#Subcutaneous Drug Delivery
Text
According to the latest research by nova one advisor, the global subcutaneous drug delivery devices market size was exhibited at USD 23.20 billion in 2023 and is projected to hit around USD 68.29 billion by 2032, growing at a CAGR of 11.4% during the forecast period 2023 to 2032.
0 notes
Text
Advancements in Technologies and Drug Delivery Systems | Market Size | 2035
The report from Roots Analysis offers a comprehensive analysis of the current and future market landscape of subcutaneously administered biologics. The study aims to identify the primary drivers of growth and estimate the market size and potential for future boosts associated with subcutaneous biologics.

0 notes
Link
Subcutaneous drug delivery has emerged as a promising and viable alternative to intravenous administration. The rising demand for subcutaneous drug delivery is attributed to the rapid development of biologics. Moreover, the subcutaneous route of administration is generally preferred, as it enables self-medication, improves quality of life, and thus reduces healthcare costs.
0 notes
Text

day 16
so instead of a drawing todays art is Babys First Embroidery
it will eventually be a patch for my Cool Patch Jacket once i get the border all filled in
it is in reference to the fact that i recently found out my state has a program that provides free naloxone to anybody who wants to carry it, no prescription necessary.
if you aren’t familiar, naloxone (brand name Narcan) is a medication that can be used to reverse an opioid overdose almost instantaneously in an emergency. in my state it is provided in the form of a nasal spray, but similar programs in other areas might offer auto-injectors, or other subcutaneous delivery methods.
BUT what i want to get across is that there are A LOT of these programs out there! if you’re curious, just google “free naloxone [your location]” and you might be able to get your hands on free or extremely reduced cost doses of naloxone to carry with you. most programs will train you how to administer the medication, and how to recognize signs of overdose.
harm reduction for drug users is important to me, regardless of who is using them, or for what purpose. and you can be part of that effort in your community SO easily by adding this to your first aid repertoire. it’s a bit like getting cpr certified! only literally so fast and cheap and easy like in my case i was in and out of the pharmacy in 15 minutes!!! you can literally save somebodys life with this, it is so so worth it.
#day 16#year 4#embroidery#harm reduction#cw drug use#cw drug overdose#i feel like a crazy person ever since i found out abt this fucking program#i have just been shouting at every person i see like HEY DID YOU KNOW YOU CAN GET FREE NARCAN#LIKE TRULY JUST FREE I WENT TO THE PHARMACY AND CAME OUT 15 MINUTES LATER WITH THE STUFF#NO PRESCRIPTION NO APPOINTMENT THEY JUST GAVE IT TO ME#there are like 7 pharmacies doing this in MY CITY ALONE like!!!!#anyway. you can literally keep a person from dying and it was so simple and so fast#its just so exciting that a program like that exists in my conservative ass state#like needle exchange programs are technically illegal here. but we have this#ANYWAY. the embroidery is busted as hell but its punk rock so who gives a shit
590 notes
·
View notes
Text
Slime HRT - First Step (Part Two)
“…and this last drug is Vasopressin, which is a standard water retention drug. Usually patients are plagued by constant thirst on this regimen, so we’ve started to prescribe this to combat it.”
This owl sure knew his stuff.
The appointment had gone perfectly, all things considered. After securing a follow-up appointment for a month in the future, Elise was walking out of the clinic with a copy of her prescription. Some of these medicines were vaguely familiar – she’d heard of salicylic acid in a chemistry class – but some of these drugs were of a fantastical nature. Myochitinase, homolipastat? These weren’t real things. You couldn’t get slime estradiol at a pharmacy.
Though…was there much use in thoughts like those anymore? She was in a city that didn’t exist, drawn by a promise from a made up company, and had been prescribed four different make-believe drugs by a six-foot-four bird in a labcoat. Reality had been blurred in the past four-ish hours, so maybe it was time to accept what she had just been given.
Plus, it wasn’t as though this city was strictly human, either. Granted, that was a majority of the population, but there were others who didn’t fit the label, not by any sense. Dragons, centaurs, other creatures of myth, and just about any kind of animal in the kingdom. Even things outside of animals, evident by the occasional dryad(?) that happened to pass her by.
Though, in spite of it all, no slimes. They could’ve just been inside, it was something like 85 degrees even here in the city. Which begged the question: what was going to change as the changes began and progressed? She’d done her research, and had asked around for advice, but the unfortunate truth was that slimes were a bit rare in this world of exotic creatures and their transspecies equivalents.
Basically, fat chance that Elise would meet someone like herself.
Such thoughts were muted as the day went on. The pharmacy in the city had the set of drugs she was in search of, and was able to set up a delivery schedule for her refills. Her medicines all looked uncannily similar to her existing HRT, but Elise could not deny that something was different about the drugs themselves, and it was hard not to describe them as ‘slimy.’
‘Well,’ she thought later that night as she took her first dose, ‘here goes nothing.’
PART TWO PART TWO GET YOUR COPY NOW
So I didn't do as much writing as part 1 (damn you writers block ;~;) so instead!!! Information Pamphlet!!
Human Replacement Therapy for Transspecies Slimefolk
Drug #1 - Myochitinase: 1mL intramuscular injection once weekly
The primary drug in slime human replacement therapy. The drug chemically changes the present myocytes (muscle cells) into chitin. Effects include increased translucence and thinning of skin, decreased muscle mass to make way for gel matrix mass, and decreased resistance to illness due to increased permeablility of the skin.
Drug #2 - Homolipastat: 100mg gel capsules once daily
The auxiliary drug in slime HRT. Similar in concept to human feminising HRT drug spironolactone and its alternatives, where homolipastat is utilised in preventing the reproduction of present myocetes. Further decreases muscle mass during conversion of muscle to gel matrix.
Drug #3 - Salicylic Acid: 30g ointment tube once weekly
Non-specific drug used to assist in the breakdown of skin cells in preparation for conversion to surface membrane.
Drug #4 - Vasopressin: 1 μL subcutaneous injection once daily
Optional antidiuretic used for water retention during initial stages of transition.
Affirming Treatments for Transspecies Slimefolk
Slime Stem Cell Therapy - Advised after one year of medical transition
Regular series of stem cell injections promoting transformation of organs to a core. Treatment includes pain relief and at-home assistance is strongly recommended.
Pigment Alteration Therapy - Optional as medical transition progresses
Pigment drugs such as melanin or other compounds may be started to change the colour of the individual later on in transition.
I LOVE YOU ALL STAY SLIMY :3 :3 :3
#slime girl#slime hrt#animal hrt#transgender#my writing#my gender#my hyperfixations#I can't wait for elise to get some tangible results and reveal my plans for her >:3
18 notes
·
View notes
Text
This includes legal drugs like nicotine, caffeine, etc.
19 notes
·
View notes
Text
Peptides – unique medicines
In contrast to proteins and small molecules, peptides represent a unique class of pharmaceutical compounds that are biochemically and therapeutically distinct from both. As intrinsic signaling molecules for many physiological functions, peptides offer an opportunity for therapeutic intervention that closely mimics natural pathways. In recent years, peptides have received increasing attention as a therapeutic approach.
The origin and development of in vitro peptide drugs
Polypeptides are amino acid derivative compounds containing at least one amide (peptide) bond. From a structural point of view, polypeptides include various types of peptides, such as linear peptides, cyclic peptides, delipidated peptides, etc. According to function, they can also be divided into antibacterial peptides and hormones. Regulatory peptides, neuroactive peptides, etc. [1].
In the early 20th century, research on peptides focused primarily on the effects of human signaling hormones. Insulin is a classic example of endogenous hormone therapy. It was the first peptide drug to be used clinically and is by far the most commercially successful [2] because it revolutionized the treatment of type I diabetes.

