#multicenter study
Explore tagged Tumblr posts
Text
youtube
#Sepsis in cancer#epidemiology#prognosis#multicenter study#prospective observational study#critical care#oncology patients#sepsis mortality#infection control#biomarkers#ICU admissions#microbial patterns#treatment outcomes#inflammatory response#cancer prognosis#immunosuppression#early detection#precision medicine#survivorship#clinical management.#Youtube
1 note
·
View note
Text
Only in the last decade, with global surveillance and advanced genomics, has the relative importance of various pathogens been reliably measured. The Global Enteric Multicenter Study, completed in 2013, was the largest-ever study of the global diarrhea burden.
"Plagues Upon the Earth: Disease and the Course of Human History" - Kyle Harper
#book quote#plagues upon the earth#kyle harper#nonfiction#passage of time#decades#10s#2010s#21st century#surveillance#genomics#pathogen#global enteric multicenter study#diarrhea#feces
0 notes
Text
Abstract Background: Women are more at risk for developing long-term symptoms after a COVID-19 infection. Only limited data are available for patients with coexisting endometriosis and/or menstrual pain symptoms.
Study Design: A total of 840 premenopausal women with menstrual pain and/or endometriosis were included in this observational cross-sectional study using an online survey platform.
Results: A total of 840 women with menstrual pain (mean age 30.7 ± 6.9, 15–54 years) were studied. Of these, 714 (84.2%) had a COVID-19 infection, 123 did not (14.5%). A total of 312 subjects had acute COVID-19 (AC) with symptoms ≤4 weeks (43.7%), 132 (18.5%) developed postacute COVID-19 syndrome (PC), and 88 (12.3%) had “long Covid” (LC). There were no statistical differences regarding number of vaccination shots between the three groups AC, PC, and LC. A total of 582 patients with surgically confirmed endometriosis (SCE) showed a twofold increased risk of LC [odds ratio (OR): 2.12, 2.18–3.84] in comparison with AC subjects. In SCE the comorbidity anxiety disorder (OR: 2.08, 1.14–3.81) and depression (OR: 2.02, 1.15–3.56) further increased the risk of LC. LC subjects had a significantly higher disturbance level of menstrual pain (p = 0.002), were more restricted in job (p < 0.001), leisure (p = 0.002), and family activities (p < 0.001), and had a higher number of endometriosis surgeries (p = 0.003).
Conclusion: Subjects with SCE had a twofold increased risk of LC (in comparison to subjects with nonconfirmed endometriosis menstrual pain). In patients with SCE concomitant diagnosis of depression or anxiety disorder further twice-fold increased risk of LC. Further studies are needed if it is possible to reduce LC risk by improving the treatment of those secondary diagnoses and whether the type of endometriosis treatment can reduce LC occurrence (holistic, coanalgetic, hormonal).
#covid#mask up#pandemic#wear a mask#covid 19#coronavirus#public health#sars cov 2#still coviding#wear a respirator#endometriosis#long covid#covid conscious#covid is not over#covidー19#covid is airborne#covid isn't over#covid pandemic#covid19
64 notes
·
View notes
Text
7 notes
·
View notes
Text
University of Florida develops AI-powered MRI tool to improve Parkinson’s diagnosis

- By InnoNurse Staff -
University of Florida researchers have developed Automated Imaging Differentiation for Parkinsonism (AIDP), a machine-learning method using MRI to accurately distinguish Parkinson’s disease (PD) from atypical parkinsonian disorders such as multiple system atrophy (MSA) and progressive supranuclear palsy (PSP).
In a multicenter study involving 249 prospective and 396 retrospective cases, AIDP analyzed diffusion MRI data from 132 brain regions, achieving 96–98% accuracy in differentiating PD, MSA, and PSP.
Compared to traditional clinical methods, AIDP improved diagnostic accuracy by 12.3%, reaching 93.9% accuracy against autopsy-confirmed cases.
Its non-invasive, scalable approach could enhance clinical care and complement existing biomarkers.

Image: Automated Imaging Differentiation for Parkinsonism (AIDP) for the precise classification of parkinsonism subtypes. Credit: JAMA Neurology (2025). DOI: 10.1001/jamaneurol.2025.0112.
Read more at Tech Xplore
///
Other recent news and insights
Researchers urge reproductive health apps to enhance data protection for users (CUNY SPH)
Polish healthtech company Jutro Medical secures €12M to develop its telehealth platform (Tech.EU)
2 notes
·
View notes
Text
Researchers in Germany have issued an alert after discovering a “striking” safety signal among children who received Covid mRNA “vaccines.”
