#MTHFR Polymorphism
Explore tagged Tumblr posts
Text
MTHFR Gene Mutation: Why It Matters & How to Get Tested
Discover the Role of the MTHFR Gene, the Impact of Its Mutations on Your Health, & How You Can Get Tested to Understand Your Genetic Risk Factors You may not have heard of MTHFR, but this enzyme plays a vital role in our body’s ability to process folate (vitamin B9) and maintain your DNA. Related to another critical B vitamin, I recently wrote a story about the global B12 epidemic. Analyzing…
#23andMe#C677T and A1298C polymorphisms#deleterious mutations#DTC Testing vs. Healthcare-Ordered Tests#elevated homocysteine levels#Folate deficiency and MTHFR#folate metabolism and methylation pathways#genetic profile#Genetic testing for MTHFR#Getting Checked to Prevent Cardiovascular Issues#holistic health strategy#homocysteine and poor B12 absorption#How to test for MTHFR mutation#Literature Review on MTHFR#methylated B12 (methylcobalamin)#Methylation and MTHFR#MTHFR and health risks#MTHFR C677T polymorphism and autism#MTHFR gene mutation#MTHFR gene mutation and pregnancy#MTHFR mutation symptoms#MTHFR mutation treatment#MTHFR Polymorphism#MTHFR testing#MTHFR’s thermolability#NVAF cardiometabolic stroke have MTHFR gene mutations#Vitamin B12 deficiency and MTHFR
0 notes
Text
If I could obliterate just one fake wellness grifting scheme, it would be the mthfr fearmongering
Why are you trying to convince everyone single woman that a gene polymorphism that 50% of the population has is the reason they have health issues
If one more snake oil selling piece of shit tries to tell me I need to take folate or to avoid gluten because my actual genuine autoimmune issues are caused a gene difference so common its not even considered a mutation any more despite my completely normal homocysteine levels, you're gonna call it MTHFR the way I motherfucking rear your head into an electrical panel. Bitch.
11 notes
·
View notes
Text
Abstract— The aim of the study was to determine an association of the UGT1A1 (rs8175347), MTHFR (rs1801133), GSTP1 (rs1695), and ITPA (rs1127354) polymorphic variants with response to platinum-based chemotherapy in patients with bladder cancer. The study group consisted of 60 patients who were treated in the National Cancer Institute. The population control groups were formed from conditionally healthy adults from different regions of Ukraine. The commercial DNA extraction kits were used to isolate genomic DNA from the blood samples of patients and the control group. Genotyping of the MTHFR, GSTP1, and ITPA alleles was carried out using PCR followed by RFLP analysis. The determination of UGT1A1 allelic variants was conducted by a method of fragment analysis of fluorescently labeled PCR products using an ALF-express II automatic laser analyzer. No significant difference was detected in the distribution of allele and genotype frequencies of the UGT1A1, MTHFR, GSTP1, and ITPA gene polymorphisms between the population sample and studied group of patients with bladder cancer. In addition, no statistically significant difference was detected in the distribution of allele and genotype frequencies for the polymorphic loci of the UGT1A1, MTHFR, and ITPA genes in codominant, dominant, and recessive models between the groups of patients with bladder cancer who had a positive response to chemotherapy and those who had no response to the therapy. It was demonstrated that the frequency of the GSTP1 gene 313G allele (0.40) was statistically significantly higher in the group of patients who responded to chemotherapy than in the group of patients who did not respond to the treatment (0.22). It was established that carriers of the GSTP1 gene 313G allele (AG and GG genotypes) have a higher probability of a positive response to chemotherapy than individuals with the AA genotype (OR = 3.05; CI 95%: 1.053–8.838). It was demonstrated that the GSTP1 gene A313G polymorphism (rs1695) is associated with a response to chemotherapy with platinum-based drugs, including cisplatin. The presence of the 313G allele in the patient genotype can indicate a better sensitivity of the tumor to platinum-based drugs.
