#Glycoconjugate structures
Explore tagged Tumblr posts
Text

GalNAc-L96 – A Key Molecule for Efficient RNA Targeted Delivery Introduction to GalNAc-L96 GalNAc-L96 (CAS 1159408-62-4) is a triantennary N-acetylgalactosamine (GalNAc) ligand primarily used for the synthesis of GalNAc-siRNA. GalNAc is a specific ligand for ASGPR (Asialoglycoprotein Receptor), enabling liver-targeted delivery, which is a crucial aspect of modern RNA delivery technology. Applications and Advantages of GalNAc-L96 - Precise Liver-Targeted Delivery: ASGPR is highly expressed on liver cell surfaces, allowing siRNA, antisense oligonucleotides (ASO), or other nucleic acid drugs conjugated with GalNAc-L96 to efficiently enter liver cells and enhance therapeutic effects. - Enhanced Bioavailability of siRNA: Traditional siRNA delivery methods (such as lipid nanoparticles, LNPs) often lead to non-specific distribution, whereas GalNAc-conjugated siRNA (GalNAc-siRNA) enables more efficient and safer RNA interference (RNAi) therapy. - Reduced Systemic Toxicity: Due to the high specificity of GalNAc delivery, compared to LNPs, GalNAc-siRNA exhibits lower immunogenicity and reduced side effects, making it a preferred strategy for RNA drug development. - Improved Stability and Optimized Pharmacokinetics: By optimizing the structure of GalNAc-L96, its affinity for ASGPR can be enhanced, thereby increasing hepatic cell uptake and prolonging in vivo half-life. Watson’s Unique Advantages in the GalNAc Field - Advanced Synthesis Techniques and Purity Control - Watson leverages cutting-edge glycochemistry and nucleic acid modification technologies to provide high-purity, high-quality GalNAc-L96 and its derivatives, ensuring optimal outcomes in RNAi and ASO drug development. - Comprehensive Support from Lab to Industrial Scale - Watson not only supplies small-scale research-grade products but also has pilot and industrial-scale production capabilities, meeting the demands from early-stage research to clinical production. - Strict adherence to quality management systems (ISO certification) ensures product quality and stability. - Strong Technical Expertise and Industry Recognition - With years of experience in glycoconjugates and RNA delivery, Watson collaborates with leading pharmaceutical and biotechnology companies worldwide, driving advancements in RNA therapeutics. - Watson’s high-end customization and innovative synthesis capabilities make it a key global partner in RNA delivery technologies. Conclusion As a core ligand for RNA-targeted delivery, GalNAc-L96 plays a vital role in siRNA and ASO therapies. With cutting-edge synthesis technologies, strict quality control, and strong customization capabilities, Watson holds a distinct competitive advantage in this field, supporting breakthroughs in RNA delivery technology. If you are interested in GalNAc-related products or RNA delivery solutions, feel free to contact Watson for more information! Sales Link GalNAc-L96 – A Key Molecule for Efficient RNA Targeted Delivery on Watson https://www.youtube.com/watch?v=4XIyWFZHIMw Read the full article
0 notes
Text
Determination of Salivary Glycoconjugates and Salivary Ros Levels in Chronic Periodontitis | Chapter 11 | New Horizons in Medicine and Medical Research Vol. 7
The research was done as a first step toward using salivary glycoconjugates and reactive oxygen species (ROS) as accurate indicators of chronic periodontitis. Glycoconjugates are crucial biological compounds that serve a variety of roles. They are made up of oligosaccharides of various sizes and complexity that are connected to a non-sugar component such as a lipid or protein. Glycoconjugate structures are frequently convoluted, with complicated biochemical routes. Overproduction of reactive oxygen species is thought to play a role in the disease's severity. ROS are extremely unstable and have a short half-life, making them difficult to detect. However, at times of stress or disease, ROS levels can skyrocket, causing severe cell structural damage. Author(S) Details Jenny Susan Roy Department of Periodontic, Mount Zion Medical College, Adoor, India. Sajith Sebastian Mount Zion Medical College, Adoor, India. Nandini Manjunath Department of Periodontics, AJ Medical College, Mangalore, India. Joanne Mary Sajith Technical and IT Assistant, India. View Book:- https://stm.bookpi.org/NHMMR-V7/article/view/6669
0 notes
Text

“The Rains’ End”
…….. okay. So. Painting on silk is evil…. Evilllll
The silk can and will eat all the paint and the fine grain weave!!!! Hhhrhhhhgg
It turned out okay in the end (just, yanno, took too many hours too paint for its size…..) Anyways due to the presence of hydroxylysine-glycosides in some of the collagen present in bone tissue I’ve furthered my evidence for my “Everything is Bread” theory (it is!!! - but basically these funky amino acids - the hydroxylysines - are structural beings to a Fibril diameter degree but they also act as docking ports for wayward carbohydrates (glycolysation!) and the resultant carbohydrate covalently bonded (here to an amino acid but it could be too a lipid or somth else too) is a glycoconjugate, or a glycan! So your bones (and various other collagen possessing tissues) contain a carbohydrate family and so you too! Are bread *tapes hydroxyl groups to you and throws breadcrumbs at Ye* GET GLYCOLSYLATED FOOL
9 notes
·
View notes
Text
What Are Peptides
Peptides Advantages - What Are They?
Content
Iaaf Values Board Choice: Timeline
Blood Sugar Level Ranges.
Peptides.
Is It Worth Taking Ostarine? Results And Advantages Of Ostarina.

Rite-Flex collagen peptides is produced via Rousselot's cutting-edge procedures with an unsinkable dedication to top quality, safety and sustainability. 80-- 90% are either type I, II or III, with kind I being one of the most common. The characteristic impacts of specific amino acid deposits on peptide fragmentation behavior have been examined carefully by Papayannopoulos. An internal piece with just a solitary side chain developed by a combination of a kind as well as y type cleavage is called an immonium ion. These ions are identified with the 1 letter code for the corresponding amino acid. Typically, these are developed by a combination of b kind andy type bosom to create the detailed framework, an amino-acylium ion. Sometimes, inner bosom ions can be formed by a mix of a type and y kind cleavage, an amino-immonium ion.
Does Vitaminhoppe sell SARMs?
SARMs are drugs and they are not dietary supplements. Using the internet one can easily find SARMs for sale. The bigger retail stores like GNC and Vitamin Shoppe do not carry them, but the smaller independently owned supplement stores are notorious for distributing SARMs.
The major teams of glycoconjugates are the glycoproteins, glycopeptides, peptidoglycans, glycolipids and lipopolysaccharides. The very first three of tbese are considered in the here and now file.
Iaaf Ethics Board Choice: Timeline
BNP dimension is utilized to look for proof of cardiac arrest as well as are used as an analysis tool. There are two different sorts of BNP levels which can be determined BNP and also NT Pro-BNP, worths for these are rather different. The normal actions of the natriuretic peptides are to help the heart in dealing with quantity overload as well as stretch. As the most plentiful healthy protein in the body, collagen serves a number of crucial architectural features. Nutritional collagen is harder for our body to absorb, as compared to hydrolysed variations.

