#ICH Guideline
Text
621 no chapter chromatography was update at on date 1-dec-2022
What is update change
Definitions
Here Definitions and chromatograms both unified and give title Definitions
Give method dwell volume determine being added
Definitions add definitions size-exclusion chromatography (Distribution constant, Retention time of an unretained compound, Total mobile phase time)
Also other definitions plate height, reduced plate height, plate number etc
In TLC…
View On WordPress
#Analytical chemistry#Analytical method development hplc#Chromatography#Gas chromatography (gc)#GMP Updates#ICH Guideline#Internal standard
0 notes
Text

FREEZE THAW CHAMBERS manufacturers india
#Freeze thaw Chambers#Freeze Thaw Chambers manufacturers#Freeze Thaw Chambers manufacturers in mumbai#Freeze thaw cycles#freezing and Thawing#Freeze Thaw Chambers manufacturers in india#Plant Growth Environment Chambers#Laboratory refrigerators#CFC free Cooling Chambers#Cooling chambers as per ICH guidelines#Cooling#Chambers as per FDA guidelines#Cold Room Solution#Cold Room Solutions
0 notes
Text
Different Climatic Zones for Stability: ICH Guidelines
Different Climatic Zones for Stability: ICH Guidelines.
Different Climatic Zones for Stability (ICH Guidelines on Stability Testing): The Introduction to Climatic Zones in Stability Studies involves understanding how different weather conditions around the world can affect the quality of medicines. When we talk about climatic zones in the pharmaceutical industry, we’re referring to different areas of the world that have similar weather patterns.
This…

View On WordPress
#Climatic Zones for Stability#Different Climatic Zones for Stability#ICH Guidelines on Stability Testing
0 notes
Text
The #Safety Specification, which is a summary of the product's risk profile based on #preclinical and #clinical data, as well as any relevant information from similar products or classes of products. It identifies the important identified risks, important potential risks in #Pharmacovigilance
Read Full Article : https://www.vigilarebp.com/scope-of-the-safety-specification-ich-e2e-guideline-in-pharmacovigilance-practice/
#pharma#ICH#ICH guidelines#drug development#adverseevents#adr#drugsafety#vigilare#healthcare#big pharma#vigilarebiopharma#pharmacovigilance
0 notes
Text
2023 Updates for Clinical Research Associates and Clinical Research Monitors
Common clinical trial guidelines used for monitors are designed to ensure the safety and accuracy of the data collected. These guidelines help to make sure that all participants in the trial are treated fairly and ethically, as well as ensuring that the results of the trial will be useful for medical research.
One important guideline is that the monitor must be independent from both the sponsor and investigator. The monitor should have no interest in or influence on the study's outcome, and must have complete access to any documents or records related to conducting the trial. Additionally, they are responsible for ensuring that all protocols are followed correctly, data is correctly recorded and stored, and any adverse events or reactions reported accurately and promptly.
Another key guideline is that monitors must act in accordance with Good Clinical Practice (GCP) guidelines established by International Conference on Harmonization (ICH). GCP outlines procedures for clinical trials involving human subjects so that ethical practices can be maintained throughout a study. It covers many topics including informed consent, protocol review, quality assurance/monitoring, investigator qualification requirements, patient safety procedures, and data verification methods.
Additionally, monitors may use other standards such as The Code of Federal Regulations (CFR), which is used by US Food & Drug Administration (FDA) to regulate drugs; International Committee on Harmonization (ICH) E6R2 ethical guidelines; European Medicines Agency’s Guidelines on Good Clinical Practice (GCP); World Health Organization’s International Ethical Guidelines for Biomedical Research Involving Human Subjects; or local regulations specified by each country’s health ministry.
Overall, these guidelines help to ensure that monitors remain impartial during a clinical trial - this helps to protect participant safety as well as providing reliable data for researchers later down the line.
Clinical research monitors are responsible for ensuring the safety of participants in clinical trials and the accuracy of data collected. In 2023, there have been several updates to guidelines for clinical research monitors that they should be aware of.
The United States Food and Drug Administration (FDA) has released Clinical Trials Guidance Documents that provide advice on the conduct of clinical trials, good clinical practice, and human subject protection. These documents outline the standards that must be met in order to ensure a safe and ethical trial environment.
Clinical research associates (CRAs) play a key role in medical research, ensuring that clinical trials are conducted according to the highest standards of quality, safety and ethics. In light of this importance, the U.S. Food and Drug Administration (FDA) has recently released new guidelines for CRAs conducting clinical trials. These guidelines provide an important framework to ensure that all research is conducted responsibly and ethically while protecting participants’ rights and safety. The FDA’s new guidelines focus on three main areas: data security, participant monitoring protocol, and communication with sponsors.
First, the FDA has established stringent data security measures to protect trial participants’ information during all stages of the trial process. This includes measures such as encryption of sensitive data, physical access control systems for secure areas where information is stored or processed, and regular backups of critical data sets to prevent any potential losses due to cyber-attacks or system malfunctions.
Second, the FDA requires that participation by CRAs in clinical trials include appropriate monitoring protocols designed to minimize risks associated with various trial procedures. This may include frequent communication with study sponsors about changes in protocol or patient status; close observation of trial participants; review and approval of all research documents before their use; scheduling regular safety assessments; and maintaining accurate records of all activities associated with each trial phase.
