#C677T and A1298C polymorphisms
Explore tagged Tumblr posts
Text
MTHFR Gene Mutation: Why It Matters & How to Get Tested
Discover the Role of the MTHFR Gene, the Impact of Its Mutations on Your Health, & How You Can Get Tested to Understand Your Genetic Risk Factors You may not have heard of MTHFR, but this enzyme plays a vital role in our body’s ability to process folate (vitamin B9) and maintain your DNA. Related to another critical B vitamin, I recently wrote a story about the global B12 epidemic. Analyzing…
#23andMe#C677T and A1298C polymorphisms#deleterious mutations#DTC Testing vs. Healthcare-Ordered Tests#elevated homocysteine levels#Folate deficiency and MTHFR#folate metabolism and methylation pathways#genetic profile#Genetic testing for MTHFR#Getting Checked to Prevent Cardiovascular Issues#holistic health strategy#homocysteine and poor B12 absorption#How to test for MTHFR mutation#Literature Review on MTHFR#methylated B12 (methylcobalamin)#Methylation and MTHFR#MTHFR and health risks#MTHFR C677T polymorphism and autism#MTHFR gene mutation#MTHFR gene mutation and pregnancy#MTHFR mutation symptoms#MTHFR mutation treatment#MTHFR Polymorphism#MTHFR testing#MTHFR’s thermolability#NVAF cardiometabolic stroke have MTHFR gene mutations#Vitamin B12 deficiency and MTHFR
0 notes
Text
Genetic Factors and Single Subsegmental Pulmonary Embolism Risk
Pulmonary embolism (PE) is a potentially life-threatening condition characterized by the obstruction of pulmonary arteries by blood clots. While PE can affect various segments of the pulmonary vasculature, single subsegmental pulmonary embolism (SSPE) has garnered increasing attention due to its unique clinical features and management challenges. In this blog post, we delve into the genetic factors influencing SSPE risk and their implications for patient care and management.
Understanding Single Subsegmental Pulmonary Embolism (SSPE)
SSPE refers to the presence of a blood clot in a single subsegmental branch of the pulmonary artery, typically detected through imaging studies such as computed tomography pulmonary angiography (CTPA). Unlike larger pulmonary emboli that may cause significant hemodynamic compromise, SSPEs are often smaller and may exhibit varying clinical presentations, ranging from asymptomatic to mild respiratory symptoms.
Genetic Factors and SSPE Risk
Recent research has shed light on the role of genetic factors in predisposing individuals to venous thromboembolism (VTE), including pulmonary embolism. VTE encompasses deep vein thrombosis (DVT) and PE, with genetic predispositions contributing to thrombus formation and propagation. Several key genetic factors are implicated in SSPE risk:
Factor V Leiden Mutation
The Factor V Leiden mutation is one of the most well-known genetic risk factors for VTE, including PE. This mutation involves a change in the Factor V protein, leading to increased resistance to inactivation by protein C, a natural anticoagulant. Individuals carrying one or two copies of the Factor V Leiden mutation have a higher risk of developing thrombotic events, including SSPE.
Prothrombin Gene Mutation (G20210A)
The prothrombin gene mutation, specifically the G20210A variant, is another genetic factor associated with increased VTE risk. This mutation results in elevated levels of prothrombin, a key protein in the coagulation cascade. The combination of Factor V Leiden and prothrombin gene mutations further amplifies the thrombotic risk, including SSPE occurrence.
MTHFR Gene Polymorphisms
Methylenetetrahydrofolate reductase (MTHFR) gene polymorphisms, particularly the C677T and A1298C variants, have been linked to altered homocysteine levels and thrombotic events. Elevated homocysteine levels are associated with endothelial dysfunction and increased clotting tendency, contributing to VTE risk, including SSPE.
Clinical Implications and Management Strategies
Understanding the genetic factors influencing SSPE risk is crucial for risk stratification, diagnosis, and management of affected individuals. Genetic testing may be considered in patients with unprovoked or recurrent VTE events, especially in younger individuals or those with a family history of thrombotic disorders. Identifying specific genetic mutations allows for personalized risk assessment and tailored anticoagulation strategies.