They found that alarming numbers of vaccinated children have been impacted with disturbing psychological, pulmonary, gastrointestinal, neurological, and dermatological side effects.
Specifically, the researchers warn that children aged between 5 and 11 who received the Pfizer-BioNTech BNT162b2 Covid mRNA “vaccine” face significant risk.
The findings were revealed during a study led by Sarah Holzwarth at the Department of Pediatrics, Faculty of Medicine, University Hospital Carl Gustav Carus, Technische Universität Dresden in Germany.
The study’s paper was published in the Springer Nature Infection journal.
The researchers conducted the study to track the safety of the Pfizer mRNA injection in children with and without comorbidities aged 5 to 11 years.
This prospective, multicenter, industry-independent cohort study involves caregivers who are vaccinated.
The caregivers participated in a robust, well-designed online questionnaire.
Potential side effects were evaluated in ten organ-related categories.
2 notes
·
View notes
Text
Sociodemographic, economic, and academic factors linked with resilience in university students during covid-19 pandemic: a Brazilian cross-sectional study
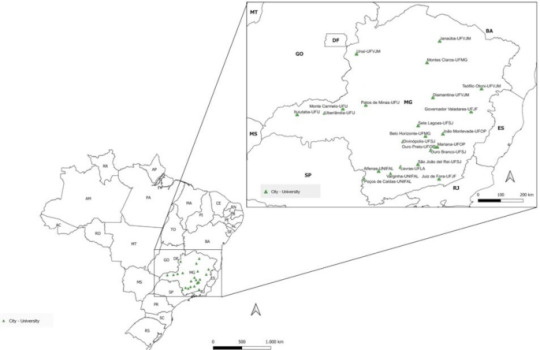
Background
Resilience is a crucial factor in students’ mental health, playing an important role in their successful adaptation to the academic environment. However, there is a lack of understanding about resilience and its associated factors in students from different undergraduate courses. This study aimed to describe the resilience profile of undergraduate students from various courses in Brazil and identify sociodemographic, economic, and academic factors associated with resilience.
Methods
This study has data from a cross-sectional multicenter study involving undergraduate students from eight Federal Institutions of Higher Education in Minas Gerais, Brazil. The dependent variable was resilience, measured using the Connor-Davidson Resilience Scale (CD-RISC) 10-item version, with sociodemographic, economic, and academic factors considered independent variables. Data was collected virtually via a self-administered questionnaire between October 2021 and February 2022 (during the covid-19 pandemic). Independent samples t-tests and ANOVAs were conducted to compare resilience scores between independent variables, and Tukey’s post-hoc test was performed when necessary. Multiple linear regression was performed to create three models.
Results
8,650 undergraduate students were included in this study. The average score on the resilience scale was 19.86 ± 8.15, with a normal distribution. The respondents ranged from 18 to 71 years old, averaging 23.9 ± 6.33. Being female, not having a religious belief, having low per capita family income, having had a decrease in the family income, not being heterosexual, or having the head of the family with a low education level were the main factors associated, individually, with low resilience scores in the sociodemographic and economic multiple linear regression model constructed. Being from linguistics, letters and arts courses, being enrolled in fewer subjects, or being from UFMG were the main factors associated, individually, with low resilience scores in the academic multiple linear regression model constructed.
Conclusions
The study’s findings revealed that sociodemographic, economic, and academic variables were significantly associated with resilience scores in undergraduates from Minas Gerais during the covid-19 pandemic. These findings can help universities develop target strategies to promote students’ resilience and reduce the risk of poor mental health among this population.
Read the paper.
2 notes
·
View notes
Text
Panax Notoginseng Saponins (PNS): Clinical Data Summary
Panax notoginseng saponins (PNS) are the primary active components extracted from the root or rhizome of Panax notoginseng (also known as Tianqi or Sanqi), containing various saponins such as ginsenosides Rg1, Rb1, Re, Rd, and notoginsenoside R1. Clinical research has primarily focused on its effects in cardiovascular and cerebrovascular diseases, anti-inflammatory activity, antioxidant properties, and tissue protection. Below is a summary of existing clinical data:
Cardiovascular and Cerebrovascular Diseases
(1) Ischemic Stroke
Clinical Studies:
Multiple randomized controlled trials (RCTs) indicate that PNS (e.g., Xuesaitong Injection or oral formulations) combined with conventional treatment (e.g., aspirin) can improve neurological deficit scores (NIHSS) and daily living ability (Barthel Index) in stroke patients, with significantly higher efficacy than control groups (PMID: 32350721).
