#Crystal structure
Explore tagged Tumblr posts
Photo

"Hematite Iron Rose" by Juan - Metallic petals form intricate layers, capturing the mineral's natural elegance.
#macro#white#metallic luster#geology#sierra alhamilla#almería#andalusia#spain#juan#hematite#natural formation#mineral#gray#crystal structure#black#photography
208 notes
·
View notes
Text

Geology Rocks: Dravite Tourmaline
Dravite Tourmaline is one of the three major types of Tourmaline, along with Schorl (black) and Elbaite (multi-colored). Dravite Tourmalines are typically brown, yellow or green, and are rich in magnesium and sodium. Tourmaline is found around the world. Dravite is one of the main varieties and comes in several colors, most notably yellow, brown and green. Notable deposits for Dravite Tourmaline include Australia, Austria, Brazil, India, Italy, Nepal, Switzerland, Tanzania, and the United States.
Spiritual Meaning:
Dravite is a strong grounding stone. It has a soothing, relaxing, and reassuring effect on your body, as well as on your heart and mind. Dravite Tourmaline gently nurtures self-compassion and healing after trauma. If we have become emotionally numb, Dravite Tourmaline helps us to feel again.
#Dravite Tourmaline#geology rocks#geology#green tourmaline#tourmaline#green gemstone#green stone#green crystals#crystal magic#crystal meanings#crystal photo#crystal structure#crystals#crystal stone#toya's tales#style#toyastales#toyas tales#art#september#minerals#rock photography#rocks#witchy#witchcore#witches#october#fall vibes#fall aesthetic#fall
141 notes
·
View notes
Text

Precision-shaped Cu₂O crystals unlock new potential for clean energy catalysts
Can the shape of a crystal really change how well it performs in clean energy technology? A new study says yes—decisively. Researchers from National Taiwan University, National Tsing Hua University, and National Yang Ming Chiao Tung University have discovered that the performance of a widely studied catalyst, cuprous oxide (Cu2O), in oxygen reduction reactions (ORR) depends heavily on which crystal face is exposed. The paper is published in the Journal of Materials Chemistry A. Oxygen reduction is a central reaction in fuel cells, which are devices that convert chemical energy into electricity. Platinum is commonly used in this role but is expensive and limited in supply. Cu2O, a more affordable alternative, has now shown surprising potential—if used with the right shape.
Read more.
#Materials Science#Science#Crystals#Copper#Oxides#Reactions#Fuel cells#Catalysts#Crystal structure#Surfaces#National Taiwan University
20 notes
·
View notes
Text
Miller Indices and Lattice Planes
Have you ever wondered how scientists talk about the microscopic building blocks of materials? Enter the world of Miller indices and lattice levels, a language that reveals the secrets of atomic arrangements within crystals. Imagine visualizing levels of atoms stacked like bricks, each level described by a unique set of numbers that reveal its orientation and spacing.
How to determine Miller Indices?
Imagine the crystal lattice with three axes: These axes represent the basic building blocks of the crystal's structure. In a cubic crystal, for example, the axes are all perpendicular to each other and of equal length.
Identify a plane: This plane can cut through the lattice in any direction, intersecting the axes at different points.
Find the intercepts: For each axis, measure the distance from the origin to the point where the plane intersects it. If the plane doesn't intersect an axis, then the intercept is considered to be "at infinity" and is represented by a zero.
Take the reciprocals: For each non-zero intercept, take the reciprocal (flip it over).
Simplify and normalize: If any of the reciprocals have a common factor, divide them all by that factor to get the smallest possible integers.
Put the numbers together: The resulting three integers, enclosed in parentheses (h k l), are the Miller indices for that plane.
Read more:
#science#materials science#materials#materials science and engineering#alloys#metals#mechanical properties#mechanical properties of materials#crystal structure#crystalline
3 notes
·
View notes
Text
For example, we have observed unprecedented spin crossover behaviour in a dicobalt complex (figure 13.44), and more recently we have determined the crystal structure of an unused mixed spin state diiron complex (figure 13.45).
