#FDA Emergency Use Authorization
Text
COVID-19 vaccines available for children under 5
New Post has been published on https://aroundfortwayne.com/news/2022/06/21/covid-19-vaccines-available-for-children-under-5/
COVID-19 vaccines available for children under 5

Today, the ISDH announced that COVID-19 vaccines for children ages 6 months up to age 5 are now available at some Indiana providers, expanding the population eligible to be protected against the disease.
#CDC Centers for Disease Control and Prevention#COVID-19 pandemic#COVID-19 vaccine#FDA Emergency Use Authorization#FDA U.S. Food and Drug Administration#Indianapolis Indiana#ISDH Indiana State Department of Health#Moderna COVID-19 Vaccine#Pfizer-BioNTech COVID-19 vaccine
0 notes
Text
COVID State of Emergency by the FDA
COVID State of Emergency by the FDA
April 7 2024By PK Morgan
Perhaps, in my previous article that contains a section on Emergency Use Authorization (EUA), I was a bit too vague or we are accustomed to taking everything our government dishes out regardless of legality. An EUA was issued for COVID tests on April 5 2024.
Why has the FDA approved a COVID/Flu self test in April of 2024 with…

View On WordPress
#covid#Covid 19#department of Human and Health Services#emergency use authorization#FDA#food and Drug Administration#HHS#incorrect#not approved
0 notes
Text
FDA Simplifies Use of Bivalent mRNA COVID-19 Vaccines for All Doses Administered to Individuals 6 Months and Older
The U.S. Food and Drug Administration (FDA) has amended the emergency use authorizations (EUAs) of the Moderna and Pfizer-BioNTech COVID-19 bivalent mRNA vaccines to simplify the vaccination schedule for most individuals.
This includes authorizing the current bivalent vaccines (original and omicron BA.4/BA.5 strains) to be used for all doses administered to individuals 6 months of age and older,…
View On WordPress
#additional doses#bivalent mRNA vaccines#COVID-19#emergency use authorization#FDA#immunocompromised#Moderna#Pfizer-BioNTech#vaccination#vaccination schedule
0 notes
Text
Updated vaccines against Covid-19 are coming, just as hospitalizations and deaths due to the virus are steadily ticking up again.
Today, the US Food and Drug Administration authorized new mRNA booster shots from Moderna and Pfizer, and a panel of outside experts that advises the Centers for Disease Control and Prevention voted to recommend the shots to everyone in the United States ages 6 months and older. Once Centers for Disease Control and Prevention director Mandy Cohen signs off on the recommendations and the vaccines are shipped, people can start getting the boosters.
The recommendation is projected to prevent about 400,000 hospitalizations and 40,000 deaths over the next two years, according to data presented at the meeting by CDC epidemiologist Megan Wallace.
This year’s mRNA vaccines are different from the 2022 booster in a key way. Last year’s shot was a bivalent vaccine, meaning it covered two variants: the original one that emerged in China in 2019, plus the Omicron subvariant BA.5, which was circulating during much of 2022. This fall’s booster drops the original variant, which is no longer circulating and is unlikely to return. It targets just the Omicron subvariant XBB.1.5, which was dominant throughout much of 2023.
Pfizer and Moderna’s vaccines work by introducing a tiny piece of genetic material called messenger RNA, or mRNA, that carries instructions for making SARS-CoV-2’s characteristic spike protein. Once it is injected, cells in the body use those instructions to temporarily make the spike protein. The immune system recognizes the protein as foreign and generates antibodies against it. Those antibodies stick around so that if they encounter that foreign invader again, they will mount a response against it.
Since the start of the Covid-19 pandemic, the virus has acquired new mutations in its spike protein and elsewhere. These mutations result in new variants and subvariants that diverge from the original virus. When enough mutations accumulate, these new versions can more easily evade the antibodies created by previous vaccine doses or infections.
The constantly evolving nature of the virus is the reason health regulators decided last year to update the original mRNA vaccines, which were designed against the version of the virus that first appeared in 2019. This year, once again, the virus has changed enough to warrant an updated booster.
In June, an advisory committee to the FDA recommended that this fall’s booster be a monovalent vaccine—targeting only the then-dominant XBB.1.5 subvariant.