Mechanism: Inhibits platelet aggregation, improves microcirculation, and reduces ischemia-reperfusion injury.
(2) Coronary Heart Disease/Angina Pectoris
Meta-analysis (including dozens of RCTs):
PNS injections (e.g., Xueshuantong) can reduce the frequency of angina attacks and improve ECG ST-T segment changes (total effective rate ~70-85% vs. 50-65% in control groups) (PMID: 31568905).
Potential mechanisms include coronary artery dilation and antithrombotic effects.
(3) Recovery After Cerebral Hemorrhage
Small-scale trials suggest PNS may promote hematoma absorption, but more high-quality evidence is needed (controversial).
Antithrombotic and Microcirculation Improvement
Clinical Observations:
PNS inhibits platelet-activating factor (PAF) and thrombin, reducing blood viscosity, and is used for hypercoagulable states or post-surgical thrombosis prevention (some studies show comparable efficacy to low-molecular-weight heparin).
Other Clinical Applications
(1) Diabetic Complications
Adjunctive therapy for diabetic peripheral neuropathy (improving numbness and pain) and retinopathy (delaying microvascular damage).
(2) Anti-inflammatory and Antioxidant Effects
In chronic kidney disease and pulmonary fibrosis, PNS may reduce inflammation by inhibiting the NF-κB pathway (limited clinical data).
(3) Wound Healing
Topical application (e.g., gel formulations) may accelerate wound healing and reduce scarring (supported by animal studies, limited human trials).
Safety Data
Adverse Reactions:
Generally well-tolerated; mild side effects include dizziness, gastrointestinal discomfort, or injection-site pain.
Injections may cause allergic reactions (e.g., rash, dyspnea)—close monitoring required.
Contraindications:
Use with caution in patients with bleeding disorders, perioperative periods, or pregnancy (due to antiplatelet effects).
Controversies and Limitations
Quality of Evidence:
Most studies are from China, with limited international recognition; some trials have small sample sizes or design flaws (e.g., lack of double-blinding).
Unclear Mechanisms:
While in vitro/animal studies suggest multi-target effects, pharmacokinetics and exact mechanisms in humans require further validation.
Preparations and Dosage
Common Products:
Xuesaitong (injection/lyophilized powder), Xueshuantong capsules, Compound Danshen Dripping Pills (containing PNS).
Dosage:
Oral: Typically 100-200 mg/dose, 2-3 times daily; injections require medical supervision.
Conclusion
PNS demonstrates potential efficacy in adjuvant therapy for cardiovascular and cerebrovascular diseases (especially stroke and angina), but more high-quality international multicenter studies are needed. It should be used under medical supervision to avoid bleeding risks when combined with anticoagulants.

Panax notoginseng saponins
0 notes
Link
#AIdiagnostics#algorithmicbias#diabeticretinopathy#EU-AIAct#FDAclearance#MedicalImaging#ophthalmology#Telemedicine
0 notes
Text
Top International CME Conferences 2025 for Physicians
Continuing Medical Education is essential for physicians aiming to stay at the forefront of clinical practice. The most prestigious cme conferences 2025 offer immersive experiences in global medical hubs, featuring leading experts, groundbreaking research, and hands-on workshops. Cell Surgical Conference curates guidance on selecting conferences that align with your specialty and career goals. From groundbreaking cardiology insights in London to innovative oncology panels in Dubai, understanding which events deliver the greatest educational value ensures you invest your time and resources wisely.
How CME Conferences 2025 Enhance Clinical Knowledge Globally?
Physicians attending top CME Conferences 2025 gain exposure to cutting-edge studies that refine diagnostic accuracy and treatment protocols. Speakers present peer-reviewed data on emerging therapies, while interactive sessions allow you to discuss case studies with global thought leaders. Cell Surgical Conference emphasizes evaluating conference agendas for balanced scientific depth and practical takeaways. Networking over coffee breaks often sparks collaborative research ideas, while poster sessions showcase innovations before they reach mainstream journals.
CME Conferences 2025 Offer Cutting-Edge Research in Medicine
Leading CME events in 2025 spotlight breakthroughs in fields like immunotherapy, gene editing, and digital health. Detailed lectures dissect trial methodologies and statistical analyses, ensuring you critically appraise new findings. Cell Surgical Conference recommends identifying sessions that align with your subspecialty—whether that’s endocrinology or interventional cardiology. Access to unpublished data and early-stage drug development panels allows you to adopt promising interventions ahead of peers.