If the pores of the gel are big, the amino acids will certainly relocate at a much faster price to the electrodes contrasted to in a gel which has smaller pores. If the temperature of the system is high, the kinetic energy of the amino acids boosts and hence the amino acids take a trip to the billed electrode at a quicker rate. Amino acids that have smaller sized alkyl chains connected to them relocate at a much faster rate than amino acids that have a bigger alkyl chain attached to them. Amino acids that have a bigger fee will move at a quicker rate contrasted to the amino acids that have a smaller charge. The fee is not because of the amino acid itself but as a result of the charge on the side chain.
Blood Sugar Level Varieties.
An additional little research study discovered that taking a day-to-day collagen peptide supplement for 6 months brought about renovations in nail toughness and development. Nevertheless, this only included 25 individuals, as well as there was no sugar pill team-- which reduces the stamina of this proof. There is some emerging evidence which recommends that taking hydrolysed collagen supplements may help to reduce creases and also boost collagen degrees in the skin, in addition to skin hydration as well as skin flexibility. Nevertheless, there are problems with some of the studies around, as an example some are very small, and do not utilize a control group.
Plumps, firms as well as reduces the appearance of great lines by boosting collagen production. Oily skin can be a problem and also the hardest skin type to deal with. Typically, you are glossy with a lot of oily spots, your pores are bigger and there are a great deal of blackheads and acnes.
Peptides.
Inner pieces are labelled with their 1 letter amino acid code. In the liver the majority of collagen is either kind 1 or type 3. The removal of the propeptides advertises advancement of collagen fibrils. The pro-peptides may either be preserved in the matrix or released into the blood circulation. Fibrosis occuring in the liver leads to the deposition of collagen and launch of propeptides, mainly P3NP. In information BPC157 Italy , 52 individuals with osteo arthritis of the knee were offered 10 mg bioactive undenatured type II collagen or glucosamine hydrochloride plus chondroitin daily for three months.
DRS-Labs Launches New Research Line of SARMs - Markets Insider
DRS-Labs Launches New Research Line of SARMs.
Posted: Mon, 13 Aug 2018 07:00:00 GMT [source]
However, to date, there are no researches on the metabolic engineering of the proteolytic system from lactobacilli. Numerous strategies have actually likewise been attempted to increase the return of peptide production by CEPs.
Is It Worth Taking Ostarine? Impacts As Well As Benefits Of Ostarina.
It is also important to note that collagen supplements are usually derived from fish-- therefore any person that has a fish allergic reaction need to prevent this sort of supplement. Vegan collagen supplements are offered, which are made from genetically modified bacteria and also yeast. There is very little proof to suggest that collagen supplements boost digestive tract health and wellness.
GTx Announces Results from Preclinical Studies of SARMs in Duchenne Muscular Dystrophy Models Published in Human Molecular Genetics - Business Wire
GTx Announces Results from Preclinical Studies of SARMs in Duchenne Muscular Dystrophy Models Published in Human Molecular Genetics.
Posted: Wed, 03 May 2017 07:00:00 GMT [source]
According to the "barrel-stave version," the peptides insert perpendicularly right into the bilayer while recruitment of additional peptides subsequently results in development of a peptide-lined transmembrane pore. In this pore, the peptides are aligned with the hydrophobic side dealing with the lipid core of the membrane and the hydrophilic areas facing the indoor area of the pore. According to the "toroidal-pore version," insertion of peptides compels the phospholipid to flex continually from one brochure to the other, leading to a pore lined by both peptides and also the head groups of the phospholipids. Finally, in the "carpet model," build-up of peptides on the membrane surface area triggers tension in the bilayer that eventually causes disruption of the membrane layer and formation of micelles. AMPs are transformative saved in the genome as well as produced by all life forms, from prokaryotes to humans. In higher microorganisms, AMPs make up important elements of the innate immunity, shielding the host versus infections.
A buffer remedy resists the adjustment in the pH of the option. The barrier service is maintained the Isoelectric Factor of one known amino acid. The amino acids with Isoelectric Factors greater than the pH of the buffer solution become positively billed. The amino acids with Isoelectric Factors lower than the pH of the buffer service end up being adversely billed. The amino acids with the Isoelectric factor that amounts to the pH of the buffer option stay uncharged.
What is the safest SARM to take?
A three-week trial at Boston University demonstrated that LGD-4033, a SARM developed by Ligand Pharmaceuticals, was safe and tolerable in healthy men, producing “significant gains in muscle mass and strength” without raising levels of a protein linked to prostate cancer.
In the condensed system of symbols for sugar residues the common arrangement and ring size are indicated in the sign. Hence, Girl signifies D-galactopyranose; Man, D-mannopyranose; Fuc, L-fucopyranose; GlcNAc, 2-acetamido-2-deoxy-D-glucopyranose or N-acetyl-D-glucosamine; Neu5Ac N-acetylneuraminic acid. The symbol Sia stands for sialic acid, a general term that can likewise be utilized when the precise structure is unidentified. Whenever the setup or ring size is found to differ from the common one it should be suggested by using the ideal icons for the extensive system.
The longer the chain of the peptide, the longer the hydrolysis process will certainly happen. This is due to the fact that even more time is required to damage the peptide bond as well as other bonds like Van der Wall forces of attraction between the atoms. Isoelectric Factor is the pH worth of the amino acids when they are added in water. This is due to the dissociation of the H+ ion from the amino acids. Our collagen peptides are made from the finest pasture-raised bovine hides. Studies reveal that 5g to 10g of collagen peptides daily have shown statistically significant benefits. Peptan is the worlds leading collagen brand generated by Rousselot.
The classification of glycolipids has been the topic of an earlier record, which is now under alteration. Identification of enhanced strain versions or even metabolic engineering is an additional approach.
UK Sarms ostarine has given me excellent recomp outcomes at 10mg/day and also helped me push past weights I was previously stuck at for months.
Selective androgen receptor modulators are a class of androgen receptor medicines, which have a high capacity to be performance boosters in human and also animal sports.
In this study, we have actually clarified as well as confirmed the chemical structure of 2 major equine arylpropionamide-based SARM metabolites utilizing a mix of chemical synthesis and fluid chromatography-mass spectrometry (LC-MS) analysis.
you can find more information on workflow rules on peptides-uk crm's help pages here. was so amazed with the ostarine that I have actually recently gotten RAD-140 for my following cycle as I currently require to focus on structure muscle mass in advance of my following comp.
Arylpropionamides are among the major SARM classes as well as get swiftly metabolized substantially making complex easy detection of transgression in blood or urine example evaluation.
Particular drug-derived metabolites are called for as references due to a short half-life of the parent compound however are typically lacking.
However, these researches contrasted the effect of resistance training plus a collagen supplement, with resistance training with no type of healthy protein after training. As protein is essential for muscle mass fixing, these researches suggest that collagen is better than consuming no healthy protein after resistance training. However there isn't adequate proof to recommend that collagen is better than various other types of protein in terms of muscle mass growth and also repair work. In 2011 the European Food Security Authority reported there was insufficient proof to declare that hydrolysed collagen aids in maintaining joint wellness in energetic individuals. Since then, a few tiny research studies have located that eating collagen may aid in lowering joint discomfort related to work out. There is likewise some proof that collagen supplements can aid with minimizing pain related to osteoarthritis in the short-term. Nonetheless, these research studies don't supply proof as to whether collagen supplements help in athletic efficiency, or the recovery from joint injuries.
