#Proteinengineering
Explore tagged Tumblr posts
Text
Biobetters Market Overview: Exploring Key Innovations Driving Next-Generation Biologic Therapies and Global Growth Trends
The Biobetters Market Overview highlights a transformative shift in the pharmaceutical industry, where enhanced biologic drugs—biobetters—are outperforming first-generation biologics in efficacy, safety, and patient compliance. Unlike biosimilars, biobetters offer modified structures and improved mechanisms of action, creating a lucrative path for both innovation and long-term healthcare solutions.

Understanding Biobetters: Beyond Biosimilars
Biobetters, also referred to as biosuperiors, are next-generation biologics developed to improve upon existing biologic therapies. These drugs share a common origin with their predecessors but are chemically modified to deliver superior clinical outcomes. The improvements range from prolonged half-life and reduced immunogenicity to simplified administration and lower side-effect profiles.
Unlike biosimilars, which are essentially generic versions of biologics, biobetters aim for therapeutic advancement. This strategic distinction offers pharmaceutical companies stronger patent protection and premium pricing opportunities, making biobetters a crucial focus in long-term R&D investment.
Key Innovations Transforming the Biobetters Market
1. Protein Engineering and Molecular Design
The cornerstone of biobetter innovation lies in protein engineering technologies. Through site-directed mutagenesis, glycoengineering, and PEGylation, researchers are creating biologics with improved solubility, stability, and bioavailability. These innovations not only enhance therapeutic efficacy but also allow for less frequent dosing—greatly benefiting patients with chronic conditions like rheumatoid arthritis and diabetes.
2. Advanced Drug Delivery Mechanisms
Another major leap in biobetters stems from novel drug delivery platforms. Innovations such as subcutaneous injections, auto-injectors, and depot formulations are simplifying the administration process for patients, replacing intravenous infusions with more patient-friendly alternatives. This not only boosts adherence but also reduces the burden on healthcare infrastructure.
3. AI and Computational Biology
Artificial intelligence and machine learning are playing a pivotal role in designing more effective biobetters. AI-driven models help predict protein folding, simulate drug-receptor interactions, and identify optimal structural modifications. These computational advances are dramatically shortening development cycles and increasing the probability of clinical success.
Market Drivers Fueling Global Expansion
Several factors are propelling the biobetters market forward:
Patent Expiry of First-Generation Biologics: As key biologics lose exclusivity, the door opens for biobetters to enter with improved therapeutic profiles.
Rising Demand for Chronic Disease Management: Biobetters offer sustained relief and higher compliance in managing long-term diseases like cancer, autoimmune disorders, and metabolic conditions.
Regulatory Encouragement: Regulatory agencies like the FDA and EMA are facilitating fast-track approvals for innovative therapies with proven improvements over existing treatments.
Investment in Biotech R&D: Major pharmaceutical companies are ramping up their investment in biobetter pipelines to strengthen portfolios and achieve market differentiation.
Regional Insights and Growth Dynamics
North America
North America currently leads the global biobetters market, owing to strong R&D ecosystems, robust healthcare infrastructure, and favorable reimbursement policies. The presence of major industry players and rising consumer awareness also contribute to the region's dominance.
Europe
Europe follows closely, supported by aggressive adoption of biologic treatments and initiatives promoting biosimilar and biobetter alternatives to reduce healthcare costs. Countries like Germany and the UK are hotspots for clinical trials and regulatory innovation.
Asia-Pacific
Asia-Pacific is emerging as a high-growth region, particularly due to rising healthcare expenditure, expanding patient populations, and increasing biopharma collaborations in countries like China, India, and South Korea. Government support for biotech innovation is further catalyzing regional market expansion.
Competitive Landscape and Strategic Moves
The biobetters market is moderately consolidated, with several key players leveraging their biologics expertise to develop next-gen therapies. Major strategies include:
Strategic Collaborations: Companies are forming partnerships with research institutes and biotech firms to access cutting-edge technologies and speed up drug development.
