#cytogenetics
Text
Since I'm so busy with work, I thought, why not upload some work pics?
Dunno if it's something anyone here'll find interesting, but I think they're pretty XD
12 notes
·
View notes
Photo
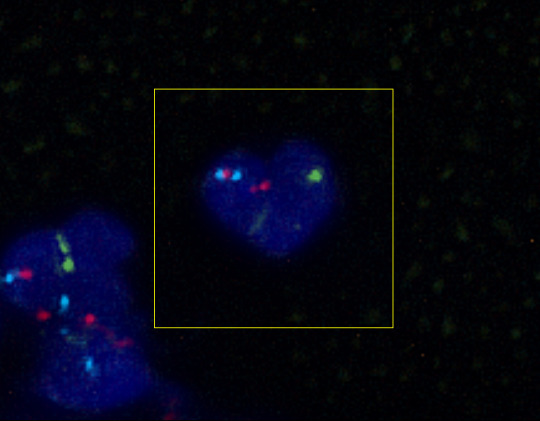
Obesity-Cancer Link
A person carrying a mutation in either of the tumour suppressor proteins BRCA1 or BRCA2 is at increased risk of developing breast cancer. So too are people with obesity and diabetes. But whether obesity could exacerbate the risk in people with BRCA mutations was unknown. Recent research suggests that indeed metabolic and genetic risk can be cumulative. The image shows nuclei (blue) of milk duct cells from a person with a BRCA mutation with evidence of DNA damage shown in red. A study of such cells revealed the extent of DNA damage in BRCA mutation carriers positively correlated with body mass index. And blocking obesity related hormone signals in these cells could lessen such damage. The new findings suggest that while maintaining a low body weight is no guarantee of preventing breast cancer, addressing lifestyle, diet and metabolic health may be especially important for people already at increased genetic risk.
Written by Ruth Williams
Image from work by Priya Bhardwaj and colleagues
Department of Medicine, Weill Cornell Medicine, New York, NY, USA
Image copyright held by the original authors
Research published in Science Translational Medicine, February 2023
You can also follow BPoD on Instagram, Twitter and Facebook
#science#biomedicine#cancer#oncology#brca1#brca2#genetic risk#breast cancer#jolie gene#immunofluorescence#cytogenetics#obesity#bmi
9 notes
·
View notes
Text
Discover Top Cytogenetics Systems for Accurate Analysis
Explore advanced Cytogenetics Systems at DSS Imagetech, designed for precise chromosome analysis and optimized sample preparations. Our Cytogenetics Laboratory Information Systems, including Autochrome, Hanabi PI, Opti Chrome Plus, and Quick Chrome, ensure high-quality results under standardized conditions. Check out now to enhance your lab's capabilities with DSS Imagetech's innovative solutions!
0 notes
Text
If Kodani was correct, then the "extra" chromosome Lejeune had detected in his patients might not be extra at all and might have nothing to do with Down's syndrome.
"In the Name of Eugenics: Genetics and the Uses of Human Heredity" - Daniel J. Kevles
#book quote#in the name of eugenics#daniel j kevles#nonfiction#masuo kodani#cytogenetics#chromosomes#jerome lejeune#downs syndrome#karyotype
0 notes
Text

𝐃𝐞𝐟𝐢𝐧𝐢𝐭𝐢𝐨𝐧: Biosensors are analytical devices that combine a biological component (such as enzymes, antibodies, or cells) with a physicochemical detector to detect the presence of a specific biological molecule or analyte.
𝐂𝐨𝐦𝐩𝐨𝐧𝐞𝐧𝐭𝐬: A typical biosensor consists of a bioreceptor (the biological component), a transducer (which converts the biological response into a measurable signal), and an interface for data processing and display.
Visit @ https://symbiosisonlinepublishing.com/biosensors-biomarkers-diagnostics/
#biosensor#biomarkers#biosensors#diagnosis#bioanalysis#Cytogenetics#molecular#MolecularDiagnostics#novelbiosensor#bioimaging#journal#journals#pubmed#peerreview#openaccess#symbiosisonlinepublishing
0 notes
Photo

Barbara McClintock was born on June 16, 1902. An American scientist and cytogeneticist who was awarded the 1983 Nobel Prize in Physiology or Medicine. She demonstrated the role of the telomere and centromere, regions of the chromosome that are important in the conservation of genetic information. She made an extensive study of the cytogenetics and ethnobotany of maize (corn) races from South America. McClintock discovered transposition and used it to demonstrate that genes are responsible for turning physical characteristics on and off. She developed theories to explain the suppression and expression of genetic information from one generation of maize plants to the next.
