#kcl electronics
Explore tagged Tumblr posts
Text










y2k tech
looking for a cd player sadly the coolest ones are always sold for parts only ♡╭╮♡
#walkman#sony#sony walkman#y2k aesthetic#nostalgia#2000s#00s#retro tech#mary kate and ashley#kcl electronics#pink aesthetic#pinkcore#brat green#brat girls#im just a girl#girl blog aesthetic#portable cd player#cds#physical media#futuristic#pastel core#kawaii aesthetic
184 notes
·
View notes
Text
The Building Blocks of Electrical Engineering: What Every Student Should Master
A solid grounding in the fundamentals is essential for every aspiring electrical engineer. Mastery of these core concepts not only enables effective problem-solving and innovation but also forms the basis for all advanced studies and professional success in the field.
Core Principles and Laws
Ohm’s Law: This fundamental law relates voltage, current, and resistance in a circuit. It states that the voltage across a conductor is directly proportional to the current flowing through it, provided the physical conditions remain constant (V = I × R).
Kirchhoff’s Laws:
Kirchhoff’s Current Law (KCL): The total current entering a junction equals the total current leaving it.
Kirchhoff’s Voltage Law (KVL): The algebraic sum of all voltages around any closed loop in a circuit is zero.
Network Theorems: Thevenin’s and Norton’s theorems are essential for simplifying complex circuits and analyzing their behavior.
Basic Electrical Quantities
Current (I): The flow of electric charge, measured in amperes. It is the movement of electrons through a conductor.
Voltage (V): The electrical potential difference that drives current through a circuit, measured in volts.
Resistance (R): The opposition to current flow, measured in ohms. It depends on the material, length, and cross-sectional area of the conductor.
Power (P): The rate of energy transfer in a circuit, calculated as P=IVP=IV, measured in watts.
Circuit Elements and Analysis
Passive Elements: Resistors, capacitors, and inductors, which absorb or store energy but do not generate it.
Active Elements: Voltage and current sources that supply energy to the circuit.
Series and Parallel Circuits: Understanding how components behave in series (same current, voltage divides) and parallel (same voltage, current divides) is crucial for circuit analysis.
Star-Delta Transformation: A technique for simplifying complex resistor networks.
Types of Circuits
DC Circuits: Circuits powered by a constant direct current source. Analysis involves the steady-state behavior of resistors, capacitors, and inductors.
AC Circuits: Circuits powered by alternating current sources. Analysis includes understanding reactance, impedance, and phase relationships.
Single-phase and Three-phase Systems: Essential for understanding power distribution and the operation of industrial equipment.
Electromagnetism and Machines
Electromagnetic Principles: Understanding magnetic fields, flux, and electromagnetic induction is foundational for working with motors, generators, and transformers.
Transformers: Devices that transfer electrical energy between circuits through electromagnetic induction. Key for voltage conversion and power distribution.
Motors and Generators: Machines that convert electrical energy to mechanical energy (motors) and vice versa (generators). Knowledge of their principles and operation is vital.
Measurement and Instrumentation
Measuring Instruments: Familiarity with devices like voltmeters, ammeters, and multimeters is essential for practical circuit analysis and troubleshooting.
Power Factor: Understanding and improving power factor is important for efficient energy use in AC systems.
Mathematics and Physics Foundations
Mathematics: Proficiency in calculus, trigonometry, and differential equations is necessary for modeling and analyzing electrical systems.
Physics: Concepts from electromagnetism and basic mechanics underpin much of electrical engineering theory and practice.
Digital and Analog Systems
Analog Circuits: Continuous signal processing; involves resistors, capacitors, inductors, and transistors.
Digital Circuits: Discrete signal processing; involves logic gates, memory systems, and microcontrollers.
Embedded Systems: Integration of hardware and software for intelligent electronic solutions.
Practical Skills and Lifelong Learning
Circuit Design and Simulation: The Ability to design, analyze, and simulate circuits using modern tools is crucial for both academic and professional success.
Project-Based Learning: Hands-on experience through projects enhances understanding and develops problem-solving skills.
Continuous Learning: The rapid evolution of technology in electrical engineering demands ongoing education and adaptability.
Concept/Area
Why It’s Essential
Ohm’s Law, KCL, KVL
Foundation for circuit analysis and design
Circuit Elements
Understanding the behavior and function of components
AC/DC Circuits
Basis for power systems, electronics, and signal processing
Electromagnetism
Underpins the operation of machines, transformers, and communication systems
Measurement & Instrumentation
Enables accurate analysis and troubleshooting
Mathematics & Physics
Provides tools for modeling and solving engineering problems
Analog & Digital Systems
Core to modern electronics and embedded systems
Lifelong Learning
Ensures relevance and adaptability in a fast-evolving field
Summary Table: Key Concepts and Their Importance
Conclusion
Mastering these fundamentals equips electrical engineering students to analyze, design, and maintain the systems that power modern society. Arya College of Engineering & I.T. is the best college of Jaipur which has a deep understanding of these core concepts fosters innovation, supports professional growth, and prepares students for the diverse challenges of an ever-evolving field.
0 notes
Text
Fwd: Workshop: KingsC_London.PythonForBiologists.Jan27-31
Begin forwarded message: > From: [email protected] > Subject: Workshop: KingsC_London.PythonForBiologists.Jan27-31 > Date: 14 January 2025 at 06:37:06 GMT > To: [email protected] > > > > > Python for Bioinformatics: 5-Day Course Overview > > Instructor: Dr. Martin Jones, in collaboration with the Hub for Applied > Bioinformatics (KCL). > > Audience: Biologists with no prior programming experience who want to > learn Python for bioinformatics. > > Course Highlights: > > Beginner-Friendly: No programming experience required; tailored for > complete beginners with a biology background. > > Practical Focus: Real-world bioinformatics examples and hands-on > exercises. > > Comprehensive Resources: Electronic copies of presentations, exercises, > data, and scripts provided. > > Goal-Oriented: Equip students to apply Python to their research and > continue learning independently. > > Who Should Attend? > > Designed for researchers and technical workers in biology who: > > Have a basic understanding of biological concepts (e.g., DNA, protein > sequences, translation, introns/exons). > > Want to learn programming from scratch. > > Requirements: A laptop with Python installed; no advanced computer > skills needed. > > Course Structure > > Session 1: Introduction and Basics > > Overview of Python and its benefits for research. > Fundamentals: terminals, variables, strings, and error handling. > Practical: Simple scripts for sequence manipulation. > > Session 2: File Handling and Slicing > > Reading/writing files and Python’s interaction with the OS. > Practical: File processing scripts using slice syntax. > > Session 3: Lists and Loops > > Handling large datasets with lists and loops. > Practical: Working with larger data files. > > Session 4: Conditions and Flow Control > > Decision-making with conditional tests and Boolean logic. > Practical: Filtering challenges with CSV files. > > Session 5: Structuring Code with Functions > > Writing reusable functions and introducing automated testing. > Practical: Creating functions for unit tests. > > Session 6: Standard Library and Regular Expressions > > Exploring Python’s standard library and regex for pattern matching. > Practical: Solving bioinformatics problems with regex. > > Session 7: Dictionaries > > Introduction to key-value data with dictionaries. > Practical: K-mer counting and DNA-to-protein translation. > > Session 8: File Management > > Automating file operations like renaming, moving, and organizing. > Practical: Managing DNA sequences by length. > > Sessions 9–10: Workshop Time > > Recap of key topics or applying Python to personal research. > > Contact Information > > For questions, email Dr. Martin Jones: [email protected]. > > For more information: > https://ift.tt/51VUqjz > > To sign up: > https://ift.tt/tZ0m4WG > > Jazmine Portch > Operations Assistant for Mathias Gautel > Administrator for Hub for Applied Bioinformatics > School of Basic and Medical Biosciences | Faculty of Life Sciences > and Medicine > > > Jazmine Portch
0 notes
Text
Harnessing the Power of Operational Amplifiers in Analog Design
Analog and signal circuit design is essential for countless electronic devices, ranging from smartphones and computers to medical instruments and automotive systems. Though digital technology often holds the utmost significance, analog circuits are necessary for processing and transmitting real-world signals with precision and efficiency. Please check out this post and understand the fundamentals of analog and signal circuit design, exploring their significance and applications.
Understanding Analog Circuit
Analog circuits are electronic circuits that process continuous signals like voltage or current whereas the binary values represent the discrete digital signals. These circuits manipulate analog signals in different ways, including amplification, filtering, modulation, and conversion. Analog circuits are specifically characterized by their ability to represent and manipulate real-world phenomena accurately. That’s why these circuits are essential for applications that require precise signal processing.
Important Principles of Analog Circuit Design
An analog circuit design requires a deep understanding of fundamental principles and components. Some important concepts include:
Ohm's Law – This law describes the relationship between voltage, current, and resistance in a circuit.
Kirchhoff's Laws – These laws administer the behavior of current and voltage in electrical circuits, including Kirchhoff's Current Law (KCL) and Kirchhoff's Voltage Law (KVL).
Component Characteristics – They allow you to understand the behavior of passive components like resistors, capacitors, and inductors, as well as active components like transistors and operational amplifiers (op-amps). These components are important for analog circuit design.
Frequency Response - Analog circuits work within certain frequency ranges and require the consideration of frequency-dependent effects like bandwidth, phase shift, and frequency response.
Understanding Signal Circuit Design
A signal circuit focuses on signal transmission and processing that can range from audio and video signals to sensor data and communication signals. These circuits are essential for various applications, including telecommunications, audio processing, instrumentation, and sensor interfacing. Signal circuit design covers different techniques and components tailored to specific signal processing needs.
Applications of Analog and Signal Circuit Design
There are so many applications of analog and signal circuit design in numerous industries and technologies:
Audio Amplification - Analog circuits are useful in audio amplifiers to enhance the amplitude of audio signals for speakers, headphones, and other audio devices.
Data Acquisition - Signal circuits are used in data acquisition systems to convert analog signals from sensors and transducers into digital data for processing and analysis.
Wireless Communication - Analog circuits are integral aspects of wireless communication systems, including radio frequency (RF) transmitters, receivers, and modulators/demodulators.
Medical Instrumentation - Analog circuits are applicable in medical devices like electrocardiographs (ECGs), ultrasound machines, and blood pressure monitors for processing and analyzing signals.
Automotive Electronics - Analog circuits are also used for automotive systems for applications like engine control, vehicle diagnostics, and entertainment systems.
Conclusion:
Analog and signal circuit design is the fundamental aspect of electrical engineering that enables the precise processing and transmission of real-world signals in a comprehensive range of applications. Using fundamental principles, components, and design techniques, Voler Systems engineers can provide analog circuit design services to accommodate the diverse demands of modern technology. Though digital systems continue to advance, the importance of analog and signal circuit design remains paramount, integrating the physical world into the digital counterpart smoothly and effortlessly.
1 note
·
View note
Text
Phản ứng hóa học là gì? Bản chất của phản ứng hóa học là gì?