Finally, CRAs must maintain open communication channels with sponsors throughout the duration of a clinical trial in order to promptly report any changes in protocol or patient status that may require further review or approval from sponsors. Additionally, CRAs need to be trained on how to effectively communicate any necessary updates or potential issues related to regulatory compliance so they can ensure effective oversight over the entire course of a study period.
The FDA's new clinical trial guidelines provide an essential reference point for CRAs responsible for conducting medical research safely and ethically while protecting participants' rights and well-being. With these comprehensive guidelines in place, CRAs now have an even greater responsibility than ever when it comes to ensuring the success of health-related studies around the world.
Electronic Systems, Electronic Records, and Electronic Signatures in Clinical Investigations: Questions and Answers 3/15/2023
Considerations for the Design and Conduct of Externally Controlled Trials for Drug and Biological Products 1/31/2023
Clinical Investigator Administrative Actions — Disqualification 12/01/2022
Acute Myeloid Leukemia: Developing Drugs and Biological Products for Treatment 10/17/2022
Tissue Agnostic Drug Development in Oncology 10/17/2022
Characterizing, Collecting, and Reporting Immune-Mediated Adverse Reactions in Cancer Immunotherapeutic Clinical Trials 10/17/2022
Ethical Considerations for Clinical Investigations of Medical Products Involving Children 09/23/2022
Submitting Documents Using Real-World Data and Real-World Evidence to FDA for Drug and Biological Products 09/08/2022
We must always review the Handbook for Good Clinical Research Practice (GCP), which provides guidance on implementation of GCP standards. Additionally, the International Council for Harmonisation (ICH) has published Efficacy Guidelines which address design, conduct, safety and reporting of clinical trials.
2023 Good Clinical Practice Guidelines for Clinical Research Associates:
Clinical research associates must stay up-to-date on the latest clinical research regulations, guidance documents, and technology advancements in order to ensure ethical and compliant clinical trial management.
Clinical research associates must establish effective communication with all members of the research team to facilitate the exchange of information regarding study updates, timelines, and protocols.
Clinical research associates are responsible for performing accurate data entry into relevant databases or case report forms (CRFs) as part of their role in documenting results from clinical trials.
Clinical research associates must ensure that informed consent is obtained from all participants in accordance with local regulations and international ethical standards.
Clinical research associates must be knowledgeable about relevant In Vitro Diagnostic (IVD) device regulations and requirements for providing evidence of conformity, accuracy, and effectiveness prior to use in a study.
Clinical research associates should create detailed visit plans for each participant in order to maximize the efficiency of visits to investigator sites during a study without compromising data quality or patient safety.
Clinical research associates should conduct regular quality assurance (QA) activities such as source document verification (SDV), query resolution, audit trails, monitoring reports review, reconciliation activities etc., ensuring data accuracy throughout the course of a study period.
During audits or inspections conducted by regulatory authorities or ethics committees, clinical research associates must be prepared to present comprehensive documentation demonstrating compliance with GCP principles and local regulations governing clinical trial conduct.
The European Medicines Agency (EMA) has also released a Clinical Trials Regulation which harmonises processes for assessment and supervision of clinical trials throughout the EU. This regulation outlines requirements to ensure patient safety during a trial as well as evaluation procedures for new drugs or treatments being tested in a trial setting. Finally, The EQUATOR Network provides study protocols such as SPIRIT and PRISMA-P; diagnostic/prognostic studies such as STARD and TRIPOD; case reports such as CARE; extensions; clinical practice guidelines such as AGREE; all aimed at enhancing quality and transparency in health research publications.
In 2022, the US Food and Drug Administration (FDA) released new clinical trial guidelines that emphasize patient safety. The guidelines mandate that all clinical trials must adhere to a rigorous set of standards in order to ensure patient safety and efficacy.
The new guidelines require research teams to obtain written informed consent from participants prior to initiating any study activity. Abuse of animals is prohibited, and investigators are expected to use only those treatments that have shown potential benefit in animal studies. Additionally, researchers must report any adverse events or reactions during the course of the trial and ensure proper follow up care for affected individuals.
Furthermore, the FDA requires that research teams perform rigorous safety monitoring throughout the course of the trial. Regular data analyses and reviews must be conducted to identify potential risks and unexpected results, which must be reported in real time. Additionally, the FDA requires research teams to implement a system for tracking participant adherence to protocols, including collecting data on missed doses, changes in medication regimens, and other protocol violations.
The FDA also mandates more frequent reporting of results throughout the course of clinical trials. They require researchers to share interim results with stakeholders every six months or whenever significant changes occur in study design or purpose. These reports should include key findings as well as basic information about participant demographics and outcomes associated with each treatment arm.
Finally, the FDA has increased their emphasis on transparency by requiring researchers to disclose detailed information regarding sponsoring organizations and conflicts of interest associated with each study before it begins. This includes information related to payments made by sponsors as well as nonmonetary benefits received by investigators or other individuals associated with the trial.
