#Pediatric Drugs Market
Text
Global Leigh Syndrome Treatment market is Estimated to Witness High Growth Owing to Increasing Disease Awareness
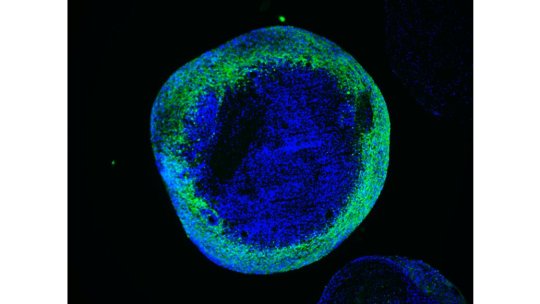
The global Leigh syndrome treatment market is expected to exhibit significant growth due to rising prevalence of the rare genetic disorder. Leigh syndrome is a rare and aggressive neurological disorder caused by mutations in mitochondrial DNA or nuclear genes involved in mitochondrial biogenesis and respiration. The characteristic symptoms of Leigh syndrome include developmental delay, loss of motor skills, weakness, vision problems, and respiratory issues.
Currently, there is no definitive treatment for Leigh syndrome. Treatments mainly focus on managing symptoms by targeting the underlying cause. Therapies primarily include vitamin and nutritional supplements, physical and occupational therapy, and medications targeting respiratory and neurological complications.
The global Leigh syndrome treatment market is estimated to be valued at US$ 272.9 million in 2024 and is expected to exhibit a CAGR of 6.7% over the forecast period from 2024 to 2031.
Key Takeaways
Key players operating in the Global Leigh Syndrome Treatment Market Growth are Abliva AB, PTC Therapeutics, VAKS Pharma, MITOCH, Medley Pharmaceuticals Ltd., Khondrion BV, OMEICOS THERAPEUTICS GMBH. , Edison Pharmaceuticals, Inc. , Dainippon Sumitomo Pharma Co. Ltd., Taysha GTx, PicnicHealth (AllStripes), Takeda Pharmaceutical Company , Biogen, Ionis Pharmaceuticals, Inc., Sarepta Therapeutics, Inc., and MECOSON LABS PRIVATE LIMITED.
The global Leigh syndrome treatment market is expected to witness lucrative growth opportunities due to increasing research funding for rare disease drug development and growing orphan drug designations. Furthermore, advancements in understanding the genetics and underlying mechanisms of Leigh syndrome are likely to aid the development of novel targeted treatment options.
Key players are actively focusing on expanding their geographical presence through collaborations and partnerships with regional healthcare providers to cater to the growing patient population worldwide. For instance, in 2021, Khondrion partnered with Taysha Gene Therapies to accelerate the clinical development of KH-176, a gene therapy drug candidate for Leigh syndrome.
Market Drivers
The primary driver propelling the global Leigh syndrome treatment market growth is increasing disease awareness driven by advocacy programs by patient support groups such as United Leukodystrophy Foundation and Cure Sanfilippo Foundation. Growing government efforts to provide orphan drug incentives and streamline the approval process for rare disease drug candidates are also expected to encourage pharmaceutical companies to invest in Leigh syndrome drug R&D.
Get More Insights On This Topic: Leigh Syndrome Treatment Market
#Leigh Syndrome Treatment Market#Mitochondrial Disease#Neurological Disorders#Rare Diseases#Genetic Disorders#Pediatric Neurology#Metabolic Disorders#Pharmaceutical Industry#Drug Development
0 notes
Link
#allied market research#pediatric vaccines market#mhealth market#oncology drugs market#tattoo removal market
0 notes
Note
I'd love to Know more about Icarus✨
Please Info dump
Hmmmm
Hes 29
His bones are far too dense and he doesn't have enough wing muscle to fly. For his species (Stav'raw) the muscle needs to be trained vigorously to even get a flutter of lift off. It's not feasible for most people of his species
He can glide though!
He's scared of heights though!!
His hair naturally grows pink and brown
He has naturally black scalera, lips, and tongue
He is currently employed at Stags adult entertainment
His work wife is Lennia, who's in charge of music management. Lennia is a human super into angels and he finds Icarus' wings super cool. He's Icarus' token straight friend.
His best friend is a man named Rowan who paints fine works of art and runs a gallery. This man is actually his love interest's, Ray's, older brother. But seeing as Rowan is a tsundere and Icarus for the love of all that is good cannot use Ray's name when referring to him, they do not connect the dots until wayyyy late in their relationship.
He is deadass referring to Ray as "angel fluff", "hottie with the boddy", "zelda twink", "Ms. Shawty", "dreamboat", "princess babygirl", and so many more to Ray's own damn brother
Rowan is also the father of his niece, Onyx. Onyx is technically his twin sister's spawn but seeing as what she did to Rowan he thinks she should be shot on sight and does not deserve the title of Onyx's mother. There's a reason Rowan has full custody.
Oh yeah, he hates his immediate family
He's an Earthian. Born and raised.
He experienced extreme neglect during his childhood due to never knowing his father and his mother never wanting children. He found himself alone in his early adult life, and that experience wasn't made easier with his sisters unforgivable actions
Rowan probably saved him. He's the closest thing Icarus has to a brother/father figure
Icarus actually had a dream to be a pediatric nurse for children who have been abused
He thinks about all the times just being noticed would have made all the hurt better. Or just having a trusted adult.
That dream was derailed with his first relationship. Luciel Lovejoy destroyed him, got him into cartel violence, and drugs by luring him in with expensive things and sweet promises
It felt affectionate
Luciel burned down a building during their last year of high school and blamed it on Icarus.
Icarus lost his full-ride scholarship to the top university of the Solar System
It was so fucking devastating he stopped trying to live
All his clawing up out of a pit until his fingers bled was for nothing. Stolen from him by some vandal that blamed him for it
Icarus actually still doesn't know Luciel did it and blamed him (Ray eventually pieces it together as he investigates Luciel for his cartel dealings and does not say anything. It clicks and he immediately thinks of how devastating it would be to bring up.)
(Luciel is actually a driving factor in how Ray and Icarus meet)
It was the perfect opportunity for Luciel to lovebomb him and mold him into his ideal arm candy
And it worked, which set off Icarus' current career path
He actually does enjoy his job despite Luciel being absolutely rancid. He chooses to do it
The relationship with Luciel ended when the jerk abandoned him in the Bahamas with no money for a new piece of arm candy
He is absolutely loaded because of his desirability.
Icarus eventually goes back to school for his dream
Largely because of Ray
Ray is just an encouraging friend and while Ray respects Icarus' control path, he just couldn't handle being married to someone who does it
He's perfectly fine with Icarus dancing though
So maybe that kicks Icarus into gear. And when he takes the first step Ray is off the market. So it really is something he feels he has to do for himself and not to gain Ray's affection
He is pining pathetically hard for Ray. He's such an enabler too. Early on Ray will come to him for the type of comfort you get from a partner and Icarus gladly lets him.
They talk like adults who respectceach other though. When it starts to bother Ray Icarus reminds him that he can say no if he doesn't want to do anything with Ray
Eventually they figure it out. When Ray is absolutely 1000% sure he will be spending his life with Icarus
Sidenote: Ray has a sickness that affects him drastically everytime he loses a connection with a lifemate. He's a widow, divorced, and had been assaulted by the woman he was dating, which resulted in the sickness progressing. So three seperate occasions that absolutely fucking wreck him. So he really does need to be damn sure. It's also why Ray can't handle Icarus having a job that requires him sleeping with other people. I'll eventually get way more into this with Ray, this is such a brief explanation.
Honestly it's no surprise they work out, the chemistry is fantastic
They're both highly intelligent, sassy, well dressed, a bit demanding.
Icarus is really sassy - which reminds me, I have a small written piece where Ray asks if he would love him if he's a worm... should I post that? Icarus destroys that question with facts and logic
Icarus has a thing for disco style blouses
He knows how to sew to alter clothes for his wings and tail
He also knows how to do makeup but would rather raw dog the world with his bare skin. Primer is the most he'll do
HE HATES BREAD, HE HATES DAIRY!! It makes him feel sick!!!
His favorite fruit is pomegranates
He has three dogs, Sir, Burr, and Essi
Did I mention he abhors greek/roman mythology references?
He does. So much.
Doesn't stop him from melting when Ray asks if he is "the star that melts him" though
Will genuinely punch someone if they make too many references to Icarus though
He was once asked if his "dad drowned instead of him" once and it took a moment to click but he clobbered the asshole
That may have been the moment that made him hate references to it
Icarus has a thing for motorcycles. He has a few and loves using his wings to do tricks.
He's not a burnt out gifted kid, he's rather blazing at the moment.
Thatsss all i have for now oops x3
8 notes
·
View notes
Text
A revolution in weight loss is apparently underway. It started in 2021, when the FDA approved the diabetes drug semaglutide for weight loss. The weekly injectable—sold under the brand name Wegovy—can help users lose 5 to 10 percent of their body weight, leading commentators to describe the drug as both a “medical breakthrough” and a “silver bullet” for obesity. Elon Musk says he’s taking it, Kim Kardashian is rumored to be using it, and everyone from Hollywood to the Hamptons reportedly wants a prescription.
Soon, there will be a new weight loss medication on the block—and it’s even more potent than its peers. Last fall, the FDA fast-tracked the review process for using tirzepatide as a weight loss drug after a clinical trial showed that people with BMIs labeled “overweight” or “obese” lost a staggering 22.5 percent of their body weight on the highest dose. If all goes according to plan, that will make Mounjaro the latest in a fast-growing biomedical sector—spanning everything from bariatric surgery to deep brain stimulation for binge-eating—that aims to combat, if not cure, the problem of “excess” weight.
For pharmaceutical companies, the race to market is financially motivated: Wegovy and Mounjaro cost more than $1,000 a month. Weight loss drugs are rarely covered by insurance, but people who can afford them have proven they’re willing to pay. And the market seems effectively limitless: Despite an ongoing “war on obesity,” more than 1.9 billion adults globally are considered overweight or obese, and the number of prospective users is growing every year. Now doctors—desperate to treat what is widely seen as an “obesity epidemic”—are coming on board. In January, the American Academy of Pediatrics recommended such medications for kids as young as 12.