Networking Opportunities Abound at CME Conferences 2025 Events
Beyond academic sessions, cme conferences 2025 thrive on interpersonal connections. Formal networking lunches, roundtable discussions, and evening receptions facilitate dialogue with renowned clinicians and industry leaders. Cell Surgical Conference underscores the importance of pre-conference planning: review attendee lists and schedule one-on-one meetings. These interactions often lead to mentorship opportunities, multicenter trial collaborations, or guest lecture invitations. Strong professional networks not only enrich your knowledge but also open avenues for leadership roles and international speaking engagements.
Hands-On Workshops Feature in CME Conferences 2025 Programs
Practical skills development is a highlight of top cme conferences 2025. Workshops on ultrasound-guided procedures, robotic surgery simulators, or advanced suturing techniques provide supervised, hands-on experience. Cell Surgical Conference notes that these sessions bridge theory and practice, bolstering your competence before returning to the clinic. Small group sizes ensure personalized feedback, while state-of-the-art equipment replicates real-world settings.
CME Conferences 2025 Include Interactive Multi-Specialty Sessions and Panels
Interdisciplinary panels at cme conferences 2025 bring together cardiologists, endocrinologists, and primary care physicians to tackle overlapping clinical challenges. Case-based discussions encourage debate on best practices for complex comorbidities, such as diabetes with cardiovascular disease. Cell Surgical Conference highlights the value of these sessions in broadening your perspective and fostering holistic patient care.
Technological Innovations Showcased at CME Conferences 2025 Forums
Emerging technologies—AI diagnostics, telemedicine platforms, and wearable health trackers—are prominent at cme conferences 2025. Exhibitors demonstrate how these tools integrate into clinical workflows, offering practical tutorials and real-time data analytics. Cell Surgical Conference recommends attending tech-focused symposia and visiting innovation booths to evaluate gadgets firsthand. Early adoption of validated technologies can streamline patient monitoring, reduce administrative burden, and support precision medicine initiatives in your practice.
Accreditation Benefits from CME Conferences 2025 Attendance Validated
Earning CME credits is crucial for licensure maintenance and board certification. Top cme conferences 2025 offer dual accreditation from multiple medical councils, ensuring broad credit acceptance. Cell Surgical Conference advises checking each conference’s accreditation status before registration. Some events also provide digital badge systems, allowing you to track credit accrual effortlessly. Accumulating credits through high-impact conferences signals your dedication to lifelong learning and excellence, enhancing your professional reputation and career advancement prospects.
Conclusion
Planning your 2025 continuing education around premier cme conferences 2025 empowers you to stay informed, skilled, and connected within the global medical community. By focusing on research depth, hands-on workshops, interdisciplinary dialogue, and technological innovation, you maximize the return on your time and investment. Cell Surgical Conference emphasizes choosing accredited events that align with your specialty and career aspirations. Through strategic selection and active participation, you not only earn valuable CME credits but also foster collaborations, adopt the latest clinical advances, and reinforce your commitment to delivering exceptional patient care.
0 notes
Text
Mastering the Phlebotomist Order of Draw: Essential Techniques for Accurate Blood Collection
Mastering the Phlebotomist Order of Draw: Essential Techniques for Accurate Blood Collection
The art of phlebotomy is a critical skill that healthcare professionals must master, especially the order of draw. This procedure guarantees the integrity of blood samples, helping clinicians make accurate diagnoses and provide effective treatment. In this comprehensive guide, we will explore everything you need to know about the phlebotomist order of draw, including essential techniques, benefits, practical tips, and real-world experiences.
Understanding the Importance of Order of Draw
The order in wich blood samples are collected is vital for minimizing contamination across the tubes used in tests. Different additives in blood collection tubes can interfere with test results if they mix. Understanding the order of draw can prevent these issues, ensuring the highest quality of patient care and accurate laboratory results.
Standard Order of Draw for Blood Collection
The standardized order of draw for blood collection is as follows:
Tube Color
Additive
Common Uses
1. Yellow
Sodium Polyethanol Sulfonate
Blood cultures
2. Light Blue
Citrate
Coagulation studies
3. Red
No additive
Seroogy,blood bank
4. Gold or Tiger Top
Gel separator
Serum tests
5. Green
Heparin
Stat tests, chemistry tests
6. Lavender
EDTA
Complete blood count, blood smears
7. Gray
Fluoride
Glucose testing
Essential Techniques for Accurate Blood Collection
1. Preparation
Verify the patient’s identity using at least two identifiers.