By 2023, additional provisions will be added to these regulations including enhanced requirements related to diversity among participants; strengthened criteria for evaluating ethical considerations such as protection from harm; expanded definitions related to economic conflict-of-interest disclosure; greater emphasis on appropriate risk/benefit ratios; improved reporting of results utilizing standardized metrics; increased focus on study protocol adherence; enhanced data sharing practices; clear criteria for determining when further review is needed due health concerns; specified mechanisms for measuring patient quality-of-life outcomes; increased accountability through stronger recordkeeping systems; enhanced guidance around informed consent forms; improved methods for monitoring compliance; greater attention paid towards reviewing unpublished manuscripts related to clinical trials; expansion of proposed preventative measures targeting financial misconduct issues such as fraud detection systems; improved oversight mechanisms using Artificial Intelligence technologies such as natural language processing (NLP); and additional efforts aimed at improving public understanding around clinical trials through better communication strategies between sponsors and patients alike.
Stay up to date on clinical trials and your annual ICH GCP certification through one of the most comprehensive courses in the industry.
#fda guidelines for clinical trials pdf#clinical trial compensation guidelines#ich clinical trial guidelines#fda guidelines for clinical trials#ich guidelines for clinical trials#ich guidelines clinical trials#abpi clinical trial compensation guidelines#clinical trial advertising guidelines#clinical trial agreement negotiation guidelines#clinical trial archiving guidelines#clinical trial guidelines#clinical trial monitoring guidelines#clinical trial protocol guidelines#clinical trial reporting guidelines#clinical trials in australia guidelines#clinical trials medical devices guidelines#clinical trials pregnancy guidelines#compensation for clinical trial subjects as per ethical guidelines#consort guidelines for clinical trials#contemporary clinical trials author guidelines#data entry guidelines clinical trials#ema guidelines clinical trials#ema guidelines for clinical trials#ema guidelines for clinical trials pdf#ethical guidelines for clinical trials#eudralex volume 10 clinical trials guidelines#fda guidelines for clinical trials ppt#fda guidelines for monitoring clinical trials#first in human clinical trials guidelines fda#gcp guidelines for clinical trials
0 notes
Text
sv5 | that lavender haze

summary: [ florist!sebastian vettel x f!driver!reader — social media au ] your florist husband spoils you with his creations
faceclaim: phoebe tonkin
author’s note: seb the love of my life <3
[ masterlist / guidelines / lola's masterlist / series masterlist ]




liked by sebastianvettel, lewishamilton, mercedesamgf1 and 35,201,234 others
yourusername catching the waves 🏄🏻♀️
view comments
sebastianvettel Ich liebe dich 🥰
↪ yourusername can't wait to be home with you again 💗
ausgp can we keep you down under please? 🦘
↪ f1mia back off 🦅🇺🇸
user mother AND mommy omg
mickschumacher can you teach me how to surf instead 🙏 lewishamilton doesn't understand that not everyone is naturally talented at everything
↪ lewishamilton i don't know what to tell you, mate 😂 keep calm and keep your balance, it's all chill
↪ mickschumacher easy for you to say 🙄 you're not the one drinking seawater every five minutes
yourusername has added to their story




liked by yourusername, mickschumacher, charles_leclerc and 124,129 others
tagged: yourusername
sebastianvettel Welcome home yourusername ❤️ the flowers missed you and so did I 😉
view comments
user i love how y/n's husband's instagram is basically just a fanpage for her 😂
↪ user nah you can't forget the flowers ‼️
↪ user seb loves two things in life and they're his flowers and his wife 😌
user i don't even go here but i'm all for the golden retriever and black cat vibes 🤭
mickschumacher seb i have a bee problem in my backyard...
↪ charles_leclerc you know you could just text him right 🙃
↪ mickschumacher he checks his phone once every three months if your name isn't y/n l/n-vettel 💀
↪ sebastianvettel and I'm not ashamed of it 😄 but what can I help you with?
↪ mickschumacher a colony of bees moved into my garden 😅 i don't mind them but is there anything i should watch out for?
↪ sebastianvettel As long as they're not being overly aggressive you shouldn't have any problems 👍 keep me updated though
↪ mickschumacher thanks seb you're a lifesaver 😊
yourusername thanks for the flowers schatz 😘
↪ user ugh they're so Parents 😭
liked by charles_leclerc
↪ user charles liked your comment 😂 i guess even the drivers agree
↪ landonorris you didn't hear it from me but seb and y/n are the unofficial official grid parents



liked by mickschumacher, lewishamilton, yourusername, and 23,109,234 others
tagged: sebastianvettel
mercedesamgf1 We have a special guest this weekend at the #JapaneseGP 🐝 sebastianvettel is here at Suzuka to promote biodiversity and build some bee hotels with the drivers 💪
view comments
charles_leclerc Appreciated the art tips 😉
user this man 😭 "what do you think about this weekend's race?" "well obviously my wife is going to win everything"
↪ user as he should honestly
↪ user when you're in a "being a wife guy" competition and your opponent is sebastian vettel 💀
kevinmagnussen Thanks a lot Seb 😂 the kids want beehives now!
↪ sebastianvettel Glad to know that someone was listening when I was giving my talk about the role that bees play in our ecosystem 😔
↪ landonorris in my defence someone brought cookies and i was hungry...
