#biopharmaceutical services
Text
Elevate Your Strategy: Unleashing the Potential of Meetings Bio for Life Science Leads

In the dynamic realm of life sciences, where advancements shape the future of healthcare and biotechnology, the journey from innovation to impact often begins with effective lead generation. Amidst the complexity of this landscape, Meetings Bio emerges as a catalyst for success, offering unparalleled solutions to elevate your strategy and unleash the full potential of life science leads.
At the core of Meetings Bio's prowess lies a deep understanding of the unique challenges and opportunities within the life sciences industry. With a team of seasoned professionals well-versed in the nuances of biotechnology, pharmaceuticals, medical devices, and beyond,
Meetings Bio brings a wealth of expertise to the table. This expertise serves as the foundation for crafting strategies that resonate with target audiences and drive meaningful results.

One of the key strengths of Meetings Bio is its ability to leverage data-driven insights to inform decision-making. By harnessing the power of advanced analytics and predictive modeling, Meetings Bio identifies trends, anticipates customer behavior, and optimizes campaign performance.
This data-driven approach not only enhances targeting precision but also enables continuous refinement and improvement, ensuring that strategies remain effective in an ever-evolving landscape. If you are looking for the best lead generation services, you can check various online sources.

Furthermore, Meetings Bio excels in crafting engaging and compelling content that captivates audiences and fosters meaningful connections. From informative blog posts and whitepapers to interactive webinars and thought leadership pieces, Meetings Bio delivers content that educates, inspires, and drives action.
By providing value at every touchpoint along the customer journey, Meetings Bio establishes itself as a trusted partner and cultivates long-term relationships with prospects and clients alike.
In addition to its expertise in content creation, Meetings Bio offers a suite of innovative technologies and tools to enhance lead-generation efforts. From AI-powered chatbots and personalized email campaigns to virtual events and interactive demos, Meetings Bio leverages cutting-edge solutions to engage audiences in meaningful ways.
By embracing innovation and staying ahead of the curve, Meetings Bio empowers clients to stay competitive and achieve their goals in an increasingly digital landscape. If you want to get more information about lead generation, you can look at this website.

Ultimately, partnering with Meetings Bio is more than just a business decision; it's a strategic investment in the future success of your life sciences endeavors. With its industry expertise, data-driven approach, engaging content, and innovative solutions, Meetings Bio provides the tools and resources needed to unlock the full potential of life science leads. So, elevate your strategy and embark on a journey of success with Meetings Bio by your side.
💼 Connect with Us Today! 💼
Our proprietary, life sciences-specific database allows for highly targeted market segmentation and buyer identification.
🏢 Business Address: 6 Liberty Square PMB 6001, Boston, MA, 02109, United States
🌐 Website: Meetings Bio Official Website
Social Media: Don't forget to follow us on social media for the latest updates, tips, and success stories:
🔗 Linkedin: Linkedin Business
Meetings. bio team extends and accelerates your sales efforts, helping you find and close more qualified biopharma opportunities.
0 notes
Text
Unleash the Potential of Your Biopharmaceuticals: Expert Consulting Services
Quantum Pharmatech Services - Your trusted partner in biopharmaceutical consulting. We provide a comprehensive suite of services to support you throughout the entire drug development lifecycle. Our team of industry offers unparalleled expertise in navigating regulatory hurdles, optimizing processes, and ensuring the highest quality standards. Contact us today for a free consultation and unlock the full potential of your biopharmaceutical endeavors.
0 notes
Text
Biopharmaceutical CMO & CRO Production Capacity Study—a Deep-dive on Firms Opting for Contract Services
CMOs are exploring the potential of mammalian cell culture production amidst soaring demand for biopharmaceutical contract manufacturing services. Incumbents, such as Charles River and Lonza have furthered investments in mammalian cells. To illustrate, in May 2021, Lonza announced pouring USD 936 million to bolster the footprint of mammalian drug substance manufacturing sites in the U.S. and Switzerland.
Biopharmaceutical firms are responding to the demand for outsourced services with bullish investments in research activities. For instance, in July 2022, Wuxi Biologics contemplated expanding its large-scale drug product & drug substance manufacturing capabilities and R&D in Singapore.
Adoption of Contract Development & Manufacturing Organization (CDMO) and Contract Manufacturing Organization (CMO) with surging demand for Monoclonal Antibodies (MAbs) products will boost the market share. The final report, along with the database, will peruse the following dynamics:
• Insights on commercial availability and annual approvals of MAb products.
• Commercially available biopharmaceuticals and biologics produced using mammalian cell lines.
• Competitive landscape with industry developments.
• CMO Mapping of 88 companies.
Get your copy or request a free sample of the report “Biopharmaceutical CMO & CRO Production Capacity Study,” compiled and published by Grand View Research.
Biopharmaceutical CMO & CRO Production Capacity Report Scope
CMO Capacity mapping (for 88 companies)
Key Players: Location Mapping & existing Capacities
Recent expansions/ Future plans
Comparative Heat Map
Get more insights from our in-depth market intelligence report, “Biopharmaceutical CMO And CRO Market Size, Share & Trends Analysis Report By Source (Mammalian, Non-mammalian), By Service (Contract Manufacturing, Contract Research), By Product, And Segment Forecasts, 2023 – 2030.”
