#carboxylic acid
Text
Table 23.1 lists several of the unbranched aliphatic carboxylic acids found in the biological world (e.g. figure 23.1), along with the common names of each.


"Chemistry" 2e - Blackman, A., Bottle, S., Schmid, S., Mocerino, M., Wille, U.
#book quotes#chemistry#nonfiction#textbook#aliphatic#carboxylic acid#70s#1670s#17th century#formica#ants#venom#formic acid#acetic acid#propionic acid#butyric acid#valeric acid#caproic acid#caprylic acid#capric acid#lauric acid#myristic acid#palmitic acid#stearic acid#arachidic acid#methanoic acid#ethanoic acid#propanoic acid#butanoic acid#pentanoic acid
4 notes
·
View notes
Photo

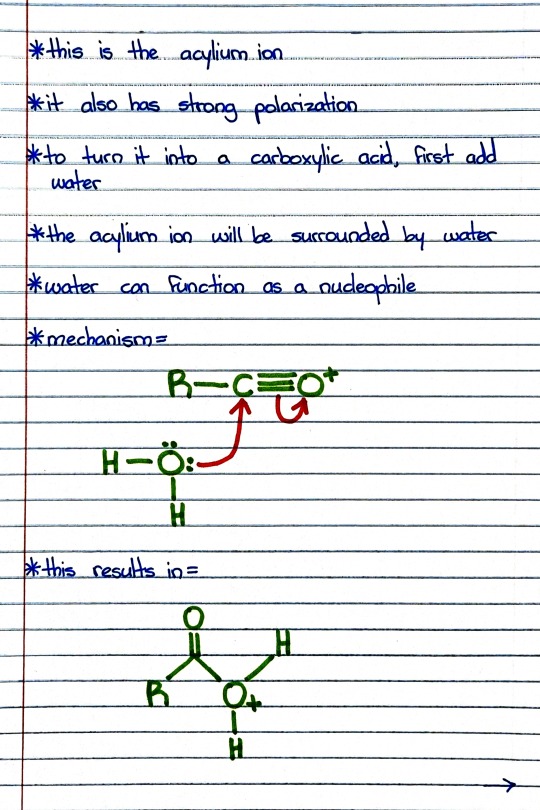

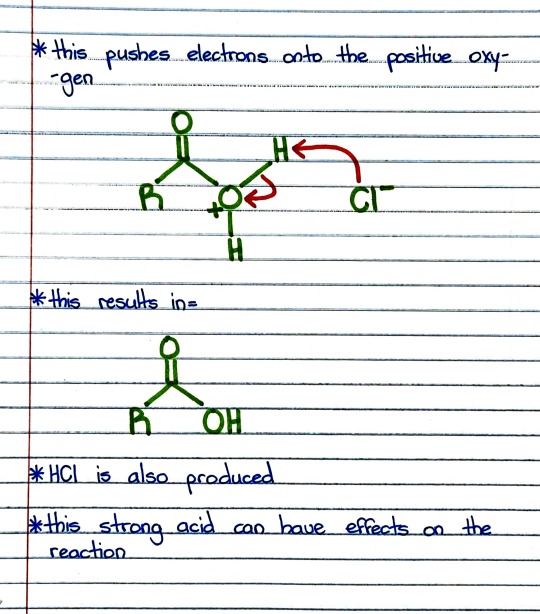
#organic chemistry#ochem#o-chem#o chem#ochem 1#ochem 2#organic chemistry 1#organic chemistry 2#mcat organic chemistry#orgo#reactions#orgo reactions#chemical reactions#orgo mcat#mcat orgo#ochem reactions#acid chloride#acyl chloride#carboxylic acid#carboxylic acid reaction#acid chloride reaction#acyl chloride reaction
28 notes
·
View notes
Text
Me to me: Bronsted Acids are those which donate H+ (or any other protons) now get that through your thick skull
#organic chemistry#bronsted acids and bases#carboxylic acid#academia#stem#pcm#pcb#jee#neet#desi academia#desi tag#studyblr stuff#high school#engineering prep
4 notes
·
View notes
Text
Because carboxylic acid salts are water soluble, we can convert water-insoluble carboxylic acids to water-soluble alkali metal or ammonium salts and then extract them into aqueous solution.