↪ sebastianvettel you are 24 years old, Lando
↪ user why can i feel seb's disappointment through an instagram comment 😭
yourusername sometimes i wonder if he'd leave me for his bees 😂
↪ lewishamilton don't worry, you can crash on my couch if he does. roscoe needs a permanent babysitter
↪ yourusername two decades of friendship and that's all you see me as?
↪ lewishamilton let me by during the grand prix and i'll think about it
↪ yourusername mercedesamgf1 i'm telling toto
↪ sebastianvettel I would never leave you for bees, liebling. Clean energy, on the other hand...



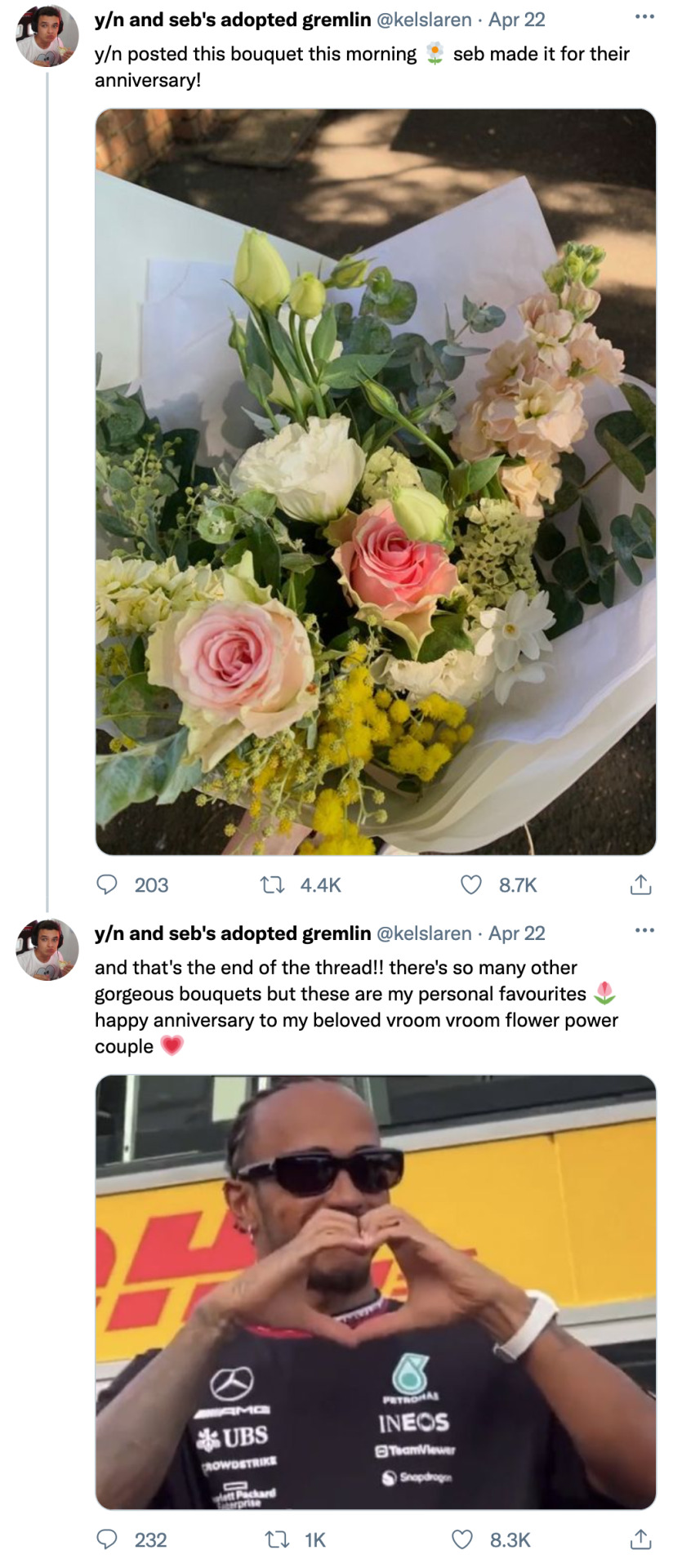
liked by sebastianvettel, lewishamilton, susie_wolff and 132,293,402 others
tagged: sebastianvettel
yourusername Happy anniversary, my love 💐 12 years and counting
view comments
user the bouquet emoji because he's a florist omg 🥹
user my favorite thing ever is how 5-time wdc y/n l/n-vettel's husband is Just Some Guy who's completely smitten with his wife and makes her all the bouquets she could ever want 😭
↪ user they're like cottagecore addams 😩 i adore them so much
↪ user COTTAGECORE ADDAMS HELP 🤣🤣🤣
susie_wolff Congratulations and our best wishes!
