#sporozoites
Text
drunk; kim mingyu




mingyu x reader // words : 1k approx.
genre : teeth-rotting fluff.
warnings : too many kisses and life cycle of malaria, very slightly jealous gyu too // unedited.

you stumbled as you spilled water on your shirt, a dopey smile on your face as you tried to close the recyclable plastic bottle- only to turn the cap in wrong direction and dropping the cap in the process.
"shit-" you giggled as mingyu stared up at you, his eyes wide like saucers as his mouth fell agape on your ministerings.
"are you alright?" he asked, his eyes on your wet shirt as he looked up at you quizzically.
"mingyu~" you whined, a grin on your face as you stagger towards him, a stumble in your unbalanced steps as you tripped; mingyu was fast on his feet, his arms wrapped around your torso as he pulled you on his lap while you giggled, making his lips quirk up in a smile too.
"are you drunk?" he laughed as you squished his face between your hands, you lips making a pout as you attached your lips with his, "mwah!"
"why do i think that you're drunk?"
"pak pak pakak!"
his eyebrows shot up in amusement as a huge laughter bellowed out of his chest, his eyes lining with tears as he endearingly laughed on your quite accurate representation of a hen.
"hehe~" you giggled as you buried your face in the crook of his neck.
"i don't smell any alcohol on you though-" mingyu said, tucking back a stray strand of hair behind your ear.
"i am not drunk- that's whyyyyyy," you whispered in his ears.
"it sure doesn't seem like that-"
"won't you believe me?" you pouted, your eyes crinkling up in a perfect puppy eyes as mingyu cooed at you, nuzzling your nose with his, "i believe you."
"as you should!" you grinned up at him, "i am just very happy- i get like this when i am happy."
"what made you so happy then?" mingyu asked, his eyes soft as he admired your face, his heart beating at a rapid pace as he noticed just how close you are to him; mingyu had heard that sometimes you did get extremely silly and almost in a drunken like state when you were extremely happy and it was so contrasting to your normal calm personality that mingyu couldn't help but dote on you- at every given second.
"just-" you started saying but stopped, your brows scrunched up as you leaned in and connected his lips with yours for a short kiss again, your silly smile returning back to your face as you continued, "just because i am happy!"
"you are?" he asked, caressing your cheek.
"yes, i am! and oh! do you know the life cycle of plasmodium vivax?"
"life cycle of what-"
you rolled up your sleeves and puckered your lips, coughing to switch to your singing voice as you started, "when a mosquito bites a human! ow ow, she spits sporozoites in his blood! ew ew! then the little rascals travel up to our liver cells and multiiiiply till our cell bursts!"
"bursts!?"
"oh yeah! then they travel up to our rbcs~ travel yeah! reproducing asexually to bursts our blood cell so that we get a fever!"
"reproduce in our blood!?"
"mhm! and when the female mosquito bite bites us- fertilization take place in her intestines!"
"this isn't rhyming."
"then she bites us again and dun dun! you have malaria!"
"i love you," he said, his eyes soft as he stared at you, a genuine smile lacing his lips.
your lips froze as you grin up at him, engulfing him in your hug and squirming in his lap you said, "i love you too~", it seemed as if your senses had returned after his sweet confession and now heat rose to your neck and cheeks as you melted in his touch, hiding your face in his chest as you murmur, "sorry- sorry if i was being annoying."
when mingyu had not replied for a few seconds you looked up at him, a frown evident on his face as he looked away from you, "you would never annoy me."
"then why are you upset?" you asked as you tilted his face towards you; his face still in a pout and a frown as he sighed.
"how could you ever annoy me? and-" he paused.
"and?"
"and..." he breathed, his cheeks slightly red as he asked, "just how many have seen you in such adorable state?"
your eyebrows and lips quirked up in amusement as a smile broke out on your face, chuckling you made him face you, momentarily taken aback at how freaking handsome your boyfriend was.
"how many?" he asked again, his voice in a whisper as you almost cried because of how cute he was.
"many people- my friends, family members and some of my exes have seen me like this," you said, noticing his frown deepening as you kissed his jaw, "but..." you said.
"but?"
"you will be the one to see it the most, and the only one from now," you smiled at him as you saw his frown morph into a grin as he giggled alongside you, stretching his pinkie towards you he asked, "promise?"
you intertwined your pinkie with his, "promise."




#seventeen#caratsland#kflixnet#caratlibrary#svt#kim mingyu#kim mingyu fluff#mingyu#mingyu x reader#seventeen mingyu#kpop#kpop fluff#nish recs#kim mingyu x reader#kim mingyu x you#mingyu fanfiction#mingyu x you#nishloves#svt imagines#seventeen imagines#seventeen fluff#svt mingyu#svt fluff
704 notes
·
View notes
Text
Just like we are multi-cellular organisms
Just like we are multi-cellular organisms, similarly unicellular protozoa organisms are also present on earth. Nowadays India, America and China are worried about the increase in human population. This population is creating disturbance in nature. When bacteria and viruses increase their population in our body, disturbances are created in our body too, which we call diseases. And just like due to the decrease in human population, we think of controlling reproduction or try to filter other planets, satellites or stars. In our body, protozoa prevent bacterial growth by eating bacteria. Is protozoa something like medicine?