"Chemistry" 2e - Blackman, A., Bottle, S., Schmid, S., Mocerino, M., Wille, U.
#book quote#chemistry#nonfiction#textbook#carboxylic acid#solubility#chemical reactions#alkali metal#ammonium#salts#extraction#aqueous solution
0 notes
Text
Scientists Find Origin-of-Life Molecule in Space for First Time
— By Jess Thomson | August 8, 2023

NASA image of the Tarantula Nebula star-forming region taken by Webb’s Near-Infrared Camera. A molecular cloud in interstellar space has been found to contain carbonic acid for the first time. NASA, ESA, CSA, AND STSCI
molecule common to Earth and usually associated with life has been detected in the depths of space by scientists.
Carbonic acid (HOCOOH), which you may know as the chemical that makes your soda fizzy, was discovered lurking near the center of our galaxy in a galactic center molecular cloud named G+0.693-0.027, a study published in The Astrophysical Journal revealed.
This marks the third time that carboxylic acids—this class of chemicals, often thought to be some of the building blocks of life—have been detected in space, after acetic acid and formic, and the first time that an interstellar molecule has been found to contain three or more oxygen atoms.
"Our observations have allowed us to know that carbonic acid, which until now had remained invisible to our eyes, is relatively abundant in space, which makes it an essential piece to understand the interstellar chemistry of carbon and oxygen, two of the fundamental chemical elements in any prebiotic process," Víctor M. Rivilla, a researcher at the Spanish Center for Astrobiology and co-author of the study, told German broadcaster Deutsche Welle (DW).
"This result confirms that the path we have chosen is the right one to search for, and detect more molecules that we suspect were key to the appearance of life on our planet," he concluded.
Carboxylic acids are a type of organic compound characterized by a carbon (C) atom doubly bonded to an oxygen (O) atom and singly bonded to a hydroxyl group (―OH). Carbonic acid in particular is formed when CO2 is dissolved in water, meaning that it is present in increased concentrations in our seas due to CO2 in the atmosphere.
Many theories as to how life on Earth evolved suggest that primitive life may have emerged from a primordial soup of chemicals when our planet was very young. Some have suggested that these chemicals, including carboxylic acids, may have arrived on Earth from space, traveling via comets and meteorites to the forming planet.
Carbonic acid has been previously detected on other astronomical bodies, including the icy moons of Jupiter, in some meteorites and comets, and even on Mars and Mercury, but until now, has not been seen in interstellar space.
The authors explained that the discovery of these more complex molecules in the interstellar medium may reveal clues about the origins of our planet and the life upon it.
"The presence of prebiotic COMs within extraterrestrial material thus firmly suggests the existence of carboxylic acids of increasing complexity in the ISM (interstellar medium), including amino acid–related species. Within this context, considerable efforts have been devoted to hunting for other acids, such as propenoic or acrylic acid, propanoic acid, cyanoacetic acid, glycolic acid, hydantoic acid, and glycine, whose identification in the [interstellar medium] remains elusive."
#Tech & Science#Origion | Life Molecule#Space#NASA | ESA | CSA | STSCI#Tarantula Nebula#Corbonic Acid (HOCOOH)#Astrobiology#Víctor M. Rivilla#Deutsche Welle (DW)#CO2#Carboxylic Acid#Moon | Jupiter | Mars | Mercury#Earth#Prebiotic COMs#ISM (Interstellar Medium)#Acids: Acrylic | Propanoic | Cyanoacetic | Glycolic | Hydantoic | Glycine
0 notes
Text
youtube
#functional groups organic chemistry#nomenclature of functional groups class 12#Carboxylic Acid Functional Group Structure#carboxylic acid#R-COOH functional group structure#Naming of Carboxlic acid#iupac of carboxylic acid#iupac nomenclature of carboxylic acid#naming of carboxylic acid#puneet sir etoos india#Nomenclature of Functional Groups by puneet sir#naming of iupac by puneet sir#Youtube
1 note
·
View note
Text
carboxylic acid
3D Amino Acids Augmented Reality Application. Amino acids are organic compounds that contain aminoa and carboxylic acid (−CO2H) functional groups, along with a side chain (R group) specific to each amino acid. The elements present in every amino acid are carbon (C), hydrogen (H), oxygen (O), and nitrogen (N) (CHON). In addition sulfur (S) is present in the side chains of cysteine and methionine, and selenium (Se).