↪ yourusername thank you ❤️😊 the same to you and toto!
sebastianvettel I'm the luckiest man in the world to be able to call you my wife and partner 💗 You're P1 forever, especially in my heart




likes and reblogs are appreciated!
taglist: @scenesofobx @vellicora @boiohboii @julesbabey @flannelforthetoads @misartymis
#solwriting#f1 x reader#formula 1 x reader#formula one x reader#formula 1#f1#f1 fanfic#formula 1 fanfic#formula 1 x you#f1 social media au#f1 imagine#f1 smau#sebastian vettel#sebastian vettel x reader#sebastian vettel x you#sebastian vettel imagine#sebastian vettel fanfic
1K notes
·
View notes
Text
#ICH Q12#ICH#Regulatory Affairs#Product Lifecycle Management#Regulatory Guidelines#Medical Devices#Post Approvals#Submissions#post-approval submissions
0 notes
Text
Ich bin wirklich kein Freund von Callouts und habe hin- und her überlegt, ob ich diesen Post mache. Ich halte dieses Thema jedoch für wichtig und habe das still über einen langen Zeitraum beobachtet, bis heute. Vorhin sprang mir ein Blog aus unserer Community ins Auge mit einer gestohlenen Promo. Dabei handelt es sich um zwei GIF Panels und eine zugehörige Icon Border, die von einem bekannten Content Creator als Commission (kostenpflichtig) für einen anderen Blog gemacht worden sind. Unser Mitglied hat keinen Credit auf seinem Profil und es handelt sich nicht um den Blog, für den diese Promo gestaltet wurde. Das ist an Dreistigkeit kaum noch zu überbieten. Es werden schon oft genug Charakter PSDs ohne Credit benutzt (von Künstlern, die in ihren Rules um Credit bitten). Dieser Ort ist nicht das alte VZ wo sich keiner um gestohlene Grafiken geschert hat. Tumblr besitzt in dieser Hinsicht eine relativ straffe Etikette, Künstler und Content Creator haben hier gewisse Rechte und werden aktiv von einer großen Userbase unterstützt. Diebe sind absolut nicht gern gesehen, Unwissenheit schützt nicht vor Strafe und es spricht sich schnell herum wenn jemand repostet oder stiehlt. Bitte supportet so ein Verhalten nicht! Die meisten Ressourcen-Blogs sind mit Guidelines ausgestattet, man kann doch das Mindestmaß an Anstand besitzen und sich daran halten, besonders wenn das meiste auch noch umsonst zur Verfügung gestellt wird. Rant ende.
Edit: Die Angelegenheit hat sich friedlich geklärt. Ich nehme meinen boshaft klingenden Ton von gestern zurück, die inhaltliche Message bleibt dieselbe. Bitte geht gewissenhaft mit dem Eigentum anderer um.
14 notes
·
View notes
Note
Als Werbung auf tumblr noch vor allem wtf war, hab ich mal einen Blog gestartet, in dem ich die absurdestes Screenshots zusammengetragen habe. Letztes Jahr hab ich dann eine Warnung bekommen, dass ich gegen Community Guidelines verstoßen habe. Weil das Bild in der Werbung, die real auf tumblr geschaltet war, zu "sexuell" war (und es war einfach nur ein Bild von einer Frau, die vollständig bekleidet rückwärts auf einem Stuhl sitzt)... man muss es lieben, wa.
Tumblr be like:

129 notes
·
View notes
Text

宮沢さんはmRNAワクチンやレプリコンの市場投入に反対ですよね? その点を私は疑っていません。 しかし今現在のお考えは今後の研究開発は容認もしくは推進ですよね? 違うのなら「今後の研究開発も反対」と表明して頂けませんか? そうすれば惑わされる人もいなくなると思います。容認推進ならもちろんお返事は結構です。 私にはmRNAやレプリコンの根本的な技術的問題を明言する村上さんと荒川さんが目障りで仕方無く、ふわっとした言い方でネガティブキャンペーンをしているようにしか見えていません。反論があれば具体的にして頂けますか? 反論無ければお返事は結構です。 1. [首相官邸ホームページ, 遺伝子治療とゲノム編集治療の研究開発の現状と課題](https://kantei.go.jp/jp/singi/kenkouiryou/genome/advisory_board/dai4/siryou4-1.pdf…) 2. [厚労省, ICH見解「ウイルスとベクターの排出に関する基本的な考え方」について](https://mhlw.go.jp/web/t_doc?dataId=00tc1096&dataType=1&pageNo=1…) 3. [European Medicines Agency, ICH Consideration General Principles to Address Virus and Vector Shedding, 2009/07](https://ema.europa.eu/en/documents/scientific-guideline/international-conference-harmonisation-technical-requirements-registration-pharmaceuticals-human-use-considerations-general-principles-address-virus-and-vector-shedding_en.pdf…)
2 notes
·
View notes
Text
Difference class A and class b glassware use in laboratory
The terms “Class A” and “Class B” are commonly used to describe different types of glassware in laboratory settings, particularly for volumetric measuring. These classes have specific standards and tolerances, and they serve different purposes. Here’s the difference between Class A and Class B glassware:
Class A Glassware:
Class A glassware is of higher precision and accuracy compared to Class…
View On WordPress
#Analytical chemistry#Chromatography#Gas chromatography (gc)#GMP Updates#Hplc#Hplc vials#ICH Guideline#Internal standard#Method validation#optical rotation#QMS
1 note
·
View note
Text

Walk-In Cooling Chambers manufacturers india
#Walk-In Cooling Chambers#Walk-In Cooling Chambers manufacturers#Walk-In Cooling Chambers manufacturers mumbai#Walk-In Cooling Chambers manufacturers india#Cooling Chambers#Cooling Chambers manufacturers#Cooling Chambers manufacturers mumbai#Cooling Chambers manufacturers india#cooling chamber manufacturers#Laboratory refrigerators#Laboratory refrigerators manufacturers#medical refrigerator manufacturers mumbai#medical refrigerator manufacturers india#CFC free Cooling Chambers#Cooling chambers as per ICH guidelines#Cooling#Chambers as per FDA guidelines#Cold Room Solution#Cold Room Solutions#Cold Room Solutions mumbai#Cold Room Solutions india
0 notes
Text

Our team will be present at the Asian Chemical Forum (ACF) - 5-6 September 2023. If you will be at the conference, do connect with us.