Hóa học là bộ môn bắt buộc, bắt đầu từ chương trình học lớp 8. Trong hóa học chủ yếu nói về các phản ứng xảy ra giữa các chất với nhau. Vậy bạn hiểu phản ứng hóa học là gì? Có những loại nào? Bản chất? Vai trò của phản ứng hóa học là gì? Hãy cùng với muahangdambao.com tìm hiểu nhé!
Phản ứng hóa học là gì? Cho ví dụ
Khái niệm Phản ứng hóa học hiểu đơn giản là quá trình mà các chất tham gia (gọi là chất phản ứng) tương tác với nhau để tạo ra được các chất mới (gọi là chất sản phẩm). Trong phản ứng hóa học thì liên kết giữa các nguyên tử trong phản ứng bị phá vỡ và hình thành nên các liên kết mới để tạo ra các chất sản phẩm mới.

Phản ứng hóa học Phản ứng hóa học thường sẽ được biểu diễn bằng các phương trình hóa học. Trong đó các chất phản ứng được viết ở phía bên trái mũi tên và các chất sản phẩm được viết ở phía phải mũi tên. Phương trình hóa học được biểu thị cân bằng với số lượng nguyên tử, điện tích giữa các chất phản ứng và chất sản phẩm. Cụ thể: Tên các chất tham gia phản ứng → Tên chất sản phẩm. Ví dụ Phản ứng cháy của hidro và oxi được biểu diễn bằng phương trình hóa học như sau: 2H2 + O2 → 2H2O Cụ thể: Trong phản ứng này 2 phân tử hidro tương tác với 1 phân tử oxi để tạo thành 2 phần tử nước là chất sản phẩm. Các liên kết hidro và oxi có trong chất phản ứng sẽ bị phá vỡ và các liên kết giữa hidro và oxi trong nước sẽ được hình thành. Phản ứng hóa học có thể được diễn ra trong nhiều điều kiện khác nhau và nó được điều khiển bởi một yếu tố như: nhiệt độ áp suất, nồng độ chất phản ứng cũng như có sự có mặt của chất xúc tác. Phân loại Phản ứng hóa hợp Phản ứng hóa hợp là phản ứng hóa học trong đó chỉ có một chất mới được hình thành từ hai hay nhiều hợp chất ban đầu. Ví dụ: 4P + 5O2 → 2P2O5 3Fe + 2O2 → Fe3O4 Phản ứng phân hủy Phản ứng phân hủy là phản ứng hóa học trong đó thì một chất sẽ sinh ra hai hoặc là nhiều chất mới. Ví dụ: KMnO4 → K2MNO4 + MNO2 + O2 KClO3 → KCl + O2

