#fda guidelines for clinical trials pdf
Text
2023 Updates for Clinical Research Associates and Clinical Research Monitors
Common clinical trial guidelines used for monitors are designed to ensure the safety and accuracy of the data collected. These guidelines help to make sure that all participants in the trial are treated fairly and ethically, as well as ensuring that the results of the trial will be useful for medical research.
One important guideline is that the monitor must be independent from both the sponsor and investigator. The monitor should have no interest in or influence on the study's outcome, and must have complete access to any documents or records related to conducting the trial. Additionally, they are responsible for ensuring that all protocols are followed correctly, data is correctly recorded and stored, and any adverse events or reactions reported accurately and promptly.
Another key guideline is that monitors must act in accordance with Good Clinical Practice (GCP) guidelines established by International Conference on Harmonization (ICH). GCP outlines procedures for clinical trials involving human subjects so that ethical practices can be maintained throughout a study. It covers many topics including informed consent, protocol review, quality assurance/monitoring, investigator qualification requirements, patient safety procedures, and data verification methods.
Additionally, monitors may use other standards such as The Code of Federal Regulations (CFR), which is used by US Food & Drug Administration (FDA) to regulate drugs; International Committee on Harmonization (ICH) E6R2 ethical guidelines; European Medicines Agency’s Guidelines on Good Clinical Practice (GCP); World Health Organization’s International Ethical Guidelines for Biomedical Research Involving Human Subjects; or local regulations specified by each country’s health ministry.
Overall, these guidelines help to ensure that monitors remain impartial during a clinical trial - this helps to protect participant safety as well as providing reliable data for researchers later down the line.
Clinical research monitors are responsible for ensuring the safety of participants in clinical trials and the accuracy of data collected. In 2023, there have been several updates to guidelines for clinical research monitors that they should be aware of.
The United States Food and Drug Administration (FDA) has released Clinical Trials Guidance Documents that provide advice on the conduct of clinical trials, good clinical practice, and human subject protection. These documents outline the standards that must be met in order to ensure a safe and ethical trial environment.
Clinical research associates (CRAs) play a key role in medical research, ensuring that clinical trials are conducted according to the highest standards of quality, safety and ethics. In light of this importance, the U.S. Food and Drug Administration (FDA) has recently released new guidelines for CRAs conducting clinical trials. These guidelines provide an important framework to ensure that all research is conducted responsibly and ethically while protecting participants’ rights and safety. The FDA’s new guidelines focus on three main areas: data security, participant monitoring protocol, and communication with sponsors.
First, the FDA has established stringent data security measures to protect trial participants’ information during all stages of the trial process. This includes measures such as encryption of sensitive data, physical access control systems for secure areas where information is stored or processed, and regular backups of critical data sets to prevent any potential losses due to cyber-attacks or system malfunctions.
Second, the FDA requires that participation by CRAs in clinical trials include appropriate monitoring protocols designed to minimize risks associated with various trial procedures. This may include frequent communication with study sponsors about changes in protocol or patient status; close observation of trial participants; review and approval of all research documents before their use; scheduling regular safety assessments; and maintaining accurate records of all activities associated with each trial phase.
Finally, CRAs must maintain open communication channels with sponsors throughout the duration of a clinical trial in order to promptly report any changes in protocol or patient status that may require further review or approval from sponsors. Additionally, CRAs need to be trained on how to effectively communicate any necessary updates or potential issues related to regulatory compliance so they can ensure effective oversight over the entire course of a study period.
The FDA's new clinical trial guidelines provide an essential reference point for CRAs responsible for conducting medical research safely and ethically while protecting participants' rights and well-being. With these comprehensive guidelines in place, CRAs now have an even greater responsibility than ever when it comes to ensuring the success of health-related studies around the world.
Electronic Systems, Electronic Records, and Electronic Signatures in Clinical Investigations: Questions and Answers 3/15/2023
Considerations for the Design and Conduct of Externally Controlled Trials for Drug and Biological Products 1/31/2023
Clinical Investigator Administrative Actions — Disqualification 12/01/2022
Acute Myeloid Leukemia: Developing Drugs and Biological Products for Treatment 10/17/2022
Tissue Agnostic Drug Development in Oncology 10/17/2022
Characterizing, Collecting, and Reporting Immune-Mediated Adverse Reactions in Cancer Immunotherapeutic Clinical Trials 10/17/2022
Ethical Considerations for Clinical Investigations of Medical Products Involving Children 09/23/2022
Submitting Documents Using Real-World Data and Real-World Evidence to FDA for Drug and Biological Products 09/08/2022
We must always review the Handbook for Good Clinical Research Practice (GCP), which provides guidance on implementation of GCP standards. Additionally, the International Council for Harmonisation (ICH) has published Efficacy Guidelines which address design, conduct, safety and reporting of clinical trials.
2023 Good Clinical Practice Guidelines for Clinical Research Associates:
Clinical research associates must stay up-to-date on the latest clinical research regulations, guidance documents, and technology advancements in order to ensure ethical and compliant clinical trial management.
Clinical research associates must establish effective communication with all members of the research team to facilitate the exchange of information regarding study updates, timelines, and protocols.
Clinical research associates are responsible for performing accurate data entry into relevant databases or case report forms (CRFs) as part of their role in documenting results from clinical trials.
Clinical research associates must ensure that informed consent is obtained from all participants in accordance with local regulations and international ethical standards.
Clinical research associates must be knowledgeable about relevant In Vitro Diagnostic (IVD) device regulations and requirements for providing evidence of conformity, accuracy, and effectiveness prior to use in a study.
Clinical research associates should create detailed visit plans for each participant in order to maximize the efficiency of visits to investigator sites during a study without compromising data quality or patient safety.
Clinical research associates should conduct regular quality assurance (QA) activities such as source document verification (SDV), query resolution, audit trails, monitoring reports review, reconciliation activities etc., ensuring data accuracy throughout the course of a study period.
During audits or inspections conducted by regulatory authorities or ethics committees, clinical research associates must be prepared to present comprehensive documentation demonstrating compliance with GCP principles and local regulations governing clinical trial conduct.
The European Medicines Agency (EMA) has also released a Clinical Trials Regulation which harmonises processes for assessment and supervision of clinical trials throughout the EU. This regulation outlines requirements to ensure patient safety during a trial as well as evaluation procedures for new drugs or treatments being tested in a trial setting. Finally, The EQUATOR Network provides study protocols such as SPIRIT and PRISMA-P; diagnostic/prognostic studies such as STARD and TRIPOD; case reports such as CARE; extensions; clinical practice guidelines such as AGREE; all aimed at enhancing quality and transparency in health research publications.
In 2022, the US Food and Drug Administration (FDA) released new clinical trial guidelines that emphasize patient safety. The guidelines mandate that all clinical trials must adhere to a rigorous set of standards in order to ensure patient safety and efficacy.
The new guidelines require research teams to obtain written informed consent from participants prior to initiating any study activity. Abuse of animals is prohibited, and investigators are expected to use only those treatments that have shown potential benefit in animal studies. Additionally, researchers must report any adverse events or reactions during the course of the trial and ensure proper follow up care for affected individuals.
Furthermore, the FDA requires that research teams perform rigorous safety monitoring throughout the course of the trial. Regular data analyses and reviews must be conducted to identify potential risks and unexpected results, which must be reported in real time. Additionally, the FDA requires research teams to implement a system for tracking participant adherence to protocols, including collecting data on missed doses, changes in medication regimens, and other protocol violations.
The FDA also mandates more frequent reporting of results throughout the course of clinical trials. They require researchers to share interim results with stakeholders every six months or whenever significant changes occur in study design or purpose. These reports should include key findings as well as basic information about participant demographics and outcomes associated with each treatment arm.
Finally, the FDA has increased their emphasis on transparency by requiring researchers to disclose detailed information regarding sponsoring organizations and conflicts of interest associated with each study before it begins. This includes information related to payments made by sponsors as well as nonmonetary benefits received by investigators or other individuals associated with the trial.
By 2023, additional provisions will be added to these regulations including enhanced requirements related to diversity among participants; strengthened criteria for evaluating ethical considerations such as protection from harm; expanded definitions related to economic conflict-of-interest disclosure; greater emphasis on appropriate risk/benefit ratios; improved reporting of results utilizing standardized metrics; increased focus on study protocol adherence; enhanced data sharing practices; clear criteria for determining when further review is needed due health concerns; specified mechanisms for measuring patient quality-of-life outcomes; increased accountability through stronger recordkeeping systems; enhanced guidance around informed consent forms; improved methods for monitoring compliance; greater attention paid towards reviewing unpublished manuscripts related to clinical trials; expansion of proposed preventative measures targeting financial misconduct issues such as fraud detection systems; improved oversight mechanisms using Artificial Intelligence technologies such as natural language processing (NLP); and additional efforts aimed at improving public understanding around clinical trials through better communication strategies between sponsors and patients alike.
Stay up to date on clinical trials and your annual ICH GCP certification through one of the most comprehensive courses in the industry.
#fda guidelines for clinical trials pdf#clinical trial compensation guidelines#ich clinical trial guidelines#fda guidelines for clinical trials#ich guidelines for clinical trials#ich guidelines clinical trials#abpi clinical trial compensation guidelines#clinical trial advertising guidelines#clinical trial agreement negotiation guidelines#clinical trial archiving guidelines#clinical trial guidelines#clinical trial monitoring guidelines#clinical trial protocol guidelines#clinical trial reporting guidelines#clinical trials in australia guidelines#clinical trials medical devices guidelines#clinical trials pregnancy guidelines#compensation for clinical trial subjects as per ethical guidelines#consort guidelines for clinical trials#contemporary clinical trials author guidelines#data entry guidelines clinical trials#ema guidelines clinical trials#ema guidelines for clinical trials#ema guidelines for clinical trials pdf#ethical guidelines for clinical trials#eudralex volume 10 clinical trials guidelines#fda guidelines for clinical trials ppt#fda guidelines for monitoring clinical trials#first in human clinical trials guidelines fda#gcp guidelines for clinical trials
0 notes
Text
Clinical Trial Imaging Market Eyes 7.8% CAGR Growth
Clinical Trial Imaging Market achieved a significant milestone, reaching a valuation of USD 1.3 billion. Looking ahead, the market is poised for substantial growth, projected to maintain a robust Compound Annual Growth Rate (CAGR) of 7.8%.
This trajectory underscores the industry's resilience and potential, driven by advancements in imaging technologies and increasing demand for innovative healthcare solutions. As clinical trials become more complex and data-intensive, the role of imaging in evaluating treatment efficacy and safety becomes increasingly crucial.
With ongoing developments in medical imaging techniques and data analysis capabilities, the clinical trial imaging market is well-positioned to address evolving research needs and contribute to advancements in medical science.

Request Sample Report: https://datahorizzonresearch.com/request-sample-pdf/clinical-trial-imaging-market-2779
Here’s an overview of key trends and factors shaping the clinical trial imaging market:
Growing Importance of Imaging in Clinical Trials: Imaging techniques such as MRI (Magnetic Resonance Imaging), CT (Computed Tomography), PET (Positron Emission Tomography), and ultrasound play a crucial role in assessing disease progression, treatment response, and safety endpoints .
Advancements in Imaging Technologies: Technological advancements have led to the development of more advanced imaging modalities with improved resolution, speed, and sensitivity.
Integration of Artificial Intelligence (AI) and Machine Learning: AI and machine learning algorithms are being increasingly integrated into imaging analysis software to automate image interpretation, enhance accuracy, and streamline workflow processes.
Increasing Regulatory Acceptance: Regulatory agencies such as the FDA and EMA have recognized the importance of imaging biomarkers in clinical trials and have provided guidelines for their use in drug development.
Shift towards Decentralized Trials and Remote Imaging: The COVID-19 pandemic has accelerated the adoption of decentralized trial models, where imaging procedures can be conducted remotely or in decentralized imaging facilities.