Assemble all necessary supplies before starting the procedure.
Ensure proper patient positioning — usually sitting or lying down may reduce the risk of dizziness or fainting.
2. Site Selection and Cleansing
Choose a suitable vein, typically in the antecubital fossa.
Clean the venipuncture site with an antiseptic swab in a circular motion to reduce the risk of contamination.
Allow the site to dry completely before inserting the needle.
3. Performing the Venipuncture
Hold the needle at a 15-30 degree angle to the skin for effective insertion.
Gently engage the vein and adjust the needle as necessary.
Follow the order of draw strictly, switching tubes without excess movement to maintain blood integrity.
4. Post-Collection procedures
Apply pressure to the collected site and place a bandage onc bleeding stops.
Label tubes correctly and instantly after collection to prevent mix-ups.
Transport samples promptly to the lab, maintaining the correct temperature and conditions.
Benefits of Mastering the Order of Draw
Accuracy: Following the correct order substantially minimizes the chances of cross-contamination.
efficiency: Reduces the need for re-collections, saving time for both healthcare providers and patients.
Patient Trust: Demonstrates skill and attention to detail, enhancing the patient experience and trust in the clinical process.
Practical Tips for Phlebotomists
Here are some practical tips for phlebotomists to enhance their blood collection skills:
Stay updated with guidelines from the Clinical and Laboratory Standards Institute (CLSI).
Practise proper hand hygiene and sanitization techniques regularly.
Participate in ongoing training to refine your techniques and learn about new developments in phlebotomy.
Communicate clearly with patients to help alleviate anxiety and build rapport.
First-Hand experience: A Case Study
Meet Sarah, a dedicated phlebotomist with over five years of experience. One day, she collected samples for a multicenter trial involving numerous tests. Remembering to adhere to the order of draw was crucial to Sarah. She carefully sequenced her collection, successfully avoiding cross-contamination between samples.
Later, when the lab results came in, they reflected accurate values, with no re-tests required. Sarah received compliments from her supervisor, highlighting the importance of the order of draw in ensuring the reliability of clinical data. Her experience reiterated how mastering this fundamental skill can significantly impact patient care and laboratory efficiency.
Conclusion
Mastering the phlebotomist order of draw is essential for anyone involved in blood collection. By understanding the correct sequence, preparing diligently, and applying the techniques outlined in this guide, healthcare professionals can ensure accurate testing and enhance patient trust. The art of phlebotomy goes beyond simply drawing blood; it’s about providing quality care and reliability in healthcare practices. Equip yourself with these skills, and embody the role of a competent and confident phlebotomist who makes a real difference in patient outcomes.
youtube
https://phlebotomycareertraining.net/mastering-the-phlebotomist-order-of-draw-essential-techniques-for-accurate-blood-collection/
0 notes
Text
Biobanking: The Future of Precision Medicine with the aid of Clinfinite Solutions

In the swiftly advancing landscape of healthcare and clinical studies, biobanking has emerged as a foundational pillar using innovation, discovery, and personalised medicine. At Clinfinite Solutions, we appreciate the crucial function biobanking plays in bridging the gap between today’s clinical understanding and tomorrow’s scientific breakthroughs. This weblog explores the idea of biobanking, its significance, applications, and the way Clinfinite Solutions is leveraging this powerful device to revolutionize the world of healthcare.
What is Biobanking?
Biobanking refers to the approach of accumulating, processing, storing, and coping with natural samples, which consist of blood, tissues, DNA, and one-of-a-type physical fluids to be used in studies and clinical studies. Those samples are meticulously cataloged and maintained in specially managed environments to ensure long-term viability and report integrity.
Each sample saved in a biobank is commonly related to comprehensive affected person statistics, which include medical information, way of life, demographics, and genetic information. This holistic repository permits researchers to conduct enormous studies on sickness development, remedy efficacy, genetic tendencies, and much more.
The Role of Biobanking in Modern Medicine
The significance of biobanking extends a long way beyond storage. It serves as a cornerstone for several medical and medical endeavors:
Disease Research: Biobanks offer the organic substances crucial for investigating the causes and development of diseases, consisting of most cancers, diabetes, Alzheimer’s, and rare genetic issues.
Personalized Medicine: Access to properly-annotated biological samples allows researchers to pick out biomarkers and tailor treatments based on the person affected.
Drug Development: Pharmaceutical businesses depend on biobanks to test drug responses and reduce risks in early-stage trials.
Public Health and Epidemiology: Biobanks help track the spread of infectious illnesses and screen vaccine responses across populations.
