#RNA Interference
Explore tagged Tumblr posts
Text

Exploring RNA Interference
Imagine a molecular switch within your cells, one that can selectively turn off the production of specific proteins. This isn't science fiction; it's the power of RNA interference (RNAi), a groundbreaking biological process that has revolutionized our understanding of gene expression and holds immense potential for medicine and beyond.
The discovery of RNAi, like many scientific breakthroughs, was serendipitous. In the 1990s, Andrew Fire and Craig Mello were studying gene expression in the humble roundworm, Caenorhabditis elegans (a tiny worm). While injecting worms with DNA to study a specific gene, they observed an unexpected silencing effect - not just in the injected cells, but throughout the organism. This puzzling phenomenon, initially named "co-suppression," was later recognized as a universal mechanism: RNAi.
Their groundbreaking work, awarded the Nobel Prize in 2006, sparked a scientific revolution. Researchers delved deeper, unveiling the intricate choreography of RNAi. Double-stranded RNA molecules, the key players, bind to a protein complex called RISC (RNA-induced silencing complex). RISC, equipped with an "Argonaut" enzyme, acts as a molecular matchmaker, pairing the incoming RNA with its target messenger RNA (mRNA) - the blueprint for protein production. This recognition triggers the cleavage of the target mRNA, effectively silencing the corresponding gene.
So, how exactly does RNAi silence genes? Imagine a bustling factory where DNA blueprints are used to build protein machines. RNAi acts like a tiny conductor, wielding double-stranded RNA molecules as batons. These batons bind to specific messenger RNA (mRNA) molecules, the blueprints for proteins. Now comes the clever part: with the mRNA "marked," special molecular machines chop it up, effectively preventing protein production. This targeted silencing allows scientists to turn down the volume of specific genes, observing the resulting effects and understanding their roles in health and disease.
The intricate dance of RNAi involves several key players:dsRNA: The conductor, a long molecule with two complementary strands. Dicer: The technician, an enzyme that chops dsRNA into small interfering RNAs (siRNAs), about 20-25 nucleotides long. RNA-induced silencing complex (RISC): The ensemble, containing Argonaute proteins and the siRNA. Target mRNA: The specific "instrument" to be silenced, carrying the genetic instructions for protein synthesis.
The siRNA within RISC identifies and binds to the complementary sequence on the target mRNA. This binding triggers either:Direct cleavage: Argonaute acts like a molecular scissors, severing the mRNA, preventing protein production. Translation inhibition: RISC recruits other proteins that block ribosomes from translating the mRNA into a protein.
From Labs to Life: The Diverse Applications of RNAi
The ability to silence genes with high specificity unlocks various applications across different fields:
Unlocking Gene Function: Researchers use RNAi to study gene function in various organisms, from model systems like fruit flies to complex human cells. Silencing specific genes reveals their roles in development, disease, and other biological processes.
Therapeutic Potential: RNAi holds immense promise for treating various diseases. siRNA-based drugs are being developed to target genes involved in cancer, viral infections, neurodegenerative diseases, and more. Several clinical trials are underway, showcasing the potential for personalized medicine.
Crop Improvement: In agriculture, RNAi offers sustainable solutions for pest control and crop development. Silencing genes in insects can create pest-resistant crops, while altering plant genes can improve yield, nutritional value, and stress tolerance.
Beyond the Obvious: RNAi applications extend beyond these core areas. It's being explored for gene therapy, stem cell research, and functional genomics, pushing the boundaries of scientific exploration.
Despite its exciting potential, RNAi raises ethical concerns. Off-target effects, unintended silencing of non-target genes, and potential environmental risks need careful consideration. Open and responsible research, coupled with public discourse, is crucial to ensure we harness this powerful tool for good.
RNAi, a testament to biological elegance, has revolutionized our understanding of gene regulation and holds immense potential for transforming various fields. As advancements continue, the future of RNAi seems bright, promising to silence not just genes, but also diseases, food insecurity, and limitations in scientific exploration. The symphony of life, once thought unchangeable, now echoes with the possibility of fine-tuning its notes, thanks to the power of RNA interference.
#science sculpt#life science#science#molecular biology#biology#biotechnology#dna#double helix#genetics#artists on tumblr#rna#rna sequencing#RNA interference#cell biology#cells#biomolecules#illustrates#scientific illustration#illustration#illustrative art#scientific research
12 notes
·
View notes
Text
Investor Alert: Why the Gene Silencing Market Could Be the Next Big Thing

Introduction
The global gene silencing market is experiencing unprecedented growth, driven by advancements in genetic research, increasing prevalence of genetic disorders, and the rising adoption of gene-silencing technologies in therapeutics and drug discovery. Valued at approximately USD 3.7 billion in 2024, the gene silencing market is projected to expand at a CAGR of over 17.6% from 2025 to 2032, reaching a valuation exceeding USD 15.9 billion by the end of the forecast period. This rapid expansion is fueled by the increasing demand for RNA interference (RNAi), CRISPR-based therapies, and antisense oligonucleotides (ASOs) for targeted gene modulation.
Breakthroughs in gene-editing tools, AI-driven bioinformatics, and nanoparticle-based delivery systems are accelerating innovation and adoption. The use of gene silencing in treating cancer, neurological disorders, and rare genetic diseases, alongside the expansion of personalized medicine and cell and gene therapy applications, continues to strengthen market growth. Additionally, strategic partnerships between biotech firms, pharmaceutical companies, and research institutions are propelling advancements in gene-based therapies.
Request Sample Report PDF (including TOC, Graphs & Tables): https://www.statsandresearch.com/request-sample/40640-global-gene-silencing-market
Gene Silencing Market Dynamics
Key Drivers
Growing Adoption of Gene Silencing in Therapeutics
Increasing application of RNAi, CRISPR-Cas9, and ASOs in gene therapy.