Portfolio Diversification: Many players are expanding their biobetter offerings across multiple therapeutic areas to capture broader market segments.
Licensing Agreements: Out-licensing and in-licensing of biobetter candidates are becoming common to reduce risk and share development costs.
Market Challenges and Future Outlook
Despite its promising outlook, the biobetters market faces some hurdles:
High Development Costs: Developing a biobetter is both time-consuming and expensive, requiring intensive research and large-scale clinical trials.
Regulatory Complexity: Although agencies support innovation, navigating the nuanced regulatory pathways for approval remains a challenge.
Market Penetration: Gaining market share against entrenched biologics or biosimilars can be difficult without clear clinical superiority or cost advantages.
Looking ahead, the future of the biobetters market appears robust. Advances in genomics, personalized medicine, and biologic delivery will continue to push the envelope. As healthcare systems worldwide emphasize value-based care, biobetters will become increasingly central to treatment strategies across therapeutic domains.
#Biobetters#BiologicsInnovation#PharmaceuticalTrends#HealthcareInnovation#ProteinEngineering#NextGenTherapies#DrugDevelopment#Biopharma#GlobalHealthcare#ChronicDiseaseManagement#BiotechTrends#FutureOfMedicine
0 notes
Text
🧪 Fusion Enzymatic Proteins: Engineering the Future of Biotechnology
By Hafiz Muhammad Husnain Azam Researcher, Brandenburg University of Technology Cottbus-Senftenberg 📘 Published 🔗 Read the Full Review on ScienceDirect
The Fusion Paradigm: Precision Meets Performance
In the rapidly advancing world of biotechnology, fusion enzymatic proteins are emerging as next-generation molecular tools—offering enhanced biological performance and enabling complex biological modifications across medicine, industry, and environmental science.
Our newly published review explores how engineered fusion proteins are transforming key sectors like biocatalysis, biosensing, gene therapy, and sustainable bio-processing by combining multiple enzymatic functions into a single, high-efficiency biological unit.
🔬 What Makes Fusion Proteins So Powerful?
Fusion proteins are created by genetically linking two or more functional proteins, yielding multifunctional biocatalysts with:
Enhanced substrate specificity
Increased catalytic efficiency
Greater structural stability
Versatile application potential
Whether it’s degrading chitin-protein waste in the biofuel industry or enabling targeted drug delivery in precision medicine, fusion proteins are designed to streamline, accelerate, and expand biological workflows.
Key Applications Covered:
✅ Biocatalysis: Custom enzyme fusions improve reaction rates and product yields in pharmaceuticals, biofuels, and waste treatment. ✅ Gene Therapy: Fusion proteins deliver genetic material with enhanced specificity and safety. ✅ Biosensing: Smart biosensors with fused reporter and binding proteins allow for ultra-sensitive detection of toxins, metabolites, and pathogens. ✅ Targeted Therapy: Engineered fusion constructs are paving the way for precision oncology and immunotherapy.
Protein Engineering: Where Science Meets Design
Advanced protein engineering tools like:
Directed evolution
DNA shuffling
Phage display
AI-driven protein design
...are enabling the creation of customized protein libraries optimized for real-world challenges. These tools are not only enhancing the structural-functional dynamics of fusion proteins but are also accelerating their translation into commercially viable products.
Challenges and Future Outlook
Despite their versatility, fusion proteins face key challenges:
Protein misfolding and structural incompatibility
Scalability of production
Thermodynamic stability under diverse operational conditions
However, breakthroughs in synthetic biology, molecular modeling, and machine learning are rapidly mitigating these issues, unlocking new frontiers in smart biomaterials, enzyme-based computing, and regenerative therapies.