About Us
Grand View Research, Inc. is a market research and consulting company that provides off-the-shelf, customized research reports and consulting services. To help clients make informed business decisions, we offer market intelligence studies ensuring relevant and fact-based research across a range of industries, from technology to chemicals, materials and energy. With a deep-seated understanding of varied business environments, Grand View Research provides strategic objective insights.
Find More information @ https://www.grandviewresearch.com/info/trend-reports
#CRO Production Capacity Report#biopharmaceutical industry#Market Trend Reports#Biopharmaceutical CMO#Biopharmaceutical Firms#contract services
0 notes
Link
The Lyophilization Services for Biopharmaceuticals Market exploration report is an intelligence report that includes precise and precious data on request size, development countries, request share, and profit vaticinations through 2028.
#Lyophilization Services for Biopharmaceuticals Market Outlook#Lyophilization Services for Biopharmaceuticals Market Analysis
0 notes
Text
Importance Of Competitive Marketing Intelligence In the Biopharmaceutical Industry
It's imperative to stay current with all business-relevant industry advancements and other market factors if one wants to succeed in the pharmaceutical sector. Moreover, businesses compete fiercely to keep their pipelines full despite fast innovation, fluctuating patient needs, and patent expiration. As a result, it's critical to continually keep an eye on the competitors in such a dynamic market. However, for pharma's Competitive Marketing Intelligence to be effective, it must be incredibly thorough, trustworthy, and customized to the demands of the particular firm.
A pharmaceutical competitive market intelligence program's major objectives are to help the industry learn about pharmaceutical market information, make tactical choices, and eventually boost revenue. To decide things that direct product development and chart the course for profitable growth, company CEOs and other leaders need timely insights. Finding answers to the problems in the area's obstacles and limitations is the key to success. This can be achieved through a variety of competitive intelligence services, including primary competitor & potential customer identification, market demand analysis, business investor monitoring, etc. These services are offered by various competitive intelligence organizations in the form of competitive intelligence reports & competitive intelligence Biopharmaceutical Companies' consulting services.
1 note
·
View note
Text

NASA’s SpaceX CRS-30 MISSION
Cartilage repair, retinal gene therapies, neurological disease treatments, and technology testing on external platforms among investigations flying on NASA’s SpaceX CRS-30
The next resupply mission to the International Space Station (ISS) will carry more than 40 payloads sponsored by the ISS National Laboratory®, including in-space production applications projects, technology demonstrations, life science experiments, and student-led inquiries. These investigations, launching on SpaceX’s 30th Commercial Resupply Services (CRS) mission, funded by NASA, aim to improve life on Earth through space-based research and foster a sustainable economy in low Earth orbit (LEO).
Below highlights a sample of those payloads, and findings could lead to advances in technology for future spaceflight and the development of novel therapeutics for use both on Earth and in space.
Redwire Corporation is partnering with pharmaceutical company Eli Lilly & Company and Butler University for two investigations leveraging Redwire’s Pharmaceutical In-space Laboratory (PIL-BOX), a platform to crystallize organic molecules in microgravity. Results from this research could lead to improved therapeutics to treat an array of conditions. This research continues Eli Lilly’s space journey, as the company has launched a variety of investigations to the orbiting laboratory over the years for the benefit of patient care on Earth.
A collaboration between Boeing and CSIRO (an Australian government agency responsible for scientific research) will test the ability of a Multi-Resolution Scanner to create 3D maps of the space station. To do this, the project will use Astrobee, an autonomous free-flying robotic system on station. This scanner technology could be useful in future exploration efforts and in remote environments for manufacturing and maintenance tasks, such as identifying leaks or checking for damage to systems.
The National Stem Cell Foundation will continue to examine the mechanisms behind neuroinflammation, a common feature of neurodegenerative diseases. To carry out this study, the research team created 3D brain models derived from induced pluripotent stem cells of patients with Alzheimer’s and Parkinson’s diseases as well as primary progressive multiple sclerosis.
Airbus U.S. Space & Defense is launching an enhancement to the station’s Bartolomeo platform. Called ArgUS, the external mechanical platform has added capabilities for hosting payloads in LEO. Once ArgUS is installed, it will host multiple payloads on this mission, including SpaceTV-1, an optical video system designed to livestream high-definition views of Earth and the space station.
A project from the University of Connecticut will examine the feasibility of producing Janus base nanomaterials in microgravity that could help repair cartilage and reduce joint inflammation. Through this project, researchers aim to advance in-space manufacturing concepts for these materials, which could significantly improve patient care for orthopedic injuries and degenerative joint diseases like arthritis, as there is currently no way to repair damaged cartilage.
Additionally, two investigations flying on NASA’s SpaceX CRS-30 mission were selected through the Technology in Space Prize, funded by Boeing and the Center for the Advancement of Science in Space™ (CASIS™), manager of the ISS National Lab, as part of the MassChallenge startup accelerator program.
An investigation from biopharmaceutical company Oculogenex will use the space station to test a novel gene therapy to prevent and possibly even reverse vision loss from age-related macular degeneration (AMD). Findings will help advance the company’s therapeutic, which can potentially treat AMD-related symptoms in millions of Americans.
A project from biomedical startup Encapsulate aims to leverage the microgravity environment of the space station to validate an automated tumor-on-a-chip system that grows patient-derived cancer cells to test chemotherapy drugs. The company seeks to use precision diagnostics for personalized cancer treatments.