Expansion of precision medicine initiatives leveraging gene silencing for personalized treatment.
Rising Prevalence of Genetic Disorders and Cancer
Escalating demand for innovative treatments for hereditary diseases, cancer, and neurodegenerative disorders.
Emerging gene silencing applications in rare genetic conditions and metabolic disorders.
Advancements in Gene-Editing Technologies
Enhancements in CRISPR-Cas systems for precise genetic modifications.
AI-driven bioinformatics for target identification and therapeutic development.
Strategic Collaborations and Investments in R&D
Increased funding for gene therapy research from public and private sectors.
Expansion of contract research organizations (CROs) and biotech partnerships.
Get up to 30%-40% Discount: https://www.statsandresearch.com/check-discount/40640-global-gene-silencing-market
Gene Silencing Market Challenges
Regulatory and Ethical Constraints
Stringent guidelines governing gene-editing technologies.
Ethical concerns regarding genetic modifications and long-term effects.
Complexities in Gene Delivery Mechanisms
Challenges associated with targeted delivery and minimizing off-target effects.
Development of safe and efficient non-viral and nanoparticle-based delivery systems.
Gene Silencing Market Segmentation
By Technology
RNA Interference (RNAi) – Dominating with a 45.6% gene silencing market share in 2024, growing at a CAGR of 18.4%.
CRISPR-Cas9 – Fastest-growing segment at a CAGR of 21.2%.
Antisense Oligonucleotides (ASOs) – Significant adoption in genetic therapeutics.
DNA Methylation-Based Silencing – Emerging applications in epigenetic modifications.
By Delivery Method
Nanoparticle-Based Delivery – Leading with a 42.7% market share, growing at 19.6% CAGR.
Viral Vector-Based Delivery – Expanding at a CAGR of 18.9%.
Electroporation & Physical Delivery Methods – Increasing adoption in clinical applications.
Chemical Delivery Methods – Advancements in stability and efficacy.
By Disease Type
Cancer – Leading with a 38.9% market share, projected to grow at a CAGR of 19.7%.
Neurodegenerative Diseases – Fastest-growing at a CAGR of 20.3%.
Hereditary and Infectious Diseases – Expanding clinical applications.
Cardiovascular Diseases and Others – Rising demand for novel gene therapies.
By Application
Therapeutics – Dominating with a 60.4% market share, growing at 19.9% CAGR.
Research & Development – Expanding as biotech firms invest in preclinical research.
By End-User
Biotechnology & Pharmaceutical Companies – Holding a 48.7% market share, growing at 18.8% CAGR.
Academic & Research Institutes – Increasing focus on CRISPR-based studies.
Contract Research Organizations (CROs) – Expanding service offerings.
Hospitals & Diagnostic Centers – Rising adoption of gene silencing diagnostics.
By Region
North America – Leading with a 46.2% market share, fueled by R&D investments and regulatory approvals.
Asia Pacific – Fastest-growing at a CAGR of 20.8%, driven by biotech innovations in China, Japan, and India.
Europe, South America, and Middle East & Africa – Steady market expansion.
Competitive Landscape
Key industry players include:
Alnylam Pharmaceuticals – Expanding RNAi-based therapeutic portfolio.
Benitec Biopharma Inc. – Partnering for next-generation RNAi therapies.
Phio Pharmaceuticals – Advancing RNAi-based cancer immunotherapy.
Avidity Biosciences, Riboxx GmbH, Integrated DNA Technologies (IDT), Dyne Therapeutics, Bit Bio, Comanche Biopharma, Thermo Electron Corporation, Temasek Life Sciences Laboratory Ltd., WuXi AppTec – Innovating in RNA-based therapeutics and strategic collaborations.
Emerging Trends and Future Outlook
Key Gene Silencing Market Trends
Expansion of Personalized Gene Therapies – Advancements in tailored treatments based on genetic profiling.
AI-Driven Drug Discovery – Integration of machine learning for gene target identification.
Development of Non-Viral Delivery Methods – Enhancing safety and efficiency.
CRISPR-Based Diagnostics and Therapeutics – Rapidly evolving applications in precision medicine.
Blockchain for Genetic Data Security – Addressing concerns related to data privacy and patient confidentiality.
Purchase Exclusive Report: https://www.statsandresearch.com/enquire-before/40640-global-gene-silencing-market
Future Projections
With continued advancements in genetic research, evolving regulatory frameworks, and increasing global investments in genomic medicine, the gene silencing market is set for sustained expansion. Companies investing in breakthrough RNA-based therapeutics, innovative gene-editing tools, and AI-driven bioinformatics will lead the next phase of growth. As clinical trial success rates improve and new partnerships emerge, gene silencing technologies will play an integral role in reshaping the future of medicine.