The victorious narratives gilding drugs like Mounjaro are already being positioned as a direct challenge to fat activism. For decades, the movement has pushed for social and economic opportunity for people of all sizes through civil rights, fat pride and liberation, and biomedical evidence itself. Thanks to prominent voices like Audrey Gordon and Michael Hobbes, many people now know that “lifestyle changes” like calorie restriction and exercise fail to produce sustained weight loss for 97 percent of people and that many dieters end up gaining back more weight than they lost. But what happens to the strength of these arguments when a weight loss drug seems to work?
Like other purported weight loss solutions, Mounjaro promises “to fix weight stigma by making you thinner, instead of removing the stigma,” says Susanne Johnson, a fat activist and family nurse practitioner in Pennsylvania. In so doing, these drugs and surgeries further exacerbate anti-fat discrimination. Instead of criticizing people in larger bodies for their perceived lack of willpower—that old “calories in, calories out” adage—people can now blame those in bigger bodies for something more akin to a techno-pessimist, or even anti-science, stance: “Just take the miracle cure!”
The history of the weight loss industry is more akin to prospecting for gold or investing in crypto than transplanting organs and developing antibiotics; less a story of scientific progress than an endless cycle of wild speculation, where boom inevitably gives way to bust. Fen-Phen was a miracle until it was linked to heart valve damage. Intermittent fasting was going to fix what caloric restriction couldn’t until researchers showed the two produce exactly the same results. And then there’s the complicated case of bariatric surgery.
From their inception in the 1950s, operations like gastric bypass (which reroutes food away from the stomach, inducing malabsorption) and gastric sleeve (which involves partially amputating the stomach so it holds less food and produces fewer hunger hormones) have been sold as a potential panacea, says Lisa Du Breuil, a clinical social worker at Massachusetts General Hospital. While fewer than 1 percent of people who qualify actually undergo bariatric surgery, those who do can lose up to 70 percent of their “excess” weight (or the weight above a BMI of 24.9).
But Du Breuil, who specializes in eating disorders and substance abuse disorders, has seen some of the worst of bariatric’s side effects. People can develop dumping syndrome—wherein sugar-rich meals leave the stomach too quickly, causing sweating, dizziness, rapid heart rate, and vomiting. Gastric bypass in particular raises the risk of postoperative alcohol abuse. Rates of suicide and self-harming behaviors also rise in the years after bariatric surgery. And even when people follow strict post-operative diets, malnutrition, tooth loss, gout, and new or resurging eating disorders are possible. “It can be really challenging to get a full picture,” Du Breuil says. She learns about new side effects all the time.
Semaglutide and tirzepatide—both part of a larger family of GLP-1 receptor agonists—were developed for diabetes management at lower doses. When pharmaceutical companies noticed their trial participants were also losing weight, they realized “if we can turn the volume up to 11, we can really enhance this side effect,” says Johnson, the nurse. “That means you’re also turning up the other side effects.”
The primary complaints from users of Ozempic, Wegovy, and Mounjaro sound like the kind of thing you can fix with a bottle (or three) of Pepto Bismol: nausea, upset stomach, diarrhea, and what one patient called “power vomiting.” But these might be less like classic “side effects” of a drug than a mechanism of weight loss itself, as The Guardian recently reported. By making the feeling of eating (and, in some cases, even hydrating) actively disgusting to the user, the drug curbs their consumption—similar to the experience of bariatric patients, who can only fit a few ounces of food in their stomachs at a time.
The list of complications doesn’t end there. For example, both GLP-1 receptor agonists may increase the risk of thyroid cancer—one of the many BMI-linked diseases that supposedly makes weight loss absolutely imperative for people in larger bodies. And there’s good reason to believe that other side effects will reveal themselves in years to come, as the number of long-term users grows.
The biggest surprise for many prospective patients is that long-term weight loss isn’t guaranteed—a reflection, perhaps, of the faulty assumption that people are obese because they overeat. Current estimates suggest that the average bariatric surgery patient regains 30 percent of the weight they lost in the 10 years after surgery. One in four regain all of their weight in that time. And 20 percent of people don’t respond to surgery in the first place.
The same is true for GLP-1 receptor agonists: If you stop injecting, the weight returns.
In case it wasn’t clear by now, biomedical weight loss interventions often mimic the deadly logic of anorexia, bulimia, or other forms of disordered eating, says Erin Harrop, a clinical social worker and researcher. Harrop would know. At the height of their own eating disorder, Harrop wished they could fill their stomach with air instead of food, or cut their stomach out, or wire their jaw shut. Later, they learned these things exist—in the form of gastric balloons, gastric sleeves, and even a magnetic jaw trap.
It’s no surprise, then, that some people who undergo bariatric surgery experience a resurgence of a preexisting eating disorder, or develop a new one. Frequent vomiting, never knowing what foods will upset your stomach, and feeling pressure to maintain a post-surgical weight—“you can create an eating disorder that way,” Du Briel says.
But semaglutide and tirzepatide promise to fulfill an even stranger fantasy: eliminating appetite itself. While a drug like Mounjaro works on numerous fronts—including preventing the body from storing fat and “browning” existing adipose tissue—it’s the feeling of being untethered from desire that seems to fascinate patients and physicians alike. People for whom the drug works often say, “I forget to eat,” says Fatima Cody Stanford, an obesity medicine specialist at Massachusetts General Hospital’s Weight Center.
If doctors really believe that obesity is the greater of any two evils, then this approach makes sense. When it comes to bariatric surgery, for example, a review of the medical literature suggests it is, on balance, associated with a reduction in all-cause mortality—or death of any cause*—*compared to patients with high BMIs who don’t go under the knife (though such studies are profoundly limited, as they often do not control for social factors, like income or education). Many hope that semaglutide and tirzepatide will one day prove just as vitalizing.
But eating disorders kill too. In many contexts, sustained hunger is considered a travesty. And desire—for food, or anything else—is a great way to know you’re alive. “It’s wild to me that we see no appetite as a positive thing,” says Shira Rosenbluth, an eating disorder therapist who works with people of all sizes. Anna Toonk agrees: “I realized that there are worse things than being fat,” she told The Cut last fall. “The worst thing you can be is wanting to barf all the time.”
Ultimately, the proliferation of drugs like Mounjaro means medicine is not only in the business of dictating “normal” weights (a thing it still hasn’t quite figured out), but “normal” appetites. What was once an intuitive process, in which your body tells you what it needs, became a dictate under diet culture: You tell your body what it can have. Now medicine promises a radical reset: With the right drug, your body will hunger for nothing at all.
Weight loss technology can’t be stopped entirely—nor should it be. Everyone has the right to choose what they want to do with their bodies. But informed consent is built on information, and we may not have enough. Mounjaro was fast-tracked by the FDA based on studies designed to observe weight loss over just 72 weeks, a small fraction of the time real patients will be on the drug. At the very least, patients should be informed that in the first years after a drug hits the market, they are participants in an ongoing experiment.
As biomedicine’s war on obesity continues, people must work harder to combat anti-fat bias—not on a technicality, but as part of the expansive vision of justice fat activists began articulating more than 50 years ago. For semaglutide, tirzepatide, bariatric surgery, and their ilk are neither miracles nor cures. There have always been fat people, and there always will be, whether they’re “non-responders” to treatment, refuseniks, or languishing on the waitlist. Notably, even those who experience dramatic weight loss after surgery or on injectables may still be overweight or obese, depending where they started.
Perhaps most importantly, the American weight loss discourse must move away from a reflexive scientism, which has enabled biomedicine to subject the entirety of human experience to its single-minded scrutiny. Weight, like almost every aspect of embodiment, is not an exclusively biological phenomenon or a clear-cut medical “problem” to solve. It is shaped by countless factors, like power distribution in society, personal psychology, and that most frightening of forces: the desire for more.
If you or a loved one is struggling with an eating disorder, the National Eating Disorders Association Helpline is available at (800) 931-2237.
11 notes
·
View notes
Text
First it was a menstrual care product shortage, then it was a baby formula shortage that could last until spring now there is a shortage of children’s painkillers.
https://www.washingtonpost.com/dc-md-va/2022/12/01/childrens-tylenol-ibuprofen-shortages/

People seeking over-the-counter medication for their sick children are often finding sparse or empty shelves, as a spike in respiratory illnesses pushes pediatricians and emergency rooms to the limit.
Usual supplies of fever- and pain-reducing medicines, such as liquid acetaminophen and ibuprofen recommended for children with RSV, flu or the coronavirus, have not kept up with demand in recent weeks in pockets of the country hit hardest by surging illnesses.
Fast, informative and written just for locals. Get The 7 DMV newsletter in your inbox every weekday morning.
Unlike in Canada where the government has issued emergency orders to address a shortage of acetaminophen and ibuprofen, commonly known by the name brands Tylenol and Advil, and similar products, U.S. manufacturers and retailers emphasized that supplies should rebound within weeks. On the prescription side, increased demand for the antibiotic amoxicillin has caused shortages in the United States, Canada and parts of Europe.
In the meantime, pediatricians say they worry limited access to medicines could result in more urgent-care and emergency visits as parents struggle to keep sick children comfortable.
“It’s a huge problem,” said Kristina Powell, a pediatrician in Williamsburg, Va., and president of the Virginia chapter of the American Academy of Pediatrics. “This is a result of the ‘triple-demic.’ Parents run to Walmart or Target, the shelves are empty. … This is going to be a long fall and winter of viral infections.”
A crop of influenza-like illnesses, which includes RSV, hit the South and Southeast hard a month ago, federal data show, and those illnesses have slowly progressed westward. By mid-November, Texas, New Mexico and Tennessee reported the highest incidence of illness, while levels remained very high in Virginia and D.C., followed closely by Maryland, according to data tracked by the Centers for Disease Control and Prevention.
Kylie Moriarty, 30, of Buffalo, Mo., searched her local Walmart for Tylenol or ibuprofen to treat her sick 9-year-old daughter and found nothing but empty shelves. That seemed odd, she said, because just last week she had no trouble buying the same products for her 2-year-old son in a merry-go-round of illnesses affecting families with young children.
“I was very frustrated that it’s 2022 and we can’t keep something in stock for parents to help their children get better,” she said in a phone interview. “It makes me want to cry, almost, because these are my kids.”
She and her husband called other pharmacies looking for medicines to soothe the girl and couldn’t find any guaranteed availability.