#barbara mcclintock#genetics#telomeres#chromosomes#cytogenetics#transposition#genes#Nobel Prize#nobel prize winners#women in science#women in history#science#science history#science birthdays#on this day#on this day in science history
0 notes
Text
#Anatomical Pathology#Automated Tissue Image Analysis#Cellular Pathology#Chemical pathology#Clinical biochemistry#Clinical forensic pathology#Clinical microbiology#Clinical Pathology#Cooperative Human Tissue Network#Cytogenetics
1 note
·
View note
Text
https://sparktv.net/read-blog/41347_molecular-cytogenetics-market-size-analysis-and-forecast-2031.html
The Molecular Cytogenetics Market in 2023 is US$ 3.07 billion, and is expected to reach US$ 6.73 billion by 2031 at a CAGR of 10.32%.
#Molecular Cytogenetics Market#Molecular Cytogenetics Market Trends#Molecular Cytogenetics Market Growth
0 notes
Text
Molecular Cytogenetics Market worth $4.9 billion by 2028
Molecular Cytogenetics Market in terms of revenue was estimated to be worth $3.1 billion in 2023 and is poised to reach $4.9 billion by 2028, growing at a CAGR of 9.9% from 2023 to 2028 according to a new report by MarketsandMarkets™. The high prevalence of cancer and genetic disorders significantly impacts the increased demand for molecular cytogenetics market. Cancer patients require genetic testing in order to chromogenic changes associated with the tumor. This will lead to an increased need for molecular cytogenetics tests. Thus, the escalating demand for cancer diagnosis has led to a pressing need for technologically advanced devices, which is thereby expected to support the growth of the molecular cytogenetics market.
Attractive Opportunities in the Molecular Cytogenetics Market

Download an Illustrative overview:
Browse in-depth TOC on "Molecular Cytogenetics Market"
139 - Tables
45 - Figures
240 – Pages
In 2022, the kits & reagents segment held the largest share of the molecular cytogenetics market by product segment.
The molecular cytogenetics market is segmented into kits & reagents, instruments, consumables and software & services based on products. The kits & reagents segment accounted for the largest share of the molecular cytogenetics market in 2022. This segment covers the most commonly used products, such as probes, testing kits, fluorescent affinity reagents, scanners, software and amongst others. These products are commonly used in clinical laboratories, research laboratories, academic institutes and pharma & biotech companies. As technologies continue to evolve, the efficiency and accuracy of these products contribute significantly to molecular cytogenetics.
The comparative genomic hybridization segment held the largest share of the molecular cytogenetics market by technique segment in 2022.
The global molecular cytogenetics market is differentiated into comparative genomic hybridization, fluorescence in-situ hybridization, chromogenic in-situ hybridization and other techniques based on techniques. In 2022, the comparative genomic hybridization segment dominated the molecular cytogenetics market. The molecular cytogenetics market in cancer is expected to grow at a high rate owing to the growing focus on targeted cancer treatment.
The cancer segment held the largest share of the molecular cytogenetics market by application segment in 2022.
The global molecular cytogenetics market is differentiated into genetic disorders, cancer, personalized medicine and other applications. In 2022, the cancer segment dominated the molecular cytogenetics market. The molecular cytogenetics market in cancer is expected to grow at a high rate owing to the rising incidence of cancer. With a burgeoning global population, the need for cancer diagnosis has significantly increased due to the increased number of cancer diagnoses and research. Diagnostic and research laboratories are at the forefront of this surge, as they cater to routine use of molecular cytogenetics.
The clinical & research laboratories segment held the largest share of the molecular cytogenetics market by end-user segment in 2022.