#bladdercancer#pharmacologicalmarkers#responsetochemotherapy#UGT1A1#MTHFR#GSTP1#ITPA#cancer#chemotherapy
0 notes
Text
Penetrance of Methylene Tetrahydrofolate Reductase C677T Gene Polymorphism and Karyotypic Variations Associated Increase Genetic Susceptibility in the Cases of Congenital Heart Defects
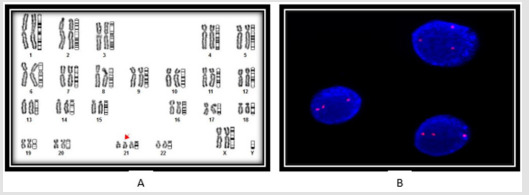
Penetrance of Methylene Tetrahydrofolate Reductase C677T Gene Polymorphism and Karyotypic Variations Associated Increase Genetic Susceptibility in the Cases of Congenital Heart Defects in Biomedical Journal of Scientific & Technical Research
Congenital heart disease (CHD), a multifaceted disorder occurs during embryogenesis due to exposure of environmental mutagens (teratogens) exposed antenatally leading to high-risk of infant mortality. Present study has been designed with the aims to evaluate the frequency of chromosome variation and corelate to methylene tetrahydrofolate reductase (MTHFR) C677T gene polymorphism as “risk factor” in clinically diagnosed cases of CHD using lymphocytes cultures and ARMS PCR, respectively. FISH analysis was carried for confirmation of chromosome-21. Interestingly, cytogenetics study shows variation in the frequency of structural and numerical chromosome aberrations with frequency in all the cases of CHD. Case-1, showing deletion of short arm of chromosome-18 and Robertsonian translocation between G/G chromosome association (24.00%), Case-2 showing numerical variation (trisomy-21), Case-3, includes dicentric, chromatid break in chromosome-2, deletion of short arm in chromosome-5, reciprocal translocation involving chromosome-6 and 10 and reporting first time appearance of ring of Y-chromosome. Case-4 showing structural variations (16.00%) including dicentric, chromatid breaks and trisomy of chromosome-21. The most common dominant frequency was observed in karyotype trisomy-21(58.30%) in all the four cases of CHD as an end point for genetic bio maker and showing significant differences (p < 0.001) using X2- test between total number of chromosomes and trisomy-21 MTHFR (C677T) gene polymorphism reveals (25.00%) of genetic heterozygosity of CT alleles and 75.00% cases shows homozygosity of wild type (CC) alleles, suggesting the variations in the frequency either in karyotypes or MTHFR C677T alleles are due to unconstitutional penetrance of gene in the genome of CHD cases and increase genetic susceptibility to make the disease more complex.
For more articles in Journals on Biomedical Sciences click here bjstr
Follow on Twitter : https://twitter.com/Biomedres01 Follow on Blogger : https://biomedres01.blogspot.com/ Like Our Pins On : https://www.pinterest.com/biomedres/
#Biomedical Open Access Journals#Open Access Journals on Surgery#Journals on Medical Drug and Therapeutics#journal of biomedical research and reviews#journal of biomedical sciences research review
0 notes
Text
0 notes
Text
Genetic Factors and Single Subsegmental Pulmonary Embolism Risk
Pulmonary embolism (PE) is a potentially life-threatening condition characterized by the obstruction of pulmonary arteries by blood clots. While PE can affect various segments of the pulmonary vasculature, single subsegmental pulmonary embolism (SSPE) has garnered increasing attention due to its unique clinical features and management challenges. In this blog post, we delve into the genetic factors influencing SSPE risk and their implications for patient care and management.
Understanding Single Subsegmental Pulmonary Embolism (SSPE)
SSPE refers to the presence of a blood clot in a single subsegmental branch of the pulmonary artery, typically detected through imaging studies such as computed tomography pulmonary angiography (CTPA). Unlike larger pulmonary emboli that may cause significant hemodynamic compromise, SSPEs are often smaller and may exhibit varying clinical presentations, ranging from asymptomatic to mild respiratory symptoms.
Genetic Factors and SSPE Risk
Recent research has shed light on the role of genetic factors in predisposing individuals to venous thromboembolism (VTE), including pulmonary embolism. VTE encompasses deep vein thrombosis (DVT) and PE, with genetic predispositions contributing to thrombus formation and propagation. Several key genetic factors are implicated in SSPE risk:
Factor V Leiden Mutation
The Factor V Leiden mutation is one of the most well-known genetic risk factors for VTE, including PE. This mutation involves a change in the Factor V protein, leading to increased resistance to inactivation by protein C, a natural anticoagulant. Individuals carrying one or two copies of the Factor V Leiden mutation have a higher risk of developing thrombotic events, including SSPE.