Is protozoa used in medicine?
Prior use of Protozoa in Disease Therapy
It is little known today, but there is precedence for the use of protozoa in medical practice. Before the advent of antibiotics, patients in the end stages of syphilis (neurosyphilis) were sometimes treated by malariatherapy (3).
Protozoa: Pathogenesis and Defenses
National Institutes of Health (NIH) (.gov)
https://www.ncbi.nlm.nih.gov › books › NBK8043
by JR Seed · 1996 · Cited by 21 — Resistance to parasitic protozoa appears to be similar to resistance against other infectious agents,
Many protozoan parasites grow and divide within host cells. For example, Plasmodium parasites grow first in hepatocytes and then in red blood cells. Leishmania and Toxoplasma organisms are capable of growing in macrophages; one genus of parasitic protozoa, Theilera, not only multiplies in lymphocytes but appears even to stimulate the multiplication of the infected lymphocytes. Although some parasites, such as Plasmodium, are restricted to a limited number of host cell types, others, such as T cruzi and Toxoplasma, appear to be able to grow and divide in a variety of different host cells.
An intracellular refuge may protect a parasite from the harmful or lethal effects of antibody or cellular defense mechanisms. For example, Plasmodium may be susceptible to the actions of antibody only during the brief extracellular phases of its life cycle (the sporozoite and merozoite stages). It should be remembered that Plasmodium actually resides in a membrane-bound vacuole in the host cell. Thus, plasmodia are shielded from the external environment by at least two host membranes (the outer cell membrane and an inner vacuole membrane). Although intracellular plasmodia are very well protected from the host's immune response early in their growth, this strategy does create physiologic problems for the parasite. For example, the parasite must obtain its nutrients for growth through three membranes (two host and one parasite), and must eliminate its waste products through the same three membranes. Plasmodia solve this problem by appropriately modifying the host cell membranes. Parasitic proteins are incorporated into the red blood cell outer membrane. The host eventually responds to these antigens, and this response ultimately leads to the increased removal of infected host cells.
The existence of extracellular phases in the malaria life cycle is important, since immunization against these stages is the rationale for the development of our current vaccine candidates. The protective antigens on these extracellular stages have been purified as potential antigens for a vaccine. However, this approach has problems. For example, the sporozoite stage is exposed to protective antibody for only a brief period, and even a single sporozoite that escapes immune elimination will lead to an infection. Second, the antigenic variability of different isolates and the ability of different strains to undergo antigenic variation are not fully known. Therefore, the effectiveness of the vaccine candidates must still be demonstrated. However a large synthetic peptide containing antigenic sequences from 3 different proteins of P falciparum has been shown to reduce the clinical incidence of malaria by 31% in field trials. There is therefore optimism that a vaccine against P falciparum may be available in the near future.
A number of parasitic protozoa reside in macrophages. Although these organisms are protected from external immune threats, they must still evade digestion by the macrophage. Three strategies have been suggested. First, the parasite may prevent the fusion of lysosomes with the phagocytic vacuole. The actual mechanism responsible for this inhibition is not yet understood, but it has been shown to occur in cells infected with Toxoplasma. A second mechanism is represented by the ability of T cruzi to escape from the phagocytic vacuole into the cytoplasm of the macrophage. Finally, it is possible that some parasites can survive in the presence of lysosomal enzymes, as can the leprosy bacillus. One of the best-studied examples of a protozoan parasite able to survive in the phagolysosome is Leishmania. It has been suggested that the resistance of this parasite to the host's hydrolytic enzymes is due to surface components that inhibit the host's enzymes and/or to the presence of parasitic enzymes that hydrolyze the host's enzymes. As previously noted, at least one protozoan parasite, Theilera, is capable of growing directly in lymphocytes. Therefore, this parasite may escape the host's immune response by growing inside the very cells required for the response.
Translate Hindi
जैसे हम बहुकोशिकीय जीव है
उसी तरह एककोशिकीय प्रोटोजोआ जीव भी मौजूद है पृथ्वी में
आजकल इंडिया अमेरिका चायना परेशान है इंसानी आबादी बढ़ जाने हेतू
यही आबादी प्रकृति मे हलचल फैला रहा है
जब बैक्टीरिया वायरस हमारी शरीर में आबादी बढ़ा देते है तब हमारी शरीर में भी हलचल पैदा हो जाते है जिसे हम रोग कहा करते है
और जैसे इसानी आबादी को घटाने कारण हम प्रजनन कंट्रोल करने की सोचते है या दूसरी ग्रहों उपग्रहों या तारों को छानने की कोशिश करते है
हमारी शरीर में प्रोटोजोआ बैक्टीरिया को खाकर बैक्टीरिया वृद्धि को रोकथाम करते है
क्या प्रोटोजोआ मेडिसिन जैसे ही कुछ है
क्या प्रोटोजोआ का उपयोग चिकित्सा में किया जाता है?