The global molecular cytogenetics market is differentiated into clinical & research laboratories, academic research institutes, pharmaceutical & biotechnology companies and other end users. In 2022, the clinical & research laboratories segment dominated the molecular cytogenetics market. The molecular cytogenetics market in clinical & research laboratories is expected to grow at a high rate owing to the increasing penetration of molecular cytogenetics in clinical pathological testing. The expanding network of healthcare institutions directly translates to a substantial market share.
North America is the largest regional market for molecular cytogenetics market.
The market for molecular cytogenetics has been divided into five key geographical regions: North America, Europe, Asia Pacific, Latin America, and the Middle East & Africa. In 2022, North America held the predominant portion of the molecular cytogenetics market. The dominant share of the molecular cytogenetics market resides in North America, thanks to its advanced healthcare infrastructure and significant investments in healthcare. These factors play a pivotal role in driving the demand for molecular cytogenetics products. Additionally, the anticipated increase in the elderly population and subsequent rise in chronic diseases are expected to further fuel the market's expansion. As the demand for advanced cancer and genetic testing rises, so does the need for precise molecular cytogenetics products to ensure successful and safe procedures.
Request Sample Pages:
Molecular Cytogenetics Market Dynamics:
Drivers:
Increasing incidence of cancer and genetic disorders
Restraint:
High cost of advanced instruments
Opportunities:
Untapped emerging markets
Challenge:
Transition from FISH to array-based techniques
Key Market Players of Molecular Cytogenetics Industry:
The major players operating in this market are Thermo Fisher Scientific Inc. (US), Illumina Inc. (US), Danaher Corporation (US), F. Hoffmann-La Roche Ltd. (Switzerland), Revvity (US), Abbott Laboratories (US), Agilent Technologies, Inc. (US), Pacific Biosciences (US), Bio-Rad Laboratories, Inc. (US), Bio-Techne Corporation (US), GeneDx (US), Oncocyte Corporation (US), BioView (Israel), Oxford Gene Technology IP, Limited. (UK), Applied Spectral Imaging, Inc. (US), Cyto-Test Inc. (US), KromaTiD, Inc. (US), Genial Genetic Solutions, Ltd. (UK), Cytognomix, Inc. (Canada), MetaSystems (Germany), SciGene (US), Biomodal (UK), Biocare Medical (US), BioDot (US), OncoDNA (Belgium).
The break-up of the profile of primary participants in the molecular cytogenetics market:
By Company Type: Tier 1 - 40%, Tier 2 - 30%, and Tier 3 – 30%
By Designation: C-level - 27%, D-level - 18%, and Others - 55%
By Region: North America - 51%, Europe - 21%, Asia Pacific - 18%, Latin America – 6%, and Middle East & Africa- 4%
Get 10% Free Customization on this Report:
Molecular Cytogenetics Market - Key Benefits of Buying the Report:
The report aims to assist market leaders and new entrants by offering close approximations of revenue figures for both the overall digital therapeutics market and its subsegments. Stakeholders can leverage this report to comprehend the competitive landscape, acquire insights for strategic business positioning, and formulate effective go-to-market strategies. Additionally, it provides stakeholders with the means to assess the market dynamics and furnishes information on crucial market opportunities, restraints, drivers, and challenges.
Reasons to Buy the Report:
The report will help the market leaders/new entrants in this market with information on the closest approximations of the revenue numbers for the overall molecular cytogenetics market and the subsegments. This report will help stakeholders understand the competitive landscape and gain more insights to position their businesses better and plan suitable go-to-market strategies. The report also helps stakeholders understand the pulse of the market and provides them with information on key market drivers, restraints, opportunities and challenges.
The report provides insights on the following pointers:
Analysis of key drivers (increasing incidence of cancer and genetic disorders, growing focus on targeted cancer treatment, rapid growth in aging population and subsequent increase in prevalence of chronic diseases, increasing penetration of molecular cytogenetics in clinical pathological testing), restraints (high cost of advanced instruments, unfavorable reimbursement scenario), opportunities (untapped emerging markets in Asia) and challenges (transition from FISH to array-based techniques) influencing the growth of the molecular cytogenetics market.
Product Development/Innovation: Detailed insights on upcoming technologies, research & development activities, and new product launches in the molecular cytogenetics market.