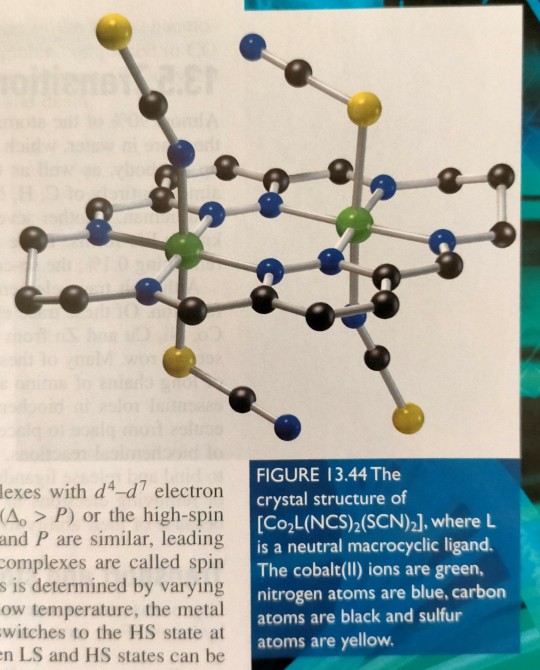
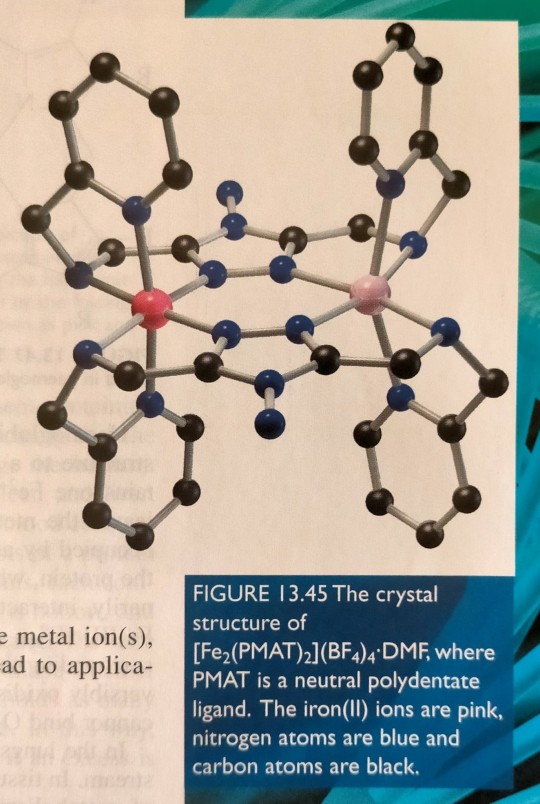
"Chemistry" 2e - Blackman, A., Bottle, S., Schmid, S., Mocerino, M., Wille, U.
#book quotes#chemistry#nonfiction#textbook#spin#electrons#electron configuration#crystal structure#cobalt#iron#nitrogen#carbon#sulfur
1 note
·
View note
Text
How can electrons can split into fractions of themselves?
New Post has been published on https://thedigitalinsider.com/how-can-electrons-can-split-into-fractions-of-themselves/
How can electrons can split into fractions of themselves?


MIT physicists have taken a key step toward solving the puzzle of what leads electrons to split into fractions of themselves. Their solution sheds light on the conditions that give rise to exotic electronic states in graphene and other two-dimensional systems.
The new work is an effort to make sense of a discovery that was reported earlier this year by a different group of physicists at MIT, led by Assistant Professor Long Ju. Ju’s team found that electrons appear to exhibit “fractional charge” in pentalayer graphene — a configuration of five graphene layers that are stacked atop a similarly structured sheet of boron nitride.
Ju discovered that when he sent an electric current through the pentalayer structure, the electrons seemed to pass through as fractions of their total charge, even in the absence of a magnetic field. Scientists had already shown that electrons can split into fractions under a very strong magnetic field, in what is known as the fractional quantum Hall effect. Ju’s work was the first to find that this effect was possible in graphene without a magnetic field — which until recently was not expected to exhibit such an effect.
The phenemonon was coined the “fractional quantum anomalous Hall effect,” and theorists have been keen to find an explanation for how fractional charge can emerge from pentalayer graphene.
The new study, led by MIT professor of physics Senthil Todadri, provides a crucial piece of the answer. Through calculations of quantum mechanical interactions, he and his colleagues show that the electrons form a sort of crystal structure, the properties of which are ideal for fractions of electrons to emerge.
“This is a completely new mechanism, meaning in the decades-long history, people have never had a system go toward these kinds of fractional electron phenomena,” Todadri says. “It’s really exciting because it makes possible all kinds of new experiments that previously one could only dream about.”