On top of that, pathogen insult will bring about growth of dendritic cells and succeeding initiation of flexible resistance. Up- or down-regulation of feedbacks by AMPs is shown by environment-friendly arrows. Remarkably, the membrane-destabilizing activity of AMPs is likewise made use of in so called Artilysins, which have actually just recently revealed potential to properly target immune and persistent Gram-negative infections.
youtube
A small number of human studies have actually also discovered that taking collagen supplements may assist to maintain and enhance bone mass. However, comparable to the research studies associated with muscular tissue mass, we can not rule out that the favorable effect of collagen in these studies may result from a rise in total protein consumption, as opposed to a specific advantage pertaining to collagen.
As an example, enzymatic immobilization (cross-linked CEP accumulations) was established to increase task and also security of CEPs. This not only supports the concept that lactobacilli can produce a huge selection of BAPs, however also highlights the need to carefully pick the pressures that will be used for BAP manufacturing. The rapid bactericidal activity of AMPs makes them appealing prospects for therapeutic anti-infectives. Schematic illustration of immunomodulatory tasks of AMPs.
Although icons such as Gal as well as Man work in representing oligosaccharide structures they ought to not be made use of in the text to stand for monosaccharides. Various sorts of substance containing carbs covalently related to various other types of chemical component are categorized under the general name of glycoconjugates.
#BPC157 EU#BPC157 Europe#information BPC157 EU#information BPC157 Europe#EU BPC157 how does it work#Europe BPC157 how does it work
1 note
·
View note
Text
Carbohydrate Synthesis & Peptide Synthesis
In most cases, the definition of synthesis identifies the combining of two or more entities, material or abstract, to make something new; or in a broader sense, synthesis also identifies the creation of something. We mention, more regularly than not, this term- synthesis- mainly in chemistry and biochemistry.
Carbohydrate Synthesis
If in chemistry, we call it chemical synthesis which covers a wide range of categories. Chemical synthesis is quite critical to numerous chemistry types of research as based on its new compounds with new physical or biological properties could be discovered. Also, it's indispensable in drug discovery if new drugs should be created.
Among all classifications of chemical synthesis, carbohydrate synthesis is one of them, such as the synthesis of some complicated compounds like oligosaccharide, glycan, sugar amino acid, glycopeptide, glycoconjugate, etc.
Carbohydrate is a multi-task player in living organisms, from the primary supply of energy to cell-cell interactions to cancer metastasis. Therefore, it's fair to express that carbohydrate plays a significant role in lots of buy SARMs credit card processes. However, they're hard to be synthesized due to their structural complexity.

According to a guide titled Carbohydrate Chemistry, Biology and Medical Applications, the difficulties of carbohydrate synthesis lie in these regards:
a) It's hard to distinguish the exact hydroxyl group at a certain position from other hydroxyl groups as every one of these hydroxyl groups shares similar properties.
b) Compared with other synthesis, glycosylation reactions are much difficult and consequently, will have poor yields.
c) The largest challenge is how to accomplish the required glycosides linkage in a stereo selective manner.
Peptide Synthesis
If in biochemistry, one important kind of synthesis is peptide synthesis or, quite simply, the production of peptides. Peptides are organic compounds consists of multiple amino acids linked via amide bonds.
Peptides are synthesized by coupling the carboxyl group of one amino acid to the amino group of another amino acid molecule. Due to the likelihood of unintended reactions, protecting groups are often necessary.
As to the buy peptides usa, you will find normally two types: liquid-phase peptide synthesis and solid-phase peptide synthesis (or short for SPPS). The latter one is more widely used in labs nowadays.
SPPS was pioneered by Robert Bruce Merrifield and quickly caused a paradigm shift within the peptide synthesis circle. It has transformed into the first choice to synthesize peptides and proteins in the lab.
SPPS makes it possible for the synthesis of natural peptides, the incorporation of unnatural amino acids, peptide/protein backbone modification, and the synthesis of D-proteins, which include D-amino acids.
1 note
·
View note
Text
THE EXCELLENT RANGE OF LECTIN CONJUGATES AND THEIR APPLICATION
WHAT ARE LECTIN CONJUGATES?
Proteins (building blocks) that attach to cells and specific carbohydrate groups on proteins or cell membrane proteins are known as lectin conjugates. They are further classified according to their amino acid sequences and biological characteristics. Lectins contain 120 amino acids that are involved in carbohydrate binding.
Because of its carbohydrate binding, lectin is employed in glycobiology to analyse cell surface glycoproteins. Lectins are synthesised in laboratories after being extracted from plant or animal components.
The capacity of Lectins to form precipitates with glycoconjugates is due to the fact that most lectin proteins are composed of non-covalently linked subunits. Agglutination of cells by Lectins is uncommon in nature and thus extremely difficult to detect.
Lectins enable scientists to investigate a wide range of biological structures and functions. Some Lectins bind to mannose or glucose residues, while others bind only to galactose residues due to their complex binding requirements. Some Lectins also require sugar-binding at specific oligosaccharide sites.
LECTINS APPLICATION
Lectins are being used in clinical laboratories to type blood cells. There is the extensive usage of Lectin in specialist applications such as-
• As chemotherapeutic agents
• In fractionation of animal cells as mitogens.
• While investigating cellular surfaces
• Lectins isolate specific cells or viruses with a mixture and study determined processes amongst several.
LECTIN IN ANIMALS
Regulate cell adhesion
• Glycoprotein synthesis is regulated by Lectins
• They can also regulate blood protein levels.
• Recognition of galactose residues on the surface of mammalian liver cells responds better to Lectins.
LECTIN IN PLANTS
Plants are naturally rich in lectins. Dietary lectins are found in protein sources such as beans and legumes, peanuts, lentils, wheat, uncooked kidney beans, fruits, and vegetables. Conversely, lectin-free diet items include pasture-raised meats, cooked sweet potatoes, cruciferous vegetables, asparagus, garlic, and onion.
Lectin activity and function in plants are both unknown. The content of Lectin in plant seeds decreases as they mature. Plant Lectin has the ability to recognise hydrophobic noncarbohydrate ligands.
Adenine, auxins, cytokinin, indole-3-acetic acid, and water-soluble porphyrins are examples. Because these compounds behave as phytohormones, their interactions may be psychologically significant.
Furthermore, the plasma membranes of human EL4 tumour cells are labelled with horseradish peroxidase-conjugated wheat-germ agglutinin. After the labelled intact cells are disrupted, plasma-membrane refinement is observed by ultrastructural examination of the various fractions for positive effect product on the membrane vesicles.
LECTINS AND OTHER CARBOHYDRATE-BINDING PROTEINS
Cellular proteoglycans, glycoproteins, and glycolipids include a wide range of oligosaccharides. Fluorescent carbohydrate-binding derivativesMicroscopy PROTEINS and flow cytometry both use proteins to identify intracellular glycoconjugates. This is done to isolate glycoproteins on protein blots and cause agglutination of specific cell types. Lectins can also be used to detect cancer since they have changed surface glycoproteins.
LECTINS INTERCONNECTING

Biotechnology has narrowed down biorecognition molecules with diagnostic potentials in light of the different diseases that impact the human species. Particular Lectin content binds with mono- or oligosaccharides with high affinity via no covalent connection via hydrogen bonds.