Anticoagulation Therapy
Anticoagulation remains the cornerstone of SSPE management, aimed at preventing clot propagation and recurrent thrombotic events. The choice of anticoagulant agents, such as direct oral anticoagulants (DOACs) or vitamin K antagonists (VKAs), depends on individual patient factors, including genetic profile, comorbidities, and bleeding risk.
Lifestyle Modifications and Follow-Up
In addition to pharmacological therapy, lifestyle modifications such as maintaining a healthy weight, regular physical activity, and smoking cessation are essential in reducing VTE recurrence risk. Regular follow-up evaluations, including imaging studies and laboratory monitoring, help assess treatment efficacy and identify potential complications.
Genetic Insights for SSPE Management with PatientSelfTesting
In conclusion, genetic factors play a significant role in single subsegmental pulmonary embolism risk, influencing thrombotic propensity and treatment outcomes. Incorporating genetic testing into clinical practice allows for personalized risk assessment and optimized management strategies for SSPE patients.
At PatientSelfTesting, we recognize the importance of genetic insights in pulmonary embolism management, offering advanced genetic testing solutions to healthcare providers and patients. Partner with PatientSelfTesting to leverage genetic knowledge and improve outcomes in SSPE and venous thromboembolism care.
0 notes
Text
Unraveling the Mysteries of MTHFR Gene Variants and Methylation
In the intricate landscape of human genetics, the MTHFR gene holds significant sway over a vital biochemical process known as methylation. Methylation, the addition of a methyl group to DNA molecules, plays a crucial role in regulating gene expression, cellular function, and overall health. However, variations in the MTHFR gene can disrupt this process, potentially leading to a myriad of health issues. Let's delve into the world of MTHFR gene variants and methylation to understand their implications.
Understanding Methylation and Its Importance
Methylation serves as a fundamental mechanism for controlling gene activity and regulating various biochemical pathways within the body. By adding or removing methyl groups to DNA, methylation can influence gene expression, impacting processes such as cell differentiation, metabolism, and immune response. This dynamic process is essential for maintaining cellular health and overall physiological balance.
The Role of the MTHFR Gene
The MTHFR gene provides instructions for producing an enzyme called methylenetetrahydrofolate reductase. This enzyme plays a critical role in the methylation cycle by converting folate (vitamin B9) into its active form, which is necessary for proper methylation to occur. Individuals inherit one copy of the MTHFR gene from each parent, and variations in this gene can affect enzyme function and methylation capacity.

Understanding MTHFR Gene Variants
Several common variations, or polymorphisms, in the MTHFR gene have been identified, with the most studied being the C677T and A1298C variants. These variants can alter the activity of the MTHFR enzyme, leading to reduced methylation capacity and potential health implications. Individuals with these variants may have difficulty metabolizing folate and other essential nutrients, which can affect various aspects of health, including cardiovascular function, neurological health, and pregnancy outcomes.
Implications for Health and Disease
The presence of MTHFR gene variants has been associated with an increased risk of certain health conditions, including cardiovascular disease, neural tube defects, mood disorders, and infertility. Reduced methylation capacity due to MTHFR variants can contribute to elevated levels of homocysteine, an amino acid linked to inflammation and cardiovascular risk. Furthermore, impaired methylation can impact neurotransmitter synthesis, potentially predisposing individuals to mood disorders such as depression and anxiety.
Genetic Methylation Test at Home, Given the significant role of methylation in health and disease, genetic testing for MTHFR gene variants and methylation capacity has gained traction in personalized medicine. Genetic methylation tests, including those that assess MTHFR gene variants, can provide valuable insights into an individual's genetic predispositions and help tailor interventions to optimize health outcomes.
Conclusion: Navigating the Path to Wellness
In the intricate interplay between genetics and health, the MTHFR gene emerges as a key player in the methylation cycle, influencing vital biochemical processes essential for maintaining optimal health. Variations in the MTHFR gene can disrupt methylation capacity, potentially contributing to a range of health issues. However, with advances in genetic testing, individuals can gain a deeper understanding of their genetic makeup and take proactive steps to support their methylation pathways. By embracing personalized approaches to health, informed by genetic insights, individuals can navigate the path to wellness with greater clarity and empowerment.
In the journey towards holistic health, unraveling the mysteries of MTHFR gene variants and methylation marks a significant step forward, illuminating the intricate connections between genetics, biochemistry, and overall well-being.