Prothrombin Gene Mutation (G20210A)
The prothrombin gene mutation, specifically the G20210A variant, is another genetic factor associated with increased VTE risk. This mutation results in elevated levels of prothrombin, a key protein in the coagulation cascade. The combination of Factor V Leiden and prothrombin gene mutations further amplifies the thrombotic risk, including SSPE occurrence.
MTHFR Gene Polymorphisms
Methylenetetrahydrofolate reductase (MTHFR) gene polymorphisms, particularly the C677T and A1298C variants, have been linked to altered homocysteine levels and thrombotic events. Elevated homocysteine levels are associated with endothelial dysfunction and increased clotting tendency, contributing to VTE risk, including SSPE.
Clinical Implications and Management Strategies
Understanding the genetic factors influencing SSPE risk is crucial for risk stratification, diagnosis, and management of affected individuals. Genetic testing may be considered in patients with unprovoked or recurrent VTE events, especially in younger individuals or those with a family history of thrombotic disorders. Identifying specific genetic mutations allows for personalized risk assessment and tailored anticoagulation strategies.
Anticoagulation Therapy
Anticoagulation remains the cornerstone of SSPE management, aimed at preventing clot propagation and recurrent thrombotic events. The choice of anticoagulant agents, such as direct oral anticoagulants (DOACs) or vitamin K antagonists (VKAs), depends on individual patient factors, including genetic profile, comorbidities, and bleeding risk.
Lifestyle Modifications and Follow-Up
In addition to pharmacological therapy, lifestyle modifications such as maintaining a healthy weight, regular physical activity, and smoking cessation are essential in reducing VTE recurrence risk. Regular follow-up evaluations, including imaging studies and laboratory monitoring, help assess treatment efficacy and identify potential complications.
Genetic Insights for SSPE Management with PatientSelfTesting
In conclusion, genetic factors play a significant role in single subsegmental pulmonary embolism risk, influencing thrombotic propensity and treatment outcomes. Incorporating genetic testing into clinical practice allows for personalized risk assessment and optimized management strategies for SSPE patients.
At PatientSelfTesting, we recognize the importance of genetic insights in pulmonary embolism management, offering advanced genetic testing solutions to healthcare providers and patients. Partner with PatientSelfTesting to leverage genetic knowledge and improve outcomes in SSPE and venous thromboembolism care.
0 notes
Text
Unraveling the Mysteries of MTHFR Gene Variants and Methylation
In the intricate landscape of human genetics, the MTHFR gene holds significant sway over a vital biochemical process known as methylation. Methylation, the addition of a methyl group to DNA molecules, plays a crucial role in regulating gene expression, cellular function, and overall health. However, variations in the MTHFR gene can disrupt this process, potentially leading to a myriad of health issues. Let's delve into the world of MTHFR gene variants and methylation to understand their implications.
Understanding Methylation and Its Importance
Methylation serves as a fundamental mechanism for controlling gene activity and regulating various biochemical pathways within the body. By adding or removing methyl groups to DNA, methylation can influence gene expression, impacting processes such as cell differentiation, metabolism, and immune response. This dynamic process is essential for maintaining cellular health and overall physiological balance.
The Role of the MTHFR Gene
The MTHFR gene provides instructions for producing an enzyme called methylenetetrahydrofolate reductase. This enzyme plays a critical role in the methylation cycle by converting folate (vitamin B9) into its active form, which is necessary for proper methylation to occur. Individuals inherit one copy of the MTHFR gene from each parent, and variations in this gene can affect enzyme function and methylation capacity.

Understanding MTHFR Gene Variants
Several common variations, or polymorphisms, in the MTHFR gene have been identified, with the most studied being the C677T and A1298C variants. These variants can alter the activity of the MTHFR enzyme, leading to reduced methylation capacity and potential health implications. Individuals with these variants may have difficulty metabolizing folate and other essential nutrients, which can affect various aspects of health, including cardiovascular function, neurological health, and pregnancy outcomes.
Implications for Health and Disease
The presence of MTHFR gene variants has been associated with an increased risk of certain health conditions, including cardiovascular disease, neural tube defects, mood disorders, and infertility. Reduced methylation capacity due to MTHFR variants can contribute to elevated levels of homocysteine, an amino acid linked to inflammation and cardiovascular risk. Furthermore, impaired methylation can impact neurotransmitter synthesis, potentially predisposing individuals to mood disorders such as depression and anxiety.