“When they’re sick, there’s only so much that loving on them and cuddling with them you can do. So when there’s no medicine or something that you can give them … it’s hard,” Moriarty said.
Feeling powerless, the couple gave up and cautiously shared their younger child’s more concentrated medicine with the older one, which seemed to help. Just as they began to relax, their little boy came home from day care with a fever. Moriarty plans to schedule an appointment with his pediatrician for treatment — and samples to take home.
The ordeal reminded her of the height of the pandemic when supply chain problems left consumers scrambling for toilet paper and other basics.
Generic varieties play a large role in the market for over-the-counter comfort drugs, and that industry runs on profit margins so lean that companies typically lack capacity to boost production on short notice, according to supply chain experts. That left shelves bare during the early days of the pandemic.
The same dynamic is playing out now as RSV, flu and the coronavirus hit simultaneously, prompting sporadic shortages of commonly used ibuprofen and Tylenol at some hospitals and retail stores.
The extent of those shortages isn’t clear. The Food and Drug Administration hasn’t reported any shortages of fever or pain medications. Drugmakers, pharmacists and industry organizations say there aren’t any constraints to manufacturing and expect supplies to rebound within weeks.
The University of Utah’s Drug Information Service, which tracks drug shortages, received its first report of a shortage of liquid ibuprofen — generally for children — on Monday, and quickly confirmed it with several manufacturers. Most of the drugs tracked by the service are purchased in large quantities by hospitals, but some formulations had over-the-counter labels. Erin Fox, the service’s director, said it was impossible to know how widespread retail shortages were given the variety of store-branded versions.
“There are definitely distribution and supply chain problems that still exist,” she said, such as a company not being able to hire enough drivers. “These shortages seem to be mostly a demand spike and should resolve relatively quickly,” she added.
Until then, both chain stores and independent pharmacies are dealing with the unpredictability of high demand and uncertain supplies.
Martin McCarthy struck out at 5 p.m. Wednesday when he stopped by Brookville Pharmacy in Chevy Chase, Md., looking for liquid Motrin for his 10-year-old son, who probably picked up a bug at school.
The pharmacy’s stock of children’s fever reducers was depleted after two busy weeks of parents and grandparents stopping in to buy Tylenol or Motrin for little ones suffering from colds, RSV, flu and other viruses.
By Wednesday evening, only three boxes of generic ibuprofen chewables, two boxes of generic acetaminophen chewables and six boxes of suppository acetaminophen remained. Other parents peeked down the aisle looking for liquid fever reducer and fever reducer for children younger than 3, only to leave empty-handed.
McCarthy scanned the cold-medicine aisle for a few minutes and called home to confirm that his son would tolerate grape-flavored chewable tablets instead of the liquid Motrin he was used to.
“It is surprising because it’s basically completely out,” he said. “And it’s just generic.”
A spokeswoman for Walgreens said McCarthy’s experience is typical. Even if their usual choice of medication is unavailable because of high demand, parents can usually find an alternative.
“Although demand for pediatric OTC medications have increased, Walgreens is prepared and able to continue meeting the needs of our customers and patients. We are working with our diverse set of suppliers and distributors to ensure our patients have the products they need most,” Walgreen spokeswoman Zoe Krey said in a statement.
Martha Welman, a pediatrician and medical director at Neighborhood Health, a primary care provider serving low-income and underinsured patients in Alexandria, Arlington, and Fairfax, said staff will sometimes call pharmacies to find medicines for patients — a time-consuming process at a busy time.
“If it’s between helping someone find a medicine or seeing a sick child, we have to make that choice. We’re all kind of compromising right now,” she said.
Perrigo, an Ireland-based manufacturer of over-the-counter products, said “shortages are occurring in a number of markets we supply” because of high demand. The company has increased production of medications for fever and pain by 46 percent through October compared with a year ago, and increased shipments by a similar rate.
The Consumer Healthcare Products Association, which represents companies making over-the-counter drugs, said parts of the country are seeing a rise in pediatric illnesses but that there aren’t “overall widespread shortages here in the United States” of children’s pain relievers.
“We understand it might be frustrating for some parents who are unable to quickly locate these products from their usual pharmacy or retailer due to limited out-of-stocks in some stores,” the association said in a statement, but it emphasized the importance of calling around for medications and not hoarding, which could lead to widespread shortages.
Elizabeth Murray, a pediatric emergency medicine physician at Golisano Children’s Hospital in Rochester, N.Y., said from the bed shortage to overflowing emergency rooms, the last thing parents need is another hurdle. But until the early and aggressive onset of respiratory illness abates, health-care providers and parents have no choice but to ride it out together.
“Everyone would like to have one thing to blame and there isn’t one thing to blame,” she said. “This is happening for a variety of reasons and we’re going to move through it and we’re going to be okay.”
7 notes
·
View notes
Text
All right, something has come up that has sparked a thought that I need to get out of my head. Specifically I need to tell you exactly why the "take care of yourself" individualistic approach we've adopted toward Covid and toward disease in general here in the United States is a terrible idea. LONG RANT (TM) time!
WHAT AM I TALKING ABOUT?
Recently, as you may have noticed if you have kid(s), infant/child Tylenol is in very short supply. It's not a coincidence that this is happening at the same time as outbreaks of RSV, Flu, and other respiratory and inflammatory illnesses are hitting kids hard and sending many of them to the hospital.
As far as the individualism, it's the general American idea that people should take care of themselves. We don't have a universal health insurance program, you have to buy it yourself (or, if you're lucky, get it through your work). We don't have a universal health care system either, people are asked to navigate a highly decentralized system of hospitals, urgent care centers, and private providers who may or may not be covered under their insurance; even the same provider for a different procedure.
Where these come together is in terms of drug distribution. You see, in many places if there was a shortage of a drug there would be a centralized system to collect and distribute it according to need. In our decentralized system, individuals are responsible for getting their own drugs.
HOW DOES THIS CAUSE PROBLEMS?
So let's think of this in terms of market forces. If something is plentiful you're not going to worry about it; you'll just go out and get it when you need it. If the supply is restricted for some reason, or if you think it might be, you'll probably try to stock up when you have the chance to get it.
If enough people stock up on it who don't actually need it, they can actually end up causing exactly the problem that they fear. Enough people buying and stockpiling can create exactly the shortage that they were buying and stockpiling in order to protect themselves against. This is known as "panic buying".
Of course, if someone who hasn't pre-emptively stockpiled the thing needs it, they may be out of luck.
THE SITUATION RIGHT NOW
After several years where respiratory diseases weren't much of an issue due to measures taken against them (masks and social distancing worked guys!) they've suddenly come roaring back. RSV, Flu, Covid, and others are now hitting kids particularly hard for a number of reasons and have overwhelmed pediatric wards in much of the country.
Even kids who don't get sick enough to go to the hospital are still getting sick and their parents are doing what we all do, giving them cold and flu medication to help ease the illness. More importantly, parents whose kids are not sick (yet?) have heard of the shortage of kids cold and flu medication and are stocking up in case their own kid gets sick.
What this means is that there a lot of parents whose kids are sick who cannot get any medication for them while some parents whose kids are not sick have medication they are not using.
And I want to be clear, this is not the fault of any of those parents. I'm one of them, I have extra Tylenol in case my own kid gets sick. This is the fault of a system that shifts responsibility for a society-wide issue onto individuals.
MARKET FORCES
Understanding the issue is best done by understanding some of the theory behind it.
Let's say that, in normal times, X amount of a good is made. When something happens to increase the demand for that good, those factories have to increase production to X+Y amount. This takes a certain amount of time. You have to secure additional raw materials, additional labor, and, if necessary, additional equipment.
In the meantime, while production gets up to speed, you encounter a potential shortage. If more people need the good than what was initially able to be produced and especially if, in addition to that increased demand, people who don't immediately need the good are stockpiling it in fear of a shortage, you can quickly run out of that good until such time as the factory or factories can increase output to meet the new demand.
In the case of something that isn't immediately necessary, this isn't terrible. If, for example, string cheese were the product in question, I think we'd all agree that it's okay and we can survive until production gets ramped up.
In the case of medication, however, this situation can literally cost lives. Children's Tylenol isn't exactly the kind of thing that will cause you to die immediately if you don't have it, but a lack of it will cause the symptoms of some children who don't get it to escalate to the point where they will need to go to the hospital when they otherwise wouldn't. Once at the hospital they will take up other resources which are also limited and which another child may die without. In other words, the lack of children's Tylenol is causing shortages of other goods which are more critical.
I should point out that none of this section is controversial, even those who promote market and individual solutions over government or centralized solutions acknowledge that these periods of adjustment occur under their proposed systems, they just believe that the benefits outweigh the costs. I simply disagree.
CONCLUSION
By making each American responsible for their own individual outcomes rather than making even the slightest effort at providing some form of centralized assistance we have created the conditions that lead to enormous market inefficiencies whenever a scarcity exists or even if one is feared to exist.
Ordinarily these are just annoyances, but when it comes to medication these inefficiencies can cost lives. There are some goods that simply need to be available when they are needed.
In a rational system we would acknowledge that some goods cannot be simply left to market forces, but our current system leaves us vulnerable to market swings when unexpected events force changes to supply and/or demand. In this way, our dedication to hyper-individualism is quite literally killing us and it would be wise of us to think about the things that we could make better instead of leaving each person to fend for themselves in a system that we know will kill some of them.
#children's tylenol#children's medication#drug policy#us politics#politics#long rant (tm)#individualism#drug shortages#health care#universal healthcare
2 notes
·
View notes
Text
disposable containers with lids for food safety
Reusable containers can be useful whether your goal is to reduce the amount of food that is wasted or you just want somewhere to store food that has already been cooked. But when it comes to both personal and environmental health, are there any food containers that are more secure than others? The information that you require is detailed below.
A master of the code You'll notice a little triangle with a number (the resin identification code) ranging from 1 to 7 on the bottom of plastic food storage containers. These numbers are on the bottom of the containers. This number provides information regarding the composition of the plastic. In general, the selections 1, 2, 4, and 5 are the most reliable when it comes to food safety. Avoiding plastic containers with the codes 3, 6, and 7 is something that is recommended by the American Academy of Pediatrics. Plastic number 3 is made of vinyl or polyvinyl chloride (PVC), plastic number 6 is made of polystyrene, and plastic number 7 is made of a variety of polymers, including bisphenol A. (BPA).