Advantages, disadvantages and new attempts of peptide drugs
The key factors for the success of peptide drugs are the effectiveness, specificity and safety of the mode of action of the peptide [3]. The rapid clearance of peptides from the body means that they do not accumulate in tissues and are relatively less toxic to the human body [4]. However, the limitations of peptide drugs are as obvious as their advantages.
Since peptide drugs are easily cleared from the serum, this also results in low bioavailability of peptide drugs. Furthermore, peptides generally have poor cell membrane permeability, which limits their use in targeting intracellular targets. Therefore, the development of peptide therapeutics has mainly focused on extracellular targets. Moreover, because they cannot penetrate the intestinal mucosa and need to be administered subcutaneously or intravenously, the convenience and compliance of peptide drugs in actual treatment are reduced [4].
Improving the bioavailability and efficacy of peptide drugs is also a popular research area. There have also been advances in universal and reproducible oral administration, as well as intracellular delivery of peptide drugs [4]. Cyclic peptides, a category of peptide drugs, are an emerging form of drugs designed to solve problems.
Cyclotides—a new form of peptide drugs
Cyclic peptides (including cyclodeposition peptides and bicyclic peptides) have many favorable properties as therapeutic agents and research tools. Compared with linear peptides, cyclic peptides have better proteolytic resistance and structural stability.

Currently, several cyclic peptides have become highly successful drugs, including vancomycin (antibacterial), daptomycin (antibacterial), cyclosporine A (transplantation immunosuppressant), and caspofenside (antifungal). Inspired by natural products, chemists have developed many methods to prepare cyclic peptides via N-to-C, side chain to side chain, or main chain to side chain cyclization. Some synthetic cyclic peptides, such as eptifibatide (used to treat heart disease), octreotide (a somatostatin mimetic used to treat acromegaly and diarrhea), cyclic RGD peptide, and linalotide Peptides have also been approved by the FDA for clinical or late-stage clinical trials [5].
Peptidomimetics – chemically synthesized peptide drugs
In terms of new drug strategies, in order to overcome the instability defects of peptides, in addition to modifying polypeptides to varying degrees like cyclic peptides, peptidomimetic compounds are also another reasonable means.
Peptidomimetic compounds are a class of compounds whose pharmacophore simulates natural peptides or proteins in three-dimensional space and retains the ability to interact with biological targets and produce the same biological effects [6]. The difference is that peptoids avoid the inherent defects of natural polypeptides and improve biological activity and stability.

Peptide drugs, whether isolated from the innate immunity of various species (including mammals, amphibians, fish, insects, plants and bacteria), or designed based on structure-activity relationship research, serve as a new structural drug , all have great potential [7]. Here we introduce a high-throughput method that can quickly identify and find suitable drugs – constructing a peptide library.

Currently, there are nearly a hundred peptide drugs on the global market, and research on new peptide therapeutic drugs continues at a steady pace, with more than 100 peptides in the clinical development stage and another 400-600 peptides in the preclinical research stage [2 ]. The utilization of peptides as therapeutics has evolved over time and continues to evolve as drug development and treatment paradigms change.
references
Luca Gentilucci. Chemical modifications designed to improve peptide stability: incorporation of non-natural amino acids, pseudo-peptide bonds, and cyclization. Curr Pharm Des. 2010;16(28):3185-203.
Markus Muttenthaler. Trends in peptide drug discovery. Nat Rev Drug Discov. 2021 Apr;20(4):309-325.
Keld Fosgerau. Peptide therapeutics: current status and future directions. Drug Discov Today. 2015 Jan;20(1):122-8.
Antoine Henninot. The Current State of Peptide Drug Discovery: Back to the Future?. J Med Chem. 2018 Feb 22;61(4):1382-1414.
Patrick G Dougherty. Understanding Cell Penetration of Cyclic Peptides. Chem Rev. 2019 Sep 11;119(17):10241-10287. Epub 2019 May 14.
Josef Vagner. Peptidomimetics, a synthetic tool of drug discovery. Curr Opin Chem Biol. 2008 Jun; 12(3): 292–296.
Sylvie E Blondelle. Optimization and high-throughput screening of antimicrobial peptides. Curr Pharm Des. 2010;16(28):3204-11.
B Guixer. Chemically synthesized peptide libraries as a new source of BBB shuttles. Use of mass spectrometry for peptide identification. J Pept Sci. 2016 Sep;22(9):577-91.
Website: https://www.ks-vpeptide.com
3 notes
·
View notes
Note
What information do you have about Johnson & Johnson?
Alza filed an NDA for the transdermal opioid analgesic product in December 1987 for post-operative use and for the relief of chronic cancer pain. Developed by J&J subsidiary Janssen Pharmaceutica, fentanyl has been marketed since 1968 under the Sublimaze brand. The drug is currently approved for I.V. administration and used primarily as a short-acting analgesic during anaesthesia. "This new drug delivery system will make it possible for the first time to use fentanyl . . . outside the operating room to control moderate to severe pain." Source
US opioids: Johnson and Johnson and drug distributors offer $26bn to end thousands of lawsuits. Source
The drug company Johnson & Johnson (J&J) has expressed regret after court documents unsealed in talcum powder litigation showed that it funded a 1971 study in which Pennsylvania prison inmates, most of them black, were injected subcutaneously with asbestos. Source
Asbestos Prisoner Study May Spell More Problems for Johnson & Johnson
WASHINGTON - Global health care giant Johnson & Johnson (J&J) and its subsidiaries will pay more than $2.2 billion to resolve criminal and civil liability arising from allegations relating to the prescription drugs Risperdal, Invega and Natrecor, including promotion for uses not approved as safe and effective by the Food and Drug Administration (FDA) and payment of kickbacks to physicians and to the nation’s largest long-term care pharmacy provider. The global resolution is one of the largest health care fraud settlements in U.S. history, including criminal fines and forfeiture totaling $485 million and civil settlements with the federal government and states totaling $1.72 billion. Source
Johnson & Johnson paused all clinical trials of its experimental COVID-19 vaccine after a study participant became sick with an "unexplained illness."
Johnson & Johnson has suspended international trials of a drug in the same class as an experimental drug made by Portuguese pharmaceutical company Bial, whose tests in France left one person brain dead and five others hospitalised. Source
1982 - McNeil
Product Recalled - Tylenol (acetaminophen) capsules
Reason for Recall - Medicine laced with potassium cyanide (poison) resulting in several patient deaths.
2009 to 2011 - McNeil
Product Recalled - Several OTC medicines including Tylenol, Motrin, Benadryl, St. Joseph aspirin, Sudafed, Pepcid, Mylanta, Rolaids, Zyrtec, Zyrtec Eye Drops (tens of millions of bottles)
Reason for Recall - Unpleasant smells causing nausea; tiny metal shards in liquid medicines; wrong ingredient levels
2010 - DePuy [Pinnacle Systems]
Product Recalled - ASR Hip Resurfacing System and ASR XL Acetabular System (metal-on-metal hip implants)
Reason for Recall - Metal poisoning (metallosis); loosening of the implant or joint dislocation; additional surgeries
2012 - Ethicon
Product Recalled - Gynecare Prolift Kit, Gynecare Prolift+M Kit, Gynecare TVT Secure and Gynecare Prosima Pelvic Floor Repair System Kit (transvaginal mesh implants)
Reason for Recall - Perforation of organs; vaginal bleeding and scarring; mesh erosion; severe pain
2014 - Ethicon
Product Recalled - Power Morcellators
Reason for Recall - Spread of uterine cancer; rapid progression of the disease; death
2019 – Johnson & Johnson
Product Recalled – 33,000 bottles of Johnson’s Baby Powder
Reason for Recall – The FDA found a small amount of asbestos — a known carcinogen — in a sample
Xarelto
Number of Lawsuits - 13,511
Injuries - severe, sometimes deadly bleeding events, blood clots, wound leaks, infection
J&J was involved in seven of 2017’s top ten health-care-related verdicts.
The company was also involved in the third-largest pharmaceutical settlement with the U.S. Department of Justice. In 2013, J&J paid the Justice Department more than $2.2 billion. The settlement resolved civil and criminal allegations involving Risperdal, Invega and Natrecor.
May 2017
J&J paid $33 million to most U.S. states and the District of Columbia. The states charged J&J with misrepresenting the manufacturing practices behind certain drugs. This included its Motrin products. These products were later recalled.
Oz
4 notes
·
View notes
Text
Psychopharmacology, pt. 2: Pharmacokinetics
Bio-availability: amount of drug in the blood that is free to bind at target sites.
Pharmacokinetic factors determining drug action:
1. Routes of administration
2. Absorption
3. Distribution
4. Binding/effects
5. Inactivation (metabolism)
6. Excretion
Routes of Administration
Drugs must get into the nervous system to have an effect.
The way that a drug enters and passes through the body to reach its target is called route of administration.
oral
injection
inhalation
topical application
transdermal
To bypass the blood-brain barrier: injection in the cerebro-spinal fluid or directly in the brain.

