Link
Extra-Life Charity is this weekend! Come help my team raise money for the Children’s Miracle network!
To donate, simply click on one of the team members (I am Claire Dearing) and then click the “Support Me” button at the top of the page.
Any and all donations can help, and there is a “custom amount” option at the bottom of the list for those who have something other than the bracketed amounts. Even if I only got a dollar from each of my followers, we’d be able to give nearly $300 to help kids with cancer and other life-threatening diseases.
Please help if you can!
Have a cat picture.

3 notes
·
View notes
Note
Hi, If serotonin is dissolved in ether, what reagent could you add to make it move to an aqueous layer(water)? Show the reaction witht the appropriate reagent (Hint: Look up the structure. The side chain is the most reactive). Plz help!!

This looks like the equation you’re working with, and the hint is that the side chain will be the most reactive, so you want to add something to make it break off. Something to sever the bonds and bind it to something like water, perhaps?
I finished the class ages ago and kind of did a brain-wipe, so I’m probably not the best person to ask anymore… ^^“
66 notes
·
View notes
Text
I have an AU that is GOLD!
430 notes
·
View notes
Text
Don’t post your negativity on a positive post.
755K notes
·
View notes
Text
Orgo Made Simple
Orgo Made Simple is a website that was created to make organic chemistry easier and more understandable for students everywhere. The site is completely free and contains chapter summaries, practice problems, and practice quizzes. Whether you feel completely lost with the material or feel you just need a little extra practice, this is the site for you. We have worked to make orgo made simple as clear as possibly without all the technical textbook language. It is the site we, as former organic chemistry students, wish we had when we were taking the class. Please feel free to check out our facebook page and website (the links are below). Don't forget to let us know what you think of the site and if you have any suggestions for new content!
https://www.facebook.com/pages/Orgo-Made-Simple/262741027267949
http://orgomadesimple.com
Thanks ,
The Orgo Made Simple Team
218 notes
·
View notes
Conversation
Schoolwork:
Me: "I love science!"
Me: *half a page into a lab report*
Me: "I don't want to be a scientist anymore..."
160 notes
·
View notes
Text
Using Formal Charge To Determine Major And Minor Contributors (Among Other Things)
Now that we know how to determine the Formal Charge, we can decide what is a Major Contributor and what is a Minor Contributor when it comes to structure.
The Major Resonance Contributor is the most stable resonance structure that exists for a molecule. The other resonance structures will be Minor Contributors.
In general, the Major Resonance Contributor:
has octets on as many atoms as possible
has reduced charge separation on the molecule (formal charges of 0 are best)
has negative charges on the more electronegative elements
has positive charges on the less electronegative elements
places the charge of carbo-cations on the more substituted carbon atom
places the charge of the carbo-anions on the less substituted carbon atom
Knowing this, the first thing you are going to want to do is look for something that does not have Formal Charges. If only one resonance structure has a Formal Charge of 0, that is the resonance structure that will be your Major Contributor, and the rest will be Minor Contributors.
If there is more than one resonance structure with a Formal Charge of 0, then you will have to look at the other bulleted points and determine which meets the criteria of a stable resonance structure best. This will be your Major Contributor. The rest will be Minor Contributors.
17 notes
·
View notes
Text
Finding Formal Charge ( To Determine Major and Minor Contributors)
So now that you know what major and minor contributors are, let's show how to calculate which one of them any given resonance structure is. The first part of doing this is determining the formal charge.
Formal charge = group number of the atom - (lone pair electrons + (1/2) Bonding electrons)
What this basically means is that you go to the periodic table and find whatever atom you are trying to find the formal charge of in the molecule. How many valence electrons does it have? This is your group number. Carbon would be 4. Nitrogen would be 5. Etc.
For the second number in the equation you find it by taking the number of lone pair electrons and adding them to half the number of bonding electrons. First, look at the molecule you have been given. How many things is the atom bonded to? Each bond is two electrons, so (1/2) bonding electrons would essentially give you the same number as the number of bonds the atom has. Because of this, I generally like to just count the bonds and add them to the lone pair electrons.
