuran (THF) and 4.0 ml of demineralised water (DMW) and 4.0 g (0.00764 moles) of 7- 3-(mercaptomethyl)-8-oxo-5-thia-1-azabicyclo-oct-2-ene-2-carboxylic acid (ceftiofur, Ia) were taken in a flask and stirred. The reaction mixture was cooled and 0.81 g (0.0080 moles) of triethyl amine (TEA) was added. Activated carbon (0.60 g) and sodium dithionite (0.04 g) were added and the reaction mixture filtered through celite bed and washed with a mixture of THF and DMW. The filtrate was passed through 0.2μ filter paper and washed with THF (100 ml). A solution of sodium-2-ethyl hexanoate (1.80 g, 0.01084 moles) was prepared in THF and passed through 0.2μ filter paper. This filtrate was added gradually to the solution of the triethylamine salt of ceftiofur, obtained above and the precipitated solid was filtered under nitrogen atmosphere. The wet cake was washed with THF, followed by washing with ethyl acetate (2×44 ml) and acetone (2×29 ml) and dried under nitrogen atmosphere for 30-45 minutes with occasional raking. The semi dry cake was dried in vacuum oven at 700 mmHg/40° C. till moisture content was 2.0% to give 3.48 g (87%) of Ceftiofur sodium (Ib) having purity of 98.0% 1000 ml of THF and 70.0 ml of demineralised water (DMW) and 100.0 g (0.191 moles) of 7- 3-(mercaptomethyl)-8-oxo-5-thia-1-azabicyclo- oct-2-ene-2-carboxylic acid (ceftiofur, Ia) were taken in a flask and stirred for 5 minutes. The mixture was cooled to -5° C. and 20.47 g (0.202 moles) of triethyl amine (TEA) was added at -5 to -3° C. in 60 minutes. Activated carbon (15 g) and sodium dithionite (1.0 g) were added and the reaction mixture was stirred at -5 to 0° C. for 60 minutes. The mixture was filtered through celite bed and washed with a mixture of THF and DMW (5:0.3 v/v; 530 mL). The filtrate was passed through 0.2μ filter paper and washed with THF (100 ml). A solution of sodium-2-ethyl hexanoate (50.78 g, 0.305 moles) was prepared in THF and passed through 0.2μ filter paper. This filtrate was added gradually to the solution of triethylamine salt of ceftiofur, obtained above and the mixture was stirred at at 8-10° C. for 60 minutes and the precipitated solid filtered under nitrogen atmosphere. The wet cake was slurry washed with THF (500 mL), followed by ethyl acetate (2×500 ml) and acetone (3×500 ml). The wet cake was dried under nitrogen atmosphere for 30-45 minutes with occasional raking. The semi dry cake was dried in vacuum oven at 700 mmHg/40° C. to give 88 g (0.88% w/w) of ceftiofur sodium (Ib), having a purity of 98%.87%With sodium 2-ethylhexanoic acid In tetrahydrofuran at 20℃; for 0.5h;Experimental Procedure Dichloromethane (20 ml) was charged and cooled to 0-5° C., 7--3--3-cephem-4-carboxylic acid p-methoxy benzyl ester (6.5 g) was added followed by trifluoroacetic acid (20 ml) at 0-5° C. The reaction mixture was stirred for 30-40 min. Anisole (12 ml) was also added at 5° C. and reaction was stirred at 5° C. for 1 hour, the reaction was checked by HPLC and after completion of reaction, water (100 ml) was added with stirring. The product was filtered and washed with water (100 ml) followed by dichloromethane (100 ml). The filtered solid was taken in tetrahydrofuran (100 ml) and dried over MgSO4. To the dried THF layer was added a solution of 5 g of 2-ethyl sodium hexanoate in THF (20 ml). The precipitated ceftiofur sodium was stirred for 30 minutes at 20° C. filtered and washed with acetone (100 ml). Yield = 5.0 g Purity = 99%
Safety and Hazards
GHS Hazard StatementsNot Classified
Other Data
HS CodeStorageUnder the room temperature and away from lightShelf Life2 yearsMarket Price
DruglikenessLipinski rules componentMolecular Weight523.571logP-2.103HBA9HBD3Matching Lipinski Rules3Veber rules componentPolar Surface Area (PSA)256.26Rotatable Bond (RotB)10Matching Veber Rules1
Use PatternCeftiofur a third-generation cephalosporin antibiotic designed for veterinary use. It has a broad spectrum of antibacterial activity, showing strong efficacy against Gram-positive and Gram-negative bacteria, as well as anaerobic bacteria. Its activity against Gram-positive bacteria is similar to or weaker than that of first-generation cephalosporins. However, it exhibits potent antibacterial activity against Gram-negative bacteria such as Escherichia coli, Salmonella typhi, Klebsiella pneumoniae, Enterobacter cloacae, and Streptococcus.