रोग चिकित्सा में प्रोटोजोआ का पूर्व उपयोग
आज यह बहुत कम जाना जाता है, लेकिन चिकित्सा पद्धति में प्रोटोजोआ के उपयोग की मिसाल है। एंटीबायोटिक दवाओं के आगमन से पहले, सिफलिस (न्यूरोसिफिलिस) के अंतिम चरण में रोगियों का कभी-कभी मलेरिया थेरेपी (3) द्वारा इलाज किया जाता था।
प्रोटोजोआ: रोगजनन और बचाव
राष्ट्रीय स्वास्थ्य संस्थान (NIH) (.gov)
https://www.ncbi.nlm.nih.gov › पुस्तकें › NBK8043
जेआर सीड द्वारा · 1996 · 21 द्वारा उद्धृत - परजीवी प्रोटोजोआ के प्रति प्रतिरोध अन्य संक्रामक एजेंटों के प्रति प्रतिरोध के समान प्रतीत होता है,
कई प्रोटोजोआ परजीवी मेजबान कोशिकाओं के भीतर बढ़ते और विभाजित होते हैं। उदाहरण के लिए, प्लास्मोडियम परजीवी पहले हेपेटोसाइट्स में और फिर लाल रक्त कोशिकाओं में बढ़ते हैं। लीशमैनिया और टोक्सोप्लाज्मा जीव मैक्रोफेज में बढ़ने में सक्षम हैं; परजीवी प्रोटोजोआ का एक जीनस, थिलेरा, न केवल लिम्फोसाइटों में गुणा करता है बल्कि संक्रमित लिम्फोसाइटों के गुणन को उत्तेजित भी करता प्रतीत होता है। हालांकि कुछ परजीवी, जैसे प्लास्मोडियम, सीमित संख्या में मेजबान कोशिका प्रकारों तक ही सीमित होते हैं, अन्य, जैसे टी क्रूज़ी और टोक्सोप्लाज्मा, विभिन्न मेजबान कोशिकाओं की एक किस्म में बढ़ने और विभाजित होने में सक्षम प्रतीत होते हैं। एक इंट्रासेल्युलर शरण एक परजीवी को एंटीबॉडी या सेलुलर रक्षा तंत्र के हानिकारक या घातक प्रभावों से बचा सकती है। उदाहरण के लिए, प्लास्मोडियम केवल अपने जीवन चक्र के संक्षिप्त बाह्य चरणों (स्पोरोजोइट और मेरोजोइट चरणों) के दौरान एंटीबॉडी की क्रियाओं के लिए अतिसंवेदनशील हो सकता है। यह याद रखना चाहिए कि प्लास्मोडियम वास्तव में मेजबान कोशिका में एक झिल्ली से बंधे रिक्तिका में रहता है हालाँकि, इंट्रासेल्युलर प्लास्मोडिया अपने विकास के शुरुआती दौर में मेजबान की प्रतिरक्षा प्रतिक्रिया से बहुत अच्छी तरह से सुरक्षित होते हैं, लेकिन यह रणनीति परजीवी के लिए शारीरिक समस्याएँ पैदा करती है। उदाहरण के लिए, परजीवी को विकास के लिए अपने पोषक तत्व तीन झिल्लियों (दो मेजबान और एक परजीवी) के माध्यम से प्राप्त करने चाहिए, और अपने अपशिष्ट उत्पादों को उन्हीं तीन झिल्लियों के माध्यम से समाप्त करना चाहिए। प्लास्मोडिया मेजबान कोशिका झिल्लियों को उचित रूप से संशोधित करके इस समस्या का समाधान करते हैं। परजीवी प्रोटीन लाल रक्त कोशिका की बाहरी झिल्ली में शामिल हो जाते हैं। मेजबान अंततः इन एंटीजन के प्रति प्रतिक्रिया करता है, और यह प्रतिक्रिया अंततः संक्रमित मेजबान कोशिकाओं को हटाने में वृद्धि करती है। मलेरिया जीवन चक्र में बाह्यकोशिकीय चरणों का अस्तित्व महत्वपूर्ण है, क्योंकि इन चरणों के विरुद्ध टीकाकरण हमारे वर्तमान वैक्सीन उम्मीदवारों के विकास का औचित्य है। इन बाह्यकोशिकीय चरणों पर सुरक्षात्मक एंटीजन को वैक्सीन के लिए संभावित एंटीजन के रूप में शुद्ध किया गया है। हालाँकि, इस दृष्टिकोण में समस्याएँ हैं। उदाहरण के लिए, स्पोरोज़ोइट चरण केवल थोड़े समय के लिए सुरक्षात्मक एंटीबॉडी के संपर्क में आता है, और यहाँ तक कि एक भी स्पोरोज़ोइट जो प्रतिरक्षा उन्मूलन से बच जाता है, वह संक्रमण का कारण बन जाएगा। दूसरा, विभिन्न आइसोलेट्स की एंटीजेनिक परिवर्तनशीलता और विभिन्न उपभेदों की एंटीजेनिक भिन्नता से गुजरने की क्षमता पूरी तरह से ज्ञात नहीं है। इसलिए, वैक्सीन उम्मीदवारों की प्रभावशीलता को अभी भी प्रदर्शित किया जाना चाहिए। हालांकि पी फाल्सीपेरम के 3 विभिन्न प्रोटीनों से एंटीजेनिक अनुक्रमों वाले एक बड़े सिंथेटिक पेप्टाइड को फील्ड परीक्षणों में मलेरिया की नैदानिक घटनाओं को 31% तक कम करने के लिए दिखाया गया है। इसलिए आशावाद है कि पी फाल्सीपेरम के खिलाफ एक टीका निकट भविष्य में उपलब्ध हो सकता है।