Market Development: Comprehensive information about lucrative markets – the report analyses the molecular cytogenetics market across varied regions.
Market Diversification: Exhaustive information about new products, untapped geographies, recent developments, and investments in the molecular cytogenetics market
Competitive Assessment: In-depth assessment of market shares, growth strategies, and service offerings of leading players like F. Hoffmann-La Roche Ltd. (Switzerland), Danaher Corporation (US), Agilent Technologies, Inc. (US), Abbott Laboratories (US), Thermo Fisher Scientific Inc. (US), among others in the molecular cytogenetics market strategies.
#Molecular Cytogenetics Market#Molecular Cytogenetics Market Size#Molecular Cytogenetics Market Trends#Molecular Cytogenetics Industry
0 notes
Text

FISH Hybridization System LFHS-A10 is programmable system and humidifying that automates the steps in a
slide-based FISH procedure. It offers capacity of 12 slides and temperature control ranges from RT+5°C to 99.9°C.
Designed with touch screen easy to read, program and adopts four operation modes: denaturation/hybridization,
hybridization fixed temperature, custom, In-situ PCR. Two-side heating of slides that allows achieving exact
correspondence of the set and actual temperature and maintaining uniform temperature across all slide positions.
It is easy to use and reduces hands-on time by more than 50% while ensuring overall precision and accuracy in all
slide-based assays. It is ideally suited for the needs of cytogenetic laboratories to provide a good instrument.
0 notes
Text
0 notes
Text
Molecular Cytogenetics Market Is Estimated To Witness High Growth Owing To Increasing Demand for Genetic Testing

The global Molecular Cytogenetics Market is estimated to be valued at US$ 4,211.4 million in 2023 and is expected to exhibit a CAGR of 24.5% over the forecast period 2023-2030, as highlighted in a new report published by Coherent Market Insights.
A) Market Overview:
Molecular cytogenetics is a branch of genetics that focuses on the study of chromosomes and their abnormalities at a molecular level. It combines cytogenetic techniques with molecular biology to investigate the structure and function of chromosomes, as well as the genetic abnormalities associated with various diseases. The market offers a wide range of products such as fluorescent in situ hybridization (FISH) probes, comparative genomic hybridization (CGH) arrays, and karyotyping reagents, which are extensively used in research laboratories and diagnostic centers for genetic testing.
B) Market Dynamics:
The Molecular Cytogenetics Market is driven by two key factors. Firstly, there is a growing demand for genetic testing, especially in the field of personalized medicine, where genetic information is utilized to tailor treatment plans for individual patients. Genetic tests help in identifying genetic abnormalities that may cause diseases, enabling healthcare practitioners to make informed decisions regarding patient care. Moreover, advancements in technologies such as FISH and CGH arrays have made genetic testing more efficient and accurate, further contributing to market growth.
Secondly, there is a rising incidence of genetic disorders worldwide. According to the World Health Organization (WHO), birth defects are the leading cause of infant mortality and contribute significantly to childhood morbidity and disability. In addition, genetic disorders can also lead to adult-onset diseases such as cancer and neurodegenerative disorders. With an increasing awareness of the importance of early diagnosis, genetic testing has become an integral part of healthcare systems worldwide, driving the demand for molecular cytogenetics products.
C) Market Key Trends:
One key trend in the Molecular Cytogenetics Market is the increasing adoption of non-invasive prenatal testing (NIPT). NIPT is a genetic screening test that can detect the risk of certain chromosomal abnormalities in the fetus, such as Down syndrome, without the need for invasive procedures such as amniocentesis. The test is based on analyzing cell-free fetal DNA present in the maternal blood. NIPT offers several advantages over traditional methods, including higher accuracy, lower risk of complications, and earlier detection. This trend is expected to boost the demand for molecular cytogenetics products in the coming years.
D) SWOT Analysis:
Strengths:
- Advanced technologies such as FISH and CGH arrays offer high sensitivity and specificity in genetic testing.
- Increasing adoption of genetic testing in personalized medicine.
Weaknesses:
- High cost associated with molecular cytogenetics tests and equipment.
- Lack of awareness and accessibility in developing regions.