Genetic Methylation Test at Home, Given the significant role of methylation in health and disease, genetic testing for MTHFR gene variants and methylation capacity has gained traction in personalized medicine. Genetic methylation tests, including those that assess MTHFR gene variants, can provide valuable insights into an individual's genetic predispositions and help tailor interventions to optimize health outcomes.
Conclusion: Navigating the Path to Wellness
In the intricate interplay between genetics and health, the MTHFR gene emerges as a key player in the methylation cycle, influencing vital biochemical processes essential for maintaining optimal health. Variations in the MTHFR gene can disrupt methylation capacity, potentially contributing to a range of health issues. However, with advances in genetic testing, individuals can gain a deeper understanding of their genetic makeup and take proactive steps to support their methylation pathways. By embracing personalized approaches to health, informed by genetic insights, individuals can navigate the path to wellness with greater clarity and empowerment.
In the journey towards holistic health, unraveling the mysteries of MTHFR gene variants and methylation marks a significant step forward, illuminating the intricate connections between genetics, biochemistry, and overall well-being.
0 notes
Text
Calcium L-Methylfolate as a Source of Folate
In the realm of nutritional science, the significance of folate, a B-vitamin crucial for cell development, cannot be overstated. Folic acid, the synthetic form of folate, is utilized in various supplements, including L-Methylfolate Calcium, to address and prevent folate deficiencies. This blog aims to explore the vital role of Calcium L-Methylfolate as a source of folate, delving into its applications, dosage, regulations, and potential interactions with other drugs. Let's embark on a comprehensive journey into the world of folate and its synthetic counterpart, L-Methylfolate Calcium.
Understanding Folate and Folic Acid:
Folate, a naturally occurring B-vitamin found in certain foods, is essential for the formation of healthy cells, particularly red blood cells. Folic acid, on the other hand, is the synthetic form of folate commonly used in supplements. These supplements, including L-Methylfolate Calcium, are prescribed to treat or prevent low folate levels, which can lead to various health issues such as anemia.
Conditions leading to low folate levels encompass a range of factors, from poor dietary choices and alcoholism to pregnancy and specific medical conditions like liver disease or kidney dialysis. Notably, women of childbearing age are advised to ensure sufficient folic acid intake, either through their diet or supplements, to prevent spinal cord birth defects in infants.
How to Use L-Methylfolate Calcium:
The administration of L-Methylfolate Calcium is a critical aspect of its efficacy. Whether taken with or without food, it is imperative to follow the doctor's instructions, typically once daily. Over-the-counter products also come with specific directions on the package, emphasizing the importance of adhering to guidelines provided by healthcare professionals. Dosage adjustments should only be made under the supervision of a medical practitioner, and regular intake is crucial for optimal benefits.
Folate Regulation and Genetic Polymorphisms:
The term "folate" encompasses B vitamins, including folic acid and active pteroylglutamates. The hydrolysis of folates occurs in the intestinal jejunum and the liver, leading to the active circulating form, L-Methylfolate. Individuals with genetic polymorphisms for genes coding methylenetetrahydrofolate reductase (MTHFR) may face challenges in metabolizing folic acid efficiently, affecting the vitamin B12 dependent methylation cycle.
It is essential to note that folate, including reduced forms such as folinic acid, may obscure pernicious anemia above certain doses, necessitating supervision by a licensed medical practitioner during administration.
Indications, Contraindications, and Warnings:
L-Methylfolate Calcium 7.5 mg is specifically indicated for addressing the nutritional requirements of patients determined by a licensed medical practitioner. However, it is contraindicated in individuals with known hypersensitivity to any of its ingredients.
Caution is recommended for patients with a history of bipolar illness. Folate therapy alone is insufficient for treating vitamin B12 deficiency, and it is crucial to screen patients with depressive symptoms for potential bipolar disorder risks.