SpaceX’s Falcon 9 rocket will launch these investigations and more no earlier than Thursday, March 21, 2024, at 4:55 p.m. EDT from Space Launch Complex 40 at Cape Canaveral Space Force Station in Florida.
2 notes
·
View notes
Text
Biopharmaceutical Contract Manufacturing Market Landscape: Trends, Drivers, and Forecast (2023-2032)

The global demand for biopharmaceutical contract manufacturing was valued at USD 8.8 Billion in 2022 and is expected to reach USD 17.9 Billion in 2030, growing at a CAGR of 9.5% between 2023 and 2030.
Biopharmaceutical Contract Manufacturing Market refers to the sector within the pharmaceutical industry where third-party manufacturers are contracted to produce biopharmaceutical products. Biopharmaceuticals are medical drugs produced using biotechnology, including proteins, nucleic acids, and living cells. Contract manufacturing organizations (CMOs) provide a range of services such as cell culture, fermentation, purification, and analytical testing. This market is driven by factors such as the increasing demand for biopharmaceuticals, the need for cost efficiency, and the complexity of manufacturing processes which require specialized facilities and expertise. The market includes various segments based on product type, service type, scale of operation, and end-users, encompassing small biotech firms to large pharmaceutical companies seeking to outsource their manufacturing needs.
CMOs play a crucial role in this industry by providing the necessary infrastructure, technical know-how, and compliance with regulatory standards set by authorities such as the FDA and EMA. They support companies that may not have the capacity or capability to carry out manufacturing in-house, thus enabling them to focus on their core competencies such as research and development, and market commercialization. The market for biopharmaceutical contract manufacturing is driven by the increasing demand for biopharmaceuticals, the need for cost efficiency, and the high complexity and cost of setting up in-house manufacturing facilities.
Furthermore, the market is segmented based on product type, service type, scale of operation, and end-users. Each segment addresses specific needs within the industry, from small-scale production for clinical trials to large-scale manufacturing for commercial distribution. CMOs also offer critical services such as fill and finish operations, packaging, labeling, and regulatory support, ensuring that products are market-ready and compliant with global standards.
The industry faces several challenges including maintaining quality control, managing complex supply chains, protecting intellectual property, and keeping pace with technological advancements. Despite these challenges, the biopharmaceutical contract manufacturing market is poised for growth, driven by innovations in biotechnology, expanding biopharmaceutical pipelines, and increasing outsourcing trends. Regions like North America and Europe dominate the market due to their advanced biopharmaceutical sectors and regulatory frameworks, while the Asia-Pacific region is emerging as a significant player due to lower manufacturing costs and increasing investments in biotechnology. This dynamic and rapidly evolving market is integral to the success and scalability of the global biopharmaceutical industry.
Services Provided:
Cell culture
Fermentation
Purification
Analytical testing
Key Drivers:
Increasing demand for biopharmaceuticals
Need for cost efficiency
Complexity of manufacturing processes requiring specialized facilities and expertise
Market Segments:
Product Type: Various biopharmaceutical products
Service Type: Different manufacturing and testing services
Scale of Operation: Small to large-scale manufacturing
End-Users: Ranges from small biotech firms to large pharmaceutical companies
Benefits:
Access to advanced manufacturing technologies
Reduced time to market for new drugs
Flexibility in production scaling
Cost savings in terms of infrastructure and operational expenses
Growth Factors:
Advancements in biotechnology
Expanding biopharmaceutical pipeline
Increased outsourcing by pharmaceutical companies to focus on core competencies
The Biopharmaceutical Contract Manufacturing Market exhibits significant regional variations, driven by differing levels of technological advancement, regulatory landscapes, and market demands. North America, particularly the United States, dominates the market due to its robust biopharmaceutical industry, extensive R&D activities, and presence of major biopharmaceutical companies.
Europe follows closely, with countries like Germany, Switzerland, and the UK being key players, bolstered by strong regulatory frameworks and a focus on innovation. The Asia-Pacific region is experiencing rapid growth, driven by increasing investments in biotechnology, lower manufacturing costs, and government initiatives to boost biopharmaceutical production in countries such as China, India, and South Korea. Latin America and the Middle East & Africa, while currently smaller markets, are gradually expanding due to improving healthcare infrastructure and rising demand for biopharmaceuticals. These regional insights underscore the global nature of the market and the importance of regional strategies for market participants.
Key Players:
Boehringer Ingelheim GmbH
Lonza
Inno Biologics Sdn Bhd
Rentschler Biotechnologie GmbH
JRS Pharma
AGC Biologics
ProBioGen
Fujifilm Diosynth Biotechnologies U.S.A., Inc.
Toyobo Co., Ltd.
Samsung BioLogics
Thermo Fisher Scientific, Inc.
Binex Co., Ltd.
WuXi Biologics
AbbVie, Inc
Novartis AG
ADMA Biologics, Inc.
Catalent, Inc
More About Report- https://www.credenceresearch.com/report/biopharmaceutical-contract-manufacturing-market
Biopharmaceutical Contract Manufacturing Market Challenges
Regulatory Compliance:
Adhering to stringent regulations and quality standards set by health authorities (e.g., FDA, EMA).
Ensuring consistent product quality and safety across different production batches.
Quality Control and Assurance:
Maintaining high-quality standards in complex biopharmaceutical production processes.