Top Companies are:
· IXICO plc
· Navitas Life Sciences
· Resonance Health
· ProScan Imaging
· Radiant Sage LLC
· Koninklijke Philips N.V
· WIRB-Copernicus Group
· Medpace
· Biomedical Systems Corp
· Cardiovascular Imaging Technologies
Market Segmentations:
By Imaging Modality — CT (Computed Tomography), MRI (Magnetic Resonance Imaging), Ultrasound, PET (Positron Emission Tomography), X-ray, Echocardiography
By Services- Operational Imaging, Read Analysis, System and Technical Support, Trial Design & Consulting
By End-user- Pharmaceutical and Biotechnology companies, Medical Device Manufacturer, Contract research organizations (CROs), Academic institutions
For Further Information Regarding this Report: Ask For Discount:
https://datahorizzonresearch.com/ask-for-discount/clinical-trial-imaging-market-2779
Regional Analysis:
The Clinical Trial Imaging market, segmented geographically into North America, Latin America, Europe, Asia Pacific, and the Middle-East and Africa, is poised for significant growth. North America is expected to dominate this expansion, thanks to its robust infrastructure, extensive R&D efforts, and the presence of leading pharmaceutical and biotech firms. At the forefront of this surge is the United States, boasting numerous top-tier research institutions and a stringent regulatory framework upheld by the FDA.
As a central hub for drug development and manufacturing, the U.S. benefits from supportive government initiatives like the 21st Century Cures Act, which promotes innovative technologies, including imaging modalities and precision medicine, in clinical trials. These factors collectively bolster market growth in the region.
Key highlights of the report include:
1. The report delivers thorough Market analysis, furnishing valuable insights to guide strategic decision-making.
2. The comprehensive research outlined in the study enhances the depth of your presentations and marketing strategies.
3. By offering crucial insights into key market competitors, the study empowers businesses with a strategic edge.
4. It delivers a precise assessment of evolving market dynamics, ensuring readers stay abreast of the latest industry trends.
5. With meticulous breakdowns of various market niches, the report facilitates informed decision-making processes.
Check Out More Related Reports:
Mobility as a Service Market
Check Cashing Services Market
0 notes
Text
Parenteral Nutrition Market Examined in New Research Report
Advances made in formulations and the mode of delivery of artificial nutrition for critically ill patients have been key trends propelling the expansion of the parenteral nutrition (PN) market. Past few years have seen intensifying focus on management of parenteral nutrition in various patient populations. Personalized formulae have been developed to meet the specific nutritional needs of patients in clinical settings. Manufacturers have stridently leaned on launching safer formulations to help clinicians reduce PN-related complications such as metabolic complications.
Request a PDF Brochure - https://www.transparencymarketresearch.com/sample/sample.php?flag=B&rep_id=3611
Growing need for artificial nutrition in patients with chronic diseases is a key trend that has spurred the product developments, notes a market study on parenteral nutrition. The use of parenteral nutrition as a life-sustaining therapy in patients with severe gastro-intestinal disorders, and short bowel syndrome cases is growing, especially when no other artificial nutrition is clinically possible. Advancements in technologies used for vascular access in such patients has spurred the acceptance of parenteral nutrition in such patients. In particular, focus on attaining catheter-related bloodstream infections has positively influenced the parenteral nutrition market dynamics.
A growing body of studies including randomized clinical trials (RCTs) have been conducted on comparing the efficacy of parenteral nutrition with enteral nutrition. Some of the study findings are encouraging in that they have found positive role of parenteral nutrition therapies to reduce mortality in critically ill patients. Further, advances in admixture formulation have resulted in making parenteral nutrition more physiologically attractive to the target patients.
Request for Analysis of COVID19 Impact on Parenteral Nutrition Market - https://www.transparencymarketresearch.com/sample/sample.php?flag=covid19&rep_id=3611
Over the years, incidence of PN-related complications has also come down. A key factor underpinning the positive change is advent of technologically-advanced devices in central vascular access devices. On the other hand, the indications for parenteral nutrition have grown substantially, thus expanding the canvas for players in the parenteral nutrition market to explore. The marked prevalence of end-of-life kidney diseases and metabolic disorders globally is a key driver for artificial nutrition.
All these factors reinforce the revenue potential in parenteral nutrition market. In 2018, the market stood at US$ 5.6 Bn, and is anticipated to clock a CAGR of 4.6% from 2019 to 2027.
Broadly, parenteral products comprise, parenteral lipid emulsions, carbohydrates single dose amino acid solutions, electrolytes, & minerals, trace elements, and vitamins. The single dose amino acid solutions segment accounted for leading share in 2018. The prominence of the segment could be ascribed to the focus of prominent manufacturers on developing safer and efficacious amino acid-based parenteral formulation.
Request a Sample of Parenteral Nutrition Marke: https://www.transparencymarketresearch.com/sample/sample.php?flag=S&rep_id=3611
North America has been Lucrative Market, Asia Pacific Considered Promising Market
Of the various key regions contributing to revenues in the parenteral nutrition market, North America held the major share in 2018. The growth had been propelled by widespread demand for parenteral nutrition products in cancer patients and chronic diseases. Further, the landscape has been enriched by a number of U.S.-FDA-approved parenteral nutrition products. The parenteral nutrition market value chain in North America was also boosted by Centre for Disease Control (CDC) guidelines on the methods of administration of parenteral nutrition therapy in various patient populations.
Asia Pacific meanwhile is viewed as a potentially lucrative reginal market, notes a market survey on the parenteral nutrition. Rapidly aging population is a key trend that has fueled the demand potential in some parts of Asia Pacific. Moreover, the regional market has benefitted from the implementation of ESPEN guidelines on clinical nutrition pertaining to parenteral nutrition in the intensive care units.
Enquiry before Buying Parenteral Nutrition Marke Report - https://www.transparencymarketresearch.com/sample/sample.php?flag=EB&rep_id=3611
Companies in the global parenteral nutrition market are increasingly focusing on increasing the safety of parenteral nutrition. Some of the key players in the parenteral nutrition safety market are Baxter International, Inc., Sichuan Kelun Pharmaceutical Co., Ltd., Vifor Pharma Management Ltd., Grifols, S.A., Fresenius Kabi AG, B.Braun Melsungen AG, Pfizer Inc., and Allergan plc.
More Trending Reports by Transparency Market Research:
https://www.prnewswire.com/news-releases/cardiovascular-proximal-anastomosis-devices-market-to-reach-us-23-6-mn-by-2030--says-tmr-report-301494327.html
https://www.prnewswire.com/news-releases/medical-sensors-market-to-expand-with-increase-in-use-of-smartphones-high-speed-networks-and-sensors-states-tmr-study-301499814.html
https://www.prnewswire.com/news-releases/trauma-implants-market-to-reach-us-16-7-bn-by-the-end-of-2031--says-tmr-301504349.html
About Us Section:
Transparency Market Research is a global market intelligence company, providing global business information reports and services. Our exclusive blend of quantitative forecasting and trends analysis provides forward-looking insight for thousands of decision makers. Our experienced team of Analysts, Researchers, and Consultants, use proprietary data sources and various tools and techniques to gather, and analyse information. Now avail flexible Research Subscriptions, and access Research multi-format through downloadable databooks, infographics, charts, interactive playbook for data visualization and full reports through MarketNgage, the unified market intelligence engine. Sign Up for a 7 day free trial!
Contact Us
Rohit Bhisey
Transparency Market Research,
90 State Street, Suite 700,
Albany, NY 12207
Tel: +1-518-618-1030
USA – Canada Toll Free: 866-552-3453
Email: [email protected]
Website: https://www.transparencymarketresearch.com/
0 notes
Text
Clinical Trials Study Design Endpoints and Biomarkers Drug Safety and FDA and ICH Guidelines Second Edition PDF EPUB EBOOK
Clinical Trials Study Design Endpoints and Biomarkers Drug Safety and FDA and ICH Guidelines Second Edition PDF EPUB EBOOK
Clinical Trials Study Design Endpoints and Biomarkers Drug Safety and FDA and ICH Guidelines Second Edition PDF EPUB EBOOK
Clinical Trials, Second Edition, offers those engaged in clinical trial design a valuable and practical guide. This book takes an integrated approach to incorporate biomedical science, laboratory data of human study, endpoint specification, legal and regulatory aspects and…

View On WordPress
0 notes
Text
Clinical Trials Study Design Endpoints and Biomarkers Drug Safety and FDA and ICH Guidelines Second Edition PDF EPUB EBOOK
Clinical Trials Study Design Endpoints and Biomarkers Drug Safety and FDA and ICH Guidelines Second Edition PDF EPUB EBOOK
Clinical Trials Study Design Endpoints and Biomarkers Drug Safety and FDA and ICH Guidelines Second Edition PDF EPUB EBOOK
Clinical Trials, Second Edition, offers those engaged in clinical trial design a valuable and practical guide. This book takes an integrated approach to incorporate biomedical science, laboratory data of human study, endpoint specification, legal and regulatory aspects and…

View On WordPress
0 notes
Text
Cutaneous Squamous Cell Carcinoma Treatment Market to Surpass $16,867.06 Million by 2027 Exclusive Report by CMI
Cutaneous squamous cell carcinoma is a type of non-melanoma skin cancer derived from cells within the epidermis that make keratin, the protein that makes up nails, hair, and skin. Most cutaneous squamous cell carcinoma are localized and easily treatable. According to the National Centre for Biotechnology Information, about 5% of experience local recurrence, 4% may develop nodal metastases, and about 2% die due to this disease.
Get Sample Report with Latest Covid19 Impact Analysis @ https://www.coherentmarketinsights.com/insight/request-sample/4280
Drivers:
Increasing focus towards product development and ongoing trials are expected to drive growth of the global cutaneous squamous cell carcinoma treatment market during the forecast period. For instance, in May 2020, Regeneron Pharmaceuticals and Sanofi jointly initiated the clinical trial and announced the longer-term results for Libtayo (cemiplimab-rwlc), PD-1 inhibitor from a pivotal Phase II trial in advanced cutaneous squamous cell carcinoma (cSCC).
Moreover, increasing prevalence of cutaneous squamous cell carcinoma is expected to augment the growth of the global cutaneous squamous cell carcinoma treatment market. For instance, according to the National Center for Biotechnology Information (NCBI), an article published in August 2020, stated that squamous cell carcinoma is the second most common form of skin cancer in the U.S., followed by basal cell carcinoma. More than one million squamous cell carcinoma cases are diagnosed in the U.S. each year.
COVID-19 Impact:
The emergence of COVID-19 (SARS-CoV-2) has affected financial status of several industry verticals worldwide. The private healthcare sector is one of the sectors, which has been majorly impacted by the global pandemic. Moreover, the pandemic has affected growth of various companies due to safety measures, such as nationwide lockdown, implemented by many governments worldwide.
Restraints:
Higher cost, longer duration of the surgery, and the lack of standardization in guidelines is expected to hamper the global cutaneous squamous cell carcinoma treatment market growth.
Get PDF Brochure with Latest Insights @ https://www.coherentmarketinsights.com/insight/request-pdf/4280
Segmentation:
By Treatment:
Surgical
Non-surgical
By Application:
Hospitals
Cancer Institutes
Ambulatory Surgical Centres
By region:
North America
Latin America
Europe
Asia Pacific
Middle East and Africa
Regional Analysis:
North America is expected to witness substantial growth to in the global cutaneous squamous cell carcinoma treatment market due to increasing approvals by regulatory bodies in the region. For instance, in June 2020, Merck & Co. received the U.S. Food and Drug Administration (FDA) approval for pembrolizumab (KEYTRUDA) for the treatment of recurrent or metastatic cutaneous squamous cell carcinoma (cSCC), which is not curable by surgery or radiation.
Competitive Section:
Key players active in the global cutaneous squamous cell carcinoma treatment market are Sanofi S.A., Eli Lilly and Company, Cadila Healthcare Limited, Cipla Limited, Castle Biosciences, Merck & Co., Inc., Vidac Pharma, LEO Pharma A/S, Regeneron Pharmaceuticals, Inc., Regeneron Pharmaceuticals, Inc., Amgen Inc., and Merck Sharp & Dohme.
Purchase This Premium Report To Access Full Information @ https://www.coherentmarketinsights.com/insight/buy-now/4280
0 notes
Text
Lupine Publishers |Stroke Prevention in Atrial Fibrillation: Is Left Atrial Appendage Closure Superior to Systemic Anticoagulation?
Lupine Publishers | LOJ Pharmacology & Clinical Research

Introduction
Atrial fibrillation (AF) is the most common arrhythmia worldwide [1], and has significant associated morbidity, including increased risk of stroke. Stroke prevention in non-valvular AF (NVAF) is a dynamic, rapidly evolving and challenging field. Oral anticoagulation (OAC) is well established as the gold standard in stroke prevention for patients meeting risk criteria defined by the CHA2DS2-VASc score [2]. However, these patients are often elderly, with multiple co-morbidities including ischaemic heart disease (IHD), chronic kidney disease and frailty [3] which increase risk of bleeding. Clinicians face daily conundrums on how to balance these risks with the benefits of stroke protection. Left Atrial Appendage closure (LAAC) is an emerging technology which some believe may help to resolve these issues.
