#eProtocol
Explore tagged Tumblr posts
Text
automated protocol management | ai protocol generator | protocol automation
Turn your study synopsis into high-quality protocol drafts in record time with the power of GenAI. Clinion’s eProtocol Automation uses cutting-edge AI to supercharge protocol drafting—delivering unmatched speed, accuracy, and regulatory compliance. Experience the future of protocol drafting, where 90% of your content is generated automatically in minutes, freeing your team to focus on innovation and excellence.
0 notes
Text
Electronic data capture software | EDC software for clinical trials
Electronic Data Capture software has revolutionized clinical trials, enabling clinical research teams to streamline data management and enhance operational efficiency. However, the true potential of EDC is realized when coupled with advanced features that leverage AI and machine learning (ML). Among the sea of options, Clinion electronic data capture software stands out as a game-changer.
Discover how Clinion EDC’s advanced AI-enabled features like eProtocol Automation, Global Libraries, AI Medical Coding and Remote SDV Automation set it apart from the competition, making it the ideal choice for accelerating your trials.
0 notes
Text
eProtocol | IRB System | Key Solutions

Key Solutions eProtocol IRB system automates the management of research with human subjects. The entire application process – including development, submission etc. Key Solutions’ eProtocol IRB system automates the management of research with human subjects. The entire application process – including development, submission, tracking, review, approval, audit, and so on – is performed in a streamlined, paperless format, providing flexibility and ensuring error-free compliance with regulations.
For more information please visit here: https://www.keyusa.com/eprotocol.html
0 notes
Text
I just got diety of research...bruh I JUST finished all my IRB eProtocol attachments and am about to send it all to my mentor......I’m like one day away from submitting for approval and after that my project is finally gonna be in the data collection date
here's a random word generator--whatever word it gives you is now the thing you are the deity of
299K notes
·
View notes
Text
automated protocol management | ai protocol generator | protocol automation
Turn your study synopsis into high-quality protocol drafts in record time with the power of GenAI. Clinion’s eProtocol Automation uses cutting-edge AI to supercharge protocol drafting—delivering unmatched speed, accuracy, and regulatory compliance. Experience the future of protocol drafting, where 90% of your content is generated automatically in minutes, freeing your team to focus on innovation and excellence.
0 notes
Text
Simplifying Clinical Trial Protocol with Generative AI
AI-driven eProtocol generation represents a significant technological advancement in clinical trial protocol development. By leveraging machine learning, utilizing standardized templates, and automation, these tools are advancing the creation of high-quality protocols, enhancing efficiency, and reducing errors. As AI technology continues to evolve, its role in clinical research is expanding, offering new opportunities for improving the design and execution of clinical trials.
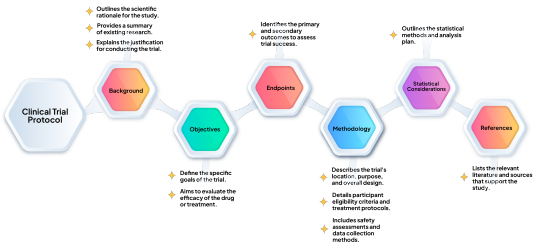
Read more: Simplifying Clinical Trial Protocol with Generative AI
0 notes
Text
Post Approval Monitoring IACUC | Key Solutions Inc

Key Solutions offers Post-Approval Monitoring which facilitates regular inspection of protocols and enables immediate correction if required.
After the approval of a protocol, an organization needs to continuously monitor its activities to confirm that they are in line with the compliance application. If there are any discrepancies, the inspection team should either prescribe corrective action or, in extreme cases, suspend the research protocol. Our PAM module streamlines this entire process.
For more information please visit here: https://www.keyusa.com/post-approval-monitoring.html
0 notes
Text
Electronic Data Capture | EDC Software for Clinical Trials | Electronic Data Capture System
Electronic Data Capture software has revolutionized clinical trials, enabling clinical research teams to streamline data management and enhance operational efficiency. However, the true potential of EDC is realized when coupled with advanced features that leverage AI and machine learning (ML). Among the sea of options, Clinion electronic data capture software stands out as a game-changer.
Discover how Clinion EDC’s advanced AI-enabled features like eProtocol Automation, Global Libraries, AI Medical Coding and Remote SDV Automation set it apart from the competition, making it the ideal choice for accelerating your trials.
0 notes
Text
EDC software | Electronic data capture software | EDC software for clinical trials
Electronic Data Capture (EDC) software has revolutionized clinical trials, enabling clinical research teams to streamline data management and enhance operational efficiency. However, the true potential of EDC is realized when coupled with advanced features that leverage AI and machine learning (ML). Among the sea of options, Clinion electronic data capture software stands out as a game-changer.
Discover how Clinion EDC’s advanced AI-enabled features like eProtocol Automation, Global Libraries, AI Medical Coding and Remote SDV Automation set it apart from the competition, making it the ideal choice for accelerating your trials.