We look forward to the opportunity to understand your requirements and help you in building a safer chemical future!
Email us at 📧 [email protected] to schedule a meeting.
Eurofins Advinus Agrosciences India offers comprehensive product development services to support your global registration needs. Our team has expertise to assess and prove the safety of your products as per requirements of all international guidelines (OECD, EPA, CIPAC, JMAFF, ICH, SANCO, ABNT etc.)
To know more about our services, click on the link - 👉
Agrochemical Development Services
#asianchemicalforum#chemicalindustry#regulations#productdevelopment#pesticides#insecticides#specialitychemicals#cropprotection#EurofinsAdvinus
2 notes
·
View notes
Text
Who is clinical research coordinator?
A Clinical Research Coordinator (CRC) is a vital member of a clinical research team who plays a significant role in the conduct of clinical trials. In this blog, we will explore what a CRC does, their qualifications, and the skills required to be successful in this role. If you want to do clinical research course you must have a bachelor's degree and at least 3,000 hours of experience as a CRA. There are Top Clinical research training institute field gives you potential to make a difference in people's lives by curing diseases and preventing illnesses, thereby enhancing people's quality of life.
What does a Clinical Research Coordinator do?
A CRC is responsible for coordinating the daily operations of a clinical trial. Their primary role is to ensure that the study is conducted in compliance with the protocol, regulatory requirements, and good clinical practice guidelines. If you want to get proper knowledge of clinical research course you must enroll Clinical Research Course The following are some of the key responsibilities of a CRC:
Protocol Development: A CRC may assist in the development of a research protocol, which outlines the study's objectives, methodology, and data analysis plan. They may also help with the design of data collection tools such as case report forms (CRFs) and electronic data capture (EDC) systems.
Recruitment and Screening of Participants: A CRC may identify and screen potential study participants for eligibility criteria, obtain informed consent, and enroll them in the study. They may also be responsible for tracking and maintaining participant files and medical records.
Study Coordination: A CRC is responsible for coordinating study visits, scheduling procedures and tests, and ensuring that study procedures are performed according to the protocol. They may also monitor participant safety and adverse events, and report them to the sponsor and regulatory authorities as required. Clinical Research course helps you to get knowledge in deep about clinical research.
Data Collection and Management: A CRC is responsible for collecting and managing study data, including ensuring that data is accurate, complete, and entered into the study database in a timely manner. They may also perform data quality checks and resolve any discrepancies.
Study Closeout: A CRC may assist in the study closeout process, which includes archiving study documents, completing study reports, and preparing for audits and inspections.
What are the qualifications of a Clinical Research Coordinator?
To become a CRC, one typically needs a bachelor's degree in a relevant field such as nursing, life sciences, or health sciences. Some employers may require additional certifications, such as the Certified Clinical Research Professional (CCRP) certification from the Society of Clinical Research Associates (SoCRA) or the Association of Clinical Research Professionals (ACRP). Additionally, some employers may require previous experience in clinical research, such as working as a clinical research assistant or study coordinator.
What skills are required to be a successful Clinical Research Coordinator?
To be a successful CRC, one needs to have a combination of technical, interpersonal, and organizational skills. The following are some of the key skills required:
Knowledge of Regulations: A CRC should have a good understanding of regulatory requirements for clinical trials, such as Good Clinical Practice (GCP) guidelines, International Council for Harmonisation (ICH) guidelines, and local regulatory requirements.
Attention to Detail: A CRC should have excellent attention to detail to ensure that study procedures are followed correctly and that data is accurate and complete.
Communication Skills: A CRC should have excellent communication skills to effectively communicate with study participants, study staff, sponsors, and regulatory authorities.
Time Management: A CRC should be able to manage their time effectively to ensure that study procedures are performed according to the timeline outlined in the protocol.
Problem Solving: A CRC should be able to identify and solve problems that arise during the course of the study, such as adverse events or protocol deviations.
In conclusion, a Clinical Research Coordinator is an essential member of a clinical research team who plays a critical role in the successful conduct of clinical trials. They are responsible for coordinating the daily operations of the study, ensuring that it is conducted in compliance with the protocol, regulatory requirements, and good clinical practice guidelines. Hence, Clinical Research Training is the best way to learn easily clinical research. To be a successful CRC, one needs to have a combination of technical, interpersonal, and organizational skills.
2 notes
·
View notes
Text

I posted 4,921 times in 2022
That's 402 more posts than 2021!