मैक्रोफेज में कई परजीवी प्रोटोजोआ रहते हैं। हालांकि ये जीव बाहरी प्रतिरक्षा खतरों से सुरक्षित हैं, फिर भी उ��्हें मैक्रोफेज द्वारा पाचन से बचना चाहिए। तीन रणनीतियों का सुझाव दिया गया है। सबसे पहले, परजीवी लाइसोसोम को फागोसाइटिक रिक्तिका के साथ संलयन को रोक सकता है। दूसरा तंत्र टी क्रूज़ी की फैगोसाइटिक रिक्तिका से मैक्रोफेज के कोशिका द्रव्य में भागने की क्षमता द्वारा दर्शाया गया है। अंत में, यह संभव है कि कुछ परजीवी लाइसोसोमल एंजाइम की उपस्थिति में जीवित रह सकते हैं, जैसा कि कुष्ठ रोग बेसिलस कर सकता है। प्रोटोजोआ परजीवी के सबसे अच्छे अध्ययन किए गए उदाहरणों में से एक जो फैगोलिसोसोम में जीवित रहने में सक्षम है, वह है लीशमैनिया। यह सुझाव दिया गया है कि मेजबान के हाइड्रोलिटिक एंजाइमों के लिए इस परजीवी का प्रतिरोध सतह के घटकों के कारण होता है जो मेजबान के एंजाइमों को रोकते हैं और/या परजीवी एंजाइमों की उपस्थिति के कारण होता है जो मेजबान के एंजाइमों को हाइड्रोलाइज करते हैं। जैसा कि पहले उल्लेख किया गया है, कम से कम एक प्रोटोजोआ परजीवी, थेलेरा, लिम्फोसाइटों में सीधे बढ़ने में सक्षम है। इसलिए, यह परजीवी प्रतिक्रिया के लिए आवश्यक कोशिकाओं के अंदर बढ़ने से मेजबान की प्रतिरक्षा प्रतिक्रिया से बच सकता है।
0 notes
Link
1 note
·
View note
Text
Invisible Predators: Understanding Eimeria and Poultry Losses
Avian coccidiosis, caused by protozoan parasites of the genus Eimeria, poses a significant threat to poultry health and productivity. Despite their microscopic size, these parasites wield immense power, inflicting devastating losses on poultry farms worldwide. In this in-depth exploration, we will unravel the biology and behavior of Eimeria parasites, shedding light on their role as invisible predators in the poultry industry and the profound economic consequences of their presence.
Eimeria parasites are the primary culprits behind avian coccidiosis, a debilitating disease that affects poultry species such as chickens, turkeys, and ducks. These obligate intracellular parasites have evolved sophisticated mechanisms to invade and colonize the intestinal lining of their avian hosts, leading to a cascade of pathological changes that compromise gut health and nutrient absorption. Despite their diminutive size, Eimeria parasites wield considerable influence over the economic viability of poultry production systems, making them formidable adversaries for poultry producers worldwide.
The Biology of Eimeria Parasites
Eimeria parasites exhibit a complex lifecycle characterized by multiple stages of development, each tailored to exploit specific niches within the avian digestive tract. Upon ingestion of sporulated oocysts, the infectious stage of the parasite, Eimeria undergoes a series of asexual and sexual reproductive cycles, culminating in the production of thousands of infective sporozoites. These sporozoites invade the intestinal epithelial cells, where they undergo further replication and differentiation, leading to the formation of characteristic intracellular structures known as schizonts, gametocytes, and oocysts. The rupture of host cells releases new generations of infective stages, perpetuating the cycle of infection and causing extensive tissue damage in the process.