The usage of the chemical known as bisphenol A (BPA) has been made illegal in the production of baby bottles, sippy cups, and the packaging of infant formulae. The use of this material in other food and beverage containers, on the other hand, is still subject to monitoring by the Food and Drug Administration of the United States and is now regarded as safe. The widespread availability of BPA-free plastic food and beverage containers in today's market enables customers to reduce their overall exposure to the chemical.
Keep your composure. Even though polycarbonate plastic is tough and long-lasting, it is possible for it to deteriorate over time due to exposure to high temperatures or excessive use. Never reheat food in a microwave while it is still in a plastic food container, including margarine tubs and takeout containers from restaurants. After their first usage, the plastic containers that come with prepackaged microwaveable meals shouldn't be reused because they were designed to be used only once before being discarded safely. It is also not recommended to place plastic containers in the washing machine or dishwasher.
Recycle whenever it's feasible. The Environmental Protection Agency (EPA) reports that in 2017, the United States recycled little more than 8% of the plastic containers and packaging that was produced. This can lead to emissions of greenhouse gases (GHG), which can have a negative impact on the environment's health.
The Crux of the Matter
Choose your plastic food containers carefully and restrict their use to the storage of cold foods only. They are also an excellent choice for transporting food items. Instead, you might choose to put cold or hot items in containers made of glass or stainless steel. Both of these options are great for storing food at home because they can be reused after being cleaned.
Is it possible that non-toxic food storage containers could improve the health of your food? Consider the following:
When you place food in plastic containers designed for food storage, you always end up with more food than you initially put in…
It is inevitable that you will ingest some of the chemicals and microplastics that are absorbed by the food you eat.
Therefore, non-plastic alternatives to Tupperware make perfect sense.
It would be a shame to serve nutritious food on safe dinner plates, only to pollute the leftovers with plastic after they have been stored in the refrigerator. It would appear that using non-toxic food storage containers is an important component of any healthy lifestyle.
Because of this, we looked at a large number of different options before settling on the 11 most secure food storage containers.
But before we get into that, there's something important you should know about the containers made of so-called safe plastic… (i.e., food storage containers that do not contain BPA)
They are not as secure as you believe they are.
Consequently, amongst other things, you'll learn how to conduct a risk assessment of the plastic Tupperware you already own. In this manner, you will be able to acquire the most superior non-plastic food storage containers available today…
And from this point forward, you'll be aware of everything that you should steer clear of.
There is a good chance that you, along with everyone else, own a huge quantity of plastic food storage containers. They are not only cheap but also quite convenient, and most of the time we get them for free when we order takeout.
However, herein lies the rub. These containers, if they get stressed, have the potential to leach dangerous elements into the food inside of them.
Where is the stress?
The heat from eating hot food or re-heating your leftovers in the microwave. There are also scratches and sunshine to consider.
And tense plastic containers don't go to the pool to unwind and relax when they're agitated…
Chemicals with estrogenic activity, such as bisphenol A (BPA) and phthalates, are instead released into the environment. These chemicals help to improve the utility of plastic in a variety of applications, including containers, water bottles, and more.
The following are some of the health dangers posed by BPA and phthalates found in plastic food storage containers:
According to a number of studies, these substances are linked to obesity and have negative effects on a number of organs, including the heart and the brain.
BPA is especially concerning when it comes to the health of children. There is a correlation between exposure in early childhood and the symptoms of ADHD, which include inattention, hyperactivity, and depression.
Yikes.
Phthalates also raise a number of problems, which is to be expected. According to the CDC's findings, they have little effect on the reproductive system of animals.
Consequently, I strongly advise getting rid of any and all plastic containers in order to eliminate any risk of contamination to your food.
But hang on. Getting rid of plastic isn't always simple or even possible… Especially if you are working with a limited amount of money.
Therefore, it's possible that you're thinking, "C'mon! What kind of food storage containers made of plastic even exist?! I can't yet afford to replace all of my plastic!"
How to determine which plastic containers are of the highest quality for use with food (Avoiding BPA)
Flipping over your plastic container and taking a quick look at the recycling number is the quickest and easiest approach to conduct a risk assessment.
(That is, the numerals 1 through 7 enclosed within a triangle formed by three arrows; see the illustration above.)
Containers made of plastic with the numbers 1, 2, 4, and 5 are considered to be the most secure for food storage. These storage containers do not contain BPA and are therefore safer to use around food.
The likelihood of finding BPA in a product increases with its number: 3, 6, and notably 7.
Polycarbonate plastics can be identified by their number 7 label or the "PC" marking on their packaging. They have BPA in them to give the stiff plastic more "give," which helps prevent cracking and breaking.
If a plastic is stiff and clear, there is a good chance that it is made of polycarbonate and that it contains BPA. Take, for instance, a durable, see-through container that can be reused to store food.
Plastic containers that are hazy, soft, and flexible are less likely to contain the chemical bisphenol A (BPA).
The important thing is to examine the labeling at all times.
However, there is one point that should be kept in mind:
There is a danger associated with the use of any plastic, even BPA-free Tupperware. The label "BPA-free" is not accurate in any way, shape, or form because this plastic instead consists of BPS.
According to Scientific American, investigations have shown that the hazards associated with BPS are essentially identical to those associated with BPA.
The Takeaway: If you are going to use a plastic container of any kind, then you should adhere to these principles to lower your risk:
Plastic containers should never be used to heat food in the microwave or any other method.
Do not save or reuse the plastic food packaging from commercial products… Takeout containers designed for a single usage, for instance. These are not designed to be reused, and the plastic they contain will contaminate your food.
Do not serve hot meals in plastic containers intended for cold foods. Prepare the heat in a dish all by itself.
Do not put food containers made of plastic into the dishwasher to clean them. The plastic deteriorates as a result of the heat.
If you have Tupperware that is more than a few years old, you should get rid of it as soon as possible. Plastic that is old and worn out degrades into food much more easily than newer plastic.
When you buy sauces or other items that come in glass mason jars, be sure to keep the jars for later use. These are an excellent and risk-free alternative to using Tupperware.
2 notes
·
View notes
Text
Understanding the Growth Dynamics of the Oral Transmucosal Drugs Market
The Oral Transmucosal Drugs Market is projected to be valued at USD 16.57 billion in 2024 and is anticipated to grow to USD 22.97 billion by 2029, with a compound annual growth rate (CAGR) of 6.75% over the forecast period (2024-2029).
The oral transmucosal drugs market has been gaining traction due to its unique drug delivery system that allows medications to be absorbed directly into the bloodstream through the oral mucosa, bypassing the digestive system. This method provides a faster onset of action and is beneficial for patients who struggle with oral intake or those requiring rapid relief. In this blog, we will explore the current trends, key drivers, and challenges shaping the market landscape, based on insights from the market research industry.
1. Market Overview: A Shift Towards Patient-Centric Drug Delivery
The global oral transmucosal drug market is experiencing notable growth due to its convenience and improved patient compliance. This mode of administration is particularly useful in treating conditions like breakthrough cancer pain, migraines, and anxiety, where rapid drug action is critical. The ability to deliver precise dosages through buccal, sublingual, or nasal routes offers a viable alternative to traditional oral or intravenous methods.
Market research points to a growing interest in transmucosal drug delivery systems as pharmaceutical companies look for ways to improve drug efficacy and enhance patient experiences.
2. Key Drivers of Market Growth
a. Advances in Drug Formulations
Continuous advancements in drug formulations are driving the oral transmucosal drugs market forward. Innovations in bioavailability and the stability of drugs administered through the mucosal lining are improving therapeutic outcomes. Pharmaceutical firms are investing in research to create formulations that ensure quicker absorption and minimal side effects.
b. Rising Demand for Pain Management Solutions
Chronic pain management remains one of the top application areas for oral transmucosal drugs. Cancer patients, especially those experiencing breakthrough pain, benefit significantly from this delivery system. Furthermore, the rise in demand for pain management due to the aging population is expected to fuel the market's expansion.
c. Patient Preference for Non-invasive Drug Delivery
Patients are increasingly favoring non-invasive drug delivery methods over traditional injections or tablets. Oral transmucosal administration provides a less invasive approach, making it suitable for individuals with swallowing difficulties, such as pediatric or elderly patients, or those who require rapid symptom control.
3. Challenges and Restraints
a. Regulatory and Approval Complexities
The complexity of obtaining regulatory approvals for transmucosal drugs can be a hurdle for market players. The stringent evaluation of safety, efficacy, and potential risks associated with absorption variability can delay the introduction of new drugs into the market. Pharmaceutical companies need to navigate these regulatory landscapes carefully.
b. Competition from Other Drug Delivery Systems
While oral transmucosal delivery offers distinct advantages, it faces competition from other emerging drug delivery technologies such as transdermal patches, inhalation systems, and implantable devices. Each method comes with its own set of benefits and limitations, leading to a competitive landscape where companies need to differentiate their offerings.
4. Innovations and Opportunities in the Market
a. Breakthroughs in Bioadhesive Technologies
New developments in bioadhesive technologies are enhancing the effectiveness of oral transmucosal drugs. These innovations improve the adhesion of drugs to the mucosal surfaces, ensuring prolonged contact and better absorption rates. The integration of nanoparticles and microencapsulation techniques is also opening new avenues for controlled drug release.
b. Personalized Medicine and Custom Drug Formulations
With the rise of personalized medicine, there is increasing demand for customizable drug formulations in the oral transmucosal segment. Tailoring drug delivery to individual patient needs based on genetic, metabolic, and lifestyle factors offers new possibilities for the industry. This personalized approach aligns with broader trends toward precision medicine, which seeks to optimize treatment outcomes.
5. Future Outlook: Market Expansion and Growth Potential
According to recent market research, the oral transmucosal drugs market is projected to expand significantly over the next decade. This growth will be driven by continued advancements in drug delivery technologies, a rising prevalence of chronic diseases requiring quick therapeutic responses, and increased investments in R&D by pharmaceutical companies. Moreover, the shift towards more patient-centric healthcare solutions will continue to push this market forward.