Các loại phản ứng hóa học Phản ứng oxi hóa khử Phản ứng oxi hóa khử là phản ứng hóa học trong đó sẽ xảy ra đồng thời sự oxi hóa cũng như sự khử. Nói cách khác, phản ứng oxi hóa khử chính là phản ứng có sự dịch chuyển electron giữa các chất có trong phản ứng. Hay phản ứng có sự thay đổi của số oxi hóa của một số nguyên tố. Chất khử chính là chất nhường electron và chất oxi hóa chính là chất nhận electron. Phản ứng thế Phản ứng thế là phản ứng hóa học xảy ra giữa đơn chất và hợp chất, trong đó thì nguyên tử của đơn chất sẽ thay thế cho nguyên tử của một nguyên tố khác trong hợp chất. Ví dụ: Zn + 2HCl → ZnCl2 + H2 Fe + H2SO4 → FeSO4 + H2
Bản chất, đặc điểm của phản ứng hóa học là gì?
Các đặc điểm chính của phản ứng hóa học như sau: Tính chất đối tác: Phản ứng hóa học được xảy ra giữa các chất phản ứng còn gọi là đối tác. Các chất phản ứng này có thể làm các nguyên tố phân tử hoặc là ion. Tính chất sản phẩm: Phản ứng hóa học tạo ra các chất mới được gọi là chất sản phẩm. Các chất sản phẩm có thể là các nguyên tố phân tử hoặc là ion khác so với chất phản ứng ban đầu. Điều kiện phản ứng: Phản ứng hóa học thường phụ thuộc vào các điều kiện như nhiệt độ, áp suất, nồng độ, chất phản ứng, ánh sáng cũng như sự có mặt của chất xúc tác. Những điều kiện này có thể ảnh hưởng đến tốc độ cũng như hiệu suất của phản ứng.

Điều kiện xảy ra phản ứng Điện tích: Trong một số phản ứng hóa học có sự chuyển đổi điện tích. Xảy ra điều này có thể dẫn đến việc hình thành nên các ion hoặc thay đổi trạng thái oxi và khử của các nguyên tử trong phản ứng. Định luật bảo toàn khối lượng: Trong một phản ứng hóa học thì khối lượng không bị thay đổi. Điều này được biểu thị thông qua định luật bảo toàn khối lượng. Tức là tổng khối lượng của chất phản ứng sẽ bằng với tổng khối lượng của chất sản phẩm. Định luật bảo toàn nguyên tử: Trong một phản ứng hóa học thì số lượng nguyên tử của mỗi nguyên tố không bị thay đổi. Điều này được phản ánh thông qua định luật bảo toàn nguyên tử. Tức là tổng số nguyên tử của mỗi nguyên tố trong chất phản ứng sẽ bằng với số nguyên tử của mỗi nguyên tố trong chất sản phẩm.

Ví dụ định luật bảo toàn nguyên tử Tốc độ phản ứng: Tốc độ phản ứng hóa học sẽ thể hiện tốc độ cũng như các chất phản ứng tương tác với chế độ thành chất xúc tác. Tốc độ phản ứng có thể ảnh hưởng bởi nhiều yếu tố như: nhiệt độ, áp suất, nồng độ chất phản ứng và sự có mặt của chất xúc tác. Đổi màu và thay đổi nhiệt độ: Trong một số phản ứng hóa học thì có thể xảy ra sự thay đổi màu hoặc là thay đổi nhiệt độ. Điều này có thể làm cho phản ứng trở nên dễ nhìn thấy cũng như cảm nhận được sự thay đổi trong điều kiện vật lý.
Vai trò của phản ứng hóa học là gì?
Các phản ứng hóa học đóng vai trò quan trọng trong quá trình tự nhiên, công nghiệp và trong cuộc sống hàng ngày của chúng ta. Cụ thể một số vai trò của phản ứng hóa học: Sản xuất và chế biến hóa chất: Phản ứng hóa học được ứng dụng trong sản xuất cũng như chế biến các hợp chất quan trọng trong các ngành công nghiệp như: công nghiệp thực phẩm, công nghiệp hóa chất, công nghiệp luyện kim và nhiều ngành khác sử dụng phản ứng hóa học để sản xuất ra các sản phẩm và chất liệu cần thiết.

Phản ứng hóa học để sản xuất ra hóa chất Năng lượng: Phản ứng hóa học đóng vai trò quan trọng trong việc chuyển đổi cũng như sử dụng năng lượng. Các phản ứng hóa học như là phản ứng cháy, phản ứng điện hóa và phản ứng hạt nhân đều được sử dụng để tạo ra năng lượng điện nhiên liệu hay nguồn năng lượng tái tạo. Kiểm soát và xử lý ô nhiễm: Phản ứng hóa học được sử dụng để kiểm soát và xử lý ô nhiễm ở trong môi trường. Các quá trình xử lý nước, xử lý chất thải và khử trùng đều dựa trên các phản ứng hóa học để loại bỏ đi các chất ô nhiễm, tạo ra môi trường sống sạch hơn. Quá trình tự nhiên: Phản ứng hóa học xảy ra trong tự nhiên và nó đóng vai trò quan trọng trong các quá trình sinh học hay môi trường. Ví dụ như phản ứng quang hợp trong cây xanh sử dụng ánh sáng mặt trời để tạo ra năng lượng và tổng hợp nên các chất hữu cơ. Hay các phản ứng hóa học trong đất và nước cũng đóng vai trò quan trọng trong chu trình chuyển hóa các chất hóa học trong tự nhiên.