0 notes
Text
i have a suspicion that i have a MTHFR gene mutation and i’ve been making MTHFR enzymes all fucked up. because folic acid was in both supplements i was taking and the MTHFR enzyme converts folic acid to L-methylfolate (available to cells) so if i hypothetically had a MTHFR gene mutation i would have reduced methylation and be unable to efficiently process folic acid so it would be in there unmetabolised and circulating. MTHFR mutations can contribute to anaemia. MTHFR deficiency increases the likelihood of lower folate levels while also increasing the possibility of higher non-methylated forms of folate. Lower levels of vitamin B12 are also common in those with MTHFR mutations. B12 and folate are crucial in producing red blood cells A deficiency of folate or vitamin B12 reduces the body’s ability to synthesize purines and thymidylate, reducing DNA synthesis. This leads to cell death and anemia from ineffective red blood cell production.
The A1298C polymorphism of the MTHFR gene has also been linked to a higher risk of iron deficiency anemia due to deficient regulation of iron. The MTHFR enzyme is required for the metabolism of cysteine, one of the essential factors in iron regulation.
there are also confirmed blood related issues on my mum’s side of the family: my mum also has anemia, confirmed leidin factor V gene mutation (linked to blood clots), has had severe blood clots and has deep vein thrombosis.
I found one study on factor V Leiden and MTHFR mutations as a combined risk factor for hypercoagulability. from results: “FVL mutation was found to be present in 10% (19/190) in our study population. Of these, 18 patients were heterozygous and 1 was homozygous for this mutation. Of which 25% (19/76) patients with deep vein thrombosis were positive for variants of FVL. 74% (20/27) of the patients screened for MTHFR were found to be positive (5 for C677T, 4 were compound heterozygous & 11 for A1298C). 2 out of 4 patients who were positive for both FVL and C677T MTHFR mutations had poor prognosis and died.” 74% of patients with both FVL and deep vein thrombosis possessed a MTHFR mutation my mum has both FVL and deep vein thrombosis so it’s likely that she also has a MTHFR gene mutation and this very well could have been passed to me. im writing this here for Me & My future reference but thanks for reading anyway xoxo
5 notes
·
View notes
Text
Juniper Publishers- Open Access Journal of Case Studies
Autoimmune Polyglandular Syndrome Type 3 with Multiple Genetic Alterations in a Young Male Patient with Type 1 Diabetes Mellitus
Authored by Abel Decmann
Abstract
Introduction: Type 1 diabetes mellitus (T1DM) is one of the most common chronic diseases among young and adolescent patients. A genetically predisposed person develops autoantibodies against beta-cells after certain environmental stimuli. Patients with T1DM can develop other organ-specific autoantibodies, most often causing autoimmune thyroiditis, coeliac disease or pernicious anaemia. Patients with multiple autoimmune diseases might be diagnosed with one of the autoimmune polyglandular syndromes. Case presentation: We present a 32-year-old male patient with type 1 diabetes mellitus and Hashimoto-thyroiditis. The habitus and laboratory tests taken at admission lead us to further investigations. The patient could be diagnosed with pernicious anaemia and hyperhomocysteinemia, as well. The latter disease raises the already higher cardiovascular risk of the diabetic patient. Conclusion: Patients with autoimmune type 1 diabetes mellitus should undergo screening for other autoimmune diseases, most importantly, autoimmune thyroid disease, pernicious anaemia and primary adrenal insufficiency.