0 notes
Text


1 April 2024
Welp, my faculty's organic chemists have officially lost it and it unfortunately isn't just a joke. I'm only now realizing the amount of material for the upcoming test (test!!!) could easily make up a whole exam. Not sure how I'm supposed to retain it all in like a week (with classes and other uni work in between???) and rn the situation is tragic. Screaming crying etc. How am I not to hate ochem when this course is sheer chaos run by what seems to be three mischievous racoons in disguise? 😭
#nervous headache incoming#honestly it's just a *test*#why the hell does it cover everything from alkanes to carboxylic acid derivatives??#that's nearly all of ochem??#🥲🥲🥲#mine#studyblr#chemblr#chemistry#sciblr#stemblr
97 notes
·
View notes
Text
Hi, this may be the “finals drawing near and studying melting my brain” talking but im losing my mind over the twin rings and it has begun to actually hurt my brain. And félix didnt get blorbo status in my brain until recently so i never really thought too deeply about his actions???
I know there’s people out there who have put huge amounts of thought into the amok lore, because i saw u in the wilds of tumblr a while ago and then proceeded to ignore it because i was too busy with my Other Blorbos, and now idk where to find posts, so, if you’re out there, here are some questions, i am imploring you for your wisdom
—why did gabe use the ring to threaten félix in the episode félix. “You know what i could do with a snap of my fingers,” or whatever. Was he talking about the peacock? The peacock wasn’t even fixed yet. It was still broken. Could he use the peacock to kill félix? I thought you needed the amok to do that???
—WHY did félix give adrien’s ring BACK to gabriel in risk. What the fuck benefit did that give him. He had blackmail and ALL of those miraculous????? He was already winning why was he like “and THIS, i wont be needing it anymore 😏” what the HECK félix.
—“anymore.” Why did he need it to BEGIN with then. Like. Is he protecting adrien with the peacock??? Is that even a thing????? He spent a while in hiding doing nothing why did he not swoop in to protect adrien sooner??? Did he even DO anything to protect adrien when he had the other amok????? Was he just constantly whispering like “adrien u can make your own choices youre free” into the ring and we didnt see it?????
—WHY IS HE SO BAD AT BARGAINING????? This isnt a lore question this is just. Still not over it. Félix what the fuck. Do you know how bargaining works. You dont play all of your cards at once. I get that he’s impulsive but SERIOUSLY my GUY. You are so STUPID why did you OFFER the ring to gabriel you dumb bitch
—amelie being like “you can keep it félix, i just wanted the ring back on our side of the family 🥰” at the end of félix makes me genuinely insane now. WHY. Amelie what did you want it on your side of the family for. Amelie couldve been like “oh we gotta give that to adrien” but instead she was like “yeah my sweet little murder boy will take good care of his cousin who he bullies”
—by the way this is not me complaining and this is not intended to be a space to complain,,, this is a space to go “omg these fictional guys are soooooo stupid and messed up (affectionate)” and try to unpack and come up with an explanation for what could’ve possibly made them act that way. Félix makes me more insane the more i study him . i hate him so much (affectionate)
(Also please please please please do not reference any leaks i am avoiding them okay thank u)
#ml spoilers#ml s5 spoilers#sentiadrien#sentifelix#ml felix#felix fathom#also while im begging tumblr for help#if nitriles and carboxylic acids and the way that ph interacts with both of those makes sense to anyone#and u have some kinda secret knowlege that makes it eadier to understand#please send help
68 notes
·
View notes
Text
ordered a streetcar for my exam and the guy making it goes, “oof, that kinda test huh?”