Implementing rigorous testing and validation procedures to ensure product efficacy and safety.
Supply Chain Management:
Managing a complex supply chain that includes raw materials, intermediates, and finished products.
Ensuring timely delivery and availability of critical raw materials.
Technological Complexity:
Keeping up with rapid advancements in biopharmaceutical manufacturing technologies.
Integrating new technologies and processes while maintaining operational efficiency.
Intellectual Property Protection:
Protecting sensitive data and proprietary manufacturing processes.
Ensuring confidentiality agreements and intellectual property rights are upheld.
Scalability:
Scaling up production processes from laboratory to commercial scale without compromising product quality.
Managing production capacity to meet fluctuating demand.
Cost Management:
Balancing the high costs associated with biopharmaceutical production with the need to remain competitive.
Investing in advanced manufacturing facilities and skilled workforce.
Partnership and Communication:
Establishing effective communication and collaboration between contract manufacturers and their clients.
Managing expectations and maintaining transparency throughout the manufacturing process.
Risk Management:
Identifying and mitigating risks associated with biopharmaceutical production, such as contamination and process failures.
Implementing robust risk management strategies to handle unforeseen challenges.
Global Competition:
Competing with established and emerging contract manufacturing organizations worldwide.
Differentiating services and capabilities to attract and retain clients.
Segmentation:
By Source
Mammalian
Non-mammalian
By Service
Process Development
Downstream
Upstream
Fill & Finish Operations
Analytical & QC studies
Packaging
By Product
Biologics
Monoclonal antibodies (MABs)
Recombinant Proteins
Vaccines
Antisense, RNAi, & Molecular Therapy
Others
Biosimilars
Browse the full report – https://www.credenceresearch.com/report/biopharmaceutical-contract-manufacturing-market
Browse Our Blog: https://www.linkedin.com/pulse/biopharmaceutical-contract-manufacturing-qonef
Contact Us:
Phone: +91 6232 49 3207
Email: [email protected]
Website: https://www.credenceresearch.com
0 notes
Text
Unlocking the Power of Antibody Purification Services
In the realm of biotechnology and medical research, antibodies are invaluable tools. These Y-shaped proteins play a pivotal role in immune response, recognizing and neutralizing foreign substances such as bacteria and viruses. However, their utility extends far beyond the body's defense mechanisms. Scientists have harnessed the specificity of antibodies to develop diagnostic tests, therapeutic treatments, and even tools for molecular biology research.
Yet, before antibodies can be utilized in various applications, they often require purification. Antibody purification services play a crucial role in isolating and refining these proteins to ensure their effectiveness and reliability. Let's delve into the significance of Antibody Services and how they contribute to advancements in science and medicine.
The Importance of Antibody Purification
Antibodies are typically produced in complex biological systems such as animals or cell cultures. As a result, the mixture in which they are present contains numerous other proteins and contaminants. Purification is essential to isolate the desired antibodies from this mixture, ensuring high specificity and reducing the risk of unwanted side effects.
Moreover, purified antibodies are essential for numerous applications:
Research:
In molecular biology and biotechnology research, purified antibodies serve as critical tools for probing and understanding biological systems. They enable scientists to detect specific proteins in cells, tissues, or biochemical samples with high precision, shedding light on various biological processes.
Diagnostics:
Purified antibodies are the backbone of diagnostic assays used to detect diseases, pathogens, and biomarkers. Whether it's a pregnancy test or a sophisticated immunoassay for detecting infectious diseases, the purity of antibodies directly impacts the accuracy and sensitivity of these tests.
Therapeutics:
Antibodies have emerged as a promising class of therapeutics for treating various diseases, including cancer, autoimmune disorders, and infectious diseases. Purification ensures that therapeutic antibodies meet stringent quality standards, minimizing the risk of adverse reactions and maximizing efficacy.
The Process of Antibody Purification
Antibody purification involves several steps designed to separate the target antibodies from other proteins and contaminants:
Capture:
The first step typically involves capturing the antibodies from the crude mixture using a specific binding interaction. This could involve affinity chromatography, where antibodies bind to a ligand immobilized on a solid support, or protein A/G purification, which exploits the affinity of antibodies for certain bacterial proteins.
Wash:
Once the antibodies are captured, the next step is to wash away any remaining contaminants. This ensures that only the desired antibodies remain bound to the purification matrix, reducing non-specific binding and background noise.
Elution:
Finally, the purified antibodies are eluted from the purification matrix, typically by changing the conditions to disrupt the binding interaction. Elution can be achieved through changes in pH, salt concentration, or the addition of competitive ligands.
The Role of Antibody Purification Services
While the concept of antibody purification may seem straightforward, the process can be complex and labor-intensive, requiring specialized equipment and expertise. This is where antibody purification services play a vital role.
By outsourcing antibody purification to specialized service providers, researchers and biopharmaceutical companies can access state-of-the-art purification techniques and facilities without the need for significant investment in infrastructure and personnel. These services offer:
Expertise:
Experienced scientists with expertise in protein purification and biopharmaceutical development oversee the purification process, ensuring high-quality results and compliance with regulatory standards.
Customization:
Antibody purification services can be tailored to meet the specific requirements of each project, including the choice of purification method, scale of purification, and desired purity level.
Scalability:
Whether it's a small-scale research project or a large-scale production run, antibody purification services can accommodate varying throughput and scale requirements.