The team’s study appeared last week in the journal Physical Review Letters. Two other research teams — one from Johns Hopkins University, and the other from Harvard University, the University of California at Berkeley, and Lawrence Berkeley National Laboratory — have each published similar results in the same issue. The MIT team includes Zhihuan Dong PhD ’24 and former postdoc Adarsh Patri.
“Fractional phenomena”
In 2018, MIT professor of physics Pablo Jarillo-Herrero and his colleagues were the first to observe that new electronic behavior could emerge from stacking and twisting two sheets of graphene. Each layer of graphene is as thin as a single atom and structured in a chicken-wire lattice of hexagonal carbon atoms. By stacking two sheets at a very specific angle to each other, he found that the resulting interference, or moiré pattern, induced unexpected phenomena such as both superconducting and insulating properties in the same material. This “magic-angle graphene,” as it was soon coined, ignited a new field known as twistronics, the study of electronic behavior in twisted, two-dimensional materials.
“Shortly after his experiments, we realized these moiré systems would be ideal platforms in general to find the kinds of conditions that enable these fractional electron phases to emerge,” says Todadri, who collaborated with Jarillo-Herrero on a study that same year to show that, in theory, such twisted systems could exhibit fractional charge without a magnetic field. “We were advocating these as the best systems to look for these kinds of fractional phenomena,” he says.
Then, in September of 2023, Todadri hopped on a Zoom call with Ju, who was familiar with Todari’s theoretical work and had kept in touch with him through Ju’s own experimental work.
“He called me on a Saturday and showed me the data in which he saw these [electron] fractions in pentalayer graphene,” Todadri recalls. “And that was a big surprise because it didn’t play out the way we thought.”
In his 2018 paper, Todadri predicted that fractional charge should emerge from a precursor phase characterized by a particular twisting of the electron wavefunction. Broadly speaking, he theorized that an electron’s quantum properties should have a certain twisting, or degree to which it can be manipulated without changing its inherent structure. This winding, he predicted, should increase with the number of graphene layers added to a given moiré structure.
“For pentalayer graphene, we thought the wavefunction would wind around five times, and that would be a precursor for electron fractions,” Todadri says. “But he did his experiments and discovered that it does wind around, but only once. That then raised this big question: How should we think about whatever we are seeing?”
Extraordinary crystal
In the team’s new study, Todadri went back to work out how electron fractions could emerge from pentalayer graphene if not through the path he initially predicted. The physicists looked through their original hypothesis and realized they may have missed a key ingredient.
“The standard strategy in the field when figuring out what’s happening in any electronic system is to treat electrons as independent actors, and from that, figure out their topology, or winding,” Todadri explains. “But from Long’s experiments, we knew this approximation must be incorrect.”
While in most materials, electrons have plenty of space to repel each other and zing about as independent agents, the particles are much more confined in two-dimensional structures such as pentalayer graphene. In such tight quarters, the team realized that electrons should also be forced to interact, behaving according to their quantum correlations in addition to their natural repulsion. When the physicists added interelectron interactions to their theory, they found it correctly predicted the winding that Ju observed for pentalayer graphene.
Once they had a theoretical prediction that matched with observations, the team could work from this prediction to identify a mechanism by which pentalayer graphene gave rise to fractional charge.
They found that the moiré arrangement of pentalayer graphene, in which each lattice-like layer of carbon atoms is arranged atop the other and on top of the boron-nitride, induces a weak electrical potential. When electrons pass through this potential, they form a sort of crystal, or a periodic formation, that confines the electrons and forces them to interact through their quantum correlations. This electron tug-of-war creates a sort of cloud of possible physical states for each electron, which interacts with every other electron cloud in the crystal, in a wavefunction, or a pattern of quantum correlations, that gives the winding that should set the stage for electrons to split into fractions of themselves.
“This crystal has a whole set of unusual properties that are different from ordinary crystals, and leads to many fascinating questions for future research,” Todadri says. “For the short term, this mechanism provides the theoretical foundation for understanding the observations of fractions of electrons in pentalayer graphene and for predicting other systems with similar physics.”
This work was supported, in part, by the National Science Foundation and the Simons Foundation.
