#US Biosimilar Market share
Text
Biosimilars Unleashed: The Future of Healthcare in the US
Buy Now
What is the Size of US Biosimilar Industry?
US Biosimilar Market is expected to grow at a CAGR of ~ % between 2017-2022 and is expected to reach ~USD Bn by 2028. Biosimilars enhance patient access to essential treatments, especially in therapies with high demand, like oncology, by providing more affordable options. Additionally, Growing evidence of biosimilars' comparable efficacy and safety fosters trust among healthcare professionals, driving adoption.

Click here to Download a sample Report
Biosimilars offer cost savings compared to originator biologics, addressing the need for affordable healthcare solutions in the face of rising medical costs. Favorable regulatory frameworks, like the BPCIA, streamline biosimilar approval processes, encouraging manufacturers to invest in development.
Furthermore, The expiration of patents for numerous reference biologics creates opportunities for biosimilar entry, leading to increased competition and market expansion. Pharmaceutical companies are investing in biosimilar R&D and production, expanding the pipeline and market availability. Supportive healthcare policies and reimbursement models incentivize biosimilar adoption, creating a favorable environment for market growth.
US Biosimilar Market by drug class
The US Biosimilar market is segmented by Monoclonal Antibodies, Recombinant Hormones, Immunomodulators, Anti-inflammatory agents and Others. Based on drug class, Monoclonal Antibodies segment dominates the bio similar market in 2022.
Monoclonal antibodies have diverse applications across various therapeutic areas. From cancer treatment to autoimmune diseases, biosimilar Mabs addressed a wide range of medical needs, leading to a broad and growing market. Biosimilars, with their potential for cost savings while maintaining comparable efficacy and safety, gained significant attention as viable alternatives.
US Biosimilar Market by application
In US Biosimilar market, they are segmented by application into Oncology, Blood disorders, Chronic diseases and autoimmune conditions and Others. On the basis of application, Oncology segment was the dominant in 2022.
The increasing prevalence of cancer and the high cost of traditional biologics used in oncology treatment have created a strong incentive for the adoption of biosimilars. Biosimilars offer the potential to provide similar therapeutic outcomes at a lower cost, making them an attractive option for both healthcare providers and patients.
Additionally, the rigorous clinical trials and regulatory processes that biosimilars undergo to gain approval provide reassurance to healthcare professionals and patients regarding their safety and efficacy. This has led to increased acceptance and adoption of biosimilars in oncology.
US Biosimilar by Region
The US Biosimilar market is segmented by Region into North, East, West and South. In 2022, the dominance region is North region in US Biosimilar market.
The North region benefits from a concentration of healthcare providers and academic institutions that are at the forefront of adopting and integrating biosimilars into their treatment protocols. These institutions are more likely to have the expertise to evaluate and incorporate biosimilars effectively, driving their adoption among healthcare professionals and patients.
Click here to Download a Custom Report
Competition Scenario in US Biosimilar Market
The US biosimilar market has witnessed an evolving competitive landscape, with several key players competing for market share. Prominent pharmaceutical companies such as Amgen, Pfizer, Sandoz (Novartis), and Boehringer Ingelheim have been actively involved in developing and marketing biosimilar products. These established players have utilized their expertise in biologics and significant resources to navigate the regulatory landscape and compete effectively.
The competition in the US biosimilar market is characterized by a balance between established pharmaceutical giants and emerging biotech companies. While the major players possess the advantage of resources and experience, smaller biotech firms are also contributing to the market with innovative approaches and niche biosimilar offerings.
What is the Expected Future Outlook for the Overall US Biosimilar Market?
The US Biosimilar market was valued at USD ~Million in 2022 and is anticipated to reach USD ~ Billion by the end of 2028, witnessing a CAGR of ~% during the forecast period 2022- 2028. The US biosimilar market is likely to experience significant growth in the coming years, driven by several factors. Biosimilars are biologic drugs that are highly similar to already approved reference biologics. They offer potential cost savings, increased competition, and improved patient access to crucial treatments.
Firstly, the regulatory environment is becoming more favorable for biosimilars. The Biologics Price Competition and Innovation Act (BPCIA) established a pathway for biosimilar approval in the US, allowing for a smoother regulatory process. As more biosimilars receive approval, competition in the market is expected to intensify.
Secondly, patents for several blockbuster biologics are expiring or have already expired. This creates opportunities for biosimilar manufacturers to enter the market with more affordable alternatives, offering healthcare systems and patients a choice in treatment options.
Thirdly, as healthcare costs continue to rise, biosimilars present an attractive solution for reducing expenses. Their potential to offer cost savings without compromising therapeutic efficacy could lead to increased adoption by healthcare providers, insurers, and patients alike.
Physician and patient education are crucial, as misconceptions about biosimilars' safety and effectiveness might hinder their adoption. Additionally, legal and market access barriers, including patent litigation and complex distribution systems, could slow down the growth of the biosimilar market.
The biosimilar market witness consolidation as larger pharmaceutical companies acquire or partner with smaller biotech firms to bolster their biosimilar portfolios. This will lead to more resources being devoted to biosimilar development and marketing. Changes in healthcare policies, such as reimbursement models and value-based care initiatives, can influence the biosimilar market's growth. Favourable policies that incentivize biosimilar adoption drives their market growth.
#US Biosimilar Market#US Biosimilar Industry#US Biosimilar Sector#United States Biosimilar Market#US Biosimilar Market forecast#US Biosimilar Market analysis#US Biosimilar Market trends#US Biosimilar Market share#US Biosimilar Market key players#US Biosimilar Market revenue#US Biosimilar Market growth#Monoclonal Antibodies in biosimilar market US#Recombinant Hormones in biosimilar industry US#Oncology in bio similar market US#Blood disorders in biosimilar market US#Research institutes in Biosimilar market US#US similar biotherapeutics products market#Hospitals in Biosimilar market US#Investors in Biosimilar market US#US comparable biologics products industry#US recombinant biosimilars industry#US replicate biologics sector#US analog biologics market#US homologous biologics market#US oncology biosimilar market#US immunology biosimilar sector#US insulin biosimilar industry#US Generics Biologics market challenges#US leading Biosimilar drug providers#US leading Biosimilar drug manufacturers
0 notes
Text
An Overview of Protein A Resin: Applications and Benefits in Bioprocessing
The global protein A resin market size is expected to reach USD 4.12 Billion in 2032 and register a steady revenue CAGR of 11.4%during the forecast period, according to latest analysis by Emergen Research. Development and launch of highly necessary biosimilar by key biopharmaceutical companies is a key factor driving market revenue growth in. Protein A resins are used for purification and fragmentation of immunoglobulins from biological fluids & cell culture media, immunoprecipitation of proteins, and antigens. For instance, on 13 June 2022, Bio-Rad Laboratories, Inc., a pioneer in life science research as well as clinical diagnostic items introduced CHT-prepacked Foresight Pro Columns. These columns are designed to facilitate downstream process-scale chromatography application areas across various stages of biological drug development and manufacturing.
The recent advancements in the Protein A Resin industry and trends driving the growth of the market. It is an investigative study covering analysis of market drivers, restraints, challenges, threats, and growth prospects in the global Protein A Resin market. The global Protein A Resin market report is a methodical research of the Protein A Resin market done by extensive primary and secondary research. The fundamental purpose of the Protein A Resin market report is to offer an accurate and strategic analysis of the Protein A Resin business sphere.
Get Download Pdf Sample Copy of this Report@ https://www.emergenresearch.com/request-sample/1724
Competitive Terrain:
The global Protein A Resin industry is highly consolidated owing to the presence of renowned companies operating across several international and local segments of the market. These players dominate the industry in terms of their strong geographical reach and a large number of production facilities. The companies are intensely competitive against one another and excel in their individual technological capabilities, as well as product development, innovation, and product pricing strategies.