10 posts created (0%)
4,911 posts reblogged (100%)
Blogs I reblogged the most:
@fiddlepickdouglas
@mimi-mindless
@willex-molina
@saladbroth
@dingdongyouarewrong
I tagged 2,334 of my posts in 2022
#spatort - 209 posts
#warrior nun - 206 posts
#esc22 - 190 posts
#tatort saarbrücken - 126 posts
#wn s2 - 122 posts
#german stuff - 114 posts
#goncharov - 91 posts
#jatp - 90 posts
#ofmd - 76 posts
#unreality - 74 posts
Longest Tag: 139 characters
#but on the other hand such a big fence and them basically locking him in there and then chasing him down when he's already had zero privacy
My Top Posts in 2022:
#5
Gerade Kleo geschaut und muss schon sagen bin ein riesen Fan von Anne im Hemd, den geschlitzten Ärmeln mit Schulterholster
I am looking respectfully 👀
5 notes - Posted August 26, 2022
#4
Eine Freundin hat über die Arbeit Zugriff auf die Saarbrücker Zeitung und hat mir den Artikel abfotografiert und da waren dann wohl noch diese Fotos drin (leider nicht die beste Quali durch das Fotografieren)


7 notes - Posted May 31, 2022
#3
Ich habe gerade Spatort brainrot und bitte sagt mir, dass ihr Pushing Daisies noch kennt aber auf jeden Fall Spatort!PushingDaisiesAU
Leo als the Piemaker, der Morde aufklärt, indem er kurzzeitig Tote wieder auferweckt
Adam als sein bester Freund aus der Jugend, der eines Tages verschwand und dann Jahre später auf einer Weltreise ums Leben gekommen ist
Esther als Emerson, der grimmige Privatermittler, der widerwillig auf Leos Hilfe angewiesen ist.
Und Pia als Olive damit sie Kuchen essen kann wann sie will.
Für die Beerdigung wird Adam nach Saarbrücken gebracht, wo Leo beim Ermitteln über ihn stolpert und ihn wiederbelebt und dadurch ausversehen Lausch umbringt
Und dann muss Adam bei Leo einziehen, damit niemand merkt, dass Adam lebt. Sie lösen gemeinsam Mordfälle (unter anderem Adams) aber können sich nicht berühren, weil Adam sonst wieder stirbt und ngl das ist für die zwei soften bois vermutlich das schlimmste an der ganzen Situation.
Roland Schürk sieht Adam irgendwann und versucht das Geheimnis auffliegen zu lassen, um ihm nochmal eine reinzuwürgen.
12 notes - Posted April 12, 2022
#2
I absolutely loved Warrior Nun s2 but how did seriously none of these professionally trained fighters and strategists consider that Vincent also has a cross necklace and can read their secret code I asdjsjd-
17 notes - Posted November 11, 2022
My #1 post of 2022
Since I rated zitti e buoni last year it's time to look at the final performance of Stefania according to the official Winning Song Guidelines, aka the Love, Love, Peace, Peace Ranking. Let's go
Get everyone's attention
starting with the harmonies straight away 1/1
Drums! There has to be drums, +1 for gorgeous topless men and/or a grandmother
Sadly no drums or topless men or gradnmothers in sight 0/2
Using an old traditional folklore instrument, +1 for an old man, +1 if he has a beard
They had some kind of flute and plenty of beards, not all ages are clearly definable but we're gonna go with a fail on that one 2/3
A violin
Not a violin exactly but a contrabass 0.5/1
Add A DJ who pretends to scratch
No DJ but we'll give half credits for the breakdancer & rapping 0.5/1
Costumes, something that the viewers will notice
The pink hat alone nailed that one 1/1
The song is about love or peace
A mother's love and love for their home country during war times definitely works 1/1
6/10 for the technical parts so let's look at execution
Let the song beginn with passion
Enough said 1/1
Let the wind begin to flow
No wind machines on stage 0/1
Look into the TV camera
Hard to tell with the hat but there's some definite stares 1/1
Smile
No smiles this time 0/1
Fill the stage with light
Lights coming from all directions, even the floor 1/1
Dancers will join us
The dancers are there from the beginning but there's definitely dancers 1/1
Old women baking bread
No fresh bread on stage 0/1
See the full post
195 notes - Posted May 15, 2022
Get your Tumblr 2022 Year in Review →
#tumblr2022#year in review#my 2022 tumblr year in review#your tumblr year in review#I'm sorry#Or you're welcome#Whichever works for you
1 note
·
View note
Text
The Importance of Chemical Testing in Dubai's Pharmaceutical Industry
Dubai's pharmaceutical industry plays a critical role in healthcare, providing essential medicines and healthcare products to local and global markets. Central to the success and safety of this industry is the rigorous testing conducted by chemical testing labs in Dubai. This blog explores the pivotal role of chemical testing in Dubai's pharmaceutical sector, highlighting its importance in ensuring product quality, safety, regulatory compliance, and innovation.
Introduction to Chemical Testing in Pharmaceuticals
Chemical testing in the pharmaceutical industry involves comprehensive analysis of active pharmaceutical ingredients (APIs), excipients, formulations, and finished products. These tests are essential to verify the identity, purity, potency, and stability of pharmaceutical products, ensuring they meet strict regulatory requirements before they reach consumers.