The route of administration affects the dosage of a drug, i.e. the amount of drug needed to have a psychoactive effect.
Ex. amphetamine:
1000 mg - orally
100 mg - injected or inhaled
10 mg - injected into the CSF
1 mg - injected directly into the brain

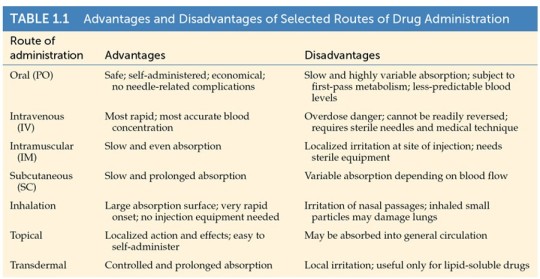
Oral Administration
PO = per os (Latin, “by mouth”)
Pros:
Safest, easiest, and most common way to take drugs
Longest route
Cons:
Affected by how much food is in the stomach
Delivery often erratic and incomplete
Most difficult pathway to the brain, because the drug has to...
survive stomach acid and enzymes
be absorbed by intestine
enter into the blood stream
pass through the blood-brain barrier
Example: Insulin is not resistant to stomach acid and enzymes, so it cannot be given orally.

Injections
Before a drug (including cocaine, amphetamines, barbiturates, morphine, and heroin) can be injected, it must be liquid.
Vehicle:
normal saline (0.9% NaCl)
viscous oil (often sesame oil) for depot injections
Intravenous (IV) - into the blood stream
Intramuscular (IM) - into a muscle
Subcutaneous (SC) - under the skin
In the CSF
Intracranial (IC)
Intravenous Injections
Pros:
fastest and most accurate method; drug reaches brain instantly
fewer barriers to pass
Cons:
painful, invasive
quick onset of drug effect can be a hazard, because you have little time to correct an overdose or allergic reaction. the drug cannot be removed from the body as it can by stomach pumping.
lack of sterility can result in infectious diseases, including HIV (this risk is reduced with free needle exchanges)
Intramuscular Injections
Pros:
slower than IV, more even absorption over period of time
absorption can be slowed by combining the drug with another drug that constricts blood vessels, or with vegetable oil
Cons:
injection solution can be highly irritating, causing significant muscle discomfort
Subcutaneous Injection
- drug injected just below the skin
- absorption is slow but can be variable; can be slowed by vegetable oils or implantation of a pellet or delivery device
Ex. SUBLOCADE (buprenorphine XR) is used for subcutaneous injection only. It is designed to deliver buprenorphine at a controlled rate over a one month period.
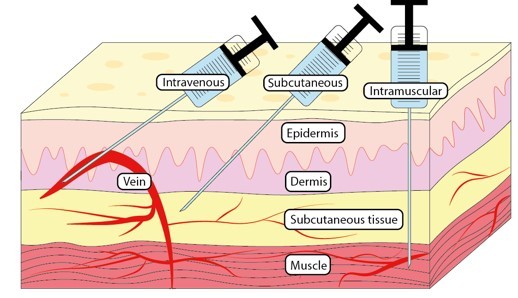
Inhalation
- smoke of burning dried plant material (tobacco, cannabis), aerosol/vapor, gases can be inhaled and absorbed through the lungs
- takes effect quickly
- rapid absorption because the lungs have large surface area and many capillaries
Topical Application
- drug is applied to mucous membranes in the eyes, nose, colon, or vagina
- mostly local effects, but drug can be absorbed into the bloodstream and have systemic effects
- oral topical application: drugs that may be chewed, but not swallowed (ex. chewing tobacco)
Intranasal Administration/Insufflation
- can cause local effects such as relieving nasal congestion, but can also have systemic effects
- drug effect peaks in 15 to 30 minutes
- powdered drugs (ex. cocaine), medicinal nasal sprays (ex. ketamine for depression)
Transdermal Administration
- administration via skin patches: controlled and sustained delivery of drug
- nicotine patches: to treat nicotine use disorder
- fentanyl patches: to alleviate pain
Absorption: movement of the drug from site of administration to the blood circulation.

Factors that Influence Absorption:
drug solubility
stomach contents
concentration of the drug (generally high concentrations are absorbed more rapidly than low concentrations)
size and sex of individual (a larger person has more body fluid to dilate a drug; AFAB people tend to have less body fluid than AMAB people)
circulation to side of absorption (increased blood flow due to massage or local application of heat enhances absorption)
area of absorbing surface (drugs are absorbed very rapidly in regions with large surface areas such as the lungs and the intestines)

Fat-soluble drugs: can easily pass through the cellular membranes by passive diffusion.
Cell membranes are primarily phospholipids, which have a negatively charged region (hydrophilic) and two uncharged tails (hydrophobic), arranged in a bilayer.

Heroin vs. Morphine
Heroin is derived from morphine; they stimulate the same receptors.
However, heroin is 2-3x more potent than morphine, because it is more fat-soluble.

Distribution
- highest concentration of a drug will occur where blood flow is greatest
- blood brain barrier limits movement of ionized molecules
- Because the brain receives about 20% of the blood that leaves the heart, lipid-soluble drugs are readily distributed to brain tissue.
Blood-Brain Barrier
Cerebrospinal fluid (CSF) fills the subarachnoid space around the brain and spinal cord, ventricles, and canals.
Blood-brain barrier: the separation between brain capillaries and the brain/CSF.
Many substances that diffuse out of the blood do not enter the CSF.
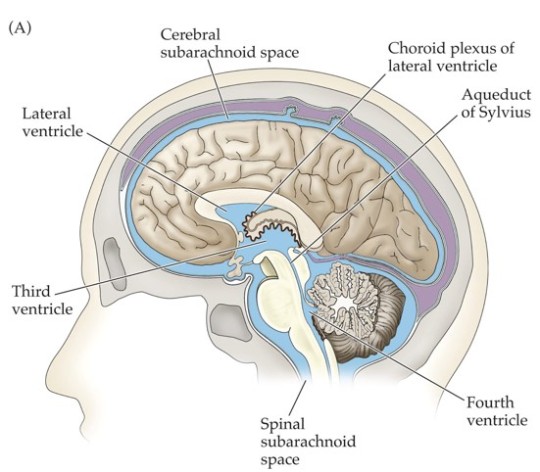
Typical capillaries are designed to allow movement of materials between the blood and surrounding cells.
Brain capillaries have no clefts, movement of water-soluble molecules is minimized.
Astrocytes help maintain tight junctions between capillary endothelial cells.