The lone pair electrons will be drawn on the atom (black dots around it). Just add these up.
Let's calculate the Formal Charge of the Nitrogen in the molecule Pyrrole:
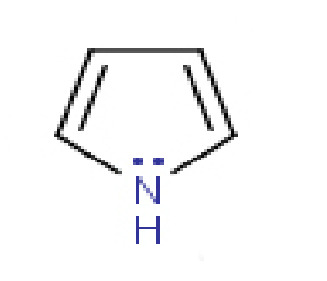
On the periodic table, Nitrogen has 5 valence electrons. This is our group number. Now, let's count the bonds. The Nitrogen here is bonded to the Hydrogen and single-bonded to the Carbons on either side of it. That's three bonds. This means six electrons total, so (1/2) of the six would be three. It also has a lone pair of electrons above it. That's 2 more electrons.
This brings the total for the second number in the equation to 5.
Remembering that the group number is 5, we fill in the numbers of our equation:
Formal Charge = (group number of 5) - (combined bonding electrons and lone pairs adding up to 5)
Formal Charge = (5) - (5)
Formal Charge = 0
In this molecule, the Formal Charge of the Nitrogen is 0, so the Nitrogen does not have a formal charge.
16 notes
·
View notes
Text
Frost Diagram For A 4-Sided Shape
Here's where things get a bit more complex than the Frost Diagrams we have been doing so far. The first few steps are the same as the others:
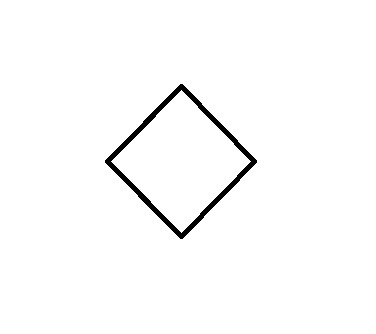
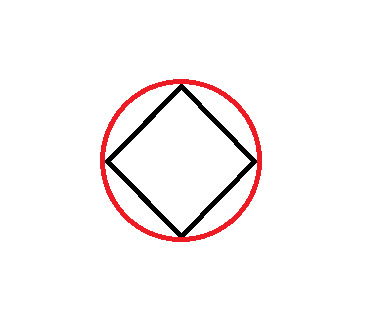
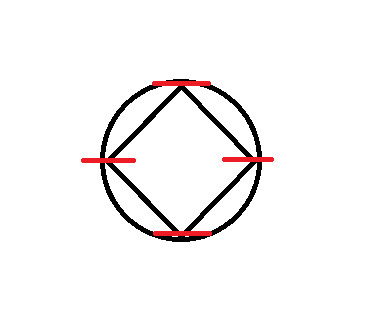
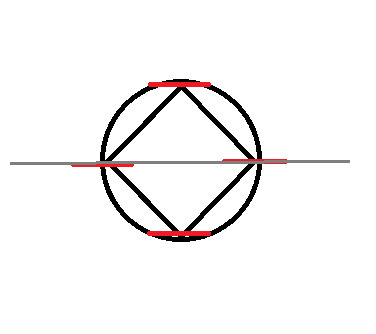
Now you'll notice here that our grey line dividing the circle in half goes through two of the points where the corners touch the circle. These cannot be classified as bonding or anti-bonding. They are non-bonding. This is signified with an "n":
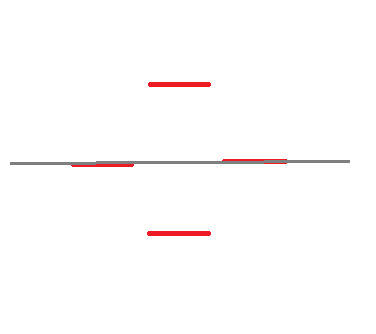
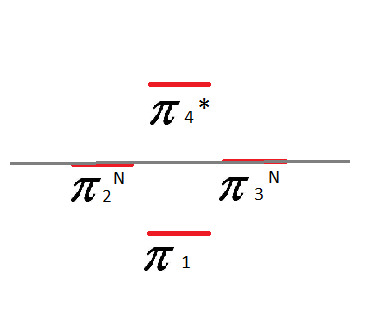
When it comes to assigning the four valence electrons, we start with the bottom and put 2 on each line, like with the Benzene.