#2023#agents#arrangement#atom#atoms#Behavior#boron nitride#california#carbon#carbon atoms#Carbon materials#chicken#Cloud#crystal#crystal structure#crystals#data#electron#electronic#electrons#experimental#explanation#form#Foundation#Future#graphene#harvard#History#how#interference
0 notes
Text
helena eagan both defying and exceeding my expectations by losing so hard and so immediately... having an even worse relationship with her dad than expected... filming a youtuber apology with an embarrassingly obvious fake cover story... reacting to the cctv footage Like That
#ceruleanrambling#severance#severance spoilers#it's way more immediate vulnerability than i expected. which makes total sense in retrospect bc she's been crystallized mid s1#for two years in my mind. and tv shows need character arcs or whatever.#also looking incredible. obviously.#i hope someone makes a gif asap of her by the lockers bc it's so damn clever to have#the big structured coat that she takes off and suddenly she transforms into Helly in Helly's Outfit#ummm i loved devon. liked the glimpses of other people's lives. would have liked a bit more forward motion
2K notes
·
View notes
Text
These crystal structures can be described such that all the atoms in a unit cell are associated with the lattice points, i.e. the number of atoms in the unit cell and the number of lattice points will be identical.
"Chemistry" 2e - Blackman, A., Bottle, S., Schmid, S., Mocerino, M., Wille, U.
0 notes
Text



A Linked Universe meets The Dark Crystal AU! I don't even remember what started it at this point. I remembered that the Dark Crystal and Age of Resistance are things I like, blinked, and woke up three days later with an AU and a bunch of art.
The designs and the story are a wip and for fun so expect a lot of variation! (I have a few different beginnings, ideas for different designs, etc)! :D
In addition to #linked universe I'll be using the tags #the dark crystal lu au and #courage of the dark crystal!
#linked universe#tdc aor#the dark crystal lu au#courage of the dark crystal#lu au au#lu legend#lu hyrule#lu four#lu wind#I've made an au of the au I've gone too far help help-#I gotta get better at drawing gelfling! Their facial structures are very distinct#ALSO I went really big with the ears here lmao#the hugest ears ever seen on gelfling#TRANS ROOLIE TRANS ROOLIE TRANS ROOLIE YIPPEE!!!!#I drew this last month (except for Four) sO HAPPY PRIDE! Roolie gets WINGS! :D#I'm SO pleased with Wind's design! he's a lil fishy! and Four with the horned headband/armor in place of the lil ups in his bangs#gonna try to put the aureyal or symbol of the conjunction and triangles on all of them#IF YOU HAVE QUESTIONS ABOUT THE AU PLS ASK THEM#I'd love to tackle some worldbuilding mayhaps?!#I'm thinking of placing them somewhere before the first battle of stone-in-the-wood in the arathim wars#or after the events of the comics with Kensho and Thurma somewhere#and just figure out another reason for the crystal to be shattered. so many possibilities!#where's the crystal shard this time and how can I split it between them? >:3 niiiiine shards made whole >:3#quest for the ~~triforce~~ crystal#Hello from summer camp also! Lots of shenanigans!#I'm surprised I was able to draw Four at all last weekend I've been so busy!!!#having fun tho!!! we're having a lunch cookout at archery and campfire is tonight!!! It's going good! see ya!!!
559 notes
·
View notes
Text


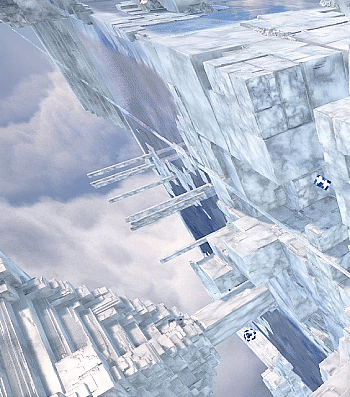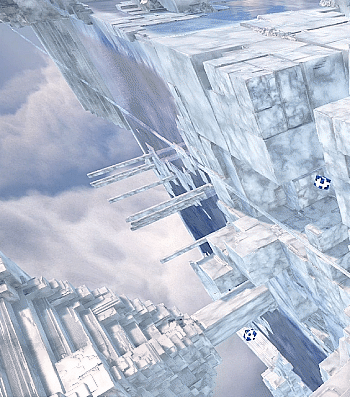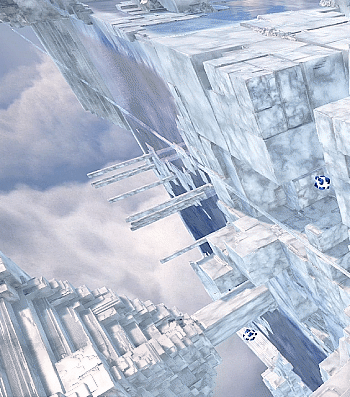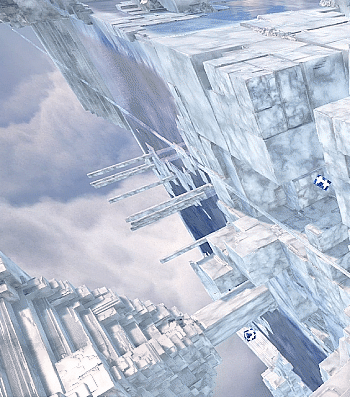
Kingdom Hearts 3 - The Final World
#kingdom hearts 3#kh3#the final world#scenery#my gif#i wish you could still speak with the stars but they're gone when you reenter this world#would've been nice to see them twinkling in the background#do we know what these structures are made of? they look like sodium chloride crystals to me#not sure what the significance of that is unless they just wanted it to look cool#because it does look very cool