Our Services:
On-Demand Reports: https://www.statsandresearch.com/on-demand-reports
Subscription Plans: https://www.statsandresearch.com/subscription-plans
Consulting Services: https://www.statsandresearch.com/consulting-services
ESG Solutions: https://www.statsandresearch.com/esg-solutions
Contact Us:
Stats and Research
Email: [email protected]
Phone: +91 8530698844
Website: https://www.statsandresearch.com
#Gene Silencing Market#Gene Silencing#RNA Interference#siRNA Market#CRISPR Technology#Epigenetics Market#RNA Therapeutics#Genetic Research#Biotechnology Trends#Gene Therapy#Molecular Biology#Genome Editing#Bioinformatics#Life Sciences#Drug Development#Targeted Therapies#Pharmaceutical Innovations#Genetic Disorders#Biotechnology Market#Healthcare Research#Biomedical Research
1 note
·
View note
Text
youtube
#Nanomaterials in cancer#leukemia treatment#targeted therapy#nanomedicine#nanoparticles#drug delivery#cancer therapy#precision oncology#nanotechnology#CRISPR cancer#RNA interference#liposome nanoparticles#gold nanoparticles#polymeric nanoparticles#antibody-drug conjugates#biomarker targeting#personalized medicine#drug resistance#smart drug delivery#cancer nanotechnology.#Youtube
0 notes
Text
Celebrating World RNA Day: Exploring the Wonders of Ribonucleic Acid 🌍🔬
Happy World RNA Day! 🌍🔬 Celebrate the incredible world of RNA and its vital role in biology and medicine. Learn about RNA, support research, and promote STEM education. #WorldRNADay #RNAResearch
Introduction Happy World RNA Day! 🌍🔬 Celebrated annually on August 1st, World RNA Day is dedicated to recognizing and appreciating the crucial role of RNA (ribonucleic acid) in biology and medicine. RNA is essential for numerous biological processes, including protein synthesis and gene regulation. Today, we celebrate the scientific discoveries and innovations surrounding RNA and its profound…
#biotechnology#CRISPR-Cas9#gene regulation#Katalin Karikó#molecular biology#mRNA vaccines#RNA interference#RNA research#STEM education#World RNA Day
0 notes
Text
Galectina-1: non solo marker ma anche potenziale bersaglio per contrastare il cancro al fegato
Il carcinoma epatico (HCC) o cancro al fegato, è uno dei tumori più comuni al mondo. E i numeri sono in aumento, con tassi di incidenti più che triplicati rispetto agli anni ’80. La malattia può essere anche piuttosto mortale: negli stadi avanzati il tasso di sopravvivenza a cinque anni è inferiore al 20%. I ricercatori del Davis Comprehensive Cancer Center dell’Università della California hanno…
View On WordPress
0 notes
Text
Ecofemminismo della petunia
Del giardino mi piace soprattutto quanto sia vicino all’imponderabile, a quel punto ineffabile in cui natura e cultura, fisica e metafisica, tangibile e simbolico coabitano in un tempo che è dentro e fuori le cose. Un tempo pieno di sorprese. Non ponderabile significa non misurabile ma non per questo inesistente. In passato quando il colore, la luce, il calore, l’elettricità, il magnetismo non…

View On WordPress
#Andrew Z. Fire#cambiamenti climatici#Craig C. Mello#ecofemminismo#ecologia#femminismo#genetica#habitat#Harry Potter#HIV#Il lavoro culturale#J. K. Rowling#Jung#Mano#Nobel#petunia#Petunia Addmas#Petunia Evans Dursley#RNA interference#sincronicità#Viviana Scarinci#zia Petunia
0 notes
Text
RNA Interference (RNAi) Drug Delivery Market Share, Overview, Competitive Analysis and Forecast 2031
#RNA Interference (RNAi) Drug Delivery Market#RNA Interference (RNAi) Drug Delivery Market Scope#RNA Interference (RNAi) Drug Delivery Market Report#RNA Interference (RNAi) Drug Delivery Market Research
0 notes
Note
Hello! First of all, thank you for the wonderful content! It's a real joy, and an enrichment, food for both the brain and the heart! I was wondering if through your treasures, you could find some writing notes/words/concepts/vocabulary relating to genetic engineering? Like...creating a virus, and a vaccine for it, modifying the virus so it has certain specific effects.... Thank you in advance!
Writing Notes: Virus & Vaccine
References How Viruses Work; Replication Cycle; Mutation, Variants, Strains, Genetically Engineering Viruses; Writing Tips; Creating your Fictional Virus & Vaccine
Virus - an infectious microbe consisting of a segment of nucleic acid (either DNA or RNA) surrounded by a protein coat.
It is a tiny lifeform that is a collection of genes inside a protective shell. Viruses can invade body cells where they multiply, causing illnesses.
It cannot replicate alone; instead, it must infect cells and use components of the host cell to make copies of itself. Often, a virus ends up killing the host cell in the process, causing damage to the host organism.
Well-known examples of viruses causing human disease include AIDS, COVID-19, measles and smallpox. Examples of viruses:
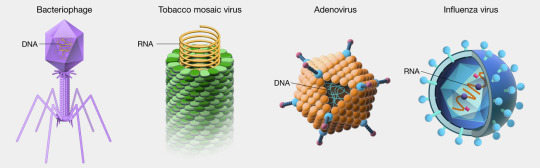
Viruses are even smaller than bacteria and can invade living cells—including bacteria. They may interfere with the host genes, and when they move from host to host, they may take host genes with them.
Bacteriophages (also known as phages)—viruses that infect and kill bacteria.
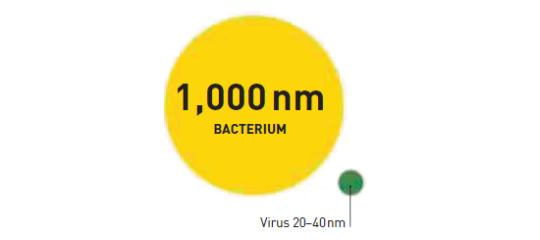
Size differential between virus and bacterium
Viruses are measured in nanometers (nm).
They lack the cellular structure of bacteria, being just particles of protein and genetic material.
How Viruses Work
Viruses use an organism’s cells to survive and reproduce.
They travel from one organism to another.
Viruses can make themselves into a particle called a virion.
This allows the virus to survive temporarily outside of a host organism. When it enters the host, it attaches to a cell. A virus then takes over the cell’s reproductive mechanisms for its own use and creates more virions.