For the second layer - the non-bonding pi-bonds - we fill in the electrons one at a time, going back and forth between the two sides until they are used up. In this case, that leaves us with one electron in each one:
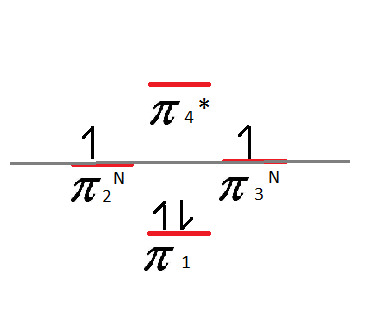
This is a highly unstable compound due to these non-bonding electrons.
This also makes it anti-aromatic.
12 notes
·
View notes
Text
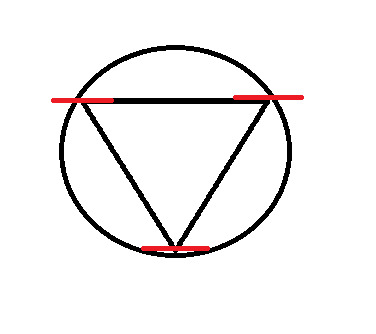
Frost Diagram For A 3-Sided Shape
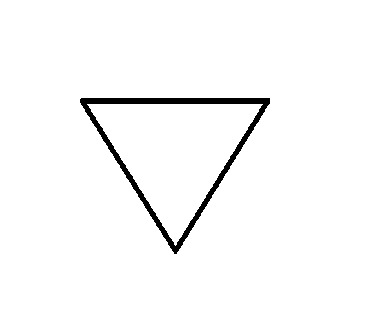
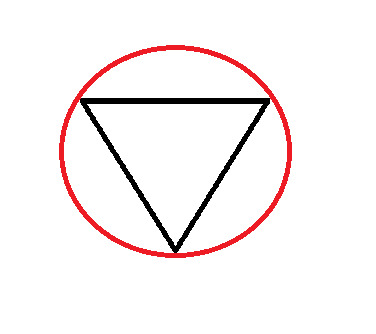
5 notes
·
View notes
Text
Frost Diagram For A 5-Sided Shape
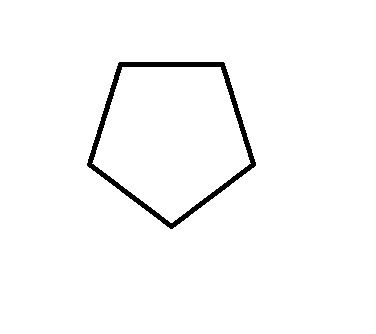
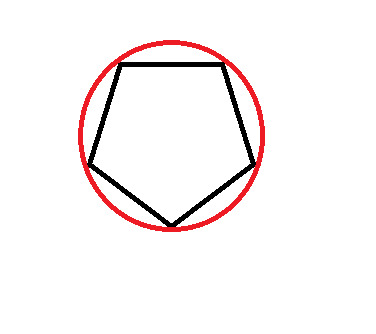
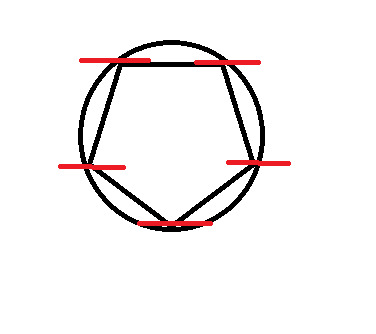
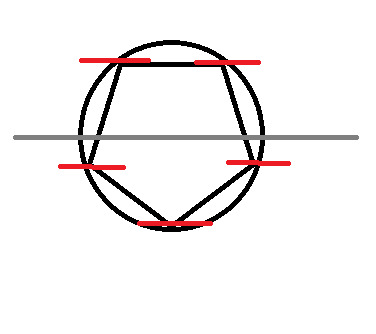
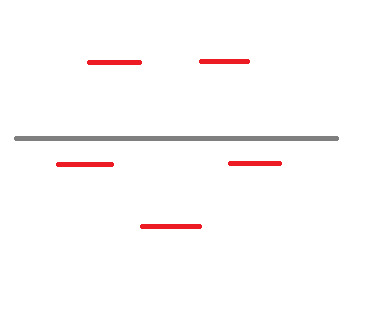
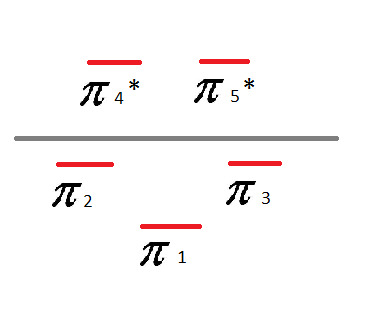
8 notes
·
View notes