Lectins from viruses, bacteria, fungi, algae, mammals, and plants recognise carbohydrates in cells, tissue sections, and biological fluids. These are useful tools for diagnostic purposes. To find medicines and inhibitors, sialic acid-specific lectins such as Influenza Virus Hemagglutinin are being studied. These can remove or inhibit sialic acid in host cells, preventing it from binding.
Similarly, strong anti-HIV activity in vitro has been associated with bacterial Lectins. Large levels of algal Lectins are attracting interest for biomedical uses such as anti-HIV, anti-inflammatory, antibacterial, and antinociceptive properties. Animal Lectins are important in psychological processes such metastatic cancer, apoptotic pathways, and immunomodulation.
LECTIN INDUCED MECHANISMS OF INFLAMMATION RESPONSES
Immune systems act in two specific ways called; innate and adaptive responses. These responses are activated by a group of cells and molecules that promote the destruction of aggressive agents. Neutrophils, eosinophils, basophils, and mono/macrophages can generate and release molecules called cytokines.
These molecules modulate the activation of immune cells, inflammation, and humoral responses. Biomolecules like these are the answer for adjustment of immune conditions and therapeutic applications in regards to immune response-related diseases.
Lectins are thought to contribute to the development of diseases such as celiac disease, autoimmune diseases, rheumatoid arthritis, obesity, cardiovascular disease, and type 2 diabetes. This happens through translocation across the intestinal barrier and activation of the adaptive immune system. Common high-lectin foods include grains, legumes, and nightshades.
Lectins aren’t digestible. They bind to cell membranes lining the digestive tract, where they may disrupt metabolism and cause damage. Lectin sensitivity is the body’s delayed immune response that can occur some hours to even days after these foods are consumed.
Symptoms associated with lectin sensitivities include:
•Bloating and abdominal cramps
•Painful or swollen joints
•Tiredness
•Skin problems
•Hormonal fluctuations
•Nausea
•Allergies or allergy-like symptoms
•Neurological symptoms
The highest concentrations of lectins are found in healthy foods like legumes, grains, and nightshade vegetables. Fortunately, there are ways to reduce the lectin content of these healthy foods to make them safe to eat.
Research studies have shown that by cooking, sprouting, or fermenting foods high in lectins, their lectin content can easily be reduced to negligible amounts.
Foods That Are High in Lectins
1. Red kidney beans
Raw kidney beans contain high levels of a lectin called phytohaemagglutinin. Eating them raw or undercooked can cause severe nausea, vomiting, and diarrhea. As few as five beans can cause a response.
A hemagglutinating unit (hau) is a measure of lectin content. When in raw form, red kidney beans contain 20,000–70,000 hau. Once cooked, however, they contain only 200–400 hau, which is considered a safe level.
In cooked form, they are valuable and nutritious food.
2. Soybeans
Soybeans have several health benefits but are another food that also contains high levels of lectins.
As with red kidney beans, cooking soybeans almost eliminates their lectin content, provided they are cooked at high temperatures. Studies show that soybean lectins are almost completely deactivated when at 100°C for at least 10 minutes.
3. Wheat
Raw wheat, including wheat germ, is high in lectins, with around 300 mcg of wheat lectins per gram. (Whole-wheat flour has a much lower lectin content at about 30 mcg per gram). Lectins are almost completely eliminated by cooking and processing, and as most whole-wheat products consumed are cooked, it’s not likely that lectins pose a major risk to health.
4. Tomatoes
Tomatoes are part of the nightshade family. They are high in fiber, a good source of potassium and vitamin K1, and high in vitamin C. (One tomato provides about 20% of the daily recommended value.
Tomatoes also contain lectins, though there is little evidence that they have any adverse effects on humans. Some people have linked tomatoes and other nightshade vegetables to inflammation, such as arthritis. No formal research has supported this link.
5. Potatoes
Potatoes are also members of the nightshade family and a good source of vitamins and minerals. Potato skins are particularly high in antioxidants, such as chlorogenic acid, which has been linked to a reduced risk of heart disease and type 2 diabetes. As with tomatoes, adverse effects have been experienced by some when eating potatoes. Studies have shown that this could be linked to lectins.
6. Peanuts
Peanuts are an excellent source of protein, unsaturated fats, and many vitamins and minerals.
Peanuts do contain Lectin, and one study found that peanut lectins increased growth in cancer cells. With evidence that peanut lectins can enter the bloodstream, this has led some people to believe that lectins could increase the risk of cancer spreading in the body. However, the above study was carried out using very high doses of pure lectins placed directly onto cancer cells.
No studies have investigated their exact effects on humans. Evidence of their health benefits appears to be stronger than that of any risks.
DRUG DELIVERY USING LECTIN SOURCE OF PROTEIN
Chemical agent therapies often come across as barriers when the need for increasing dosages and action of metabolism reduces the effectiveness of treatments. Delivery of drugs to specific targets requires a new and an effective strategy to combat side effects and chemical reactions.
Lectin medicated bio adhesion constitutes specified interactions with receptor-like structures in the cell membrane, binding directly to targeted cells. Therefore Lectins can interact differently with distinct cells and act as drug carriers to desired tissue and cells. For it to be a tool in drug delivery Lectins, need to be avid binding, low toxicity, and site-specific molecules.
To conclude, Lectins from diversified sources with distinct carbohydrate recognition events play a vital role in many biotechnological applications/disease therapies. The uses in vitro and in vivo display Lectins with protective effects against viruses and microorganisms. Lectins are a highly potent modulator of an immune response, mitosis, proli9, healing, drug delivery therapies, and cancer regression.
Histochemistry, biosensors, detect diseases, and infections against glycans alterations on cells or tissue surfaces, and serum samples can be isolated using Lectin-based technology and techniques. There is potential to unravel new interpretations in the biological effects, pathways, and biotechnological potential of Lectins. They are focusing on their achievements in therapeutic applications and health effects.
Want to learn more about Lectin Conjugate, its usage, and health benefits? Contact our experts at Helvetica Health Care today!
1 note
·
View note
Text
10385-50-9 Synthesis and biological activity of some 1-N-substituted 2-acetamido-2-deoxy-beta-D-glycopyranosylamine derivatives and related analogs.
Several 1-N-substituted derivative [haloacetyl-, glycyl-, (dimethyl)amino-acetyl-, azidoacetyl-, trifluoroacetyl-, and trifluoromethylsulfonyl-] of 2-acetamido-2-deoxy-3,4,6-tri-O-acetyl-beta-D-glucopyranosylamine (1) were synthesized as potential metabolic inhibitors of cellular-membrane glycoconjugates. Several fully acetylated derivatives were found to inhibit growth of mouse mammary adenocarcinoma TA3, leukemia L1210, or leukemia P-288 cells at 1-0.01 mM concentration in vitro. Some of these derivatives were less active after O-deacetylation. Analogs of 1 in which NH2-1 was replaced by OH- or OAc-1 were also active on the same cell systems. The growth-inhibitory activity was correlated with inhibition of the incorporation of 2-amino-deoxy-D-glucose and L-leucine into a macromolecular fraction.