0 notes
Text
Elevate Your Strategy: Unleashing the Potential of Meetings Bio for Life Science Leads

In the dynamic realm of life sciences, where advancements shape the future of healthcare and biotechnology, the journey from innovation to impact often begins with effective lead generation. Amidst the complexity of this landscape, Meetings Bio emerges as a catalyst for success, offering unparalleled solutions to elevate your strategy and unleash the full potential of life science leads.
At the core of Meetings Bio's prowess lies a deep understanding of the unique challenges and opportunities within the life sciences industry. With a team of seasoned professionals well-versed in the nuances of biotechnology, pharmaceuticals, medical devices, and beyond,
Meetings Bio brings a wealth of expertise to the table. This expertise serves as the foundation for crafting strategies that resonate with target audiences and drive meaningful results.

One of the key strengths of Meetings Bio is its ability to leverage data-driven insights to inform decision-making. By harnessing the power of advanced analytics and predictive modeling, Meetings Bio identifies trends, anticipates customer behavior, and optimizes campaign performance.
This data-driven approach not only enhances targeting precision but also enables continuous refinement and improvement, ensuring that strategies remain effective in an ever-evolving landscape. If you are looking for the best lead generation services, you can check various online sources.
Furthermore, Meetings Bio excels in crafting engaging and compelling content that captivates audiences and fosters meaningful connections. From informative blog posts and whitepapers to interactive webinars and thought leadership pieces, Meetings Bio delivers content that educates, inspires, and drives action.

By providing value at every touchpoint along the customer journey, Meetings Bio establishes itself as a trusted partner and cultivates long-term relationships with prospects and clients alike.
In addition to its expertise in content creation, Meetings Bio offers a suite of innovative technologies and tools to enhance lead-generation efforts. From AI-powered chatbots and personalized email campaigns to virtual events and interactive demos, Meetings Bio leverages cutting-edge solutions to engage audiences in meaningful ways.
By embracing innovation and staying ahead of the curve, Meetings Bio empowers clients to stay competitive and achieve their goals in an increasingly digital landscape. If you want to get more information about lead generation, you can look at this website.

Ultimately, partnering with Meetings Bio is more than just a business decision; it's a strategic investment in the future success of your life sciences endeavors. With its industry expertise, data-driven approach, engaging content, and innovative solutions, Meetings Bio provides the tools and resources needed to unlock the full potential of life science leads. So, elevate your strategy and embark on a journey of success with Meetings Bio by your side.
💼 Connect with Us Today! 💼
Our proprietary, life sciences-specific database allows for highly targeted market segmentation and buyer identification.
🏢 Business Address: 6 Liberty Square PMB 6001, Boston, MA, 02109, United States
🌐 Website: Meetings Bio Official Website
Social Media: Don't forget to follow us on social media for the latest updates, tips, and success stories:
🔗 Linkedin: Linkedin Business
Meetings. bio team extends and accelerates your sales efforts, helping you find and close more qualified biopharma opportunities.
1 note
·
View note
Text
Navigate the Future of Biotech with Quantum Pharmatech's Management Consulting
Quantum Pharmatech offers expert biotech management consulting to help your company thrive in the ever-evolving life sciences industry.
0 notes
Text
Biopharmaceutical Tubing Market Global Rising Demand & Huge Scope Till 2028
The Insight Partners recently announced the release of the market research titled Biopharmaceutical Tubing Market Outlook to 2030 | Share, Size, and Growth. The report is a stop solution for companies operating in the Biopharmaceutical Tubing market. The report involves details on key segments, market players, precise market revenue statistics, and a roadmap that assists companies in advancing their offerings and preparing for the upcoming decade. Listing out the opportunities in the market, this report intends to prepare businesses for the market dynamics in an estimated period.
Is Investing in the Market Research Worth It?
Some businesses are just lucky to manage their performance without opting for market research, but these incidences are rare. Having information on longer sample sizes helps companies to eliminate bias and assumptions. As a result, entrepreneurs can make better decisions from the outset. Biopharmaceutical Tubing Market report allows business to reduce their risks by offering a closer picture of consumer behavior, competition landscape, leading tactics, and risk management.
A trusted market researcher can guide you to not only avoid pitfalls but also help you devise production, marketing, and distribution tactics. With the right research methodologies, The Insight Partners is helping brands unlock revenue opportunities in the Biopharmaceutical Tubing market.
If your business falls under any of these categories – Manufacturer, Supplier, Retailer, or Distributor, this syndicated Biopharmaceutical Tubing market research has all that you need.
What are Key Offerings Under this Biopharmaceutical Tubing Market Research?
Global Biopharmaceutical Tubing market summary, current and future Biopharmaceutical Tubing market size
Market Competition in Terms of Key Market Players, their Revenue, and their Share
Economic Impact on the Industry
Production, Revenue (value), Price Trend
Cost Investigation and Consumer Insights
Industrial Chain, Raw Material Sourcing Strategy, and Downstream Buyers
Production, Revenue (Value) by Geographical Segmentation
Marketing Strategy Comprehension, Distributors and Traders
Global Biopharmaceutical Tubing Market Forecast
Study on Market Research Factors
Who are the Major Market Players in the Biopharmaceutical Tubing Market?