Text
Frost Diagrams
Frost Diagrams are used to help draw the Molecular Orbitals for Valence Bond Structures.
For example, say I have Benzene, and I want to know how many bonding electrons vs. non-bonding electrons it has. Our Valence Bond Structure looks like this:
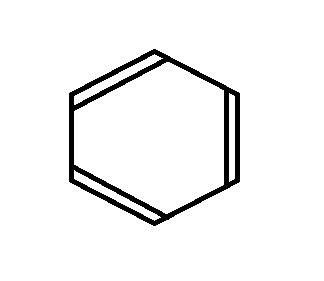
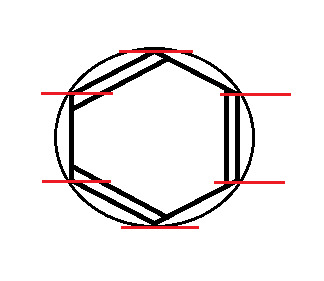
The first thing we do is draw the shape, with the point (any point) facing down. Then, we draw a circle around it, so that the tips of the shape are touching the edge of the circle.
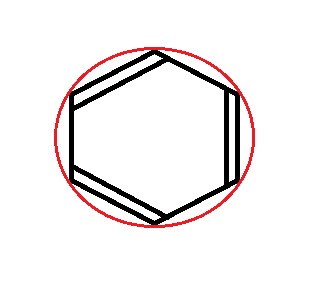
At every point where the two touch, draw a vertical line.
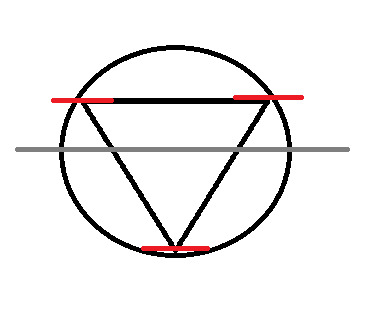
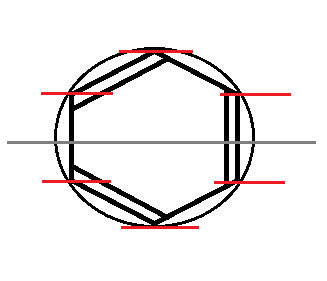
Now, split the circle in half, horizontally
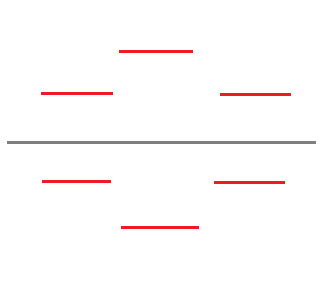
At this point, we no longer need the original shape, so I'm going to remove it.