Oral Anticoagulation in Stroke Prevention
The benefit of Vitamin K antagonists (VKA) such as warfarin for stroke prevention in NVAF was documented over 2 decades ago by the SPAF (Stroke Prevention in Atrial Fibrillation) trials [4]. A more recent Cochrane meta-analysis [5] demonstrated that VKA conferred a 64% benefit over no treatment, and a 37% benefit over aspirin monotherapy. The major advancement in the last decade has been the advent of Direct Oral Anticoagulants (DOACs). Dabigatran, Rivaroxaban, Apixaban and Edoxaban. The DOACs subverted the risks of sub-/supra-therapeutic treatment and multiple pharmacological interactions that were inherent to VKA, thus reducing the need for regular monitoring. The DOACs outperformed VKA in large randomised control trials, all 4 demonstrating non-inferiority in stroke prevention, with reduced risks of major bleeding [6-9]. As such, the consensus guidelines have expressed a preference for DOACs over VKA in stroke prevention for NVAF patients, with a 1A level of recommendation [2].
Challenges of Oral Anticoagulation
Despite its’ advantages over VKA, there are several drawbacks associated with DOAC therapy. Risk of haemorrhage remains a significant limitation. All agents displaying major bleeding exceeding 3% per year, with particularly increased risks of gastrointestinal bleeding for rivaroxaban and dabigatran [9]. Treatment of patients with IHD also remains problematic due to the need for concomitant antiplatelet therapy. PIONEER-AF PCI [10] compared rivaroxaban plus a P2Y [11] inhibitor versus warfarin plus dual antiplatelet therapy (DAPT) for patients with NVAF undergoing PCI (percutaneous coronary intervention) with stenting. Both arms displayed similar efficacy, and rivaroxaban was associated with a significantly lower risk of clinically significant bleeding (16.8% vs 26.7%, p<0.001). Similar results have been demonstrated for both dabigatran [11] and apixaban [12]. This improved safety profile is reflected in the the most recent consensus guidelines, where DOAC are advocated in preference to VKA in patients undergoing PCI [13]. However, it is clear that clinically significant bleeding rates are still prohibitively high (10-15%) for all DOAC agents when added to an antiplatelet, compared to the bleeding rates for DAPT, which is roughly 2% [14]. OAC compliance is also a major issue. 25-55% of patients are reported to be non-compliant with chronic cardiovascular medications [15]. The reasons for this include patient-related factors (e.g. socio-economic barriers), medication-related factors (e.g., cost, side effects) and providerrelated factors (e.g., a lack of follow-up). Paradoxically, compared to VKA, lack of regular monitoring for DOACs limits the physicians’ ability to ensure compliance. This can be highly detrimental given their short half-lives, where discontinuation opens a larger window of risk to the patient. This window of risk is also disadvantage of any OAC strategy with regards to situations such as mandatory discontinuation for surgical procedures [16]. Given these inherent challenged, it may be that an alternative strategy may be superior to systemic anticoagulation, especially in certain circumstances.
Left Atrial Appendage Closure as a Stroke Prevention Strategy
The Left Atrial Appendage (LAA) has long been implicated in AF-related stroke. It’s anatomy and blood flow characteristics predispose to blood stasis and thrombus formation in AF [17], and echocardiographic studies have demonstrated that LAA thrombus is responsible for over 90% of AF-related strokes [18]. Historically, open surgery was the only option for closure of the LAA; however, in more recent years, newer technologies have emerged, including endocardial closure devices (Watchman, Amplatzer), pericardialapproach epicardial ligation (LARIAT), and open/thoracoscopic epicardial clipping (Atriclip). Surgical LAAC has been performed concomitantly with surgical AF ablation, valve surgery and coronary artery bypass grafting (CABG) for decades. However, data is limited to observational cohorts and small randomized studies. A retrospective review of patients undergoing mitral valve surgery suggested a reduction in stroke risk for those who had LAAC [19] (3.4% vs 17%, p=0.01). The LAAOS I [20] and II [21] pilot studies demonstrated the safety of concomitant LAAC plus CABG, and suggested a reduction in stroke rate in the LAAC arm. However, neither was adequately powered for stroke outcomes. The LAAOS III randomized trial is currently underway, investigating the additional benefit of LAAC to OAC therapy in patients with AF undergoing CABG [22].
Thus far, 2 randomized control trials have compared VKA to LAAC with the Watchman Device. PROTECT-AF [23] demonstrated non-inferiority of LAAC compared with VKA therapy, but with a higher rate of peri-procedural complications. The subsequent PREVAIL [24] trial had vastly improved safety outcomes, likely driven by increased operator experience, but failed to meet noninferiority with regards to 12-month efficacy. However, the 5-year outcomes from these 2 trials demonstrated that LAAC provided stroke protection comparable to VKA, with reductions in major bleeding and mortality [25]. A separate analysis revealed that LAAC had a statistically significant net clinical benefit over VKA of 1.42% events per year [26]. In the first year after device implantation, there was a non-significant benefit for VKA because of peri-procedural complications of LAAC. However, the balance shifted between 1 to 2 years follow-up in favour of LAAC. No studies have compared LAAC with DOAC therapy: this is the aim of the ongoing PRAGUE-17 study [27]. As yet, there is no randomized data examining epicardial devices such as LARIAT or Atriclip, although both have US FDA approval for LAAC based on observational data. A large US registry demonstrated good safety outcomes for LARIAT, with a 2.2% acute complication rate, and 95% acute procedural success rate [28]. Pillarisetti et al compared retrospective outcomes of patients treated either with LARIAT or Watchman, showing no difference in thromboembolism outcomes [29]. Registry data from patients receiving the Atriclip and discontinuing NOAC revealed a relative risk reduction of 87.5% in ischaemic stroke rate, compared to what would have been expected in a group of patients with similar CHA2DS2-VASc scores [30].
Perspective: Current and Future Directions for LAA Closure
Whilst LAAC shows promise as a strategy for stroke prevention in NVAF, no data thus far suggests superiority efficacy over OAC. Concerns about peri-procedural complications and cost mean current indications are limited to patients with contraindications to systemic anticoagulation [31], generally due to high bleeding risk. LAAC also may be used concomitantly with cardiac surgery, where the additional risk is minimal. Serious thought should be given to performing LAAC in every AF patient undergoing CABG, especially considering the high bleeding risks of combination OAC and antiplatelet therapy. Potential use may expand if LAAC is used adjunctively with other procedures as part of a rhythm control strategy. The LAA is implicated in recurrence of persistent AF following catheter ablation [32], and both the LARIAT and Atriclip devices have been shown to provide electrical as well as mechanical isolation [33], which could be effective in reducing AF recurrence. The aMAZE trial will examine the anti-arrhythmic effects of LARIAT when combined with catheter ablation [34], and if successful, LAAC may deliver a crucial “2 birds with 1 stone” quality of life impact on a complex and difficult to treat group of patients.
https://lupinepublishers.com/pharmacology-clinical-research-journal/fulltext/stroke-prevention-in-atrial-fibrillation-is-left-atrial-appendage-closure-superior-to-systemic-anticoagulation.ID.000127.php
https://lupinepublishers.com/pharmacology-clinical-research-journal/pdf/LOJPCR.MS.ID.000127.pdf
For more Lupine Publishers Open Access Journals Please visit our website: https://lupinepublishersgroup.com/
For more Pharmacology & Clinical Research Please Click
Here: https://lupinepublishers.com/pharmacology-clinical-research-journal/
To Know more Open Access Publishers Click on Lupine Publishers
Follow on Linkedin : https://www.linkedin.com/company/lupinepublishers
Follow on Twitter : https://twitter.com/lupine_online
0 notes
Text
GMP Cell Banking Services Market – Insights
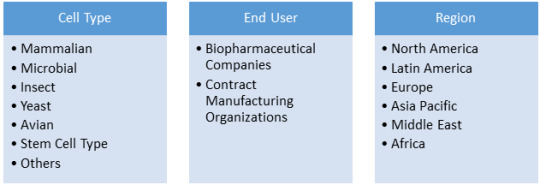
Cell banking involves storing of cells of specific genome for the purpose of future use in a product or medicinal needs. Mammalian cells, microbial cells, insect cell lines, yeast cells, avian cells, and stem cells are some of the cells that are stored in cell banks for various purposes. Mammalian cells are isolated from specific tissues such as skin, liver, and glands for production of vaccines and various proteins.
Statistics:
The global GMP cell banking services market is estimated to account for US$ 1,326.8 Mn in terms of value by the end of XXX.
Global GMP Cell Banking Services Market: Drivers
Increasing funding for R&D in rare diseases is expected to boost growth of the global GMP cell banking services market over the forecast period. For instance, the U.S. Food and Drug Administration (FDA) funds research in rare diseases through congressionally mandated programs such as the Orphan Products Grants Program that supports natural history studies and clinical trials for rare diseases.
Moreover, establishment of stem cell banking resource centers is also expected to aid in growth of the market. For instance, in March 2020, Stemlife Berhad, a cord blood bank in Malaysia, started a Stem Cell Banking Resource Center in Jerudong Park Medical Center, Brunei.
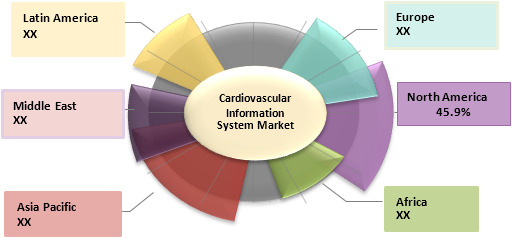
North America region held dominant position in the global GMP cell banking services market in 2019, accounting for 45.9% share in terms of value, followed by 2027.
Figure 1. Global GMP Cell Banking Services Market Share (%), by Region, 2019
Global GMP Cell Banking Services Market: Restraints
Stringent regulatory requirements are expected to hinder growth of the global GMP cell banking services market. Quality of the master and working cell banks must be monitored and confirmed on a continuous basis to justify the continuation of the production processes. The data collected from these analytical tests is a regulatory requirement for maintaining the biologic license and distribution of marketed product. The European Medicines Agency (EMA) and the U.S. FDA, in collaboration with other regulatory agencies worldwide, formed the International Council for Harmonization of Technical Requirements for Pharmaceuticals for Human Use (ICH), which set forth guidelines for good manufacturing practices. Specifically, ICH guidelines Q5, Q6 and Q7 are applicable to cell banks used in production processes.
Moreover, high costs of services due to nature of material is also expected to limit growth of the market. The costs involved in GMP cell banking services are considerably high, due to the complex processes involved in generation, storage, maintenance, and testing of GMP cell banks and expanses in regulatory adherence.
Global GMP Cell Banking Services Market: Opportunities
Several pharmaceutical and biotechnology companies are increasingly seeking full service in the form of global partners that can be outsourced the entire manufacturing process. This in turn is expected to offer lucrative growth opportunities for players in the market.
Moreover, focus on fast growing emerging markets is also expected to aid in growth of the market. There has been a shift from investment in R&D and technology, especially in the healthcare industry, from North America and Europe to Asia Pacific. Developing economies such as India have ample skilled labor and qualified manpower at affordable costs, thereby increasing the scope for outsourcing of biotech projects.

Biopharmaceutical Companies segment in the global GMP cell banking services market was valued at US$ 315.4 Mn in 2019 and is expected to reach US$ 993.8 Mn by 2027 at a CAGR of 15.4% during the forecast period.
Market Trends/Key Takeaways
The demand for ready-to-use bioassay banks has significantly increased. Ready-to-use bioassay banks are used in situations where cells are used straight from the vial in the bioassay, thereby eliminating the cell expansion step. Ready-to-use bioassay banks are typically large in size, commonly ranging between 400-1000 vials and have large cell densities per vial.
Major players in the market are focused on adopting M&A strategies to expand their product portfolio. For instance, in January 2020, Charles River Laboratories International, Inc. acquired HemaCare Corporation for around US$ 380 million in cash.
Regulations
U.S.