It is important to know whether a drug can cross the blood-brain barrier.
Physostigmine crosses the blood-brain barrier.
- blocks AChE and increases availability of the neurotransmitter acetylcholine
- the antidote for anticholinergic poisoning with scopolamine, atropine
Neostigmine does not cross the blood-brain barrier.
- also blocks AChE and increases acetylcholine, but only peripherally
- treatment for diseases such as myasthenia gravis (muscle weakness) without CNS side effects
Oxycodone crosses the blood-brain barrier:
binds to opioid receptors
painkiller
can produce euphoria
side effects include constipation
Loperamine (Imodium) does not cross the blood-brain barrier:
binds to peripheral opioid receptors in the intestines
treatment for diarrhea
Drug depots: binding at inactive sites where no biological effect is initiated (plasma proteins, muscle, fat)
Drug molecules tied up in these depots cannot reach active sites or be metabolized by the liver, but binding is reversible.
Depot binding affects magnitude and duration of drug action: it reduces concentration of drug at its sites of action and delays effects.
Depot binding can result in drugs remaining in the body for extended periods.
- Ex. Tetrahydrocannabinol (THC) can be detected in urine for several days after a single dose.
Thiopental is a rapid-onset, short-acting barbiturate (administered as general IV anesthetic)
- used for medically induced coma, euthanasia, status elipepticus
- previously the first of three drugs administered during most lethal injections in the USA
- due to depot binding, thiopental brain levels drop within 5 minutes
Inactivation and Elimination
- molecules of drugs are transformed (metabolized) with the help of enzymes
Metabolism: the processes involved in transforming/destructing drug molecules.
Drugs are broken down in the kidneys, livers, and intestines. Most biotransformation occurs in the liver. Metabolites are excreted.
Drug clearance from the blood is usually exponential (first-order kinetics).
Only a small fraction of clearance sites are occupied, so the rate is concentration-dependent.
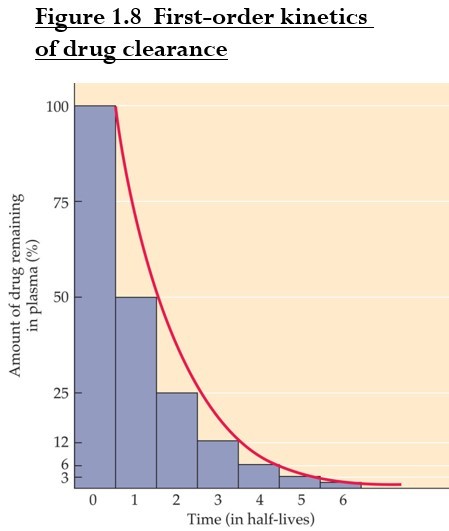
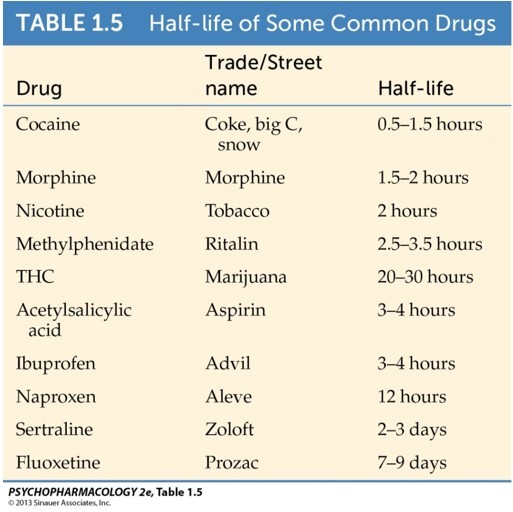
Half-life: amount of time required for removal of 50% of the drug (t 1/2).
Half-life affects interval between doses. A drug with a shorter half-life should be taken more often.
Drug almost eliminated after 4-5x half-life.
Goal: maintain concentration of a drug in blood plasma at a constant level.
Steady state plasma level: absorption/distribution phase is equal to the metabolism/excretion phase.
Target therapeutic concentration is achieved only after multiple doses, usually 4-5.

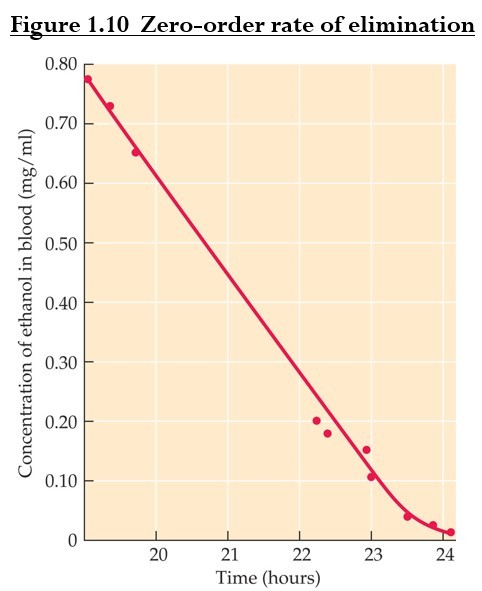
Some drugs are eliminated according to zero-order kinetics: molecules are cleared at a constant rate regardless of concentration.
It occurs when drug levels are high and routes of metabolism or elimination are saturated (ex. alcohol).
First Pass Metabolism
First pass metabolism of drugs that are taken orally, swallowed and absorbed from the digestive system occurs mostly in the liver.
Alternative routes of administration avoid the first-pass effect (e.g. intravenous, inhalation, transdermal and sublingual)
Some therapeutic drugs must be administered by injection, or in high doses.

Drugs can be modified through oxidation, reduction, hydrolysis, or synthetic reactions.
Microsomal enzymes: liver enzymes that metabolize psychoactive drugs.
They lack strict specificity and can metabolize a wide variety of chemicals.
The cytochrome P450 (CYP450) enzyme family are responsible for oxidizing most psychoactive drugs.
Factors that modify biotransformation capacity include:
Enzyme induction
Enzyme inhibition
Drug competition
Individual differences in age, gender, and genetics
Enzyme induction: repeated use of a drug increases number of enzyme molecules and speeds biotransformation.
Repeated drug use ----> Increase in enzymes ---> Higher metabolism ---> Lower bioavailability
Drug can induce its own enzymes. In this case, drug tolerance happens = drug becomes less effective with repeated use.
Sometimes enzymes for the other drugs are induced. For example, cigarette smoking increases CYP450 enzymes. People who are heavy smokers may need higher doses of drugs such as antidepressants and caffeine that are metabolized by the same enzyme.
Enzyme inhibition: a drug may inhibit an enzyme, also reducing metabolism of other drugs. Effects are more intense or prolonged; toxicity is possible.
Example: Antidepressant drugs monoamine oxidase inhibitors (MAOIs) inhibit enzyme MAO. MAO metabolizes monoamines, including tyramine, which is found in red wine, beer, some cheeses, etc. When individuals who are taking these antidepressants eat foods rich in tyramine, dangerous high blood pressure and cardiac arrhythmias can occur, making normal foods potentially life threatening.