Keywords:Diabetes mellitus type 1; Pernicious anaemia; Hashimoto-thyroiditis; Homocysteine; Autoimmune polyglandular syndrome
Abbreviations: APS: Autoimmune Polyendocrine/Polyglandular Syndrome; APECED: Autoimmune Polyendocrinopathy, Candidiasis, Ectodermal Dystrophy/Dysplasia; T1DM: Diabetes Mellitus Type 1
Introduction
Type 1 diabetes mellitus (T1DM) is one of the most common chronic diseases in young and adolescent patients which. After certain environmental stimuli a genetically predisposed persons develop autoantibodies which lead to the immune-mediated destruction of pancreatic beta-cells. People with HLA (human leukocyte antigen) genotypes of HLA-DR4-DQ8 or DR3-DQ2 have the highest genetic risk for developing T1DM [1]. The incidence of the disease varies between geographical regions, from 1-3 per 100.000/year in China and Asia to 30-60 per 100.000/year in Scandinavia [2,3]. No environmental factor has been found that is clearly associated with the development of T1DM, so far [4]. Patients with T1DM are usually not obese and develop diabetes in early childhood or in relatively young age compared to type 2 diabetes mellitus (T2DM). Characteristic circulating, islet-specific pancreatic autoantibodies may be present [against insulin, glutamic acid decarboxylase 65 (GAD65), zinc transporter 8 (ZnT8), 40k fragment of tyrosine phosphatase (IA2)] – however their absence does not necessarily rule out T2DM [5] – and lead to insulin deficiency. Patients with T1DM can develop other organ-specific autoantibodies, most often causing autoimmune thyroiditis, coeliac disease or pernicious anaemia. At least two organ-specific autoimmune diseases can be part of autoimmune polyendocrine/polyglandular syndromes (APS). APS type 1 [formerly called Autoimmune PolyEndocrinopathy-Candidiasis-Ectodermal Dystrophy/Dysplasia (APECED)] is due to a mutation in the autoimmune regulator (AIRE) gene, it has an autosomal recessive inheritance pattern. Usually it affects children and has a prevalence of 1:9.000-14.400 [6]. A patient must have two from the three characteristic manifestations (chronic mucocutaneous candidiasis, hypoparathyroidism, autoimmune adrenal insufficiency) [7]. Other diseases can be part of this syndrome: T1DM, pernicious anaemia, hypothyroidism, ectodermal dysplasia, autoimmune hepatitis, primary hypogonadism, alopecia, malabsorption and vitiligo [8]. APS type 2 is relatively rare, affects young adults and its prevalence is 1-5:100.000 population [9,10]. Obligatory features of the syndrome are autoimmune primary adrenal insufficiency (Addison’s disease) and autoimmune thyroid disease and/or T1DM. Additional autoimmune disease, such as vitiligo, autoimmune hepatitis, alopecia, pernicious anaemia, primary hypogonadism may also accompany it [10,11]. No clear inheritance pattern has been described so far. APS type 3 is characterised by autoimmune thyroid disease and at least one other autoimmune organ-specific disorder (T1DM, primary hypogonadism, pernicious anaemia, coeliac disease, vitiligo, alopecia, psoriasis etc.) that is not Addison’s disease or hypoparathyroidism [12]. The inheritance pattern of APS3 is yet to be determined.
Case Presentation
A 32-year-old male patient with diabetes mellitus presented to ambulatory care because of suboptimal blood sugar levels, alleged weight loss, vague low-back pain and supposed malabsorption. The patient history contained 3 years’ history of T1DM, 2 years’ history of thyroid disease and mitral and aortic valve prolapse. The patient’s mother had Hashimoto-thyroiditis and myasthenia gravis. On physical examination, the patient was underweight (height 180cm, weight 52kg, BMI: 16kg/m2). His posture, his long limbs and fingers suggested marfanoid habitus. HbA1c level of the patient was 8.2% (normal range: 4-6), with a fasting glucose level of 11mmol/l (4.1-5.9). Antibodies against glutamic acid decarboxylase [1745IU/ml (<10)] and against ZnT8 [17IU/ml (<15)] tested positive and antibodies against tyrosine phosphatase-A2 [2IU/ml (<20)] and insulin [6IU/ml (<12)] tested negative, result of islet-cell antibody was uncertain. Based on low BMI, relatively young age and islet-specific antibody positivity we could confirm the diagnosis of T1DM.
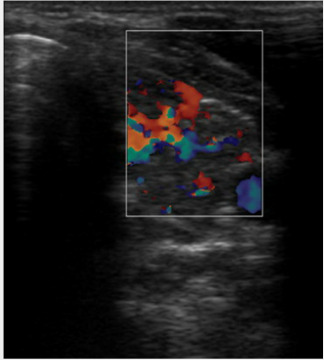
We evaluated the thyroid function of the patient. TSH (thyroid stimulating hormone) [3.9mU/l (0.35-4.9)], fT4 [12.97pmol/l (9-23.2)] and fT3 [5.11pmol/l (2.63-5.7)] were in normal range without therapy. Anti-thyroid peroxidase antibody level was highly elevated [1196U/ml (<5.6)]. Subsequent thyroid sonography showed inhomogeneous, hypervascularized lobes of thyroid gland. These findings were consistent with a euthyroid Hashimoto-thyroiditis (Figure 1).