Drug Interactions and Precautions:
The drug interactions and precautions associated with L-Methylfolate Calcium are critical aspects that healthcare professionals must consider when prescribing this supplement. Understanding these interactions ensures the safety and efficacy of the treatment, preventing potential adverse effects. Let's delve deeper into the specific drug interactions and precautions associated with L-Methylfolate Calcium:

Antiepileptic Drugs (AED):
L-Methylfolate Calcium may interact with antiepileptic drugs, such as phenytoin, carbamazepine, primidone, valproic acid, fosphenytoin, valproate, phenobarbital, and lamotrigine.
Caution is warranted due to the potential impairment of folate absorption and increased metabolism of circulating folate. Enhanced phenytoin metabolism may lead to lower blood levels, potentially allowing breakthrough seizures.
Capecitabine:
Interaction with capecitabine may increase its toxicity when combined with L-Methylfolate Calcium. Caution is advised to avoid adverse effects and ensure patient safety during cancer treatment.
Cholestyramine:
The co-administration of cholestyramine with L-Methylfolate Calcium may reduce folic acid absorption and serum folate levels. Monitoring and adjustments in dosage may be necessary to maintain optimal folate levels.
Phenytoin and Other Anticonvulsants:
Caution is particularly emphasized when prescribing L-Methylfolate Calcium alongside phenytoin and other anticonvulsants. Enhanced phenytoin metabolism may compromise the therapeutic levels of antiepileptic medications, necessitating close monitoring and potential dosage adjustments.
Healthcare professionals should thoroughly review a patient's medical history, current medications, and potential drug interactions before prescribing L-Methylfolate Calcium. Individualized treatment plans and close monitoring are crucial to ensuring the safety and efficacy of this supplement, especially in populations susceptible to interactions or with specific medical conditions. As with any prescription, open communication between healthcare providers and patients is essential to address concerns, monitor for adverse effects, and optimize the overall success of the treatment regimen.
The blog also details interactions with oral contraceptives, nonsteroidal anti-inflammatory drugs (NSAIDs), and other medications, emphasizing the importance of medical supervision and thorough screening before prescribing L-Methylfolate Calcium.
Pregnancy and Nursing Considerations:
While L-Methylfolate Calcium 7.5 mg is not intended as a prenatal/postnatal multivitamin for lactating and non-lactating mothers, its use during pregnancy and lactation should be discussed with a medical practitioner. The reduced form of B vitamin in the product necessitates caution, and consultation with healthcare professionals is crucial for pregnant or lactating individuals.
Adverse Reactions and Dosage Recommendations:
Allergic sensitization to folic acid has been reported, warranting attention during oral or parental administration. The recommended adult dose is 7.5 to 15 mg daily, administered with or without food, as directed by a licensed medical practitioner.
Product Supply and Storage:
L-Methylfolate Calcium 7.5 mg is available in coated, round, blue tablets supplied in bottles of 30 and 90 tablets. Proper storage at controlled room temperature (15°-30°C) is essential to maintain its efficacy.
Conclusion:
In conclusion, Calcium L-Methylfolate proves to be a vital and targeted solution for individuals facing folate deficiencies, offering a synthesized form of the essential B-vitamin necessary for cell development. This comprehensive exploration has shed light on the multifaceted aspects of L-Methylfolate Calcium, emphasizing its role as a source of folate and its applications in diverse medical scenarios.
Understanding the distinction between natural folate and synthetic folic acid becomes imperative, especially when addressing conditions that lead to low folate levels. The necessity of such supplementation is highlighted in cases ranging from poor dietary choices and pregnancy to complex medical conditions like liver disease and kidney dialysis. Notably, the blog emphasizes the critical role of folic acid, particularly in women of childbearing age, to prevent spinal cord birth defects in infants, underscoring the significance of timely intervention.
The dosage and administration guidelines stressed throughout the blog reinforce the importance of medical supervision. Whether taken with or without food, adherence to prescribed dosages is crucial for optimal effectiveness. Moreover, the cautionary notes regarding potential interactions with various drugs, including antiepileptic medications and NSAIDs, stress the need for meticulous screening and supervision by healthcare professionals.
The discussion on folate regulation, genetic polymorphisms, and the potential obscuring of pernicious anemia by certain forms of folate provides valuable insights into the complexity of folate metabolism. The blog urges practitioners to exercise caution and vigilance, especially in populations with specific genetic variations.