0 notes
Text
The Ultimate Guide To Glycobiology

Glycobiology is the study of the structure, function, and biosynthesis of saccharides (carbohydrates and carbohydrates containing molecules). Carbohydrates that are covalently linked to other chemical species such as proteins, peptides, lipids, and saccharides are known as glycoconjugates. Glycoconjugates and polysaccharides play important structural roles in connective tissues.
According To Coherent Market Insights " The global glycobiology market is estimated to be valued at US$ 1,165.3 million in 2020 and is expected to exhibit a CAGR of 12.8 % during the forecast period (2020-2027). "
When several groups competed to determine the crystal structure of concanavalin A in the 1970s, structural glycobiology was born. Since this early work, a wealth of structural information on lectins, carbohydrate-processing enzymes, and glycosyltransferases has become available. The lectins have unquestionably proven to be the most diverse group of proteins among these three classes. This is not surprising given the broad definition of a lectin: a protein of nonimmune origin that binds carbohydrates without enzymatically altering them.
Read more @ https://cmiinfoistic.blogspot.com/2022/04/glycobiology-is-multidisciplinary.html
0 notes
Photo

Olá pessoal! Hoje a Sra. Proteína vai ensinar um pouco para vocês sobre LECTINAS. Esse grupo de proteínas apresenta várias aplicabilidades biomédicas e biotecnológicas as quais têm sido alvo de diversos estudos realizados pelo nosso grupo de pesquisa. Vocês podem conferir os trabalhos desenvolvidos lá no nosso site (link disponível na bio)! Referências: COELHO, L.C.B.B. et al. Lectins, Interconnecting Proteins with Biotechnological/Pharmacological and Therapeutic Applications. Evidence-Based Complement. Altern. Med., v. 2017, p. 1–22, 2017. MISHRA, A. et al. Structure-function and application of plant lectins in disease biology and immunity. Food Chem. Toxicol., v. 134, 110827, 2019. NASCIMENTO, K. S. et al. Dalbergieae lectins: A review of lectins from species of a primitive Papilionoideae (leguminous) tribe. Inter. J. Biol. Macromol., v. 144, p. 509–526, 2020. TSANEVA, M.; VAN DAMME, E.JM. 130 years of plant lectin research. Glycoconjugate Journal, p. 1-19, 2020. #lectins #lectinas #proteinas #proteínasbioativas #carboidratos #atividadesbiológicas #pesquisa #research #bioquimica #bioquimicadeproteinas #bioquimicademacromoleculas https://www.instagram.com/p/CRgnlObMwfC/?utm_medium=tumblr
#lectins#lectinas#proteinas#proteínasbioativas#carboidratos#atividadesbiológicas#pesquisa#research#bioquimica#bioquimicadeproteinas#bioquimicademacromoleculas
0 notes
Text
Glycobiology Market – Insights
The term glycobiology is a branch of biology that deals with study of structure, function, and biosynthesis of saccharides (carbohydrates and carbohydrates containing molecules). Glycoconjugates is the general classification for carbohydrates covalently linked with other chemical species such as proteins, peptides, lipids and saccharides. Glycoconjugates and polysaccharides play a vital structural role in connective tissues.
The global glycobiology market is estimated to account for US$ 1,007.4 Mn in terms of value in 2020 and is expected to reach US$ 2,397.5 Mn by the end of 2027.
Global Glycobiology Market: Drivers
Glycans impact tumor proliferation, hematogenous metastasis, invasion and angiogenesis. Therefore, increasing prevalence of cancer is expected to propel growth of the global glycobiology market over the forecast period. For instance, according to the World Health Organization, around 18.1 million new cases and 9.6 million deaths were registered due to cancer worldwide in 2018.
Moreover, high incidence of HIV/AIDS is also expected to aid in growth of the market. For instance, according to Joint United Nations Program on HIV/AIDS, 37.9 million people globally were living with HIV at the end of 2018.
North America region held dominant position in the global glycobiology market in 2018, accounting for 44.3% share in terms of value, followed by Europe.

Global Glycobiology Market: Restraints
Complex structure of glycans is expected to hinder growth of the global glycobiology market. Unlike DNA or proteins, glycans do not have linear structure. Conventional sequence comparison methods cannot be applied directly to glycan comparison as structure and mechanism of glycans are completely different from DNA or proteins, which makes it more complex for analyzing. DNA or proteins have template sequences to synthesize primary structure, which are uniquely identified by template sequences. However, glycans do not have a template structure. Structure of glycan is determined by substrate specificity of glycosyl-transferases.
Moreover, high costs of spectrometry and high performance liquid chromatography are also expected to limit growth of the market.
Global Glycobiology Market: Opportunities
R&D in glycans is expected to offer lucrative growth opportunities for players in the global glycobiology market. For instance, in February 2020, researchers from University of Minho, Portugal, reported development of a neurocompatible strategy to image temporal distribution of glycoproteins during neuronal development using Cu-free click chemistry.
Moreover, increasing focus on manufacturing anti-cancer drugs is also expected to aid in growth of the market. For instance, in January 2020, researchers from Universidade do Porto, Portugal, reported assessment of the role of O-glycosylation using glycoengineered gastric cancer models in the detection of CD44v9 – a major protein splice variant isoforms expressed in human gastrointestinal cancer cells—by monoclonal antibodies.
Enzymes segment in the global glycobiology market was valued at US$ 487.1 Mn in 2019 and is expected to reach US$ 1,371.0 Mn by 2027 at a CAGR of 13.6% during the forecast period.
Market Trends/Key Takeaways
Increasing adoption of viscosupplementation and facial aesthetic using hyaluronic acid is aiding growth of the market. Hyaluronan, a natural occurring glycosaminogen, is used in viscosupplementation and tissue fillers for volume restoration in facial rejuvenation.
Replacement of conventional glycoanalysis approaches with advanced techniques is also boosting growth of the market. Conventional methods for analysis of glycoforms in complex glycoproteins such as gel electrophoresis and elemental analysis are rapidly getting replaced by new approaches such as nuclear magnetic resonance (NMR) spectroscopy and mass spectrometry (MS).
https://www.coherentmarketinsights.com/insight/request-sample/3639
https://www.coherentmarketinsights.com/insight/request-pdf/3639
Global Glycobiology Market: Competitive Landscape
Major players operating in the global glycobiology market include, Merck KGaA, Bio-Techne Corporation, Bruker Corporation, Thermo Fisher Scientific, Inc., Shimadzu Corporation, Plexera Bioscience LLC, New England Biolabs Inc., Agilent Technologies, Inc., and ProZyme, Inc.
Global Glycobiology Market: Key Developments
Major players in the market are focused on adopting various marketing strategies to expand their customer base. For instance, in February 2020, New England Biolabs demonstrated its next generation sequencing portfolio at 2020 Advances in Genome Biology and Technology (AGBT) General Meeting, held in the U.S.
Players in the market are also focused on adopting partnership strategies to expand their product portfolio. For instance, in November 2019, MOBILion Systems, Inc. partnered with researchers at the University of Georgia, for R&D in glycans and glycoproteins.
About Us:
Coherent Market Insights is a global market intelligence and consulting organization focused on assisting our plethora of clients achieve transformational growth by helping them make critical business decisions.