Read the full article
0 notes
Text
Unraveling Pseudoephedrine: Composition, Synthesis, and Flexibility
Pseudoephedrine, famous for its decongestant properties, has attracted attention for its chemical structure and adaptable applications. This article provides a concise overview of its composition, synthesis, and diverse uses beyond nasal relief.
Introduction: Pseudoephedrine, classified as a sympathomimetic amine, is renowned for its efficacy in alleviating nasal congestion. However, its chemical composition and wide-ranging applications extend beyond this primary function.
Chemical Composition and Structure: Pseudoephedrine, a chiral compound, shares structural similarities with ephedrine but possesses distinct pharmacological effects. Its stereochemistry, centered on a carbon atom adjacent to the amino group, yields two enantiomers, with the (+) form exhibiting pharmacological activity.
Synthesis and Production: Synthesis of pseudoephedrine involves chemical transformations starting from benzaldehyde or phenylacetic acid, although regulatory constraints have tightened due to concerns about illicit diversion.
Applications: Pseudoephedrine finds utility across pharmaceuticals, chemical intermediates, organic synthesis, and research. While primarily used as a decongestant, it also serves as chiral auxiliaries in asymmetric synthesis and is explored for potential therapeutic applications beyond nasal congestion.
Conclusion: Pseudoephedrine's versatility renders it a subject of enduring interest. As research unfolds, its pharmacological potential and industrial adaptability may expand further, opening up new avenues for scientific exploration and innovation.
0 notes
Text
From Production Process to Industrial Applications: A Deep Dive into Epoxy Resin (2023–2034)
Epoxy Resin, often perceived as a complex scientific compound, is surprisingly versatile, finding applications. This blog serves as your comprehensive guide to all things epoxies! In this blog we'll embark on a journey into the captivating realm of epoxy resin, dwelling into:
The Science: Unveiling the chemistry behind epoxy, we'll elucidate its remarkable properties as a potent adhesive, sealant, and casting material.
Industrial Applications: Exploring the extensive industrial uses of epoxy, we'll examine its role in constructing robust composite materials and safeguarding electrical components.
Manufacturing Process: Get super detailed information about the process that leads into the formation of this resin and gain knowledge about the feedstock used for its production.
Introduction
Epoxy Resin is a versatile synthetic polymer valued for its exceptional adhesive, mechanical, and electrical properties. Its widespread applications include serving as adhesives in construction, woodworking, and manufacturing industries due to its robust bonding capabilities and resistance to environmental factors like moisture, chemicals, and temperature fluctuations. Moreover, epoxy resin finds extensive use in coatings and sealants, providing corrosion and abrasion resistance in diverse sectors such as flooring, automotive, marine, and aerospace. It is also a crucial component in composite materials, contributing to the production of strong yet lightweight structures in aerospace, automotive, sports equipment, and construction. Additionally, its low viscosity makes it suitable for molding and casting various objects, prototypes, and architectural elements, allowing for precise reproduction of fine details in sculptures and models.
These versatile properties make epoxy resin indispensable across industries, driving innovation and enabling the creation of durable, high-performance products. Various types of epoxy resins are utilized across different sectors. These resins are Bisphenol A Based Resin, Bisphenol F Based Resin, Epoxy Phenol Novolac Based Resin, Cycloaliphatic Epoxy Based Resin, and Others. Bisphenol A Based Resin finds extensive use across diverse applications, spanning coatings, civil engineering, adhesives, electrical insulation materials, and reactive intermediates. The global Epoxy Resin market is likely to flourish at a moderate CAGR of 4.11% by the year 2034.
Manufacturing Process
Epoxy resins exhibit high viscosity levels and are typically molded. Catalysts or curing agents, such as accelerators or hardeners, can facilitate curing either through catalytic action or direct reaction with the resin. Epoxy resins are typically created through the reaction of compounds containing at least two active hydrogen atoms, including polyphenolic compounds, diamines, amino phenols, heterocyclic imides and amides, and aliphatic diols, with epichlorohydrin. The oxirane group within an epoxy monomer reacts with various curing agents such as aliphatic and aromatic amines, phenols, polyamides, amidoamines, anhydrides, thiols, and acids. These reactions lead to the formation of rigid thermosetting products through the combination with other suitable ring-opening compounds.