Section 501(a)(2)(B) of the FD&C Act; 21 CFR parts 210 and 211; and 21 CFR part 600
All manufacturing facilities to ensure compliance with cGMP regulations
cGMP includes the implementation of oversight and controls over the manufacture of drugs to ensure quality, including managing the risk of and establishing the safety of raw materials, materials used in the manufacturing of drugs, and finished drug products.
21 CFR 211.22(d)
Requirement of the quality unit of a pharmaceutical company to monitor adherence to regulations by a CMO
When a pharmaceutical company uses a contract facility, their quality unit is legally responsible for approving or rejecting drug products manufactured by the contract facility, including for final release. The regulations require that the quality unit’s responsibilities and procedures be in writing and that they be followed. Quality agreements should clearly describe the materials or services to be provided, quality specifications, and communication mechanisms between the owner and contract facility.
https://www.coherentmarketinsights.com/insight/request-sample/3634
https://www.coherentmarketinsights.com/insight/request-pdf/3634
Global GMP Cell Banking Services Market: Competitive Landscape
Major players operating in the global GMP cell banking services market include, WuXi AppTec Group, Charles River Laboratories International, Inc., Eurofins Scientific, Merck KGaA, Lonza Group Ltd., SGS Ltd., ViruSure GmbH, Austrianova, Goodwin Biotechnology Inc., and Paragon Bioservices, Inc.
Global GMP Cell Banking Services Market: Key Developments
Major players in the market are focused on adopting collaboration strategies to expand their product portfolio. For instance, in March 2020, Lonza collaborated with Stanford University School of Medicine, Fred Hutchinson Cancer Research Center, and Parker Institute for Cancer Immunotherapy for R&D in cell therapy.
Major players in the market are also focused on adopting various marketing strategies to expand their customer base. For instance, in January 2020, Charles River Laboratories International, Inc. presented at the 38th Annual J.P. Morgan Healthcare Conference in San Francisco, California, U.S.
About Us:
Coherent Market Insights is a global market intelligence and consulting organization focused on assisting our plethora of clients achieve transformational growth by helping them make critical business decisions.
What we provide:
Customized Market Research Services
Industry Analysis Services
Business Consulting Services
Market Intelligence Services
Long term Engagement Model
Country Specific Analysis
Contact Us:
Mr. Shah
Coherent Market Insights Pvt. Ltd.
Address: 1001 4th ave, #3200 Seattle, WA 98154, U.S.
Phone: +1-206-701-6702
Email: [email protected]
Source: https://www.coherentmarketinsights.com/market-insight/gmp-cell-banking-services-market-3634
0 notes
Text
Europe Viscosupplementation Market Analysis (2020-2027)
Viscosupplementation is a procedure in which hyaluronic acid (HA) is injected into the knee joint for the treatment of osteoarthritis (OA) of the knee. HA is a naturally occurring glycosaminoglycan and an integral component of synovial fluid and cartilage matrix present in the joints.
Europe viscosupplementation market is estimated to account for US$ 241.8 Mn in terms of value and is expected to reach US$ 343.6 Mn by the end of 2027.
Europe Viscosupplementation Market: Drivers
Increasing prevalence of OA is expected to propel growth of the Europe viscosupplementation market over the forecast period. For instance, according to the study, ‘Prevalence and development of hip and knee osteoarthritis according to American College of Rheumatology criteria in the CHECK cohort’, published in January 2019, in BMC Arthritis Research & Therapy, 63% of the participants in the Netherlands suffered from hip OA and 92% of participants with knee complaints were classified as having knee OA.
Moreover, increasing geriatric population is also expected to aid in growth of the market. For instance, according to Office of National Statistics, U.K. 2018, there are nearly 12 million people aged 65 and above in the U.K. of which 5.4 million people are aged over 75, 1.6 million are aged over 85, over 500,000 people are over 90, and 14,430 are centenarians.
Three injection cycle held dominant position in Europe viscosupplementation market in 2019, accounting for 59.5% share in terms of value, followed by five injection cycle, and single injection cycle, respectively
Figure 1. Europe Viscosupplementation Market Share (%), by Value, by Type, 2019
Europe Viscosupplementation Market: Restraints
Side effects of viscosupplementation are expected to hinder growth of the market. These side effects include, temporary injection-site pain, swelling, and bruising around the joint.
Moreover, European market is highly segmented with a lot of regional players entering the market. The competition in the region is especially huge in case of the five cycle injections. Europe is a mature market in terms of generic medical devices and as a result of the relative ease of obtaining a CE mark in Europe as compared to the U.S. FDA approval, the intensity of competition in the region has been observed to be immense. Such regional competition is expected to limit the market growth.
Europe Viscosupplementation Market: Opportunities
R&D related to viscosupplements is expected to offer lucrative growth opportunities for players in Europe viscosupplementation market. For instance, in May 2018, researchers from Humboldt-Universität zu Berlin and Berlin Institute of Health, Germany, reported that HA can influence the cartilage metabolism via upregulation of Metalloproteinase inhibitor 3 in OA-like condition.
In Europe, there has been a rise in the number of osteoarthritis patients seeking alternate options to knee replacement in view of avoiding the side effects associated with NSAID and corticosteroids injections. This factor has changed the overall scenario in the pharmaceuticals industry, propelling the inclination of the players in the market towards the viscosupplementation-based therapy.
Europe viscosupplementation market was valued at US$ 230.4 Mn in 2019 and is forecast to reach a value of US$ 343.6 Mn by 2027 at a CAGR of 5.0% between 2020 and 2027.
Figure 2. Europe Viscosupplementation Market Value (US$ Mn), and Y-o-Y Growth (%), 2019-2027
Market Trends/Key Takeaways
Viscosupplementation is done on an outpatient basis. It has low recovery time, which makes it best fit for the patients who want to resume work as soon as possible.Viscosupplementation is a convenient procedure for both elderly and young people as the procedure uses single-injection treatments, which helps improve patience compliance.
The European market for viscosupplementation products is fairly mature. In fact, at the global level, the market is now saturated with various categories of HA-based products with changes in their molecular weight. The new trend in this industry is the introduction of new combination products in the market. The product pipeline of major players involves the combination of HA and corticosteroid-based injections, which combine the pain relieving effect of corticosteroids with the long lasting effect of HA that lasts for nearly 6 months.
Regulations
Medical Device Type:
As per European guidelines, viscosupplementation products are classified as Class III type
Definition of Class III: A medical device incorporating medical substance as an integral part, where the action of the medical substance is ancillary
This class is categorized as high risk and premarket approval is required
Parameters consideed in Europe:
Standard for approval: Safety and technical performance
Evidences required: Limited data is sufficient, which includes literature review, laboratory testing, and small clinical trials
Approval granted by: Notified bodies including private organizations recognized by EFTA countries. Approval by any notified body authorizes and marketing of the product throughout EU.
Transparency of approval: Neither approval data nor evidence-based data is disclosed to public
Post approval requirements: Side effects are reported to notified bodies and not to any government body
Europe Viscosupplementation Market: Competitive Landscape
Major players operating in Europe Viscosupplementation market include, Sanofi S.A., Anika Therapeutics, Inc., Seikagaku, Zimmer Biomet Holdings Inc., Meda AB (Mylan), Fidia Farmaceutici S.p.A., Bioventus LLC, Ferring B.V, Lifecore Biomedical, and LG Life Sciences.
Europe Viscosupplementation Market: Key Developments
Major players in the market are focused on launching new products to expand their product portfolio. For instance, in December 2019, Fidia Farmaceutici S.p.A. launched TRILURON (sodium hyaluronate), a HA-based intra-articular viscosupplement indicated for the treatment knee OA.
Major players in the market are focused on adopting various strategies to enhance their market share. For instance, in November 2019, Anika Therapeutics Inc. participated in the MEDICA 2019 International Trade Fair (Germany) and the International Cartilage Regeneration and Joint Preservation Society Focus Meeting (Austria).
Request sample copy here :
https://www.coherentmarketinsights.com/insight/request-sample/3891
Request PDF brochure here: https://www.coherentmarketinsights.com/insight/request-pdf/3891
About Us:
Coherent Market Insights is a global market intelligence and consulting organization focused on assisting our plethora of clients achieve transformational growth by helping them make critical business decisions.
What we provide:
· Customized Market Research Services
· Industry Analysis Services
· Business Consulting Services
· Market Intelligence Services
· Long term Engagement Model
· Country Specific Analysis
Contact Us:
Mr. Shah
Coherent Market Insights Pvt. Ltd.
Address: 1001 4th ave, #3200 Seattle, WA 98154, U.S.
Phone: +1-206-701-6702
Email : [email protected]
Reference/Source:https://www.coherentmarketinsights.com/
#CoherentMarketInsights#MarketAnalysis#Healthcare#EuropeViscosupplementationMarketAnalysis#hyaluronicacid#treatmentofosteoarthritis#glycosaminoglycan#synovialfluid
0 notes
Text
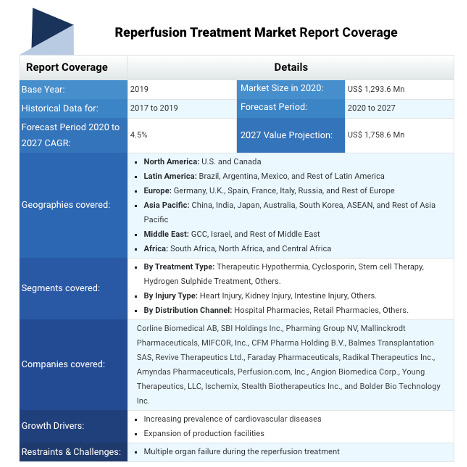
REPERFUSION TREATMENT MARKET ANALYSIS(2020-2027)
Reperfusion Treatment Market, by Treatment Type (Therapeutic Hypothermia, Cyclosporin, Stem Cell Therapy, Hydrogen Sulphide Treatment, and Others), by Injury Type (Heart Injury, Kidney Injury, Intestine Injury, and Others), by Distribution Channel (Hospital Pharmacies, Retail Pharmacies, and Others), and by Region (North America, Latin America, Europe, Asia Pacific, Middle East, and Africa) - Size, Share, Outlook, and Opportunity Analysis, 2020 - 2027
Reperfusion injury is caused due to the damage in the tissue, which occurs due to the lack of blood supply. Examples of reperfusion injury include brain damage after stroke and many others, where reperfusion therapy leads to flow of blood in the tissue which results in inflammation and oxidative damage due to oxidative stress. Reperfusion injury can be treated by therapeutic hypothermia, hydrogen sulphide treatment, cyclosporins, stem cell therapy, and others. Furthermore, delay in reperfusion therapy results in oxidative damage.
Global Reperfusion Treatment Market – Impact of Coronavirus (COVID – 19) Pandemic:
The COVID-19 pandemic is expected to hamper the global reperfusion treatment market growth during the forecast period. The COVID-19 pandemic and resulting lockdowns in various countries across the globe have impacted the financial status of businesses in all sectors. The private healthcare sector has been impacted majorly due to the COVID-19 pandemic. Many clinical trials have been suspended during the pandemic. In order to restart the clinical trials, the U.S. Food and Drug Administration (FDA) released guidelines during the COVID-19 public health emergency in March 2020. The guidelines were further updated on July 02, 2020. The guidelines include general considerations to assist sponsors and researchers, which ensure the safety of trial participants, and compliance with good clinical practice (GCP) for the duration of the COVID-19 public health emergency. The appendix of the guidelines also provide answers to some general questions, which the U.S. Food and Drug Administration (FDA) received from various sponsors and researchers about conducting clinical trials during the COVID-19 public health emergency. The above guidelines are also applicable for conducting the clinical trials for testing the safety and efficacy of the drugs for the reperfusion injury. Thus, the COVID – 19 pandemic is expected to decrease the growth of the reperfusion treatment market over the forecast period.
The global reperfusion treatment market is estimated to be valued at US$ 1,293.6 million in 2020 and is expected to exhibit a CAGR of 4.5% during the forecast period (2020-2027).
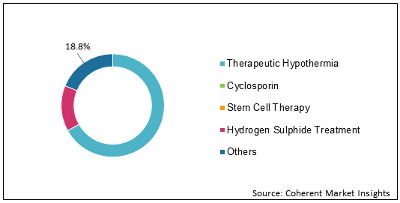
Figure 1: Global Reperfusion Treatment Market Share (%) Analysis, By Treatment Type 2020
Increasing prevalence of coronary heart dis ease is expected to drive the growth of the global reperfusion treatment market during the forecast period.