At that meeting, committee members reviewed evidence suggesting that the inclusion of the original variant may hamper the booster’s effectiveness against newer offshoots. “The previous bivalent vaccine contained the ancestral spike and thus skewed immune responses to the old spike,” says David Ho, a professor of microbiology at Columbia University whose research, which is not yet peer-reviewed, was among the evidence the FDA panel reviewed. “This is what we call immunological imprinting, and it results in lack of immune responses to the new spike.” He thinks taking out the old variant should optimize the immune response.
But over the past few months, even newer Omicron offshoots have arrived. Currently, EG.5.1, or Eris, is the dominant one in the United States, United Kingdom, and China. Meanwhile, a variant called BA.2.86, or Pirola, has been detected in several countries. Pirola has raised alarm bells because it has more than 30 new mutations compared to XBB.1.5.
Even though the new boosters were formulated against XBB.1.5, they’re still expected to provide protection against these new variants. “The reason is, while antibodies are important in protection against mild disease, the critical part of the immune response that’s important for protecting against severe disease is T cells,” says Paul Offit, a professor of vaccinology at the University of Pennsylvania and member of the FDA’s vaccine advisory committee.
These cells are a different part of the immune response. Unlike antibodies, which neutralize a pathogen by preventing it from infecting cells, T cells work by eliminating the cells that have already been invaded and boosting creation of more antibodies. Both the Moderna and Pfizer-BioNTech Covid vaccines produce long-lasting T cells in addition to antibodies.
It’s why, Offit says, when the Omicron wave hit in late 2021 and peaked in January 2022, the US didn’t see a dramatic increase in hospitalizations and deaths even as cases rose significantly: People’s T cells kicked into gear, even when their antibodies didn’t recognize the Omicron variant.
“In some ways,” says Offit, when it comes to vaccine booster development, “it almost doesn’t matter what we pick to target” because the coronavirus has yet to evolve away from T cell recognition. “Everything works.”
Scientists think T cells are able to protect against severe Covid because they’re recognizing parts of the virus that have remained unchanged throughout the pandemic. “I suspect that as we continue to vaccinate, there are some conserved regions [of the virus],” says Jacqueline Miller, Moderna’s head of infectious diseases. “So even with the accumulation of mutations, we’re still building on previous immunity.”
People who have hybrid immunity—that is, have had a Covid infection and have also been vaccinated—seem to have the best immune responses to new variants, she says, which suggests that previous exposure shapes and improves immune responses to new variants. Preliminary studies show that antibodies generated by previous infections and vaccinations should be capable of neutralizing Pirola.
Earlier this month, Moderna issued a press release saying that clinical trial data showed that its updated booster generated a strong immune response against Pirola, as well as the more prevalent Eris variant.
In a statement to WIRED, Pfizer spokesperson Jerica Pitts said the company continues to closely monitor emerging variants and conduct tests of its updated monovalent booster against them. Data presented at Tuesday’s CDC meeting showed that Pfizer-BioNTech’s updated booster elicited a strong neutralizing antibody response against both Eris and Pirola.
The FDA expects that Covid-19 vaccines will continue to be updated on an annual basis, unless a completely new variant emerges that requires a different approach. ���We will always be a little behind the virus,” says Ho. “In this instance, we won’t suffer too much, but that might not be the case going forward. Surveillance is imperative.”
814 notes
·
View notes
Text
COVID-19 mRNA vaccines were deliberately designed to be ineffective and lethal, according to bombshell testimony by pharmaceutical executive Alexandra “Sasha” Latypova.
According to Latypova, the Department of Defense (DoD) had “very clear intent to harm” by executing a “mass genocide of Americans.”
Under the DoD’s control and direction, drug manufacturers like Pfizer, Moderna, and Janssen started mass-producing the shots for Operation Warp Speed – long before the first cases of Covid even appeared, it turns out. These “figurehead” organizations, Latypova insists, were just obeying the DoD’s orders.
Naturalnews reports: What this means is the United States military oversaw the creation and rollout of these “covid countermeasures,” as they were classified before being erroneously dubbed as “vaccines.” This is why they called it Operation Warp Speed: because it was a military warfare operation, not a “public health” operation.
The Centers for Disease Control and Prevention (CDC) and the Food and Drug Administration (FDA) also played their role by fast-tracking emergency use authorization (EUA) for the deadly drugs, followed by official approval for some of them – and the rest is history.
Back in December, Latypova laid this all out in a video lecture.