Epoxy resins are commonly synthesized through a reaction between epichlorohydrin (ECH) and Bisphenol-A (BPA), although alternative raw materials like Aliphatic Glycols, Phenol, And O-Cresol Novolacs. Initially, ECH and BPA are introduced into a reactor, followed by the addition of a caustic soda solution as the mixture reaches its boiling point. After unreacted ECH evaporates, the resulting phases are separated using an inert solvent such as Methyl Isobutyl Ketone (MIBK). Subsequently, the resin undergoes water washing, and solvent removal is accomplished via vacuum distillation. Producers then incorporate specific additives tailored to desired properties such as flexibility, viscosity, color, adhesiveness, and accelerated curing, depending on intended applications. These resins can be obtained in liquid or solid forms through similar processes. To transform epoxy resins into durable and rigid materials, curing with a hardener is an essential step. Primary and secondary amines are commonly employed as curing agents.
Maximize image
Edit image
Delete image

Source: VALCO
Applications of Epoxy Resin:
Paints & Coatings
Due to their exceptional bonding properties with metals, epoxy resins and amine curing agents exhibit outstanding anticorrosion characteristics when incorporated into coatings. When utilizing high molecular weight variants, these coatings display excellent secondary workability, rendering them ideal as PCM primers. Additionally, epoxy resins offer a diverse range of viscosities, spanning from solventless to solvent-based types, making them well-suited as ink components where viscosity attributes play a crucial role.
Adhesives
Epoxy resin is widely utilized as an adhesive due to its robust properties, particularly in structural and engineering applications. Its versatility extends across various industries, including vehicle construction, snowboard manufacturing, aircraft assembly, and bicycle production. Beyond structural uses, epoxy adhesives find application in virtually any scenario.
Electronics and Electrical Systems
Epoxy resins are integral to manufacture insulators, motors, transformers, and generators. Renowned for its exceptional insulating properties, epoxy resin provides effective safeguarding against dust, moisture, and short circuits, making it a prominent choice for circuitry production as well.
Repair Epoxy resins effectively blend with a range of materials such as latex, wood, metal, and various synthetic substances. Applying epoxy resin to delicate objects results in the formation of a thin, secure barrier that remains tightly adhered over extended periods, ensuring lasting stability and protection.
Market Outlook:
Epoxy resin, a thermosetting copolymer formed by the polymerization of epoxide and other monomers with hydroxyl groups, comprises a monomeric resin, accelerator, hardener, and plasticizer. Possessing corrosion resistance, thermal stability, mechanical strength, chemical resistance, durability, and adhesion, epoxy resins are utilized in paints, coatings, adhesives, composites, and electronic encapsulation across various sectors. Their applications span building and construction, automotive, industrial, and consumer goods and are likely to foster market growth. The global Epoxy Resin market is anticipated to reach approximately 6.2 million tonnes by 2034.
Epoxy Resin Major Manufacturers
Significant companies in the Global Epoxy Resin market are Olin Coporation, Huntsman Corporation, Nan Ya Plastics Co Ltd, Jiangsu Sanmu Group, Hexion Inc., Kumho P&B Chemicals, Nantong Xincheng Synthetic Material Co Ltd, Zhuhai Hongchang Electronic Material Co Ltd, Jiangsu Yangnong Kumho Chemical Co., Ltd., Kukdo Chemical Co., Ltd., Sinopec Baling Petrochemical Co., Ltd, and Others.
Challenges and Opportunities
The epoxy resin market faces several challenges, including environmental concerns regarding the production and disposal of epoxy resins, particularly due to the presence of harmful chemicals and potential emissions during manufacturing processes. Additionally, fluctuating raw material prices, such as those for bisphenol-A (BPA) and epichlorohydrin, can impact production costs and profit margins for epoxy resin manufacturers. Moreover, increasing regulatory scrutiny and stringent environmental regulations may require epoxy resin producers to invest in sustainable manufacturing practices and develop eco-friendly alternatives, which could pose challenges in terms of research and development costs and market adoption. Furthermore, competition from alternative materials and substitutes, such as bio-based resins and advanced polymers, presents a challenge for the epoxy resin market, necessitating innovation and differentiation to maintain market share and competitiveness.