Biopharmaceutical Tubing market is all set to accommodate more companies and is foreseen to intensify market competition in coming years. Companies focus on consistent new launches and regional expansion can be outlined as dominant tactics. Biopharmaceutical Tubing market giants have widespread reach which has favored them with a wide consumer base and subsequently increased their Biopharmaceutical Tubing market share.
Report Attributes
Details
Segmental Coverage
Type
Plastic
Metal
and Silicone
Application
Pharmaceutical {Single Use Technology
and Others
Medical Devices
Research and Development
and Others
Regional and Country Coverage
North America (US, Canada, Mexico)
Europe (UK, Germany, France, Russia, Italy, Rest of Europe)
Asia Pacific (China, India, Japan, Australia, Rest of APAC)
South / South & Central America (Brazil, Argentina, Rest of South/South & Central America)
Middle East & Africa (South Africa, Saudi Arabia, UAE, Rest of MEA)
Market Leaders and Key Company Profiles
W. L. Gore and Associates, Inc.
Saint-Gobain Sekurit
Freudenberg Group
RAUMEDIC AG
TEKNI-PLEX
NewAge Industries, Inc.
Optinova
Zeus Industrial Products, Inc.
NORDSON CORPORATION
Other key companies
What are Perks for Buyers?
The research will guide you in decisions and technology trends to adopt in the projected period.
Take effective Biopharmaceutical Tubing market growth decisions and stay ahead of competitors
Improve product/services and marketing strategies.
Unlock suitable market entry tactics and ways to sustain in the market
Knowing market players can help you in planning future mergers and acquisitions
Visual representation of data by our team makes it easier to interpret and present the data further to investors, and your other stakeholders.
Do We Offer Customized Insights? Yes, We Do!
The The Insight Partners offer customized insights based on the client’s requirements. The following are some customizations our clients frequently ask for:
The Biopharmaceutical Tubing market report can be customized based on specific regions/countries as per the intention of the business
The report production was facilitated as per the need and following the expected time frame
Insights and chapters tailored as per your requirements.
Depending on the preferences we may also accommodate changes in the current scope.
Author’s Bio:
Sunil Jadhav
Senior Market Research Expert at The Insight Partners
0 notes
Text
Global Top 5 Companies Accounted for 73% of total SUS (Single Use System) for Biopharma Process market (QYResearch, 2022)
Single-use systems (SUS) refers to biopharmaceutical manufacturing (bioprocessing) equipment designed to be used once (or for a single manufacturing campaign) and then discarded.
This report focuses on devices and consumables, mainly including single-use bioreactors, disposable sterile bags, and disposable filtration systems.

According to the new market research report “Global SUS (Single Use System) for Biopharma Process Market Report 2023-2029”, published by QYResearch, the global SUS (Single Use System) for Biopharma Process market size is projected to reach USD 15.47 billion by 2029, at a CAGR of 19.0% during the forecast period.
Figure. Global SUS (Single Use System) for Biopharma Process Market Size (US$ Million), 2018-2029
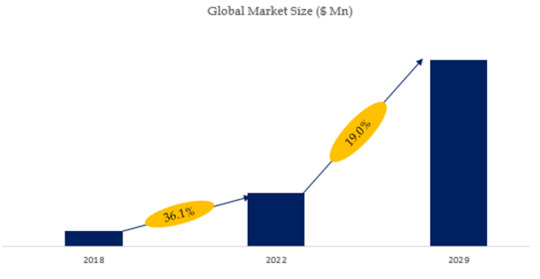
Figure. Global SUS (Single Use System) for Biopharma Process Top 11 Players Ranking and Market Share (Ranking is based on the revenue of 2022, continually updated)
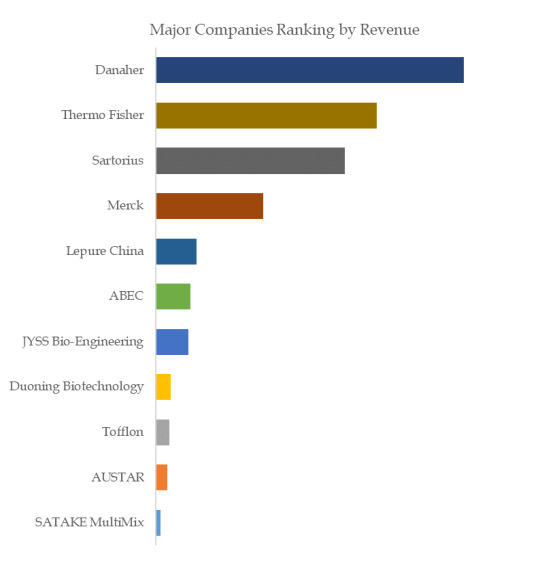
The global key manufacturers of SUS (Single Use System) for Biopharma Process include Danaher, Thermo Fisher, Sartorius, Merck, Lepure China, ABEC, JYSS Bio-Engineering, Duoning Biotechnology, Tofflon, AUSTAR, etc. In 2022, the global top five players had a share approximately 73.0% in terms of revenue.
About QYResearch
QYResearch founded in California, USA in 2007.It is a leading global market research and consulting company. With over 16 years’ experience and professional research team in various cities over the world QY Research focuses on management consulting, database and seminar services, IPO consulting, industry chain research and customized research to help our clients in providing non-linear revenue model and make them successful. We are globally recognized for our expansive portfolio of services, good corporate citizenship, and our strong commitment to sustainability. Up to now, we have cooperated with more than 60,000 clients across five continents. Let’s work closely with you and build a bold and better future.