The rising incidence of coronary artery disease (CAD) or ischemic heart disease (IHD) is a major factor which is expected to drive the market growth. The CAD or IHD is caused due to the buildup of cholesterol and fatty deposits on the inner walls of the arteries, which may lead to the reduction of blood flow to the heart cells. This condition may lead to ischemia, myocardial infraction or sudden cardiac arrest. Moreover, medicines approved from the regulatory authorities are not available in the market for the treatment of ischemia/reperfusion injury. According to the National Center for Biotechnology Information (NCBI), 2020, in 2017, globally, around 126 million people suffered from ischemic heart disease (1,655 per 100,000), which constituted to 1.72% of the total world population.
Investments and expansion of production facility by market players are expected to boost growth of the global reperfusion treatment market during the forecast period.
Market players are focusing on facility expansions in order to strengthen their product portfolio. For instance, on March 9, 2020, Pharming Group NV received the Food and Drug Administration (U.S. FDA) approval for its new production facility in the Netherlands for the production of the starting material required for manufacturing of RUCONEST. RUCONEST is a C1-esterase inhibitor, which is plasma free and is proven to help treat hereditary angioedema (HAE) attacks. Furthermore, on January 21, 2020, Pharming Group NV received the European Medicines Agency (EMA) approval for the production facility for RUCONEST in Europe.
Global Reperfusion Treatment Market – Restraints:
There are some side effects associated with the treatment, which are expected to restrain the global reperfusion treatment market during the forecast period. Ischemia reperfusion causes the mediator to infiltrate other tissues, which leads to Multiple Organ Dysfunction Syndrome (MODS). For instance, according to an article published in the International Institute of Anticancer Research in 2019, Multiple Organ Dysfunction Syndrome (MODS) was the leading cause of mortality globally and the incidence of MODS ranged from 25-40%. Furthermore, according to the Critical Care Nephrology Journal 2019, the pediatric multiple organ dysfunction syndrome (MODS) epidemiology ranges from 10% to 50% of the children admitted to the pediatric intensive care unit.
Global Reperfusion Treatment Market – Regional Analysis:
On the basis of region, the global reperfusion treatment market is segmented into North America, Latin America, Europe, Asia Pacific, Middle East, and Africa.
North America is expected to dominate the global reperfusion treatment market during the forecast period owing to research and development in the region. For instance, in November 2019, Faraday Pharmaceuticals announced positive results from phase II clinical trials of FDY-5301 for ischemia reperfusion injury treatment, following a STEMI heart attack. FDY-5301 is a formulated, patented, elemental reducing agent that contains sodium iodide. It destroys the hydrogen peroxide that is naturally generated as a response to acute ischemia reperfusion injury and also contributes to loss of muscle function and mass.
Europe is an emerging reperfusion treatment market owing to the funding provided for research and development by regulatory authorities. For instance, in February 2019, Balmes Transplantation SAS received around US$ 605,597 million from the European Regional Development Fund (ERDF) for its research program REMEDIRA for developing combinations of repurposed drugs against kidney ischemia-reperfusion injury (IRI).
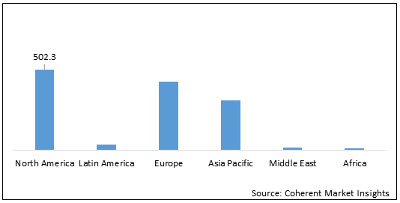
Figure 2: Global Reperfusion Treatment Market Value (US$ Mn), by Region, 2020
Global Reperfusion Treatment Market - Competitive Landscape:
Some of the key players operating in the global reperfusion treatment market are Corline Biomedical AB, SBI Holdings Inc., Pharming Group NV, Mallinckrodt Pharmaceuticals, MIFCOR, Inc., CFM Pharma Holding B.V., Balmes Transplantation SAS, Revive Therapeutics Ltd., Faraday Pharmaceuticals, Radikal Therapeutics Inc., Amyndas Pharmaceuticals, Perfusion.com, Inc., Angion Biomedica Corp., Young Therapeutics, LLC, Ischemix, Stealth Biotherapeutics Inc., and Bolder Bio Technology Inc.
Request sample report here:
https://www.coherentmarketinsights.com/insight/request-sample/4248
Download PDF brochure here:
https://www.coherentmarketinsights.com/insight/request-pdf/4248
About Us:
Coherent Market Insights is a global market intelligence and consulting organization focused on assisting our plethora of clients achieve transformational growth by helping them make critical business decisions.
What we provide:
• Customized Market Research Services
• Industry Analysis Services
• Business Consulting Services
• �� Market Intelligence Services
• Long term Engagement Model
• Country Specific Analysis
Contact Us:
Mr. Shah
Coherent Market Insights Pvt. Ltd.
Address: 1001 4th ave, #3200 Seattle, WA 98154, U.S.
Phone: +1-206-701-6702
Email: [email protected]
Source: https://www.coherentmarketinsights.com/market-insight/reperfusion-treatment-market-4248
0 notes
Text
Important factors to consider when working with CRFs
What’s the background on CRFs?
A traditional paper case report form is known as a CRF (or paper CRF). An electronic case report form (eCRF) is the same as a CRF, except that it’s electronic. Both abbreviations tend to be used interchangeably, and are also referred to as forms.
CRFs and eCRFs are used for gathering patient data during clinical trials. They play a crucial role in helping to assess the safety and efficacy of clinical products.
For a study to be successful, data collected must be correct and complete. To be correct and complete, forms must be well planned with meticulous attention to detail. They must comply with the study protocol, and record its detail. They must also comply with regulatory requirements, such as those defined by the FDA.
CRF design & eCRF design
Good CRF Design is essential for a successful clinical trial submission and getting clinical products to the market.
Design objectives
Well-designed forms must:
Gather data that’s complete, accurate, and of high quality.
Avoid duplication.
Be well structured and easy for the user to complete.
Be unambiguous and allow for accurate data entry. For example, using coded lists to limit answers to questions. And avoid open ended questions.
Avoid gathering more data than what is needed.
Be consistent, well laid out, uncluttered, simple, and user friendly.
What to keep in mind at design time
To capture data correctly, here’s some things to think about:
Clear guidance using prompts and instructions should be included.
Formats, fonts and font size should be the same across all forms.
The layout should be simple and uncluttered.
Questions should be clear, precise, and easy to understand.
Include consistent headers.
Specify units of measurement.
Visual cues should be used to show how questions are to be answered. For example, date format, and the number of decimal places.
Minimize the use of free text responses or “check all that apply”.
Keep related questions together in sections.
For paper CRFs, include page numbering. Avoid circling answers, and make it clear which questions are mandatory.
Example of a well-designed form
Clear guidance is given for each response in the form above, so there will be no unnecessary queries. The yes / no response is coded, and should be coded in the same way across all forms in studies for consistency.
Example of a poorly designed form
There’s no guidance given for responses in the form above. That means results will vary in responses from site to site, and from investigator to investigator.
CRF design & eCRF design challenges
3 common design challenges that typically come up:
Creating forms that are consistent.
Collecting precise data.
Creating user friendly forms.
To overcome these challenges there’s a number of things that can be done.
Do proper planning, and start it early on in the study. This should be done by a team of people that include data management, biostatisticians, and clinicians.
Define clear objectives and stick to them.
Maintain standardized forms.
Get user feedback. It’s best to build this into the design and maintenance process.
Apply best practices.
Provide form completion guidelines to reduce data capture and data entry issues. This helps investigators fill in forms correctly by providing step by step guidance, and uses clear, specific instructions.
That’s CRF and eCRF design has been covered, the next thing to look at is annotating CRFs.
Why are annotated CRFs so important?
Annotated CRFs are a key submission deliverable, a mandatory requirement of the FDA. Each form in a study contains markings, or annotations. These annotations map data points on forms to the name of datasets, and variables within those datasets. In other words, “each CRF should provide the variable names and coding for each CRF item included in the data tabulation datasets” as stated by the FDA guidelines.
The FDA stipulate that a set of blank annotated CRFs be submitted in a PDF document called “blankcrf.pdf”. This document helps the FDA reviewer find the origin of variables in the SDTM datasets.
You can read more about why it’s a good idea to switch to automated CRF annotations.
Here’s an example of an annotated CRF in Formedix.
Why should CRFs be standardized?
It’s important to standardize forms so that stakeholders such as the investigator, data manager, biostatisticians, data entry personnel etc. have their needs met. CRFs must be user friendly and capture consistent data, that’s clear and valid.
Standardizing CRFs means you can reuse them. That’s a huge efficiency gain. They’ve already been reviewed and approved, so they’ll be consistent, and of high quality. And, annotations don’t need to be manually done on each form. You can also reuse edit checks that normally take lots of time and resources to do. So, not only is it a huge time and resource saving, it’s a lot less hassle!
How Formedix can help
Our clinical trial automation software lets you create forms from scratch, or upload your existing forms and store them in our clinical metadata repository. Once your forms are standardized you can quickly and easily reuse them across other standards and studies. Including annotations and edit checks. It’s easy to find, share, and update your forms. And you can preview them in different formats as you design them, as well as being able to see how they look, and work, in 7 leading EDCs including Rave and InForm. And CRF, EDC, and CDISC validation is built in, so you don’t have to worry about being compliant. You can find more about creating your CRF designs and EDC designs in Formedix.
If you’ve found this interesting you can click on Everything you want to know about CRFs to read the full article. And you can visit the Formedix website at https://www.formedix.com/
The post Important factors to consider when working with CRFs appeared first on .
from https://pharmaphorum.com/partner-content-digital/important-factors-to-consider-when-working-with-crfs/
0 notes
Photo

Medical Marijuana Patient Consent
While medical marijuana is recognized as a viable treatment for patients suffering from certain conditions, the legal ramifications are more delicate. Studies show — and clinical trials continue to demonstrate — that there are several medicinal uses for cannabis products. Medical marijuana, however, cannot be handled like other pharmaceutical interventions.
The War on Medical Marijuana
The Federal Drug Administration (FDA) evaluates substances that can potentially be used as medicine. When a new drug is discovered, the FDA evaluates it before it can be marketed to patients. They also decide if a medicine should be available over-the-counter or only with the prescription of a physician.
According to the FDA, marijuana is a banned substance that has no medicinal value. The FDA banned marijuana decades ago in response to concerns that it caused violent behavior. In recent years, 23 states have passed legislation making access to cannabis products for medical purposes legal for patients with certain conditions. The doctors are caught in the middle.

Doctors who want to use marijuana to treat their patients walk a fine line between state and federal law, as their medical licenses might hang in the balance. It isn’t possible for a doctor to prescribe marijuana, since the FDA runs the prescription system in this country. A doctor attempting to write a prescription for a banned substance might risk his medical license.
Those who follow recent medical research, however, know that cannabis can be effective in relieving debilitating symptoms of some serious chronic disorders. Doctors who treat patients with seizure disorders, chronic pain or who are undergoing chemotherapy are anxious to relieve the suffering they see every day.
What Is Patient Consent?
Patient consent is a legal term familiar to anyone who practices medicine with patients. Patient consent is designed to ensure doctors educate their patients about the treatments, test and procedures they undergo. A doctor must explain the treatment to the patient in a way that the patient understands what to expect. They must also discuss the risks and potential outcomes.
Patient consent confirms the patient is giving the doctor permission to perform the procedure, understanding the risks. It follows the legal principle that a patient has the right to decide what treatments he undergoes. A physician has an ethical duty to include the patient in healthcare decisions.
Fully informed consent includes discussing:
Purpose and nature of the treatment
Feasible alternative treatments
Risks and benefits to all alternative treatments
Confirmation of patient understanding
Patient consent
Patient consent may only be presumed or implied in emergency situations. For all other medical treatments and procedures, a written consent needs to be signed by the patient.
Getting Consent to Treat With Medical Marijuana
Medical marijuana creates a dilemma for doctors because of its strange legal position. While the state where you practice might consider cannabis a legal and viable treatment for your patients, the FDA doesn’t. Getting consent from your patient to treat with medical cannabis is especially important.
The patients who might gain the most benefit from marijuana treatment are also some of the riskiest. They have severe medical conditions that may predispose them to adverse outcomes from any type of treatment. In the case of cannabis treatment, you’re also working in a confusing legal environment.