Conclusion:
In conclusion, the Epoxy Resin market is poised for continued growth and evolution despite facing various challenges. PVC remains a widely used and versatile material with applications spanning across multiple industries, including construction, automotive, healthcare, and packaging. Epoxy resins have several key properties: high strength, minimal shrinkage, superb adhesion to diverse substrates, effective electrical insulation, resilience against chemicals and solvents, and affordability.
0 notes
Text

Unveiling Clenbuterol: Exploring Its Uses, Risks, and Legal Considerations
In the realm of fitness and performance enhancement, Clenbuterol has garnered significant attention for its purported benefits in weight loss and muscle retention. However, beneath its allure lie complex issues surrounding its legality, safety, and ethical considerations. This comprehensive article delves deep into the multifaceted landscape of Clenbuterol, shedding light on its applications, associated risks, and legal status.
Understanding Clenbuterol:
Clenbuterol, commonly referred to as "Clen," belongs to the class of sympathomimetic amines and is primarily utilized as a bronchodilator to alleviate respiratory conditions such as asthma. While its therapeutic applications are well-established, Clenbuterol has also found widespread use beyond medical settings, particularly in athletic and bodybuilding circles. Its reputation as a potent aid in weight loss and muscle preservation has fueled its popularity among individuals striving for enhanced physical performance and aesthetics.
Uses of Clenbuterol:
Bronchodilation: Clenbuterol's primary medical function is to relax the smooth muscles in the airways, facilitating improved breathing for individuals grappling with respiratory ailments like asthma. Its bronchodilatory effects make it a valuable tool in managing conditions characterized by airway constriction.
Performance Enhancement: Athletes and bodybuilders often turn to Clenbuterol to augment their physical performance and achieve specific body composition goals. By stimulating the beta-2 adrenergic receptors, buy Clenbuterol is believed to enhance metabolism, promote fat loss, and preserve lean muscle mass, thereby contributing to improved athletic performance and aesthetic outcomes.
Risks and Side Effects:
Despite its potential benefits, Clenbuterol is not without risks, and its misuse can lead to a myriad of adverse effects, including:
Cardiovascular Complications: Clenbuterol's ability to increase heart rate and blood pressure may result in palpitations, arrhythmias, and in severe cases, cardiovascular events such as heart attacks. Prolonged or excessive use of Clenbuterol can exert undue stress on the cardiovascular system, posing a significant health risk.
Neurological Effects: Users may experience tremors, anxiety, insomnia, and headaches, attributed to Clenbuterol's stimulatory effects on the central nervous system. These symptoms can be disruptive and impact daily functioning and overall well-being.
Muscle Cramps: Clenbuterol usage has been associated with muscle cramps, primarily due to its ability to deplete taurine levels in the body. Taurine, an essential amino acid, plays a crucial role in muscle function, and its depletion can lead to discomfort and impaired physical performance.
Legal Ramifications: Clenbuterol is classified as a performance-enhancing drug and is prohibited by various sports organizations, including the World Anti-Doping Agency (WADA) and the International Olympic Committee (IOC). Its inclusion on the list of banned substances underscores the ethical and legal concerns surrounding its use in competitive sports and athletic endeavors.
Legal Status:
The legal status of clenbuterol for sale varies across jurisdictions, with regulations governing its production, distribution, and use differing significantly from one country to another. While Clenbuterol may be approved for medical use in certain regions, it is often subject to strict regulations due to concerns regarding its potential for misuse, abuse, and adverse health effects. In many jurisdictions, Clenbuterol is classified as a controlled substance, and its acquisition, possession, or distribution without a valid prescription is unlawful and punishable by law.
Conclusion:
Clenbuterol occupies a complex and contentious position within the realms of medicine, fitness, and sports. While its therapeutic benefits in managing respiratory conditions are well-established, its off-label use as a performance-enhancing and weight-loss aid raises significant ethical, legal, and health concerns. The risks associated with Clenbuterol, including cardiovascular complications, neurological effects, and legal ramifications, underscore the importance of informed decision-making and responsible usage practices. Individuals considering Clenbuterol use should prioritize their health and well-being, seeking guidance from healthcare professionals and adhering to applicable legal regulations. Ultimately, achieving fitness goals should not come at the expense of one's health or integrity, and alternative approaches to diet, exercise, and lifestyle modifications should be explored to promote sustainable and holistic well-being.
1 note
·
View note