The virions destroy the cell as they burst out of it to infect more cells.
Viral shedding - when an infected person releases the virus into the environment by coughing, speaking, touching a surface, or shedding skin.
Viruses also can be shed through blood, feces, or bodily fluids.
Virus Replication Cycle
While the replication cycle of viruses can vary from virus to virus, there is a general pattern that can be described, consisting of 5 steps:
Attachment – the virion attaches to the correct host cell.
Penetration or Viral Entry – the virus or viral nucleic acid gains entrance into the cell.
Synthesis – the viral proteins and nucleic acid copies are manufactured by the cells’ machinery.
Assembly – viruses are produced from the viral components.
Release – newly formed virions are released from the cell.
Mutations, Variants, and Strains
Not all mutations cause variants and strains. Below are definitions that explain how mutations, variants, and strains differ.
Mutation - errors in the replication of the virus’s genetic code; can be beneficial to the virus, deleterious to the virus, or neutral
Variants - viruses with these mutations are called variants; the Delta and Omicron variants are examples of coronavirus mutations that cause different symptoms from the original infection
Strains - variants that have different physical properties are called strains; these strains may have different behaviors or mechanisms for infection or reproduction
Genetically Engineering Viruses
Using reverse genetics, the sequence of a viral genome can be identified, including that of its different strains and variants.
This enables scientists to identify sequences of the virus that enable it to bind to a receptor, as well as those regions that cause it to be so virulent.
Vaccine - a special preparation of substances that stimulate an immune response, used for inoculation
Vaccines & Fighting Viruses with Viruses
Common pathogenic viruses can be genetically modified to make them less pathogenic, such that their virulent properties are diminished but can still be recognized by the immune system to produce a robust immune response against. They are described as live attenuated.
This is the basis of many successful vaccines and is a better alternative than traditional vaccine development which typically includes heat-mediated disabling of viruses that tend to be poorer in terms of immunogenicity.
Viruses can also be genetically modified to ‘fight viruses’ by boosting immune cells to make more effective antibodies, especially where vaccines fail. Where vaccines fail, it is often due to the impaired antibody production by B-cells, even though antibodies can be raised against such viruses – including HIV, EBV, RSV & cold-viruses.
Related Articles: Modified virus used to kill cancer cells ⚜ Genetic Engineering ⚜ Engineering Bacterial Viruses ⚜ Benefits of Viruses
A Few Writing Tips
As more writers look to incorporate infectious diseases into their work, there are quite a few things writers should keep in mind:
Don’t anthropomorphize. Really easy to do, but scientifically wrong. Viruses don’t want to kill you; bacteria don’t want to infect you; parasites don’t want to make your blood curdle. None of these things are big enough to be sentient to want to do anything. They just do it (or don’t do it).
Personal protective equipment. This includes wearing gloves, lab coats, safety glasses, and tying your hair back if it’s long. It is the same as Edna Mode’s “no capes.” Flowing hair looks cool all the way to the explosive ball of flames that engulfs someone’s head.
Viruses are small. You can’t see viruses down a normal microscope—they need a special microscope called an electron microscope. These are highly specialized and take a long time to make the preparations to be able to see the virus. Normally viruses are detected by inference—measuring part of them using an assay that can amplify tiny amounts of material, for example PCR.
Viruses don’t really cause zombie apocalypses.
Vaccines work. But they take time. The best vaccine in the world will still only prevent infections two weeks after it is given. Drugs are quicker, but still take some time. But the good news is an infection is not going to kill you (or turn you into a zombie) quickly, so they both have time to work.
Scientists use viruses as a vector to introduce healthy genes into a patient’s cells:
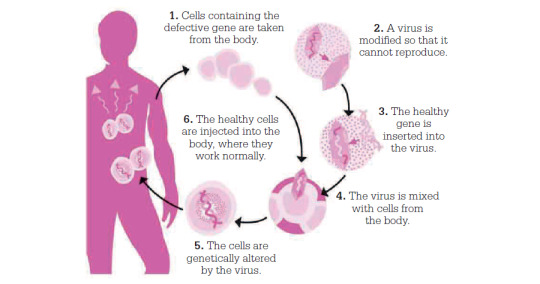
Your Fictional Virus & Vaccine
When creating your own fictional virus, research further on the topic and consider choosing a specific one as your basis/inspiration.
Here's one resource. For some of them, you'll need a subscription to access, but those that are available give you a good overview of the virus, as well as treatment options.
You can do the same for creating your fictional vaccine:
Here's one resource. And here's one on vaccine developments.
Sources: 1 2 3 4 5 6 7 8 9 10 11 12 13 ⚜ Writing Notes & References
Lastly, here's an interesting article on how science fiction can be a valuable tool to communicate widely around pandemic, whilst also acting as a creative space in which to anticipate how we may handle similar future events.
Thanks so much for your kind words, you're so lovely! Hope this helps with your writing. Would love to read your work if it does :)
#writing notes#virus#vaccine#writeblr#dark academia#spilled ink#writing reference#writing prompt#literature#science#writers on tumblr#creative writing#fiction#novel#light academia#lit#writing ideas#writing inspiration#writing tips#science fiction#writing advice#writing resources
132 notes
·
View notes
Text
New Safer RNA Insecticide Can Target Only the Devastating Potato Beetles and No Other Bugs https://www.goodnewsnetwork.org/new-safer-rna-insecticide-can-target-only-the-devastating-potato-beetles-and-no-other-bugs/
#good news#insecticides#environmentalism#science#environment#pesticides#nature#conservation#RNA#agriculture#agritech#green technology
56 notes
·
View notes
Text
By Nicolas Hulscher, MPH
A comprehensive literature review by Mathilde Debord titled “COVID-19 mRNA vaccines can induce cancer in 17 distinct ways, according to over 100 studies” was just published in Le Point Critique. Drawing from over 100 peer-reviewed studies, it outlines 17 distinct biological mechanisms by which the injections may initiate, accelerate, or reactivate malignant processes.