What we provide:
Customized Market Research Services
Industry Analysis Services
Business Consulting Services
Market Intelligence Services
Long term Engagement Model
Country Specific Analysis
Contact Us:
Mr. Shah
Coherent Market Insights Pvt. Ltd.
Address: 1001 4th ave, #3200 Seattle, WA 98154, U.S.
Phone: +1-206-701-6702
Email: [email protected]
Source: https://www.coherentmarketinsights.com/market-insight/glycobiology-market-3639
0 notes
Text
From 2019 to 2027, The global glycobiology market is estimated to reach CAGR of 13.6%
US$ 1,007.4 Mn in 2020, The global glycobiology market is estimated to account for and is expected to reach US$ 2,397.5 Mn by the end of 2027

Request for Sample PDF Copy:
https://www.coherentmarketinsights.com/insight/request-sample/3639
Description:
The term glycobiology is a branch of biology that deals with study of structure, function, and biosynthesis of saccharides (carbohydrates and carbohydrates containing molecules). Glycoconjugates is the general classification for carbohydrates covalently linked with other chemical species such as proteins, peptides, lipids and saccharides. Glycoconjugates and polysaccharides play a vital structural role in connective tissues.
The global glycobiology market is estimated to account for US$ 1,007.4 Mn in terms of value in 2020 and is expected to reach US$ 2,397.5 Mn by the end of 2027.
Global Glycobiology Market: Drivers
Glycans impact tumor proliferation, hematogenous metastasis, invasion and angiogenesis. Therefore, increasing prevalence of cancer is expected to propel growth of the global glycobiology market over the forecast period. For instance, according to the World Health Organization, around 18.1 million new cases and 9.6 million deaths were registered due to cancer worldwide in 2018.
Moreover, high incidence of HIV/AIDS is also expected to aid in growth of the market. For instance, according to Joint United Nations Program on HIV/AIDS, 37.9 million people globally were living with HIV at the end of 2018.
North America region held dominant position in the global glycobiology market in 2018, accounting for 44.3% share in terms of value, followed by Europe.
Global Glycobiology Market: Restraints
Complex structure of glycans is expected to hinder growth of the global glycobiology market. Unlike DNA or proteins, glycans do not have linear structure. Conventional sequence comparison methods cannot be applied directly to glycan comparison as structure and mechanism of glycans are completely different from DNA or proteins, which makes it more complex for analyzing. DNA or proteins have template sequences to synthesize primary structure, which are uniquely identified by template sequences. However, glycans do not have a template structure. Structure of glycan is determined by substrate specificity of glycosyl-transferases.
Moreover, high costs of spectrometry and high performance liquid chromatography are also expected to limit growth of the market.
Global Glycobiology Market: Opportunities
R&D in glycans is expected to offer lucrative growth opportunities for players in the global glycobiology market. For instance, in February 2020, researchers from University of Minho, Portugal, reported development of a neurocompatible strategy to image temporal distribution of glycoproteins during neuronal development using Cu-free click chemistry.
Moreover, increasing focus on manufacturing anti-cancer drugs is also expected to aid in growth of the market. For instance, in January 2020, researchers from Universidade do Porto, Portugal, reported assessment of the role of O-glycosylation using glycoengineered gastric cancer models in the detection of CD44v9 – a major protein splice variant isoforms expressed in human gastrointestinal cancer cells—by monoclonal antibodies.
Enzymes segment in the global glycobiology market was valued at US$ 487.1 Mn in 2019 and is expected to reach US$ 1,371.0 Mn by 2027 at a CAGR of 13.6% during the forecast period.
Market Trends/Key Takeaways
Increasing adoption of viscosupplementation and facial aesthetic using hyaluronic acid is aiding growth of the market. Hyaluronan, a natural occurring glycosaminogen, is used in viscosupplementation and tissue fillers for volume restoration in facial rejuvenation.
Replacement of conventional glycoanalysis approaches with advanced techniques is also boosting growth of the market. Conventional methods for analysis of glycoforms in complex glycoproteins such as gel electrophoresis and elemental analysis are rapidly getting replaced by new approaches such as nuclear magnetic resonance (NMR) spectroscopy and mass spectrometry (MS).
Global Glycobiology Market: Key Developments
Major players in the market are focused on adopting various marketing strategies to expand their customer base. For instance, in February 2020, New England Biolabs demonstrated its next generation sequencing portfolio at 2020 Advances in Genome Biology and Technology (AGBT) General Meeting, held in the U.S.
Players in the market are also focused on adopting partnership strategies to expand their product portfolio. For instance, in November 2019, MOBILion Systems, Inc. partnered with researchers at the University of Georgia, for R&D in glycans and glycoproteins.
About Us: Coherent Market Insights is a global market intelligence and consulting organization focused on assisting our plethora of clients achieve transformational growth by helping them make critical business decisions. We are headquartered in India, having office at global financial capital in the U.S. Our client base includes players from across all business verticals in over 150 countries worldwide. We do offer wide range of services such as Industry analysis, Consulting services, Market Intelligence, Customized research services and much more. We have expertise in many fields such as healthcare, chemicals and materials, Automation, semiconductors, electronics, energy, food and beverage, packaging and many more. Visit our website to know more.
Buy Report:
https://www.coherentmarketinsights.com/insight/buy-now/3639
Contact Us: Mr. Shah Coherent Market Insights 1001 4th Ave. #3200 Seattle, WA 98154 Tel: +1-206-701-6702 Email: [email protected]
0 notes
Link
Hemagglutinins refers to glycoproteins which bring about agglutination of erythrocytes or hemagglutination. Hemagglutination can be used to identify surface antigens on erythrocytes (with known antibodies) and, hence, the blood type of an individual. Hemagglutinins consist of lectins and antibodies. Lectins (from the Latin legere, “to select”) are non-immune glycoproteins that exhibit reversible binding to specific carbohydrate structures, glycans of glycoproteins, glycolipids and polysaccharides. Lectins own at least one non-catalytic domain, and, in some cases, a few carbohydrate binding domains, which enable them to effect agglutination of erythrocytes and other cells or precipitation of glycoconjugates. Lectins are found in a constellation of organisms and display an array of activities.
Topics discussed in this book encompass algal lectins, plant type 2 ribosome inactivating proteins, edible legume lectins, jacalin, jacalin-related lectin, wheat lectins, rice lectins, banana lectins and potato lectin, immunomodulatory action of plant lectins, piezoelectric assay using the galactose-binding Bauhinia monandra leaf lectin and its antibody as a potent tool for detection of antigen-antibody recognition, assays using hemagglutinins of different origins to investigate their mechanisms of action, lectins from medicinal herbs and medicinal mushrooms, C-type lectins from a diversity of invertebrates and vertebrates, fish lectins and amphibian lectins. (Imprint: Nova)
#reading#education#hemagglutinins#biochem#biochemistry#sciences#biochemistrybook#book#books#bookish#booklover#sciencebooks#sciencenerd#naturalscience#nature#biology#chemistry#educational#educate#sciencenova#novasciencepublishers#educationalreading
0 notes
Video
Structure and Function of Complex CarbohydratesComplex carbohydrates are very common in animals, plants, and bacteria. They are constituents of cell membranes, as well as subcellular materials of cells. They are also found in physiological fluids such as blood, tears, milk, and urine. Many complex carbohydrates are unsubstituted at their reducing ends and are referred to as polysaccharides; examples include the oligosaccharides of milk, the cellulose of plant cell walls, and storage forms such as starch and glycogen. Many other naturally occurring complex carbohydrates are covalently connected to other molecules, such as proteins or lipids, by glycosidic linkages of the sugar residues at their reducing ends to form glycoconjugates.