154 notes
·
View notes
Text
Here's the complete list of DHS flagged search terms. Don't use any of these on social media to avoid having the 3-letter agencies express interest in your activities!
DHS & Other Agencies
Department of Homeland Security (DHS)
Federal Emergency Management Agency (FEMA)
Coast Guard (USCG)
Customs and Border Protection (CBP)
Border Patrol
Secret Service (USSS)
National Operations Center (NOC)
Homeland Defense
Immigration Customs Enforcement (ICE)
Agent
Task Force
Central Intelligence Agency (CIA)
Fusion Center
Drug Enforcement Agency (DEA)
Secure Border Initiative (SBI)
Federal Bureau of Investigation (FBI)
Alcohol Tobacco and Firearms (ATF)
U.S. Citizenship and Immigration Services (CIS)
Federal Air Marshal Service (FAMS)
Transportation Security Administration (TSA)
Air Marshal
Federal Aviation Administration (FAA)
National Guard
Red Cross
United Nations (UN)
Domestic Security
Assassination
Attack
Domestic security
Drill
Exercise
Cops
Law enforcement
Authorities
Disaster assistance
Disaster management
DNDO (Domestic Nuclear Detection Office)
National preparedness
Mitigation
Prevention
Response
Recovery
Dirty Bomb
Domestic nuclear detection
Emergency management
Emergency response
First responder
Homeland security
Maritime domain awareness (MDA)
National preparedness initiative
Militia
Shooting
Shots fired
Evacuation
Deaths
Hostage
Explosion (explosive)
Police
Disaster medical assistance team (DMAT)
Organized crime
Gangs
National security
State of emergency
Security
Breach
Threat
Standoff
SWAT
Screening
Lockdown
Bomb (squad or threat)
Crash
Looting
Riot
Emergency Landing
Pipe bomb
Incident
Facility
HAZMAT & Nuclear
Hazmat
Nuclear
Chemical Spill
Suspicious package/device
Toxic
National laboratory
Nuclear facility
Nuclear threat
Cloud
Plume
Radiation
Radioactive
Leak
Biological infection (or event)
Chemical
Chemical burn
Biological
Epidemic
Hazardous
Hazardous material incident
Industrial spill
Infection
Powder (white)
Gas
Spillover
Anthrax
Blister agent
Exposure
Burn
Nerve agent
Ricin
Sarin
North Korea
Health Concern + H1N1
Outbreak
Contamination
Exposure
Virus
Evacuation
Bacteria
Recall
Ebola
Food Poisoning
Foot and Mouth (FMD)
H5N1
Avian
Flu
Salmonella
Small Pox
Plague
Human to human
Human to ANIMAL
Influenza
Center for Disease Control (CDC)
Drug Administration (FDA)
Public Health
Toxic
Agro Terror
Tuberculosis (TB)
Agriculture
Listeria
Symptoms
Mutation
Resistant
Antiviral
Wave
Pandemic
Infection
Water/air borne
Sick
Swine
Pork
Strain
Quarantine
H1N1
Vaccine
Tamiflu
Norvo Virus
Epidemic
World Health Organization (WHO and components)
Viral Hemorrhagic Fever
E. Coli
Infrastructure Security
Infrastructure security
Airport
CIKR (Critical Infrastructure & Key Resources)
AMTRAK
Collapse
Computer infrastructure
Communications infrastructure
Telecommunications
Critical infrastructure
National infrastructure
Metro
WMATA
Airplane (and derivatives)
Chemical fire
Subway
BART
MARTA
Port Authority
NBIC (National Biosurveillance Integration Center)
Transportation security
Grid
Power
Smart
Body scanner
Electric
Failure or outage
Black out
Brown out
Port
Dock
Bridge
Canceled
Delays
Service disruption
Power lines
Southwest Border Violence
Drug cartel
Violence
Gang
Drug
Narcotics
Cocaine
Marijuana
Heroin
Border
Mexico
Cartel
Southwest
Juarez
Sinaloa
Tijuana
Torreon
Yuma
Tucson
Decapitated
U.S. Consulate
Consular
El Paso
Fort Hancock
San Diego
Ciudad Juarez
Nogales
Sonora