Below is a summary of the 17 mechanisms identified (the references supporting these statements can be found in the article):
1. Genome Instability
mRNA may be reverse-transcribed and integrated into host DNA, triggering mutations that initiate cancer.
2. Immune Escape
The spike protein binds and inhibits tumor suppressor genes like p53 and BRCA1, shielding cancer cells from immune destruction.
3. Impaired DNA Repair Mechanism
The spike protein interferes with essential DNA repair enzymes, increasing the risk of unchecked mutations.
4. Chronic Inflammation
Lipid nanoparticles and spike protein cause long-lasting inflammation, a well-known driver of cancer.
5. Dysregulation of the Immune System
Suppression of T cells and type I interferon weakens cancer surveillance and promotes immune evasion.
6. RNA Disruption
Codon optimization disrupts microRNA networks, destabilizing cell growth regulation and apoptosis.
5 notes
·
View notes
Text
Medical innovations and scientific advances at Harvard Medical School through the decades (Part 2 of 2)
1995 Triple-organ transplant; kidney disease blood glucose levels
1996 How cells sense oxygen; Alzheimer's treatments; immune system advances
1997 p73 gene; aspirin
1998 Adult live-donor liver transplant
1999 Fluorescent molecular probes
2000 Brain abnormalities associated with abuse and neglect
2001 Circadian clock
2002 Rheumatoid arthritis pathway; C-reactive protein
2003 Multi-drug-resistant tuberculosis treatment; source of pre-eclampsia
2004 Blood stem cells; protein transfer
2005 Prenatal nutrition; herpes vaccine candidate
2006 Cholesterol mechanism; DNA sequencing techniques
2007 Cellular switch; rheumatoid arthritis gene; brown-fat cell switch
2008 RIPKI inhibitors; metastatic melanoma remission
2009 LIN28 protein; RNA interference; cancer cells' starvation; brown fat
2010 Enhancer transcription
2011 Kidney failure markers; cancer cell vulnerability; global health care budget models
2012 Tumour suppressor gene p53; ancient migration; infectious disease diagnostics
2013 Cardiac hypertrophy reversal; cathepsin k pathways
2014 Hematopoietic stem cells; pancreatic stem cells
2015 Bioartificial replacement limb; PD-1 pathway; The Lancet Commission on Global Surgery; pseudogene; damaged protein disposal; multiple sclerosis; somatic mutations; deafness gene therapies
2016 Sigma-1 receptor structure; Zika vaccine candidate; circadian rhythm-bipolar disorder link; microbiome
2017 Unlocking the blood-brain barrier; deciphering the structure of a scissor like enzyme
2018 The 'graying' of T cells; From one cell, a detailed road map
2019 Finding herpes' Achilles' heel; viral peptides critical to natural HIV control
2020 How COVID causes loss of smell; obesity fuels tumour growth; heart muscle dysfunction
2021 SARS-CoV-2 vaccine; immune evasion; AI gene interpretation; radiation vulnerability
2022 Fruit fly cell atlas; viral infection on video; boot camp for immune cells
2023 How the brain senses infection; new origin of breast cancer; the microbiome and cancer immunotherapy
3 notes
·
View notes
Text
How the innate immune system manages to cope with antibody resistant SARS2 varieties
December 17, 2024 Radagast
"So, as I have been documenting over the past few years now, we’ve seen a situation in which the new coronavirus, SARS-COV-2, become forced to evolve first into increasingly infectious variants (Alpha, Delta) with higher ACE2 affinity and then into highly antibody evasive variants (the Omicron variants). This then results in a population that has a relatively wide range of antibodies, to a wide range of Spike epitopes.
That results in a situation, where SARS-COV-2 becomes increasingly forced to increase its inherent antibody resistance. That involves the accumulation of sugar molecules (glycans) on the N-Terminal Domain, that prohibit the antibodies from binding that are now necessary for neutralization. This interplay between the vaccine, the immune system and the virus, is a process that takes many years to unfold.
What critical thinkers would ask themselves, is why we don’t just see every virus that regularly reinfects humans develop a bunch of glycans on its surface, if that allows viruses to render an antibody response useless. Logic would suggest there has to be some sort of cost involved for a virus, in covering a viral protein in these glycans that prohibit antibodies from binding to the protein.
This is a correct assessment. The innate immune system evolved various mechanisms to recognize basic patterns that pathogens and misbehaving cells in our bodies tend to display. As one example, our cells are forced to display small bits of proteins they’re producing in their MHC molecule on their surface. This allows your T cells to inspect whether they’re producing the right proteins, or whether their protein factory was hijacked by a virus.
Many viruses thus evolved mechanisms to interfere in this phenomenon, by stopping cells from displaying the MHC molecule on their surface altogether, so that the T cells can’t inspect what’s going on. The human immune system of course has to have ways to deal with that behavior of viruses. So what you see is that our Natural Killer cells, a population part of the innate immune system, treat it as suspicious when a cell fails to produce the MHC molecule, and weigh it as a factor part of their complex calculation on whether a cell should be killed or not.
The innate immune system has various other such clever mechanisms. There are specific molecules it produces, that allow it to recognize proteins that are unusually densely covered in these antibody-blocking glycans. These molecules are called Lectins. Lectins are what we call carbohydrate binding proteins that seek out sugar groups part of bigger molecules.