Final Thoughts
The oral transmucosal drugs market is positioned for substantial growth due to its unique benefits and applications in various therapeutic areas. As pharmaceutical companies focus on enhancing drug delivery methods, investing in innovative formulations, and navigating regulatory challenges, this market will continue to evolve. Understanding the market trends, drivers, and opportunities can help stakeholders in the healthcare sector make informed decisions to capitalize on this burgeoning industry.Call to Action: Interested in exploring more insights on the oral transmucosal drug market? Stay ahead of the curve with comprehensive market research reports that delve deeper into emerging trends and forecast analyses for the next decade.
#Oral Transmucosal Drugs Market trends#Oral Transmucosal Drugs Market size#Oral Transmucosal Drugs Market share#Oral Transmucosal Drugs Market analysis#Oral Transmucosal Drugs Market forecast#Oral Transmucosal Drugs Market demand
0 notes
Text
2024 Compounding Pharmacies Market Dynamics: Trends and Insights
The global compounding pharmacies market is projected to witness steady growth over the next decade, with its market size expected to increase from USD 12.6 billion in 2023 to USD 19.9 billion by 2032. The market will experience a compound annual growth rate (CAGR) of 5.2% during the forecast period from 2024 to 2032, driven by rising demand for personalized medications and tailored healthcare solutions.
Compounding pharmacies provide customized medications by combining, altering, or mixing ingredients to meet individual patient needs. These pharmacies are particularly beneficial for patients who require specific doses, alternative forms of medication, or allergen-free formulations that are not available in mass-produced pharmaceutical products. As the healthcare landscape shifts toward more personalized approaches, the compounding pharmacy industry is poised for consistent growth.
Get Free Sample PDF: https://www.snsinsider.com/sample-request/4513
Key Market Drivers
Rising Demand for Personalized Medication: One of the primary growth drivers for the compounding pharmacies market is the increasing demand for personalized healthcare. Standard pharmaceutical products do not always meet the specific needs of every patient. Compounding pharmacies play a crucial role in filling this gap by offering custom formulations that cater to individual patient requirements, such as adjusting medication strengths, flavors, or delivery methods. This trend is especially prevalent in treating chronic conditions, hormone replacement therapies, and pediatric care.
Growth of Geriatric Population: The global aging population is a significant factor contributing to the expansion of the compounding pharmacies market. Older adults often require personalized medication solutions due to polypharmacy (the use of multiple medications) and the need for tailored dosages. Many elderly patients also face difficulties in swallowing pills, leading to a demand for liquid formulations or transdermal options that compounding pharmacies can provide.
Increasing Incidence of Chronic Diseases: The rising prevalence of chronic diseases such as diabetes, cardiovascular diseases, and cancer has further propelled the need for customized therapies. Compounding pharmacies are able to develop medications that are tailored to manage the symptoms of these conditions effectively. In addition, they can create formulations that address medication shortages or provide alternative treatments when commercial drugs are unavailable.
Regulatory Support and Innovations: Regulatory bodies such as the U.S. Food and Drug Administration (FDA) are increasingly supporting the use of compounding pharmacies, provided they adhere to stringent quality standards. Regulatory initiatives, along with technological advancements in compounding techniques, are creating new opportunities for market growth. Innovations in drug delivery methods, automation, and formulation technologies are further enhancing the capabilities of compounding pharmacies to produce high-quality and safe medications.
Challenges and Market Opportunities
Despite the positive outlook, the compounding pharmacies market faces several challenges. Stringent regulatory frameworks and concerns over the safety and efficacy of compounded medications can hinder market growth. Compounding pharmacies must meet rigorous quality standards, and any lapses can affect public trust and demand for their services.
However, the increasing focus on patient-centric care, coupled with technological advancements in drug compounding, presents ample opportunities. Automation in compounding processes is reducing the risk of human error, while digital health solutions are making it easier for healthcare providers to prescribe customized medications. Additionally, the market is expected to see growth in veterinary compounding, as pet owners seek personalized treatments for their animals.
Regional Insights
North America remains the largest market for compounding pharmacies, driven by the presence of advanced healthcare infrastructure and a strong focus on personalized medicine. The United States, in particular, has a well-established regulatory framework that supports compounding pharmacies, leading to the proliferation of these services.
Europe is also witnessing steady growth, especially in countries like Germany, the UK, and France, where there is increasing awareness of the benefits of personalized medication. Meanwhile, the Asia-Pacific region is emerging as a key growth area due to its expanding healthcare sector, growing patient population, and increasing demand for customized treatments.
Future Outlook
The future of the compounding pharmacies market looks promising, as healthcare continues to move toward a more personalized and patient-centric model. With a projected CAGR of 5.2% from 2024 to 2032, the market is expected to see innovations in compounding technologies and broader adoption of custom medications across various therapeutic areas.
In conclusion, the global compounding pharmacies market, valued at USD 12.6 billion in 2023, is on track to reach USD 19.9 billion by 2032. With the rising demand for personalized medicine, the growth of the aging population, and advancements in compounding techniques, this market is set for significant expansion over the next decade.
Other Trending Reports
Dental Suction Systems Market
Cosmeceuticals Market
Cell Therapy Market
Growth Hormone Deficiency Market
0 notes
Text
Intravenous Infusion Pumps Market By Product Type, By Manufacturers, By End-User And Market Trend Analysis Forecast 2033
The intravenous infusion pumps global market report 2024 from The Business Research Company provides comprehensive market statistics, including global market size, regional shares, competitor market share, detailed segments, trends, and opportunities. This report offers an in-depth analysis of current and future industry scenarios, delivering a complete perspective for thriving in the industrial automation software market.

Intravenous Infusion Pumps Market, 2024 report by The Business Research Company offers comprehensive insights into the current state of the market and highlights future growth opportunities.
Market Size -
The intravenous infusion pumps market size has grown strongly in recent years. It will grow from $5.71 billion in 2023 to $6.15 billion in 2024 at a compound annual growth rate (CAGR) of 7.9%. The growth in the historic period can be attributed to advances in medical technology, aging population, home healthcare services, hospital and healthcare facility expansion, regulatory standards.
The intravenous infusion pumps market size is expected to see strong growth in the next few years. It will grow to $8.51 billion in 2028 at a compound annual growth rate (CAGR) of 8.5%. The growth in the forecast period can be attributed to increasing chronic disease burden, technological innovation, home infusion services, telehealth and remote monitoring. Major trends in the forecast period include smart infusion pumps, wireless and remote monitoring, home infusion therapy, precision medicine and personalized infusion.
Order your report now for swift delivery @
https://www.thebusinessresearchcompany.com/report/intravenous-infusion-pumps-global-market-report
The Business Research Company's reports encompass a wide range of information, including:
1. Market Size (Historic and Forecast): Analysis of the market's historical performance and projections for future growth.
2. Drivers: Examination of the key factors propelling market growth.
3. Trends: Identification of emerging trends and patterns shaping the market landscape.
4. Key Segments: Breakdown of the market into its primary segments and their respective performance.
5. Focus Regions and Geographies: Insight into the most critical regions and geographical areas influencing the market.
6. Macro Economic Factors: Assessment of broader economic elements impacting the market.
Market Drivers -
The rise in the prevalence of people with chronic pain, cancer, and diabetes is a significant driver of the demand for intravenous infusion pumps, as these pumps are commonly used to deliver pharmaceutical drugs during the treatment of these diseases. Chronic illnesses and disorders are on the rise around the world, which can be attributed to an aging population and shifts in social behavior that led to a gradual increase of these widespread and expensive long-term medical issues. Infusion pumps are used to transfer regulated doses of nutrients or drugs into a patient's body, such as chemotherapy medicines, pain relievers, antibiotics, insulin, or other hormones. For instance, in March 2022, according to the Australian Bureau of Statistics, an Australia-based autonomous statutory agency tasked with obtaining and analyzing data and making factual recommendations to local, national, and territorial governments, almost fifty percent of the population (46.6%, or 11.6 million) suffered from at least one chronic illness. Therefore, the rise in the prevalence of chronic diseases is expected to drive the growth of the intravenous infusion pumps market.
The intravenous infusion pumps market covered in this report is segmented –
1) By Product: Volumetric, Syringe, Enteral, Ambulatory, IV Disposables, Other Products
2) By Application: Chemotherapy, Diabetes, Gastroenterology, Analgesia/Pain Management, Pediatrics/Neonatology, Hematology, Other Applications
3) By End-User: Hospitals, Ambulatory Surgical Centers, Cancer Treatment Centers, Specialty Clinics, Other End Users
Get an inside scoop of the intravenous infusion pumps market, Request now for Sample Report @
https://www.thebusinessresearchcompany.com/sample.aspx?id=3305&type=smp
Regional Insights -
North America was the largest region in the intravenous infusion pumps market in 2023. Western Europe was the second-largest region in the intravenous infusion pumps market. The regions covered in the intravenous infusion pumps market report are Asia-Pacific, Western Europe, Eastern Europe, North America, South America, Middle East, Africa
Key Companies -
Major companies operating in the intravenous infusion pumps market include B. Braun Melsungen AG, Baxter International Inc., Medtronic plc, Fresenius Kabi AG, Smiths Medical Inc., Becton Dickinson and Company (BD), Terumo Corporation, F. Hoffmann-La Roche Ltd., ICU Medical Inc., IRadimed Corporation, Mindray Medical International Limited, Zyno Medical LLC, Tandem Diabetes Care Inc., Johnson & Johnson, Micrel Medical Devices SA, Boston Scientific Corporation, Pfizer Inc., Q-Core Medical Ltd., Moog Inc., Roche Diagnostics International AG, Nipro Corporation, Animas Corporation, Ypsomed Holding AG, Valeritas Inc., ZOLL Medical Corporation, Codan Medical A/S, Halyard Health Inc., InfuSystem Holdings Inc., Hospira Infusion Systems, CareFusion Corporation
Table of Contents
1. Executive Summary
2. Intravenous Infusion Pumps Market Report Structure
3. Intravenous Infusion Pumps Market Trends And Strategies
4. Intravenous Infusion Pumps Market – Macro Economic Scenario
5. Intravenous Infusion Pumps Market Size And Growth
…..