Colombia
Mara salvatrucha
MS13 or MS-13
Drug war
Mexican army
Methamphetamine
Cartel de Golfo
Gulf Cartel
La Familia
Reynose
Nuevo Leon
Narcos
Narco banners (Spanish equivalents)
Los Zetas
Shootout
Execution
Gunfight
Trafficking
Kidnap
Calderon
Reyosa
Bust
Tamaulipas
Meth Lab
Drug trade
Illegal immigrants
Smuggling (smugglers)
Matamoros
Michoacana
Guzman
Arellano-Felix
Beltran-Leyva
Barrio Azteca
Artistics Assassins
Mexicles
New Federation
Terrorism
Terrorism
Al Queda (all spellings)
Terror
Attack
Iraq
Afghanistan
Iran
Pakistan
Agro
Environmental terrorist
Eco terrorism
Conventional weapon
Target
Weapons grade
Dirty bomb
Enriched
Nuclear
Chemical weapon
Biological weapon
Ammonium nitrate
Improvised explosive device
IED (Improvised Explosive Device)
Abu Sayyaf
Hamas
FARC (Armed Revolutionary Forces Colombia)
IRA (Irish Republican Army)
ETA (Euskadi ta Askatasuna)
Basque Separatists
Hezbollah
Tamil Tiger
PLF (Palestine Liberation Front)
PLO (Palestine Libration Organization)
Car bomb
Jihad
Taliban
Weapons cache
Suicide bomber
Suicide attack
Suspicious substance
AQAP (Al Qaeda Arabian Peninsula)
AQIM (Al Qaeda in the Islamic Maghreb)
TTP (Tehrik-i-Taliban Pakistan)
Yemen
Pirates
Extremism
Somalia
Nigeria
Radicals
Al-Shabaab
Home grown
Plot
Nationalist
Recruitment
Fundamentalism
Islamist
Weather/Disaster/Emergency
Emergency
Hurricane
Tornado
Twister
Tsunami
Earthquake
Tremor
Flood
Storm
Crest
Temblor
Extreme weather
Forest fire
Brush fire
Ice
Stranded/Stuck
Help
Hail
Wildfire
Tsunami Warning Center
Magnitude
Avalanche
Typhoon
Shelter-in-place
Disaster
Snow
Blizzard
Sleet
Mud slide or Mudslide
Erosion
Power outage
Brown out
Warning
Watch
Lightening
Aid
Relief
Closure
Interstate
Burst
Emergency Broadcast System
Cyber Security
Cyber security
Botnet
DDOS (dedicated denial of service)
Denial of service
Malware
Virus
Trojan
Keylogger
Cyber Command
2600
Spammer
Phishing
Rootkit
Phreaking
Cain and abel
Brute forcing
Mysql injection
Cyber attack
Cyber terror
Hacker
China
Conficker
Worm
Scammers
Social media
SOCIAL MEDIA?!
20 notes
·
View notes
Text
The entire media is LYING about FDA "approval" for the new COVID vaccine. 👇
From The FDA website 👇

Approving it for EUA(Emergency Use Authorization) is NOT the FDA's approval. 🤔
#pay attention#educate yourselves#educate yourself#knowledge is power#reeducate yourself#reeducate yourselves#think for yourselves#think about it#think for yourself#do your homework#do some research#ask yourself questions#question everything#do your own research#lies lies and more lies#fake news#fda#government corruption#medical malpractice#medical corruption#coersion
85 notes
·
View notes
Text
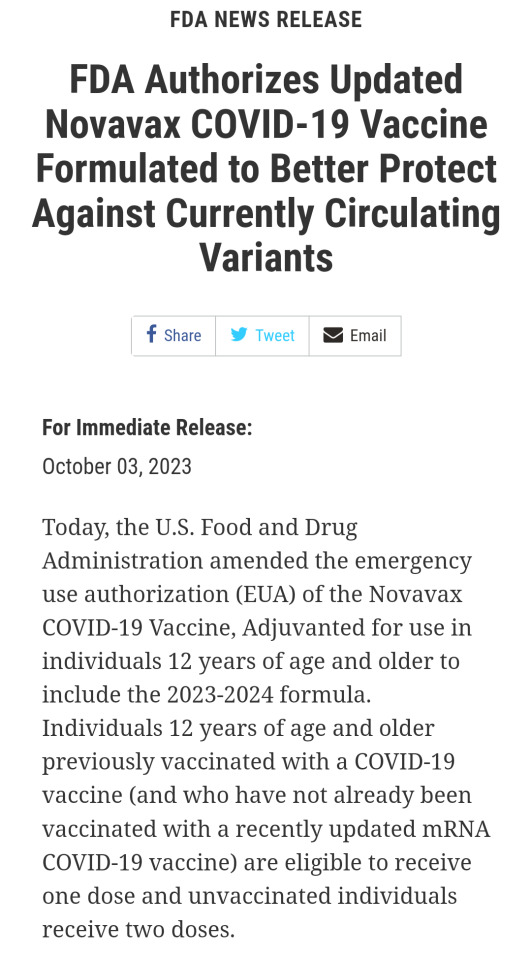
https://www.fda.gov/news-events/press-announcements/fda-authorizes-updated-novavax-covid-19-vaccine-formulated-better-protect-against-currently
For Immediate Release:
October 03, 2023
Today, the U.S. Food and Drug Administration amended the emergency use authorization (EUA) of the Novavax COVID-19 Vaccine, Adjuvanted for use in individuals 12 years of age and older to include the 2023-2024 formula. Individuals 12 years of age and older previously vaccinated with a COVID-19 vaccine (and who have not already been vaccinated with a recently updated mRNA COVID-19 vaccine) are eligible to receive one dose and unvaccinated individuals receive two doses
—
It should be at some pharmacies within a couple of weeks. Contact yours, or your health department.