Phản ứng hóa học xảy ra trong tự nhiên Sản phẩm hàng ngày: Các phản ứng hóa học cũng đóng vai trò trong cuộc sống hàng ngày của con người. Ví dụ như việc nấu ăn, tiêu thụ thuốc, sử dụng xà phòng, chất tẩy rửa hay nhiều sản phẩm khác thì đều có liên quan đến các phản ứng hóa học. Nghiên cứu và phát triển: Phản ứng hóa học cung cấp cơ sở để nghiên cứu cũng như phát triển các phương pháp công nghệ, vật liệu mới… Có thể bạn quan tâm: Phản ứng thế là gì? Chia sẻ từ A – Z về phản ứng thế và ví dụ minh họa Kiến thức: Chất khử là gì? Ý nghĩa của phản ứng oxi hoá khử Phản ứng hóa học có nhiều ứng dụng quan trọng và rộng rãi trong đời sống hàng ngày cũng như trong các ngành công nghiệp. Hy vọng bài viết này sẽ giúp bạn có cái nhìn tổng quan hơn về phản ứng hóa học là gì và biết cách áp dụng trong học tập, nghiên cứu! Read the full article
0 notes
Text
A Glimpse into the Significance of Ag/AgCl Reference Electrodes and Pt Counter Electrodes in Electrochemistry
In the realm of electrochemistry, careful selection of electrodes is crucial for precise results. Two essential components in this field are the Ag/AgCl reference electrode and the Pt counter electrode. Let's delve into how these electrodes play a vital role in electrochemical experiments.
Ag/AgCl Reference Electrode:
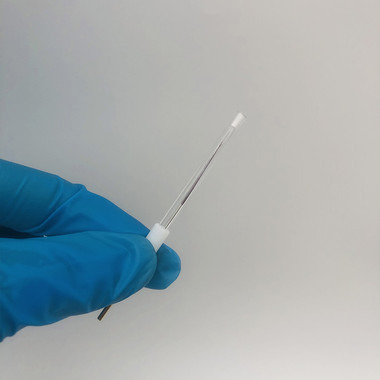
The Ag/AgCl reference electrode is a cornerstone of electrochemistry. Comprised of a silver wire coated with silver chloride (AgCl) submerged in a saturated KCl solution, this electrode is known for its stability, thanks to the reversible Ag/AgCl redox reaction.
One of its primary advantages is its consistent potential, which remains unchanged throughout experiments. This stable potential makes it ideal for measuring and controlling electrochemical reactions accurately.
Moreover, Ag/AgCl reference electrodes are non-toxic, making them suitable for various applications, including biological and environmental research. Their versatility and reliability make them a vital asset in the field of electrochemistry.
Pt Counter Electrode:
The platinum (Pt) counter electrode is a crucial component that complements the Ag/AgCl reference electrode. Its primary function is to ensure a continuous flow of current between the working and reference electrodes, facilitating desired electrochemical reactions.

Pt counter electrodes are favored for their excellent electrical conductivity, inertness, and resistance to corrosion. These properties promote efficient electron transfer and maintain a stable electrical connection during experiments.
Additionally, platinum counter electrodes are adaptable, accommodating a wide range of electrolytes and reaction conditions. This versatility allows researchers to work in various environments, from acidic to alkaline, with precision.
In summary, Ag/AgCl reference electrodes and Pt counter electrodes are indispensable in electrochemistry. They provide stability, accurate measurements, and efficient electrochemical reactions. Together, they contribute to a deeper understanding of chemical and biological processes, making significant contributions to scientific research.
0 notes
Text

Current Electricity
Introduction
Current electricity is a fundamental concept in physics and plays a crucial role in various fields, including medicine, engineering, and technology. For NEET (National Eligibility cum Entrance Test) aspirants, having a solid understanding of current electricity is essential as it forms a significant part of the physics syllabus. In this blog, we will delve into the world of current electricity, exploring its key principles, formulas, and real-world applications.
Understanding Current Electricity
Current electricity refers to the flow of electric charge through a conducting medium. The conductors, such as metals, allow the movement of charged particles, typically electrons, resulting in the flow of current. This flow occurs due to the presence of an electric potential difference or voltage across the conductor.
Ohm's Law
A cornerstone of current electricity is Ohm's Law, which establishes a relationship between current, voltage, and resistance. According to Ohm's Law, the current passing through a conductor is directly proportional to the voltage across it and inversely proportional to the resistance offered by the conductor. The Mathematical form of the expression for Ohm's Law is:
V = IR
Where: V represents the voltage (in volts) I represents the current (in amperes) R represents the resistance (in ohms)
This simple equation allows us to analyze and calculate various electrical quantities in a circuit.
Circuits and Components
Electric circuits consist of interconnected components that facilitate the flow of current. Key components include resistors, capacitors, inductors, and various types of power sources such as batteries or generators. Understanding the behavior of these components and their interactions within a circuit is crucial for solving problems related to current electricity.
Series and Parallel Circuits
The components in a series circuit are connected one after the other in a single path. The current flowing through each component remains the same, while the voltage is divided among them. The total resistance in a series circuit is the sum of individual resistances.
In parallel circuits, the components are connected across the same two points, creating multiple paths for the current. The voltage across each component remains the same, while the total current is divided among them.
Kirchhoff's Laws
Kirchhoff's laws are essential tools for analyzing complex circuits. They provide a systematic approach to determining the currents and voltages at various points within a circuit.
1. Kirchhoff's Current Law (KCL): The total current entering a junction is equal to the total current leaving that junction. In other words, the algebraic sum of currents at a node in a circuit is zero.
2. Kirchhoff's Voltage Law (KVL): The total sum of the voltage drops and rises in a closed loop within a circuit is zero. This law is based on the principle of conservation of energy.
Applications of Current Electricity
Current electricity finds application in various fields, and its understanding is crucial for aspiring medical professionals. Here are a few notable applications:
1. Medical Devices: Current electricity is extensively used in medical devices such as electrocardiograms (ECGs), electroencephalograms (EEGs), and defibrillators, which aid in the diagnosis and treatment of various medical conditions.
2. Bioelectrical Impedance Analysis (BIA): BIA is a technique that measures the body's composition by sending a small current through the body and analyzing its impedance. It helps determine parameters like body fat percentage and hydration levels.
3. Electrotherapy: Current electricity is used in electrotherapy for pain management, muscle stimulation, and tissue repair. Techniques like transcutaneous electrical nerve stimulation (TENS) and electrical muscle stimulation (EMS) rely on electrical currents for therapeutic purposes.
Conclusion
A comprehensive understanding of current electricity is indispensable for NEET aspirants pursuing a career in the medical field. Mastering the principles of current flow, Ohm's Law, circuit analysis, and Kirchhoff's laws will not only help in scoring well on the physics section of the NEET exam but also lay a strong foundation for future endeavors in the field of medicine. At DR Academy, we strive to provide you with the best NEET coaching in Bangalore and Hyderabad, equipping you with the knowledge and skills necessary to excel in your medical entrance exams. We, as the best institute for NEET preparation in Bangalore, encourage you to continue your exploration of physics and its applications, as it forms an integral part of the NEET syllabus.
0 notes
Text
Title:- What are ionic compounds?
Introduction:-
What are ionic compounds?is our main discussion today. I am just trying to focuss on the chemistry topic What are ionic compounds?which will be very helpful for class (ix) and class(x) school children, I think.
Explanation:-
To start this discussion, let us first know some basic definitions of chemistry,
what are elements?
Answer:- The matters which are composed of only one type of particles are known as elements.e.g; Sodium (Na), Magnesium(Mg), Carbon(C).....
How elements are classified?
Answer:-Elements are classified in three types:-(i) Metals (ii) Non metals. (iii) Metalloids. Metals:- Sodium(Na), Magnesium (Mg), Gold (Au)..... Non metals :-Carbon (C), Sulphur(S),Nitrogen (N).... Metalloids :- Boron(B), Silicon(Si), Arsenic(As)....
What are molecules?
Answer:- When two or more than two similar or different types of atoms combine together chemically they form molecules e.g; H + H —----> H₂ Hydrogen molecule(Diatomic molecule) Cl + Cl —-----> Cl₂
Chlorine molecule(Diatomic molecule) H₂ + S —------> H₂ S
Hydrogen sulphide(Triatomic molecule)
What are compounds?
Answer:- When two or more molecules or atoms chemically react themselves under some definite conditions and combines then they form compounds. e.g; C + ½ O₂ —---------> CO (atom) (molecule) (Carbon monoxide is a gas/compound) C + O₂ —--------> CO₂ (atom) (molecule) (Carbon dioxide is a gas/compound)
Finally I will discuss on todays topic
What are ionic compounds?
Answer:- The compounds which are formed by the transfer electrons from metal atom to non metal atom are known as ionic compounds. e,g; Potassium Chloride (Kcl), Aluminium oxide (Al₂O₃) Now we will learn a little more on ionic compounds .
How following ionic compounds are formed?
(a) Sodium Chloride (Nacl)
Answer:- Na is a metal, the electronic configuration of sodium (Na) = 2,8, 1 to complete octet and become stable sodium needs to collect 7 electrons from other element to full fill its outer most shell i.e its third shell or 'M' shell to get its nearest noble gas Argon (2,8,8) and to full fil octet and become stable or Na has to give up its only valence shell electron (1) to get 2,8 electronic configuration of Neon as sodium is a metal so according to metals chemical property , all metals give up electrons they do not accept electrons or they are unable to accept electrons due to strong inter molecular force, thats why sodium is also unable to accept 7 electrons.
Again, chlorine (Cl) is a non metal and its electronic configuration is 2,8,7 so to fulfil its octet chlorine needs to accept one(1)electron or must have to give up its valence shell's 7 electrons to get either its nearest noble 2,8,8(Argon) or 2,8 (Neon) gas configuration to become stable due to non metals chemical property non metals never give up electrons, so chlorin is also unable to give up electrons.So in chemical reaction between sodium(Na) and chlorine ,Sodium (Na) gives up its lone valence electron to get its nearest noble gas Neon's configuration(2,8) and become stable in the mean time chlorine accepts the lone electron which is given up by sodium(Na) to get its nearest noble gas Argon's (2,8,8)configuration and become stable and by the process of electrons transfer they form ionic compound (Nacl) The formation can be given by:-