At Clinfinite Solutions, our biobanking offerings are structured to assist all these essential regions, making us a trusted associate in each public and private sector research.
How Clinfinite Solutions Enhances Biobanking
As a forward-thinking scientific research agency, Clinfinite Solutions integrates era, fine assurance, and regulatory compliance to supply industry-leading biobanking services. Here's how we stand apart:
1. Advanced Sample Management
Our modern-day biobank facilities use computerized structures and easy data platforms to manipulate samples with precision. Temperature-managed environments, real-time tracking, and barcode-based inventory ensure pattern integrity from collection to retrieval.
2. Ethical and Regulatory Compliance
Ethics and privacy are non-negotiable in our biobanking system. All pattern collections at Clinfinite Solutions are conducted below strict knowledgeable consent protocols, making sure participant rights are upheld. We follow international standards, including ICH-GCP, GDPR, and ISO 20387.
3. Custom Biobanking Solutions
From single-site studies to multicenter trials, our biobanking services may be customized to meet the precise needs of researchers, academic establishments, hospitals, and pharma corporations. Whether it’s long-term storage, sample cargo, or fact analytics, we provide give up-to-give up support.
Applications of Biobanking Across Industries
The versatility of biobanking has led to its integration across numerous sectors:
Academic Research: Universities use biobanks to conduct groundbreaking studies in genomics and molecular biology.
Healthcare Providers: Hospitals integrate biobanks to personalize patient care and create focused remedy protocols.
Pharmaceuticals: Drug development pipelines increasingly depend on biobank assets for global evidence and pharmacogenomics.
Public Health Agencies: Biobanks help prepare for and respond to pandemics by storing viral traces and affected person samples for destiny analysis.
Clinfinite Solutions actively collaborates with partners in each of these domains, maximizing the price and effect of every saved specimen.
Challenges in Biobanking – And Our Solutions
Despite its mammoth capacity, biobanking comes with its share of challenges—sample degradation, data protection, ethical dilemmas, and logistical hurdles. Clinfinite Solutions addresses those demanding situations head-on with:
Cutting-side preservation technology
Robust records encryption and cybersecurity
Transparent ethical governance
Expert logistical coordination for pattern shipping
These practices ensure the very best requirements of sample great and reliability, empowering our partners to behavior impactful, reproducible studies.
The Future of Biobanking: AI, Big Data, and Beyond
As we appear in advance, biobanking is set to evolve along with improvements in artificial intelligence, machine learning, and big data analytics. This technology will allow researchers to extract deeper insights from biological samples and associated metadata, opening new frontiers in diagnostics and therapeutics.
Clinfinite Solutions is actively making an investment in digital equipment and structures in an effort to combine biobanking with predictive modeling and international information. Our aim is to create smarter, extra responsive study ecosystems where organic samples are not simply stored, but activated for discovery.
Conclusion: Partner with Clinfinite Solutions for Biobanking Excellence
In a world where customized healthcare and statistics-driven studies are becoming the norm, biobanking stands as a transformative resource. Clinfinite Solutions is proud to be at the forefront of this evolution, offering at-ease, scalable, and technology-sponsored biobanking offerings to drive innovation across healthcare, academia, and life sciences.
Whether you are engaging in a scientific trial, developing a new remedy, or exploring genomics, Clinfinite Solutions is your dependable partner in building and managing a biobank that meets the highest medical and moral standards.
Read More:
Value Of Clinical Development
Clinical Trials Near Me
#healthcare technology companies#specimen collection in healthcare#clinfinite solutions#value of clinical development#blood collection methods#clinical research jobs in hyderabad#clinical research specialists#biobanking#clinical care solutions#sample collection tubes
0 notes
Text
Reference preserved in our archive (Daily updates!)
This study shows the importance of masking and distancing not just on covid, but all common respiratory infections. Even if we're wrong about covid, we're right about 7 other diseases. Mask up. Keep everyone more healthy.
Abstract Background. Microbiologic confirmation of respiratory tract infections gained importance during the coronavirus disease 2019 (COVID-19) pandemic. This study retrospectively evaluated seasonal distribution, clinical presentation, and complications of respiratory viral infections (RVIs) other than COVID-19 in children with cancer during and after the pandemic lockdown.
Methods. Two hundred and sixty-five inpatient and outpatient RVI episodes in 219 pediatric cancer patients confirmed by multiplex reverse transcriptase polymerase chain reaction (RT-PCR) panels from 13 centers were enrolled.