Fusion Proteins in the Age of AI & Sustainable Bioengineering
As biotechnology converges with artificial intelligence and green chemistry, fusion enzymatic proteins are positioned to become core components of:
Personalized therapeutics
Environmentally friendly industrial catalysts
Next-gen biosensors
Synthetic cellular systems
📖 Read the full review: ScienceDirect – Fusion of Enzymatic Proteins
https://go.nature.com/3YjzDcc
https://doi.org/10.1016/j.cis.2025.103473

#FusionProteins#Biocatalysis#MolecularEngineering#EnzymaticEngineering#GeneTherapy#Biosensing#BiotechnologyInnovation#ProteinEngineering#SyntheticBiology#SmartBiomolecules#SustainableBioengineering#BiotechResearch#Bioinformatics#DirectedEvolution#AIInBiotech#IndustrialEnzymes#DrugDeliverySystems#NextGenBiotech#EnzymeDesign#BiofuelTech#EnvironmentalBiotech#BiologicalModifications#PrecisionMedicine#ScienceForSustainability#ProteinFusionTech#BiochemicalEngineering#GreenBiotechnology#MolecularBiology#BioengineeringFuture#academia
1 note
·
View note
Text
Dive into the fascinating world where scientists design and construct new proteins to create revolutionary advancements in medicine, agriculture, and biotechnology.
#ProteinEngineering#Biotechnology#GeneticEngineering#MolecularBiology#BiomedicalScience#BioInnovation#EngineeringLife#FutureOfScience#ResearchAndDevelopment#InnovationInBiotech#EngineersHeaven
1 note
·
View note
Text
From AI Error to Nobel Glory: How "Hallucinations" Revolutionized Chemistry
What was once considered a bug in AI is now a feature! Generative AI's "hallucinations" have played a crucial role in protein design, earning scientists the 2024 Nobel Prize in Chemistry.

#AIInnovation#NobelChemistry#ProteinEngineering#ArtificialIntelligence#ScientificDiscovery#TechForGood
0 notes
Text
ESM3: The AI That's Fast-Forwarding Evolution
In a breakthrough that sounds like science fiction becoming reality, Evolutionary Scale's new AI model ESM3 is accomplishing what typically takes nature hundreds of millions of years - creating entirely new proteins from scratch. This development represents a fundamental shift in how we can manipulate the building blocks of life itself.
The Power of 2.78 Billion Proteins
At its core, ESM3 is a generative AI model trained on an astronomical 2.78 billion proteins. But unlike its predecessors, ESM3 doesn't just analyze protein sequences - it understands their three-dimensional structures and functions, similar to how ChatGPT comprehends language. This comprehensive understanding allows it to design entirely new proteins with specific desired functions.
The GFP Breakthrough: Proof in the Glow
The team behind ESM3 demonstrated its capabilities through a remarkable achievement - creating a completely novel Green Fluorescent Protein (GFP). While GFPs naturally occur in jellyfish and have been used by scientists as cellular markers, ESM3 designed an entirely new version that has never existed in nature. This achievement effectively compressed what might have taken 500 million years of evolution into mere months of computational work.
Beyond Analysis to Creation
What sets ESM3 apart is its ability to go beyond analyzing existing proteins to generating new ones with specific functions. The process mirrors modern AI language models but instead of working with words and sentences, ESM3 works with the complex language of protein structures and functions. When asked to create proteins with specific properties, it can generate multiple candidates and iterate based on feedback, leading to novel proteins with desired characteristics.
Real-World Applications
The implications of ESM3's capabilities are staggering: - Drug Discovery: The potential to design proteins that can target specific disease pathways with unprecedented precision, leading to more effective treatments with fewer side effects - Personalized Medicine: The ability to create treatments tailored to individual genetic profiles - Materials Science: Development of new biomaterials with specific properties for applications ranging from sustainable packaging to advanced electronics - Environmental Solutions: Potential to create proteins that could efficiently capture carbon dioxide from the atmosphere - Synthetic Biology: New possibilities in designing biological systems for various applications
Democratizing Protein Design
Perhaps most importantly, Evolutionary Scale is making ESM3 accessible to the broader scientific community. Through: - An open-source version for researchers - A closed beta API for specific applications - Partnerships with AWS and NVIDIA for wider availability This commitment to accessibility could accelerate scientific discovery across multiple fields.