QYResearch is a world-renowned large-scale consulting company. The industry covers various high-tech industry chain market segments, spanning the semiconductor industry chain (semiconductor equipment and parts, semiconductor materials, ICs, Foundry, packaging and testing, discrete devices, sensors, optoelectronic devices), photovoltaic industry chain (equipment, cells, modules, auxiliary material brackets, inverters, power station terminals), new energy automobile industry chain (batteries and materials, auto parts, batteries, motors, electronic control, automotive semiconductors, etc.), communication industry chain (communication system equipment, terminal equipment, electronic components, RF front-end, optical modules, 4G/5G/6G, broadband, IoT, digital economy, AI), advanced materials industry Chain (metal materials, polymer materials, ceramic materials, nano materials, etc.), machinery manufacturing industry chain (CNC machine tools, construction machinery, electrical machinery, 3C automation, industrial robots, lasers, industrial control, drones), food, beverages and pharmaceuticals, medical equipment, agriculture, etc.
For more information, please contact the following e-mail address:
Email: [email protected]
Website: https://www.qyresearch.com
0 notes
Text
Fresher Pharmacovigilance Vacancies at Soterius Life Sciences Pvt. Ltd., Delhi
Are you passionate about ensuring patient safety in the pharmaceutical industry? Soterius Life Sciences Pvt. Ltd., a subsidiary of Soterius Inc., is seeking enthusiastic and energetic individuals for Drug Safety Associate positions in Delhi.
Role Overview
Title: Drug Safety Associate/ Senior Drug Safety Associate/ Team Lead (based on experience)
Role Type: Full-time
Location: On-site at our office in Nehru Place, New Delhi
Company Overview
Soterius Life Sciences Pvt. Ltd. is a leading outsourced service provider with a focus on clinical safety, pharmacovigilance, and medical affairs. Our client base includes top global biopharmaceutical companies, and we are known for our innovative and customer-centric solutions.
Key Responsibilities
Record and review adverse reactions in the safety database for post marketing and clinical trial reports in accordance with applicable regulatory standards, with high accuracy and quality.
Assessment and review of literature abstracts and full-text articles.
Communicate with required stakeholders, as necessary, to carry out the operational responsibilities.
Carry out necessary administrative duties required for the job.
Support effective functioning of PV projects (including but not limited to case processing, Literature Monitoring, Aggregate Reporting, Medical Writing, Signal Detection and Management, Medical Information and Risk Management etc.) in compliance with agreements and applicable regulations and guidelines.
Have ownership of assigned projects and tasks and ensure adherence to timelines and quality.
Management of quality, compliance and documentation across PV projects.
Continuously work with internal and external stakeholders to ensure compliance with agreements, applicable regulation and guidance’s.
Train and mentor personnel, as needed.
Respond to/ represent department/ function during audits and inspections.
Author and review SOPs and other documents relevant for department/ function.
Other responsibilities as assigned by the Management.
Qualifications & Experience
B.Pharm / M.Pharm / BDS or equivalent qualification recognized by the Pharmacy Council of India.
0-8 years of experience in drug safety / pharmacovigilance.
How to Apply
Apply online through our careers portal: Apply Now
[caption id="attachment_75342" align="aligncenter" width="1200"] Fresher Pharmacovigilance Vacancies in Delhi: Drug Safety Associate[/caption]
0 notes
Text
Global Perspective: Regional Markets and Emerging Trends in Cell Expansion
The Cell Expansion Market is experiencing significant growth propelled by advancements in regenerative medicine, cell therapy, and biotechnology, alongside the growing demand for cell-based therapies for various diseases and injuries. Cell expansion involves the propagation and amplification of cells in vitro, enabling the production of large quantities of cells for therapeutic applications, drug discovery, and research.
Request for a free Sample: https://www.marketdigits.com/request/sample/3954
One of the primary drivers of market growth is the rising prevalence of chronic and degenerative diseases, such as cancer, cardiovascular disorders, and neurological conditions, which have fueled the demand for cell-based therapies. Cell therapies, including stem cell therapies, chimeric antigen receptor (CAR) T-cell therapies, and dendritic cell therapies, offer promising treatment options by harnessing the regenerative and immunomodulatory properties of cells to restore tissue function, modulate immune responses, and target cancer cells. As a result, pharmaceutical companies, biotech firms, and academic research institutions are investing heavily in cell expansion technologies to support the development and commercialization of cell-based therapies.
Major vendors in the global cell expansion market: Akadeum Life Sciences, Bio-Techne, Corning, Cytiva, DH Life Sciences, LLC, GE HealthCare, Lonza, Merck KGaA, Miltenyi Biotec, Sartorius AG, STEMCELL Technologies., TERUMO BCT, INC., Thermo Fisher Scientific Inc. and Others.
Furthermore, advancements in cell culture techniques, bioreactor systems, and cell expansion media have expanded the capabilities and scalability of cell expansion processes. Traditional two-dimensional (2D) cell culture methods have been supplemented or replaced by three-dimensional (3D) culture systems, microcarrier-based cultures, and suspension cultures, which provide a more physiologically relevant environment for cell growth and differentiation. Bioreactor systems enable the controlled cultivation of cells in large-scale production settings, facilitating the expansion of cells for commercialization and clinical use. Additionally, cell expansion media formulations have been optimized to support the growth, viability, and functionality of various cell types, enhancing the efficiency and reproducibility of cell expansion processes.