0 notes
Text
EDC software | Electronic data capture software | EDC clincial trials software
Electronic Data Capture (EDC) software has revolutionized clinical trials, enabling clinical research teams to streamline data management and enhance operational efficiency. However, the true potential of EDC is realized when coupled with advanced features that leverage AI and machine learning (ML). Among the sea of options, Clinion electronic data capture software stands out as a game-changer.
Discover how Clinion EDC’s advanced AI-enabled features like eProtocol Automation, Global Libraries, AI Medical Coding and Remote SDV Automation set it apart, making it the ideal choice for accelerating your trials.
0 notes
Text
EDC software | Electronic data capture (EDC) software | EDC clincial trials software
Electronic Data Capture (EDC) software has revolutionized clinical trials, enabling clinical research teams to streamline data management and enhance operational efficiency. However, the true potential of EDC is realized when coupled with advanced features that leverage AI and machine learning (ML). Among the sea of options, Clinion electronic data capture software stands out as a game-changer.
Discover how Clinion EDC’s advanced AI-enabled features like eProtocol Automation, Global Libraries, AI Medical Coding and Remote SDV Automation set it apart from the competition, making it the ideal choice for accelerating your trials.
0 notes
Text
Accelerate Clinical Trials with Clinion's AI-Powered Electronic Data Capture(EDC) Software
Electronic Data Capture (EDC) software has revolutionized clinical trials, enabling clinical research teams to streamline data management and enhance operational efficiency. However, the true potential of EDC is realized when coupled with advanced features that leverage AI and machine learning (ML). Among the sea of options, Clinion electronic data capture software stands out as a game-changer.
Discover how Clinion EDC’s advanced AI-enabled features like eProtocol Automation, Global Libraries, AI Medical Coding and Remote SDV Automation set it apart from the competition, making it the ideal choice for accelerating your trials.
0 notes
Text
Accelerate Your Clinical Trials with Clinion EDC
Electronic Data Capture (EDC) software has revolutionized clinical trials, enabling clinical research teams to streamline data management and enhance operational efficiency. However, the true potential of EDC is realized when coupled with advanced features that leverage artificial intelligence (AI) and machine learning (ML). Among the sea of options, Clinion EDC software stands out as a game-changer.
Discover how Clinion EDC’s advanced AI-enabled features like eProtocol Automation, Global Libraries, AI Medical Coding and Remote SDV Automation set it apart from the competition, making it the ideal choice for accelerating your trials.
0 notes
Text
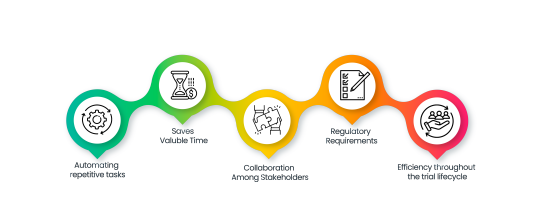
eProtocol Automation – Accelerating Protocol Design and Execution
Complementing its existing EDC capabilities, Clinion brings eProtocol Automation, enabling CROs and pharma companies to streamline protocol design and execution. This feature revolutionizes the creation, modification, and deployment of protocols, offering an intuitive interface and AI-driven suggestions.
0 notes
Text
Clinical trial protocol design & Development | eprotocol automation in clinical trials
Clinical trials are critical for bringing new treatments and therapies to market, but they are also time-consuming, expensive, and complex. Protocol development is a crucial component of the clinical trial process, but it can be a bottleneck that delays study start times. In recent years, eProtocol automation has emerged as a powerful tool for streamlining the protocol creation process, reducing errors, and improving efficiency.
https://www.clinion.com/insight/unlocking-faster-study-start-with-eprotocol-automation/
0 notes
Text
IACUC Compliance Ensure that all your animal research protocols are compliant with IACUC regulations.
Our IACUC consistence module encourages the audit and observing of each examination convention including creatures, both USDA species and something else.
Highlights:
Exhaustive Review: Animal research needs to agree to USDA guidelines and authoritative strategies for the consideration and utilization of creatures. Our item encourages head specialists to make and submit definite convention applications for endorsement from the IACUC Committee.
Convention Amendments:
Research approach frequently develops over the span of an examination study. Head Investigators can then rapidly add a correction to their endorsed convention and get acknowledgment from the IACUC Committee. This streamlined procedure limits interference to the progressing research.
iacuc compliance software
Record Version Control:
A primary specialist may adjust the convention application a few times dependent on the criticism from the IACUC Committee or change in look into system. The application keeps up a background marked by totally submitted forms of the convention application with the goal that the exploration organizers can get to any emphasis for reference.
irb regulations
Joining with Other Modules:
The IACUC convention consistence module can be coordinated with other eProtocol modules, for example, IBC, SCRO, RSC, CSC, and CS. Past consistence, we additionally bolster the creature examine process with our Facility Management (LARS) and Lab Animal Health Program (LAHS) modules.img
Contact Key Solutions : research compliance software
#irb compliance software#IRB software#iacuc compliance software#iacuc software#eprotocol#iacuc solutions#iacuc protocol solutions
0 notes