The leading market contenders listed in the report are:
GE HealthCare, Merck KGaA, Repligen Corporation, Thermo Fisher Scientific Inc., Tosoh Corporation, Purolite, Novasep Holding SAS, Agilent Technologies, Inc., GenScript, and PerkinElmer Inc
Key market aspects studied in the report:
Market Scope: The report explains the scope of various commercial possibilities in the global Protein A Resin market over the upcoming years. The estimated revenue build-up over the forecast years has been included in the report. The report analyzes the key market segments and sub-segments and provides deep insights into the market to assist readers with the formulation of lucrative strategies for business expansion.
Competitive Outlook: The leading companies operating in the Protein A Resin market have been enumerated in this report. This section of the report lays emphasis on the geographical reach and production facilities of these companies. To get ahead of their rivals, the leading players are focusing more on offering products at competitive prices, according to our analysts.
Report Objective: The primary objective of this report is to provide the manufacturers, distributors, suppliers, and buyers engaged in this sector with access to a deeper and improved understanding of the global Protein A Resin market.
Emergen Research is Offering Limited Time Discount (Grab a Copy at Discounted Price Now)@ https://www.emergenresearch.com/request-discount/1724
Market Segmentations of the Protein A Resin Market
This market is segmented based on Types, Applications, and Regions. The growth of each segment provides accurate forecasts related to production and sales by Types and Applications, in terms of volume and value for the period between 2022 and 2030. This analysis can help readers looking to expand their business by targeting emerging and niche markets. Market share data is given on both global and regional levels. Regions covered in the report are North America, Europe, Asia Pacific, Latin America, and Middle East & Africa. Research analysts assess the market positions of the leading competitors and provide competitive analysis for each company. For this study, this report segments the global Protein A Resin market on the basis of product, application, and region:
Segments Covered in this report are:
Product Outlook (Revenue, USD Billion; 2019–2032)
Agarose-based Protein A
Glass/Silica-based Protein A
Organic Polymer-based Protein A
Type Outlook (Revenue, USD Billion; 2019–2032)
Recombinant Protein A
Natural Protein A
Application Outlook (Revenue, USD Billion; 2019–2032)
Antibody Purification
Immunoprecipitation
Browse Full Report Description + Research Methodology + Table of Content + Infographics@ https://www.emergenresearch.com/industry-report/protein-a-resin-market
Major Geographies Analyzed in the Report:
North America (U.S., Canada)
Europe (U.K., Italy, Germany, France, Rest of EU)
Asia Pacific (India, Japan, China, South Korea, Australia, Rest of APAC)
Latin America (Chile, Brazil, Argentina, Rest of Latin America)
Middle East & Africa (Saudi Arabia, U.A.E., South Africa, Rest of MEA)
ToC of the report:
Chapter 1: Market overview and scope
Chapter 2: Market outlook
Chapter 3: Impact analysis of COVID-19 pandemic
Chapter 4: Competitive Landscape
Chapter 5: Drivers, Constraints, Opportunities, Limitations
Chapter 6: Key manufacturers of the industry
Chapter 7: Regional analysis
Chapter 8: Market segmentation based on type applications
Chapter 9: Current and Future Trends
Request Customization as per your specific requirement@ https://www.emergenresearch.com/request-for-customization/1724
About Us:
Emergen Research is a market research and consulting company that provides syndicated research reports, customized research reports, and consulting services. Our solutions purely focus on your purpose to locate, target, and analyse consumer behavior shifts across demographics, across industries, and help clients make smarter business decisions. We offer market intelligence studies ensuring relevant and fact-based research across multiple industries, including Healthcare, Touch Points, Chemicals, Types, and Energy. We consistently update our research offerings to ensure our clients are aware of the latest trends existent in the market. Emergen Research has a strong base of experienced analysts from varied areas of expertise. Our industry experience and ability to develop a concrete solution to any research problems provides our clients with the ability to secure an edge over their respective competitors.
Contact Us:
Eric Lee
Corporate Sales Specialist
Emergen Research | Web: www.emergenresearch.com
Direct Line: +1 (604) 757-9756
E-mail: [email protected]
Visit for More Insights: https://www.emergenresearch.com/insights
Explore Our Custom Intelligence services | Growth Consulting Services
Trending Titles: Geocell Market | Pancreatic Cancer Treatment Market
Latest Report: Ceramic Tiles Market | Life Science Analytics Market
0 notes
Text
Biological Safety Testing Products and Services Market 2024 | Upcoming Trend in Biological Safety Testing Products and Services Industry by an Expert
The global biological safety testing products and services market is on a robust growth trajectory, valued at $4.42 billion in 2023 and projected to reach $10.51 billion by 2032. This remarkable growth reflects a compound annual growth rate (CAGR) of 10.10% over the forecast period from 2024 to 2032, driven by the increasing demand for safety and efficacy testing across the biopharmaceutical and healthcare sectors.
Biological safety testing is essential for ensuring that medical products, including pharmaceuticals, vaccines, and medical devices, meet stringent safety and efficacy standards before reaching the market. The market encompasses a wide range of testing services, including sterility testing, endotoxin testing, and biocompatibility assessments, all critical for regulatory compliance.
Key Market Drivers
Increasing Biopharmaceutical R&D Activities: The rising investment in biopharmaceutical research and development is a significant factor propelling the market. As the industry focuses on innovative therapies, including monoclonal antibodies, gene therapies, and cell therapies, the need for rigorous biological safety testing becomes paramount. These testing services help ensure that new products are safe for human use, fostering trust in the healthcare system.
Regulatory Compliance and Safety Standards: Stringent regulatory requirements from agencies such as the U.S. Food and Drug Administration (FDA), European Medicines Agency (EMA), and other global health authorities are driving the adoption of biological safety testing services. Compliance with these regulations is critical for companies looking to launch new medical products, creating a steady demand for testing services that ensure product safety and efficacy.
Growing Concerns About Contamination and Quality Assurance: The increasing prevalence of product recalls due to contamination and safety issues has heightened awareness about the importance of biological safety testing. Companies are now more vigilant in their quality assurance processes, recognizing that thorough testing is essential to maintain product integrity and safeguard public health.
Expansion of the Healthcare Sector: The ongoing expansion of the healthcare sector, particularly in emerging markets, is creating new opportunities for biological safety testing services. With the growth of healthcare facilities and the increasing production of biologics and biosimilars, the demand for reliable testing solutions is expected to rise significantly.
Access Free Sample Report: https://www.snsinsider.com/sample-request/4483
Challenges and Opportunities
While the market shows strong potential, it faces challenges such as the high cost of testing services and the need for specialized expertise. However, advancements in technology, including automation and digitalization, are likely to streamline testing processes, reduce costs, and enhance the accuracy of results.
Moreover, the increasing adoption of 3D cell culture systems and organ-on-a-chip technologies offers opportunities for innovative testing solutions. These advancements are expected to improve the efficiency and effectiveness of biological safety testing, providing companies with the tools they need to ensure product safety.
Regional Insights
North America holds the largest share of the biological safety testing market, driven by the presence of leading biopharmaceutical companies, advanced research facilities, and stringent regulatory standards. Europe follows closely, with significant investments in healthcare and biotechnology sectors. The Asia-Pacific region is expected to experience the highest growth rate during the forecast period, supported by the expansion of healthcare infrastructure and increasing research activities in countries such as China, India, and Japan.
Future Outlook
As the demand for innovative biopharmaceutical products continues to rise, the biological safety testing products and services market is set for significant growth. With a projected CAGR of 10.10% from 2024 to 2032, the market is poised to see substantial advancements in testing technologies, helping to meet the increasing demand for safety and efficacy in healthcare products.
In conclusion, the biological safety testing products and services market is entering a dynamic phase of growth, with a valuation expected to rise from $4.42 billion in 2023 to $10.51 billion by 2032. This growth is driven by regulatory compliance, the expansion of biopharmaceutical R&D, and the need for rigorous quality assurance in an increasingly complex healthcare landscape
Other Trending Reports
Dental Suction Systems Market Size
Cosmeceuticals Market Size
Cell Therapy Market Size
Growth Hormone Deficiency Market Size
0 notes
Text
Prefilled Syringes: A Game-Changer for Drug Delivery

Introduction
The Prefilled Syringes Market is experiencing rapid growth as healthcare providers and patients increasingly prefer prefilled syringes over traditional vial-and-syringe methods. Prefilled syringes offer convenience, accuracy, and safety, reducing the risk of dosing errors and contamination. They are used for a variety of therapeutic applications, including vaccines, biologics, and anticoagulants. The market is expanding globally, driven by increasing chronic disease prevalence, technological advancements in injectable drugs, and the growing demand for self-administration.