When it comes to the immune system, C-type Lectins appear to be the most relevant in our defense. These are proteins expressed by most cells part of the innate immune system. There are many different types of C-type Lectins and they tend to look specifically for proteins that have a high density of glycans.
That is, the recognition is density dependent. A normal protein part of our body may have some glycans, but a very high density of glycans on a protein reveals to the innate immune system that something weird may be going on that requires intervention.
As I have explained a few times before, natural immunity results in the expansion of the population of plasmacytoid dendritic cells, which recognize viral RNA and/or DNA. This is only possible when the first exposure occurs in the absence of an adaptive immune response induced by previous vaccination, as otherwise the B cells will just deal with an infection, before the plasmacytoid dendritic cells ever get to see the virus and proliferate in response.
When the plasmacytoid dendritic cells detect viral RNA/DNA, through their toll like receptors, they start to produce large amounts of Interferon alpha, which is a molecule that evolved to interfere in just about every step of the viral reproductive cycle. However, how much Interferon alpha they produce, is also dependent on secondary factors.
One of these factors, is whether their own specialized C-type lectin receptors like CLEC4C, recognized some protein that’s densely covered in glycans. If that is the case, they boost their interferon alpha production. For the plasmacytoid dendritic cells it becomes easier to realize it’s time to do their job, when the glycan density on the Spike protein starts to increase.
Another place where you see the innate immune system respond differently in breakthrough infections versus natural immunity, is in the brain. What you see here is that a population of monocytes gets to enter the brain upon infection, that does not get to enter the brain if someone was vaccinated before being infected. You also see an increase in Natural Killer cells and Dendritic cells in the brain.
The natural killer cells recognize whether a cell is infected by the virus and then decide whether the infected cells should be killed or not. But the monocytes and the dendritic cells also have an important job: Their job is to “eat” viral particles.
The dendritic cells try to capture viral particles, so that they can then degrade the viral particles with their lysosomes. But how do the denritic cells capture viral particles? They use their C-type lectin receptors for that!
In other words, what you would expect to see, is that as the dendritic cells now become faced with variants of SARS-COV-2 with more glycans on the Spike protein, they start to be able to do their job more effectively.
In essence, what’s currently happening is that SARS-COV-2 is being forced by the mass vaccination experiment, to evolve in a direction that makes it easier for the innate immune system to recognize the virus.
This is good for young people, as their innate immune system tends to be strong and capable. After all, it has to be able to protect them against all sorts of pathogens, as they normally don’t have any adaptive immunity yet against most of the pathogens that circulate (except for the passive adaptive immunity from breastfeeding).
You would expect this to cause problems however, for people whose adaptive immune system is mainly responsible for suppressing this virus. After vaccination, antibody concentration are about fifty times higher than normally seen after infection.
Constant breakthrough infections have not stimulated innate immunity. Rather, they just recall and broaden the adaptive immune response developed as a consequence of vaccination with non-live vaccines.
Once antibodies against the Receptor Binding Domain became unable to solve the problem, the immune system developed a type of antibody that targets part of the Receptor Binding Domain and part of the N-Terminal Domain (the N1 loop), to which the virus then responded with BA.2.86, which has a unique insertion mutation exactly in the part where these antibodies bind.
This BA.2.86 lineage wiped out all other lineages, revealing that most of the world’s population depends very strongly on the antibody response to keep the virus under control. The body then developed antibodies to this new version of the N1 loop, to which the virus then began to respond by putting the glycans on the N1 loop.
This is why you’re dealing with a situation where everyone keeps catching SARS-COV-2 and getting sick as a result.
All these elegant receptors our innate immune cells have to recognize glycoproteins like the Spike protein, like the C type lectin receptors, tend to depend on the Spike protein not being covered by antibodies. If there are antibodies on the Spike protein, those receptors bump into the antibodies, rather than managing to bind the Spike protein.
This is important to understand: If the antibodies are already on the job, they have to solve the job. And so when the virus has mutated to make the antibodies that bind to it of poor quality and to mainly keep around enhancing antibodies, that bind in places where they won’t stop the Spike protein from correctly binding to the ACE2 receptor, the immune system is forced to start targeting more and more regions of the Spike protein (immune refocusing).
Worst of all perhaps, some of these antibodies directed against SARS-COV-2, seem to cross-react with other respiratory viruses, like Influenza, where they bind to the glycans, but don’t neutralize the protein. So, these antibodies against SARS-COV-2, seem to be making it more difficult for the immune system to deal with other respiratory viruses too, because it’s just much harder for the C-type lectin receptors of the innate immune cells to bind to a protein when it already has these antibodies on it, particularly on its glycans.
You see an epidemic of various respiratory viruses around the world right now, sickening people at abnormally high levels. You need to be asking yourself, what the cause of that is. Some of it may be damage to the immune system, some of it may be due to antibodies against SARS-COV-2 interfering in the innate immune system’s ability to deal with those viruses. I already warned about this long ago.
The point I wish to make clear however with this post, is that it’s inappropriate to expect that the evolution of SARS-COV-2 towards a glycan-covered antibody resistant virus would increase its inherent virulence for everyone.
Instead, what you would expect to see, is that as these glycans accumulate on the Spike protein, the virus will increasingly begin to sicken people who depend on an adaptive immune response against it, whereas when the innate immune system handles the response to this virus, the impact on people’s health will start to decline.
Who cares about any of these details? Well, I’m explaining this for a reason. Immunologists are currently in the process of developing new types of SARS-COV-2 vaccines, that manage to evade recalling the original antigenic sin antibodies and encourage the development of new antibodies instead.
BUT THIS IS THE WRONG APPROACH!
You are very clearly dealing with a virus, that is increasing its glycan density!