Drug competition for an enzyme: elevated levels of one drug reduces metabolism of the second, causing potentially toxic levels.
- Example: alcohol + sedatives (such as Valium) compete for cytochrome P450.
Excretion: the processes of eliminating waste products.
Drugs are excreted in urine, feces, sweat, breast milk, and exhaled air.
Urine is the most important route for drug elimination.
- The kidneys filter materials from the blood, unless they are large or bound to plasma proteins.
Drugs can be excreted changed and/or unchanged.

3 notes
·
View notes
Text
How does the veterinary B-ultrasound machine control the reproduction of ewes?
When satisfactory results cannot be obtained under natural breeding conditions, the reproductive cycle of sheep can be artificially manipulated by veterinary ultrasound machines.

Veterinary Ultrasound Imaging Hormonal Control: The common method of inducing estrus in non-cycling ewes is progesterone-based therapy. Progesterone prevents ewes from returning to estrus and ovulation. It is produced by the corpus luteum (CL) of the ovary after ovulation and maintains pregnancy. When progesterone is introduced artificially, they trick the body into thinking it is pregnant and the animal will not ovulate or go into estrus (heat. When the source of progesterone is completely removed, the body realizes it is no longer pregnant and will ovulate at a very predictable period. Progestogens refer to synthetic compounds that have the properties of progesterone. These substances mimic the function of CL. Progestogens (synthetic analogs of progesterone) can be delivered by feeding (MGA), subcutaneous implants (Synchro-Mate B?), sponges (or pessaries) inserted into the vagina, or plastic delivery devices (CIDRs) inserted into the vagina.
Intravaginal sponges (or pessaries) have been the traditional method of inducing and/or synchronizing estrus in ewes. They contain progesterone at a lower dose than natural progesterone. Two types of sponges are Chronogest (FGA) and Veramix? (MAP). Intravaginal sponges are usually inserted for 9 to 19 days and are used in conjunction with PMSG, which is injected at the time of sponge removal or 48 hours before sponge removal. Intravaginal sponges have a high retention rate (> 90%), and females typically show estrus 24 to 48 hours after removal. Responses to the intravaginal sponge vary, depending on breed, protocol, co-handling, management, and mating system. The CIDR™ (controlled internal drug release) device is made of a medical silicone elastomer impregnated with progesterone and was developed in New Zealand. Protocols for using the CIDR™ device are generally the same as those for the intravaginal sponge. Studies have shown that the CIDR™ device and the intravaginal sponge produce similar results. The CIDR™ device was recently approved for use in sheep in the U.S. Synchro-mate-B™ is a bovine implant containing 6 mg of synthetic progesterone. One-third or one-half of the Synchro-mate-B™ implant is typically used in ewes. The implantation period is 9 to 14 days. An injection of PMSG and/or PGF2a is typically given two days before the end of the implantation period.
An orally active synthetic progestin used to suppress estrus in heifers on feed. Use of this product requires feeding a supplement containing MGA® once or twice daily for 8 to 14 days. Regimens typically include co-treatment with PMSG, PG600®, or Ralgro® (zeranol). Ralgro® is a commercially available growth promoter for cattle and sheep that has estrogen-like effects on LH and FSH concentrations. It is the only veterinary grade source of PMSG available in the United States. The response of estrus to MGA feeding as detected by veterinary ultrasound varies but is generally higher with co-treatment. Prostaglandin-based regimens are only suitable for cycling ewes and are limited to use during the breeding season. Two commonly used products are Lutalyse® (PGF2a) and Estrumate® (cloprostenol). Prostaglandins cause regression of the CL, telling the body than no pregnancy exists. The ewes will ovulate at a very predictable time. When a flock of cycling ewes is given a single prostaglandin treatment, 60% to 70% of the flock will begin synchronized estrus 30 to 48 hours later. A double injection system (11 days apart) is most common in sheep. Melatonin Melatonin treatment has been shown to be an effective method for inducing estrus in non-cycling ewes. Melatonin is known as the "darkness hormone" because it is released by the pineal gland at night. Therefore, melatonin treatment simulates the short fall day and induces estrus after a minimum of about 35 days of treatment. It is important to note that most of the above drug treatments have been approved by the U.S. Food and Drug Administration for use in sheep, although they may be available to producers in other countries. Light Control Controlled lighting can be used to initiate estrus. Short-day breeders like sheep can be programmed to cycle if they are kept in a light-tight building, with ewe breeding tapering off over a period of 8 to 12 weeks. Rams should be exposed to the same light regiment for high fertility. Light control is usually impractical for most producers.
0 notes
Text
Home Infusion Therapy Market Size, Share, Growth, Analysis Forecast to 2030
Home Infusion Therapy Industry Overview
The global home infusion therapy market size was valued at USD 35.96 billion in 2023 and is expected to grow at a compound annual growth rate (CAGR) of 8.1% from 2024 to 2030.
Home infusion therapy involves delivering therapeutic treatments, medications, or fluids directly into a patient's bloodstream through intravenous (IV) infusion, usually in the comfort and convenience of their home.
The growth of the market is driven by several key factors, including the expanding geriatric population characterized by decreased mobility, a rising preference for home care, and the swift evolution of technological advancements. Infusion therapy, encompassing essential components like IV therapy and IV hydration therapy, plays a crucial role in addressing conditions such as immune deficiencies, cancer, and congestive heart failure, where oral medication is not a viable treatment option. The increasing demand for these therapies stems from the need for long-term treatment among patients, positioning home infusion therapy as a notably cost-effective alternative to hospital-based care. The incorporation of IV therapy and IV hydration therapy serves as a driving force, providing patients with enhanced accessibility to effective and personalized medical solutions in the comfort of their homes
Gather more insights about the market drivers, restrains and growth of the Home Infusion Therapy Market
The home infusion market experienced a positive shift during the COVID-19 pandemic, with home infusion becoming a crucial necessity as healthcare facilities faced a surge in COVID patients. Despite the challenges posed by regional and country-wide lockdowns, causing disruptions in operations and supply chains, the market witnessed a substantial increase in 2020. As reported by Medtech Dive in October 2020, Baxter disclosed third-quarter sales of USD 2.97 billion, marking a 4% growth attributed to the rising demand for its COVID-related medical products. Furthermore, Baxter reported operational sales growth of 6% (reaching 3.2 billion) in Q3 2021 compared to 3.0 billion in Q3 2020, indicating a sustained recovery from the pandemic's impact.
Moreover, the market's expansion is propelled by the enhanced outcomes observed in patients and the cost-effectiveness and convenience provided by home infusion therapy. The increasing demographic of baby boomers struggling with diminished mobility due to conditions such as paralysis, osteoarthritis, and diabetes is expected to amplify the demand for home infusion therapy. The growing imperative to reduce the duration of inpatient stays is a pivotal factor poised to contribute significantly to the market's growth. Remarkably, continuous subcutaneous (SC) apomorphine infusion emerges as an exceptionally effective treatment for Parkinson's disease (PD), with diverse drug formulations available for the management of PD through subcutaneous delivery. In response to the mounting burden of PD, there is a notable surge in the demand for subcutaneous infusion therapy. For instance, in line with the Parkinson's Foundation's 2022 data update, approximately 90,000 individuals receive a PD diagnosis annually in the U.S. Furthermore, the anticipated number of people living with PD in the country is projected to soar to nearly 1.2 million by the year 2030.
Browse through Grand View Research's Medical Devices Industry Research Reports.
• The global knee braces market size was valued at USD 1.12 billion in 2023 and is projected to grow at a CAGR of 7.7% from 2024 to 2030.
• The global western blotting market size was valued at USD 986.2 million in 2023 and is projected to grow at a CAGR of 6.1% from 2024 to 2030.
Key Companies & Market Share Insights
Some of the key players operating in the market include Baxter, BD, Smiths Medical, Terumo Corporation, ICU Medical, etc
Baxter International Inc., commonly known as Baxter, is a global healthcare company that specializes in providing a wide range of medical products, therapies, and technologies. With a rich history dating back to the 1930s, Baxter has evolved into a leading player in the healthcare industry. The company develops innovative solutions for critical medical needs, including renal care, medication delivery, pharmaceuticals, and various therapeutic areas.
Becton, Dickinson and Company (BD) is a global medical technology company. BD specializes in developing and manufacturing medical devices, laboratory equipment, and diagnostic products aimed at advancing the diagnosis and treatment of various medical conditions. With a commitment to improving healthcare outcomes, BD focuses on delivering solutions in areas such as medication management, infection prevention, diagnostics, and biosciences.
Key Home Infusion Therapy Companies:
CVS/Coram
Option Care Health
BriovaRx/Diplomat (UnitedHealth Optum)
PharMerica
Fresenius Kabi
ICU Medical, Inc.
B. Braun Melsungen AG
Baxter
BD
Caesarea Medical Electronics
Smiths Medical
Terumo Corporation
JMS Co. Ltd.
Recent Developments
In June 2023, Baxter International, an American healthcare company, introduced Progressa+ Next Gen ICU bed for addressing critical needs of patients at their homes. This technology makes it easier for nurses to take care of patients, while supporting therapy at home.
In May 2023, Fresenius Kabi, a global healthcare company, initiated an agreement with Premier, Inc., an American healthcare company, that resulted in pricing and term benefits for the Ivenix Infusion System. This system is designed to advance the reliability and simplicity of infusion pumps.
In May 2023, Option Care Health, a healthcare service provider, created an independent platform for home care services in collaboration with Amedisys Inc., a leading provider of home health services. This platform comprises pharmacists, dieticians, therapists, social workers, and others for providing high quality healthcare services at home.
In April 2023, CareFusion, currently owned by Becton Dickinson, an American medical technology company, launched an advanced ultrasound technology to provide clinicians with optimal IV insertions. More than 90% hospitalized patients receive the IV therapy, thus contributing towards the market growth of home infusion therapy.
In January 2022, ICU Medical, a California-based global operations company, finalized the acquisition of Smiths Medical from Smiths Group Plc for creating a leading infusion therapy company with a combined revenue of USD 2.5 billion.
In November 2021, Terumo Corporation, a global medical device company, developed a smartphone device for controlling insulin pump. This device can be utilized as a home infusion therapy by patients for harmonizing the insulin therapy at home.
Order a free sample PDF of the Home Infusion Therapy Market Intelligence Study, published by Grand View Research.
0 notes
Text
Advancements in Technologies and Drug Delivery Systems | Market Size | 2035
The report from Roots Analysis offers a comprehensive analysis of the current and future market landscape of subcutaneously administered biologics. The study aims to identify the primary drivers of growth and estimate the market size and potential for future boost associated with subcutaneous biologics. The report delves into the opportunities and challenges in this market segment and provides insights on the competitive landscape and technological advancements.