(b) Magnesium Oxide (MgO)
Answer:- Magnesium is a metal,the electronic configuration magnesium(Mg) is 2,8,2 and these electrons occupies s,p,and d orbits. To become stable or get its nearest noble gas ,argon's(Ar) configuration(2,8,8) magnesium need 6 more electrons to fill up its d-orbit and to become stable but due to inter molecular force of electronic attraction magnesium is unable to take 6 more electrons from other atoms. On the other hand oxygen is a non metal and electronic configuration of oxygen is 2,6 ; which occupies its s and p orbits.To get its nearest noble gas, Neon's electronic configuration (2,8)oxygen need 2 more electrons to fill up its p-orbit and to get (2,8) neon gas configuration and to become stable. So in the chemical reaction between magnesium and oxygen one magnesium(Mg) atom gives up its lone pair(2) electrons to get neons configuration(2,8) and become stable also produces Mg⁺² ion ,in the same time one oxygen atom accepts 2 electrons which are given up by magnesium (Mg) aton also to get its nearest noble gas neon gas configuration (2,8) and become stable also produces o²⁻ion. Due to electro atomic force of attraction between Mg⁺² ion and o²⁻ion they combines themselves and produces magnesium oxide (MgO) The formation can be given by :- Mg - 2e —-------> Mg⁺² (Magnesium ion) (2,8,2) (2,8) neon gas O + 2e —-------> O²⁻(Oxygen ion) (2,6) (2,8) neon gas Now Mg⁺² + O²⁻ —------>Mg⁺²O²⁻ —->MgO
(magnesium oxide)
(c) Potassium Oxide (K₂O)
Answer:- Potassium is a metal,the electronic configuration magnesium(K) is 2,8,8,1 and these electrons occupies s,p, d and f orbit. To become stable or get its nearest noble gas ,krypton's(kr) configuration(2,8,8,18) potassium need 17 more electrons to fill up its f-orbit and to become stable but due to inter molecular force of electronic attraction magnesium is unable to take 17 more electrons from other atoms. On the other hand oxygen is a non metal and electronic configuration of oxygen is 2,6 ; which occupies its s and p orbits.To get its nearest noble gas, Neon's electronic configuration (2,8)oxygen need 2 more electrons to fill up its s-orbit and to get (2,8) neon gas configuration and to become stable. So in the chemical reaction between potassium and oxygen two potassium(K) atom gives up its lone (1) electron each to get argons configuration(2,8,8) and become stable also produces K⁺ ion ,in the same time one oxygen atom accepts 2 electrons which are given up by two potassium (K) aton also to get its nearest noble gas neon gas configuration (2,8) and become stable also produces o²⁻ion. Due to electro atomic force of attraction between 2K⁺ ion and o²⁻ion they combines themselves and produces potassium oxide (K₂O) The formation can be given by :-

Conclusion:-
Ionic compounds are formed by the transfer of electrons from metal atom to non metal atom......
To read more....
4 notes
·
View notes
Photo

Potassium chloride crystal
The crystal of potassium chloride is similar to that of sodium chloride and both salts adopt a face-centered cubic crystal structure. Each potassium atom loses one electron to form a unipositive potassium ion and the chlorine atom gains that electron to form a uni-negative chloride ion. The two oppositely charged ions are held together by the electrostatic force of attraction to form crystalline potassium chloride salt. for more about potassium chloride (KCl)
2 notes
·
View notes
Text
The first ionization potential of K is 4.34eV, the electron affinity of Cl is 3.82 eV and the equilibrium separation of KCl is 0.3 nm. The energy required to dissociate a KCl molecule into a K and a Cl atom is
1. 8.62 eV
2.8.16 eV
3. 4.28 eV
4. 4.14 eV
#ionisation#potential#potassium#electron#affinity#chlorine#equilibrium#molecule#dissociation#energy#atom#volt
1 note
·
View note
Text
The Importance of water testing