Market Dynamics
Drivers
Growing Prevalence of Chronic Diseases: The rise in conditions such as diabetes, cardiovascular diseases, and autoimmune disorders has increased the demand for injectable medications, fueling the growth of the prefilled syringes market.
Patient Convenience and Safety: Prefilled syringes offer a more convenient and safer alternative to traditional methods, especially for patients requiring frequent injections.
Challenges
High Production Costs: The manufacturing of prefilled syringes requires stringent quality control and high precision, leading to increased production costs.
Regulatory Compliance: Prefilled syringes must meet rigorous regulatory standards, particularly for biological drugs, creating challenges for manufacturers.
Opportunities
Biologics and Biosimilars: The growing market for biologic drugs presents significant opportunities for the prefilled syringes market, as these drugs often require injectable delivery.
Homecare and Self-Administration: With the increasing demand for home healthcare solutions, prefilled syringes are becoming more popular for self-administration of medications, offering a major growth avenue.
Regional Analysis
North America: The largest market for prefilled syringes, driven by the high prevalence of chronic diseases and advanced healthcare infrastructure.
Europe: Europe is a major market, with countries like Germany and the U.K. leading in the adoption of biologics and injectable therapies.
Asia-Pacific: The region is witnessing rapid growth due to rising healthcare expenditure, increasing chronic disease burden, and expanding access to healthcare in countries like China and India.
Sample pages of Report: https://www.infiniumglobalresearch.com/form/193?name=Sample
Market Segmentation
By Material:
Glass Syringes: Traditionally dominant, but gradually being replaced by plastic due to concerns about breakage and contamination.
Plastic Syringes: Gaining popularity due to their lightweight and durable nature.
By Application:
Diabetes: Prefilled syringes are widely used for insulin administration.
Vaccines: Increasingly used for vaccine delivery due to their convenience and precision.
Competitive Landscape
How much share do large players hold? Companies like BD (Becton, Dickinson and Company), Gerresheimer, and SCHOTT dominate the market, holding a significant share due to their established presence and advanced manufacturing capabilities.
Do big players control the price? Yes, large players with sophisticated manufacturing processes and partnerships with pharmaceutical companies have significant control over pricing in the market.
Do small and mid-size companies challenge the large companies domestically? While smaller companies are entering the market with niche products and regional focus, they face challenges in competing with the scale and pricing power of larger players.
Report Overview : https://www.infiniumglobalresearch.com/market-reports/global-prefilled-syringes-market
Future Outlook
Does new product development really help companies? Yes, innovations in materials, such as plastic prefilled syringes, and the development of safety features like needle shields have helped companies gain market share.
Do sustainable products hold strong customers' minds? Sustainability is becoming increasingly important, with healthcare providers and patients showing a preference for eco-friendly packaging and materials in prefilled syringes.
Conclusion
The prefilled syringes market is poised for substantial growth, driven by increasing chronic disease prevalence, the rise of biologics, and the growing demand for patient convenience. While challenges around cost and regulatory compliance remain, innovations in materials and safety features are expected to drive the market forward.
0 notes
Text
Prefilled Syringes Market worth $13.1 billion by 2030 driven by Rising Chronic Illness Rates | MarketsandMarkets™
The global Prefilled Syringes Market is expected to grow from USD 7.1 billion in 2024 to USD 13.1 billion by 2030, at a CAGR of 10.8%. Prefilled syringes are a convenient and precise medical device for delivering single doses of medication, reducing drug waste and extending product lifespan. They are increasingly adopted due to rising chronic illnesses, demand for effective drug delivery, and regulatory support for safer injection methods. Commonly used for biologics, vaccines, and other therapeutic products, these syringes enhance patient safety by minimizing errors and improving dosing accuracy. The market is expanding due to the prevalence of chronic diseases and the growing trend towards self-administration. However, challenges such as product recalls and competition from alternative drug delivery methods may impact growth. The market is driven by rising demand for biologics and biosimilars, with glass and single-chamber syringes expected to dominate. North America leads the market due to its advanced healthcare infrastructure and high demand for precise drug delivery solutions. Key players include BD, Gerresheimer AG, SCHOTT, and West Pharmaceutical Services, among others.

Download PDF Brochure:
Prefilled Syringes Market Dynamics
Drivers
· Rising target disease population
· Rapid growth in generic products
· Rising adoption of self-medication and digitalization
· Affordable cost with increased efficiency of prefilled syringes
· Technological advancements
Restraints
· Dearth of prefilled syringes with integrated safety features
Opportunities
· Growing healthcare infrastructure across emerging markets
· Surge of biologics and biosimilars in biopharma industry
· Increasing adoption of wearable drug delivery technologies
Challenges
· Availability of cheaper alternatives
· Challenges associated with manufacturing of prefilled syringes
Key Market Players
Becton, Dickinson and Company (US), Gerresheimer (Germany), SCHOTT AG (Germany), West Pharmaceutical Services, Inc. (US), Baxter International Inc (US), Ompi (Italy), Catalent, Inc. (US), Weigao Group (China), Vetter Pharma International GmbH (Germany), Nipro Corporation (Japan), Elcam Medical (Israel), YPSOMED (Switzerland), Oval Medical Technologies (UK), SHL Medical AG (Switzerland), Terumo (Japan).
Request Sample Pages
North America accounted for the largest market share of the global prefilled syringes industry, by region in the forecast period.
The pre-filled syringe market is anticipated to be dominated by the North American region due to a number of factors, including an advanced healthcare infrastructure, a high prevalence of chronic diseases, and a focus on patient safety and technological innovation. Its dominant position in the market is also a result of the region’s well-established pharmaceutical industry and rising demand for practical and precise drug delivery solutions. Furthermore, continued R&D expenditures and favorable regulatory environments in North America contribute to the expansion and use of pre-filled syringes.
Recent Developments
· In October 2018, Becton, Dickinson and Company launched the BD Intevi 1mL two-step disposable autoinjector.
· In July 2020, Becton, Dickinson and Company entered into an partnership with Biomedical Advanced Research and Development Authority (BARDA) (US)
· In July 2018, Becton, Dickinson and Company acquired Teva Medical Inc. (US)
Content Source:
0 notes
Text
The Generic Sterile Injectables Market poised for strong growth driven by increasing demand for affordable healthcare
The generic sterile injectables market encompasses pharmaceutical formulations such as vials, ampoules, bottles, syringes and bags, which are administered parenterally into the body for treatments. They offer effective and affordable alternatives to branded sterile injectable drugs across therapeutic areas including oncology, cardiovascular diseases, infectious diseases and autoimmune diseases. The growing prevalence of chronic diseases and increasing healthcare expenditure have boosted the demand for generic sterile injectables globally.
The global generic sterile injectables market is estimated to be valued at US$ 46.33 Bn in 2024 and is expected to exhibit a CAGR of 10% over the forecast period 2024 to 2031.
Key Takeaways
Key players operating in the generic sterile injectables market are Baxter International Inc., AstraZeneca plc, Merck and Co., Inc., Pfizer Inc., Fresenius Kabi, Novartis International AG, Teva Pharmaceuticals, Hikma Pharmaceuticals, Dr. Reddy's Laboratory, Mylan N.V., Sun Pharmaceutical Industries Ltd. The key players dominate the market with their wide array of products in various dosages.
The increasing prevalence of chronic diseases and aging population has amplified the demand for affordable healthcare solutions. The rising healthcare costs have prompted patients and providers to shift towards cost-effective generic injectable drugs from branded equivalents. This has accelerated the growth of the global generic sterile injectables market.
With rising healthcare expenditures, healthcare providers are boosting investments in emerging markets of Asia Pacific, Latin America, Middle East and Africa for expansion of their generic sterile injectables portfolio. Generic Sterile Injectables Market Trends is expected to drive during the forecast period.