#yeah brother it’s about the derivatives of carboxylic acids and their reaction mechanisms#the iupac names are like lamda-3 dimethylbutanoic acid like
2 notes
·
View notes
Text
(Recall from chapter 11 that the smaller the pKa the more acidic is the group. At lower pH, carboxylic acids are found in the RCOOH form and amines are found in the RNH3+ form. At higher pH, the opposite is true; carboxylic acids are present as the salt RCOO- and amines are present as uncharged RNH2. Figure 24.3 on p. 1061 shows how this looks at different pH.)
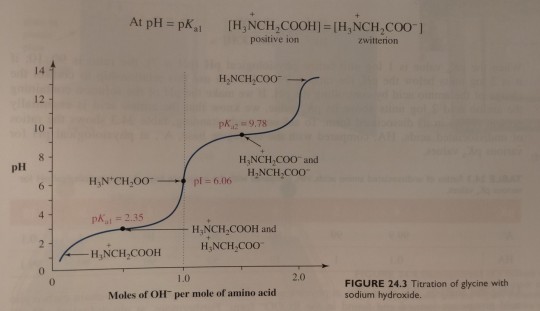
Next, the solution is titrated with 1.00 M NaOH; the volume of base added and the pH of the resulting solution are recorded and then plotted as shown in figure 24.3. (...) By examining the titration curve (figure 24.3), you can see that the isoelectric point for glycine falls halfway between the pKa values for the carboxyl groups and the ammonium ion:
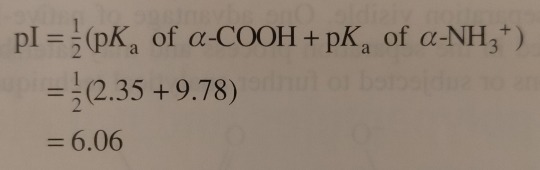
"Chemistry" 2e - Blackman, A., Bottle, S., Schmid, S., Mocerino, M., Wille, U.
#book quotes#chemistry#nonfiction#textbook#acidic#carboxylic acid#ph#salt#amine#titration#glycine#sodium hydroxide#carboxyl#ammonium#ions
1 note
·
View note
Text
ew i rly don't want to learn those 20 amino acids ://
#a few days ago i learned 35 carboxylic acids so like give me a break#but i gotta learn these too :/#at least some of the 3 letter codes are funny tho
6 notes
·
View notes
Text
Substitution at the α-carbon with an atom or a group of atoms of higher electronegativity than carbon increases the acidity of carboxylic acids, often by several orders of magnitude.
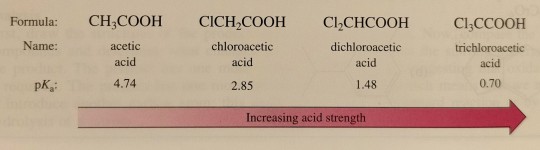
"Chemistry" 2e - Blackman, A., Bottle, S., Schmid, S., Mocerino, M., Wille, U.
#book quote#chemistry#nonfiction#textbook#substitution#alpha#electronegativity#carbon#acidity#carboxylic acid#acetic acid#chloroacetic acid#dichloroacetic acid#trichloroacetic acid
0 notes
Photo

#carboxylic acid#boiling point#aldehydes#chemistry#solutions#ketone#alcohol#molecular mass#van der waals#force#attraction#carboxylate ion#hydrogen bond#intermolecular#intramolecular#noncavalent#interactions
1 note
·
View note
Text
CAS No : 93940-19-3| Product Name : Tranexamic Acid - Impurity A| Chemical Name : (1r,4r,1′r,4′r)-4,4′-[Azanediylbis(methylene)]di(cyclohexane-1-carboxylic Acid) | Pharmaffiliates
Buy highly pure Tranexamic Acid - Impurity A, CAS No : 93940-19-3, Mol. Formula : C16H27NO4, Mol. Weight : 297.39, from Pharmaffiliates. Login as registered user for prices, availability and discounts.
#CAS No : 93940-19-3#Product Name : Tranexamic Acid - Impurity A#impurities#intermediates#pharmaceutical standards#fine chemicals#metabolites#Chemical Name : (1r#4r#1′r#4′r)-4#4′-[Azanediylbis(methylene)]di(cyclohexane-1-carboxylic Acid)
0 notes