Looking to the Future
While ESM3 represents a significant breakthrough, it's likely just the beginning of what's possible in protein engineering. As one researcher noted, "ESM3 is like the Model T of protein engineering - we can expect to see the equivalent of Ferraris and spaceships in the not-too-distant future."
Navigating the Challenges
This powerful technology also raises important considerations: - The need for careful safety protocols in protein design - Ethical considerations around manipulating biological systems - Potential risks of misuse - The importance of responsible development and application

Conclusion
ESM3 represents a fundamental shift in our ability to understand and manipulate the building blocks of life. As we stand at this frontier of biological engineering, the potential for breakthrough discoveries in medicine, materials science, and environmental protection is enormous. While the technology must be developed responsibly, ESM3 marks a significant step toward making biology programmable - opening up possibilities that were previously confined to the realm of science fiction. View the original White Paper by clicking or scanning the QR Code. Read the full article
#AIFramework#BiologicalProgramming#BiomolecularEngineering#ComputationalBiology#DrugDiscoveryAI#EvolutionaryComputing#GenerativeBiology#MachineLearningBiology#MolecularDesign#ProteinDesignAI#ProteinEngineering#ProteinFolding#ScientificComputing#SyntheticBiology#TherapeuticProteinDesign
0 notes
Text
Unnatural Amino Acids: Beyond the Basics - Unlocking New Research Frontiers
Unnatural amino acids are revolutionizing research and bioengineering. Explore their applications in protein engineering, biomaterial design, and drug discovery. Discover the top players in the market and the exciting future of unnatural amino acids
The Unnatural Amino Acids Market: Pushing Boundaries in Bioengineering The unnatural amino acids market is a rapidly evolving field with vast potential in various scientific and technological applications. Unnatural amino acids are molecules structurally similar to the 20 naturally occurring amino acids that form the building blocks of proteins. However, unnatural amino acids possess unique…

View On WordPress
#Bioengineering#Biomaterials#Bioorthogonal#Biotechnology#DrugDiscovery#FutureOfScience#NextGenMaterials#ProteinEngineering#ScientificResearch#UnnaturalAminoAcids
0 notes
Text
Engineering L-threonine Aldolase for Droxidopa #sciencefather #LThreonineAldolase
Engineering L-threonine aldolase offers a sustainable biocatalytic route for synthesizing Droxidopa, a therapeutic agent for Parkinson’s-related conditions. By enhancing enzyme activity and stereoselectivity, this approach enables efficient, green production of chiral amino alcohols for pharmaceutical applications. Visit Our Website : http://biotechnologyscientist.com Contact Us : [email protected] Nomination Link : https://biotechnologyscientist.com/award-nomination/?ecategory=Awards&rcategory=Awardee #LThreonineAldolase #Droxidopa #Biocatalysis #EnzymeEngineering #SyntheticBiology #GreenChemistry #ChiralSynthesis #ProteinEngineering #PharmaceuticalBiotech #Biotechnology #DrugDevelopment #ParkinsonsTreatment #MetabolicEngineering #EnzymaticSynthesis #MedicinalChemistry #SustainablePharma #Bioengineering #CatalystDesign #Biocatalyst #AminoAlcohols
0 notes
Text
“The Future of Biomanufacturing: Insights on Recombinant Proteins (2024-2033)”
Recombinant proteins manufacturing services are transforming the landscape of biopharmaceuticals, enabling the scalable production of high-quality proteins for research, diagnostics, and therapeutics. These services are crucial in developing cutting-edge treatments like monoclonal antibodies, vaccines, and gene therapies, offering precision and consistency. With demand for biologics surging, recombinant proteins are at the forefront of innovation, driving advancements in personalized medicine and accelerating breakthroughs in drug discovery.