185 notes
·
View notes
Text





Dream Demon (1988), dir. Harley Cokeliss
#when i see things and they're right....#just like the structure of crystals#even on letterboxd i only saw one person mentioning how gay this was like how does this fly over your head??? the REPRESSION#dream demon#harley cokeliss#lesbian subtext#film stills#horror#*
94 notes
·
View notes
Text
Me every time I check out the dead boy detectives tag: *rolls up sleeves* welp. Looks like it’s time for another Crystal Palace defence post
#crystal palace#crystal palace surname von hoverkraft#dead boy detectives#long one might be incoming tbh#cause like seriously ‘we need more complex women’ ‘you can’t even handle one traumatized teenage girl’#and some takes I’ve seen are genuinely baffling#like it’s even one thing not to like her but to consider her a superfluous character is just factually incorrect#and requires a level of ignoring so much of the plot#as well as plot structure; themes; character arcs; basic narrative…#like girl what#anyways yeah long post might be coming but I am NOT going to write it here in the tags I am NOT#dbda
67 notes
·
View notes
Text
Researchers at New York University have devised a mathematical approach to predict the structures of crystals -- a critical step in developing many medicines and electronic devices -- in a matter of hours using only a laptop, a process that previously took a supercomputer weeks or months. Their novel framework is published in the journal Nature Communications. Organic molecular crystals are an important class of materials in many industries, from pharmaceuticals and agriculture to electronics and explosives. Crystals are the building blocks that make up many over-the-counter and prescription drugs, insecticides to fight mosquitoes, explosives such as TNT, semiconductors, and light-emitting technologies used in television screens and cell phones.
Read more.
25 notes
·
View notes
Text
Crystalline imperfections of materials
In reality, crystals are never perfect, and they contain various types of imperfections and defects that affect many of their physical and mechanical properties, which in turn affect many important engineering properties of materials such asthe cold formability of alloys, the electronic conductivity of semiconductors, the rate of migration of atoms in alloys (Diffusion), and the corrosion of metals.
Crystalline Imperfections
Crystalline imperfections, also known as crystal defects or lattice defects, are deviations from the ideal atomic arrangement in a crystalline material. Crystalline materials have a regular and repeating three-dimensional atomic or molecular structure. However, in reality, perfect crystals are rare, and most materials contain various types of imperfections. These imperfections can significantly impact the properties and behavior of the material.
Types of imperfections in crystals
Crystalline imperfections are classified according to their geometry and shape into:
Zero-dimensional or point defects.
One-dimensional or line defects (dislocations).
Two-dimensional or Planar defects.
Three-dimensional volume defects.
Read more:
#science#materials science#engineering#materials science and engineering#mechanical properties of materials#defects#Imperfections#crystalline defects#crystal structure#Crystalline imperfections of materials
0 notes
Text







#aesthetic#grunge#fashion#crystal castles#gothic#fashion model#model inspo#model instagram#model icons#pyramidsofgiza#structure#egypt#alt girl
23 notes
·
View notes
Text
More durable metals for fusion power reactors
New Post has been published on https://thedigitalinsider.com/more-durable-metals-for-fusion-power-reactors/
More durable metals for fusion power reactors


For many decades, nuclear fusion power has been viewed as the ultimate energy source. A fusion power plant could generate carbon-free energy at a scale needed to address climate change. And it could be fueled by deuterium recovered from an essentially endless source — seawater.