0 notes
Text
Exploring the Future of Injectable Drug Delivery Systems: Innovations and Impact on Healthcare
The field of healthcare is evolving rapidly, and one area seeing significant advancements is the injectable drug delivery system. This technology plays a crucial role in the treatment of chronic diseases, vaccinations, and emergency medical care. It allows for precise, rapid, and efficient delivery of medications directly into the bloodstream, offering several benefits over traditional methods.
What Are Injectable Drug Delivery Systems?
Injectable drug delivery systems involve the administration of drugs through needles, directly into the body’s tissues or bloodstream. These systems include intramuscular, intravenous, subcutaneous, and intradermal injections, which are selected based on the medication's purpose and the desired absorption rate. They ensure the drug reaches the bloodstream quickly, providing fast relief, especially in critical conditions.
Download PDF Brochure
Benefits of Injectable Drug Delivery Systems
High Bioavailability: Since the drug is delivered directly into the body, it bypasses the digestive system, preventing any loss of efficacy and ensuring higher bioavailability.
Targeted Treatment: These systems allow for localized delivery, targeting specific areas within the body, which is particularly beneficial in cancer therapies and localized infections.
Rapid Onset of Action: Injectable drugs can provide quick relief in emergency situations, making them indispensable in cases like anaphylaxis, heart attacks, or severe infections.
Controlled Release: Innovations in injectable formulations allow for controlled release, meaning a single injection can offer therapeutic benefits over an extended period. This is particularly useful in the treatment of chronic conditions such as diabetes or arthritis.
Types of Injectable Drug Delivery Systems
Conventional Syringes and Needles: The most common method, suitable for a variety of drugs including vaccines, insulin, and antibiotics.
Prefilled Syringes: Preloaded with medication, they offer increased convenience, safety, and accuracy, reducing the risk of contamination or dosing errors.
Injectable Pens: Commonly used for insulin, injectable pens offer ease of use, portability, and precise dosing, making them ideal for long-term conditions that require regular medication.
Auto-Injectors: Designed for emergency use, auto-injectors like EpiPens administer a predetermined dose of medication and are essential in treating allergic reactions.
Needle-Free Injectors: A newer development, these systems use high-pressure technology to deliver drugs through the skin without needles, reducing pain and the risk of needle-stick injuries.
Request Sample Pages
Innovations in Injectable Drug Delivery
The injectable drug delivery system market has witnessed groundbreaking innovations aimed at improving patient experience and outcomes. Key trends include:
Biodegradable Implants: These injectables release drugs slowly over time, eliminating the need for multiple doses.
Smart Injections: Equipped with sensors and monitoring technologies, these injectables can record dosage and patient adherence, transmitting data to healthcare providers for real-time monitoring.
Microneedles: Tiny needles that minimize pain while delivering drugs more effectively, often used in vaccination efforts.
Impact on Healthcare
Injectable drug delivery systems have significantly transformed patient care by improving the efficiency and precision of treatments. They are crucial for managing chronic diseases, cancer therapies, and emergency medical interventions. Additionally, advancements in drug delivery technologies are enhancing patient compliance, offering solutions that require fewer injections, or even reducing the need for hospitalization.
Challenges and Future Outlook
While injectable drug delivery systems offer many advantages, they also face challenges such as the need for skilled administration and potential discomfort associated with needle use. However, ongoing research and technological advancements promise to address these issues. The development of needle-free injectors and automated devices is expected to make injectable therapies more patient-friendly, increasing adoption and furthering the reach of modern medicine.
In conclusion, the future of injectable drug delivery systems looks promising, with innovations making treatments more effective, accessible, and comfortable for patients worldwide. As these technologies continue to evolve, they will likely play an even greater role in improving health outcomes across various medical fields.
Content Source:
0 notes
Text
Multiple Sclerosis Drugs Market - Forecast(2024 - 2030)
Multiple Sclerosis Drugs Market Overview
The Multiple Sclerosis Drugs Market size is estimated to reach $31.3 billion by 2028, growing at a CAGR of 3.7% during the forecast period 2023-2028. Treatment of multiple sclerosis may involve immunosuppressants, immunomodulators and monoclonal antibodies. Interferons are disease-modifying drugs that assist in decreasing relapses in people enduring multiple sclerosis. Intramuscular injections are utilized when additional kinds of delivery techniques like oral, intravenous and subcutaneous are not suggested. As per a novel clinical trial in August 2022, an experimental antibody therapy for multiple sclerosis can reduce symptom flare-ups by half, as compared to standard treatment. Interferon beta-1a has been certified by the U.S. Food and Drug Administration (FDA) to treat relapsing forms of multiple sclerosis and it has been assessed in clinical trials for the treatment of COVID-19. According to the National Multiple Sclerosis Society, more than 2.8 million people are living with Multiple Sclerosis worldwide. The burgeoning focus of firms on pipeline products for multiple sclerosis is set to drive the Multiple Sclerosis Drugs Market. The recommended continuous medicines for multiple sclerosis-like interferons to decrease the requirement for hospitalization due to COVID-19 are set to propel the growth of the Multiple Sclerosis Drugs Industry during the forecast period 2023-2028. This represents the Multiple Sclerosis Drugs Industry Outlook.
Multiple Sclerosis Drugs Market Report Coverage
The “Multiple Sclerosis Drugs Market Report - Forecast (2023-2028)” by IndustryARC, covers an in-depth analysis of the following segments in the Multiple Sclerosis Drugs Market.
By Drug Class: Immunomodulators, Immunosuppressants, Interferons and Others.
By Route Of Administration:Oral, Injection (Intramuscular, Subcutaneous and Intravenous).
By Geography: North America (the US, Canada and Mexico), Europe (Germany, France, the UK, Italy, Spain, Russia and the Rest of Europe), Asia-Pacific (China, Japan, South Korea, India, Australia & New Zealand and the Rest of Asia-Pacific), South America (Brazil, Argentina, Chile, Colombia and the Rest of South America) and the Rest of the World (the Middle East and Africa).
Request Sample
Key Takeaways
Geographically, North America (Multiple Sclerosis Drugs market share) accounted for the highest revenue share in 2022. It is poised to dominate the market over the period 2023-2028 owing to the increasing predominance of multiple sclerosis involving immunomodulators in the North American region.
The growth of the Multiple Sclerosis Drugs Market is being driven by considerable financing of large pharmaceutical firms in the drug development procedure and surging interferon treatment of Multiple Sclerosis. However, the soaring cost of medications is one of the major factors hampering the growth of the Multiple Sclerosis Drugs Market.
The Multiple Sclerosis Drugs Market Detailed Analysis of the Strengths, Weaknesses and Opportunities of the prominent players operating in the market would be provided in the Multiple Sclerosis Drugs Market report.
Multiple Sclerosis Drugs Market Segment Analysis - by Drug Class