The laboratory tests performed at admission showed mild, macrocytic anaemia (Table 1). Vitamin B12 level was below reference range with 72pmol/l (138-652). Gastroscopy showed type A atrophic gastritis and histologic evaluation revealed severe chronic gastritis with intestinal metaplasia without H. pylori infection. Immunological tests were also performed. Anti-parietal cell antibodies tested positive, while antibodies against intrinsic factor were negative. According to the abovementioned results, the diagnosis of pernicious anaemia could be established.
Clearly, the patient had vitamin B12 malabsorption. However, iron-, lipid-, protein- and calcium/vitamin D3-metabolism were without marked alterations. Coeliac disease could also be excluded because of the negativity of antibodies against tissue transglutaminase and deamidated gliadin peptides. The gastroscopy showed no typical signs of coeliac disease in the small intestine. There were no ionic disturbances [serum sodium 142mmol/l (135-146), potassium 4.4mmol/l (3.5-5.1)], fasting serum cortisol level was within the normal range [cortisol 465.3nmol/l (220-690)] and no clinical signs of hypoadrenalism were detected, therefore we could rule out clinically manifested Addison’s disease.
Concurrent Hashimoto-thyroiditis, T1DM and pernicious anaemia, and the absence of Addison’s disease and hypoparathyroidism suggested the diagnosis of autoimmune polyendocrine syndrome type 3. Maternal anamnesis of Hashimoto-thyroiditis and myasthenia gravis that could be diagnosed also as APS3, strengthens the diagnosis.
We performed further tests because of the marfanoid habitus and valvular prolapses of the patient. Because of the vague lowback pain and the characteristic phenotype of the patient, we performed tests to evaluate ankylosing spondylitis. There was no clear sign of sacroiliitis on plain pelvic x-ray (Figure 2). From the 11 characteristic ankylosing spondylitis symptoms, the patient showed only 1, namely HLA-B27 positivity. According to the relevant guideline, sacroiliitis on MRI would further strengthen the diagnosis, but it is itself not diagnostic [13]. Because of vitamin B12 deficiency homocysteine level was measured and was found to be extremely elevated [homocysteine 100.2μmol/l (5.4- 16)]. Homocysteine is produced by complex enzymatic activity from dietary methionine. B12 and folate-dependent enzymatic conversions are responsible for keeping homocysteine levels relatively low by conversion to methionine or cysteine [14]. We, therefore, performed genetic analysis of the two most frequent mutations of the methylenetetrahydrofolate reductase (MTHFR) gene. We have found the A1298C mutation in homozygous variant [MTHFR (methylenetetrahydrofolate reductase) C677T normal variant, MTHFR A1298C homozygous variant)]. Therefore, we have concluded that elevated homocysteine level may be explained by vitamin B12 deficiency and the patient’s genetic predisposition.
Discussion
The young patient has multiple autoimmune conditions (T1DM, pernicious anaemia, Hashimoto-thyroiditis), that can together be classified as autoimmune polyendocrine syndrome type 3. At the time of hospitalization, the patient did not need any thyroid hormone replacement therapy but needed insulin therapy and replacement of vitamin B12. However, the cardiovascular risk of the patient is higher because of the T1DM. We measured homocysteine levels and performed genetic testing for hyperhomocysteinaemia. Elevated homocysteine levels are associated with higher cardiovascular risk and more frequent thromboembolic events, moreover, homocysteine is an independent risk factor of cardiovascular diseases [15,16]. Approximately, 10% of the US population are homozygous for the thermolabile variant of MTHFR (C677T), and 30% are heterozygous for another polymorphism of MTHFR gene, A1298C is found in approximately 10% of Caucasian population [17]. There is no clear consensus on whether reducing homocysteine level through vitamin B12 replacement lowers the cardiovascular risk [18]. However, there are articles that discuss the role of vitamin D3 replacement in patients with hyperhomocysteinaemia to lower homocysteine levels [19]. We observed genetic alteration independent from the comorbidities of type 1 diabetes mellitus.