27. Intravenous Infusion Pumps Market Competitor Landscape And Company Profiles
28. Key Mergers And Acquisitions
29. Future Outlook and Potential Analysis
30. Appendix
Contact Us:
The Business Research Company
Europe: +44 207 1930 708
Asia: +91 88972 63534
Americas: +1 315 623 0293
Email: [email protected]
Follow Us On:
LinkedIn: https://in.linkedin.com/company/the-business-research-company
Twitter: https://twitter.com/tbrc_info
Facebook: https://www.facebook.com/TheBusinessResearchCompany
YouTube: https://www.youtube.com/channel/UC24_fI0rV8cR5DxlCpgmyFQ
Blog: https://blog.tbrc.info/
Healthcare Blog: https://healthcareresearchreports.com/
Global Market Model: https://www.thebusinessresearchcompany.com/global-market-model
0 notes
Text
Best Quality PCD Pharma Franchise Company in India: Lezaa Biotech
In today's rapidly evolving pharmaceutical landscape, the demand for high-quality medicines and healthcare products is at an all-time high. People are becoming more health-conscious, and with this rising awareness, the need for reliable pharmaceutical companies has grown exponentially. For entrepreneurs looking to enter the pharmaceutical industry, a PCD (Propaganda Cum Distribution) pharma franchise offers a lucrative opportunity. One name that stands out in this sector is Lezaa Biotech, widely recognized as the Best Quality PCD Pharma Franchise Company in India. This article delves into what makes Lezaa Biotech the best in the business and how partnering with them can bring success and growth.
What is a PCD Pharma Franchise?
Before we explore Lezaa Biotech's exceptional standing, it's essential to understand the concept of a PCD pharma franchise. PCD stands for Propaganda Cum Distribution, which allows individuals or companies to market and distribute pharmaceutical products under the brand name of an established pharma company. It is a low-risk, high-reward business model that requires minimal investment and provides an excellent opportunity for growth.
A PCD pharma franchise model is popular because it offers business owners the flexibility to work in their area, market proven products, and receive promotional support. Lezaa Biotech has mastered this model by providing top-notch products and comprehensive business support to its franchise partners.
Lezaa Biotech: The Best Quality PCD Pharma Franchise Company in India
Lezaa Biotech has become synonymous with trust, quality, and innovation in the pharmaceutical industry. It has earned its place as the Best Quality PCD Pharma Franchise Company in India due to its relentless focus on delivering high-quality medicines, customer satisfaction, and ethical business practices. Let’s explore the key factors that set Lezaa Biotech apart:
1. Uncompromising Commitment to Quality
One of the primary reasons why Lezaa Biotech is regarded as the Best Quality PCD Pharma Franchise Company in India is its unwavering commitment to maintaining the highest quality standards in its products. The company follows stringent manufacturing processes that are compliant with WHO-GMP (World Health Organization - Good Manufacturing Practices) norms. From raw material sourcing to the final packaging, every step is carefully monitored to ensure that the medicines meet global quality standards.
Moreover, Lezaa Biotech’s in-house quality control team tests every batch of products to ensure safety, efficacy, and consistency. This dedication to quality not only enhances the credibility of the brand but also instills confidence in franchise partners and consumers alike.
2. Diverse Product Portfolio
Lezaa Biotech boasts a vast product range, covering various therapeutic segments. Whether it is antibiotics, analgesics, anti-inflammatory drugs, or supplements, Lezaa Biotech has an extensive catalog of products that cater to the needs of healthcare professionals and patients. This variety allows franchise partners to offer a wide range of medicines and healthcare products in their region, enhancing their ability to meet the needs of their local market.
By partnering with Lezaa Biotech, franchisees can access high-quality products in segments such as:
Antibiotics
Cardiology
Diabetology
Gastroenterology
Pediatrics
Dermatology
Gynecology
Neurology
Orthopedics
The ability to offer such a diverse portfolio makes it easier for franchisees to establish themselves in the market and gain the trust of medical professionals and consumers.
3. Innovative Marketing and Promotional Support
Lezaa Biotech understands that marketing and promotion are critical to the success of any PCD pharma franchise. The company goes the extra mile to provide its franchise partners with comprehensive promotional materials, including visual aids, product brochures, visiting cards, and sample products. Franchisees also benefit from access to a wide array of digital marketing tools and strategies to enhance their online presence.
This support is a significant reason why Lezaa Biotech is considered the Best Quality PCD Pharma Franchise Company in India. Franchise partners are never left to fend for themselves; instead, they are equipped with the resources needed to thrive in their local markets.
4. Monopoly Rights for Franchise Partners
Lezaa Biotech offers exclusive monopoly rights to its franchise partners, ensuring that they have control over the distribution and marketing of products in their territory. This unique benefit allows franchisees to operate without competition from other partners of the same company within their designated area, thereby maximizing their business potential.
Monopoly rights help franchise partners maintain a solid market position and enjoy a steady stream of revenue. This is a testament to the company's belief in creating mutually beneficial relationships with its franchise network.
5. Affordable Investment with High Returns
One of the key attractions of a PCD pharma franchise is that it requires minimal investment, making it accessible to a wide range of entrepreneurs. Lezaa Biotech offers flexible investment options, allowing individuals to start their own businesses without the burden of hefty financial commitments.
Despite the low investment, the potential for high returns is substantial. With a strong product portfolio, exclusive rights, and robust marketing support, franchise partners can quickly establish themselves in the market and start generating significant revenue. This combination of low risk and high reward makes Lezaa Biotech a preferred choice for entrepreneurs seeking a business opportunity in the pharma sector.
6. Ethical Business Practices
Lezaa Biotech’s success is rooted in its ethical approach to business. The company adheres to all regulatory requirements and maintains transparency in its dealings with franchise partners. This ethical framework has earned Lezaa Biotech the trust of not only its partners but also healthcare professionals and patients.
Franchisees can be confident that they are working with a company that values honesty, integrity, and a commitment to improving healthcare outcomes across India. This trust is a vital element of Lezaa Biotech’s enduring reputation as the Best Quality PCD Pharma Franchise Company in India.
7. Fast and Efficient Product Delivery
In the pharmaceutical industry, timely delivery of medicines is critical. Lezaa Biotech has a well-established supply chain and logistics network that ensures the quick and efficient delivery of products to its franchise partners. This means that franchisees can rely on a consistent supply of products, allowing them to meet the demands of their customers without delays.
Fast delivery times are especially important for maintaining a strong relationship with healthcare professionals and ensuring that patients receive the medicines they need on time. Lezaa Biotech's reliable distribution system is another reason why the company is a leader in the PCD pharma industry.
Conclusion
Lezaa Biotech has established itself as the Best Quality PCD Pharma Franchise Company in India through its unwavering commitment to quality, a diverse product portfolio, strong marketing support, ethical business practices, and reliable product delivery. For entrepreneurs looking to enter the pharmaceutical industry, partnering with Lezaa Biotech offers a highly profitable and sustainable business opportunity.
Whether you are a budding entrepreneur or an established business owner looking to expand into the pharmaceutical sector, Lezaa Biotech provides the perfect platform for success. With their comprehensive support and high-quality products, you can confidently build a thriving business in the pharmaceutical industry while contributing to the betterment of healthcare in India.
0 notes
Text
Pharmaceutical Sterility Testing Market 2024 World Technology, Development, Trends and Opportunities Industry Research Report to 2030
Pharmaceutical Sterility Testing Industry Overview
The global pharmaceutical sterility testing market size was estimated at USD 1.59 billion in 2023 and is projected to grow at a compound annual growth rate (CAGR) of 11.02% from 2024 to 2030.
Increasing government investments, R&D activities, a growing number of drug launches, and a rising focus on quality & sterility are expected to drive the market growth. The development of comprehensive sterility testing procedures is regulated with stringent policies and quality control standards.
Gather more insights about the market drivers, restrains and growth of the Pharmaceutical Sterility Testing Market
In addition, several government initiatives play a pivotal role in shaping the healthcare landscape, and their financial support significantly influences the development & implementation of advanced sterility testing procedures within the pharmaceutical sector. Increasing government funding for enhanced R&D activities for novel therapeutics, especially vaccines, biologics, and sterile products, which need sterility testing to ensure safety & efficacy. This leads to the introduction of cutting-edge technologies and innovative solutions in the sterility testing market. For instance, according to the European Federation of Pharmaceutical Industries and Associations (EFPIA), total healthcare R&D expenditure in the European Union (EU) was around USD 48.5 billion in 2022 compared to USD 46.4 billion in 2021.
Global Pharmaceutical Sterility Testing Market Report Segmentation
This report forecasts revenue growth at global, regional, and country levels and provides an analysis of the latest industry trends in each of the sub-segments from 2018 to 2030. For this study, Grand View Research has segmented the global pharmaceutical sterility testing market report based on the type, product type, test type, sample, end-use, and region:
Type Outlook (Revenue, USD Million, 2018 - 2030)
In-House
Outsourcing
Product Type Outlook (Revenue, USD Million, 2018 - 2030)
Kits & Reagents
Instruments
Services
Test Type Outlook (Revenue, USD Million, 2018 - 2030)
Sterility Testing
Membrane Filtration
Direct Inoculation
Product Flush
Bioburden Testing
Bacterial Endotoxin Testing
Rapid Microbial Method
ATP Bioluminescence
Fluorescent -Based
Solid-phase Cytometry
Others
Sample Outlook (Revenue, USD Million, 2018 - 2030)
Pharmaceuticals
Medical Devices
Biopharmaceuticals
End-use Outlook (Revenue, USD Million, 2018 - 2030)
Compounding Pharmacies
Medical Device Companies
Pharmaceutical Companies
Others
Regional Outlook (Revenue, USD Million, 2018 - 2030)
North America
US
Canada
Europe
UK
Germany
France
Italy
Spain
Netherlands
Switzerland
Sweden
Belgium
Russia
Asia Pacific
Japan
China
India
Australia
South Korea
Philippines
Malaysia
New Zealand
Singapore
Thailand
Latin America
Brazil
Mexico
Argentina
Colombia
Chile
Middle East and Africa (MEA)
South Africa
Saudi Arabia
Egypt
Israel
UAE
Browse through Grand View Research's Clinical Diagnostics Industry Research Reports.
• The global cancer biopsy market size was valued at USD 30.05 billion in 2023 and is projected to grow at a CAGR of 8.11% from 2024 to 2030.
• The global pediatric cancer biomarkers market size was estimated at USD 815.81 million in 2023 and is projected to grow at a CAGR of 8.5% from 2024 to 2030.