A raised complete broke up solids (TDS) fixation isn't a wellbeing danger. The TDS fixation is an optional drinking water standard and, thusly, is directed in light of the fact that it is a greater amount of a tasteful as water testing opposed to a wellbeing danger.The centralization of the disintegrated particles might make the water be destructive with a pungent or a harsh taste as well as result in scale development, diminishing the productivity of boiling water warmers.
It would likewise propose that there are numerous particles that are over the Essential or Auxiliary Drinking Water Guidelines, like a raised degree of nitrate, arsenic, aluminum, copper, lead, and so forth.The estimation of all out disintegrated solids doesn't show that there is explicit wellbeing hazard or danger, yet it tends to be utilized to give knowledge into the status or the water over the long run and as an advance notice indication of an expected issue.
Ordinarily, we prescribe water clients to get an extensive starting test that ought to incorporate various boundaries including the all out broke down solids of the water and the conductivity of the water. Conductivity and all out broke up solids are in a roundabout way related, however these are two devices to follow the difference in your water quality with time. As a general rule, a TDS of under 50 mg/L ought to raise a worry for a potential consumption issue, a TDS of more than 250 mg/L ought to raise a worry about the hardness, iron, manganese, alkalinity, chloride, sulfate, nitrate, and general salt substance, and north of 500 mg/L ought to raise a worry about different salts (bromide, lithium, aluminum, different metals, and the scale framing capability of the water). An all out broke up solids north of 1000 mg/L ought to raise a worry about the potential for a man-had direct effect or a saline water effect on the source.
For stylish reasons, the EPA has set an optional drinking water standard of < 500 mg/L (milligrams per liter) . A few states have laid out this as a drinking water standard.
Not at all like numerous impurities, there are advance notice signs that there might be an issue with your drinking water related or brought about by having to little or an excess of stuff in your water. Indeed Stuff-the all out broke up solids test doesn't let you know the stuff, however assuming that you connect your perceptions with some at-home water testing or screening, educational testing, or business confirmed testing you can distinguish this stuff, foster an arrangement to make a financially savvy arrangement, and execute an answer that safeguards yourself, your family, your home, and the frameworks in your home.
The all out disintegrated solids focus can be connected with the conductivity of the water, yet the relationship is definitely not a consistent. The connection between complete disintegrated solids and conductivity is a component of the sort and nature of the broke down cations and anions in the water and potentially the idea of any suspended materials. For instance, a NaCl arrangement and KCl arrangement with a conductivity of 10000 umhos/cm won't have similar convergence of NaCl or KCl and they will have an alternate complete broke up solids focus. Conductivity is estimated using a meter and is generally multiple times the complete cations or anions communicated as reciprocals and the all out broke down solids (TDS) in ppm as a rule goes from 0.5 to 1.0 times the electrical conductivity.
All out Broke down Solids can be estimated in the field utilizing an electronic pen. A considerable lot of these gadgets really measure the conductivity of the water, i.e., the capacity of the water to convey a charge, and not the genuine all out broke down solids. These gadgets then, at that point, compute the all out broke up solids expecting that the essential disintegrated minerals are either a mix of NaCl or KCl. In this manner, the estimation of absolute broken down solids by these gadgets are not a precise measure, but rather a guess. In the event that you are considering involving these gadgets for an undertaking, we would suggest buying a conductivity pen which estimates the conductivity of the water and finishing a lab test to KNOW the proportion of TDS to Conductivity that ought to be utilized as a remedy factor.
1 note
·
View note
Text
Electrical Engineering Questions and Answers for Competitive Exams
I can provide some information on electrical engineering topics that are commonly asked in competitive exams.
What is Ohm's law? Ohm's law states that the current through a conductor between two points is directly proportional to the voltage across the two points, and inversely proportional to the resistance between them.
What is Kirchhoff's laws? Kirchhoff's laws are two fundamental laws of circuit analysis that describe the conservation of charge and energy in an electrical circuit. The first law, Kirchhoff's current law (KCL), states that the total current entering a junction must equal the total current leaving the junction. The second law, Kirchhoff's voltage law (KVL), states that the sum of the voltages around a closed loop in a circuit must equal zero.
What is the purpose of a diode? A diode is a two-terminal electronic component that allows current to flow in only one direction, acting as a one-way valve for electric current. Diodes are commonly used in rectifier circuits to convert AC to DC, as voltage stabilizers, and to protect circuits from high-voltage transients.
What is the difference between AC and DC? AC (Alternating Current) changes direction periodically, while DC (Direct Current) flows in only one direction. AC is used in the electrical grid because it is more efficient to transmit over long distances, while DC is used in batteries and electronic devices.
What is a transistor and what is its function? A transistor is a three-terminal semiconductor device that can be used as an amplifier or switch. The transistor controls the flow of current between the input and output terminals by adjusting the voltage applied to the third terminal, the base. Transistors are widely used in electronic circuits for amplification, switching, and voltage regulation.
What is an inductor and how does it work? An inductor is a passive electrical component that stores energy in a magnetic field when electric current flows through it. Inductors oppose changes in current and can be used to filter, store, or regulate energy in an electrical circuit.
What is a capacitor and how does it work? A capacitor is a passive electrical component that stores energy in an electric field when a voltage difference exists between its plates. Capacitors can be used to store energy, filter signals, and regulate voltages in an electrical circuit.
What is a microcontroller?
0 notes
Text
Price: [price_with_discount] (as of [price_update_date] - Details) [ad_1] Product Description Storite 3 in 1 Screen Cleaning Kit with Microfiber Cloth and Brush for Electronic Screens (100 ml) Brand: Storite Microfiber cloth Size - 13cm x 13cm. Storite KCL-1005 dust free screen cleaning kit has the ultimate essentials for your electronic cleaning needs and provides an effective and safe way to clean your electronic devices. Conventional screen cleaners use alcohol and other chemicals which can resonate into electronic screens causing fading and depreciation. Our non-streak cleaner boasts an odorless, non-toxic solution.Our solution has been tested and is non-static, non-streak and free of alcohol and other toxic substances. Just spray the cleaner onto the included microfiber cleaning cloth, and say goodbye to dust, stains and fingerprints.Screen cleaner can not only removes fingerprints, oil and smudges, but also leaves a layer of ultra-thin coating on your screen. It resists fingerprint and being scratched, easier to clean and looks great Product Specification: 1. Could Clean The Dust Most Effectively and Completely, Without any Water Track, no cauterization, Cleaner Can Keep the LCD Screen Bright and Clean Like new one, There will be s film on the surface of LCD screen after use this Cleaner, to Keep Screen anti-static and dustproof. 2. The Superfine fiber cloth was made of special high-technology, can clean the oleic dust out. As the fibers are so thin to increase the surface area, can absorb more water and stubborn dust. dust together with special cleaner can clean up stubborn dust better. Easy To Use: Can be used to clean the TFT display, screen, notebook screen, PDA Screen, Printer Screen and CD safely. Will be no scratch and track left and won’ t hurt the screen coat. Remove Germs, Bacteria, Fingerprints: This Screen Cleaning kit includes a variety of cleaning tools to make sure that your DSLR, compact camera or action camera is always spotless and ready to use. MULTI-PURPOSE. Ideal for touchscreen mobiles, mac, android tablets, and PC monitors, even your spectacles! MMicrofiber cleaning cloths and lens tissues: Both of these items can be used to gently remove dust and dirt from your lens. Microfiber cloth Size - 14.
5cm x 14.5cm. Can Clean all kinds of cd,fold the Cloth and Clean the CD With The Center Of cloth, Clean The CD from the Center to the edge, Without any scratch and dust left. Liquid Volume 120 ML 100 ML 200 ML 100 ML Micro Fiber Cloth ✓ ✓ ✓ ✓ Dust Blower ✓ NO NO NO Pack 1 1 2 2 Solution Bottle Capacity: 100 ml Compatible Devices: LCD, LED TV, Laptops and All Electronic Screens This LCD Cleaning Kit cleans Messy Finger Prints, Dirt and Dust, Stains. Gives a good clean finish afterwards The Kit contains special liquid, special cloth and soft brush to clean corners and sides of the screen [ad_2]
0 notes
Text
IIT JAM Chemistry Syllabus 2022 [Updated]