Conclusion
In conclusion, in patients with type 1 diabetes mellitus and with autoimmune polyglandular syndrome who have higher cardiovascular risk the presence of other genetically known risk factors, such as hyperhomocysteinaemia or spondylarthritis should be considered, in order to initiate, if possible, preventive measures.
To know more about Juniper Publishers please click on: https://juniperpublishers.com/manuscript-guidelines.php
For more articles in Open Access Journal of Case Studies please click on: https://juniperpublishers.com/jojcs/index.php
#juniper publishers contact info#juniper publishers journals#Obstetrics and Gynaecology#Otolaryngology#Transplantation Medicine
0 notes
Text
Folate pathway genetic polymorphisms modulate methotrexate-induced toxicity in childhood acute lymphoblastic leukemia
Abstract
Background
Acute lymphoblastic leukemia (ALL) is one of the major malignancies affecting children in Jordan. Methotrexate (MTX) is the cornerstone of chemotherapy for ALL, and works by targeting enzymes involved in the folate pathway. We hypothesize that genetic polymorphisms of the folate pathway are associated with MTX toxicity in children with ALL.
Methods
A total of 64 children with ALL were included in this study; 31 (48.4%) boys and 33 (51.6%) girls aged 2–16 years. The folate pathway genes were genotyped using polymerase chain reaction followed by sequencing and studying the association between genetic polymorphisms and MTX toxicity.
Results
The immunophenotype was B-lineage in 55 patients (85.9%) and T-lineage in nine patients (14.1%). All genetic polymorphisms, except for dihydropyrimidine dehydrogenase polymorphisms, were associated with hematological toxicities and did not appear to precipitate any non-hematological adverse events. Patients with ALL carrying dominant alleles of methylene tetrahydrofolate (MTHFR) C677T and dihydrofolate reductase 19 bp deletion were at a higher risk of developing severe leucopenia [OR (95% CI) = 4.5 (1.2–17), p = 0.03; 5.4 (1.6–17.8); p = 0.006] while minor allele carriers of MTHFR A1298C were more likely to develop neutropenia [OR (95% CI) = 6.1 (1.3–29.5); 0.04]. Furthermore, dominant allele carriers of thymidylate synthase 1494 del6 were at a higher risk of developing neutropenia [OR (95% CI) = 6 (1.2–31.1); p = 0.04].
Conclusion
Genetic polymorphisms of the folate pathway may modulate MTX-induced toxicity in childhood ALL.
http://bit.ly/2WicXYq
0 notes
Text
Genetic Associations With Anxiety and Depression
youtube
Today we are discussing genetics and their associations to depression as well as anxiety. With the mapping of the human genome significant information has come to the surface regarding convergent functional genomics and the interplay between these and environment. We hope that you find today’s broadcast informative.
Please forward us any comments here on our website or go to our Power Health Facebook page http://powerhealthreno.com/Facebook
References:
1. What do the genetic association data say about the high risk of suicide in people with depression? A novel network-based approach to find common molecular basis for depression and suicidal behavior and related therapeutic targets. Bozorgmehr A, Alizadeh F, Ofogh SN, Hamzekalayi MRA, Herati S, Moradkhani A, Shahbazi A, Ghadirivasfi M. J Affect Disord. 2018 Jan 8;229:463-468. doi: 10.1016/j.jad.2017.12.079. [Epub ahead of print] PMID: 29331709 [PubMed – as supplied by publisher] Similar articles
2. Understanding and predicting suicidality using a combined genomic and clinical risk assessment approach. Niculescu AB, Levey DF, Phalen PL, Le-Niculescu H, Dainton HD, Jain N, Belanger E, James A, George S, Weber H, Graham DL, Schweitzer R, Ladd TB, Learman R, Niculescu EM, Vanipenta NP, Khan FN, Mullen J, Shankar G, Cook S, Humbert C, Ballew A, Yard M, Gelbart T, Shekhar A, Schork NJ, Kurian SM, Sandusky GE, Salomon DR. Mol Psychiatry. 2015 Nov;20(11):1266-85. doi: 10.1038/mp.2015.112. Epub 2015 Aug 18. PMID: 26283638 [PubMed – indexed for MEDLINE] Free PMC Article Similar articles