The Multiple Sclerosis Drugs Market based on drug class can be further segmented into Immunomodulators, Immunosuppressants, Interferons and Others. The Immunomodulators Segment held the largest share of the Multiple Sclerosis Drugs market in 2022. This growth is fueled by the surging application of immunomodulators for the treatment of multiple sclerosis and its connected symptoms. Interferon beta and glatiramer acetate (GA) were the earliest immunomodulators certified for the treatment of relapsing-remitting multiple sclerosis (MS) and clinically isolated syndromes. The greater prescription rates are further propelling the growth of the Immunomodulators segment.
Furthermore, the Immunosuppressants segment is estimated to grow at the fastest CAGR of 4.3% during the forecast period 2023-2028 owing to the typical application of immunosuppressants like azathioprine, cyclophosphamide, methotrexate and mitoxantrone for the treatment of Multiple Sclerosis as well as application of immunosuppressants as combination therapy or monotherapy.
Inquiry Before Buying
Multiple Sclerosis Drugs Market Segment Analysis - by Route Of Administration
The Multiple Sclerosis Drugs Market based on the route of administration can be further segmented into Oral, Subcutaneous and Injection. The Injection Segment held the largest share of the multiple Sclerosis Drugs market in 2022. This growth is fueled by the surging count of approvals for multiple sclerosis medications as injections for subcutaneous application in the treatment of ailment. The injection segment is sub-segmented into intramuscular, subcutaneous and intravenous. The increasing application of intravenous (IV) infusions like OCREVUS to treat relapsing or primary progressive forms of Multiple Sclerosis is further propelling the growth of this segment.
Furthermore, the Oral segment is estimated to grow at the fastest CAGR of 5.5% during the forecast period 2023-2028 due to the growing introduction of novel products, assistance in patient satisfaction, boosting therapeutic compliance and the soaring inclination toward oral medications.
Multiple Sclerosis Drugs Market Segment Analysis - by Geography
North America (Multiple Sclerosis Drugs Market) dominated the Multiple Sclerosis Drugs market with a 40% share of the overall market in 2022. The growth is driven by the existence of key players in the North American region. The approval by regulatory authorities is further propelling the growth of the Multiple Sclerosis Drugs Industry, thereby contributing to the Multiple Sclerosis Drugs Industry Outlook, in the North American region. Furthermore, the Asia-Pacific region is estimated to be the region with the fastest CAGR over the forecast period 2023-2028. This growth is fuelled by the factors like enhanced distribution networks of pharmaceutical firms in emerging economies in the Asia-Pacific region. The surging government initiatives are further fueling the progress of the Multiple Sclerosis Drugs Market in the Asia-Pacific region.
Schedule a Call
Multiple Sclerosis Drugs Market Drivers
Surging Approvals for Intramuscular Injections:
As per Healthline, current findings from the National MS Society estimate that almost 1 million people in the U.S. are living with Multiple Sclerosis. In February 2021, the U.S. Food and Drug Administration (FDA) certified an intramuscular injection formulation of Plegridy (peginterferon beta-1a) to treat patients with relapsing forms of multiple sclerosis (MS). This formulation, for injection directly into the muscle, is what is usually utilized to convey the flu shot. As per Biogen, Plegridy’s developer, treatment provided through intramuscular injection is as efficient as the subcutaneous injection formulation. This novel formulation was also currently certified by the European Commission. The surging approvals for intramuscular injections are therefore fueling the growth of the Multiple Sclerosis Drugs Market during the forecast period 2023-2028.
Soaring Treatment Involving Immunomodulators and Immunosuppressants:
As per Healthline, a supposed 2.5 million people live with Multiple Sclerosis globally. Medications certified for application in multiple sclerosis that decrease the frequency of intensifications or gradual infirmity advancement are termed disease-modifying drugs (DMDs). These DMDs can be further categorized as immunomodulators or immunosuppressants. Teriflunomide is an oral immunomodulator that causes anti-inflammatory impacts by restricting dihydroorotate dehydrogenase, a mitochondrial enzyme included in pyrimidine synthesis. It is designated for relapsing forms of MS. The most typically utilized immunosuppressants in Multiple Sclerosis are azathioprine, cyclophosphamide, methotrexate and mitoxantrone. The soaring treatment involving immunomodulators and immunosuppressants is fueling the growth of the Multiple Sclerosis Drugs Industry, thereby contributing to the Multiple Sclerosis Drugs Industry Outlook during the forecast period 2023-2028.
Multiple Sclerosis Drugs Market Challenges
Side Effects of Interferons:
As per MS Discovery Forum, an approximated 200 novel cases are diagnosed every week in the U.S. Interferon beta (IFNbeta) decreases the relapse rate and activity as assessed by serial MRI scanning and ailment advancement of Multiple Sclerosis. Therapy with IFNbeta may be connected with numerous unfavorable reactions. Comparatively repeated side effects involve flu-like symptoms, transient laboratory abnormalities, menstrual ailments and raised spasticity. Dermal injection site reactions happen subsequent to subcutaneous application of IFNbeta-1b and IFNbeta-1a. Likely side effects of IFNbeta involve different autoimmune reactions, capillary leak syndrome, anaphylactic shock, thrombotic-thrombocytopenic purpura, insomnia, headache, alopecia and depression. These issues are thus hampering the growth of the Multiple Sclerosis Drugs Market.
Buy Now
Multiple Sclerosis Drugs Industry Outlook
More focus on pipeline drugs for multiple sclerosis and emerging R&D financing in the pharmaceutical industry are key strategies adopted by players in the Multiple Sclerosis Drugs Market. The top 10 companies in the Multiple Sclerosis Drugs market are:
Bayer AG
Teva Pharmaceutical Industries Ltd.
Novartis AG
Sanofi Inc.
F. Hoffmann-La Roche Ltd.
Celgene Corporation
Acorda Therapeutics, Inc.
Biogen, Inc.
Actelion Pharmaceuticals Ltd. (Johnson & Johnson)
Merck Serono (Merger between EMD Serono and Merck KGaA)
Recent Developments
In October 2021, Novartis declared that it would introduce 41 abstracts at the upcoming 37th Congress of the European Committee for Treatment and Research in Multiple Sclerosis (ECTRIMS). The data being introduced covered an all-inclusive MS portfolio. This stressed the firm’s dedication in enhancing the quality of life for people residing with MS at all phases of the ailment.
In June 2021, Novartis marked a collaboration agreement and alternative to acquiring Cellerys. Cellerys is a Zurich-based startup, conducting research on a therapy to combat Multiple Sclerosis (MS).
In January 2020, Novartis favorably finished the acquisition of The Medicines Company. This included a possibly first-in-class, investigational cholesterol-lowering therapy - inclisiran. The Medicines Company proposed the New Drug Application (NDA) for inclisiran to FDA in December 2019.
#Multiple Sclerosis Drugs Market#Multiple Sclerosis Drugs Market Share#Multiple Sclerosis Drugs Market Size#Multiple Sclerosis Drugs Market Forecast#Multiple Sclerosis Drugs Market Report#Multiple Sclerosis Drugs Market Growth
0 notes
Text
Subcutaneous Drug Delivery Devices Market Demand By Product, Distribution Channel, Region And Forecast To 2030: Grand View Research Inc.
San Francisco, 23 Aug 2024: The Report Subcutaneous Drug Delivery Devices Market Size, Share & Trends Analysis Report By Product (Prefilled Syringes, Pen Injectors, Wearable Injectors), By Distribution Channel, By Region, And Segment Forecasts, 2024 – 2030
The global subcutaneous drug delivery devices market size is expected to reach USD 51.8 billion by 2030, registering to grow at a CAGR of…