Results. Eighty-six (32.5%) of the total 265 episodes occurred in 16 months corresponding to the lockdowns in Türkiye, and the remaining 67.5% in 10 months thereafter. Human rhinovirus/enterovirus (hRE) (48.3%) was the most common agent detected during and after lockdown. Parainfluenza virus (PIV) (23.0%), influenza virus (9.8%), and respiratory syncytial virus (RSV) (9.1%) were the other common agents. The 28.7% of episodes were lower respiratory tract infections (LRTIs), and complications and mortality were higher than upper respiratory tract infections (URTIs) (25.0% vs 5.3%). Bacteremia was identified in 11.5% of culture-drawn episodes. Treatment delay in one-third and death within four weeks after RVI in 4.9% of episodes were observed.
Conclusion. During the pandemic, fewer episodes of RVIs occurred during the lockdown period. Respiratory viruses may cause complications, delays in treatment, and even death in children with cancer. Therefore, increased awareness of RVIs and rapid detection of respiratory viruses will benefit the prevention and, in some cases, abrupt supportive and some antiviral treatment of RVI in children with cancer.
#mask up#covid#pandemic#wear a mask#public health#covid 19#wear a respirator#still coviding#coronavirus#sars cov 2#influenza#common cold#RSV
53 notes
·
View notes
Text
"Globalization of Clinical Research: Opportunities and Regulatory Challenges"
In the last few decades, the globalization of clinical research has significantly reshaped the way medical science evolves. What was once confined largely to a handful of developed nations is now a worldwide effort, spanning continents, cultures, and regulatory systems. This shift has opened up a host of opportunities while also bringing a set of complex regulatory challenges that must be navigated with caution, responsibility, and a patient-first approach.
The term clinical research refers to the scientific studies conducted to evaluate the safety, effectiveness, and benefits of medical treatments, interventions, or diagnostics. As the industry becomes more global, it is essential to understand what this globalization means for stakeholders—sponsors, regulators, investigators, and most importantly, patients.
🌍 Opportunities in Global Clinical Research
The globalization of clinical research has created numerous advantages that benefit both the scientific community and patients across the globe. Some of the most notable opportunities include:
1. Diverse Patient Populations
Conducting trials across multiple countries ensures access to a variety of genetic backgrounds, lifestyles, and environmental influences.
This diversity helps produce more reliable and generalizable results for global patient populations.
2. Faster Patient Recruitment
In regions where healthcare access is limited, clinical trials can be a way for patients to receive advanced therapies, resulting in quicker and more enthusiastic recruitment.
This helps reduce delays in drug development timelines.
3. Cost Efficiency
Running clinical trials in developing or emerging countries often lowers operational and logistical costs.
Cost savings can be reinvested into research and development, expediting drug innovation.
4. Accelerated Drug Approvals
Simultaneously conducting studies across different regions may speed up global regulatory approvals.
Companies are able to bring life-saving medications to market more efficiently.
5. Strengthened Global Collaboration
The collaborative nature of international trials encourages knowledge sharing between researchers across the globe.
This fosters innovation, capacity-building, and scientific progress that benefits all nations.
⚖️ Regulatory Challenges of Global Clinical Research
While the expansion of clinical research across borders presents exciting prospects, it also poses significant regulatory and ethical challenges. Every country has its own legal framework, and reconciling these differences is often complex.
1. Varying Regulatory Standards
Each country’s drug regulatory authority (e.g., FDA in the U.S., EMA in Europe, CDSCO in India) has distinct requirements and timelines.
Harmonizing documentation, ethics approvals, and safety reporting becomes a tedious and often slow process.
2. Ethical Considerations
Vulnerable populations in low-income countries may be at risk of exploitation if proper oversight isn't maintained.
Ensuring informed consent, post-trial access to medications, and fair compensation are critical ethical concerns.
3. Data Integrity and Quality Control
Ensuring consistent data collection and quality across multiple international sites requires robust monitoring systems.
Differences in infrastructure and training may lead to data discrepancies or non-compliance.
4. Intellectual Property and Data Privacy
Countries vary in their data protection laws, which can impact data sharing and storage.
Concerns around intellectual property rights can also slow collaboration.
5. Regulatory Delays
Differences in submission processes and timelines often lead to extended approval periods.
Sponsors must carefully plan multicenter trials to avoid bureaucratic bottlenecks.
🧭 Navigating the Way Forward
To balance the opportunities and regulatory challenges, stakeholders must adopt a thoughtful, ethical, and strategic approach. Here’s how:
Invest in Regulatory Intelligence: Understand the local and international guidelines that govern clinical trials. Being proactive reduces delays.