And when a virus is rapidly increasing its glycan density, the immune system becomes increasingly dependent on the innate immune response to deal with it, as it just becomes easier to recognize it through the C-type lectins, while the most important parts of the virus for antibody mediated neutralization become inaccessible due to the glycans!
You have to figure out how to suppress the adaptive immune response, allowing the innate immune system to take over and do its job. I have seen just one approach that looks viable to me: Cannabinoids like CBD can suppress adaptive immunity, while encouraging NK cell activity.
It’s not coincidence, that you see better immunological functioning in HIV infected people with strong cannabis use. You see a DECREASED VIRAL RESERVOIR, in cannabis using HIV infected people. Because HIV rapidly mutates and establishes persistent infections, an antibody response is the wrong tool for the job. HIV already covers itself in a dense glycan shield.
Heavy cannabis use has the effect in HIV infected people of shifting their immune response to HIV more towards dependence on the innate immune system. For a respiratory virus like SARS-COV-2, which is still mostly targeting the lungs of vaccinated people, vaporized cannabis would seem like a proper candidate to me, to reduce the immunological abnormalities that were induced by vaccination. The terpenes are also known to have beneficial stimulating effects on the innate immune system.
Look, I understand this is just a weird blog, but look around you. People are coughing everywhere. They’re collapsing on stage. The hospitals are overwhelmed, there’s an epidemic of “walking pneumonia”, at record levels that have never been seen before since we started measuring in the 90’s. People don’t have to believe me, you can just connect the dots yourself.
This is not just some inherent trait of SARS-COV-2, it is mostly a consequence of provoking an inappropriate immune response towards SARS-COV-2. It really doesn’t have to be like this."
#covid-19 vaccine#covid-19#innate immune system#glycans#steric immune refocusing#C-type Lectins#print this off later
2 notes
·
View notes
Photo
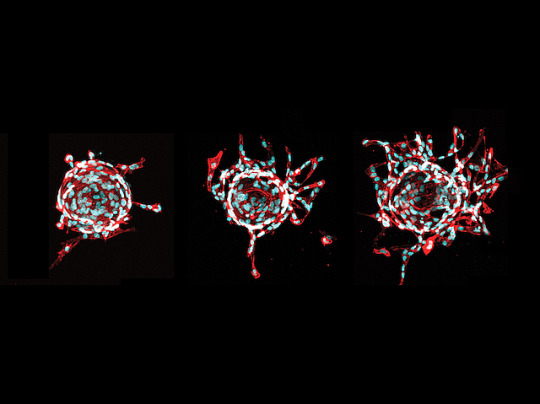
Stopping Sprouts
Like roads in a city, veins in your body need careful mapping. When they don’t stick to the plan, sporadic vascular malformation – malformed veins – occur, causing pain and disfigurement. These veins form lesions containing genetically faulty cells (mutants). Currently, lesions often recur after treatment. Researchers now search for new therapeutics by growing mutant endothelial cells (ECs), collected from the veins of patients, in dishes. RNA sequencing revealed mutant ECs produced the signalling molecule TGFa. Fluorescence microscopy revealed when supporting cells from lesions (stromal cells) were grown alongside normal ECs, they triggered more EC sprouting (pictured, middle, right) compared with non-lesion stromal cells (left). Next, by grafting mutant ECs into mice, they found EC-produced TGFa triggered stromal cells to release the signalling molecule VEGFA. This caused EC sprouting and malformed veins. Treating mice with afatinib, a drug that interferes with VEGF signalling, decreased lesions, suggesting it may be a useful therapeutic.
Written by Lux Fatimathas
Image from work by Suvi Jauhiainen and Henna Ilmonen, and colleagues
A.I. Virtanen Institute for Molecular Sciences, University of Eastern Finland, Kuopio, Finland
Image originally published with a Creative Commons Attribution 4.0 International (CC BY 4.0)
Published in eLife, May 2023
You can also follow BPoD on Instagram, Twitter and Facebook
#science#biomedicine#angiogenesis#blood vessels#vegf#immunofluorescence#veins#endothelial cells#capillaries#afatinib
15 notes
·
View notes
Text
I love talking to non biologists about my research because I get to think of fun and creative ways to explain oxidative stress or RNA interference or freeze tolerance and usually I'm pretty successful at conveying it and that make me feel good :) a couple weeks ago a sociologist asked me what a model organism is and then why we would want to work with something that isn't a model organism and that was a fun brain exercise too
6 notes
·
View notes
Text
Japan's 30-year recession and innovation (Essay)

Professor Kaliko (m-RNA vaccine inventor)
Since the bubble economy collapsed in the 1990s, Japan has been stuck in a 30-year recession. Workers' wages are shrinking, and Japan is the only developed country in the world to experience subsidence. There are various reasons for this phenomenon, but it is probably due to the government's wrong policies (the Liberal Democratic Party).
What is most troubling to the population is the ultra-low interest rate policy introduced after Abenomics, launched by the exiled politician Shinzo Abe. However, the underlying cause is much deeper. The real culprit is Prime Minister Junichiro Koizumi. This man believed in neoliberalism and applied this false economics to the world of education. -- ``Selection and concentration,'' placing fixed rankings on universities, sparing research funding, and only allowing research that would produce immediate results. Researchers atrophied, and original research faded into obscurity. There are almost no Japanese Nobel Prize winners in science anymore.
This hindered innovation, and Japan no longer developed novel science and technology. Look, isn't the USA, with its active innovation, currently leading the world? The interference of science and technology amateurs in this field is the cause of Japan's current stagnation. Junichiro Koizumi's sin is serious.