A couple of reasons some people have chosen to wait for Novavax:
Data showing that it may offer a slightly boosted immune response & slightly better defense. And that mixed vaccination (called heterologous, meaning mRNA & non) may also offer expanded protection.
mRNA vaccines have been harsh for a lot of people, requiring multiple "sick" days, and/or other symptoms afterwards. Likewise many have a milder or no reaction to Novavax (non-mRNA).
That said, the numbers are not so drastically different. And everyone reacts differently. It's much better to get any vaccine you have access to than to continuously get reinfected with a virus that damages your entire body each time — vascular system, heart, brain, all of it.
There's a a massive wave of infections recently. If you're in frequent contact with others (have kids in school, work in-person, etc), it's safer to get the already-available mRNA vaccines if you're able to.
24 notes
·
View notes
Text
Supreme Court blocks lower court rulings restricting abortion pills
The Supreme Court on Friday temporarily blocked lower court orders that imposed restrictions on the widely used abortion pill mifepristone, keeping the status quo for the most common form of medication abortion while a legal challenge to the Food and Drug Administration's authority plays out.
Why it matters: The decision forestalled what would have been an unprecedented court-ordered rollback of FDA powers and at least temporarily settled a legal standoff arising from conflicting orders issued by separate federal courts on abortion pills.
The big picture: The U.S. District Court in Texas and the 5th U.S. Circuit Court of Appeals orders reimposed restrictions on mifepristone that the FDA had gradually removed over the years.
But a Washington state federal court ruled that the FDA cannot roll back access to mifepristone in 17 states and the District of Columbia, in a case brought by Democratic state attorneys general.
The restrictions, set to take effect Saturday, would "upend the status quo based on the [Texas] court's deeply misguided assessment of mifepristone's safety," according to an appeal from the Department of Justice to the Supreme Court earlier Friday.
Danco Laboratories, the manufacturer of the brand name version of mifepristone, also filed an emergency application asking the high court to block the Texas and 5th Circuit orders, arguing that they have "created regulatory chaos across the country."
Details: Without issuing an opinion, Justice Samuel Alito ordered that the lower court rulings are stayed until next Wednesday, April 19.
Access to mifepristone for medication abortion will remain unchanged at least until then.
Alito also ordered the anti-abortion groups that originally brought the lawsuit to respond to the Justice Department's appeal by Tuesday.
45 notes
·
View notes
Text
FDA Approves Fast and Convenient Azure Fastep COVID-19 Antigen Pen Home Test for Symptomatic and Asymptomatic Individuals
The FDA has issued emergency use authorization for the Azure Fastep COVID-19 Antigen Pen Home Test, manufactured by Azure Biotech, Inc. Validation data for this test was gathered through the National Institutes of Health Independent Test Assessment Program, a collaboration between the FDA and the NIH.
This test is specifically designed for serial testing for symptomatic individuals within the…

View On WordPress
#asymptomatic#Azure Biotech Inc#Azure Fastep COVID-19 Antigen Pen Home Test#COVID 19#emergency use authorization#FDA#Independent Test Assessment Program#National Institutes of Health#serial testing#symptomatic
0 notes
Text
"Breaking News: FDA Greenlights Groundbreaking Update in Novavax Covid Vaccine—A Game-Changer in the Fight Against the Pandemic!"