Pathogenesis and Clinical Manifestations
The pathogenesis of avian coccidiosis is multifaceted, involving a complex interplay between parasite virulence factors and host immune responses. Eimeria parasites exert their pathological effects primarily through the destruction of intestinal epithelial cells, leading to villous atrophy, mucosal inflammation, and impaired nutrient absorption. Clinical manifestations of coccidiosis vary depending on the species and virulence of the infecting Eimeria strain, ranging from subclinical infections with mild diarrhea to severe cases characterized by bloody droppings, dehydration, and increased mortality rates. The economic impact of these clinical signs extends beyond the immediate costs of treatment and mortality, affecting feed conversion efficiency, growth rates, and overall flock performance.
The Economic Consequences of Eimeria Infections
Beyond their direct effects on poultry health and welfare, Eimeria infections impose significant economic burdens on poultry producers through various channels. The costs associated with mortality, morbidity, and treatment expenses represent tangible losses that directly impact the profitability of poultry operations. Additionally, subclinical infections, which often go unnoticed, can still exert a substantial toll on productivity by reducing feed efficiency, impairing weight gain, and predisposing birds to secondary infections. Moreover, the emergence of drug-resistant Eimeria strains further complicates control efforts, necessitating the development of alternative strategies to manage and mitigate the economic impact of coccidiosis outbreaks.
For more information visit us:
0 notes
Text
📆 Jul 2019 📰 T cell-mediated immunity to malaria 🗞 Nature Immunology Reviews
The lead subunit vaccine candidate, RTS,S (Mosquirix™) [G] provides only short-lived, partial protection against malaria2, 3, 4. Thus, despite the current conceptual frameworks for αβ and γδ T cell-mediated protection against Plasmodium, we still lack sufficient mechanistic understanding of their formation and function, which has hampered the design of efficacious vaccines that can be deployed in malaria-endemic regions. The development of innovative anti-malarial vaccine platforms and the potential application of immunotherapies that stimulate or enhance resistance to malaria will require deeper insights into the cellular and molecular mechanisms that govern anti-Plasmodium T cell responses.
Identifying immunodominant subsets of CD4+ and CD8+ T cells will necessitate a greater emphasis on deciphering immunodominant T cell epitopes that are targeted by liver- or blood-stage-specific T cells. Notably, some dominant T cell epitopes targeted by substantial populations of T cells do not represent protective epitopes; this is likely due to large numbers of precursor T cells bearing T cell receptors (TCRs) that can recognize the epitope presented by professional antigen-presenting cells (APCs), but a lack of this epitope on infected cells.
Determining which of the detectable epitope-specific T cell responses are the most protective represents a major goal.

In experimental malaria, CD8+ T cells specific for sporozoite antigens, liver-stage antigens (the pre-erythrocytic stages) and blood-stage antigens (erythrocytic stage) (Fig. 2) have been described after infection or vaccination. Although CD8+ T cells are primed against the various pre-erythrocytic stages of malaria in the vertebrate host, their relevance to protection in a primary infection is contentious. This is largely because an infected mosquito delivers only a few hundred sporozoites into the host dermis, leading to a very low proportion of infected hepatocytes prior to release of blood-stage merozoites. Further, liver-stage malaria offers a short window of opportunity (~7 days in humans and ~2 days in mice), to mount optimally functional effector CD8+ T cell responses.
Additionally, repeated exposure to Plasmodium infections does not generate sufficient immunity against the liver-stages in humans despite eliciting disease-limiting humoral immunity against the pathogenic blood stage148, 149, 150; the precise reasons for this remain a major knowledge gap.
For a long time it was assumed that CD8+ T cell responses against pre-erythrocytic stages of Plasmodium were primed by infected hepatocytes. Yet, the unlikelihood of rare naïve CD8+ T cells, encountering infrequently infected hepatocytes in the liver made this event improbable based on the existing paradigms of T cell priming mechanisms.
Early studies revealed that CD11c+ dendritic cells (DCs) played a vital role in priming CD8+ T cell responses to pre-erythrocytic developmental stages of Plasmodium.
The precise mechanisms by which CD8+ T cells function to limit liver-stage infection in human malaria remain unknown, and a better understanding of those will help further our ability to tailor sterilizing immunity to malaria.
The field of malaria subunit vaccines is desperately in need of new target antigens to evaluate and the capacity to carry out whole parasite immunization studies in human volunteers could be a fertile basis for such antigen discovery. Importantly, recent work in animal models shows that detection of a T cell response to a Plasmodium antigen does not ensure that antigen will elicit T cells that can protect against infection.
0 notes
Text
First evidence for development of Plasmodium relictum (Grassi and Feletti, 1891) sporozoites in the salivary glands of Culex modestus Ficalbi, 1889
http://dlvr.it/Sn7Vbh
0 notes
Link
Scientists from Sanaria Inc., and collaborators at multiple institutions publish results in Nature reporting “In vitro production of infectious Plasmodium falciparum sporozoites.”
0 notes
Text
Schnakenstich-Impfung: Wissenschaftler haben eine Methode entwickelt, Moskitos zum Impfstoff unzufunktionieren.