Listening to the statements made by the Japanese government, the central bank (Bank of Japan), politicians, and the Japan Federation of Economic Organizations (Keidanren), I find that while they mention money redistribution, they rarely say anything about innovation in science and technology. Today, Japan is dominated by people with liberal arts backgrounds, not science and engineers. Because they are ignorant of science and technology, they have no idea that innovation determines a country's rise and fall. Japan is on the path to becoming a second-rate country. The Bank of Japan now (Head: Kazuo Ueda) is a group of idiots. No matter how much they twist finance, it will not lead to innovation.
Rei Morishita
日本の30年不況とイノベーション
1990年代にバブル経済が破綻して以降、日本は30年にわたる不況から抜け出せない。労働者の賃金は目減りし、先進国では唯一地盤沈下している。この現象の要因は、いろいろ言われるが、もとは政府=自民党の誤った諸政策に起因するであろう。
もっとも人口に膾炙しているのは、亡国政治家安倍晋三が始めたアベノミクス以降の超低金利性政策だが、深層はもっと根深い。ずばり、真犯人は小泉純一郎首相である。この男は新自由主義の信奉者で、教育の世界にも、この誤った経済学を適用した。――「選択と集中」、大学に固定した順位をつけ、研究費を出し惜しみして、すぐに結果のでる研究しか認めなかった。研究者は委縮し、独創的な研究は影を潜めた。もう、科学におけるノーベル賞受賞者は、日本人からはほとんどでないだろう。
これはイノベーションを阻害し、日本には斬新な科学技術は生まれなくなった。見よ、現在世界をリードしているのはイノベーションが活発なUSAではないか。科学技術の素人がこの分野に口を出したことが今の日本の停滞の元凶なのである。小泉純一郎の罪は重い。
日本の政府、中央銀行(日本銀行)、政治家、経団連の発言を聞いていると、お金の再配分のことは言及しても、科学技術のイノベーションについての発言はほとんどない。今の日本を支配しているのは、理系の科学技術者ではなく、文科系の出身者ばかりである。彼らは科学技術に無知であるから、国の興亡を左右するのがイノベーションであることが全く理解できない。日本は2流国への道をまっしぐらである。日本銀行もバカ集団だ。金融をいくらこねくりまわしても、イノベーションにはつながらない。
#Japan's 30-year recession and innovation#essay#rei morishita#Kaliko#Prime Minister Junichiro Koizumi#neoliberalism#innovation#The Bank of Japan#finance
4 notes
·
View notes
Text
interesting! it's not clear from the pull quote, but these vaccines are "RNA-based" in a very different way than the mRNA vaccines we've been using for COVID-19.
mRNA vaccines introduce mRNA sequences that code for a viral protein, causing the body's cells to produce these proteins which then trigger an immune response, most importantly activating B and T immune cells and antibodies, some of which will stick around and be able to specifically recognize that viral protein in the future and attack viruses with that protein. one problem with these vaccines is that, if those viral proteins mutate too much, this protection will be weakened or disappear.
the vaccines discussed in this article, on the other hand, are live viruses altered to get rid of their ability to suppress the body's antiviral RNA interference (RNAi). this is a totally different mechanism of the immune system—and unfortunately my one immunology class only quickly touched on it, so I don't know how it works in great detail. basically, the body's cells recognize that there's viral RNA and produce their own little bits of RNA ("small interfering RNA," or siRNA) that match up with that viral RNA. these are then used in a system that recognizes viral RNA when it enters a cell and stops it from being translated into proteins. I think it's a recent discovery that these antiviral siRNAs continue to circulate in the bloodstream after an infection is cleared. this means that when the mice in this study were vaccinated with viruses altered to be particularly susceptible to RNAi, (1) these viruses didn't harm them because RNAi was able to clear the infection, and (2) the siRNAs that continued to circulate protected them from subsequent infections for at least 90 days.
my (non-expert) takeaways: this is really exciting as a plausible new mechanism for long-lasting immune protection which could also work for people deficient in the B cell/T cell/antibody aspects of the immune system, and in infants! as the article says, it's also more likely to work for different strains of a virus because pieces of siRNA will be produced which match (as far as I can tell) all/most of the viral RNA genome, including sections which aren't likely to mutate. a caveat: this is a proof of concept in mice and with a virus which doesn't actually infect humans. the article notes that humans produce siRNA in reponse to influenza, so it seems likely that this strategy could work against the flu. I don't know the current state of research on RNA interference in humans, so I'm curious whether we know, for example, how long siRNA remains circulating or how effective an RNA interference response is against flu.
the "universal vaccine" statement is a little misleading: a different vaccine would have to be produced for a different virus (but using the same strategy), and I don't think this could work at all against DNA viruses (e.g. chickenpox, herpes, HPV). but definitely a cool approach that I hope works out!
Scientists at UC Riverside have demonstrated a new, RNA-based vaccine strategy that is effective against any strain of a virus and can be used safely even by babies or the immunocompromised. Every year, researchers try to predict the four influenza strains that are most likely to be prevalent during the upcoming flu season. And every year, people line up to get their updated vaccine, hoping the researchers formulated the shot correctly. The same is true of COVID vaccines, which have been reformulated to target sub-variants of the most prevalent strains circulating in the U.S. This new strategy would eliminate the need to create all these different shots, because it targets a part of the viral genome that is common to all strains of a virus. The vaccine, how it works, and a demonstration of its efficacy in mice is described in a paper published today in the Proceedings of the National Academy of Sciences. “What I want to emphasize about this vaccine strategy is that it is broad,” said UCR virologist and paper author Rong Hai. “It is broadly applicable to any number of viruses, broadly effective against any variant of a virus, and safe for a broad spectrum of people. This could be the universal vaccine that we have been looking for.”
Continue Reading.
22K notes
·
View notes