Decades of work and billions of dollars in research funding have yielded many advances, but challenges remain. To Ju Li, the TEPCO Professor in Nuclear Science and Engineering and a professor of materials science and engineering at MIT, there are still two big challenges. The first is to build a fusion power plant that generates more energy than is put into it; in other words, it produces a net output of power. Researchers worldwide are making progress toward meeting that goal.
The second challenge that Li cites sounds straightforward: “How do we get the heat out?” But understanding the problem and finding a solution are both far from obvious.
Research in the MIT Energy Initiative (MITEI) includes development and testing of advanced materials that may help address those challenges, as well as many other challenges of the energy transition. MITEI has multiple corporate members that have been supporting MIT’s efforts to advance technologies required to harness fusion energy.
The problem: An abundance of helium, a destructive force
Key to a fusion reactor is a superheated plasma — an ionized gas — that’s reacting inside a vacuum vessel. As light atoms in the plasma combine to form heavier ones, they release fast neutrons with high kinetic energy that shoot through the surrounding vacuum vessel into a coolant. During this process, those fast neutrons gradually lose their energy by causing radiation damage and generating heat. The heat that’s transferred to the coolant is eventually used to raise steam that drives an electricity-generating turbine.
The problem is finding a material for the vacuum vessel that remains strong enough to keep the reacting plasma and the coolant apart, while allowing the fast neutrons to pass through to the coolant. If one considers only the damage due to neutrons knocking atoms out of position in the metal structure, the vacuum vessel should last a full decade. However, depending on what materials are used in the fabrication of the vacuum vessel, some projections indicate that the vacuum vessel will last only six to 12 months. Why is that? Today’s nuclear fission reactors also generate neutrons, and those reactors last far longer than a year.
The difference is that fusion neutrons possess much higher kinetic energy than fission neutrons do, and as they penetrate the vacuum vessel walls, some of them interact with the nuclei of atoms in the structural material, giving off particles that rapidly turn into helium atoms. The result is hundreds of times more helium atoms than are present in a fission reactor. Those helium atoms look for somewhere to land — a place with low “embedding energy,” a measure that indicates how much energy it takes for a helium atom to be absorbed. As Li explains, “The helium atoms like to go to places with low helium embedding energy.” And in the metals used in fusion vacuum vessels, there are places with relatively low helium embedding energy — namely, naturally occurring openings called grain boundaries.
Metals are made up of individual grains inside which atoms are lined up in an orderly fashion. Where the grains come together there are gaps where the atoms don’t line up as well. That open space has relatively low helium embedding energy, so the helium atoms congregate there. Worse still, helium atoms have a repellent interaction with other atoms, so the helium atoms basically push open the grain boundary. Over time, the opening grows into a continuous crack, and the vacuum vessel breaks.
That congregation of helium atoms explains why the structure fails much sooner than expected based just on the number of helium atoms that are present. Li offers an analogy to illustrate. “Babylon is a city of a million people. But the claim is that 100 bad persons can destroy the whole city — if all those bad persons work at the city hall.” The solution? Give those bad persons other, more attractive places to go, ideally in their own villages.
To Li, the problem and possible solution are the same in a fusion reactor. If many helium atoms go to the grain boundary at once, they can destroy the metal wall. The solution? Add a small amount of a material that has a helium embedding energy even lower than that of the grain boundary. And over the past two years, Li and his team have demonstrated — both theoretically and experimentally — that their diversionary tactic works. By adding nanoscale particles of a carefully selected second material to the metal wall, they’ve found they can keep the helium atoms that form from congregating in the structurally vulnerable grain boundaries in the metal.
Looking for helium-absorbing compounds
To test their idea, So Yeon Kim ScD ’23 of the Department of Materials Science and Engineering and Haowei Xu PhD ’23 of the Department of Nuclear Science and Engineering acquired a sample composed of two materials, or “phases,” one with a lower helium embedding energy than the other. They and their collaborators then implanted helium ions into the sample at a temperature similar to that in a fusion reactor and watched as bubbles of helium formed. Transmission electron microscope images confirmed that the helium bubbles occurred predominantly in the phase with the lower helium embedding energy. As Li notes, “All the damage is in that phase — evidence that it protected the phase with the higher embedding energy.”