As we navigate through the intricacies of L-Methylfolate Calcium, the blog concludes by emphasizing its role in meeting distinct nutritional requirements under licensed medical supervision. Its contraindications, warnings, and precautions underscore the need for a personalized and cautious approach in its prescription. Overall, this exploration into Calcium L-Methylfolate as a source of folate illuminates its significance in the realm of nutritional science, offering targeted solutions for those in need, while also highlighting the importance of informed and supervised use in diverse healthcare contexts.
Source: https://sites.google.com/view/adorshea01/calcium-l-methylfolate-as-a-source-of-folate
0 notes
Text
MTHFR is a key enzyme. It can have polymorphisms that lead to differential pathway preference. If people’s MTHFR is deficient, giving them folic acid causes that enzyme to choose the 5,10methylene THF pathway, increasing promotion of DNA and cell proliferation (this is the opposite of anti-cancer procedure). Excessive folic acid administration can promote cancers. Because of the polymorphisms, you don’t know which side you’re favoring. Thus, blindly using excessive folic acid isn’t a good thing.
(Increasing folic acid was thought to LOWER homocysteine)
page 102 in Advanced Food Science: An Ecological Approach by Gregoire
1 note
·
View note
Text
We now extend that phenotype to apparent vulnerability to inflammatory muscle disease in a spectrum from JRA to fibromyalgia (FMS) and specific behavioral subsets of ADD, PTSD, and specific late onset neurological syndromes (FTD-PD and PPA). High and low risk FMS subsets can be defined using A1AT, MTHFR and APOE genotyping. Clinical diagnoses associated with A1AT polymorphisms included fibromyalgia, JRA/JIA, bipolar disorder, PTSD, primary progressive aphasia and FTDPD, but not most Alzheimer Disease subtypes. These results support an extended phenotype for A1AT mutation carriers beyond liver and lung vulnerability to selective advantages: ICE phenotype and disadvantages: fibromyalgia, affective disorders, and selected late onset neurological syndromes.
what
45 notes
·
View notes
Text
the results are in and i’m heterozygous for the C677T MTHFR polymorphism. which is quite common apparently and makes me think something else is involved since folic acid causes such intense neurological side effects in me
6 notes
·
View notes
Text
Potential effects of genetic polymorphism on anesthesia use for COVID-19 infected patients at intensive care unit
Background: New coronavirus disease is considered one of the most widely spreading viral infections all over the world. Increased numbers of severe COVID-19 cases are growing up. Severe cases require ICU mechanical ventilation and hence anesthesia requirement.
Aim: Reviewing of different genetic polymorphisms which might affect patient clinical response, safety and tolerability to different types of anesthesia used in severe COVID-19 patients requiring mechanical ventilation.
Main body of the abstract: Severity of COVID-19 infection resulted from cytokine storm that leads to Acute Respiratory Distress Syndrome (ARDS) contribute in ICUs mechanical ventilation and anesthesia. Genetic polymorphisms showed to contribute in wide variation in anesthetic responses. Different polymorphic genes of RYR1, CACNA1S, MTHFR, OPRM1, ABCB1, CYP2B6 and others, play a main role in such variations. Different types of anesthesia as sevoflurane, midazolam, suxamethonium, nitrous oxide, fentanyl, and propofol showed altered pharmacokinetics and/or dynamics leading to a lack of anesthetic effect and incidence of life-threatening adverse effects as malignant hyperthermia, myocardial infarction, dyspnea, and others.
Short conclusion: Genetic screening is a serious step to take into consideration to identify genetic polymorphic types that may alter the anesthetic effect in ICUs ventilation. Besides, it will avoid possible adverse effects and different sedation response variations. Sevoflurane, Fentanyl, and propofol can be taken into consideration as a safe choice for use in ICUs taking into consideration genetic polymorphic variants.
https://www.peertechzpublications.com/articles/SJGGT-8-120.php
0 notes
Text
Why Bioactive B Vitamins Are Critical For Promoting Brain Health
Why Bioactive B Vitamins Are Critical For Promoting Brain Health
While converting folate to 5-MTHF isn’t entirely impossible with an MTHFR gene variation, this unique polymorphism can reduce active folate production up to 70%, which can cause real health concerns down the road. However, when supplements feature B vitamins that are already in their bioactive forms (and for folate and vitamin B12, that means their methylated forms), folks with an MTHFR gene…

View On WordPress
0 notes
Text
Activate Energy with Activated B's | what does Activated vitamin B mean?