Opportunities:
- Growing application of molecular cytogenetics in oncology for cancer diagnosis and prognosis.
- Emerging markets such as Asia-Pacific offer significant growth opportunities.
Threats:
- Stringent regulatory requirements for genetic testing.
- Ethical concerns related to prenatal genetic testing.
E) Key Takeaways:
Paragraph 1: The global molecular cytogenetics market is expected to witness high growth, exhibiting a CAGR of 24.5% over the forecast period, due to increasing demand for genetic testing and rising incidence of genetic disorders.
Paragraph 2: North America is expected to dominate the market, followed by Europe, due to the presence of key players and a well-established healthcare infrastructure. However, the Asia-Pacific region is projected to be the fastest-growing market, owing to a growing population, rising healthcare expenditure, and increasing awareness about genetic testing.
Paragraph 3: Key players operating in the global molecular cytogenetics market include Abbott Laboratories, Affymetrix, Inc., Agilent Technologies, Inc., Applied Spectral Imaging, Inc., Bio-Rad Laboratories, and Thermo Fisher Scientific Inc., among others. These players focus on product innovation, collaborations, and acquisitions to sustain their market position and expand their product portfolios.
0 notes
Text
Molecular Cytogenetics Market Outlook On The Basis Of Application, Product, End Use, Technology, Region And Forecast to 2025: Grand View Research Inc.
San Francisco, 28 April 2023: The Report Molecular Cytogenetics Market Analysis Report By Application (Oncology, Personalized Medicine), By Product, By End Use, By Technology (FISH, Immunochemistry, Karyotyping), And Segment Forecasts, 2018 – 2025
The global molecular cytogenetics market size is expected to reach USD 3. 8 billion by 2025, according to a new report by Grand View Research, Inc.,…

View On WordPress
#Molecular Cytogenetics Industry#Molecular Cytogenetics Market#Molecular Cytogenetics Market 2025#Molecular Cytogenetics Market Revenue#Molecular Cytogenetics Market Share#Molecular Cytogenetics Market Size
0 notes
Text
In February 1956, Polani attempted to persuade cytogeneticist to help him; the man declined because he was unconvinced by Polani's arguments that the Turner's women might be XO.
"In the Name of Eugenics: Genetics and the Uses of Human Heredity" - Daniel J. Kevles
#book quote#in the name of eugenics#daniel j kevles#nonfiction#february#50s#1950s#20th century#paul polani#cytogenetics#declined#unconvinced#turner syndrome#karyotype#chromosomes
0 notes
Text
De novo acute B-cell acute Lymphoblastic Leukemia with BCL2/IGH and BCR/ABL1 rearrangements by Pier Paolo Piccaluga in Journal of Clinical Case Reports Medical Images and Health Sciences

ABSTRACT
T(14;18)(q32,q21) and t(9;22)(q34;q11) translocations, leading to BCL2/IGH and BCR/ABL1 rearrangements, respectively, are common genetic aberrations in hematological malignancies. Particularly, t(14;18)(q32;q21) is the genetic hallmark of follicular lymphoma, while t(9;22)(q34;q11) is commonly rearranged in acute lymphoid leukemia (ALL) and chronic myeloid leukemia. Nevertheless, their association has never been described. We report the first case of acute lymphoid leukemia (ALL) in which both BCL2/IGH and BCR/ABL1 rearrangements were present. The patient presented with pre-B ALL, achieved molecular complete remission with intensified chemotherapy, then reinforced with autologous stem cell transplantation, relapsed after a few months, and unfortunately died 17 months after diagnosis. Of note, only BCL2/IGH but not BCR/ABL1 was detected at relapses.
Key words: B-acute lymphoid leukemia, BCR/ABL1, t(14;18)(q32,q21), BCL2, Philadelphia chromosome, apoptosis, Imatinib, targeted therapy
INTRODUCTION
The t(14;18)(q32;q21) translocation is the most common translocation in B-cell malignancies; in particular, it is found in about 90% of follicular lymphomas, being the chromosomal hallmark of this tumor, and in about 20-25% of diffuse large B-cell lymphomas(1–4). Only a few cases of de novo B-acute lymphoid leukemia (B-ALL) carrying t(14;18)(q32;q21) have been described(5–13). Most of these cases presented with additional chromosomal abnormalities, often involving band 8q24 and/or MYC rearrangement and had a very aggressive clinical course(5,6,8,9,12). Central nervous system (CNS) involvement seems to be a frequent event, despite of adequate prophylaxis. The association between t(14;18)(q32;q21 ) and BCR/ABL1 rearrangement has never been described in ALL. We report on a de novo B-ALL carrying both t(14;18)(q32;q21) with BCL2/IGH fusion and BCR/ABL1 rearrangement.