ScienceFiles:»Was halten Sie davon? “Genetically engineered live Plasmodium falciparum sporozoites constitute a potential platform for creating consistently attenuated, genetically defined, whole-parasite vaccines against malaria through targeted gene deletions. Such genetically attenuated parasites (GAPs) do not require attenuation by irradiation or concomitant drug treatment. We previously developed a P. falciparum (Pf) GAP with deletions in P52, […] http://dlvr.it/SZRGfN «
0 notes
Text
PLASMODIUM (MALARIAL PARASITE)
INTRODUCTION
• Plasmodium (Malarial Parasite) belongs to the subphylum Sporozoan of the phylum Protozoa. All sporozoites are parasites. They are intracellular or extracellular parasites of both vertebrates and invertebrates.
• Among sporozoans, Plasmodium is the best known and most important species that causes malaria fever. Of these, about 60 species are known among humans and other animals.…

View On WordPress
0 notes
Text
there’s nothing in the extended HCU [Homeric Cinematic Universe] that DISproves my interpretation where, when Helen and Menelaus ended up in Egypt for a while, it took them forever to get back to Greece because Menelaus nearly died of malaria and spent several months convalescing
#Helen is immune to malaria because of her divine blood mosquitoes don't bite her and sporozoites cannot enter her hepatocytes#the iliad
26 notes
·
View notes
Photo
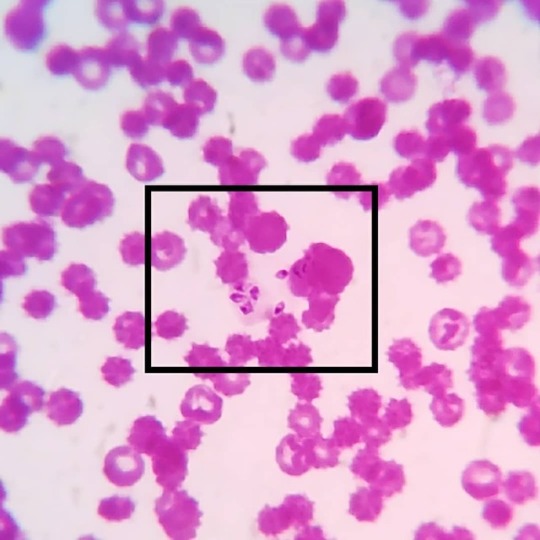
#merozoite 's of #babesiacanis in a #caninebloodsmear: Babesia canis are #intercellular #haemoprotozoa belonging to the family of #piroplasmida e. The babesial organisms are transmitted by the dog #browntick #rhipicephalussanguineus , which are present as #sporozoites in the salivary glands. During their blood meal the sporozoites are passed on to the vertebrate host and attaches itself on the erythrocytic membranes. The parasites enter the RBCs and multiply by asexual reproduction to form merozoites which are seen as leaf like structures. While feeding again the organisms are transferred to the ticks and the organisms enter the gut mucosa and undergo gametogony to form male and female forms which reproduce by sexual and by asexual reproduction to form more sporozoites. And the cycle continues!! Most common babesial organisms seen in canine are Babesia canis and Babesia Gibsoni. Babesia Gibsoni are more smaller, seen as small ring/ circular structures and lack a pyriform shape. Babesia canis on the other hand have large pyriform merozoites and pointed at one end. #vetpathology #vetpath #vetparasitology #veterinaryparasitology #veterinaryparapathologist #bloodparasite #vetcasestudy #vetcases #vetstudies #vetmed #vetmedicine #vetlife #vetknowledge #vetclinicalpathology #vetcliniclife #vetdiagnosis #vetlabdiagnostic https://www.instagram.com/drdashvetpath/p/Bw82BXIh07-/?utm_source=ig_tumblr_share&igshid=15ay9kvz7f12y
#merozoite#babesiacanis#caninebloodsmear#intercellular#haemoprotozoa#piroplasmida#browntick#rhipicephalussanguineus#sporozoites#vetpathology#vetpath#vetparasitology#veterinaryparasitology#veterinaryparapathologist#bloodparasite#vetcasestudy#vetcases#vetstudies#vetmed#vetmedicine#vetlife#vetknowledge#vetclinicalpathology#vetcliniclife#vetdiagnosis#vetlabdiagnostic
0 notes
Text
biology test didnt go too badly actually...
#like it was. alright#one question I had no clue but no one else did either so its fine#apparently the answer was#Following an infective mosquito bite sporozoites travel via the blood to the liver.#Here they develop into schizonts which then burst and release merozoites into the blood leading to the clinical symptoms of malaria.#In some species of parasite particularly P. vivax some sporozoites become hypnozoites.#so. did not get that#wtf that's not even on the spec??? did she take it from an a level paper or what#actually maybe i'll get a mark because I did talk abt the symptoms of malaria and how the protist goes into the blood after a bite#im so dumb tho I should have mentioned the liver like it was in the question#i think i'll get one or two marks if shes generous#everything else was mostly okay so..#anyways there were 66 marks so i can afford to get some wrong
3 notes
·
View notes
Photo
https://www.malariavaccine.org/malaria-and-vaccines/vaccine-development/life-cycle-malaria-parasite
The malaria parasite develops both in humans and in the female Anopheles mosquitoes. The size and genetic complexity of the parasite mean that each infection presents thousands of antigens (proteins) to the human immune system.