0 notes
Text
LECTIN CONJUGATES' APPLICATIONS IN MOLECULAR AND CELLULAR BIOLOGY

Lectins are carbohydrate-binding proteins found in almost all plants and a few mammals. The term Lectins is derived from the Latin word 'Legree,' which meaning to choose or select. Lectins do not cause antigenic activation inside the immune system, but they do have the ability to bind analogously to an antibody.
LECTIN CONJUGATES
Lectin Conjugates are proteins (building blocks) that bind to cells and specific lectin carbohydrate groups on proteins or cell membrane proteins. They are further classified based on their amino acid sequences and biological characteristics. Lectins contain 120 amino acids that are responsible for carbohydrate binding.
Lectins proteins with a high degree of stereo specificity that recognise diverse sugar structures and generate reversible connections when they engage with glycoconjugate complexes. These are abundant in plant lectins, animal lectins, and many other species known to agglutinate numerous erythrocyte blood types.
Because of its carbohydrate binding, lectin is employed in glycobiology to analyse cell surfaces glycoproteins. Lectins are synthesised in laboratories after being extracted from either plant or animal components.
Lectins enable researchers to explore a wide range of biological activities and processes. Because of their complex binding requirements, certain Lectins bind to mannose or glucose residues, whilst others bind only to galactose residues. Some lectins also require sugar-binding in specific locations in oligosaccharides.
Lectin proteins are widely employed in biomedical applications due to their ability to recognise carbohydrates via carbohydrate-binding sites, which identify glycans linked to cell surfaces, glycoconjugates, or free sugars, detecting aberrant cells and disease biomarkers.
A variety of infectious agents and diseases afflict the human species, causing a chain reaction of repercussions. The biotechnological area has looked for biorecognition molecules with diagnostic and therapeutic potential from natural or recombinant sources. Numerous algal lectins have also received significant interest for biological uses such as antinociceptive, anti-HIV, antitumoral, anti-inflammatory, and antibacterial properties.
MOLECULAR AND CELLULAR BIOLOGY APPLICATIONS
1. Lactin-Induced Immunological and Inflammatory Responses: Immunological and inflammatory responses are important in protecting the organism from invading agents and altered cells. The immune system functions in two ways: innate immunity and adaptive immunological responses, which are activated by a variety of cells and molecules and promote the destruction or inactivation of a hostile agent.
Basophils, neutrophils, eosinophils, and monocytes/macrophages all have specialised tasks and the ability to release and create chemicals known as cytokines. Cytokines can influence immune cell inflammation, humoral response, and activation. Lectins are also being studied for their ability to protect the host and vectors against parasites and viruses.
2. Many medical researchers have observed therapeutic benefits and effects promoted by lectins. Healing is the process of repairing tissue following harm. A monitored group of cells and molecules initiates ordered phases that result in morphological and functional healing of wounded tissues.
The importance of lectins as a healing agent is not totally understood; nonetheless, lectins may influence the immune response, inflammatory response, cytokine generation, and cell antiproliferative action throughout the healing process. Lectins have stimulated wound healing and scarring modification, with outstanding results and therapeutic potential.
3. Lectins for Drug Delivery: Therapies based on chemical agents face some challenges, primarily in terms of rising dosages and metabolic activity, which reduces treatment effectiveness. Systems for delivering medications to a specified target may be interesting and practical solutions for troubleshooting these issues and minimising undesirable side effects. To be a promising drug delivery method, lectins must be avid mannose-binding lectins with low toxicity and site-specific molecules.
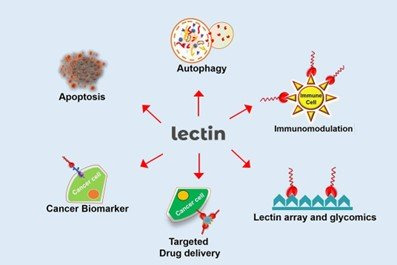
4. Histochemical Markers of Lectins: Glycan moieties on cell surfaces play a role in a variety of physiological and pathological processes. Disruptions in the cell environment caused by illnesses typically cause changes in glycans]. This technique has been used in the study, diagnosis, and prognosis of human disorders characterised by changed cells in tissues, such as cancer.
Lectin histochemistry is an appealing method for identifying altered tissues and clinical processes such as metastasis. Differential lectin binding patterns may discriminate between normal, benign, and malignant tumours of varying degrees. Lectins used to study the glycan profile in altered tissues are valuable tools for cancer detection and prognosis.
5. Lectin-Based Biosensors for Disease Detection: Glycans can be identified and quantified using lectin-based biosensors. These systems work by translating lectin-carbohydrate interactions into a quantifiable signal on a surface, allowing biomarkers to be measured.
Biosensing and transduction methods can be electrochemical, optical, mass, and thermal in accordance with a single kind. However, the electrochemical biosensor is more appealing since it is a practical, quick, user-friendly, and simple-to-use assay with different designs and analytical performance. Electrochemical lectin-positioned biosensors are more appealing as analytical tools for glycans and their application in detecting pathogens and diagnosing diseases via biomarker identification.
6. Lectins as Anticancer Agents: Lectins from many sources have cytotoxic effects on cancer cells, such as activation of cell death pathways and inhibition of proliferation. Furthermore, several anticancer lectins have poor cytotoxicity against nontransformed cells. This is most likely due to the differential expression of glycans on the surfaces of cancer and normal cells, which allows lectins to recognise malignant cells uniquely.
CONCLUSIONS
Many biotechnological applications and medical therapeutics rely on lectins from various sources with varying carbohydrate recognition events. Lectins do have antimicrobial and antiviral properties; it is a powerful modulator of mitosis, immune response, proliferation, drug-delivery therapies, cancer regression, and healing.
Using lectin-based techniques such as biosensors and histochemistry, which can detect illnesses and infectious agents, changed glycans on tissue or cell surfaces and serum samples can be identified. As a result, the accomplishments attributed to lectins are focused on biotechnological/pharmacological and therapeutic applications, serving as a significant resource for future research into the biological effects, routes, and biotechnological potential of lectins.
Contact the experts at Helvetica Health Care for more information on the molecular and cellular application of lectins conjugates.
0 notes
Text
PowerPoint 演示文稿
Carbohydrates and glycobiology
Tymoczko et al. Biochemistry – A Short Course Chapter 10
Glycobiology: the study of the structure, function and biology of carbohydrates
• Carbohydrate structure and function • Lectins • Roles of glycobiology in human health and disease
Carbohydrates: any carbohydrates (monomers, oligomers, polymers or glycoconjugates)
Glycans: the carbohydrates attached to a wide variety of biological molecules through glycosylation
Glycosylation: the process of attaching carbohydrates to other molecules such as proteins and lipids
Protein glycosylation is the enzyme-catalysed covalent attachment of carbohydrates to a protein
Approximately half of all proteins typically expressed in a cell undergo this modification
From genome to proteins, and protein glycosylation
Carbohydrates in proteins impart an additional level of 'information content' to underlying protein structures
It is estimated that for most genomes about 1% of the coding regions are glycosyltransferases and glycosidases
Glycosyltransferases: to add sugars on Glycosidases: to cleave sugars off
Why glycosylation for proteins?