METHODS
Cytogenetics
Short term cultures from bone marrow samples were performed at diagnosis and during the follow-up. Metaphases were analyzed after G-banding with Wright’stain. Karyotype was described according to the International System for Human Cytogenetic Nomenclature (ISCN 1995)(14–16).
FISH
FISH was performed on fixed cells. Directly labeled BCR and ABL probes (Vysis, Inc), producing a split of red signal when ABL is involved in genetic rearrangements. FISH data were collected with a fluorescence microscope (E 1000, Nikon Instruments) equipped with a CCD camera and Genikon software (Nikon Instruments). Two hundred nuclei/cells were analyzed for each experiment.
Molecular evaluation of BCL2/IGH rearrangement
Molecular evaluation was based on nested PCR(17). Mononuclear cells from BM and PB samples were obtained by Ficoll-Hypaque density gradient centrifugation. Genomic DNA was isolated from mononuclear cells using the QIAamp DNA mini kit (Qiagen, Hilden, Germany)(18). DNA integrity was assessed by amplifying a 510 bp fragment of the Beta-globin gene. Samples positive for Beta-globin were then investigated for the BCL2/IGH rearrangement using a nested PCR specific for MBR and mcr breakpoints. The first round of amplification was done using 1 microg of genomic DNA and the following primers: 5’–CAGCCTTGAAACATTGATGG–3’(forward, for MBR), 5’– CGTGCTGGTACCACTCCTG–3’ (forward, for mcr) and 5’–ACCTGAGGAGACGGTGACC–3’ (reverse, for the JH consensus region). An initial denaturation step of 5 min at 95° C was followed by amplification for 30 cycles (denaturation: 40 sec at 95° C; annealing: 40 sec at 55° C (MBR) or 58°C (mcr); extension: 50 sec at 72° C) and final extension for 7 min at 72° C. Reamplification of a 1 microL aliquot from a 1:50 dilution of the first PCR product was then performed using the internal primers: 5’–ATGGTGGTTTGACCTTTAG–3’ (forward, for MBR), 5’–GGACCTTCCTTGGTGTGTTG–3’ (forward, for mcr), 5’–ACCAGGGTCCCTTGGCCCCA–3’ (reverse, for the JH consensus region), and the following
PCR conditions: initial denaturation step of 5 min at 95° C; amplification for 35 cycles (denaturation: 40 sec at 95° C; annealing: 40 sec at 56° C (MBR) or 59°C (mcr); extension: 50 sec at 72° C); final extension for 7 min at 72° C. All PCR experiments were performed in 50 microL final volume containing 1U of Taq Gold DNA Polymerase (PE Applied Biosystems, San Francisco, USA), 10x PCR buffer, 100 mM of each dNTP, 2.5mM MgCl2, and 1 microM of each primer. Samples were tested twice, and both positive and negative controls were included in all experiments. A patient-specific positive control was also included in every follow-up experiment to compare the BCL2/IGH fragment length with the PCR product obtained at the time of diagnosis. Amplified products were visualized on a 2% agarose gel stained with ethidium bromide. The sensitivity of the assay for the detection of BCL2/IGH rearrangement was routinely =10-4.