3. Discovery and validation of blood biomarkers for suicidality. Le-Niculescu H, Levey DF, Ayalew M, Palmer L, Gavrin LM, Jain N, Winiger E, Bhosrekar S, Shankar G, Radel M, Bellanger E, Duckworth H, Olesek K, Vergo J, Schweitzer R, Yard M, Ballew A, Shekhar A, Sandusky GE, Schork NJ, Kurian SM, Salomon DR, Niculescu AB 3rd. Mol Psychiatry. 2013 Dec;18(12):1249-64. doi: 10.1038/mp.2013.95. Epub 2013 Aug 20. PMID: 23958961 [PubMed – indexed for MEDLINE] Free PMC Article Similar articles
4. [Folates in the treatment of depression]. Erbe S, Pellert UN. Fortschr Neurol Psychiatr. 2014 Feb;82(2):78-83. doi: 10.1055/s-0033-1356123. Epub 2014 Feb 11. Review. German. PMID: 24519190 [PubMed – indexed for MEDLINE] Similar articles
5. Methylenetetrahydrofolate Reductase A1298C Polymorphism and Major Depressive Disorder. Cho K, Amin ZM, An J, Rambaran KA, Johnson TB, Alzghari SK. Cureus. 2017 Oct 1;9(10):e1734. doi: 10.7759/cureus.1734. Review. PMID: 29209581 [PubMed] Free PMC Article Similar articles
6. Association of C677T polymorphism (rs1801133) in MTHFR gene with depression. Rai V. Cell Mol Biol (Noisy-le-grand). 2017 Jul 31;63(6):60-67. doi: 10.14715/cmb/2017.63.6.13. PMID: 28968218 [PubMed – in process] Similar articles
7. The C677T variant in <i>MTHFR</i> modulates associations between brain integrity, mood, and cognitive functioning in old age. Roussotte FF, Hua X, Narr KL, Small GW, Thompson PM; Alzheimer’s Disease Neuroimaging Initiative. Biol Psychiatry Cogn Neurosci Neuroimaging. 2017 Apr;2(3):280-288. doi: 10.1016/j.bpsc.2016.09.005. PMID: 28435933 [PubMed] Free Article Similar articles
The post Genetic Associations With Anxiety and Depression appeared first on Power Health Talk.
Genetic Associations With Anxiety and Depression
0 notes
Text
Analysis of genetic polymorphisms associated with leukoaraiosis in the southern Chinese population: A case-control study.
PubMed: Related Articles Analysis of genetic polymorphisms associated with leukoaraiosis in the southern Chinese population: A case-control study. Medicine (Baltimore). 2016 Aug;95(35):e3857 Authors: Huang WQ, Ye HM, Li FF, Yi KH, Zhang Y, Cai LL, Lin HN, Lin Q, Tzeng CM Abstract Leukoaraiosis (LA) is a frequent neuroimaging finding commonly observed on brain MRIs of elderly people with prevalence ranging from 50% to 100%. Multiple susceptibility genes or genetic risk factors for LA have been identified in subjects of European descent. Here, we report the first replication study on several common and novel genetic variations in the Chinese population. In this study, a total of 244 subjects (201 LA patients and 43 controls) were enrolled according to our new and strict definition for LA. Subsequently, 6 genetic variants at 5 genes, rs3744028 in TRIM65, rs1055129 in TRIM47, rs1135889 in FBF1, rs1052053 in PMF1, and rs1801133 (C677T) and rs1801131(A1298C) in MTHFR, were selected for genotyping using polymerase chain reaction (PCR)-based pyrosequencing and restriction fragment length polymorphism (RFLP) together with capillary electrophoresis (CE) and agarose gel electrophoresis. Finally, Pearson's χ and multivariate logistic regression tests were used to examine the associations between the genotypes and LA. Among these candidate polymorphisms, except for rs1052053 and rs1801131, rs1135889 (P = 0.012) showed significant associations with LA in the dominant model, and the other 3 SNPs, rs3744028 (P = 0.043), rs1055129 (P = 0.038), and rs1801133 (P = 0.027), showed significant associations with LA in the recessive model. However, these differences no longer remained significant after adjusting for age, gender, hypertension, and diabetes mellitus and applying Bonferroni correction or Sidak correction for multiple testing. These results suggest that the above-mentioned genetic variants are not associated with LA risk. In summary, the study did not replicate the susceptibility of rs3744028, rs1055129, and rs1135889 at the Chr17q25 locus for LA nor did it find