What all the fuss is about with activated B vitamins, you may have wondered? If you take activated B vitamins, you might be wondering whether they are superior to conventional B vitamins and whether you should continue taking them.
Understanding more about B vitamins in general, what they do, why they’re vital for our bodies, and the distinction between conventional B vitamins and activated B vitamins will help with these queries.
Let’s take a look:
B complex vitamins, which consist of the water-soluble vitamins B1, B2, B3, B5, B6, B12, and folate, are crucial for:
Energy production
Cell metabolism
Red blood cell health
Cardiovascular system health, and
Emotional wellbeing [1]
About B Vitamins
There are three important aspects to B vitamins that you may not be aware of:
Because B vitamins are water soluble and are typically not kept by the body, frequent dietary replenishment is necessary.
You need a balanced intake of all the B vitamins to promote overall health and wellbeing since, while sharing a name, they are chemically separate substances that act together synergistically in the body while also providing their own individual benefits.
Before they are helpful, B vitamins must undergo some sort of metabolic or enzymatic change.
Activation of a B vitamin
Each B vitamin contributes to the body’s process of producing energy. B vitamins circulate in the bloodstream after being digested by food until they go through an activation process that puts them in a state where they may be absorbed and used by the body. Specific enzymes are needed for this process, along with healthy liver and digestive systems.
B vitamins are typically not stored by the body and are quickly excreted after consumption. Therefore, slow conversion to their active form can result in their elimination from the body before they can exert their full benefits, providing a lesser effect and possibly affecting the amount of energy they help to provide [2].
By consuming a version of some B vitamins that has already undergone activation, this process can be avoided. A B vitamin that has been activated allows for quicker absorption into the body, reducing the chance of elimination and allowing for greater gains.
For certain people, a variety of factors, such as intestinal health and age, can impair how easily their systems convert B vitamins from their non-active to their active forms.
Some people might benefit from taking a B vitamin in an activated form as opposed to a non-activated version to help avoid this activation process.
Who should take an activated B vitamin?
Activated Bs may be beneficial for those who:
consume a diet high in refined or processed foods
have absorption or digestive issues
are strict vegetarians or vegan
are elderly
have methylene-tetrahydrofolate reductase (MTHFR) polymorphisms
MTHFR polymorphisms
Those who have MTHFR polymorphisms may benefit from taking activated B vitamins[3]. The MTHFR gene produces the MTHFR enzyme, which is necessary for the proper metabolism of folate, a vitamin essential for many metabolic and neurological system functions. Methylfolate production is affected by decreased MTHFR enzyme activity[4].
Methylfolate is required for:
Maintaining brain function and health
Supporting cognitive function and memory recall
Supporting healthy foetal development
Activated B Vitamins
B vitamins are available in their activated form in Herbs of Gold Activated B Complex. The vegan-friendly, one-a-day capsule of this premium, complex mix contains the active forms of the vitamins B2, B6, folate, and B12 to support the creation of energy.
Given that B vitamins are crucial for the proper operation of the nervous system, Herbs of Gold Activated B Complex is also advantageous for promoting mental wellbeing and a balanced stress response in the body. The active form of folate, methylfolate, is present in Herbs of Gold Activated B Complex, promoting brain health and cognitive performance.
The elderly, strict vegetarians and vegans are at increased risk of vitamin B12 deficiency[5]. Herbs of Gold Activated B Complex include vitamin B12 in its active form, methylcobalamin, as a component of the total B complex, assisting in the prevention of dietary vitamin B12 insufficiency.
#health#beauty#beauty products#beautyproductonline#skincare#healthcare#beauty tips#skincareproducts#health and fitness#healthylifestyle
1 note
·
View note
Text
i have a suspicion that i have a MTHFR gene mutation and i’ve been making MTHFR enzymes all fucked up. because folic acid was in both supplements i was taking and the MTHFR enzyme converts folic acid to L-methylfolate (available to cells) so if i hypothetically had a MTHFR gene mutation i would have reduced methylation and be unable to efficiently process folic acid so it would be in there unmetabolised and circulating. MTHFR mutations can contribute to anaemia. MTHFR deficiency increases the likelihood of lower folate levels while also increasing the possibility of higher non-methylated forms of folate. Lower levels of vitamin B12 are also common in those with MTHFR mutations. B12 and folate are crucial in producing red blood cells A deficiency of folate or vitamin B12 reduces the body’s ability to synthesize purines and thymidylate, reducing DNA synthesis. This leads to cell death and anemia from ineffective red blood cell production.