Having confirmed their approach, the researchers were ready to search for helium-absorbing compounds that would work well with iron, which is often the principal metal in vacuum vessel walls. “But calculating helium embedding energy for all sorts of different materials would be computationally demanding and expensive,” says Kim. “We wanted to find a metric that is easy to compute and a reliable indicator of helium embedding energy.”
They found such a metric: the “atomic-scale free volume,” which is basically the maximum size of the internal vacant space available for helium atoms to potentially settle. “This is just the radius of the largest sphere that can fit into a given crystal structure,” explains Kim. “It is a simple calculation.” Examination of a series of possible helium-absorbing ceramic materials confirmed that atomic free volume correlates well with helium embedding energy. Moreover, many of the ceramics they investigated have higher free volume, thus lower embedding energy, than the grain boundaries do.
However, in order to identify options for the nuclear fusion application, the screening needed to include some other factors. For example, in addition to the atomic free volume, a good second phase must be mechanically robust (able to sustain a load); it must not get very radioactive with neutron exposure; and it must be compatible — but not too cozy — with the surrounding metal, so it disperses well but does not dissolve into the metal. “We want to disperse the ceramic phase uniformly in the bulk metal to ensure that all grain boundary regions are close to the dispersed ceramic phase so it can provide protection to those regions,” says Li. “The two phases need to coexist, so the ceramic won’t either clump together or totally dissolve in the iron.”
Using their analytical tools, Kim and Xu examined about 50,000 compounds and identified 750 potential candidates. Of those, a good option for inclusion in a vacuum vessel wall made mainly of iron was iron silicate.
Experimental testing
The researchers were ready to examine samples in the lab. To make the composite material for proof-of-concept demonstrations, Kim and collaborators dispersed nanoscale particles of iron silicate into iron and implanted helium into that composite material. She took X-ray diffraction (XRD) images before and after implanting the helium and also computed the XRD patterns. The ratio between the implanted helium and the dispersed iron silicate was carefully controlled to allow a direct comparison between the experimental and computed XRD patterns. The measured XRD intensity changed with the helium implantation exactly as the calculations had predicted. “That agreement confirms that atomic helium is being stored within the bulk lattice of the iron silicate,” says Kim.
To follow up, Kim directly counted the number of helium bubbles in the composite. In iron samples without the iron silicate added, grain boundaries were flanked by many helium bubbles. In contrast, in the iron samples with the iron silicate ceramic phase added, helium bubbles were spread throughout the material, with many fewer occurring along the grain boundaries. Thus, the iron silicate had provided sites with low helium-embedding energy that lured the helium atoms away from the grain boundaries, protecting those vulnerable openings and preventing cracks from opening up and causing the vacuum vessel to fail catastrophically.
The researchers conclude that adding just 1 percent (by volume) of iron silicate to the iron walls of the vacuum vessel will cut the number of helium bubbles in half and also reduce their diameter by 20 percent — “and having a lot of small bubbles is OK if they’re not in the grain boundaries,” explains Li.
Next steps
Thus far, Li and his team have gone from computational studies of the problem and a possible solution to experimental demonstrations that confirm their approach. And they’re well on their way to commercial fabrication of components. “We’ve made powders that are compatible with existing commercial 3D printers and are preloaded with helium-absorbing ceramics,” say Li. The helium-absorbing nanoparticles are well dispersed and should provide sufficient helium uptake to protect the vulnerable grain boundaries in the structural metals of the vessel walls. While Li confirms that there’s more scientific and engineering work to be done, he, along with Alexander O’Brien PhD ’23 of the Department of Nuclear Science and Engineering and Kang Pyo So, a former postdoc in the same department, have already developed a startup company that’s ready to 3D print structural materials that can meet all the challenges faced by the vacuum vessel inside a fusion reactor.
This research was supported by Eni S.p.A. through the MIT Energy Initiative. Additional support was provided by a Kwajeong Scholarship; the U.S. Department of Energy (DOE) Laboratory Directed Research and Development program at Idaho National Laboratory; U.S. DOE Lawrence Livermore National Laboratory; and Creative Materials Discovery Program through the National Research Foundation of Korea.
#000#3d#3D printers#ADD#advanced materials#agreement#approach#atom#atomic#atoms#bubbles#carbon#ceramics#challenge#change#climate#climate change#comparison#continuous#crystal#crystal structure#Department of Energy (DoE)#development#DMSE#easy#electricity#electron#energy#engineering#experimental
0 notes