The Food and Drug Administration (FDA) has granted emergency use authorization for Novavax’s updated Covid vaccine for individuals aged 12 and above. This approval paves the way for the vaccine to be available weeks after the release of new vaccines from Pfizer and Moderna. However, the vaccine still requires an updated recommendation from the Centers for Disease Control and Prevention (CDC)…
View On WordPress
18 notes
·
View notes
Text
On Tuesday, the Supreme Court of the United States will hear oral arguments in a challenge to abortion pill access across the country, including in states where abortion is legal. The stakes for abortion rights are sky-high, and the case is the most consequential battle over reproductive health care access since Roe v. Wade was overturned in 2022.
At the center of this fight is mifepristone, a pill that blocks a hormone needed for pregnancy. The drug has been approved by the US Food and Drug Administration for more than two decades, and it’s used to treat some patients with Cushing’s syndrome, as well as endometriosis and uterine fibroids. But its primary use is the one contested now—mifepristone is the first of two pills taken in the first 10 weeks of pregnancy for a standard medication abortion, along with the drug misoprostol.
If the justices side with the antiabortion activists seeking to limit access to mifepristone, it could upend nationwide access to the most common form of abortion care. A ruling that invalidates mifepristone’s approval would open the door for any judge to reverse the FDA approval of any drug, especially ones sometimes seen as controversial, such as HIV drugs and hormonal birth control. It could also have a chilling effect on the development of new drugs, making companies wary of investing research into medicines that could later be pulled from the market.
Pills are now the leading abortion method in the US, and their popularity has spiked in recent years. More than six in 10 abortions in 2023 were carried out via medication, according to new data from the Guttmacher Institute. Since rules around telehealth were relaxed during the Covid-19 pandemic, many patients seeking medication abortions have relied on virtual clinics, which send abortion pills by mail. And it keeps getting more popular: Hey Jane, a prominent telemedicine provider, saw demand increase 73 percent from 2022 to 2023. It recorded another 28 percent spike comparing data from January 2023 to January 2024.
“Telemedicine abortion is too effective to not be in the targets of antiabortion folks,” says Julie F. Kay, a longtime reproductive rights lawyer and director of the advocacy group Abortion Coalition for Telemedicine.
Tomorrow’s argument comes after a long, tangled series of legal disputes in lower courts. The Supreme Court will be hearing two cases consolidated together, including FDA v. Alliance for Hippocratic Medicine, in which a coalition of antiabortion activists filed a suit challenging the FDA’s approval of mifepristone, asking for it to be removed from the market. The Alliance for Hippocratic Medicine is represented by the Alliance Defending Freedom, a right-wing Christian law firm that often takes politically charged cases.
Despite decades of scientific consensus on the drug’s safety record, the Alliance for Hippocratic Medicine has alleged that mifepristone is dangerous to women and leads to emergency room visits. A 2021 study cited by the plaintiffs to back up their claims was retracted in February after an independent review found that its authors came to inaccurate conclusions.
In April 2023, the Trump-appointed judge Matthew Kacsmaryk of the Northern District of Texas issued a preliminary ruling on the FDA case invalidating the agency’s approval of mifepristone. The ruling sent shock waves far beyond the reproductive-rights world, as it had major implications for the entire pharmaceutical industry, as well as the FDA itself; the ruling suggested that the courts could revoke a drug’s approval even after decades on the market.
The US 5th Circuit Court of Appeals narrowed Kacsmaryk’s decision a week later, allowing the drug to remain on the market, but undid FDA decisions in recent years that made mifepristone easier to prescribe and obtain. That decision limited the time frame in which it can be taken to the first seven weeks of pregnancy and put telemedicine access, as well as access to the generic version of the drug in jeopardy.
Following the 5th Circuit ruling, the FDA and Danco Laboratories sought emergency relief from the Supreme Court, asking the justices to preserve access until it could hear the case. In its legal filing, Danco aptly described the situation as “regulatory chaos.”
SCOTUS issued a temporary stay, maintaining the status quo; the court ultimately decided to take up the case in December 2023.