Market Key Trends
Increased Research & Development and manufacturing capabilities of emerging players: With growing demand for affordable and effective biologics, emerging players are investing significantly in R&D and expanding their sterile injectables manufacturing infrastructure. This has led to increased competition and entry of more affordable biologics in the market.
Porter’s Analysis
Threat of new entrants: Low barriers to entry make it easy for new companies to enter the market. However, regulations and requirement of high capital to set-up sterile facilities pose challenges.
Bargaining power of buyers: Large group purchasing organizations and hospital networks have significant influence on prices. However, need for essential medicines keeps bargaining power in check.
Bargaining power of suppliers: Few major global players supply key starting materials and APIs. However, potential for forward integration limits suppliers' bargaining power.
Threat of new substitutes: Limited threat as generics have few major therapeutic substitutes. Biosimilars pose a potential long-term threat in certain disease segments.
Competitive rivalry: Intense competition on pricing and new product development. Major players compete by improving quality, reliability of supply and enhancing portfolios. Frequent litigation and regulatory issues also impact competition.
The United States dominates the Generic Sterile Injectables Market Regional Analysis accounting for over 40% revenue share in 2024. Strong payer system, sizable healthcare spending and increasing generic adoption to contain costs drive high growth.
China sterile injectables market is projected to grow at over 12% till 2031, making it the fastest growing regional market. This can be attributed to rising living standards, healthcare reforms focusing on essential medicines and initiatives to expand domestic sterile manufacturing capabilities.
Get more insights on Generic Sterile Injectables Market
Get More Insights—Access the Report in the Language that Resonates with You
French
German
Italian
Russian
Japanese
Chinese
Korean
Portuguese
Alice Mutum is a seasoned senior content editor at Coherent Market Insights, leveraging extensive expertise gained from her previous role as a content writer. With seven years in content development, Alice masterfully employs SEO best practices and cutting-edge digital marketing strategies to craft high-ranking, impactful content. As an editor, she meticulously ensures flawless grammar and punctuation, precise data accuracy, and perfect alignment with audience needs in every research report. Alice's dedication to excellence and her strategic approach to content make her an invaluable asset in the world of market insights.
(LinkedIn: www.linkedin.com/in/alice-mutum-3b247b137 )

#Coherent Market Insights#Generic Sterile Injectables Market#Generic Sterile Injectables#Generic Pharmaceuticals#Sterile Injections#Injectable Medications#Pharmaceutical Industry#Generic Drugs#Injectables#Sterile
0 notes
Text
India Single-use Bioprocessing Probes And Sensors Market To Reach $191.0 Million By 2030
The India single-use bioprocessing probes and sensors market is anticipated to reach USD 191.0 million by 2030 and is anticipated to grow at a CAGR of 12.61% during the forecast period from 2024 to 2030, according to a new report by Grand View Research, Inc. The increasing demand for biopharmaceuticals and the growing popularity of disposable systems in preclinical trials are key factors driving the growth of the single-use bioprocessing probes and sensors market in India. The need for faster and more efficient drug development processes and the rising demand for personalized medicines contribute to the increased implementation of single-use bioprocessing systems.
The implementation of single-use technology (SUT) in biomanufacturing processes offers advantages such as reduced risk of cross-contamination and ensuring product purity and integrity, which is crucial for the growing domestic biosimilars and biologics market. It enhances operational efficiency, leading to shorter turnaround times and increased productivity - a key benefit for Indian manufacturers to scale and meet the rising domestic and global demand for biopharmaceuticals.
Furthermore, the commercial advantages of single-use sensors, such as streamlined operations, enhanced flexibility, and improved regulatory compliance and product quality, make them an attractive option for Indian biomanufacturers. The adoption of these technologies can help Indian companies lower their capital expenditure and achieve faster turnaround times.

Request a free sample copy or view report summary: India Single-use Bioprocessing Probes And Sensors Market Report
India Single-use Bioprocessing Probes And Sensors Market Report Highlights
The pH sensors type segment held the largest revenue share of 20.24% in 2023 and is expected to grow at the fastest CAGR over the forecast period. The growing demand for precise process monitoring, driven by the expanding biopharmaceutical industry, drives the adoption of single-use pH sensors. The oxygen sensors segment is expected to register a significant CAGR over the forecast period.
The upstream segment dominated the segment with a market share of 73.49% in 2023 and is anticipated to grow at the fastest CAGR over the forecast period. It is driven by the increasing demand for biopharmaceuticals and the need for efficient and cost-effective manufacturing processes.
The biopharmaceutical & pharmaceutical companies dominated the segment with a market share of 41.69% in 2023. The consistent introduction of new and innovative single-use bioprocessing probes and sensors is a key driver behind the growth of this market segment.
India Single-use Bioprocessing Probes And Sensors Market Segmentation
Grand View Research has segmented the India single-use bioprocessing probes and sensors market based on type, workflow, and end use:
India Single-use Bioprocessing Probes And Sensors Type Outlook (Revenue, USD Million, 2018 - 2030)
pH Sensor
Oxygen Sensors
Pressure Sensors
Temperature Sensors
Conductivity Sensors
Flow Meter & Sensors
Other Sensors
India Single-use Bioprocessing Probes And Sensors Workflow Outlook (Revenue, USD Million, 2018 - 2030)
Upstream
pH Sensor
Oxygen Sensors
Pressure Sensors
Temperature Sensors
Conductivity Sensors
Flow Meter & Sensors
Other Sensors
Downstream
pH Sensor
Pressure Sensors
Temperature Sensors
Conductivity Sensors
Flow Meter & Sensors
Other Sensors
India Single-use Bioprocessing Probes And Sensors End-use Outlook (Revenue, USD Million, 2018 - 2030)
Biopharmaceutical & Pharmaceutical Companies
CROs & CMOs
Academic & Research Institutes
Others
List of Key Players in theIndia Single-use Bioprocessing Probes And Sensors Market
Thermo Fisher Scientific
Sartorius AG
PreSens Precision Sensing GmbH
Hamilton Company
Mettler-Toledo India Private Limited
PARKER HANNIFIN CORP
Danaher
Saint-Gobain
0 notes
Text
Antibody Production Market - Forecast(2024 - 2030)
Overview
Antibody Production Market is projected to reach revenue of $22.5 billion by 2025, and is estimated to grow at a CAGR of 12.8% during the forecast period 2019-2025. The Antibody Production market size, in 2018, is $9.7 billion. Antibody production by various biotechnology companies is to make medicines or diagnostic tests. The antibodies are mainly used to develop treatments for cancer and diseases such as arthritis, sclerosis, and others.
Request Sample
Key Takeaways
The market is estimated to grow at a CAGR of 12.8% owing to the rising rate of infectious diseases and increasing investments by biotechnology companies to develop treatment for various chronic diseases.
Companies are adopting various strategic alliances to expand in various regions. Investments in antibody production and research have increased in order to develop treatment for deadly diseases such as cancer.
Cancer treatment is observed to be more effective when multiple methods are used. Treatment methods such as stem cell transplantation along with antibody treatment has improved the survival rate of patients. Advances in biotechnology and antibody production have led to the development of various therapies for common diseases.
Process - Segment Analysis
Downstream processing is having major share by revenue in the owing to the large use of chromatography systems and resins for antibody production. This process is highly accurate, sensitive and effective, making its use popular for the production of antibodies.
Inquiry Before Buying
Type - Segment Analysis
Main types of antibodies produced are monoclonal and polyclonal antibodies. Monoclonal antibodies are clones of the parent cell and Polyclonal antibodies are made from different cells. Monoclonal antibodies are dominant segment compared to polyclonal antibodies and have a CAGR of 11.2%. They are having more demand for treatment of infections, cancer, and other chronic diseases. A common method of preparation of monoclonal antibodies is using ion exchange chromatography. This is because with this method, the process can be controlled easily and has high capacity or purification capability. Ammonium sulfate precipitation is used for monoclonal and polyclonal isolation.
End-User - Segment Analysis
Pharmaceutical and biotechnological companies are having larger share by end-user segment. This is because of the production of antibodies especially for these companies and due to the high investment for research and development by such companies.