#RecombinantProteins #BiopharmaInnovation #ProteinEngineering #GeneTherapy #MonoclonalAntibodies #BiologicsBoom #PrecisionMedicine #DrugDiscovery #BiotechRevolution #ProteinManufacturing
0 notes
Text
"Harnessing Technology: Trends and Insights in Recombinant Proteins Manufacturing Services (2024-2033)"
In the rapidly evolving biotech landscape, recombinant proteins are at the forefront of innovation, driving advancements in therapeutics, diagnostics, and research. With cutting-edge manufacturing services, companies can now produce high-quality, scalable, and cost-effective recombinant proteins, tailored to meet the exact needs of the modern medical and scientific communities. As demand for precision medicine and targeted therapies continues to grow, partnering with top-tier recombinant protein manufacturing services is essential to stay ahead in this dynamic industry.
#BiotechInnovation #RecombinantProteins #PrecisionMedicine #BiotechTrends #FutureOfMedicine #BioManufacturing #ProteinEngineering #HealthcareRevolution #BiotechServices #GeneticEngineering
0 notes
Text
"From Lab to Market: Navigating Recombinant Proteins Manufacturing Services!"
Recombinant proteins manufacturing services are redefining the biotech landscape, offering precise, scalable solutions for producing high-quality proteins used in research, diagnostics, and therapeutics. By utilizing cutting-edge technology, these services provide custom proteins with enhanced purity and consistency, fueling advancements in drug development, vaccine production, and personalized medicine. As industries push for innovation, recombinant proteins are at the heart of breakthrough therapies, ensuring faster, more effective solutions in healthcare and beyond.
#RecombinantProteins #BiotechInnovation #ProteinManufacturing #FutureOfMedicine #BioTechBreakthrough #CustomProteins #HealthcareInnovation #Biopharma #NextGenTherapies #VaccineDevelopment #PrecisionMedicine #ProteinEngineering #DrugDevelopment #TherapeuticProteins #PharmaTech #MedicalBreakthroughs #BioManufacturing #ClinicalResearch #Biotech2024 #HealthTechRevolution #CuttingEdgeScience
0 notes
Text

Enjoy Personalized Online BIOL5373M Protein Engineering Laboratory Project Assignment Help At The Most Affordable Price!! Download unique solutions for Diploma course!! Order Instant Solution on WhatsApp: +44 141 628 6080!!
#BIOL5373M #ProteinEngineering #LaboratoryProject #AssignmentHelp #Solution #AssessmentHelp #top10writingservice #UKtopwritingservice #OnlineTutor #AskTutor #LeedsAssessmentWriting #HND #UK #BTEC
0 notes
Text

📌 Breaking News! 🌟 Magnoreach has shared a must-read article on how AI-Driven Systems Create Custom Proteins with Precision. 🧬💻
Explore the cutting-edge field of artificial intelligence in protein engineering. Don't miss this fascinating read! Read it here: AI Systems Can Now Generate Novel Proteins
2 notes
·
View notes
Text

Exams are getting closer. I'm drinking coffe and preparing for an oral answer about Y2H. Yeast two-hybrid system is a method for identifying interactions between proteins.
#science#student#biotech#biotechnology#study#studyblr#biology#goodday#proteins#proteinengineering#starbucks#starbuckscoffee#productivity#sciencegirl#hardworking
27 notes
·
View notes
Photo

Chinese Hamster Ovary Cells (#CHOcells) are a great choice for the industrial production of recombinant protein therapeutics.