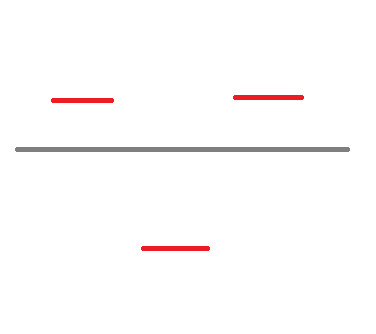
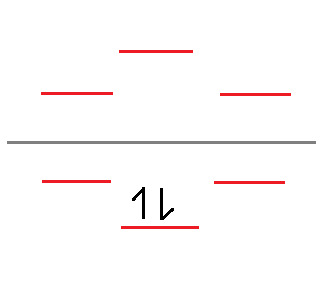
What we have here is the skeleton structure for finding the Molecular Orbitals of the structure. Now, we know that Benzene has 6 valence electrons. Let's start assigning them, 2 per line, starting at the bottom.
We draw arrows with single fishhooks on the end to indicate electrons. This is the first level done. Now we split the electrons evenly between the lines on the next level - in this case, 2 each:
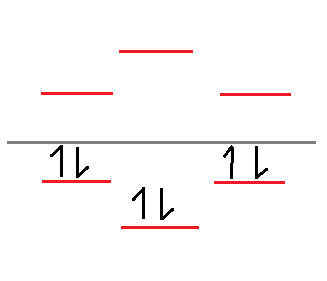
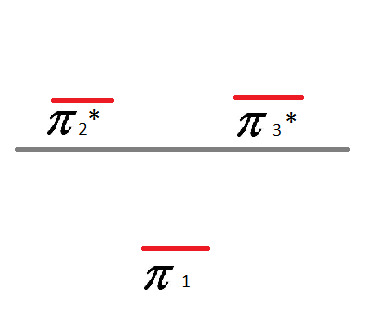
Now that we've assigned all of the valence electrons, let's label the lines. Below the line - pi bonds. Above the line - pi anti-bonds. Like so:
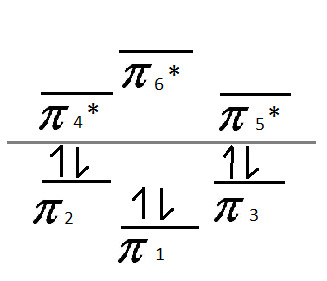
And voila! There is your Frost Diagram for Benzene.
I'll put up Frost Diagrams for some other cyclo-molecules later today.
#organic chemistry#organic chemistry 2#frost diagrams#pi bonding#anti-bonding#molecular orbitals#valence electrons
47 notes
·
View notes
Text
Aromaticity means lower energy, more stability, and less heat energy when the compound is burned.
15 notes
·
View notes
Text
Resonance Structures and De-Localization
When you draw a molecule, you are not drawing what it actually looks like. What you are really drawing is one of its resonance structures.
There are multiple ways to draw the Lewis structures for many of the chemical compounds we use, each of which is a simplification of the real molecule's structure. These are Resonance Structures.
The structure of the ACTUAL molecule is a combination of these different structures, called a Resonance Hybrid.
Different resonance structures do not mean you are dealing with a physically different molecule - it is the same molecule being drawn as a simplified form of the actual structure, because delocalized electrons are really, really, really hard to draw.
For example, Resonance Structure A might have a positive charge on the second atom, while Resonance Structure B might have the charge shown on the third atom. They are both the same molecule, and on the actual molecule the two atoms share the charge, but we draw the charge on one of the two atoms for simplicity when making Lewis Structures.
In the actual structure, the charge being spread out over many atoms is called "de-localized", because the charge is not local to any specific atom. (Think of the atoms as cities, where you are local if you live there, but you are de-local if you move around between a lot of them.)
De-localizing charges moves like charges further apart, so compounds that have resonance are more stable than compounds that do not. These are "resonance-stabilized" structures.
The stability of a specific resonance structure shows us how close it is to the actual structure of the molecule. The more stable the resonance structure, the closer it is to the actual structure. The less stable the structure, the less accurate it is when compared to the actual structure.
More stable structures: Major Contributors.
Less stable structures: Minor Contributors.
12 notes
·
View notes