Schedule a Call
Geography - Segment Analysis
North America accounts for largest Antibody Production market share with 32.3% in 2018. This is owing to the presence of various biotechnology companies present here. Also, the government expenditure for healthcare sector in North America is high. These factors contribute to the growth of the market in this region. However, Asia Pacific will have higher growth rate in the coming years due to supportive regulations and increasing innovations by the companies here.
Drivers – Antibody Production Market
· Rising rate of chronic diseasesAntibodies protect body by strengthening the immune system. They are used to prevent virus or bacteria related diseases. Antibodies are produced for various infections and are given to people as immunizations. They are also being developed for treatment of cancer and arthritis. Gastric cancer is said to be the second leading cause of global cancer-related deaths. Thus the antibody production will contribute to gastroenterology. The major gastric cancer treatment antibodies include Ramucirumab and Trastuzumab.
· Increase in expenditure by biotechnology companies
Biosimilars are gaining popularity as they serve the same purpose as the biologic and are also less expensive. With many patent expirations in the coming years, the biotechnology companies are investing in development of biosimilars. They are also increasing expenditure for research and developments for antibody production processes.
Buy Now
Challenges – Antibody Production Market
· High cost of production
Antibody production requires heavy expense by the companies. Their expenditure and investments for antibody production will be high. The high cost for antibody production will challenge the market.
Antibody Production Industry Outlook
Research & development along with strategic alliances are some of the key strategies adopted by players in the Antibody Production market. Antibody Production Market top 10 companies are Cellab Gmbh, Eppendorf Ag, Integra Bioscience Ag, Merck Kgaa, Pall Corp, Sartorius Ag, Thermo Fisher Scientific, Inc, ProteoGenix, Genentech, Inc. and BIOTEM.
Antibody Production Market Research Scope:
The base year of the study is 2018, with forecast done up to 2025. The study presents a thorough analysis of the competitive landscape, taking into account the market shares of the leading companies. These provide the key market participants with the necessary business intelligence and help them understand the future of the Antibody Production Market. The assessment includes the forecast, an overview of the competitive structure, the market shares of the competitors, as well as the market trends, market demands, market drivers, market challenges, and product analysis. The market drivers and restraints have been assessed to fathom their impact over the forecast period. This report further identifies the key opportunities for growth while also detailing the key challenges and possible threats.
#Antibody Production Market#Antibody Production Market Size#Antibody Production Market Share#Antibody Production Market Analysis#Antibody Production Market Revenue#Antibody Production Market Trends#Antibody Production Market Growth#Antibody Production Market Research#Antibody Production Market Outlook#Antibody Production Market Forecast
0 notes
Text
Leqembi (lecanemab-irmb) is a prescription drug used to treat Alzheimer’s disease. Leqembi’s cost may depend on factors such as your dosage, whether you have health insurance, and the pharmacy you use.
How much does Leqembi cost?
The price you pay for Leqembi can vary. It may depend on your treatment plan, your insurance coverage (if you have it), and the pharmacy you use. It will also depend on how much you have to pay for an office visit with your doctor to receive Leqembi infusions.*
To find out how much you’ll pay for Leqembi, talk with your doctor, pharmacist, or insurance provider. Or check out the section below to learn how much you can save by using an Optum Perks coupon.
* Leqembi comes as a liquid solution in single-dose vials. It’s given as an intravenous (IV) infusion (an injection into a vein given over time) by a healthcare professional.
Leqembi coupons and savings
To save money on your Leqembi prescription, explore these Optum Perks coupons.
FAQ about cost and Leqembi
Below are answers to some frequently asked questions about Leqembi and cost.
Is Leqembi covered by Medicare?
It’s possible. To find out whether your Medicare plan covers the cost of Leqembi, call your plan provider. Many types of Medicare plans are available. Your cost and coverage depend on your particular plan’s benefits.
Your final cost may also depend on the price to receive Leqembi infusions at a doctor’s office or clinic.
Keep in mind that your plan may have prior authorization requirements before it will cover Leqembi. (See the “Prior authorization” section below for more information.)
You can also ask your doctor about the cost of Leqembi if you have Medicare.
How does the cost of Leqembi compare with that of similar drugs, such as Aduhelm?
The price of Leqembi compared with the price of Aduhelm can depend on several factors.
These include:
whether there are any savings programs available for the drug you’re prescribed
whether you have insurance or are paying out of pocket
how long your treatment lasts
Note: Biogen, the maker of Aduhlem, made the decision in early 2024 to discontinue Aduhelm. After November 1, 2024, Aduhelm will no longer be available.
If you have questions about the cost of Leqembi compared with the cost of similar drugs, talk with your doctor or pharmacist and your insurance provider (if you have one). They can share more details on treatment costs based on your specific situation.
How can I lower my long-term drug costs?
You may be able to lower your Leqembi cost per year if you use this medication long term.
Talk with your doctor about financial assistance options if you don’t have health insurance.
Is Leqembi available as a generic or biosimilar?
Leqembi is a biologic drug, which means it’s made from parts of living organisms. It doesn’t come in a biosimilar form. Biosimilars are like generic drugs. But unlike generics, which are made for nonbiologic drugs, biosimilars are made for biologics.
Why is there such a cost difference between biologic drugs and biosimilar drugs?
Biologic drugs can be expensive because of the research and testing needed to ensure their safety and effectiveness. The manufacturer of a biologic drug can sell it exclusively for up to 12 years. When the biologic drug’s patent expires, other drugmakers can create biosimilar versions. This competition in the market may lead to lower costs for biosimilars. And because biosimilars are very similar to biologic drugs, they don’t need to be studied again. This can also lead to lower costs for biosimilars.
Can I get help paying for Leqembi?
If you need help covering the cost of Leqembi or understanding your insurance, check out these resources:
Eisai Patient Support
NeedyMeds
prescription discount programs
On these pages, you can find insurance information, details on drug assistance programs, and links to savings cards and other services.
If you have questions about how to pay for your prescription, talk with your doctor or pharmacist.
Prior authorization
If you have insurance, you may need to get prior authorization before your insurance provider will cover Leqembi. This means your insurer and your doctor will discuss Leqembi in regard to your treatment. Then the insurance company will determine whether the drug is covered. If Leqembi requires prior authorization and you don’t receive it before you start treatment, you could pay the full cost of the drug.
Be sure to ask your insurance company whether Leqembi requires prior authorization.
Disclaimer: adoctor has made every effort to make certain that all information is factually correct, comprehensive, and up to date. However, this article should not be used as a substitute for the knowledge and expertise of a licensed healthcare professional. You should always consult your doctor or another healthcare professional before taking any medication. The drug information contained herein is subject to change and is not intended to cover all possible uses, directions, precautions, warnings, drug interactions, allergic reactions, or adverse effects. The absence of warnings or other information for a given drug does not indicate that the drug or drug combination is safe, effective, or appropriate for all patients or all specific uses.
0 notes
Text
Top 5 players in US Biosimilar Market
Buy Now
STORY OUTLINE
Pfizer: Excelling in the line of Biosimilar drugs with an experience of more than 10 years with presence in over 180 countries.
Amgen: Making pharmaceutical products with an experience of over 40 years and presence in over 100 countries.
Viartis: Presence in over 165 countries, and making Biosimilar drugs in over 75 markets, this pharmaceutical company is another leading contributor of US Biosimilar market.
Coherus Biosciences: Increasing patient access to cost effective medicines with a Biosimilar drugs experience of 13 years.
Biogen: serving humanity through science with a experiences of more than 40 years in the field of biologics.
According to Ken Research, the US Biosimilar market is anticipated to grow at a CAGR of ~40% in the next five years which currently has a market size of ~USD 9.4 Bn.
The US Biosimilar market is rapidly growing and will be witnessing a significant growth in the next five years.
There are various reasons behind the rapid growth of US Biosimilar market. Some of the major reasons behind the growth of US Biosimilar market include the cost effective nature of Biosimilar drugs, rising geriatric population, rising prevalence of chronic diseases, and growing partnerships between companies to develop Biosimilar drugs.