Molecular evaluation of BCR/ABL1 rearrangement
RNA extraction was performed by phenol/chloroform using bone marrow mononuclear cells obtained by Ficoll-Hypaque density gradient centrifugation. One microg of total RNA was reverse transcribed using random hexamer primers and MMLV reverse transcriptase; briefly, RNA was prewarmed for 10 min at 70°C and subsequently cooled for a further 10 min at 25°C. The RNA solution was then incubated for 42 min at 45°C in a 20 L reaction mixture containing 10 mM Tris
HCl (pH 8.3), 50 mM KCl, 5.5 mM MgCl2, 1 mM of each deoxyribonucleotide, 20 U of RNAsin
(Pharmacia, Upsala, Sweeden), 25 microM random hexamers (Pharmacia, Upssala, Sweeden), 10 mM of DTT (Pharmacia, Upssala, Sweeden), and 100U of MoMLV reverse transcriptase (BRL, Bethesda, MD). After incubation, cDNA solution was diluted 1:5 to 50 microL final volume. The cDNA integrity was assessed by amplifying a 296 bp fragment of the ABL1 gene. Samples positive for ABL1 were then investigated for the BCR/ABL1 rearrangement by qualitative PCR. Five microLs of cDNA were PCR-amplified using the following set of primers: EA500 5’ TGTGATTATAGCCTAAGACCCGGAG 3’, and R112 5’ TTGTCGTGTCCGAGGCCACC 3’. Thirty-five cycles of PCR were performed as follows: denaturation (30 sec at 96°C), annealing (30 sec at 60°C), and extension (30 sec at 72°C). Samples were tested twice, and both positive and negative controls were included in all experiments.
Amplified products were visualized on a 2% agarose gel stained with ethidium bromide (19).REF
Case report
In July 2020, a 40-years-old woman, presenting only with moderate fatigue, was diagnosed with pre-B ALL, L2 subtype. The peripheral blood count showed: Hb 9.3 g/dl; WBC 17x109/L; PLT 56x109/L. The bone marrow aspirate was hypocellular with 80% of lymphoid blasts. The karyotype was: 46,XX, del(6)(8q21q25), t(9;9)(p11;q22), t(14;18)(q32;q21)(10/20). The immuphenotype, assessed by flow cytometry, was: CD19+, CD22+, TdT+, CD20-, CD3-, CD10-.
The molecular analysis carried out by PCR confirmed a BCL2/IGH rearrangement (mcr breakpoint) but also unveiled a BCR/ABL1 (E1-A2 /p190) rearrangement. Thus, FISH analysis was also performed. The probe for BCR/ABL1 dual fusion gene gave two green signals and two red signals as expected from samples not carrying the ABL1 rearrangement. Molecular analysis was then repeated confirming the previous results. We administered a standard induction therapy (doxorubicine, vincristine, L-asparaginase, and prednisone plus imatinib), and an intensified consolidation therapy (idarubicine and high dose cytarabine) obtaining a molecular complete remission (CR). Particularly, neither BCL/IGH nor BCR/ABL1 rearrangements were detected. Other 2 consolidation courses were then administered (BFM-B regimen, including vincristine, ifosfamide, methothrexate, teniposide, high dose cytarabine, and dexamethasone; and BFM-A regimen, including vincristine, doxorubicine, cyclophosphamide, high dose methothrexate, and dexamethasone) associated with imatinib. Bone marrow harvest and autologous bone marrow transplantation were then performed, lacking a HLA-matched donor. Twelve months after the first documentation of CR, the patient relapsed. The bone marrow aspirate was hypercellular with 90% of leukemic cells. The karyotype was: 46 XX, t(1;5)(p32;q31), del(12)(p11;p13)(14/15); the molecular analysis conducted by PCR showed the BCL2/IGH rearrangement, whereas there was no evidence of the BCR/ABL1 fusion transcript. Salvage therapy with liposomal daunorubicin and intermediate dose cytarabine (23) was then administered, obtaining a second molecular CR (disappearance of BCL2/IGH). Two months later, a second relapse occurred. The karyotype was: 46 XX, t(1,5)(p32;q31), del(12)(p11;p13)(29/30). The molecular analysis showed again only the BCL2/IGH rearrangement, without evidence of the BCR/ABL1 fusion gene. Despite of neuro-meningeal prophylaxis, there was clinical evidence of CNS involvement. Compassionate treatment with campath-1H, 30 mg/dose, for 5 doses, was administered i. v., obtaining a peripheral blood blast clearance, but not a CR. The patients eventually died 17 months after diagnosis due to leukemic progression.