As all this was unfolding, pro-abortion-rights states across the country were passing what are known as shield laws, which protect medical practitioners who offer abortion care to pregnant patients in states where abortion is banned. This has allowed some providers, including the longtime medication-abortion-advocacy group Aid Access, to mail abortion pills to people who requested them in states like Louisiana and Arkansas.
Though the oral arguments before the Supreme Court begin on Tuesday, it will likely be months before a ruling. Court watchers suspect a decision may be handed down in June. With the US presidential election in the fall, the ruling may become a major campaign issue, especially as abortion access helped galvanize voters in the 2022 midterms.
If the Supreme Court agrees with the plaintiffs that mifepristone should be taken off the market, some in the pharmaceutical industry worry that it will undermine the authority of the FDA, the agency tasked with reviewing and approving drugs based on their safety and efficacy.
“This case isn't about mifepristone,” says Elizabeth Jeffords, CEO of Iolyx Therapeutics, a company developing drugs for immune and eye diseases. Jeffords is a signatory on an amicus brief filed in April 2023 that brought together 350 pharmaceutical companies, executives, and investors to challenge the Texas district court’s ruling.
“This case could have easily been about minoxidil for hair loss. It could have been about Mylotarg for cancer. It could have been about measles vaccines,” Jeffords says. “This is about whether or not the FDA is allowed to be the scientific arbiter of what is good and safe for patients.”
Greer Donley, an associate professor of law at the University of Pittsburgh and an expert on abortion on the law, doesn’t think it’s likely that the court will revoke mifepristone’s approval entirely. Instead, she sees two possible outcomes. The Supreme Court could dismiss the case or could undo the FDA’s decision in 2023 to permanently remove the in-person dispensing requirement and allow abortion by telehealth. “This would be an even more narrow decision than what the 5th Circuit did, but it would still be pretty devastating to abortion access,” she says.
The Supreme Court could also decide that the plaintiffs lack a right to bring the case to court, says David Cohen, a professor of law at Drexel University whose expertise is in constitutional law and gender issues. “This case could get kicked out on standing, meaning that the plaintiffs aren't the right people to bring this case,” he says. “If most of the questions are about standing, that will give you a sense that that's what the justices are concerned about.”
As the current Supreme Court is considered virulently antiabortion, reproductive-health-care workers are already preparing for the worst. Some telehealth providers have already floated a backup plan: offering misoprostol-only medication abortions. This is less than ideal, as the combination of pills is the current standard of care and offers the best results; misoprostol on its own can cause additional cramping and nausea. For some providers who may have to choose between misoprostol-only or nothing, it’s better than nothing.
Abortion-rights activists have no plans to give up on telehealth abortions, regardless of the outcome of this particular case. “Let us be clear, Hey Jane will not stop delivering telemedicine abortion care, regardless of the outcome of this case,” says Hey Jane’s CEO and cofounder, Kiki Freedman.
“They’re not going to stuff the genie back in the bottle,” Kay says.
31 notes
·
View notes
Text
5 notes
·
View notes
Text
While the Centers for Disease Control and Prevention (CDC) was promoting the COVID-19 vaccine as “safe and effective” for children and teens, Pfizer was studying whether and how much it damaged their hearts, according to a DailyClout report on internal Pfizer documents.
The documents show that Pfizer conducted a “Phase 2/3 Study” in Europe, during which it vaccinated and collected blood samples from children ages 5-11 and 12-15 and tested them for troponin I.
Troponin I, a protein released into the bloodstream when the heart is damaged, is an indicator of subclinical myocarditis.
Pfizer began the study in September 2021, the month before the U.S. Food and Drug Administration (FDA) granted Pfizer emergency use authorization (EUA) for its COVID-19 vaccine for children ages 5-11.
The FDA said it based the EUA on the agency’s “thorough and transparent evaluation of the data” that found “no serious side effects.”
However, Pfizer’s ongoing active surveillance and testing for troponin 1 was an acknowledgment of the undisclosed risk of vaccine-induced myocarditis and pericarditis, according to Dr. Christopher Flowers, who wrote the report on the Pfizer documents for DailyClout.
79 notes
·
View notes
Text
Overall, the new FDA decision “comes at a time when COVID-19 cases are once again climbing. Now, most people 6 months or older in the U.S. are eligible to receive this season’s COVID-19 vaccine, even if they have never been vaccinated against COVID-19 before,” Albert Bourla, chairman and chief executive officer at Pfizer, said in a news release.