Various companies and players are contributing to their best efforts in the growth of the US Biosimilar market.
This article aims to put light on the contributions done by the major players towards the growth of the US Biosimilar market.
1.Pfizer
Click to read more about Pfizer
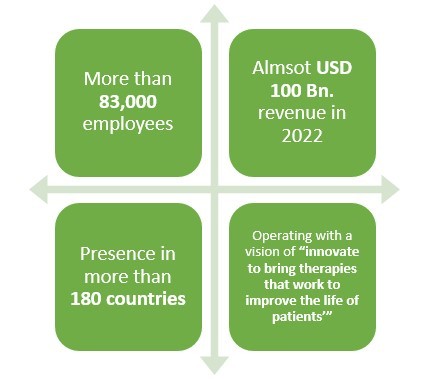
Pfizer is a leading American pharmaceutical company which is operating in the field of generics or original drugs for more than 30 years. But did you know that this pharma not only manufactures biologics but also biosimilar drugs?
Pfizer has been in the business of biosimilar drugs for more than 10 years and have been quite successful as well. With more than 83,000 employees and presence in over 180 countries, this leading pharmaceutical company made almost USD 2 Bn. revenue only from its Biosimilar drugs sale in 2021.
Recently, this pharmaceutical company also collaborated with Samsung in two deals to produce various biosimilar drugs in South Korea. The deal size between these two companies happens to be approximately USD 900 Bn.
The major Biosimilar drugs of this pharmaceutical giant are primarily
ZIRABEV (a Biosimilar of Avastin)
TRAZIMERA (a Biosimilar of Herceptin)
RUXIENCE (a Biosimilar of Rituxan)
RITACRIT (a Biosimilar of Epogen)
NVYEPRIA (a Biosimilar of Neulasta)
NIVESTYM (a Biosimilar of Neupogen)
FILGRASTIM (a Biosimilar of Neupogen).
2.Amgen
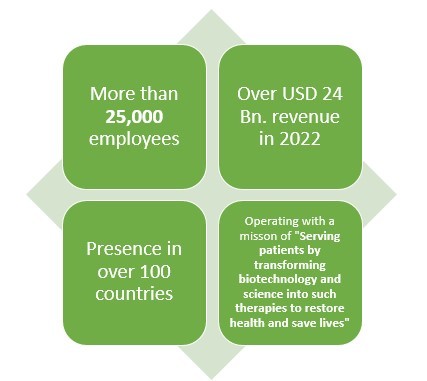
Click here to Download a Sample Report
Amgen is another leading American pharmaceutical company which not only makes Biologics or generic drugs but also Biosimilar drugs. This pharmaceutical company has more than 40 years of experience when it comes to pharmaceutical line.
With over 25000 employees and presence in over 100 countries, this pharmaceutical company earned about USD 2 Bn. from their three biosimilar drugs which are reportedly MVASI, KANJITNTI, and AMJEVITA.
This pharma giant has also invested about USD 2 Bn. in the development of Biosimilar drugs.
This pharmaceutical company has made Biosimilar drugs primarily in 4 fields which are General Medicine, Oncology, and Hematology along with, Inflammation.
EPOTEIN ALFA
AMJEVITA
AVSOLA
KANJINTI
MVASI
RIABNI
are the various Biosimilar drugs of Amgen. And, STELARA, EYLEA, SOLIRIS are in their pipeline.
Recently Amgen revealed their Biosimilar report’s 8 version. It revealed a major information which said that the pharmaceutical company saved about USD 10 Bn. through their Biosimilar drugs in the past five years.
3.Viartis

Headquartered in Canonsburg, Pennsylvania, this American pharmaceutical company was founded only in 2020 yet they have achieved massive success in the pharmaceutical products with their revenue being USD 16 ~Bn. in 2022.
With presence in 165 countries and with over 45,000 employees worldwide, this pharmaceutical company makes pharmaceutical products in 10 areas which primarily are Cardiovascular, Dermatology, ophthalmology, Oncology, Gastroenterology, Women’s health, Infectious diseases, Diabetes & Metabolism, Immunology, CNS & Anesthesiology, Respiratory diseases and allergy.
Speaking of their first Biosimilar products, their first ever Biosimilar drug was launched in 2014. They have a variety of Biosimilar drugs which are primarily
TRASTUZUMAB
INSULIN ASPART
PEGFILGRASTIM
INSULIN GLARGINE-YFGN
ADALIMUMAB
BEVACIZUMAB
Their Biosimilar drug Insulin Glargine which is known as SEMGLEE was the first ever interchangeable Biosimilar drug in the United States which was FDA approved.
Their PEGFILGRASTIM also was the first ever FDA approved drug in the United States. They have launched their Biosimilar drugs in over 75 markets worldwide.
4.Coherus Biosciences:
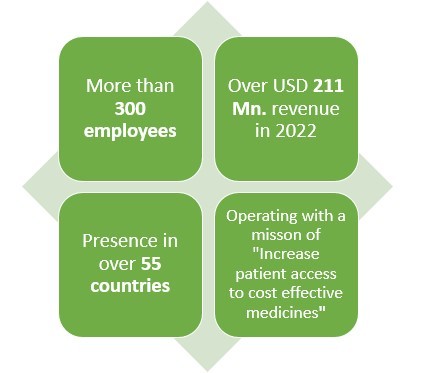
Click here to Ask for a Custom Report
Headquartered in Redwood city, California this American pharmaceutical company earned a revenue of almost USD 211 Mn. In 2022.
With presence in over 55 countries and 300+ employees worldwide, this pharmaceutical company makes products in various areas such as solid tumors, non-small lung cancers, nasopharyngeal carcinoma, small cell lung cancer and hepatocellular carcinoma.
Speaking of their Biosimilar drugs, this pharma has been in the field of creating Biosimilar drugs since 2010 which has given them almost 13 years of experience.
This pharmaceutical company also disclosed that it plans to spend at least USD 1 Tn. on medicines worldwide, out of which at least 40% will be spent on Biosimilar drugs.
Their three major Biosimilar drugs which are also FDA approved include UDENCYA, YUSIMRY, and CIMERLI.
Udencya is a Biosimilar drug of Pegfilgrastim, Yusimry is a Biosimilar drug of Ranibizumab, and Cimerli is a Biosimilar drug of Adalimumab.
5.Biogen
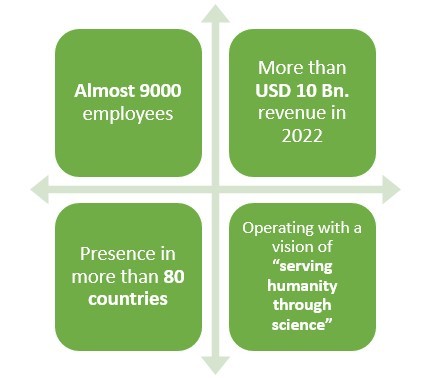
Headquartered in Cambridge, Massachusetts, this American pharmaceutical company earned a revenue of around USD 10 Bn. in 2022.
This company happens to have an experience of more than 40 years when it comes to making pharmaceutical products.
With presence in over 80 countries and more than 9000 employees worldwide, this pharmaceutical company primarily deals in Neurology, Specialized Immunology, Neuropsychiatry, Ophthalmology, and Rare Diseases.
ADUCANUMAB
LECANEMAB
TOFERSEN
ZURANOLONE
LITIFILIMAB
BENAPALI
FLIXABI
IMRALDI
are some of their Biosimilar drugs.
With their Biosimilar drugs, more than 250,000 people have gone on Anti-Tumor Necrosis Factor therapy.
Recently, this pharmaceutical company also made an agreement with Bio-Thera solutions to develop a Biosimilar drug for the treatment of Rheumatoid Arthritis.