The parasite also changes through several life stages even while in the human host, presenting different antigens at different stages of its life cycle. Understanding which of these can be a useful target for vaccine development has been complicated. In addition, the parasite has developed a series of strategies that allow it to confuse, hide, and misdirect the human immune system.
Malaria infection begins when an infected female Anopheles mosquito bites a person, injecting Plasmodium parasites, in the form of sporozoites, into the bloodstream.
The sporozoites pass quickly into the human liver.
The sporozoites multiply asexually in the liver cells over the next 7 to 10 days, causing no symptoms.
In an animal model, the parasites, in the form of merozoites, are released from the liver cells in vesicles, journey through the heart, and arrive in the lungs, where they settle within lung capillaries. The vesicles eventually disintegrate, freeing the merozoites to enter the blood phase of their development.*
In the bloodstream, the merozoites invade red blood cells (erythrocytes) and multiply again until the cells burst. Then they invade more erythrocytes. This cycle is repeated, causing fever each time parasites break free and invade blood cells.
Some of the infected blood cells leave the cycle of asexual multiplication. Instead of replicating, the merozoites in these cells develop into sexual forms of the parasite, called gametocytes, that circulate in the blood stream.
When a mosquito bites an infected human, it ingests the gametocytes, which develop further into mature sex cells called gametes.
The fertilized female gametes develop into actively moving ookinetes that burrow through the mosquito's midgut wall and form oocysts on the exterior surface.
Inside the oocyst, thousands of active sporozoites develop. The oocyst eventually bursts, releasing sporozoites into the body cavity that travel to the mosquito's salivary glands.
The cycle of human infection begins again when the mosquito bites another person.
==
“tHe wOrLd iS tOo bEaUt1fUl t0 n0t bE dEs1gNeD!1!!”
#malaria#human malaria parasite#god is incompetent#god of incompetence#incompetence#cruel god#god of cruelty#cruelty#religion#religion is a mental illness
54 notes
·
View notes
Text
📅 Aug 2023 📰 Malaria vaccine candidate appears safe and produces promising immune response in a cohort of Tanzanian infants
There is currently only one malaria vaccine, "RTS,S" that is approved by the World Health Organization and offers partial disease protection. However, in the results of the early-stage phase Ib trial conducted in Tanzania and published on August 11th in the journal Med, researchers find that targeting RH5 -- a protein that the malaria pathogen Plasmodium falciparum uses to invade red blood cells -- can generate a promising immune response that is most pronounced in an infant cohort.
"Anti-sporozoite vaccines such as RTS,S need to be 100% effective in stopping the parasite from invading the liver to prevent disease," says senior author Angela Minassian, a clinician scientist at the University of Oxford. "Even if just one parasite slips through the net, this will then go on to multiply in the liver, burst out into the bloodstream, and then infect red blood cells where the parasites then grow at an exponential rate. Having a blood-stage vaccine like RH5 on board gives you a second line of defense once the parasite has entered the bloodstream, allowing a second chance to stop malaria before it causes illness."

A person is infected with malaria when bitten by an infected mosquito, which releases Plasmodium falciparum into the body. RTS,S and many other vaccine candidates teach the immune system how to target the parasite at this sporozoite stage, before it invades the liver. Once the parasite matures and is released from the liver into the bloodstream, Plasmodium falciparum displays RH5 and infects red blood cells, which causes disease. If an anti-sporozoite and an anti-RH5 vaccine were used in combination in the future, individuals could potentially experience more effective protection against malaria for a longer period of time.
All participants were given the second dose of vaccine two months later and followed for four months after this.
The primary purpose of this study was to evaluate the safety of this vaccine in a population where malaria is endemic. Participants in both the control and treatment group reported pain at the injection site and a mild fever shortly after vaccination, but overall the vaccine was well tolerated and there were no safety concerns.
A secondary outcome of the study was whether the vaccine would promote an immune response. Researchers found that participants who received the malaria vaccine developed antibodies against RH5 in their blood upon follow-up. In the laboratory, these antibodies were able to inhibit the growth of the malaria parasite at high levels that are associated with disease protection. "These data justify onward progression to phase IIb field efficacy trials to determine whether parasite growth-inhibition levels of this magnitude can ultimately protect against clinical malaria." say the authors.
The authors note that they observed the strongest immune responses in infants under 11 months, followed by children aged 1-6 years, then adults. "Why the infants and young children vaccinated with ChAd63-MVA RH5 induced such high levels of antibody remains to be fully understood," say the authors. "Given that both anti-sporozoite and blood-stage malaria vaccine strategies necessitate very high levels of antibody to protect against parasite infection, current efforts remain focused on infants and young children."