The A1298C polymorphism of the MTHFR gene has also been linked to a higher risk of iron deficiency anemia due to deficient regulation of iron. The MTHFR enzyme is required for the metabolism of cysteine, one of the essential factors in iron regulation.
there are also confirmed blood related issues on my mum’s side of the family: my mum also has anemia, confirmed leidin factor V gene mutation (linked to blood clots), has had severe blood clots and has deep vein thrombosis.
I found one study on factor V Leiden and MTHFR mutations as a combined risk factor for hypercoagulability. from results: “FVL mutation was found to be present in 10% (19/190) in our study population. Of these, 18 patients were heterozygous and 1 was homozygous for this mutation. Of which 25% (19/76) patients with deep vein thrombosis were positive for variants of FVL. 74% (20/27) of the patients screened for MTHFR were found to be positive (5 for C677T, 4 were compound heterozygous & 11 for A1298C). 2 out of 4 patients who were positive for both FVL and C677T MTHFR mutations had poor prognosis and died.��� 74% of patients with both FVL and deep vein thrombosis possessed a MTHFR mutation my mum has both FVL and deep vein thrombosis so it’s likely that she also has a MTHFR gene mutation and this very well could have been passed to me. im writing this here for Me & My future reference but thanks for reading anyway xoxo
5 notes
·
View notes
Text
Penetrance of Methylene Tetrahydrofolate Reductase C677T Gene Polymorphism and Karyotypic Variations Associated Increase Genetic Susceptibility in the Cases of Congenital Heart Defects - BJSTR Journals

Penetrance of Methylene Tetrahydrofolate Reductase C677T Gene Polymorphism and Karyotypic Variations Associated Increase Genetic Susceptibility in the Cases of Congenital Heart Defects by Ajit Kumar Saxena Biomedical Journal of Scientific & Technical Research
https://biomedres.us/fulltexts/BJSTR.MS.ID.006103.php
Congenital heart disease (CHD), a multifaceted disorder occurs during embryogenesis due to exposure of environmental mutagens (teratogens) exposed antenatally leading to high-risk of infant mortality. Present study has been designed with the aims to evaluate the frequency of chromosome variation and corelate to methylene tetrahydrofolate reductase (MTHFR) C677T gene polymorphism as “risk factor” in clinically diagnosed cases of CHD using lymphocytes cultures and ARMS PCR, respectively. FISH analysis was carried for confirmation of chromosome-21. Interestingly, cytogenetics study shows variation in the frequency of structural and numerical chromosome aberrations with frequency in all the cases of CHD. Case-1, showing deletion of short arm of chromosome-18 and Robertsonian translocation between G/G chromosome association (24.00%), Case-2 showing numerical variation (trisomy-21), Case-3, includes dicentric, chromatid break in chromosome-2, deletion of short arm in chromosome-5, reciprocal translocation involving chromosome-6 and 10 and reporting first time appearance of ring of Y-chromosome. Case-4 showing structural variations (16.00%) including dicentric, chromatid breaks and trisomy of chromosome-21. The most common dominant frequency was observed in karyotype trisomy-21(58.30%) in all the four cases of CHD as an end point for genetic bio maker and showing significant differences (p < 0.001) using X2- test between total number of chromosomes and trisomy-21 MTHFR (C677T) gene polymorphism reveals (25.00%) of genetic heterozygosity of CT alleles and 75.00% cases shows homozygosity of wild type (CC) alleles, suggesting the variations in the frequency either in karyotypes or MTHFR C677T alleles are due to unconstitutional penetrance of gene in the genome of CHD cases and increase genetic susceptibility to make the disease more complex.
For more article on Journals on Biomedical sciences click here
bjstr
Follow on Twitter : https://twitter.com/Biomedres01 Follow on Blogger :https://biomedres01.blogspot.com/ Like Our Pins On : https://www.pinterest.com/biomedres/
#Journals on Biomedical Engineering#Journals on Biomedical Science#Journal on medical science#Open access medical journal#Free medical journal
0 notes