Protein folding Conformation stabilisation Protein function Protein secretion and targeting Protein-protein interaction Disruption of glycosylation cause disease
such as congenital muscular dystrophy (disruption of O-glycans)
Cell to cell interaction Signal transduction Microbial infection
When to think about the involvement of sugars in cell-cell interaction, we are talking about sugars on the cell membrane.
The cells are decorated with sugars
When to think about the involvement of sugars in protein transport, we are mainly talking about Intracellular events
GlcNAc Man Gal
Glc
GalNAc
Sialic acid
Glycosylation reactions
Essentials of Glycobiology
Second Edition
asparagine (N-linkage)
serine or threonine (O-linkage)
11
Draw asparagine Draw serine and threonine Draw asparagine residue in a protein
Draw serine and threonine residues in a protein
Draw asparagine linked with a glucose Draw serine and threonine linked with a glucose
Draw N-acetylglucosamine linked to asparagine in a protein Draw N-acetylgalactosamine linked to serine in a protein
ABO blood type is determined by two glycosyltransferases
O blood type
oligosaccharide chain attached to either lipid or protein on RBC
A blood type
B blood type
Glycoprotein = sugars + protein (predominated by the protein)
Proteoglycan = polysaccharide + protein (predominated by carbohydrates) Mucin (proteins in mucus) = sugars + protein (predominated by carbohydrates)
N-linked protein glycosylation in the endoplasmic reticulum (ER) is a conserved three-phase process in eukaryotic cells. It involves the assembly of an oligosaccharide on a lipid carrier, dolichylpyrophosphate and the transfer of the oligosaccharide to selected asparagine residues of polypeptides
in the lumen of the ER
glycosyltransferases oligosaccharyltransferase
O-linked glycosylation
N-linked glycosylation
Nucleotide-activated sugars
N-Linked Protein glycosylation
1. Assembly of the precursor oligosaccharide...
IMPORTANT POINTS:
– Assembly takes place on the carrier lipid dolichol, anchored in the ER membane.
– A pyrophosphate bridge joins the 1st sugar (N-acetylglucosamine) to the dolichol.
– Sugars are added singly and sequentially.
– The glycan assembly flips from the cytosolic side to the ER lumen.
– More sugar molecules are added to the assembly.
Now in the second step, attachment
2. En-bloc transfer of the oligosaccharide to the protein
One step transfer, catalyzed by oligosaccharyl transferase, which is bound to the membrane at the translocator.
Covalently attached to certain asparagines in the polypeptide chain (said to be “N-linked” glycosylation).
Attaches to NH2 side chain of Asn but only in the context:
Asn-x-Ser or Asn-x-Thr
Finally modification of oligosaccharide
3. Modification of the oligosaccharide by removal of sugars...
Transport from the ER to Golgi
• The N-linked glycoproteins leave the ER and travel to the Golgi Apparatus.
• They travel in membrane vesicles that arise from special regions of membranes that are coated by proteins.
The Golgi Apparatus has two major functions: 1. Modifies the N-linked oligosaccharides and adds O-linked oligosaccharides. 2. Sorts proteins so that when they exit the trans Golgi network, they are delivered to the correct destination.
Modification of the N-linked oligosaccharides is done by enzymes in the lumen of various Golgi compartments.
Ribosomes bind the ER
Proteins sorted by secretory pathway start synthesis on a free ribosome in cytoplasm.
Ribosome directed to rough ER docks with ER membrane & protein synthesis continues.
Newly synthesised protein transported through membrane to ER.
How does the ribosome interact with ER??
Getting proteins into the ER
Signal sequence
• Machinery required to direct ribosome to ER & to translocate nascent protein across ER membrane consists of 4 components:
1. Signal sequence – sequence of 9 – 12 hydrophobic residues near amino terminus.
Can be cleaved by signal peptidases 2. Signalrecognitionparticle–recognizessignal
sequence on nascent polypeptide chain as emerges from ribosome. It binds this sequence & ribosome & shepherds ribosome with nascent polypeptide chain to ER
Signal sequence
3. SRP receptor – Integral membrane protein. SRP binds this receptor 4. Translocon – this is a channel across the membrane.
Channel opens when translocon & ribosome bind to each other Once ribosome bound to ER, protein synthesis reactivated – nascent protein now directed through ER membrane
Where does Dolichol come from?
• Dolichol is an isoprenoid compound synthesized by the same metabolic route as cholesterol. In vertebrate tissues, dolichol contains 18-20 isoprenoid units (90-100 carbons total). Dolichol is phosphorylated by a kinase that uses CTP to form dolichol Phosphate. Dolichol phosphate is the structure upon which the carbohydrate moieties of N- linked glycoproteins are built. After assembly on dolichol phosphate, the carbohydrate structure is transferred to an asparagine residue of a target protein having the sequence Asn-x-Ser/Thr, where X is any amino acid.
Dolichols function as a membrane anchor for the formation of the oligosaccharide
O-linked glycosylation
The O-glycosidic mechanism is not as complex as that of N-glycosylation. Proteins trafficked into the Golgi are most often O-glycosylated by N-acetylgalactosamine
(GalNAc) transferase, which transfers GalNAc to the -OH group of serine or threonine,
and then may add more sugars in a step-wise manner.
O-linked glycosylation
N-linked protein glycosylation
O-linked protein glycosylation
Cotranslationally or posttranslationally
Posttranslationally
Complex oligosaccharides (assembly of oligosaccharides first, then en bloc transfer to Asn in proteins)
Simple oligosaccharides (step-wise addition of monosaccharides to Ser ot Thr in proteins)
ER and Golgi
Mainly Golgi
Attached to asparagine residue
Attached to serine or threonine residue
Consensus sequence: NXS/T
No consensus sequence
Such as antibodies
Such as mucins
Lectin
Carbohydrates-binding proteins. Therefore it binds to glycoproteins
They bind carbohydrates with specificity and promote molecular recognition
Lectin in action
0 notes
Text
Evaluation of immune responses to group B Streptococcus type III oligosaccharides containing a minimal protective epitope.
PMID: J Infect Dis. 2020 Mar 2 ;221(6):943-947. PMID: 31641758 Abstract Title: Evaluation of Immune Responses to Group B Streptococcus Type III Oligosaccharides Containing a Minimal Protective Epitope. Abstract: Recent structural studies demonstrated that the epitope recognized by a monoclonal antibody representative of the protective response against the type III group B Streptococcus polysaccharide was comprised within 2 of the repeating units that constitute the full-length native structure. In the current study, we took advantage of this discovery to design a novel vaccine based on multivalent presentation of the identified minimal epitope on a carrier protein. We show that highly glycosylated short oligosaccharide conjugates elicit functional immune responses comparable to those of the full-length native polysaccharide. The obtained results pave the way to the design of well-defined glycoconjugate vaccines based on short synthetic oligosaccharides.
read more
0 notes