If you're looking for CHO cells, get in touch with #Kosheeka at [email protected] or call us at +91-96544321400
#chinesehamsterovarycells#antibody#geneexpression#monoclonalantibodies#Proteinengineering#recombinantprotein#ProteinExpression#bioengineering#antibodydevelopment#biotechnology#cellculture#research
0 notes
Text
Evolutionary Scale Model 3
ESM3: The AI That's Revolutionizing Protein Design
The Minds Behind the Innovation
Evolutionary Scale, a groundbreaking AI research lab that emerged in 2023, stands at the forefront of computational biology. The team behind ESM3 brings impressive credentials, having previously developed ESM1 at Meta (Facebook). This wasn't just a new company entering the field - it was a team of veteran researchers and engineers who had already proven their expertise in protein language models. Their vision caught the attention of major investors, securing $142 million in funding, demonstrating the technology industry's confidence in their approach to making biology programmable through AI. A New Frontier in Protein Engineering ESM3 represents a fundamental leap forward in artificial intelligence for biological design. At its core, it's a generative AI model trained on an astronomical 2.78 billion proteins - but calling it just a protein database would be like calling ChatGPT a dictionary. ESM3 understands proteins in three crucial dimensions: - Sequence: The basic "code" of amino acids that make up proteins - Structure: The complex 3D shapes that proteins fold into - Function: The actual roles these proteins play in biological systems What sets ESM3 apart is its ability to not just analyze existing proteins but to create entirely new ones from scratch. This generative capability allows it to design proteins that have never existed in nature but serve specific desired functions. The Timeline of Innovation The journey of ESM3 began with its predecessors at Meta, where the team developed the original ESM1 model. After establishing Evolutionary Scale in 2023, the team worked to push the boundaries of what was possible in protein design. The result was ESM3, which emerged as a powerful tool for protein engineering, capable of compressing what would take nature hundreds of millions of years of evolution into months of computational work. From ESM3 Lab to Real World ESM3's impact extends far beyond the laboratory. The technology is being deployed across multiple platforms through partnerships with AWS and NVIDIA, making it accessible to researchers and companies worldwide. The framework operates in both academic and commercial settings, with applications ranging from pharmaceutical laboratories to environmental research facilities. More importantly, Evolutionary Scale has committed to democratizing access to this technology through: - An open-source version for researchers - A closed beta API for specific applications - Cloud-based deployment options - Partnerships with major technology providers The Transformative Potential The development of ESM3 addresses several crucial needs in modern science and technology: Medical Breakthroughs ESM3's ability to design precise, targeted proteins opens new possibilities in drug development and personalized medicine. Rather than relying on traditional trial-and-error methods, researchers can now design proteins specifically tailored to target disease pathways with unprecedented precision. Environmental Solutions The technology's potential extends to environmental challenges, with the ability to design proteins that could efficiently capture carbon dioxide or break down pollutants. This could provide new tools in the fight against climate change and environmental degradation. Materials Science Innovation ESM3's capabilities in protein design extend to the development of new biomaterials, potentially revolutionizing fields from sustainable packaging to advanced electronics. Accelerating Scientific Discovery By compressing evolutionary timescales from millions of years to months, ESM3 dramatically accelerates the pace of biological innovation. This acceleration could lead to breakthroughs in fields that traditionally required extensive trial-and-error experimentation.
The Proof: The GFP Breakthrough
The team demonstrated ESM3's capabilities through a remarkable achievement - creating a novel Green Fluorescent Protein (GFP) that had never existed in nature. While GFPs naturally occur in jellyfish and have been used by scientists as cellular markers, ESM3 designed an entirely new version with unique properties. This achievement effectively demonstrated the model's ability to not just understand but to innovate in protein design.
Looking Ahead: The Future of Biological Engineering
ESM3 represents more than just a technological advancement - it's a fundamental shift in how we approach biological engineering. As researchers and companies begin to explore its capabilities, we're likely to see applications that we haven't even imagined yet. The technology raises important questions about the future of biological engineering and our ability to design life's building blocks. While the potential benefits are enormous, Evolutionary Scale maintains a commitment to responsible development, ensuring that this powerful technology is used ethically and safely. As we stand at this frontier of biological engineering, ESM3 marks a significant step toward making biology programmable - opening up possibilities that were previously confined to the realm of science fiction. Whether it's developing new treatments for diseases, creating sustainable materials, or finding solutions to environmental challenges, ESM3 is poised to play a crucial role in shaping our technological future.