Foster Transparency and Ethics: Adhere to ICH-GCP (International Council for Harmonisation – Good Clinical Practice) guidelines across all study sites.
Build Strong Local Partnerships: Collaborate with local CROs (Contract Research Organizations), investigators, and institutions who understand cultural and legal nuances.
Implement Unified Technologies: Use standardized electronic data capture and remote monitoring tools to maintain data integrity.
Encourage Training and Capacity Building: Educate and support investigators in emerging regions to meet global compliance standards.
✨ Final Thoughts
The globalization of clinical research reflects a pivotal shift toward more inclusive, efficient, and collaborative healthcare innovation. When conducted responsibly, it has the power to improve lives, not just in a select few countries, but across the entire globe.
However, the road is not without its bumps. As sponsors and regulators work simultaneously to bring therapies to market, they must do so with an unwavering commitment to ethics, transparency, and patient safety.
In the end, the goal is clear: to make clinical research a tool for global good—one that respects local contexts while contributing to a shared future of health and healing.
0 notes
Text
¶ … Penington, P., Caminniti, S., Schein, J.R., Hewitt, D.J., & Nelson, W.W. (2009). Patients' Assessment of the Convenience of Fentanyl HCl Iontophoretic Transdermal System (ITS) Versus Morphine Intravenous Patient- Controlled Analgesia (IV PCA) in the Management of Postoperative Pain After Major Surgery, Pain Management Nursing, 10, 124-133 to evaluate patients' assessment of fentanyl ITS and morphine intravenous patient-controlled analgesia (IV PCA) Research question/objective/hypothesis Two previous studies used Ease of Care questionnaire in order to assess the superiority of ITS over morphine intravenous patient-controlled analgesia. However extensive analysis of the EOC data was not collected. This study intends to do just that, assessing the convenience of each patient modality using the EOC questionnaire form both previous studies. Review of the literature There is inadequate management of postoperative pain, which can impact a host of conditions including chronic pain. Patient controlled anesthesia is preferred to clinician-controlled anesthesia. The common analgesic used until now is PCA morphine, but this has its limitation. A recent PCA that has just been approved for acute post operative pain management is patient-controlled fentanyl HCl iontophoretic transdermal system ITS) (fentanyl ITS; IONSYS™; Ortho-McNeil, Raritan, NJ). Two studies (one on patients with total hip replacement surgery (THR); and the other conducted on patients undergoing abdominal or pelvic surgery (APS)), using Ease of care questionnaire have been conducted on comparison of the modalities and results have seemed to indicate superior EOC for ITS. 5. Conceptual/theoretical framework Two studies (one on each patient group) that were studies were open-label, randomized, multicenter, active-controlled phase using questionnaires (Likert scale) that were statistically analyzed. 6 Variables or key concepts studied Variables of Ease of care of ITS as compared to variables of EOC involved with 7. Significance of the problem to nursing To assess whether OTS can be used as postoperative pain management system and whether its use in all variables is better to that of current modalities. 8. Design and method Two studies, similar in design, one on patients with total hip replacement surgery (THR), the other conducted on patients undergoing abdominal or pelvic surgery (APS), using EOC questionnaires (Likert scale) and then statistically analyzed. 9. Subjects (participants) (a) patients with total hip replacement surgery (THR), (b) patients undergoing abdominal or pelvic surgery (APS). All were 18 years or older and expected to receive parenteral opioids for 24 hours and above after their surgery. 10. Instruments used The Ease of Care questionnaire scored on a 6-point Liker scale 11. Data analysis methods Statistical analysis with, depending on the nature of the variable, either an ANOVA with treatment as the factor or an uncorrected chi-square (categorical data). 12. Findings and Limitations Findings showed that for the majority of items on the survey, more patients responded in favor of ITS than they did for morphine IV PCA in both studies. Differences were particularly significant on the Movement scale. In the THR study, more patients responded for the ITS as compared to the morphine when assessing categories of Confidence with Device, Pain Control, Knowledge / Understanding, and Satisfaction. Responder rates for these scales did not differ in the APS study. Limitations included differences in gender distribution between the two studies and the mean patient age in the APS study was significantly lower than in the THR study. 14. Conclusions Fentanyl ITS is superior to morphine IV PCA for postoperative pain management, particularly in terms of mobility. https://www.paperdue.com/customer/paper/penington-p-caminniti-s-schein-jr-50475#:~:text=Logout-,PeningtonPCaminnitiSScheinJRHewitt,-Length2pages Read the full article
0 notes