IIT JAM is an examination that is conducted on a national level. The examination aims to shortlist candidates for MSc or integrated MSc programs. Institutes accept candidates into their courses through the IIT JAM examination. The test is conducted jointly by IITs and IISC. The examination is conducted by colleges that take rotational turns.
Candidates who take the IIT JAM examination can secure a seat at IISC, IITs, NITs, or CFITs. Qualifying this examination helps a student to get admission to postgraduate courses.
After completing the program successfully, candidates can get any job they aspire to in their respective specialization areas.
Students who want to pursue their post-graduation in Chemistry must be well-versed with the subject’s syllabus. Along with that, candidates must know how to prepare and not burn themselves out in the process.
In this article, we discuss IIT JAM Chemistry Syllabus. Apart from it, we discuss preparation tips as well as books that you might want for reference.
IIT JAM Chemistry Syllabus
1. Basic Mathematical Concepts
This topic includes functions; maxima and minima; integrals; ordinary differential equations; vectors and matrices; determinants; elementary statistics; and probability theory.
2. Atomic and Molecular Structure
This topic includes Fundamental particles, Bohr’s theory of hydrogen-like atoms; wave-particle duality; uncertainty principle; Schrödinger’s wave equation.
Along with the ones mentioned, you also have quantum numbers; shapes of orbitals; exclusion principle of Hund’s rule and Pauli’s; details of the electronic configuration of simple homonuclear diatomic molecules, etc.
3. Theory of Gases
The topic includes the following- Equation of state for ideal and nonideal gases; Kinetic theory of gases; distribution law of Maxwell-Boltzmann and concept of equipartition of energy.
4. Solid State
The topic includes the following- Crystals and crystal systems; X-rays; the structures of NaCl and KCl; close packing; the concept of atomic and ionic radii; radius ratio rules as well as lattice energy; Born-Haber cycle; isomorphism and heat capacity of solids.
5. Chemical Thermodynamics
The topic includes the following subtopics- Reversible and irreversible processes, the concept of first law and its application to ideal and nonideal gases, thermochemistry, the second law is in detail, Entropy and free energy, and criteria for spontaneity.
6. Chemical and Phase Equilibria
The topic includes the following- Law of mass action; Kp, Kc, Kx and Kn; effect of temperature on K; ionic equilibria in solutions; pH and buffer solutions to hydrolysis; solubility product; phase equilibria–phase rule.
Also, its application to one-component and two-component systems; colligative properties, etc. all are included here.
IIT JAM Organic Chemistry Syllabus
1. Basic concept and Stereochemistry
This topic includes electronic effects that cover resonance, inductive, and hyperconjugation. Apart from the mentioned ones, you have got optical isomerism in compounds with and without any stereocenters such as allenes, biphenyls, etc.; the conformation of acyclic systems includes substituted ethane or n-propane or n-butane as well as cyclic systems containing mono- and di-substituted cyclohexanes, etc.
2. Organic Reaction Mechanism and Synthetic Applications
This topic includes the following- Chemistry of reactive intermediates covering carbocations, carbanions, free radicals, carbenes, nitrenes, benzynes, etc.; Hofmann-Curtius-Lossen rearrangement Wolff rearrangement, Simmons-Smith reaction.
It also includes Reimer-Tiemann reaction, Michael reaction, Darzens reaction, Wittig reaction, and McMurry reaction; Pinacol-pinacolone, Favorskii, benzilic acid rearrangement, dienone-phenol rearrangement, Baeyer-Villeger reaction, and many more.
3. Qualitative Organic Analysis
This topic includes the following- Different functional groups by chemical tests along with elementary UV, IR, and 1H NMR spectroscopic techniques as tools for structural elucidation.
Syllabus for IIT JAM Inorganic Chemistry
1. The Periodic Table
This topic includes the following- Element’s periodic classification and periodicity in all the properties along with general methods of isolation and the process of purification of elements. Everything related to the periodic table is essential. You must remember all the elements by heart.
2. Chemical Bonding and Shapes of Compounds
This topic includes different types of bonding, details of VSEPR theory and shapes of molecules, hybridization, and dipole moment. It also defines ionic solids and the structure of NaCl and CsCl, diamond and graphite, lattice energy, etc.
3. Main Group Elements, especially for s and p blocks
This topic includes the following- Basic concepts on group relationships and gradation in properties; formation of electron-deficient compounds containing some elements from the primary group.
Some preparation tips for IIT JAM
Before you start preparing for your IIT JAM Chemistry paper, why don’t you go through some of the preparation tips?
1. You can start with the syllabus and exam pattern
Before beginning with the IIT JAM Chemistry preparation, you should know the complete syllabus. Knowing all the topics will help you to plan accordingly. The more clarity you have about the IIT JAM Chemistry Syllabus, the more precise you will be about proceeding with the preparation plan
2. Go through the previous year question papers
To have a grasp on your content, practicing questions is very important. So you must refer to the previous year’s question papers. Doing a lot of it can help you understand the question pattern, assess the difficulty levels, and the like.
Moreover, you will be able to identify both your weak and strong points. And this, in turn, will help you to improve a lot!
You can sign up for any coaching center that provides good guidance. It is a good idea to join some coaching institutes.
These institutes make sure to keep you in a proper drill. Such coaching centers are excellent, from conducting regular tests to updating students with all the latest news and changes.
These mock tests are helpful as you can evaluate yourself with respect to others as well as understand where you stand.
3. While studying, make sure to make notes for later reference
Making notes while you study is always recommended by teachers. You make notes that can come in handy for later use. If it is in your own language you can understand better. Also, you can highlight certain important portions which can be useful for later reference.
4. Do not jump from one chapter to the other without finishing the first one. Be systematic
You must have a systematic approach to your preparation. Make sure to complete a topic before going on to the next. Be sure what you want to start with. It is important that you plan and proceed.
5. Revise before you move on to the next the topic
Revision plays quite an important part in preparing for the IIT JAM examination. Once you are done with a topic or a chapter, revise it or practice active recall.
It is necessary to boost your confidence as well as to test your preparation and memory.
6. Last but not least, do not forget to take some time off and rest one a while. Remember, health is wealth!
Make sure you take adequate rest. You do need to have dedicated preparation for your entrance examination, but you must take some time off. Otherwise, you might have burn-out symptoms.
You might feel anxious, lethargic, nervous, on the edge, irritated, etc. And all these are likely to affect your health as well as your academics. So, it is better that you take good care of both your mental and physical health.
0 notes
Text
A Handy Buying Guide for Water Softener Solution
Hard water, which is very common in India, results in multiple problems. While many people use conventional methods of making hard water soft, these methods do not provide the expected results. A better and more productive substitute is to go for a water softener, which makes hard water soft automatically. This article will help you with all the necessary information that you have to know while buying a water softener solution.
What Is Hard Water?
The hardness of water depends on the number of GPG present in water. Sometimes, the hardness of water is also measured by the PPM of minerals. Water having more than 1 GPG of dissolved minerals like salts, magnesium, carbonate or manganese is hard water. However, in a stainless steel water tank, if the water contains up to 3.5 GPG of minerals, a water softener is not required.
Problems of Hard Water
Hard water affects your household stuff like plumbing fixtures, pottery, washroom tiles and clothes. Besides impacting your household stuff, hard water also affects your health. Hard water may result in diseases like diabetes, eczema, constipation, kidney stones, etc. Considering these issues, it is important to install a water softener to enhance water quality, provide the best water softener solution and make it perfect for use.
Types of Water Softener
Though there are many water softeners available, ion exchange water softeners are the leading water softener filter in India.
Ion Exchange Water Softeners: These are connected to the plumbing systems of your house. The water softeners also have salt that replaces magnesium, calcium and other hard minerals that make water hard. The water softeners include resin beads which soften hard water by exchanging excess magnesium, calcium and iron with salts.
Salt-Free Water Softeners: These softeners use KCl to make water soft. Though these are not as productive as the previous one, salt-free water softeners are best for those concerned about sodium intake. The water softener does not lessen the minerals that make water hard. Instead, it stops mineral deposits on the surface of the appliances.
Magnetic & Electronic Water Softeners: These are usually descalers and reverse the electromagnetic properties of minerals that make water hard. The process makes sure that the water pipes stop the hard minerals from settling to keep away from damage.
Dual Tank Water Softeners: Recharging the water softener is important to make sure it works steadily. However, while in the recharging phase, the water softeners stop working. These water softeners have two tanks that function alternatively whenever recharging is needed.
Detecting the Hardness of Water
There are some basic signs of detecting hard water. Whenever you observe stains on your sinks, washroom tiles or if your bathroom units need usual repair, it specifies that you get hard water. Besides observing the signs, you also have to get the water quality tested to know the level of hardness in the water.
Features of Water Softener
Before buying one, make sure that you think about the features and controls of the water softener. Look into the programming levels, settings in the menu and display options before buying. Moreover, you must check whether the water softener has automatic regeneration for hassle-free operation.
To Conclude
Check out the best range of best water softeners in the market and buy one for your home considering the factors mentioned above.
0 notes
Text
How To Find Lattice Energy 103
The formula for ionic lattice energy. You can try to use it.