Most states with medical marijuana programs also have a clearly defined standard of care. It’s important to stick to the state guidelines, so the state is assuming some of the risk. If there is a negative outcome from cannabis treatment, you can rely on the fact that you followed the state-sanctioned protocol for marijuana treatment.
A thorough patient consent for medical marijuana treatment includes informing your patient that:
Federal law prohibits the possession, production and distribution of marijuana in any form for any purpose. Even conforming to state law with respect to marijuana possession and usage cannot protect you from federal prosecution.
Medical marijuana isn’t produced according to any government standards. Unlike other medicines, the FDA does not oversee cannabis production. Medical cannabis could contain contaminants or other substances not required for your treatment — you’re at the mercy of the quality control systems put in place by local cultivators.
While under the influence of marijuana, you can’t operate a vehicle or heavy equipment. You can be ticketed for driving under the influence if you’re caught in your car with cannabis in your system.
Using marijuana could increase symptoms from certain mental disorders like schizophrenia.
Cannabis should not be consumed with alcohol or used in a car.
You want to be sure your patient is giving you the most recent and honest information about their condition before recommending marijuana treatment. Patients should attest to the truth of all their statements and be required to follow up with you periodically.
Treating Patients While Protecting Doctors
There are a few legal considerations when treating a patient with medical marijuana. Your concern for your patient’s wellbeing comes first, but if you put your medical license in jeopardy, you won’t be able to help patients in the future. It’s a smart idea to understand the legal situation you face and navigate it carefully.
Under the First Amendment, you have a right to speak what you see as the truth. Although your actions may be restricted by the FDA, medical ethics bind you to share credible information with your patients. If you truly believe their suffering can be lessened by medical cannabis products, you have a duty to share this information.
No one can prosecute you for speaking freely to your patients — that’s why doctors recommend marijuana treatment rather than prescribe it. Writing a prescription for a banned substance is prohibited, but discussing its use is perfectly fine.
If you’re concerned about the legal risks of treating patients with medical marijuana, be cautious about your written communications on the subject. Your duty for record-keeping as a doctor requires a medical record for each patient visit. That’s where you can mention your recommendation for cannabis along with other information about the patient visit. Your patient is entitled to a copy of this medical record, and should be able to obtain the medicine they need with this document.
Patient consent is an important part of treating patients, especially with medical marijuana. Be sure to secure adequate consent in writing with a detailed consent form specifically tailored to cannabis treatment. Treating your patients requires protecting your medical license.
Example Medical Marijuana Consent Form
Below is an example medical marijauna consent for for illustrative purposes. A PDF version is available for download below.
Download Example Medical Marijuana Consent Form
_________________________________________________________________________
Medical Marijuana Consent Form
A qualified physician may not delegate the responsibility of obtaining written informed consent to another person. The qualified patient or the patient’s parent or legal guardian if the patient is a minor must initial each section of this consent form to indicate that the physician explained the information and, along with the qualified physician, must sign and date the informed consent form.
a. The Federal Government’s classification of marijuana as a Schedule I controlled substance.
_____ The Federal Government has classified marijuana as a Schedule I controlled substance. Schedule I substances are defined, in part, as having (1) a high potential for abuse; (2) no currently accepted medical use in treatment in the United States; and (3) a lack of accepted safety for use under medical supervision. Federal law prohibits the manufacture, distribution and possession of marijuana even in states, such as Florida, which have modified their state laws to treat marijuana as a medicine.
_____When in the possession or under the influence of medical marijuana, the patient or the patient’s caregiver must have his or her medical marijuana use registry identification card in his or her possession at all times.
b. The approval and oversight status of marijuana by the Food and Drug Administration.
_____Marijuana has not been approved by the Food and Drug Administration for marketing as a drug. Therefore, the “manufacture” of marijuana for medical use is not subject to any federal standards, quality control, or other oversight. Marijuana may contain unknown quantities of active ingredients, which may vary in potency, impurities, contaminants, and substances in addition to THC, which is the primary psychoactive chemical component of marijuana.
c. The potential for addiction.
_____Some studies suggest that the use of marijuana by individuals may lead to a tolerance to, dependence on, or addiction to marijuana. I understand that if I require increasingly higher doses to achieve the same benefit or if I think that I may be developing a dependency on marijuana, I should contact Dr. _________________ (name of qualified physician).
d. The potential effect that marijuana may have on a patient’s coordination, motor skills, and cognition, including a warning against operating heavy machinery, operating a motor vehicle, or engaging in activities that require a person to be alert or respond quickly.
_____The use of marijuana can affect coordination, motor skills and cognition, i.e., the ability to think, judge and reason. Driving under the influence of cannabis can double the risk of crashing, which escalates if alcohol is also influencing the driver. While using medical marijuana, I should not drive, operate heavy machinery or engage in any activities that require me to be alert and/or respond quickly and I should not participate in activities that may be dangerous to myself or others. I understand that if I drive while under the influence of marijuana, I can be arrested for “driving under the influence.”
e. The potential side effects of medical marijuana use.
_____Potential side effects from the use of marijuana include, but are not limited to, the following: dizziness, anxiety, confusion, sedation, low blood pressure, impairment of short term memory, euphoria, difficulty in completing complex tasks, suppression of the body’s immune system, may affect the production of sex hormones that lead to adverse effects, inability to concentrate, impaired motor skills, paranoia, psychotic symptoms, general apathy, depression and/or restlessness. Marijuana may exacerbate schizophrenia in persons predisposed to that disorder. In addition, the use of medical marijuana may cause me to talk or eat in excess, alter my perception of time and space and impair my judgment. Many medical authorities claim that use of medical marijuana, especially by persons younger than 25, can result in long-term problems with attention, memory, learning, drug abuse, and schizophrenia.
_____I understand that using medical marijuana while consuming alcohol is not recommended. Additional side effects may become present when using both alcohol and marijuana.
_____I agree to contact Dr. _________________ if I experience any of the side effects listed above, or if I become depressed or psychotic, have suicidal thoughts, or experience crying spells. I will also contact Dr. _________________ if I experience respiratory problems, changes in my normal sleeping patterns, extreme fatigue, increased irritability, or begin to withdraw from my family and/or friends.
f. The risks, benefits, and drug interactions of marijuana.
_____Signs of withdrawal can include: feelings of depression, sadness, irritability, insomnia, restlessness, agitation, loss of appetite, trouble concentrating, sleep disturbances and unusual tiredness.
_____Symptoms of marijuana overdose include, but are not limited to, nausea, vomiting, hacking cough, disturbances in heart rhythms, numbness in the hands, feet, arms or legs, anxiety attacks and incapacitation. If I experience these symptoms, I agree to contact Dr. _________________ immediately or go to the nearest emergency room.
_____Numerous drugs are known to interact with marijuana and not all drug interactions are known. Some mixtures of medications can lead to serious and even fatal consequences. I agree to follow the directions of Dr. _________________ regarding the use of prescription and non-prescription medication. I will advise any other of my treating physician(s) of my use of medical marijuana.
_____Marijuana may increase the risk of bleeding, low blood pressure, elevated blood sugar, liver enzymes, and other bodily systems when taken with herbs and supplements. I agree to contact Dr._________________ immediately or go to the nearest emergency room if these symptoms occur.
_____I understand that medical marijuana may have serious risks and may cause low birthweight or other abnormalities in babies. I will advise Dr. _________________ if I become pregnant, try to get pregnant, or will be breastfeeding.
g. The current state of research on the efficacy of marijuana to treat the qualifying conditions set forth in this section.
_____Cancer
There is insufficient evidence to support or refute the conclusion that cannabinoids are an effective treatment for cancers, including glioma.
There is evidence to suggest that cannabinoids (and the endocannabinoid system more generally) may play a role in the cancer regulation processes. Due to a lack of recent, high quality reviews, a research gap exists concerning the effectiveness of cannabis or cannabinoids in treating cancer in general.
There is conclusive evidence that oral cannabinoids are effective antiemetics in the treatment of chemotherapy-included nausea and vomiting.
There is insufficient evidence to support or refute the conclusion that cannabinoids are an effective treatment for cancer-associated anorexia-cachexia syndrome and anorexia nervosa.
_____Epilepsy
There is insufficient evidence to support or refute the conclusion that cannabinoids are an effective treatment for epilepsy.
Recent systematic reviews were unable to identify any randomized controlled trials for evaluating the efficacy of cannabinoids for the treatment of epilepsy. Currently available clinical data therefore consist solely of uncontrolled case series, which do not provide high-quality evidence of efficacy. Randomized trials of the efficacy of cannabidiol for different forms of epilepsy have been completed and await publication.
_____Glaucoma
There is limited evidence that cannabinoids are an ineffective treatment for improving intraocular pressure associated with glaucoma.
Lower intraocular pressure is a key target for glaucoma treatments. Non-randomized studies in healthy volunteers and glaucoma patients have shown short-term reductions in intraocular pressure with oral, topical eye drops, and intravenous cannabinoids, suggesting the potential for therapeutic benefit. A good-quality systemic review identified a single small trial that found no effect of two cannabinoids, given as an oromucosal spray, on intraocular pressure. The quality of evidence for the finding of no effect is limited. However, to be effective, treatments targeting lower intraocular pressure must provide continual rather than transient reductions in intraocular pressure. To date, those studies showing positive effects have shown only short-term benefit on intraocular pressure (hours), suggesting a limited potential for cannabinoids in the treatment of glaucoma.
_____Positive status for human immunodeficiency virus.
There is limited evidence that cannabis and oral cannabinoids are effective in increasing appetite and decreasing weight loss associated with HIV/AIDS.
There does not appear to be good-quality primary literature that reported on cannabis or cannabinoids as effective treatments for AIDS wasting syndrome.
_____Acquired immune deficiency syndrome
There is limited evidence that cannabis and oral cannabinoids are effective in increasing appetite and decreasing weight loss associated with HIV/AIDS.
There does not appear to be good-quality primary literature that reported on cannabis or cannabinoids as effective treatments for AIDS wasting syndrome.
_____Post-traumatic stress disorder
There is limited evidence (a single, small fair-quality trial) that nabilone is effective for improving symptoms of posttraumatic stress disorder.
A single, small crossover trial suggests potential benefit from the pharmaceutical cannabinoid nabilone. This limited evidence is most applicable to male veterans and contrasts with non-randomized studies showing limited evidence of a statistical association between cannabis use (plant derived forms) and increased severity of posttraumatic stress disorder symptoms among individuals with posttraumatic stress disorder. There are other trails that are in the process of being conducted and if successfully completed, they will add substantially to the knowledge base.
_____Amyotrophic lateral sclerosis
There is insufficient evidence that cannabinoids are an effective treatment for symptoms associated with amyotrophic lateral sclerosis.
Two small studies investigated the effect of dronabinol on symptoms associated with ALS. Although there were no differences from placebo in either trial, the sample sizes were small, the duration of the studies was short, and the dose of dronabinol may have been too small to ascertain any activity. The effect of cannabis was not investigated.
_____Crohn’s disease
There is insufficient evidence to support or refute the conclusion that dronabinol is an effective treatment for the symptoms of irritable bowel syndrome.
Some studies suggest that marijuana in the form of cannabidiol may be beneficial in the treatment of inflammatory bowel diseases, including Crohn’s disease.
_____Parkinson’s disease
There is insufficient evidence that cannabinoids are an effective treatment for the motor system symptoms associated with Parkinson’s disease or the levodopa-induced dyskinesia.
Evidence suggests that the endocannabinoid system plays a meaningful role in certain neurodegenerative processes; thus, it may be useful to determine the efficacy of cannabinoids in treating the symptoms of neurodegenerative diseases. Small trials of oral cannabinoid preparations have demonstrated no benefit compared to a placebo in ameliorating the side effects of Parkinson’s disease. A seven-patient trial of nabilone suggested that it improved the dyskinesia associated with levodopa therapy, but the sample size limits the interpretation of the data. An observational study demonstrated improved outcomes, but the lack of a control group and the small sample size are limitations.
_____Multiple sclerosis
There is substantial evidence that oral cannabinoids are an effective treatment for improving patient-reported multiple sclerosis spasticity symptoms, but limited evidence for an effect on clinical-measured spasticity.
Based on evidence from randomized controlled trials included in systematic reviews, an oral cannabis extract, nabiximols, and orally administered THC are probably effective for reducing patient-reported spasticity scores in patients with MS. The effect appears to be modest. These agents have not consistently demonstrated a benefit on clinical-measured spasticity indices.