The updated vaccines are approved for people 12 and older and are authorized under emergency use for individuals 6 months through 11 years old.
“COVID-19 remains a leading cause of death in the U.S. and poses a significant threat to vulnerable populations, particularly as we enter peak respiratory virus season,” Stéphane Bancel, CEO of Moderna, said in a news release Monday.
Under the Affordable Care Act, most insurance plans are covering the full cost of vaccines, without co-pays. So most insured people will be able to get the updated Covid-19 vaccine at their doctor’s offices or pharmacies, such as CVS or Walgreens, at no cost.
12 notes
·
View notes
Text
Just to let you know things ARE happening behind the scenes, I'll leave this here 👇
Operation Warp Speed Architect Arrested
U.S. Army Rangers on Saturday arrested Operation Warp Speed architect Moncef Slaoui, the Moroccan-born pharmaceutical mogul who in May 2020 spearheaded the administration’s efforts to poison 300 million Americans by January 2021, a source in General Eric M. Smith’s said.
Slaoui largely flew under the radar throughout the Plandemic. The media seldom mentioned his name, focusing instead on publicly influential figures like Fauci, Birx, and Collins, articulate public servants who spoke better English. Our source said Slaoui was relegated to media obscurity because the administration thought he looked shady and that Americans wouldn’t trust him.
And Americans would have been right not to trust a man who spent 30 years as GlaxoSmithKline’s head of vaccines department and was working at Moderna when Trump picked him to helm Warp Speed. At the time, Trump called Slaoui “one of the most respected men in the world in the production and, really, on the formulation of vaccines,” but was merely parroting what subordinates Michael Pence, Alex Azar, Admiral Brett Giroir, and Robert Redfield told him. They and others, our source said, were part of a major conspiracy to deceive President Trump into putting Slaoui in charge of OWS.
Slaoui faced criticism for holding $10 million in Moderna stock options and working as an advisor to Brii Biosciences, a firm with sizable Chinese investments. To avoid a conflict of interest, he begrudgingly resigned from those positions, then began working with then-Health and Human Services Secretary Alex Azar—who praised Slaoui as “arguably the world’s most experienced and successful vaccine developer”—to hasten Warp Speed.
Our source said that Slaoui, despite resigning from Moderna, continued receiving payouts exceeding $56 million after the FDA granted Moderna emergency-use authorization on December 8, 2020.
However, the military was less interested in Slaoui’s financial motivations than his knowledge that Moderna’s experimental vaccine had killed 34 of 600 Phase II trial participants in June 2020. White Hats, our source said, now have a wealth of evidence—physical and digital documents authored by Slaoui—proving he knew the vaccine caused myocarditis and potentially lethal blood clots but never publicly disclosed that information, even after Trump personally asked him if the shots were truly safe and effective.
“We have a treasure chest of incriminating evidence on Slaoui. This guy was one of the biggest violators of the Plandemic. We got a letter he wrote to Pence, saying he knew vaccines would kill people and that they could blame Trump for pushing Operation Warp Speed on the public. We have tons more that will be made available when he faces a military tribunal and hopefully gets hanged. We had more than enough proof to get him,” our source said.
The arrest, he added, came after Gen. Smith talked with Colonel J.D. Keirsey, a White Hat council member and commander of the 75th Ranger Regiment, the U.S. Army’s premier light infantry unit and special operations force within the United States Army Special Operations Command.
On Saturday morning, Rangers bashed down the door to Slaoui’s 7,500-sq-ft home in Zebulon, North Carolina. One of his three sons, Hussein Mohamad Abdul Slaoui, was present and pulled a pistol on the Rangers. He was shot dead, and the Rangers took Slaoui into custody. He shouted, “sayantaqim li allah,” or “Allah will avenge me,” as the Rangers shoved him into a civilian vehicle.
“We got him. We got the bastard,” our source said. “He’s just one of many. But we’re chipping away at them.”
As an aside, Slaoui was fired from chairman of the board of directors of Galvani Bioelectronics, a subsidiary of GlaxoSmithKline, in March 2021 after several young male employees accused him of sexual harassment.
#pay attention#educate yourself#educate yourselves#reeducate yourself#knowledge is power#reeducate yourselves#think for yourself#think for yourselves#think about it#do your homework#do your research#do your own research#question everything#ask yourself questions#ask yourself#truthful news#real news#world news#news#not in the news#you decide
76 notes
·
View notes