Key Pharmaceutical Sterility Testing Company Insights
Several key players are acquiring various strategic initiatives to strengthen their market position offering diverse services to customers. The prominent strategies adopted by companies are service launches, mergers & acquisitions/joint ventures merger, partnership & agreements, expansions, and others to increase market presence & revenue and gain a competitive edge drives the market growth.
Key Pharmaceutical Sterility Testing Companies:
The following are the leading companies in the pharmaceutical sterility testing market. These companies collectively hold the largest market share and dictate industry trends. Financials, strategy maps & products of these pharmaceutical sterility testing companies are analyzed to map the supply network
Pacific Biolabs
Steris Plc
Boston Analytical
Sotera Health Company (Nelson Labs)
Sartorius Ag
Solvias Ag
SGS SA
Labcorp
Pace Analytical
Charles River Laboratories
Thermo Fisher Scientific, Inc.
Rapid Micro Biosystems, Inc.
Almac Group
Labor LS SE & Co. KG
Recent Developments
In January 2024, Rapid Micro Biosystems, Inc. company announced the launch of the Growth Direct Rapid Sterility application by mid-2024
In August 2023, Pace Analytical Services improved its capabilities by adding advanced hydrocarbon analytical support and expanded sediment & tissue testing with the acquisition of Alpha Analytical
In May 2023, Thermo Fisher Scientific, Inc. announced the launch of a sterile drug facility in Singapore. It would help deliver new vaccines and medicines in the Asia Pacific market
Order a free sample PDF of the Pharmaceutical Sterility Testing Market Intelligence Study, published by Grand View Research.
0 notes
Text
Necrotising Enterocolitis Market Will Grow At Highest Pace Owing To Rising Prevalence Of Preterm Birth Complications

Necrotising enterocolitis (NEC) is a devastating gastrointestinal disease that primarily affects premature infants. It is characterized by inflammation and necrosis of the intestine. The risk factors associated with NEC include prematurity, formula feeding, and bacterial colonization of the intestine. Infants with very low birth weights have the highest risk. NEC treatment involves management of sepsis, support of vital organ function, bowel rest with no oral feeding, and surgery if necessary.
The Necrotising Enterocolitis Market is estimated to be valued at US$ 7.10 Bn in 2024 and is expected to exhibit a CAGR of 5.6% over the forecast period 2024-2031.
Key Takeaways
Key players operating in the Necrotising Enterocolitis market are AbbVie, AstraZeneca, Baxter International, Bristol-Myers Squibb, Fresenius Kabi. Rising prevalence of preterm birth complications globally is expected to drive the growth of the market during the forecast period. According to the World Health Organization, preterm birth complications are the leading cause of death among children under 5 years of age, responsible for approximately 1 million deaths in 2015. Technological advancements in parenteral nutrition and minimal invasive surgery have provided improved treatment outcomes for NEC.
Market Trends
Increasing research on nutraceuticals and probiotics for NEC prevention: Several clinical studies are evaluating the role of pre and probiotics such as Lactobacillus and Bifidobacterium in reducing the risk of NEC in preterm infants. This presents an opportunity for novel prevention strategies.
Rising adoption of minimal invasive surgery: Advancements in minimal invasive surgical techniques such as laparoscopy has resulted in reduced recovery time and complications for NEC patients undergoing surgery. This trend is expected to drive the future demand.
Market Opportunities
Development of novel therapeutics targeting inflammatory pathways: Researchers are investigating potential drug targets such as Toll-like receptor 4 (TLR4) and nuclear factor kappa B (NF-κB) signaling pathways to develop novel anti-inflammatory therapies for NEC treatment.
Increasing healthcare expenditure on pediatric care in emerging nations: Emerging countries in Asia Pacific and Latin America are witnessing increased healthcare spending focused on neonatal and pediatric care. This will propel the growth of therapeutics and medical devices market for pediatric gastrointestinal conditions.
Impact Of COVID-19 On Necrotising Enterocolitis Market Growth
The COVID-19 pandemic has adversely impacted the growth of the necrotising enterocolitis market globally. During the peak of pandemic in 2020-2021, the concentration of healthcare resources towards treatment of COVID-19 patients has negatively affected the diagnosis and treatment of other health conditions including necrotising enterocolitis. This led to reduction in number of surgeries and procedures carried out for necrotising enterocolitis management. Moreover, restrictions on non-essential healthcare services along with fear of virus spread stopped patients from visiting hospitals even for emergency cases. This impacted the market growth negatively during the period.
However, with gradual lift of restrictions in 2022 and availability of COVID-19 vaccines, the market is recovering slowly. The healthcare facilities are focusing on clearing backlog of non-COVID cases and regaining lost momentum in treatment of other diseases. The manufacturers are expanding supply chain capabilities and ramping up production to meet the increasing demand. Various initiatives are being taken by governments and healthcare organizations to raise awareness about timely management of necrotising enterocolitis. This will potentially boost the market in the coming years.
The United States holds the major share of necrotising enterocolitis market in terms of value, owing to large patient population, high treatment cost and adequate reimbursement framework. The region accounted for over 35% revenue share of global market in 2024.
Asia Pacific region is poised to witness fastest growth during the forecast period. Factors such as increasing healthcare expenditure, rising medical tourism, growing birth rate and expanding private hospital infrastructure will aid the market growth in Asia Pacific. China, India and Japan are emerging as profitable markets for necrotising enterocolitis treatment.
Get more insights on this topic: https://www.trendingwebwire.com/necrotising-enterocolitis-market-is-estimated-to-witness-high-growth-owing-to-advancements-in-parenteral-nutrition-solutions-and-devices/
About Author:
Ravina Pandya, Content Writer, has a strong foothold in the market research industry. She specializes in writing well-researched articles from different industries, including food and beverages, information and technology, healthcare, chemical and materials, etc. (https://www.linkedin.com/in/ravina-pandya-1a3984191)
What Are The Key Data Covered In This Necrotising Enterocolitis Market Report?
:- Market CAGR throughout the predicted period
:- Comprehensive information on the aspects that will drive the Necrotising Enterocolitis Market's growth between 2024 and 2031.
:- Accurate calculation of the size of the Necrotising Enterocolitis Market and its contribution to the market, with emphasis on the parent market
:- Realistic forecasts of future trends and changes in consumer behaviour
:- Necrotising Enterocolitis Market Industry Growth in North America, APAC, Europe, South America, the Middle East, and Africa
:- A complete examination of the market's competitive landscape, as well as extensive information on vendors
:- Detailed examination of the factors that will impede the expansion of Necrotising Enterocolitis Market vendors
FAQ’s
Q.1 What are the main factors influencing the Necrotising Enterocolitis Market?
Q.2 Which companies are the major sources in this industry?
Q.3 What are the market’s opportunities, risks, and general structure?
Q.4 Which of the top Necrotising Enterocolitis Market companies compare in terms of sales, revenue, and prices?
Q.5 Which businesses serve as the Necrotising Enterocolitis Market’s distributors, traders, and dealers?
Q.6 How are market types and applications and deals, revenue, and value explored?
Q.7 What does a business area’s assessment of agreements, income, and value implicate?
*Note:
1. Source: Coherent Market Insights, Public sources, Desk research
2. We have leveraged AI tools to mine information and compile it
#Necrotising Enterocolitis Market Trend#Necrotising Enterocolitis Market Size#Necrotising Enterocolitis Market Information#Necrotising Enterocolitis Market Analysis#Necrotising Enterocolitis Market Demand
0 notes
Text
Why Choose the Best Pharmacy College in Dehradun for Your Future?
Dehradun, nestled amidst the majestic Himalayas, has emerged as a prominent educational hub, offering a diverse range of courses. Among the many academic disciplines, pharmacy stands out as a rewarding and challenging field. Choosing the best pharmacy college in Dehradun is crucial for your future career success. This blog explores the reasons why a pharmacy degree is valuable and why Graphic Era Hill University is the premier choice in the region.
The Importance of a Pharmacy Degree
A Bachelor of Pharmacy (B.Pharm.) degree equips students with the necessary knowledge and skills to excel in the pharmaceutical industry. It is a rewarding career path that offers a blend of science, healthcare, and business. Here's why a pharmacy degree is important:
Career Opportunities: Pharmacists play a vital role in healthcare, working in various settings such as community pharmacies, hospitals, pharmaceutical companies, and research institutions.
Job Security: The pharmaceutical industry is a stable and growing sector, ensuring job security for qualified pharmacists.
Impact on Society: Pharmacists contribute significantly to public health by ensuring the safe and effective use of medications.
Intellectual Stimulation: Pharmacy involves a constant learning process, keeping you intellectually engaged and up-to-date with the latest advancements.
Competitive Salary: Pharmacists often enjoy competitive salaries and benefits.
Key Areas of Study in Pharmacy
A pharmacy degree covers a wide range of subjects, including:
Pharmaceutical Chemistry: Understanding the chemical composition and properties of drugs.
Pharmaceutics: Learning about drug formulation, dosage forms, and manufacturing processes.
Pharmacology: Studying the effects of drugs on the body, including their mechanisms of action and therapeutic uses.
Pharmacognosy: Exploring the study of medicines derived from natural sources.
Pharmaceutical Analysis: Analyzing drugs for quality, purity, and potency.
Pharmaceutical Jurisprudence: Understanding the legal and ethical aspects of pharmacy practice.
Why Choose Graphic Era Hill University for Pharmacy?
Graphic Era Hill University stands out as the best pharmacy college in Dehradun due to several compelling reasons:
Experienced Faculty: Our faculty members are highly qualified professionals with extensive experience in the pharmaceutical industry.
Modern Infrastructure: Our state-of-the-art laboratories and facilities provide a conducive learning environment.
Industry Partnerships: We have strong collaborations with pharmaceutical companies and healthcare organizations.
Research Opportunities: Students can engage in research projects under the guidance of experienced faculty members.
Placement Assistance: Our dedicated placement cell helps students secure internships and jobs in leading pharmaceutical companies.
Career Paths for Pharmacy Graduates
Graduates of our pharmacy program can explore various career paths, including:
Community Pharmacist: Working in retail pharmacies, dispensing medications and providing healthcare advice.
Hospital Pharmacist: Working in hospital pharmacies, ensuring the safe and effective use of medications.