#US Biosimilar Sector#United States Biosimilar Market#US Biosimilar Market forecast#US Biosimilar Market analysis#US Biosimilar Market trends#US Biosimilar Market share#US Biosimilar Market key players#US Biosimilar Market revenue#US Biosimilar Market growth#Monoclonal Antibodies in biosimilar market US#Recombinant Hormones in biosimilar industry US#Oncology in bio similar market US#Blood disorders in biosimilar market US#Research institutes in Biosimilar market US#US similar biotherapeutics products market#Hospitals in Biosimilar market US#Investors in Biosimilar market US#US comparable biologics products industry#US recombinant biosimilars industry#US replicate biologics sector#US analog biologics market
0 notes
Text
Rheumatoid Arthritis Therapeutics Market To Reach USD 36.39 Billion By 2030

Rheumatoid Arthritis Therapeutics Market Growth & Trends
The global rheumatoid arthritis therapeutics market size is expected to reach USD 36.39 billion by 2030, expanding at a CAGR of 5.93% from 2024 to 2030, according to a new study by Grand View Research, Inc. Some of the key trends driving the market are availability of novel biologics, increase in potential clinical pipeline candidates, and rise in aging population. Thus, growing availability and awareness of safer drugs are projected to escalate rheumatoid arthritis drugs market growth.
Rheumatoid arthritis affects approximately 1% of the global population. The condition has different symptoms such as joint pain, swelling, and stiffness, which are generally accompanied by chronic pain and inability to perform daily activities. Over a prolonged period, this disorder can hamper a patient’s mobility and can lead to permanent joint damage. If left untreated, this disorder can lead to mobility impairment and risk of joint replacement.
Growing prevalence of the disease, presence of biologics & biosimilars, and well-defined regulatory guidelines are poised to provide a fillip to the market. According to the Arthritis Foundation, 64% of the population within the age group of 65 is likely to suffer from arthritis in U.S. Increasing awareness regarding disease remittance therapies and rising healthcare expenditure in developed regions are estimated to promote revenue growth.
Current treatment for rheumatoid arthritis includes generic pharmaceuticals such as NSAIDs and corticosteroids along with novel biologics such as JAK inhibitors and IL-6 antagonists, which are widely used in developed countries due to their high effectiveness. New product launches such as Infliximab and Adalimumab biosimilars are anticipated to work in favor of the market. Moreover, Eli Lilly’s Olumiant and AbbVie’s Upadacitinib are expected to cut down chances of disease remittance.
Request a free sample copy or view report summary: https://www.grandviewresearch.com/industry-analysis/rheumatoid-arthritis-therapeutics-market
Rheumatoid Arthritis Therapeutics Market Report Highlights
Biopharmaceuticals segment led the market and accounted for 88.73% of the global revenue in 2023. Biopharmaceuticals are large-molecule drugs that are manufactured, extracted, and synthesized from biological sources.
Prescription led the market with a market share of 89.64% in 2023. As rheumatologist consultations rise, the prescription segment is expected to remain dominant in the market for rheumatoid arthritis medications over the forecast period.
North America rheumatoid arthritis therapeutics market accounted for 52.31% share in 2023. The regional market continues to grow and evolve, driven by demographic shifts, advancements in medical research, regulatory changes, and an increasing emphasis on personalized healthcare.
The Asia Pacific rheumatoid arthritis therapeutics market is experiencing significant growth, driven by the increasing prevalence of rheumatoid arthritis, improving healthcare infrastructure, and advancements in treatment options.
Rheumatoid Arthritis Therapeutics Market Segmentation
Grand View Research has segmented global rheumatoid arthritis therapeutics market report based on molecule, sales channel, and region:
Rheumatoid Arthritis Therapeutics Molecule Outlook (Revenue, USD Million, 2018 - 2030)
Pharmaceuticals
NSAIDs
Analgesics
DMARDs
Glucocorticoids
Biopharmaceuticals
Biologics
TNF-α antagonists
T-cell inhibitors
CD20 antigen
JAK inhibitors
anti-IL6 biologics
Biosimilars
CD20 antigen
TNF-α antagonists
Rheumatoid Arthritis Therapeutics Sales Channel Outlook (Revenue, USD Million, 2018 - 2030)
Prescription
Over-the-Counter (OTC)
Regional Insights
North America rheumatoid arthritis therapeutics market accounted for 52.31% share in 2023.The regional market continues to grow and evolve, driven by demographic shifts, advancements in medical research, regulatory changes, and an increasing emphasis on personalized healthcare. The aging population across the region significantly impacts the demand for rheumatoid arthritis therapeutics. As people live longer, the prevalence of chronic conditions such as RA increases, boosting the need for effective treatment options. This demographic trend is a primary driver of sustained market growth.
Europe Rheumatoid Arthritis Therapeutics Market Trends
The Europe rheumatoid arthritis therapeutics market is substantially changing, influenced by healthcare reforms, research advancements, and patient-focused initiatives across different countries. The prevalence of rheumatoid arthritis is increasing due to an aging population and changing lifestyles, leading to a growing demand for effective & innovative treatment options across the continent.
Asia Pacific Rheumatoid Arthritis Therapeutics Market Trends
The Asia Pacific rheumatoid arthritis therapeutics market is experiencing significant growth, driven by the increasing prevalence of rheumatoid arthritis, improving healthcare infrastructure, and advancements in treatment options. The prevalence of rheumatoid arthritis is on the rise across Asia Pacific due to factors such as the aging population, changing lifestyles, and increasing awareness leading to early diagnosis. This rising prevalence has led to a growing demand for effective and affordable RA treatments, prompting healthcare providers & policymakers to focus on improving RA care & management.
List of Key Players of Rheumatoid Arthritis Therapeutics Market
AbbVie, Inc.
Boehringer Ingelheim International GmbH
Novartis AG
Regeneron Pharmaceuticals Inc.
Pfizer, Inc.
Bristol-Myers Squibb Company
F. Hoffmann-La Roche Ltd.
UCB S.A.
Johnson & Johnson Services, Inc.
Amgen, Inc.
Lilly (Eli Lilly and Company)
Browse Full Report: https://www.grandviewresearch.com/industry-analysis/rheumatoid-arthritis-therapeutics-market
#Rheumatoid Arthritis Therapeutics Market#Rheumatoid Arthritis Therapeutics Market Size#Rheumatoid Arthritis Therapeutics Market Share
0 notes
Text
Cardiovascular Drugs Market Size & Share to Surpass USD 195.6 billion By 2031
The global cardiovascular drugs market was valued at US$ 142.8 billion in 2022. The market is expected to expand at a CAGR of 3.8% from 2023 to 2031, reaching US$ 195.6 billion. Cardiovascular treatments may become more targeted and effective with genomics and personalized medicine advances. By identifying a patient's genetic profile and specific risks, tailored treatment can help improve outcomes and reduce side effects.
Researchers are working on new drug classes and therapies to treat cardiovascular disease, like gene therapies, RNA-based therapeutics, and stem cell therapies. By using these innovations, traditional medications could be rendered less effective and less durable. With digital health technologies like wearables and remote monitoring, cardio conditions could be better managed. Monitoring in real-time, coupled with data analytics, could lead to more proactive and personalized interventions.
Download Sample PDF of this Strategic Report @ https://www.transparencymarketresearch.com/sample/sample.php?flag=S&rep_id=33281
Research may continue into combinations of drugs with complementary mechanisms. The use of combination therapies may lead to better patient compliance and efficacy for cardiovascular diseases. Biosimilar versions may become more competitive as some cardiovascular drugs' patents expire. As a result, essential medications may be more affordable and easier to access.
Key Findings of the Market Report
Based on drug class, the anti-clotting agents (anti-coagulants and platelet aggregation inhibitors) will drive the cardiovascular drugs market.
North America accounts for the majority of market share as cardiovascular disease incidence rises.
Based on indication, the hypertension segment is anticipated to drive demand for cardiovascular drugs.
In terms of distribution channels, hospital pharmacy is expected to drive the market for cardiovascular drugs.
Global Cardiovascular Drugs Market: Regional Landscape
Cardiovascular drugs are expected to be in high demand in North America. North America continues to experience a significant burden of cardiovascular diseases (CVDs), accounting for a significant share of the overall burden of disease. Several cardiovascular diseases, including hypertension and coronary artery disease, are prevalent, resulting in a high demand for cardiovascular drugs.
Healthcare expenditures in North America are relatively high in the United States and Canada. This financial commitment to healthcare infrastructure and services enhances access to and utilization of cardiovascular drugs.