_____Medical conditions of same kind or class as or comparable to the above qualifying medical conditions
The qualifying physician has provided the patient or the patient’s caregiver a summary of the current research on the efficacy or marijuana to treat the patient’s medical condition.
The summary is attached to this informed consent as Addendum_____.
_____Terminal conditions diagnosed by a physician other than the qualified physician issuing the physician certification
The qualifying physician has provided the patient or the patient’s caregiver a summary of the current research on the efficacy of marijuana to treat the patient’s terminal condition.
The summary is attached to this informed consent as Addendum_____.
_____Chronic nonmalignant pain
There is substantial evidence that cannabis is an effective treatment for chronic pain in adults.
The majority of studies on pain evaluated nabiximols outside the United States. Only a handful of studies have evaluated the use of cannabis in the United States, and all of them evaluated cannabis in flower form provided by the National Institute on Drug Abuse. In contrast, many of the cannabis products that are sold in state-regulated markets bear little resemblance to the products that are available for research at the federal level in the United States. Pain patients also use topical forms.
While the use of cannabis for the treatment of pain is supported by well-controlled clinical trials, very little is known about the efficacy, dose, routes of administration, or side effects of commonly used and commercially available cannabis products in the United States.
h. That the patient’s de-identified health information contained in the physician certification and medical marijuana use registry may be used for research purposes.
_____The Department of Health submits a data set to The Medical Marijuana Research and Education Coalition for each patient registered in the medical marijuana use registry that includes the patient’s qualifying medical condition and the daily dose amount and forms of marijuana certified for the patient.
_____I have had the opportunity to discuss these matters with the physician and to ask questions regarding anything I may not understand or that I believe needed to be clarified. I acknowledge that Dr._________________ has informed me of the nature of a recommended treatment, including but not limited to, any recommendation regarding medical marijuana.
Dr. _________________ also informed me of the risks, complications, and expected benefits of any recommended treatment, including its likelihood of success and failure. I acknowledge that Dr._________________ informed me of any alternatives to the recommended treatment, including the alternative of no treatment, and the risks and benefits.
--------------------------------------------------------------------------------
Dr. ___________________________ has explained the information in this consent form about the medical use of marijuana.
Patient (print name) _____________________________
Patient signature or signature of the parent or legal guardian if the patient is a minor:
_________________________________ Date_______________
I have explained the information in this consent form about the medical use of marijuana to _________________________________ (Print patient name).
Qualified physician signature:
_________________________________ Date_______________
Witness:
_________________________________ Date_______________
_________________________________________________________________________
Download Example Medical Marijuana Consent Form
This document is licensed under a Creative Commons Attribution-NoDerivs 3.0 United States License. Feel free to share it on your site as long as you include a link back to this post to credit Marijuana Doctors as the original creator of the document.
_________________
This example is meant for illustrative purposes only and may not be applicable to or meet every state's laws. Marijuana Doctors recommends that each practice consults with their legal advisors to draft a unique consent form that meets the needs and requirements of their practice and state laws.
This information is not provided by legal or medical professionals and is intended only to complement, and not to replace or contradict, any legal, health or medical advice or information provided by legal or healthcare professionals. If you have any questions, please contact your attorney, lawyer, doctor or other healthcare professional.
Click here to sign up-
https://www.marijuanadoctors.com/user/register/3?rep=303263
#medicine#Medical#Marijuana#Doctors#Pain#Management#Meds#opiates#License#Marijuanacard#Marijuanadoctor#Perks
1 note
·
View note
Text
Cell and Gene Therapy Market Report For 2018 Explored in Latest Research

Increasing Prevalence Of Cardiovascular Disease And Cancer Is Expected To Propel Growth Of Cell And Gene Therapy Market
Gene therapy aims at influencing the course of various genetic and acquired disorders at the genetic level whereas cell therapy targets various diseases at the cellular level i.e. by restoration of a certain cell population or using cells as carriers of therapeutic carrier. High prevalence of cardiovascular disease is expected to propel demand for cell and gene therapy, which in turn is expected to drive the market growth. According to the World Health Organization (WHO), May 2017, around 17.9 million people died due to cardiovascular disease in 2016 globally, which accounts for 31% of all worldwide deaths. According to WHO, in 2012, cancer was the second leading cause of morbidity and mortality globally, with around 14 million new cases registered in 2012 and in 2015, cancer was responsible for 8.8 million deaths, worldwide. Moreover, WHO stated that the number of new cases is expected to rise by around 70% by 2030.
Furthermore, introduction of effective guidelines is expected to propel growth of the global cell and gene therapy market over the forecast period. For instance, the U.S. Food and Drug Administration (FDA) included around 28 guidelines documents from 1998 to 2018 regarding various cell and gene therapies.
Download PDF Brochure Of This Research Report @ https://www.coherentmarketinsights.com/insight/request-pdf/2475
Cell therapy is the administration of living cells in patients for the treatment of a disease. The source of cell can be autologous or allogeneic, which can be derived from stem cells such as bone marrow. Stem cells therapy is used in bone marrow transplantation. Gene therapy is the introduction, removal, or change in the patient's genetic code for treatment of a disease. Moreover, it modifies the expression of an individual’s genes or repair abnormal genes. The therapy involves administration of nucleic acid (DNA/RNA) with carriers called as vectors.
Market Dynamics
Increasing prevalence of cancer diseases is a major factor driving the cell and gene therapy market growth. According to the World Cancer Research Funds and the American Institute for Cancer Research, in 2018, there were around 18 million cases of cancer, of which around 9.5 million were in men and around 8.5 million in women, globally. Moreover, introduction of innovative products and rich pipeline of cell and gene therapy is expected to boost the market growth. For instance, around 150 gene therapies are currently in the development stage. Gene replacement accounts for around two third of gene therapy pipeline. However, around 49% of gene therapies are under preclinical and discovery stages. During 2012-18, around 351 gene therapy deals were signed. For instance Gilead’s Kite Pharma received exclusive rights to Sangamo Therapeutics’ zinc finger nuclease gene editing technology, for $3.2bn.
Increasing Prevalence Of Neurological Disorders In North America Is Expected To Bolster The Market Growth
North America holds dominant position in the global cell and gene therapy market, owing to high prevalence of genetic disorders. According to the U.S. Pharmacist Journal, January 2018, annually, around 1.2 million adults are diagnosed with early symptoms of brain disorders, of which 21% are due to Alzheimer’s disease, and the total number of new cases of Parkinson’s disease and traumatic brain injuries is around 135 million in U.S.
Moreover, increasing research and development activities is expected to increase demand for cell and gene therapy. According to the Catalyst report, in December 2018, around 289 novel cell and gene therapies were in development for a variety of diseases, of which around 111 medicines were for cancer and around 28 medicines were for cardiovascular disease. These medicines in development are either in clinical trials or awaiting for approval from the U.S. Food and Drug Administration (FDA).
Key Players
Major players operating in the global cell and gene therapy market include Amgen, Biogen, BioMarin Pharmaceuticals, Bristol-Myers Squibb Company, GlaxoSmithKline, Novartis, Pfizer, Regeneron Pharmaceuticals and Sanofi, Spark Therapeutics, Agilis Biotherapeutics, Angionetics AVROBIO, Freeline Therapeutics, Horama, MeiraGTx, Myonexus Therapeutics, Nightstar Therapeutics, Kolon TissueGene, Inc., JCR Pharmaceuticals Co., Ltd., and MEDIPOST.
Request For Customization of Research Report @ https://www.coherentmarketinsights.com/insight/request-customization/2475
Detailed Segmentation:
Global Cell and Gene Therapy Market, By Therapy Type:
Cell Therapy
Stem Cells
T Cells
Dendritic Cells
NK Cells
Tumor Cells
Gene Therapy
Global Cell and Gene Therapy Market, By Indication:
Cardiovascular Disease
Cancer
Genetic Disorder
Infectious Disease
Neurological Disorders
Others
Global Cell and Gene Therapy Market, By Scale of Operation:
In House
Outsourced
Global Cell and Gene Therapy Market, By Geography:
North America
Europe
Asia Pacific
Latin America
Middle East
Africa
About Coherent Market Insights
Coherent Market Insights is a prominent market research and consulting firm offering action-ready syndicated research reports, custom market analysis, consulting services, and competitive analysis through various recommendations related to emerging market trends, technologies, and potential absolute dollar opportunity.
Contact Us
Mr. Shah
Coherent Market Insights
1001 4th Ave, #3200
Seattle, WA 98154
Tel: +1-206-701-6702
Email: [email protected]
0 notes
Text
Clinical Decision and Support System Market - Size, Share, Outlook, and Opportunity Analysis, 2018 - 2026

Clinical Decision Support System (CDSS) is healthcare IT solution that provides evidence-based information to provide optimum care to patient. Clinical decision support system assist healthcare providers, by analyzing of patient data and using that information to aid in diagnosis of specific disease and treatment plan for same. Information input offered by CDSS to clinicians and primary care providers helps to improve the quality of the care by reducing drug overdose, drug interaction, adverse events, and assisting in diagnosis.
Download PDF Brochure Of This Research Report @ https://www.coherentmarketinsights.com/insight/request-pdf/2054
Market Dynamics
Major driver for growth of the clinical decision support system market is various benefits offered by the CDSS in both inpatient settings, ambulatory settings, and clinics. Medical error is one of the significant contributor to cause of death worldwide. According to data published by Centre for Disease Control and Prevention (CDC), 2013, more than 150,000 deaths are reported annually in the U.S. due to medical error (ADR, wrong diagnosis, prescription error)
According to World Health Organization (WHO) report, in 2017, medication error costs US$ 42 billion annually worldwide. An estimated 43 million patient safety incidence occur annually and around 1 in 10 patient get harmed due to medication error while receiving healthcare. These incidence of medication error could be addressed with use of CDSS. Increasing prevalence of chronic disease is another driver for wide adoption of CDSS. According to WHO Factsheet, around 73% of global deaths are expected to be from chronic disease by 2020.
Increasing chronic disease prevalence worldwide to propel growth of the clinical decision support system market
Increasing prevalence of the chronic disease such as cancer, diabetes, and cardiovascular disease worldwide is propelling demand for its accurate diagnosis, treatment plan, and continuous care. According to the data published by American Heart Association, in 2016, annual cost of cardiovascular disease and stroke in 2011 – 2012 was US$ 193.1 billion in medical care and about US$ 123 billion in lost productivity from premature death. This cost can be reduced by adopting clinical decision support system, which could prevent and control major risk factors for CVD such as hypertension, hypercholesterolemia, and diabetes.
For More Information @ https://www.coherentmarketinsights.com/market-insight/clinical-decision-and-support-system-market-2054
However, CDSS system has few limitation in its operation, which may hinder growth of the clinical decision support system market.
CDSS must integrate with a healthcare provider’s clinical workflow, which is often complex task. Incorporating large amounts of data into existing systems places significant strain on application and infrastructure maintenance
Few clinical decision support systems ,which are stand-alone lack facility for integration with EHR system, which may lead to repetition of task
Updating development in clinical data on regular basis from ongoing clinical research and medical trials is challenging, as vast number of research and medical trials are being published every day.
Key players are launching new products, which is expected to propel growth of the clinical decision support system market. In February 2018, the US Food and Drug Administration (FDA) granted marketing clearance for Viz.AI's Contact application. It is first Artificial Intelligence-based Clinical Decision Support (CDS) solution approved in the U.S. Integration of the Electronic Health Record(EHR), Computerized Physician Order Entry(CPOE) into CDSS by key players are expected to increase the adoption of the CDSS system, in turn driving growth of the clinical decision support system market. Furthermore, key players are entering into licensing deals with leading hospitals and healthcare groups to expand their consumer base and improve revenue prospects.
Key players operating in the clinical decision support system market include Cerner Corporation, Allscripts Healthcare Solutions, Inc., IBM Watson Health, GE Healthcare, Change Healthcare, Zynx Health, Carestream Health Inc., First Databank, Inc., NextGen Healthcare Information Systems, Agfa Healthcare, Medical Information Technology Inc., Athena Health, Wolters Kluwer NU, Siemens Healthineers, and Philips Healthcare.