How To Find Lattice Energy Born Haber Cycle
You can even get the proper results.

How to find lattice energy. Lattice energy is inversely proportional to the radius of the ions. This lattice energy formula is as follows: The lattice energy is exothermic, i.e., the value of δh lattice is negative because it corresponds to the coalescing of infinitely separated gaseous ions in vacuum to form the ionic lattice.
Finally calculate the lattice energy. It may or it may not work correctly. The lattice energy is defined as the change in enthalpy for the reverse reaction, rbcl (s) ?
Δ g h denotes the molar lattice enthalpy. I take you through the basic types of chemical bonds, and then show you how to calculate lattice energy with 3 example problems. Lattice energy of ionic compounds, how to find ionic energy of.
For nacl(s) sample exercise 11.2. Lif has a lattice energy of 1030 kj/mol, naf has a lattice energy of 910 kj/mol. Recall that lattice energy is the energy required to combine two gaseous ions into a solid ionic compound:
Arranging the above equation to find lattice energy, we get; The lattice enthalpy is reported as a positive value. Which ionic compound would be expected to have the highest lattice energy?
The more charge, the greater the lattice energy when they join with a suitable negative ion. The size of magnesium ion is less than calcium ion. Lattice energy refers to the energy that is released when two oppositely charged gaseous ions attract to each other and form an ionic solid.
22 related question answers found The attraction of the two ions releases energy and the process is exothermic. How do you find the lattice energy of mgf2?
We’re being asked to calculate the lattice energy of calcium chloride (cacl 2). • the lattice energy is proportional to the charge/distance. Lattice energy for lif = −1016 kj cycle 2 na (s) na (g) δh sub = 108 kj na (g) na+(g) + e− 1st ionization energy of na = 496 kj ½ cl 2 (g) cl (g) ½ bond energy of cl = 122 kj cl (g) + e− electron affinity for chlorine = cl− (g) −349 kj na+ (g) + cl− (g).
We find the ions for this example to be at a distance of 3 nm. Lattice energy can be a very complicated process but is often simplified by using coulomb’s law. Δ g u denotes the molar lattice energy.
•the sizes of the ions change and hence the distance. As you move down a group eg alkali metals, the ions get larger. Sublimation energy of `na = 26 kcal//g`atom, di asked jul 4, 2019 in chemistry by siddhichawla ( 59.5k points)
Beta version # beta test version of this item this online calculator is currently under heavy development. Lattice energy (e) increases as q increases and/or as r decreases. By comparing the lattices of substances from the same group or period, the lattice energy trend may be determined.
This causes the lattice energy (when they join to a suitable negative ion) to decrease down the group. •the same reasoning tells us that nacl has a larger. Looking for college credit for chemistry?
Table shows lattice crystal energy in kj/mol for selected ion compounds. Lattice energy (u) is the energy required to completely separate one mole of a solid ionic compound into gaseous ions. Licl = 800 nacl = +780 kcl = +710 kbr = 680.
This is possible to make the lattice energy when two oppositely charged ions in gaseous state attract each other and responsible to make the ionic solid ahead. the lattice energy of an ionic solid is a measure of the strength of bonds in that ionic compound. The molar lattice energy of an ionic crystal can be expressed in terms of molar lattice enthalpy, pressure, and change in volume via the following equation:
Hence, the lattice energy of magnesium oxide is the highest and that of lithium fluoride is the lowest. We can compute the lattice energy of nearly any ionic solid by using a modified form of coulomb’s law. Δv m is the change in volume (per mole).
The negative sign of the energy is indicative of an exothermic reaction. To completely remove the constituent ions from its crystal lattice to an infinite distance from one of crystal, some amount of energy is. Na+ (g) + cl− (g) → nacl (s) the experimental lattice energy of nacl is −787 kj/mol.
As you move across the table, positive ions become more charged eg na+,mg2+, al3+. It is usually defined as the enthalpy of formation of the ionic compound from gaseous ions and as such is invariably exothermic.
0 notes