View On WordPress
0 notes
Text
The global demand for hypodermic needles was valued at USD 2354.20 million in 2023 and is expected to reach USD 4079.84 million in 2032, growing at a CAGR of 6.30% between 2024 and 2032.The hypodermic needles market is a crucial segment of the global medical devices industry, playing a pivotal role in healthcare delivery by enabling efficient administration of medications, vaccines, and collection of blood samples. Over the years, the market for hypodermic needles has seen significant growth, driven by factors such as rising prevalence of chronic diseases, increasing demand for vaccinations, advancements in needle technology, and expanding healthcare infrastructure. This article delves into the current state of the hypodermic needles market, examining key trends, growth drivers, and the future outlook.
Browse the full report at https://www.credenceresearch.com/report/hypodermic-needles-market
Market Overview
Hypodermic needles are thin, hollow tubes used in conjunction with syringes to inject substances into the body or extract fluids. They are widely utilized in various medical procedures, including intravenous (IV) administration, intramuscular (IM) injections, and subcutaneous injections. The market is segmented based on type (safety and non-safety), application (drug delivery, vaccination, blood collection, others), and end-users (hospitals, clinics, home healthcare, and others).
Key Trends and Developments
1. Rising Demand for Safety Needles: One of the prominent trends in the hypodermic needles market is the increasing adoption of safety needles. These needles are designed to reduce the risk of needlestick injuries, which pose a significant health hazard to healthcare workers. Regulatory bodies, such as the Occupational Safety and Health Administration (OSHA) in the United States, mandate the use of safety-engineered needles, further propelling their demand.
2. Technological Advancements: Continuous advancements in needle technology have led to the development of ultra-thin needles, which cause less pain and discomfort to patients. Innovations such as retractable needles, which withdraw into the syringe barrel after use, enhance safety and compliance with waste management regulations.
3. Growth in Home Healthcare: The shift towards home-based healthcare is another significant trend influencing the hypodermic needles market. The convenience of home-based treatment for chronic conditions like diabetes has led to increased demand for user-friendly and safe hypodermic needles.
4. Increasing Vaccination Programs: The global emphasis on vaccination, especially in the wake of the COVID-19 pandemic, has resulted in a surge in demand for hypodermic needles. Mass vaccination campaigns and immunization drives necessitate the large-scale procurement of needles, bolstering market growth.
Growth Drivers
1. Prevalence of Chronic Diseases: The rising incidence of chronic diseases, such as diabetes, cancer, and cardiovascular conditions, requires frequent administration of drugs and monitoring of health parameters. Hypodermic needles are indispensable in managing these diseases, driving their demand.
2. Aging Population: An aging global population is more susceptible to chronic illnesses and requires regular medical interventions. The elderly demographic's increasing need for healthcare services, including injections and blood tests, fuels the growth of the hypodermic needles market.
3. Expansion of Healthcare Infrastructure: Emerging economies are investing heavily in healthcare infrastructure to improve access to medical services. This expansion includes establishing new hospitals and clinics, which in turn increases the demand for medical supplies, including hypodermic needles.
4. Government Initiatives and Regulations: Government initiatives promoting vaccination and immunization programs, coupled with stringent regulations ensuring the safety of healthcare workers, are significant drivers of the hypodermic needles market. Subsidies and funding for healthcare facilities also support market growth.
Future Outlook
The hypodermic needles market is poised for continued growth in the coming years, driven by technological innovations, rising healthcare expenditure, and the increasing prevalence of chronic diseases. The adoption of safety needles is expected to become more widespread, with manufacturers focusing on developing cost-effective solutions to meet regulatory requirements and address safety concerns.
Moreover, the integration of smart technologies, such as needle tracking systems and digital monitoring, could revolutionize the market, enhancing patient safety and healthcare efficiency. The growing trend of self-administration of medications at home will further drive the demand for easy-to-use hypodermic needles.
Key Players
Cardinal Health Inc.
McKesson Corporation
B. Braun Melsungen AG
Becton, Dickinson and Company
Terumo Medical Corporation
Retractable Technologies, Inc.
Exelint International Co.
Connecticut Hypodermics Inc.
Hitech Syringes
Nipro Corporation
Segmentation
By Type of Needles
Standard Hypodermic Needles
Safety Hypodermic Needles
Pen Needles
IV Cannula Needles
Blood Collection Needles
By Material
Stainless Steel Needles
Plastic Needles
By Application
Therapeutic Injections
Diagnostic Procedures
Blood Collection
By End User
Hospitals and Clinics
Diagnostic Laboratories
Home Healthcare Settings
By Region
North America
US
Canada
Mexico
Europe
Germany
France
UK
Italy
Spain
Rest of Europe
Asia Pacific
China
Japan
India
South Korea
South-east Asia
Rest of Asia Pacific
Latin America
Brazil
Argentina
Rest of Latin America
Middle East & Africa
GCC Countries
South Africa
Rest of the Middle East and Africa
Browse the full report at https://www.credenceresearch.com/report/hypodermic-needles-market
About Us:
Credence Research is committed to employee well-being and productivity. Following the COVID-19 pandemic, we have implemented a permanent work-from-home policy for all employees.
Contact:
Credence Research
Please contact us at +91 6232 49 3207
Email: [email protected]
Website: www.credenceresearch.com
0 notes