Pharmaceutics research and development are centered in North America, especially in the United States. Technological advances and drug discovery have introduced innovative cardiovascular drugs with improved efficacy and safety. In general, cardiovascular health is becoming more widely known among the public, which has led to a greater emphasis on prevention and early intervention.
Government regulatory agencies, such as Health Canada and the U.S. Food and Drug Administration (FDA), ensure cardiovascular medication safety and efficacy by overseeing drug approvals. Regulatory frameworks and government policies influence market dynamics.
Global Cardiovascular Drugs Market: Key Players
Leading companies follow market trends and launch generic heart failure drugs after patents on well-known drugs expire, per the latest cardiovascular drugs market analysis. In addition, the major drug manufacturers have established partnerships with academic institutions to develop innovative treatments and retain their competitive edge.
● ICU Medical Inc.
● Tandem Diabetes Care Inc.
● Medtronic plc
● Terumo Corporation
● F. Hoffmann-La Roche AG
● Baxter International
● BD
● Fresenius Kabi AG
● B. Braun SE
● Insulet Corporation
Key Developments
In January 2023, Lupin launched an India-based generic version of a Novartis drug for heart failure following the expiration of the patents on Valsartan and Sacubitril. In addition, it is available under the brand names Arnipin and Valentas, both of which are used for treating heart failure.
In January 2023, Glenmark Pharmaceuticals launched sacubitril + valsartan tablets for treating heart failure in India. The products are marketed as 'Sacu V'.
Global Cardiovascular Drugs Market: Segmentation
By Drug Class
Renin-Angiotensin System Blockers (ACE Inhibitors and Angiotensin Receptor Blockers)
Beta Blockers
Diuretics
Anti-Clotting Agents (Anti-Coagulants and Platelet Aggregation Inhibitors)
Antihyperlipidemics
Other Antihypertensive
Calcium Channel Blockers
Others
By Indication
Hypertension
Hyperlipidemia
Coronary Artery Disease
Peripheral Artery Disease
Arrhythmia
Others
By Distribution Channel
Hospital Pharmacy
Retail Pharmacy
Online Pharmacy
By Region
North America
Europe
Asia Pacific
Latin America
MEA
Buy this Premium Research Report: https://www.transparencymarketresearch.com/checkout.php?rep_id=33281<ype=S
About Transparency Market Research
Transparency Market Research, a global market research company registered at Wilmington, Delaware, United States, provides custom research and consulting services. Our exclusive blend of quantitative forecasting and trends analysis provides forward-looking insights for thousands of decision makers. Our experienced team of Analysts, Researchers, and Consultants use proprietary data sources and various tools & techniques to gather and analyses information.
Our data repository is continuously updated and revised by a team of research experts, so that it always reflects the latest trends and information. With a broad research and analysis capability, Transparency Market Research employs rigorous primary and secondary research techniques in developing distinctive data sets and research material for business reports.
Contact:
Transparency Market Research Inc.
CORPORATE HEADQUARTER DOWNTOWN,
1000 N. West Street,
Suite 1200, Wilmington, Delaware 19801 USA
Tel: +1-518-618-1030
USA – Canada Toll Free: 866-552-3453
Website: https://www.transparencymarketresearch.com
0 notes
Text
Biosimilars Market Projections: Future Growth and Trends

Biosimilars Market Outlook, Scope & Overview:
Industry reports indicate that the global biosimilars market was valued at USD 29.51 billion in 2023 and is projected to reach USD 108.8 billion by 2031, growing at a CAGR of 17.7% over the forecast period 2024-2031.
Regulatory Approvals and Patent Expirations to Drive Growth of Global Biosimilars Market
The increasing adoption of biosimilars will continue to influence global market revenues. Healthcare systems are turning to biosimilars as a cost-effective alternative to biologics, with the potential to significantly reduce healthcare expenditures and improve patient access to essential therapies.
As a product segment, monoclonal antibodies (mAbs) currently hold a significant share of the global biosimilars market. This segment is anticipated to grow at a year-over-year rate of 17.7% in 2024 over 2023 and reach USD 108.8 billion in revenues by 2031. The upcoming patent expirations of major biologic drugs and the increasing number of biosimilar approvals are expected to drive market growth.
Get a Free Sample Report: https://www.snsinsider.com/sample-request/2941
Biosimilars Market – Market Dynamics
Drivers:
The biosimilars market is experiencing significant growth due to the rising prevalence of chronic diseases, the increasing demand for cost-effective biologic therapies, and the expanding number of biosimilar products entering the market. Regulatory bodies in major regions, such as the FDA in the United States and the EMA in Europe, are actively supporting the development and approval of biosimilars, further driving their adoption. Additionally, the growing acceptance of biosimilars by healthcare providers and patients, along with initiatives to promote biosimilar usage, is contributing to market expansion.
Restraints:
Despite the strong growth potential, challenges such as stringent regulatory requirements, high development costs, and the complexity of manufacturing biosimilars are hindering their widespread adoption. Additionally, patent litigations, brand loyalty towards original biologics, and the need for extensive clinical trials to demonstrate biosimilarity pose additional challenges to market growth. Furthermore, the lack of awareness and education about biosimilars among healthcare professionals and patients can also limit market expansion.
Biosimilars Market – Market Outlook
The proven benefits of biosimilars in reducing healthcare costs, increasing access to biologic treatments, and improving patient outcomes have contributed to the market's growth. Biosimilars are expected to witness increased adoption across major markets, including North America, Europe, and Asia Pacific, driven by favorable regulatory frameworks, growing healthcare expenditures, and the rising focus on biologic treatments for chronic diseases.
Global Biosimilars Market
The rise in demand for biosimilars in developed and emerging markets is expected to drive market growth over the forecast period. North America currently holds a significant market share in the global biosimilars market, with the US being a key contributor to market revenues. Europe and Asia Pacific regions are also experiencing rapid adoption of biosimilars, supported by increasing biosimilar approvals, patent expirations, and initiatives to enhance biosimilar uptake.
Key Players in the Biosimilars Market
Leading companies in the biosimilars market include Pfizer Inc., Novartis AG (Sandoz), Amgen Inc., and Biocon Limited. These companies are at the forefront of developing and commercializing biosimilar products for various therapeutic areas, including oncology, immunology, and endocrinology.
In conclusion, the global biosimilars market is poised for substantial growth over the forecast period, driven by increasing regulatory support, patent expirations of major biologics, and the expanding adoption of biosimilars across diverse therapeutic areas.
Other Trending Reports
3-D Ultrasound Market Segmentation
Digital Hearing Aids Market Segmentation
Telehealth and Telemedicine Market Segmentation
Digital Radiology Market Segmentation
0 notes
Text
Prefilled Syringes Market worth $13.1 billion in 2030
The prefilled syringes market is expected to generate a revenue of USD 13.1 billion in 2030, with a revenue of USD 7.1 billion in 2024 to register a CAGR of 10.8%. A prefilled syringe is a convenient medical device for delivering parenteral medications, offering a premeasured single-dose. This syringe benefits manufacturers by minimizing drug waste and extending product lifespan, while also making self-administration easier for patients at home or outside of hospital settings. Drugs commonly packaged in prefilled syringes include biologics, vaccines, blood stimulants, therapeutic proteins, erythropoietin products, and interferons.
Download PDF Brochure:
Prefilled Syringes Market Dynamics
Driver: Increasing prevalence of chronic diseases
The prefilled syringe market growth is largely being driven by the rising prevalence of chronic diseases like diabetes, cardiovascular disease, and autoimmune disorders. Prefilled syringes provide a practical, safe, and effective alternative for patients and healthcare professionals alike, as these disorders frequently necessitate the exact and regular administration of drugs. They raise patient compliance, lower the possibility of dosage errors, and improve the standard of treatment. Prefilled syringes demand is further fueled by the growing trends in self-administration, especially with regard to biologics and biosimilars, which is driving the market's growth.
Restraint: Impact of product recalls
Product recalls impede the prefilled syringe market's growth by eroding consumer confidence in these medical products. