#Clinical Trials Market Analysis
Text
The Rise of Clinical Trial Support Services: Trends and Market Insights
The clinical trial support services industry plays a critical role in the advancement of medical research and drug development. As the demand for innovative therapies continues to grow, the need for efficient, reliable, and comprehensive clinical trial support services is more important than ever. The Clinical Trials Support Services Market Size is projected to be valued at USD 26.10 billion in 2024 and is anticipated to grow to USD 37.5 billion by 2029, reflecting a compound annual growth rate (CAGR) of 7.52% throughout the forecast period (2024-2029).
Market Overview
The clinical trial support services market has been experiencing significant growth, driven by an increasing number of clinical trials and the rising complexity of drug development processes. With a projected market size valued in billions by 2024, this industry encompasses a range of services, including project management, regulatory affairs, site management, patient recruitment, and data management. The global focus on accelerating drug approval processes and improving patient outcomes is propelling the demand for these services.
Key Trends Influencing the Industry
Increased Focus on Patient-Centric Approaches
As clinical trials evolve, there is a growing emphasis on patient engagement and recruitment. Companies are adopting patient-centric strategies that prioritize the needs and experiences of participants. This includes using digital tools for better communication, streamlining enrollment processes, and ensuring that trials are designed with patient feedback in mind.
Adoption of Technology and Digital Solutions
The integration of technology is transforming clinical trial support services. Electronic data capture (EDC), electronic patient-reported outcomes (ePRO), and telemedicine are becoming standard practices. These technologies enhance data accuracy, improve patient monitoring, and streamline trial processes, making it easier to manage large-scale studies.
Regulatory Changes and Compliance Requirements
With evolving regulatory landscapes, particularly in regions like North America and Europe, clinical trial support services must adapt to new compliance requirements. This has led to increased demand for regulatory affairs experts who can navigate complex regulations and ensure that trials meet necessary standards.
Growth in Outsourcing
Pharmaceutical and biotechnology companies are increasingly outsourcing clinical trial support services to specialized providers. This trend allows sponsors to focus on core competencies while leveraging the expertise of service providers to enhance trial efficiency, reduce costs, and accelerate timelines.
Emphasis on Data Analytics
Data-driven decision-making is becoming essential in clinical trials. Companies are investing in advanced analytics to derive insights from trial data, improving operational efficiency and enhancing the likelihood of successful outcomes. This trend is leading to better patient selection, optimized trial designs, and improved regulatory submissions.
Expansion of Global Clinical Trials
As pharmaceutical companies seek to tap into diverse patient populations and expedite timelines, global clinical trials are on the rise. Clinical trial support services are adapting to accommodate the unique challenges of conducting studies across multiple countries, including managing logistics, regulatory approvals, and cultural considerations.
Challenges Facing the Industry
Despite the promising growth outlook, the clinical trial support services industry faces several challenges. These include rising operational costs, recruitment and retention of qualified staff, and navigating complex regulatory environments. Additionally, the ongoing impact of the COVID-19 pandemic has introduced uncertainties that require adaptability and resilience.
Future Outlook
The future of the clinical trial support services industry looks promising. As healthcare continues to advance and the demand for new therapies grows, the need for efficient clinical trial processes will remain critical. Companies that can leverage technology, prioritize patient engagement, and maintain compliance will be well-positioned to thrive in this dynamic landscape.
Conclusion
The clinical trial support services industry is integral to the success of clinical research and drug development. With increasing complexity and a growing emphasis on patient-centricity, the market is poised for substantial growth. By embracing technology, fostering collaboration, and navigating regulatory challenges, stakeholders can drive innovation and improve patient outcomes in the evolving landscape of clinical trials.
For a detailed overview and more insights, you can refer to the full market research report by Mordor Intelligence https://www.mordorintelligence.com/industry-reports/clinical-trial-support-services-market
#clinical trial support services market#clinical trial support services market size#clinical trial support services market share#clinical trial support services market trends#clinical trial support services market analysis
0 notes
Text

#Clinical Trial Outsourcing Market#Clinical Trial Outsourcing Size#Clinical Trial Outsourcing Growth#Clinical Trial Outsourcing Trend#Clinical Trial Outsourcing segment#Clinical Trial Outsourcing Opportunity#Clinical Trial Outsourcing Analysis 2024#Clinical Trial Outsourcing Forecast
0 notes
Text
Virtual Clinical Trials Market Future: Trends, Challenges, and Opportunities

Virtual Clinical Trials Market Outlook, Scope & Overview:
Industry reports indicate that the global virtual clinical trials market was valued at USD 8.39 billion in 2023 and is projected to reach USD 13.17 billion by 2031, growing at a CAGR of 5.8% over the forecast period 2024-2031.
Technological Advancements to Drive Growth of Global Virtual Clinical Trials Market
The adoption of virtual clinical trial solutions will continue to influence global market revenues. The shift towards virtual and decentralized clinical trials is driven by the need for more flexible, efficient, and patient-centric trial designs that can enhance data collection and reduce operational costs.
As a product segment, remote patient monitoring and digital data collection solutions currently hold a significant share of the global virtual clinical trials market. This segment is anticipated to grow at a year-over-year rate of 5.8% in 2024 over 2023 and reach USD 13.17 billion in revenues by 2031. The increasing demand for real-time data access and the need to overcome geographical and logistical challenges in clinical trials are expected to drive market growth.
Virtual Clinical Trials Solutions – Market Dynamics
Drivers:
Virtual clinical trials are witnessing significant growth due to their ability to provide greater flexibility, enhance patient engagement, and improve the efficiency of trial processes. The advancements in digital health technologies, such as remote monitoring devices, telemedicine platforms, and electronic data capture systems, are key factors driving the adoption of virtual clinical trials. Additionally, the need for faster trial recruitment and the growing focus on patient-centric approaches are further propelling market growth.
Restraints:
Despite the growth potential, challenges such as data privacy concerns, regulatory hurdles, and the complexity of integrating virtual trial technologies with existing systems are hindering the widespread adoption of virtual clinical trials. Moreover, issues related to technology accessibility, patient engagement, and the need for robust cybersecurity measures pose additional challenges to market expansion.
Virtual Clinical Trials Solutions – Market Outlook
The proven benefits of virtual clinical trials in enhancing trial efficiency, improving patient participation, and reducing operational costs have contributed to the market's growth. Virtual clinical trials are expected to witness increased adoption across major markets, including North America, Europe, and Asia Pacific, driven by advancements in digital health technologies and the growing emphasis on decentralized trial models.
Global Virtual Clinical Trials Market
The rise in demand for virtual clinical trials in developed and emerging markets is expected to drive market growth over the forecast period. North America currently holds a significant market share in the global virtual clinical trials market, with the US being a key contributor to market revenues. Europe and Asia Pacific regions are also experiencing rapid adoption of virtual trial solutions, supported by favorable regulatory frameworks and increasing investments in digital healthcare infrastructure.
Key Players in the Virtual Clinical Trials Solutions Market
Leading companies in the virtual clinical trials solutions market include Medidata Solutions, Parexel International, Veeva Systems, and Oracle Corporation. These companies are at the forefront of developing and commercializing advanced virtual trial platforms and technologies for various clinical research applications, including remote monitoring, data management, and patient engagement.
In conclusion, the global virtual clinical trials market is poised for substantial growth over the forecast period, driven by technological advancements, increasing demand for decentralized trial models, and the expanding adoption of digital health solutions across diverse clinical research settings.
Other Trending Reports
Artificial Intelligence in Ultrasound Imaging Industry Growth
Growth Hormone Deficiency Industry Growth
Patient Registry Software Industry Growth
Contrast Media/Contrast Agent Industry Growth
#Virtual Clinical Trials Market#Virtual Clinical Trials Market Size#Virtual Clinical Trials Market Share#Virtual Clinical Trials Market Trends#Virtual Clinical Trials Market Growth#Virtual Clinical Trials Market Analysis#Virtual Clinical Trials Market Outlook
0 notes
Text
Evaluating Progress: Insights into Dry Atrophic Macular Degeneration Clinical Trials Market

Navigate the forefront of medical research with our in-depth analysis of the Dry Atrophic Macular Degeneration Clinical Trials Market. Explore the latest advancements, promising interventions, and potential breakthroughs in the quest for effective treatments against dry atrophic macular degeneration. Join us on a journey through the evolving landscape of clinical trials shaping the future of vision care.
Dry Atrophic Macular Degeneration (AMD) poses a significant challenge to vision health, necessitating continuous advancements in medical research. In this comprehensive exploration, we delve into the Dry Atrophic Macular Degeneration Clinical Trials Market, shedding light on the latest advancements, innovative interventions, and potential breakthroughs that hold promise for improved outcomes in individuals affected by this degenerative eye condition.
#dry atrophic macular degeneration Clinical Trials Market#dry atrophic macular degeneration Clinical Trials Market analysis
0 notes
Text
Clinical Trials Supplies Market: Examined in New Market Research
The research report provides detailed information on global market revenues, parent market trends, macroeconomic indicators and drivers, along with market attractiveness by market segment. The report provides an overview of the growth rate of clinical trial supplies during the forecast period, i.e. 2020-2027. More importantly, the report further identifies the qualitative impact of various market factors on market segments and geographies. The research segments the market based on product type, application, technology and region. To provide greater clarity regarding the industry, the report takes a closer look at the current state of various factors including, but not limited to, supply chain management, niche markets, distribution channel, trade, demand and supply and production capacity in different countries.
Request a sample copy of the clinical trial supplies at: https://www.theinsightpartners.com/sample/TIPRE00009672
Key vendors covered in this report:
1. Catalent, Inc.
2. Termo Fisher Scientific, Inc.
3. Almac Group
4.Parexel International Company
5. Biocair
6. UDG Healthcare plc (Sharp)
7. PCI health services
8. Owens & Minor Inc.
9. CLIFF
10. Rubicon Research Pvt. Limited.
The report outlines the key players in the industry, along with a detailed analysis of their individual positions in comparison to the global landscape. The study conducts a SWOT analysis to evaluate the strengths and weaknesses of key players in clinical trial supplies. The researcher provides in-depth analysis of the size, share, trends, overall earnings, gross revenue and profit margin of Clinical Trial Supplies to make accurate forecasts and provide expert insights to investors to keep them updated on the market trends.
Competitive scenario:
The study evaluates factors such as segmentation, description and applications of clinical trial supplies. It obtains accurate information to provide a holistic view of the dynamic characteristics of the business, including actions and profit generation, thus focusing on the critical aspects of the business.
Scope of the report
Clinical trial supplies research focuses on extracting valuable data on investment pockets, growth opportunities, and leading vendors in the market to help clients understand competitive methodologies. The research also segments clinical trial supplies by type and application for the 2020-2027 forecast period. Comprehensive analyses of critical aspects such as impact factors and competitive landscape are visualized with the help of vital resources such as graphs, charts and infographics.
The most important types of drugs supplied for clinical trials covered in this report are:
• Small molecule drugs
• Biological drugs
The main applications of clinical trial supplies covered in this report are:
• Oncology
• Cardiovascular disease
• Neurological disorders
• Respiratory disorders
Clinical Trial Supplies Segmented by Region/Country: North America, Europe, Asia Pacific, Middle East & Africa, Central & South America
sThank you for reading this article; You can also customize this report to get selected chapters or regional coverage with regions like Asia, North America, and Europe.
Who we are:
Insight Partners is a one-stop shop for market research reports and solutions for various companies across the globe. We assist our clients in their decision support system by helping them choose the most relevant and cost-effective research reports and solutions.
Contact us:
If you have any questions about this report or would like more information, please contact us:
The Insight Partners
Telephone: +1-646-491-9876
Email: [email protected]
#Clinical Trials Supplies Market#Clinical Trials Supplies Market Size#Clinical Trials Supplies Market Trends#Clinical Trials Supplies Market Forecast#Clinical Trials Supplies Market Growth#Clinical Trials Supplies Market Analysis
0 notes
Text
Global In Silico Clinical Trials Market Is Estimated To Witness High Growth Owing To Growing Demand For Virtual Clinical Trials

The global In Silico Clinical Trials Market is estimated to be valued at US$ 3,173.1 Mn in 2022 and is expected to exhibit a CAGR of 7.95% over the forecast period 2023-2030, as highlighted in a new report published by Coherent Market Insights.
A) Market Overview:
Silico Clinical Trials are virtual trials conducted using computer models and simulations to evaluate drug efficacy and safety. These trials provide several advantages over traditional clinical trials, such as reduced time and cost, ethical considerations, and the ability to collect large amounts of data.
Virtual trials eliminate the need for physical participation, making it more convenient for patients and reducing the burden on healthcare systems. They also allow for the exploration of various drug combinations and dosages, accelerating the development of personalized medicine.
B) Market Key Trends:
One key trend in the In Silico Clinical Trials Market is the growing demand for virtual clinical trials. With advancements in technology and the increasing need for efficient drug development processes, pharmaceutical companies are increasingly adopting virtual trials. These trials provide real-time data analysis, reduce patient recruitment time, and offer cost-effective solutions for drug development.
For example, Insilico Medicine Inc., a key player in the market, utilizes artificial intelligence (AI) algorithms to accelerate the drug discovery process. Their AI-based platform enables researchers to identify potential drug candidates and predict their efficacy using virtual models.
C) PEST Analysis:
Political: Regulatory frameworks play a crucial role in the adoption of virtual clinical trials. Governments need to establish guidelines and standards to ensure patient safety and data privacy.
Economic: Silico Clinical Trials offer cost-effective solutions compared to traditional clinical trials. They reduce the need for physical locations, extensive patient recruitment efforts, and travel expenses.
Social: Virtual trials provide opportunities for patients who may otherwise be unable to participate in traditional trials due to geographical constraints, physical limitations, or personal commitments.
Technological: Advancements in technology, such as AI, machine learning, and big data analytics, have facilitated the growth of virtual trials. These technologies enable researchers to analyze complex data sets and make predictions about drug efficacy and safety.
D) Key Takeaways:
Paragraph 1: The global In Silico Clinical Trials Market is expected to witness high growth, exhibiting a CAGR of 7.95% over the forecast period. This growth can be attributed to increasing demand for virtual trials due to their ability to reduce time and cost in drug development. For example, the use of virtual models and simulations enables researchers to predict drug efficacy and safety before conducting physical trials.
Paragraph 2: Regionally, North America is expected to dominate the In Silico Clinical Trials Market . The region has a well-established healthcare infrastructure, favorable regulatory environment, and a high adoption rate of advanced technologies. Additionally, collaborations between pharmaceutical companies, academic institutions, and research organizations in North America contribute to the region's growth in the market.
In conclusion, the In Silico Clinical Trials Market is witnessing significant growth due to the increasing demand for virtual trials. Advancements in technology, cost-effectiveness, and the ability to collect real-time data are driving the adoption of virtual trials in drug development. The market is dominated by key players who are continuously investing in research and development to stay ahead in the competitive landscape. As virtual trials become more widely accepted, they have the potential to revolutionize the drug development process and improve patient outcomes.
#In Silico Clinical Trials Market#Medical Devices#In Silico Clinical Trials Market Growth#In Silico Clinical Trials Market Analysis#In Silico Clinical Trials Market Forecast#In Silico Clinical Trials Market Future#In Silico Clinical Trials Market Overview
0 notes
Text
https://www.databridgemarketresearch.com/reports/global-artificial-intelligence-ai-based-clinical-trials-market
#Artificial-Intelligence-(AI)-based-Clinical-Trials-Market-by-Product#Artificial-Intelligence-(AI)-based-Clinical-Trials-Market-Global-Opportunity#Analysis-and-Industry-regional#Artificial-Intelligence-(AI)-based-Clinical-Trials-Market-Size-Share#Artificial-Intelligence-(AI)-based-Clinical-Trials-Market-Growth#Artificial-Intelligence-(AI)-based-Clinical-Trials-Market-Insights-Country-Share-Competitors-Research-Study
0 notes
Text
Clinical Trial Packaging Market Growth, Overview with Detailed Analysis 2022-2028
Clinical Trial Packaging Market Growth, Overview with Detailed Analysis 2022-2028
The Clinical Trial Packaging Market research report 2022-2030 provides an in-depth analysis of the changing trends, opportunities, and challenges influencing the growth over the next decade. The study includes a detailed summary of each market along with data related to demand, supply and distribution. The report examines Clinical Trial Packaging market growth strategies adopted by leading…
View On WordPress
#Clinical Trial Packaging#Clinical Trial Packaging forecast#Clinical Trial Packaging Industry#Clinical Trial Packaging Market#Clinical Trial Packaging price#Clinical Trial Packaging report#Clinical Trial Packaging research#Clinical Trial Packaging share#Clinical Trial Packaging trends#Covid-19 Impact Analysis
0 notes
Note
AITA for blackmailing someone and then snitching to the feds anyway?
Okay, so I work for a contract medical research lab generating quantitative image data, working closely with veterinary pathologists who provide the qualitative data. Together, we put together a report like "okay, here's what the medicine/medical device did and here's why we think it happened", and that report usually gets sent to the FDA if it looks promising enough that the sponsor wants to push for clinical trials and eventual market release.
So we get a study in and we've got (fake numbers here) 400 sections, but the quote says they only want 300 measurements done. I'm confused and go "wait, which 300 out of the 400? which 100 should I ignore?" and go to the pathologist. She also thinks it's weird and reaches out to the client, hoping it's a typo and we're about to get paid for the bonus 100.
Nope! He pressures us for it to be a phone call (no paper trail) and then not-so-subtly hints that he wants the... uglier-looking sections dropped. In other words, he wants to cherry-pick data that makes him look good. This is not only dangerous but The Most Illegal Shit. People's lives hang in the balance and they have to be able to trust the research that tells them medicines and medical devices are safe. We take that responsibility seriously.
So the pathologist gathers data and emails him like "I'm taking a REPRESENTATIVE 300 samples for analysis, my report will include scoring of the ones that make you look bad, and if I find out you doctored the reports behind my back, I'm sending everything I have directly to the FDA." (this is not how data is normally submitted in the industry. normally the report is commissioned, and then all dealings with the FDA are done by the client)
He grouses, but agrees.
And then says "if the FDA reaches out to you, don't respond."
.....What? But that's already industry standard? Why would he say that? Why would he expect the FDA to reach out to us?
Anyway the pathologist and I discuss it, and both assume he's definitely about to doctor these reports behind our back once it's submitted. So at my suggestion... the pathologist sends the communications to the FDA anyway.
Here's the thing: we don't actually know that this guy meant to do some ethics violations. We just assumed he was suspicious without real proof. Even unproven accusations in this industry can get you blacklisted for life, if not facing criminal charges. Did we risk destroying some random guy's life over bad vibes and nothing else?
What are these acronyms?
113 notes
·
View notes
Text
It’s almost like the Usher children *knew* they weren’t going to live long and so they intentially left no marks upon the world.
Camille’s speech about how none of the kids actually makes or does anything is so startling: here is a group of people given all the opportunities and access money can buy, all of whom have had this their entire adult life, and they haven’t used it to create or build anything.
You can almost sense Roderiks disappointment in them, in his speech to Perry. He has this hyper focus on what his ‘investment’ money will fund, and says that ‘Ushers change the world’. But outside of himself and Madeline, not one of them has.
Frederick took the money, if he ever got any, and probably funnelled it back into his house or the company. By the looks of it he doesn’t have anything other than his family and his job, so there’s nothing for Roderick to invest in.
Tammy funnelled the money into a lifestyle brand, but one that wouldn’t have her at the front and centre. She scathingly reveals to Bill that she selected him to be her husband based upon his brand and marketability, showing she was ready to create this new empire but with her pulling strings in the shadows. From the outside it probably looks like she hasn’t created anything at all and that it’s all Bill, using his wife’s money. On top of this, the running gag of her storyline is that her brand and ideas aren’t even original, but are ripped off of Goop. So she hasn’t made anything new, and if Goldbug has any impact at all it will be no different to another more successful, more well know product. Hardly ‘changing the world’.
Victorine has some medial training but she looks to be a supporting role to her partner within their clinic, in which Al is the talented surgeon who people come to see and Victorine is a kind of silent partner. So she decided to go into medical devices or smart medical tech, but she relies upon the ideas and skills of others. As Camille said ‘the mesh is the surgeons, that’s why she’s fucking the surgeon’. And her medical knowledge seems to be limited if she thinks just her word and some money will move their experiments into human trials. So she also hasn’t ‘changed the world’ she’s just found someone else who was trying to and co-op-ed their ideas. You could even argue she poisoned those ideas, as Al mentions that the pain medication Victorine has been supplying looks like street drugs and wouldn’t stand up in any medical paper or research study.
Camille is, like she said, spinning furiously and going nowhere. She looks skilled in her field (from the analysis scenes we get, and Madeleine’s signing off on her PR analysis post Perry’s death) but she works from the shadows and hasn’t ‘created’ anything that wasn’t there before. There have been PR spin doctors before and there will be more to come; Camille offers nothing new ans hasn’t ‘changed the world’ in any measurable way. From what little we see of her work she hasn’t recreated a PR agency, hasn’t trained up other spin doctors under her, hasn’t created a brand or company which will outlast her. She leaves nothing behind to show what her skills or talents were.
Leo is shot down quickly when he claims he makes games: he doesn’t, he gives money to people who do. So he too will leave little to nothing behind when he’s gone. His references to past boyfriends show no long lasting relationships in his life and he has no other hobbies or pursuits we know of. Like Camille he hasn’t created a company to help with game design, hasn’t trained up others within this field he claims as his own. Even with the gaming ‘world’ it sounds like he changed very little. Fredrick’s throw away comments about Leo’s flat reveal that Leo hadn’t even had input in the decoration or style of his own home: he just latches onto the styles, ideas, aesthetic of his current boyfriend and goes with their ideas and plans. It’s such a small tiny thing but he truly has no original ideas in any aspect of his life.
And finally Perry, who’s desperate for that start up money but clearly has no plans or ideas on how to use it. He’s had a year and his main idea is an exclusive whisky bar. Even this idea, for all its crude intentions, shows his lack of vision: he doesn’t understand that to get the reputation he claims his bars would have will take time. You don’t just ‘create’ a consequent free bar celebrating decadence and privilege overnight. Reputations take time and as Madeline asks ‘what will be different about this one’ to draw people in to begin with? Studio 54 (which he compares his club to). only operated for 3 years before closing: not the smartest inclusion in an investment pitch.
To be fair to Perry though, looking at what the other siblings did or didn’t do with their loan money it seems a bit unfair that his ‘Blow job whiskey bar’ was shot down so decisively and cruelty. Assuredly Leo’s ‘video game studio for just myself’, Camille’s ‘PR agency just for me with my two assistants’, Victorines ‘medical training and clinic where I help out other surgeons’, Tammys ‘subscription lifestyle brand ripped off from a celebrity’ and Fredrick’s ‘I’d just like to work with you Dad’ were all clearly given the green light. But Perry apparently wasn’t good enough. Maybe this was a reaction to Roderick getting the news he was dying as so he wanted Perrys investment at least to actually change something, but still. He might as well give him the money either way at that point.
And I think it’s probably intended as a commentary on the ultra wealthy. Like of course people with more money than most counties have no plans to leave anything for the next generation. They have achieved their high levels of success by being solely focused upon themselves and so are honestly incapable of considering others. They are solely interested in enjoying the life they are currently living and why strain themselves to fight and build something when they don’t have to?
But it also works so well as a supernatural legacy and ironic conclusion to Roderick’s deal: he agreed that none of his bloodline would outlive him, and so none of them built anything that would.
120 notes
·
View notes
Text

#Clinical Trial Outsourcing Market#Clinical Trial Outsourcing Size#Clinical Trial Outsourcing Growth#Clinical Trial Outsourcing Trend#Clinical Trial Outsourcing segment#Clinical Trial Outsourcing Opportunity#Clinical Trial Outsourcing Analysis 2024#Clinical Trial Outsourcing Forecast
0 notes
Text
Clinical Trial Management System Market Trends: Future Growth and Opportunities

Clinical Trial Management System Market Outlook, Scope & Overview:
Industry reports indicate that the global clinical trial management system (CTMS) market was valued at USD 1.82 billion in 2023 and is projected to reach USD 5.49 billion by 2031, growing at a CAGR of 14.8% over the forecast period 2024-2031.
Technological Advancements to Drive Growth of Global Clinical Trial Management System Market
The adoption of advanced clinical trial management systems will continue to influence global market revenues. Healthcare providers and research organizations are increasingly turning to CTMS solutions due to their efficiency in managing clinical trials, ensuring compliance, and enhancing data accuracy.
As a product segment, cloud-based CTMS solutions currently hold a significant share of the global clinical trial management system market. This segment is anticipated to grow at a year-over-year rate of 14.8% in 2024 over 2023 and reach USD 3 billion in revenues by 2031. The increasing complexity of clinical trials and the need for streamlined management processes are driving the demand for advanced CTMS solutions.
Clinical Trial Management Systems – Market Dynamics
Drivers:
Clinical trial management systems are witnessing significant growth in the global market due to their ability to streamline clinical trial processes, improve data management, and ensure regulatory compliance. The use of advanced technologies in CTMS solutions has enhanced their capabilities, driving demand across the healthcare and research sectors. Additionally, the growing number of clinical trials and the increasing need for efficient management systems are key factors fueling the growth of the CTMS market.
Restraints:
Despite the growth potential, challenges such as high implementation costs, data security concerns, and the need for specialized training are hindering the widespread adoption of CTMS solutions. Moreover, regulatory challenges and the complexity of integrating CTMS with existing systems in healthcare organizations can impact market growth.
Clinical Trial Management Systems – Market Outlook
The effective outcomes observed from using CTMS solutions in managing clinical trials have contributed to the market's growth. CTMS solutions are projected to witness a steady increase in demand, particularly in developed regions where the number of clinical trials is higher and the need for efficient management systems is critical.
Global Clinical Trial Management System Market
The rise in demand for CTMS solutions in North America, Europe, and the Asia Pacific regions is expected to drive market growth over the forecast period. North America currently holds a significant market share in the global CTMS market, with the US being a key contributor to market revenues. Europe and Asia Pacific regions are also experiencing a surge in demand for CTMS solutions, fueled by the increasing number of clinical trials and advancements in CTMS technologies.
Key Players in the Clinical Trial Management System Market
Leading companies in the clinical trial management system market include Oracle Corporation, Medidata Solutions, PAREXEL International Corporation, BioClinica, and Veeva Systems. These companies offer a range of CTMS solutions, including cloud-based and on-premise systems, designed to enhance the efficiency and effectiveness of clinical trial management.
In conclusion, the global clinical trial management system market is poised for significant growth over the forecast period, driven by technological advancements, the increasing number of clinical trials, and the growing need for efficient management systems in the healthcare and research sectors.
Other Trending Reports
Antipsychotic Drugs Industry Trends
Computer Vision in Healthcare Industry Trends
Bioinformatics Industry Trends
Neuroscience Industry Trends
#Clinical Trial Management System Market#Clinical Trial Management System Market Size#Clinical Trial Management System Market Share#Clinical Trial Management System Market Trends#Clinical Trial Management System Market Growth#Clinical Trial Management System Market Analysis#Clinical Trial Management System Market Outlook
0 notes
Text
How to write a good abstract

Writing a compelling and effective abstract is crucial for communicating the essence of your research succinctly and clearly. A well-crafted abstract not only summarizes your study but also emphasizes its significance, thereby attracting the attention of the intended audience, including researchers, practitioners, and policymakers. Below are essential guidelines and a structured approach to writing a high-quality abstract for scientific papers, particularly in the biomedical field, though the principles can be adapted for other disciplines.
Key Elements of a Good Abstract:

Declarative Title:
Your title should be clear and direct, reflecting the main findings of your study. It should convey the primary message accurately, ensuring that even those who only read the title understand the core outcome of your research.
2 .Introduction to the Problem:
Start with a sentence that introduces a significant problem or field of interest. In biomedical sciences, this could involve highlighting a critical health issue. The goal is to establish the relevance of your research by showing the urgency or importance of the problem.
3 . Identification of a Significant Challenge:
Clearly state the specific challenge or barrier that is hindering progress in your field. This sets the stage for your study by pinpointing the precise issue you aim to address without yet delving into your methodology.
4 . Opportunity for Advancement:
Introduce a recent advancement or opportunity that makes addressing the identified challenge feasible. This could be a technological innovation, new data availability, or a novel methodological approach that provides a fresh perspective on the problem.
5 . Description of Your Study:
Summarize the core of your study in 1–2 sentences. Describe what you did and how you leveraged the identified opportunity to tackle the challenge. This should provide a brief but comprehensive overview of your approach.
6 .Key Results:
Highlight the main findings of your study in 2–3 sentences. These results should directly support the conclusions stated in your title and demonstrate the impact of your research.
7. Implications and Broader Impact:
Conclude with a sentence on the potential impact of your findings. Explain how your results could change current practices, inform future research, or have broader implications for the field.
Example of an Abstract Using These Guidelines:
Title: Data-driven Prediction of Drug Effects and Interactions
Abstract: Adverse drug events remain a leading cause of morbidity and mortality worldwide. Many such events are undetected during clinical trials before a drug receives approval for clinical use. Regulatory agencies maintain extensive collections of adverse event reports as part of post marketing surveillance, presenting an opportunity to study drug effects using patient population data. However, confounding factors such as concomitant medications, patient demographics, medical histories, and prescribing reasons are often uncharacterized in spontaneous reporting systems, limiting quantitative signal detection methods. Here, we present an adaptive data-driven approach for correcting these confounding factors in cases with unknown or unmeasured covariates and combine this approach with existing methods to improve drug effect analyses using three test datasets. We also introduce comprehensive databases of drug effects (OffSIDES) and drug-drug interaction side effects (TwoSIDES). To demonstrate the utility of these resources, we identified drug targets, predicted drug indications, and discovered drug class interactions, corroborating 47 (P < 0.0001) interactions using independent electronic medical record analysis. Our findings suggest that combined treatment with selective serotonin reuptake inhibitors and thiazides significantly increases the incidence of prolonged QT intervals. We conclude that controlling for confounding effects in observational clinical data enhances the detection and prediction of adverse drug effects and interactions.
Investing in your academic future with Dissertation Writing Help For Students means choosing a dedicated professional who understands the complexities of dissertation writing and is committed to your success. With a comprehensive range of services, personalized attention, and a proven track record of helping students achieve their academic goals, I am here to support you at every stage of your dissertation journey.
Feel free to reach out to me at [email protected] to commence a collaborative endeavor towards scholarly excellence. Whether you seek guidance in crafting a compelling research proposal, require comprehensive editing to refine your dissertation, or need support in conducting a thorough literature review, I am here to facilitate your journey towards academic success. and discuss how I can assist you in realizing your academic aspirations. Whether you seek guidance in crafting a compelling research proposal, require comprehensive editing to refine your dissertation, or need support in conducting a thorough literature review, I am here to facilitate your journey towards academic success.
#academics#education#grad school#gradblr#phd#phd life#phd research#phd student#phdblr#study#essays#literature#writters on tumblr#my writing#writeblr#writing#writers on tumblr#thesis#dissertation#university student#university#uniblr#stanford university#case study#student life#students#studyblr#studying#studyspo#student
3 notes
·
View notes
Text
The Rise of 3D Printing in Prosthetics and Orthotics Market

The global prosthetics and orthotics market plays a vital role in improving quality of life for millions worldwide. Worth an estimated $7.2 billion in 2024, the market facilitates mobility for those with limb differences or injuries through highly customized external limb replacements and braces.
The market introduces prosthetics and orthotics—Medical devices that enhance or assist impaired body parts and mobility. Orthotics are braces or supports for joints, spine, and limbs; prosthetics externally replace missing limbs. Together they improve functionality and quality of life for users.
Major players in the prosthetics and orthotics space utilizing advanced manufacturing include Ossur, Steeper Group, Blatchford, Fillauer, Ottobock, and WillowWood Global. These industry leaders increasingly deploy cutting-edge 3D printing and customized design software to produce state-of-the-art prosthetics and braces.
Current trends in the prosthetics and orthotics market include growing utilization of 3D printing and advanced manufacturing techniques. 3D printing enables on-demand production of complex, customized devices. It reduces manufacturing costs and wait times while improving fit and comfort. Expanding material options also allow more lifelike prosthetics. As technology evolves, the market is positioned for continued growth through 2031 in facilitating mobility worldwide.
Future Outlook
The prosthetics and orthotics market is expected to witness significant advancements in the coming years. Manufacturers are constantly focusing on developing innovative technologies such as 3D printed prosthetics that provide a better fit, enhanced comfort, and unrestricted movement. There is also a rising trend of using lightweight, highly durable and comfortable materials like carbon fiber and thermoplastics to manufacture prosthetic devices. Advancements in myoelectric prosthetics with touch and motion sensors are making them more dexterous and responsive. Using pattern recognition and machine learning techniques, next-gen prosthetics could gain functionality approaching that of natural limbs.
PEST Analysis
Political: Regulations regarding clinical trials and approvals of new prosthetic technologies may affect market growth. Favorable reimbursement policies for prosthetic devices can boost adoption.
Economic: Rising disposable incomes allow more individuals to opt for higher-end prosthetics. Emerging markets present abundant opportunities for growth. Inflation and economic slowdowns can hinder market profitability.
Social: Increasing incidence of amputations and disabilities due to aging population, accidents, war injuries etc. drive market demand. Growing awareness regarding prosthetics and orthotics aids adoption. Stigma associated with limb loss poses challenges.
Technological: Advancements in materials, manufacturing techniques like 3D printing, sensors, computing power and battery technologies are enhancing functionality and usability of prosthetics/orthotics. Myoelectric and robotic prosthetics have vastly improved in recent years.
Opportunity
Rising aging population presents a huge opportunity for prosthetics and orthotics targeting mobility issues and disabilities. Over 630,000 amputations occur annually in the U.S. due to dysvascular conditions like diabetes, presenting a sizable patient pool. Expanding applications of prosthetics and orthotics beyond mobility impairment into sports and military could drive significant growth. Growing incidence of trauma and injuries globally increases the number of patients relying on these devices. Emerging markets like Asia Pacific and Latin America offer immense opportunities owing to increasing disposable incomes, expanding healthcare infrastructure and rising medical tourism. Technological advancements are constantly improving functionality and usability of prosthetic devices, fueling adoption rates. The lightweight, durable and comfortable characteristics of newer materials expand addressable indications and patient acceptance.
Key Takeaways
Growing demand from aging population: The rapid increase in aging population worldwide who are prone to mobility issues, disabilities and chronic diseases like diabetes is a key driver spurring sales of orthotic and prosthetic devices.
Global expansion into emerging markets: Emerging markets like Asia Pacific, Latin America, Eastern Europe and the Middle East offer immense opportunities owing to their large population bases and improving healthcare penetration.
Technological advancements: Constant R&D bringing advancements in areas such as 3D printing, lightweight materials,
4 notes
·
View notes
Text
Key Concepts in Frozen Shoulder Clinical Trials
When exploring and understanding Frozen Shoulder Syndrome (FSS) clinical trials, several key concepts are important to consider. These concepts provide insights into the design, implementation, and outcomes of clinical trials related to FSS.

To know more about the leading sponsors in the Frozen Shoulder Syndrome clinical trials market, download a free report sample
Here are some key concepts:
Study Phases:
Clinical trials are often conducted in phases, with each phase serving a specific purpose. Phase 1 focuses on safety, Phase 2 on efficacy, Phase 3 on effectiveness and safety in a larger population, and Phase 4 involves post-marketing surveillance. Understanding the phase of a trial helps assess its developmental stage.
Intervention Types:
Trials may involve different types of interventions, such as medications, physical therapies, surgical procedures, or a combination of these. Understanding the nature of the intervention is crucial to evaluating its potential effectiveness for treating FSS.
Randomized Controlled Trials (RCTs):
RCTs are considered the gold standard in clinical research. In these trials, participants are randomly assigned to different groups, with one group receiving the experimental treatment and another serving as a control. RCTs help establish causation and reduce bias.
Placebo-Controlled Trials:
Some trials involve a placebo group, where participants receive an inactive substance. This helps researchers assess the true impact of the intervention by comparing it to a group that does not receive the treatment.
Blinding:
Trials may be single-blind (participants are unaware of their treatment group) or double-blind (both participants and researchers are unaware). Blinding helps minimize bias in reporting and assessing outcomes.
Inclusion and Exclusion Criteria:
Trials have specific criteria for participant eligibility. Inclusion criteria define characteristics participants must have, and exclusion criteria specify factors that disqualify individuals. Understanding these criteria is essential for determining eligibility.
Primary and Secondary Endpoints:
Primary endpoints are the main outcomes the trial aims to measure, often related to efficacy. Secondary endpoints provide additional information. These endpoints guide the assessment of the intervention's impact.
Safety Monitoring:
Trials include safety monitoring to identify and assess adverse events. Robust safety measures are essential to ensure participant well-being.
Patient-Reported Outcomes (PROs):
PROs capture data directly from participants about their health status and treatment experiences. They can provide insights into the impact of FSS and the effectiveness of interventions from the patient's perspective.
Crossover Design:
Some trials use a crossover design, where participants switch from one treatment to another during the course of the study. This design helps control for individual variability.
Statistical Significance:
Statistical analysis is crucial for determining whether observed differences between groups are likely due to the intervention or occurred by chance. Results are considered statistically significant if the probability of their occurrence by chance is low.
Informed Consent:
Informed consent is a fundamental ethical requirement. Participants must be fully informed about the trial's objectives, procedures, potential risks, and benefits before providing their consent to participate.
Understanding these key concepts will enhance your ability to critically evaluate FSS clinical trials and interpret their findings. Always consult with healthcare professionals and researchers for personalized advice and insights.
2 notes
·
View notes
Text
Artificial Intelligence (AI)-based Clinical Trials Market by Product, Types, Procedure, Application, End-user Global Forecast to 2029
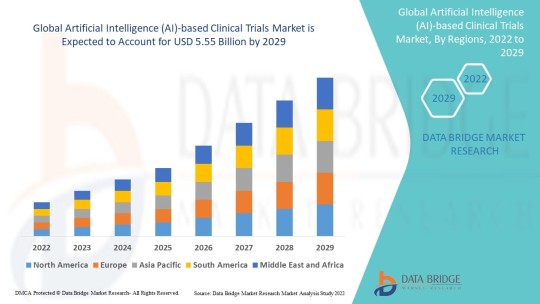
Industry Analysis
Data Bridge Market Research analyses that the artificial intelligence (AI)-based clinical trials market which was USD 1.3 billion in 2021, would rocket up to USD 5.55 billion by 2029, and is expected to undergo a CAGR of 19.90% during the forecast period 2022 to 2029.
Data Bridge market report covers an array of aspects of the market analysis which today’s businesses call for. This market document also defines a chapter on the global market and allied companies with their profiles, which provides important data pertaining to their insights in terms of finances, product portfolios, investment plans, and marketing and business strategies. This market research report is generated with a nice blend of industry insight, talent solutions, practical solutions and use of technology to advance user experience. An outstanding Data Bridge market report puts light on many aspects related to healthcare industry and market.
Market Insights and Scope
Artificial intelligence can be used to identify disease, provide healthcare services, and even develop new treatments, while improving clinical trials. The scale and speed of AI are significantly superior to any system that depends just on human activity. In many ways, this will be the biggest problem moving forward because we haven't yet reached an AI level that can run totally autonomously.
Artificial Intelligence (AI)-based Clinical Trials Market report helps the manufacturer in finding out the effectiveness of the existing channels of distribution, advertising programs, or media, selling methods and the best way of distributing the goods to the eventual consumers. Taking up such market research report is all the time beneficial for any company whether it is a small scale or large scale, for marketing of products or services. It makes effortless for healthcare industry to visualize what is already available in the market, what market anticipates, the competitive environment, and what should be done to surpass the competitor.
Industry Segmentation
The Artificial Intelligence (AI)-based clinical trials market is segmented on the basis of clinical trial phase, application and end-user. The growth amongst these segments will help you analyze meagre growth segments in the industries and provide the users with a valuable market overview and market insights to help them make strategic decisions for identifying core market applications.
Clinical Trial Phase
Phase-I
Phase-II
Phase-III
Application
Oncology
Cardiovascular Diseases
Neurological Diseases or Conditions
Infectious Diseases
End-user
Pharmaceutical Companies
Academia
Get a Free Sample of The Report: https://www.databridgemarketresearch.com/request-a-sample/?dbmr=global-artificial-intelligence-ai-based-clinical-trials-market
Market Country Level Analysis
The countries covered in the Artificial Intelligence (AI)-based clinical trials market report are
U.S., Canada and Mexico in North America, Germany, France, U.K., Netherlands, Switzerland, Belgium, Russia, Italy, Spain, Turkey, Rest of Europe in Europe, China, Japan, India, South Korea, Singapore, Malaysia, Australia, Thailand, Indonesia, Philippines, Rest of Asia-Pacific (APAC) in the Asia-Pacific (APAC), Saudi Arabia, U.A.E, South Africa, Egypt, Israel, Rest of Middle East and Africa (MEA) as a part of Middle East and Africa (MEA), Brazil, Argentina and Rest of South America as part of South America.
Get full access to the report: https://www.databridgemarketresearch.com/reports/global-artificial-intelligence-ai-based-clinical-trials-market
Industry Share Analysis
Some of the major players operating in the Artificial Intelligence (AI)-based clinical trials market are:
Phesi (India)
CONSILX (Singapore)
DEEP LENS Inc. (U.S.)
Unlearn.AI, Inc. (U.S.)
Saama Technologies, LLC (U.S.)
Antidote Technologies, Inc. (U.K.)
Innoplexus (Germany)
Mendel.ai (U.S.)
Median Technologies (France)
Symphony AI (U.S.)
BioAge Labs, Inc. (U.S.)
AiCure (U.S.)
Halo Health Systems (U.S.)
An influential Artificial Intelligence (AI)-based Clinical Trials Market research report displays an absolute outline of the market that considers various aspects such as product definition, customary vendor landscape, and market segmentation. Currently, businesses are relying on the diverse segments covered in the market research report to a great extent which gives them better insights to drive the business on the right track. The competitive analysis brings into light a clear insight about the market share analysis and actions of the key industry players. With this info, businesses can successfully make decisions about business strategies to accomplish maximum return on investment (ROI).
Get TOC Details: https://www.databridgemarketresearch.com/toc/?dbmr=global-artificial-intelligence-ai-based-clinical-trials-market
Browse Related Reports@
Global 1, 4-Cyclohexanedimethanol Dibenzoate Market
Global Plant Hydrocolloids Market
U.S. Tahini Market
Asia-Pacific Hydroxyl-Terminated Polybutadiene (HTPB) market
West Africa Shisha Tobacco Market
Global Orthostatic Hypotension Drugs Market
Europe Customer Journey Analytics Market
About Us:
Data Bridge Market Research set forth itself as an unconventional and neoteric Market research and consulting firm with an unparalleled level of resilience and integrated approaches. We are determined to unearth the best market opportunities and foster efficient information for your business to thrive in the market
Contact:
Data Bridge Market Research
Tel: +1-888-387-2818
Email: [email protected]
#Artificial Intelligence (AI)-based Clinical Trials Market-by Product-Types-Procedure-Application-End User-Global-Forecast to-2029#Artificial Intelligence (AI)-based Clinical Trials Market-Global Opportunity-Analysis and-Industry-regional#Artificial Intelligence (AI)-based Clinical Trials Market-Growth-Competition-Scenario-Outlook#Artificial Intelligence (AI)-based Clinical Trials Market-Insights-Country-Share-Competitors-Research-Study#Artificial Intelligence (AI)-based Clinical Trials Market-Demands-Size-Share-Top Trends-Report-to-2029#Artificial Intelligence (AI)-based Clinical Trials Market Value-Segmentation-CAGR rate-Future Trends#Artificial Intelligence (AI)-based Clinical Trials Market-drivers-advantages-restraints-challenges#Artificial Intelligence (AI)-based Clinical Trials Market-Leading Brands-Business-Healthcare#Artificial Intelligence (AI)-based Clinical Trials Market-Growing Popularity-Traffic-DBMR
0 notes