The Cell Expansion Market is Valued USD 17.61 billion in 2024 and projected to reach USD 56.16 billion by 2032, growing at a CAGR of CAGR of 12.32 % During the Forecast period of 2024-2032.
The cell expansion market is characterized by the presence of a diverse range of products and technologies, including cell culture media, reagents, bioreactor systems, and cell processing devices. Major players in the market include Thermo Fisher Scientific Inc., Merck KGaA, Danaher Corporation (including GE Healthcare Life Sciences), Sartorius AG, and Becton, Dickinson and Company (BD), among others. These companies offer comprehensive solutions for cell expansion, from research-grade reagents and consumables to cGMP-compliant manufacturing platforms and services, catering to the needs of academic researchers, biotech startups, and biopharmaceutical companies worldwide.
Emerging trends in the cell expansion market include the development of automated and closed-system cell expansion technologies, advanced cell culture media formulations, and integrated cell therapy manufacturing platforms. Automated cell expansion systems enable high-throughput and reproducible production of cells, reducing manual labor, minimizing contamination risks, and increasing process efficiency. Closed-system bioreactors and cell processing devices provide a controlled and aseptic environment for cell expansion, ensuring product safety and compliance with regulatory requirements. Integrated manufacturing platforms combine cell expansion, purification, and formulation steps into a single workflow, streamlining cell therapy manufacturing and reducing production costs.
Looking ahead, the cell expansion market is poised for continued growth driven by ongoing advancements in cell biology, tissue engineering, and regenerative medicine, alongside the increasing adoption of cell-based therapies in clinical practice. As stakeholders collaborate to address technical, regulatory, and commercialization challenges, cell expansion technologies will play a crucial role in supporting the development and commercialization of cell-based therapies for a wide range of indications. Collaborations between industry stakeholders, regulatory agencies, and academic research institutions will be essential in driving innovation, establishing quality standards, and ensuring the safe and effective translation of cell expansion technologies into clinical applications.
0 notes
Text
Dynamic Landscape of Pharmaceuticals: Key Trends and the Role of Contract Manufacturing

Introduction: In the dynamic realm of pharmaceuticals, staying abreast of key trends is essential for industry players seeking to innovate and adapt. From precision medicine to digital health, the landscape is constantly evolving, presenting both challenges and opportunities. In this blog post, we’ll explore the pivotal role of contract manufacturing and regulatory support amidst these trends, shedding light on their significance in driving progress and ensuring compliance in the pharmaceutical industry.
Precision Medicine and Contract Manufacturing:
Precision medicine, with its focus on personalized therapies, presents unique manufacturing challenges due to the need for small batch sizes and customized formulations. Contract manufacturing organizations (CMOs) are playing a vital role in this space by offering flexible manufacturing solutions tailored to the specific needs of precision medicine products.
CMOs specializing in advanced manufacturing technologies, such as continuous manufacturing and 3D printing, are enabling pharmaceutical companies to accelerate the development and production of personalized therapies, enhancing efficiency and scalability.
Digital Health and Regulatory Support:
The integration of digital health solutions into pharmaceutical products necessitates robust regulatory support to ensure compliance with evolving standards and guidelines. Regulatory consulting firms provide invaluable expertise in navigating the complex regulatory landscape, helping companies secure approvals for digital therapeutics, mobile health apps, and virtual clinical trials.
Regulatory support extends beyond product approvals to encompass post-market surveillance, pharmacovigilance, and compliance with data privacy regulations. By partnering with regulatory experts, pharmaceutical companies can mitigate risks, expedite market entry, and maintain compliance throughout the product lifecycle.
Biopharmaceuticals and Contract Manufacturing:
The growing demand for biopharmaceuticals, driven by their potential to address unmet medical needs, is driving expansion in the contract manufacturing market. CMOs with expertise in bioprocessing and cell culture technologies are in high demand, offering end-to-end manufacturing services for biologics, biosimilars, and cell and gene therapies.
Strategic partnerships between pharmaceutical companies and CMOs enable access to specialized capabilities and capacity, allowing for faster development and commercialization of biopharmaceutical products while mitigating capital investment risks.
Sustainability and Regulatory Compliance:
Sustainability considerations are increasingly influencing regulatory requirements in the pharmaceutical industry, with agencies emphasizing environmental stewardship and responsible manufacturing practices. Regulatory support firms assist companies in navigating sustainability regulations, ensuring compliance with environmental, health, and safety standards.
From green chemistry principles to waste reduction strategies, pharmaceutical companies are embracing sustainable manufacturing practices with the guidance of regulatory experts, aligning with global sustainability goals and enhancing their corporate social responsibility initiatives.
Conclusion:
As the pharmaceutical industry continues to evolve, the roles of contract manufacturing and regulatory support are becoming increasingly indispensable. From facilitating the production of precision medicines to navigating complex regulatory frameworks, these partners enable pharmaceutical companies to innovate responsibly and bring life-saving therapies to market efficiently. By leveraging the expertise of contract manufacturers and regulatory consultants, pharmaceutical companies can navigate the ever-changing landscape with confidence, driving progress and ensuring compliance in an era of unprecedented transformation.
0 notes
Link
0 notes