0 notes
Link
Having just seen the world develop Covid vaccines in record time, you might be wondering why it has taken so long with malaria?
Malaria is caused by a parasite which is far more insidious and sophisticated than the virus that causes Covid. Comparing them is like comparing a person and a cabbage.
The malaria parasite has evolved to evade our immune system. That's why you have to catch malaria time and time again before starting to get even limited protection.
It has a complicated life cycle across two species (humans and mosquitoes), and even inside our body it morphs between different forms as it infects liver cells and red blood cells.
Developing a malaria vaccine is like nailing jelly to a wall and RTS,S is only able to target the sporozoite form of the parasite (this is the stage between being bitten by a mosquito and the parasite getting to the liver).
It is why the vaccine is 'only' 40% effective. However, this is still a remarkable success and paves the way for the development of yet more potent vaccines.
The vaccine, developed by the pharmaceutical giant GSK, is not going to replace all the other measures for controlling malaria such as insecticide-treated bed nets. It will be used alongside them to get closer to the goal of zero deaths from malaria.
And it won't be used outside of Africa where different forms of malaria, which the vaccine can't protect against, are more prevalent.
1 note
·
View note
Link
Many people think of a Shoggoth as a giant amoeba by their general appearance and the fact that Lovecraft mentioned in At the Mountains of Madness that they reproduce through fission (simple cellular division).
However, just assuming the Shoggoth is a giant amoeba poses a number of problems. First there is a size limitation posed on cells based on the ratio of surface area to volume of the cell. In order to efficiently transfer nutrients, oxygen and food in and waste products out, the cell can not become too large. As the surface area of the cell increases, the volume of the cell increases at a faster rate. This reduces the efficient transfer of material into and out of the cell. This is why there are no giant amoebas. As we mentioned in the previous article, it is hypothesized that the Elder Ones developed multicellular life using the resident microorganisms of Earth as the raw material, which eventually lead to the differentiation of cells into tissues and organs.
In addition to the limitations associated with cell size, the reference that the Shoggoths reproduce through fission is a bit more complicated than simple cellular fission. While Lovecraft cited that Shoggoths reproduce through fission, he also mentioned several other points that are important.
First, Lovecraft mentions several times that the Shoggoths were “bred” by the Elder Ones. If a Shoggoth behaved like an amoeba it would simply feed, grow and once it attains a certain size, it would reproduce through asexual fission. However, the term bred means that some other, sexual means of reproduction was involved.
Second, the Elder Ones eventually moved onto the land due to the “difficulty in breeding and managing the Shoggoths..”. In addition, it appears that the Shoggoths acquired the ability to reproduce through fission, along with a “dangerous degree of accidental intelligence”, which caused problems for the Elder Ones.
Based on these facts, it appears that the Shoggoths were created and could not reproduce on their own. They needed “something” that the Elder Ones provided to reproduce. Thus, once the Elder Ones created the Shoggoths, they manipulated and bred them like cattle in their “nether pits” as referenced in the Fungi from Yuggoth. Such breeding programs were probably developed to ensure that the Elders Ones had control over the Shoggoths, similar to a lake manager using sterile grass carp to control nuisance aquatic plants.
One means of controlling nuisance aquatic plant growth and avoiding the use of aquatic herbicides, is to stock a lake with sterile grass carp. The carp are made sterile by pressure shocking the fertilized eggs, making them triploid (the vast majority of sexually reproducing animals, plants, fungi and protists are diploid, meaning the organism has two complete sets of chromosomes, one from each parent). Making the grass carp triploid (three sets of chromosomes) renders them sterile.
Did the Elder Ones have the ability to conduct a reverse process, where they could stimulate the typically sterile Shoggoths to reproduce? Thus, were the Shoggoths essentially created sterile and when more were needed, were they placed into the breeding pits where the Elder Ones facilitated the production of new Shoggoths?
My observation: Fission can also mean more than just binary fission.
Fission of protists
Multiple fission at the cellular level occurs in many protists, e.g. sporozoans and algae. The nucleus of the parent cell divides several times by amitosis, producing several nuclei. The cytoplasm then separates, creating multiple daughter cells.[15][16][17]
Some parasitic, single-celled organisms undergo a multiple fission-like process to produce numerous daughter cells from a single parent cell. Isolates of the human parasite Blastocystis hominis were observed to begin such a process within 4 to 6 days.[18] Cells of the fish parasite Trypanosoma borreli have also been observed participating in both binary and multiple fission.[19]
Fission of apicomplexans
In the apicomplexans, a phylum of parasitic protists, multiple fission, or schizogony, is manifested either as merogony, sporogony or gametogony. Merogony results in merozoites, which are multiple daughter cells, that originate within the same cell membrane,[20][21] sporogony results in sporozoites, and gametogony results in microgametes.
Fission of green algae
Green algae can divide into more than two daughter cells. The exact number of daughter cells depends on the species of algae and is an effect of temperature and light.[22]
2 notes
·
View notes