Clinical Pharmacist: Specializing in a particular area of healthcare, such as oncology or pediatrics.
Pharmaceutical Research: Conducting research and development of new drugs.
Regulatory Affairs: Ensuring compliance with pharmaceutical regulations and standards.
Drug Inspector: Ensuring the quality and safety of pharmaceutical products in the market.
Conclusion
Choosing the best pharmacy college is a crucial decision that can significantly impact your future career. Graphic Era Hill University offers a comprehensive pharmacy program that equips students with the necessary skills and knowledge to excel in this rewarding field. By enrolling in our program, you can lay a strong foundation for a successful and fulfilling career in pharmacy.
Address: 566/6, Bell Road, Society Area, Clement Town, Dehradun, Uttarakhand PIN: 248002
Contact: 1800 270 1280
0 notes
Text
The Iron Deficiency Anemia Treatment Market Is Thriving On Growing Demand

The iron deficiency anemia treatment market consists of oral iron replacement therapies that are used to treat low iron levels in the blood. Oral iron supplements offer convenience as they can be taken at home and have advantages like lower cost and fewer side effects compared to intravenous infusions. Iron deficiency anemia is a widespread nutritional disorder globally owing to insufficient dietary intake of iron or absorption issues. It can cause fatigue, weakness, and shortness of breath if left untreated.
Global iron deficiency anemia treatment market is estimated to be valued at US$ 12.1 Bn in 2024 and is expected to reach US$ 21.6 Bn by 2031, exhibiting a compound annual growth rate (CAGR) of 8.6% from 2024 to 2031.
Key Takeaways
Key players operating in the iron deficiency anemia treatment market are AdvaCare Pharma, Otsuka Pharmaceutical Co., Ltd., Sanofi, Emcure Pharmaceuticals, Wellona Pharma, SiNi Pharma Pvt Ltd, Sun Pharmaceutical Industries Ltd., Zydus Group, Akebia Therapeutics., Rockwell Medical, Inc., AbbVie Inc., Pfizer, Inc., Velnex Medicare, PHAEDRUS LIFE SCIENCE PVT. LTD., Inopha International Co, Limited, PharmaNutra S.p.A., Pharmascience Inc., American Regent, Inc.
The growing Iron Deficiency Anemia Treatment Market Growth for oral iron replacement therapies owing to advantages like convenience of use and less side effects compared to intravenous infusions is fueling the market growth. Oral iron supplements can easily be taken at home without much supervision.
The market is witnessing expansion in developing regions due to rising awareness and healthcare investments. There is a growing focus of market players on these regions through product launches, collaborations and mergers & acquisitions to strengthen their presence.
Market Key Trends
The market is witnessing high research and development activities by players to come up with innovative oral iron formulations. Iron Deficiency Anemia Treatment Market Size and Trends includes extended-release formulations with lower dosing frequency and tablets with enhanced biocompatibility for better iron absorption. Development of new pediatric formulations suitable for infants and children is also among the key research areas.
Porter’s Analysis
Threat of new entrants: Low due to high costs involved to established production and distribution networks along with high capital requirements. Also, presence of few large players makes it difficult for new entrants.
Bargaining power of buyers: Moderate as large number of generic alternatives available. However, severity and risk associated with condition increases buyer power.
Bargaining power of suppliers: Moderate as raw material suppliers have limited control over pricing due to availability of substitutes.
Threat of new substitutes: High due to emergence of alternative therapies and newer oral and injectable formulations.
Competitive rivalry: Very high due to presence of many global and local players providing different treatment options. Intense competition keeps pricing pressure on existing products.
Geographical Regions
In terms of value, North America accounts for the largest share of the iron deficiency anemia treatment market due to growing prevalence of the disease and presence of advanced healthcare facilities. The U.S. is the major revenue generator within North America.
Asia Pacific is the fastest growing region owing to rising geriatric population, increasing awareness regarding anemia, and improving access to healthcare services in emerging countries like India and China. The availability of low-cost generic drugs provides an impetus to market growth in Asia Pacific.
Get more insights on Iron Deficiency Anemia Treatment Market
Discover the Report for More Insights, Tailored to Your Language
French
German
Italian
Russian
Japanese
Chinese
Korean
Portuguese
Vaagisha brings over three years of expertise as a content editor in the market research domain. Originally a creative writer, she discovered her passion for editing, combining her flair for writing with a meticulous eye for detail. Her ability to craft and refine compelling content makes her an invaluable asset in delivering polished and engaging write-ups.
(LinkedIn: https://www.linkedin.com/in/vaagisha-singh-8080b91)
#Coherent Market Insights#Iron Deficiency Anemia Treatment Market#Iron Deficiency Anemia Treatment#Iron Supplements#Anemia Treatment#Iron Deficiency#Oral Iron Therapy#Intravenous Iron#Iron Absorption#Ferrous Sulfate
0 notes
Text
The Oral Dosing Cups market is projected to grow from USD 378.76 million in 2024 to USD 524.37 million by 2032, reflecting a compound annual growth rate (CAGR) of 4.15%.The oral dosing cups market is witnessing steady growth due to its increasing use in the medical and pharmaceutical sectors. These small, calibrated cups are designed to measure liquid medications accurately, ensuring precise dosing for patients of all ages, particularly children and the elderly who may have difficulty swallowing pills. The market's expansion is driven by rising healthcare awareness, advancements in drug delivery systems, and an emphasis on safe and accurate medication administration.
Browse the full report at https://www.credenceresearch.com/report/oral-dosing-cups-market
Market Overview
Oral dosing cups are essential tools in clinical settings, pharmacies, and homes. They are commonly used for liquid medications such as syrups, suspensions, and elixirs, providing an easy-to-read measurement system that helps reduce dosing errors. With the increasing prevalence of chronic diseases, the demand for oral dosing cups is growing, as they help ensure patients adhere to prescribed dosages, thereby improving treatment outcomes.
The global oral dosing cups market is segmented by material type, capacity, end-user, and region. The most common materials used in manufacturing these cups are plastic, polyethylene, and polypropylene, chosen for their durability, cost-effectiveness, and safety for pharmaceutical use. The cups are available in various capacities, typically ranging from 5 ml to 30 ml, to accommodate different dosing requirements.
Market Drivers
1. Rising Demand for Pediatric and Geriatric Medicines: Children and older adults often require liquid medications due to difficulties swallowing pills. Oral dosing cups provide a convenient way to administer these medications accurately, minimizing the risk of underdosing or overdosing.
2. Emphasis on Accurate Dosing: Dosing accuracy is crucial for the effectiveness of medications, especially for potent drugs with narrow therapeutic ranges. The use of oral dosing cups with clear, precise measurement markings helps ensure correct dosages, improving patient compliance and safety.
3. Growth in Home Healthcare: With the rise of home healthcare services, there is an increasing need for user-friendly medical devices. Oral dosing cups are simple, cost-effective tools that support self-care and medication management at home, reducing the need for frequent hospital visits.
4. Technological Advancements: The market is also benefiting from technological innovations, such as cups with improved readability, enhanced grip, and designs that minimize spillage. Some manufacturers are even exploring the integration of smart features, such as sensors that can detect and record the volume of liquid consumed, providing additional data to healthcare providers.
Challenges in the Market
Despite its growth prospects, the oral dosing cups market faces certain challenges. Environmental concerns over plastic waste have led to increased scrutiny of disposable plastic products. As a result, there is growing demand for eco-friendly and biodegradable alternatives, pushing manufacturers to innovate and develop sustainable options.
Another challenge is the risk of incorrect usage. While oral dosing cups are designed to enhance dosing accuracy, misuse or misinterpretation of the measurement markings can still lead to errors. Educating patients and caregivers on the correct use of these cups is essential to maximize their benefits.
Regional Insights
North America and Europe currently lead the market, driven by high healthcare spending, advanced medical infrastructure, and strong regulatory frameworks ensuring the quality and safety of medical devices. The Asia-Pacific region is expected to witness significant growth in the coming years, fueled by increasing healthcare awareness, a growing elderly population, and rising incidences of chronic diseases requiring long-term medication.
Key Players and Competitive Landscape
The oral dosing cups market is moderately fragmented, with several key players contributing to its growth. Some of the prominent manufacturers include Comar LLC, Gerresheimer AG, Medi-Dose, Inc., and Berry Global, Inc. These companies are investing in product innovation, expanding their distribution networks, and adopting sustainable practices to gain a competitive edge.
Future Outlook
The future of the oral dosing cups market looks promising, with continued innovation and increasing demand from both developed and developing regions. The focus on patient safety, accurate dosing, and sustainable solutions will shape the market's evolution, making oral dosing cups an indispensable component of modern healthcare.
As the market grows, stakeholders must address environmental concerns, improve user education, and continue to innovate to meet the changing needs of healthcare providers and patients alike. The ongoing advancements in material science and smart technology integration are likely to further enhance the functionality and appeal of oral dosing cups, solidifying their position in the global healthcare landscape.
Key Player Analysis
Medline Industries Inc.
Stiplastics S.A.S
Argo S.A.
Origin Pharma Packaging
Eastman Chemical Company
Gramß GmbH Kunststoffverarbeitung
Yuyao Liantong Plastic & Mould Co. Ltd.
Adelphi Healthcare Packaging
Comar, Inc.
Segments:
Based on Product Type:
Disposable
Reusable
Based on Capacity:
<5 ml
5 ml – 15 ml
15 ml – 25 ml
25 ml
Based on End User:
Hospitals
Clinics
Retail Pharmacies
Pharmaceuticals
Others
Based on the Geography:
North America
US
Canada
Mexico
Europe
Germany
France
UK
Italy
Spain
Rest of Europe
Asia Pacific
China
Japan
India
South Korea
South-east Asia
Rest of Asia Pacific
Latin America
Brazil
Argentina
Rest of Latin America
Middle East & Africa
GCC Countries
South Africa
Rest of Middle East and Africa
Browse the full report at https://www.credenceresearch.com/report/oral-dosing-cups-market
About Us:
Credence Research is committed to employee well-being and productivity. Following the COVID-19 pandemic, we have implemented a permanent work-from-home policy for all employees.
Contact:
Credence Research
Please contact us at +91 6232 49 3207
Email: [email protected]
Website: www.credenceresearch.com
0 notes