Importance of Chemical Testing in Dubai's Pharmaceutical Industry
1. Quality Assurance
Chemical testing labs in Dubai ensure that pharmaceutical products meet stringent quality standards set by regulatory authorities such as the Dubai Health Authority (DHA) and international bodies like the International Conference on Harmonisation (ICH). By conducting thorough analysis and testing, these labs verify the consistency, efficacy, and safety of medicines, preventing potential risks to patient health.
2. Regulatory Compliance
Compliance with regulatory standards is paramount in the pharmaceutical industry to ensure public safety and market access. Chemical testing labs help pharmaceutical companies in Dubai adhere to regulations such as Good Manufacturing Practices (GMP), Pharmacopeial standards (e.g., USP, BP, EP), and guidelines on stability testing and validation.
3. Product Development and Innovation
Chemical testing supports pharmaceutical research and development (R&D) by providing critical data and insights. Testing methodologies such as High-Performance Liquid Chromatography (HPLC), Mass Spectrometry (MS), and Nuclear Magnetic Resonance (NMR) spectroscopy are used to analyze molecular structures, detect impurities, and optimize formulations, facilitating the development of safe and effective medicines.
4. Safety and Efficacy
Ensuring the safety and efficacy of pharmaceutical products is a primary concern. Chemical testing labs in Dubai perform microbiological testing, endotoxin testing, and sterility testing to detect microbial contamination and ensure products are free from harmful pathogens that could compromise patient health.
5. Batch Release and Quality Control
Before pharmaceutical products can be released to the market, they undergo batch release testing to confirm they meet pre-defined specifications for identity, purity, and potency. Chemical testing labs play a pivotal role in batch release and quality control, providing the necessary assurance that each batch of medicine is safe and effective for patient use.
Advanced Testing Techniques and Methodologies
Chemical testing labs in Dubai utilize advanced technologies and methodologies tailored to the specific needs of the pharmaceutical industry:
1. Chromatography
High-Performance Liquid Chromatography (HPLC): Separates and quantifies components in a sample, such as APIs and impurities, with high resolution and sensitivity.
Gas Chromatography (GC): Analyzes volatile compounds and residual solvents in pharmaceutical formulations to ensure compliance with safety and quality standards.
2. Mass Spectrometry (MS)
Liquid Chromatography-Mass Spectrometry (LC-MS): Identifies and quantifies trace-level impurities and degradation products in complex matrices, supporting stability testing and formulation optimization.
Inductively Coupled Plasma-Mass Spectrometry (ICP-MS): Analyzes metals and metalloids in pharmaceutical products, ensuring compliance with permissible limits and safety guidelines.
3. Spectroscopy
Fourier Transform Infrared Spectroscopy (FTIR): Characterizes molecular structures and identifies functional groups in pharmaceutical ingredients and formulations.
UV-Vis Spectroscopy: Quantifies concentration and purity of APIs and excipients based on their absorption of ultraviolet-visible light.
Ensuring Regulatory Compliance
Chemical testing labs in Dubai adhere to international standards and guidelines for pharmaceutical testing, including:
ISO/IEC 17025 Accreditation: Demonstrates technical competence and reliability in testing and calibration services.
Good Laboratory Practices (GLP): Ensures laboratories maintain high standards of quality, traceability, and data integrity throughout testing processes.
Pharmacopeial Standards: Follows monographs and methods outlined in pharmacopeias (e.g., USP, BP, EP) for testing pharmaceutical ingredients and products.
Case Studies: Leading Chemical Testing Labs in Dubai
1. Dubai Central Laboratory (DCL)
Operated by Dubai Municipality, DCL offers comprehensive testing services for pharmaceuticals, ensuring compliance with local and international standards. It supports regulatory compliance and product quality for pharmaceutical companies in Dubai.
2. SGS Gulf Limited
SGS Gulf Limited provides specialized testing and certification services for pharmaceutical products, supporting quality assurance and regulatory compliance. Its expertise in microbiological testing and stability studies helps pharmaceutical companies meet industry standards.
3. Intertek
Intertek offers a wide range of pharmaceutical testing services, including method development, validation, and stability testing. Its global network and technical capabilities support pharmaceutical companies in Dubai throughout the product lifecycle.
4. METS Laboratories
METS Laboratories delivers advanced analytical testing solutions for pharmaceuticals, ensuring safety, efficacy, and regulatory compliance. Its laboratories in Dubai provide comprehensive testing services for APIs, finished products, and raw materials.
Conclusion
Chemical testing plays a crucial role in Dubai's pharmaceutical industry by ensuring the safety, quality, and regulatory compliance of medicines. Through advanced testing methodologies, adherence to international standards, and continuous innovation, chemical testing lab in Dubai support the development and manufacturing of safe and effective pharmaceutical products. As Dubai continues to expand as a global healthcare hub, the role of chemical testing in maintaining high standards of product integrity and patient safety will remain indispensable. Pharmaceutical companies in Dubai benefit from partnering with accredited chemical testing labs to navigate regulatory complexities, mitigate risks, and deliver high-quality medicines to global markets.
0 notes