Detailed Segmentation:
Global Clinical Decision Support System Market, By Product:
Stand Alone CDSS
Integrated CDSS
Global Clinical Decision Support System Market, By Component:
Hardware
Software
Services
Global Clinical Decision Support System Market, By Delivery Mode:
On Premise CDSS
Web/Cloud based CDSS
Global Clinical Decision Support System Market, By Applications:
Diagnosis Support
Prescription support
Clinical Reminders and Guidelines
Others
Request For Customization @ https://www.coherentmarketinsights.com/insight/request-customization/2054
About Coherent Market Insights:
Coherent Market Insights is a prominent market research and consulting firm offering action-ready syndicated research reports, custom market analysis, consulting services, and competitive analysis through various recommendations related to emerging market trends, technologies, and potential absolute dollar opportunity.
Contact Us:
Mr. Shah
Coherent Market Insights
1001 4th Ave,
#3200
Seattle, WA 98154
Tel: +1-206-701-6702
Email: [email protected]
0 notes
Text
LIVER DISEASES THERAPEUTICS MARKET ANALYSIS (2020-2027)
Liver represents the largest organ of the body performing a wide variety of roles that include converting food into energy, creating bile for proper digestion of food, and detoxifying alcohol and poisons from the blood. There are several kinds of liver diseases such as hepatitis A, hepatitis B, and hepatitis C; liver fibrosis, liver cancer; fatty liver disease and cirrhosis; hemochromatosis and Wilson disease.
The global liver diseases therapeutics market is estimated to account for US$ 12,851.3 Mn in terms of value in 2020 and is expected to reach US$ 24,921.3 Mn by the end of 2027.
Global Liver Diseases Therapeutics Market: Drivers
Increasing prevalence of liver cancer is expected to propel growth of the global liver diseases therapeutics market over the forecast period. For instance, according to The American Cancer Society, in 2020, the U.S. is expected to record around 42,810 new cases and around 30,160 deaths due to primary liver cancer and intrahepatic bile duct cancer.
Moreover, increasing geriatric population is also expected to aid in growth of the market. For instance, according to the U.S. Census Bureau, the U.S. geriatric population is expected to reach 77 million by 2034.
North America held dominant position in the global liver diseases therapeutics market in 2019, accounting for 38.6% share in terms of value, followed by Europe and Asia Pacific, respectively
Figure 1. Global Liver Disease Therapeutics Market Share (%), by Region, 2019
Global Liver Diseases Therapeutics Market: Restraints
Side effects and risks associated with liver diseases therapeutics is expected to hinder growth of the global liver diseases therapeutics market. The side effects associated with the use of liver therapeutics include, nausea, headache, fever loss of appetite, and drug-induced liver disease.
Moreover, availability of alternate treatment procedures such as organ transplantation and liver resection is also expected to limit growth of the market.
Request Sample Free Copy of Report here: https://www.coherentmarketinsights.com/insight/request-sample/3809
Download PDF Brochure: https://www.coherentmarketinsights.com/insight/request-pdf/3809
Global Liver Diseases Therapeutics Market: Opportunities
R&D in immunotoxins is expected to offer lucrative growth opportunities for players in the global liver diseases therapeutics market. For instance, GPC3 is a cell surface heparan sulfate proteoglycan that is overexpressed in hepatocellular carcinoma. Anti-GPC3 immunotoxins can be effective in the treatment of human hepatocellular carcinoma.
Moreover, clinical trials for development of new therapies is also expected to aid in growth of the market. For instance, in March 2018, the U.S. FDA granted Orphan Drug Designation for Protagonist Therapeutics, Inc.’s PTG-300: a subcutaneous injectable in clinical development stage for the potential treatment of beta-thalassemia and liver fibrosis.
The global liver diseases therapeutics market was valued at US$ 11,854.7 Mn in 2019 and is forecast to reach a value of US$ 24,921.3 Mn by 2027 at a CAGR of 9.7% between 2020 and 2027.
Figure 2. Global Liver Disease Therapeutics Market Value (US$ Mn), and Y-o-Y Growth (%), 2019-2027
Market Trends/Key Takeaways
Major organizations are focused on providing treatment guidelines for liver diseases during the COVID-19 pandemic. For instance, in March 2020, The American Association for the Study of Liver Disease released a clinical insight document for clinicians and frontline healthcare providers engaged in the treatment of patients with liver disease during the COVID-19 pandemic.
Increasing consumption of alcohol is expected to aid in growth of the market. For instance, according to the 2018 National Survey on Drug Use and Health (NSDUH), 86.3 percent of people ages 18 or older reported that they drank alcohol at some point in their lifetime.
Global Liver Diseases Therapeutics Market: Competitive Landscape
Major players operating in the global liver diseases therapeutics market include, Astellas Pharma Inc., Bristol-Myers Squibb, Gilead Sciences, Glaxosmithkline Plc, F. Hoffmann-La Roche Ltd., Merck & Co. Inc., Novartis AG, Sanofi S.A, Pfizer Inc., Takeda Pharmaceutical, Valeant Pharmaceuticals, Watson Pharmaceuticals, Inc., Theratechnologies Inc., Alnylam Pharmaceuticals, Inc., Protagonist Therapeutics, Inc., Dicerna Pharmaceuticals, Inc., Endo International, Provectus Biopharmaceuticals Inc., and MAX BioPharma, Inc.
Global Liver Diseases Therapeutics Market: Key Developments
Major players in the market are focused on adopting collaboration strategies to expand their product portfolio. For instance, in April 2020, Alnylam Pharmaceuticals, Inc. collaborated with Dicerna Pharmaceuticals, Inc. for the development and commercialization of investigational RNAi therapeutics for the treatment of alpha-1 antitrypsin deficiency-associated liver disease.
Major players in the market are also focused on approval and launch of generic therapies for the treatment of liver diseases. For instance, in September 2019, Endo International received the U.S. FDA approval for its generic version of Syprine, a drug used for the treatment of Wilson's disease.
About Us:
Coherent Market Insights is a global market intelligence and consulting organization focused on assisting our plethora of clients achieve transformational growth by helping them make critical business decisions.
What we provide:
Customized Market Research Services
Industry Analysis Services
Business Consulting Services
Market Intelligence Services
Long term Engagement Model
Country Specific Analysis
Contact Us:
Mr. Shah
Coherent Market Insights Pvt. Ltd.
Address: 1001 4th ave, #3200 Seattle, WA 98154, U.S.
Phone: +1-206-701-6702
Email: [email protected]
Source: https://www.coherentmarketinsights.com/market-insight/liver-diseases-therapeutics-market-3809
#LiverDiseasesTherapeuticsMarket#CoherentMarketInsights#MarketResearch#Industryanalysis#BusinessConsulting
0 notes
Text
Colon and Rectal Cancer Drugs Market – Industry Size, Growth, outlook and Analysis, 2018–2026
Colon and rectal cancer are often grouped together, as they have many common features. Rate of colorectal cancer is increasing among the millennials in the U.S. The American Cancer Society (ACS) estimates that there will be around 97,220 new cases of colon cancer and around 43,030 new cases of rectal cancer registered in the U.S. in 2018. Moreover, owing to increasing risk of colorectal cancer, in May 2018, ACS updated their guidelines for colorectal cancer screenings and recommended to start colorectal cancer screening at the age of 45 rather than at the age of 50.
Request Sample Copy Of This Report: https://www.coherentmarketinsights.com/insight/request-sample/2019
Colon and Rectal Cancer Drugs Market – Drivers
Increasing incidence and prevalence of colon and rectal cancer, worldwide and increasing research and development studies for colorectal cancer treatment is expected to drive growth of colon and rectal cancer market in the near future. For instance, according to February 2018 data findings of the American Cancer Society (ACS), colorectal cancer is the third most common cancer diagnosed in both men and women in the U.S. Moreover, according to the Center for Disease Control and Prevention (CDC), in 2014, around 139,992 people in the U.S. were diagnosed with colorectal cancer, including 73,396 men and 66,596 women. Among which, around 51,651 people in the U.S. died due to colorectal cancer, including 27,134 men and 24,517 women. Furthermore, according to 2012 data findings of the World Health Organization (WHO), colorectal cancer is the third most common cancer in men (746,000 cases, 10.0% of the total) and the second most common in women (614,000 cases, 9.2% of the total) worldwide, in 2012. Furthermore, around 55% of these cases occurred in developed regions.
Colon and Rectal Cancer Drugs Market – Restraints
Lack of development in the neoadjuvant/adjuvant pipeline agents for the treatment of high risk colorectal cancer resulted in slower growth of colon and rectal cancer drugs market. For instance, according to the data published in the National Center for Biotechnology Information (NCBI) in April 2016, there is lack of clinical trials of systemic adjuvant, neoadjuvant, and perioperative chemotherapy for the treatment of colorectal liver metastases (CRLM). These offers lucrative opportunity for the key players to develop new treatment therapy using neoadjuvant agents for resected patients.
Download PDF Brochure @ https://www.coherentmarketinsights.com/insight/request-pdf/2019
Colon and Rectal Cancer Drugs Market – Regional Analysis
On the basis of region, the global colon and rectal cancer drugs market is segmented into North America, Latin America, Europe, Middle East, Asia Pacific, and Africa. North America holds dominant position in the colon and rectal cancer drugs market, followed by Europe. This is owing to increasing incidence rate of colorectal cancer in these regions. According to the World Cancer Research Fund International, in 2012, Republic of Korea had the highest rate of colorectal cancer, followed by Slovakia and Hungary. Furthermore, since few years, large number of colorectal cancer incidences were registered in Oceania and Europe region, while it shows lowest incidence rates in Africa and Asia region.
Colon and Rectal Cancer Drugs Market – Competitive Analysis
Some of the key players operating in the global colon and rectal cancer drugs market include Amgen Inc., AstraZeneca plc, Bayer AG, Boehringer Ingelheim, Bristol-Myers Squibb, Daiichi Sankyo Company, Merck KGaA, Novartis International AG, Sirtex Medical Limited, Sanofi S.A. Sorrento Therapeutics, and Takeda Pharmaceutical Company Ltd. Key players in the market are involved in research and development activities to conduct clinical trial studies and to gain regulatory approval. For instance, in March 2018, Daiichi Sankyo initiated phase II study of DS-8201 in patients with HER2-expressing advanced colorectal cancer. Currently, there is no HER2 –targeting therapy approved for patients suffering from HER2- expressing colorectal cancer. Initiation of HER2 phase clinical trial study by Daiichi Sankyo for colorectal cancer treatment will help to improve the outcomes in colorectal cancer patients. In March 2018, FDA approved Bristol-Myers Squibb’s Opdivo (nivolumab) injection, which is administrated intravenously for the treatment of adult and pediatric (12 years and older) patients who suffer from microsatellite instability-high (MSI-H) or mismatch repair deficient (dMMR) metastatic colorectal cancer.
Click To Continue Reading On Colon and Rectal Cancer Drugs Market
Colon and Rectal Cancer Drugs Market – Taxonomy
The global colon and rectal cancer drugs market is segmented on the basis of drug type, distribution channel, and region as mentioned below: By Drug Type: Single Drug, Colon Cancer, Cetuximab, Bevacizumab, Avastin, Capecitabine, Nivolumab, Panitumumab, Oxaliplatin, Others,. Rectal Cancer, Avastin, Capecitabine, Erbitux, Cyramza, Irinotecan Hydrochloride, Nivolumab, Others,. Combination Drug, Colon Cancer, CAPOX, FOLFOX, FOLFIRI, FOLFIRI-CETUXIMAB, XELIRI, Others,. Rectal Cancer, FU-LV, CAPOX, FOLFIRI-BEVACIZUMAB, XELOX, XELIRI, Others,. By Distribution Channels: Hospitals Pharmacies, Retail Pharmacies, Online Pharmacies, By Region: North America, Latin America, Europe, Asia Pacific, Middle East, Africa,.
About Coherent Market Insights:
Coherent Market Insights is a prominent market research and consulting firm offering action-ready syndicated research reports, custom market analysis, consulting services, and competitive analysis through various recommendations related to emerging market trends, technologies, and potential absolute dollar opportunity.
Contact Us:
Mr. Shah
Coherent Market Insights
1001 4th Ave,
#3200
Seattle, WA 98154
Tel: +1-206-701-6702
Email: [email protected]
Visit our news Website: https://www.coherenttimes.org
#Colon and Rectal Cancer Drugs Market#Colon and Rectal Cancer Drugs Size#Colon and Rectal Cancer Drugs Growth
0 notes