DISCUSSION
BCR/ABL1 and BCL2/IGH rearrangements are common molecular abnormalities in B-cell malignancies. In particular, the BCR/ABL1 rearrangement is the most frequent genetic aberration in adult B-ALL(20–22). On the other hand, t(14;18)(q32;q21) with BCL2/IGH rearrangement is the most common abnormality in tumors derived from peripheral B-lymphocytes, whereas it is absolutely rare in B-cell precursor malignancies (24). However, while the biological role of BCR/ABL1 in acute leukemia is at least partially well known(25), the significance of BCL2 in ALL is still largely indefinite. BCL2 overexpression, without BCL2/IGH rearrangement, is frequent in ALL, and does not seem to be associated with a poorer prognosis (26). On the contrary, t(14;18)(q32;q21) and BCL2/IGH rearrangement are a rarity in ALL, but are associated with very aggressive tumors. Morphologically, the described cases are often L3, according to their immunophenotype of mature B-ALL, with Burkitt-like features. Notably, in all cases, complex karyotypes were observed, with almost constant involvement of the 8q24 locus and MYC deregulation(5–13). Sequential emergence of molecular abnormalities has been proposed in these cases, with progression from indolent (BCL2/IGH positive) to aggressive (BCL2/IGH and MYC positive) B-cell tumors (5–13). Therefore, they most likely represented leukemic variants of high-grade B-cell lymphomas with “double hits”. On the clinical ground, most of the patients presented with rapidly worsening general condition, fever, fatigue, night sweat, and weight loss; massive bone marrow and blood involvement, nodal and extra-nodal infiltration were also present. Clinical course was aggressive, with a median overall survival usually below than 12 months(5–13).
To the best of our knowledge, the association between t(14;18)(q32;q21) and BCR/ABL1 rearrangement has not been previously described in ALL. Nevertheless, a case of co-existing
BCR/ABL1 and BCL2/IGH rearrangements was reported in a MDS case(27). Our patient presented with a pre-B ALL, L2 subtype, carrying the t(14;18)(q32;q21) and other additional chromosomal aberrations, such as del(6)(q21;q25) and t(9;9)(p11;p22) but lacking 8q24 involvement; the BCR/ABL1 rearrangement was documented only by molecular analysis. Clinical course was aggressive, with recurrent relapses, CNS involvement, and death within seventeen months. Interestingly, at relapse, the patient presented a different karyotype [t(1,5)(p32;q31), del(12)(p11;p13), quite common as secondary abnormalities], still showing the BCL2/IGH rearrangement. Furthermore, during the clinical history of the patient, other chromosomal aberrations appeared. The relationship between the molecular events, and even a possible sequential appearance cannot be established. No peculiar morphologic or immunophenotipic patterns can be identified, to be easily associated to either one translocation, and the bad prognosis could be conferred by both the main genetic alterations; however, a dominant role of BCL2/IGH should be hypothesized, since it was always present during all disease phases. In this regard, based on the lack of cytogenetic evidence of Philadelphia chromosome we cannot exclude that BCR/ABL1 rearrangement constituted a sub-clonal lesion, cleared out by the more specific targeted therapy (chemotherapy plus imatinib).
Certainly, the treatment of t(14;18)(q32;q21) positive ALL remains a major problem, as conventional therapy are scarcely effective. Probably, the highly proliferating phenotype is made highly insensitive to chemotherapy by the antiapoptotic effect of BCL2, as observed in high-grade B-cell lymphomas with double hits.
The present case, besides its unicity, also confirmed the importance of molecular testing after cytogenetic analysis in human leukemia. Future experiences and hopefully trials will be useful to improve the current treatment of t(14;18)(q32;q21) positive ALL by adopting more rationally targeted therapies such as BCL2 inhibitors (eg venetoclax), peroxisome proliferator-activated receptor-gamma ligands (28), or others.
For more information: https://jmedcasereportsimages.org/about-us/
For more submission : https://jmedcasereportsimages.org/
#B-acute lymphoid leukemia#BCR/ABL1#t(14;18)(q32#q21)#BCL2#Philadelphia chromosome#apoptosis#Imatinib#targeted therapy#Cytogenetic Nomenclature#Genikon#DNA#Pier Paolo Piccaluga#jcrmhs
0 notes