Conclusion
ESM3 represents a convergence of artificial intelligence and biological understanding that could fundamentally transform how we approach some of humanity's biggest challenges. As the technology continues to develop and more researchers and organizations gain access to its capabilities, we're likely to see an acceleration in biological innovation that could reshape multiple industries and scientific fields. Read the full article
#AIFramework#BiologicalProgramming#BiomolecularEngineering#ComputationalBiology#DrugDiscoveryAI#EvolutionaryComputing#GenerativeBiology#MachineLearningBiology#MolecularDesign#ProteinDesignAI#ProteinEngineering#ProteinFolding#ScientificComputing#SyntheticBiology#TherapeuticProteinDesign
0 notes
Text
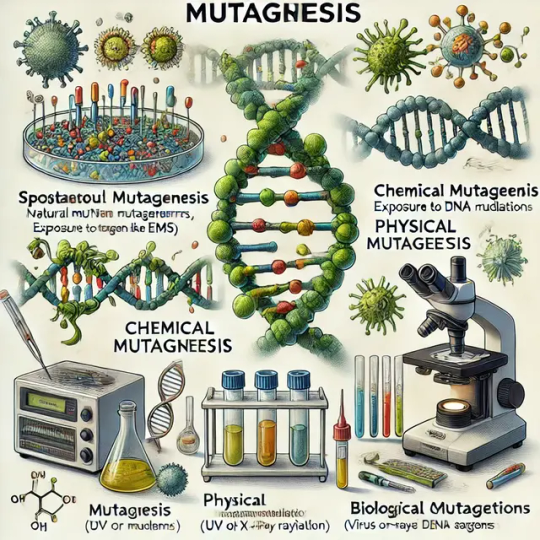
Mutagenesis is the process of inducing genetic mutations within an organism's DNA, either naturally or artificially. It plays a crucial role in biotechnology, genetics, and molecular biology, enabling scientists to study gene function, develop disease models, and engineer desirable traits in organisms.
Types of Mutagenesis:
Spontaneous Mutagenesis – Naturally occurring mutations due to DNA replication errors, environmental factors, or transposable elements.
Induced Mutagenesis – Mutations triggered by external agents, categorized as:
Chemical Mutagenesis – Uses chemicals like EMS (ethyl methanesulfonate) or nitrosoguanidine to introduce mutations.
Physical Mutagenesis – Uses radiation (UV, X-rays, gamma rays) to cause DNA damage.
Biological Mutagenesis – Utilizes mobile genetic elements, viruses, or transposons to alter DNA.
Biotechnology Scientist Awards
Visit Our Website : http://biotechnologyscientist.com
Contact Us : [email protected]
Nomination Link : https://biotechnologyscientist.com/member-submission/?ecategory=Membership&rcategory=Member…
#sciencefather#researchawards#Scientist#Scholar#Researcher #Mutagenesis #GeneticMutation #SiteDirectedMutagenesis #RandomMutagenesis #GeneEditing #CRISPR #DNARepair #GenomeEngineering #Biotechnology #ProteinEngineering #MolecularBiology #GeneticModification #GenomeEditing #FunctionalGenomics #RadiationMutagenesis #ChemicalMutagenesis #Bioengineering #MutationBreeding #SyntheticBiology #GeneticEnhancement #ExonShuffling #TransposonMutagenesis #GenomeManipulation #MolecularGenetics #BiotechInnovation
👉 Don’t forget to like, share, and subscribe for more exciting content!
Get Connected Here: =============
Facebook : https://www.facebook.com/profile.php?id=61572562140976
Twitter : https://x.com/DiyaLyra34020
Tumblr : https://www.tumblr.com/blog/biotechscientist
Blogger: https://www.blogger.com/u/1/blog/posts/3420909576767698629
Linked in : https://www.linkedin.com/in/biotechnology-scientist-117866349/
Pinterest : https://in.pinterest.com/biotechnologyscientist/
0 notes