any other significant results for rs1052053, rs1801133, and rs1801131 in the Chinese population. It strongly indicated the ethnic differences in the genetics of LA. However, the associations of rs3744028 (TRIM65), rs1055129 (TRIM47), rs1135889 (FBF1), and rs1801133 (MTHFR) with LA before Bonferroni correction and Sidak correction for multiple testing are worth highlighting. Thus, we believe that a genome-wide association study and candidate gene association studies are needed to reassess the previous findings and screen novel risk genes for LA in China. PMID: 27583843 [PubMed - indexed for MEDLINE] http://dlvr.it/NLbsxJ
0 notes
Text
Association of the C677T and A1298C polymorphisms in the 5,10 methylenetetrahydrofolate reductase gene in pati
http://dlvr.it/N2vkrs
0 notes
Text
Methylenetetrahydrofolate reductase C677T variant and hyperhomocysteinemia in subarachnoid hemorrhage patients from India
Abstract
Methylenetetrahydrofolate reductase (MTHFR) polymorphism (C677T, A1298C) has been implicated in increased plasma homocysteine (Hcy) levels. The present study was designed to investigate the association between MTHFR polymorphism and increased Hcy levels in subarachnoid haemorrhage (SAH) patients. A total of 150 subjects from North India were included in the study, comprising of 100 SAH patients and 50 healthy controls. Plasma Hcy levels was determined and MTHFR polymorphism (C677T, A1298C) was screened by High resolution melting (HRM) analysis. Plasma Hcy levels were found to be significantly higher (p < 0.001) in SAH patients than in healthy controls. No significant difference in the genotype and allele frequency of MTHFR A1298C was observed. However, frequency of MTHFR C677T genotype, CT (53% vs. 20%; p < 0.001) and TT (15% vs. 2%; p < 0.05) was significantly higher in SAH group as compared to healthy controls. The frequency of T allele (41.5% vs. 12%; p < 0.001) was also found to be higher in SAH patients in comparison to healthy controls. Furthermore, Hcy levels were higher in SAH patients with TT genotype than in patients having CT genotype, whereas CC genotype had lower Hcy levels. The study suggests that higher frequency of MTHFR C677T allele may contribute to etiopathology of SAH through increase in Hcy levels.
https://ift.tt/2xhKQgn
0 notes
Text
Polymorphisms of MTHFR C677T and A1298C associated with survival in patients with colorectal cancer treated with 5-fluorouracil-based chemotherapy
Abstract
Background
This study examined the association between methylenetetrahydrofolate reductase (MTHFR) polymorphisms and survival of patients with colorectal cancer (CRC) treated with 5-fluorouracil (5-FU)-based chemotherapy in Taiwan.
Methods
We genotyped MTHFR polymorphisms C677T (rs1801133) and A1298C (rs1801131) for 498 CRC patients treated with 5-FU-based chemotherapy after receiving surgery. Survival analyses on MTHFR polymorphisms were performed using log-rank test and Kaplan–Meier curve. Cox proportional hazards models were used to calculate the hazard ratios (HRs) and 95% confidence intervals (CIs) for the association between MTHFR genotypes and survival.
Results
Overall survival (OS) was significantly longer in CRC patients with MTHFR 677 CT+TT genotypes compared with those with 677 CC genotype (HR 0.77; 95% CI 0.60–0.98). Although the MTHFR A1298C polymorphism was not associated with OS in CRC, this polymorphism was associated with significantly shorter OS in rectal cancer. Among rectal cancer patients, OS was shorter for patients with AC+CC genotypes than for those with the AA genotype (HR 1.95; 95% CI 1.35–2.83). In haplotype analysis, better OS was found for colon cancer patients carrying the MTHFR 677T-1298A haplotype (HR 0.73; 95% CI 0.55–0.97), but worse survival was linked to rectal cancer patients carrying the MTHFR 677C-1298C haplotype (HR 1.53; 95% CI 1.08–2.18).
Conclusions
Our findings suggest that MTHFR genotypes provide prognostic information for CRC patients treated with 5-FU-based chemotherapy.
http://ift.tt/2i3yakf
0 notes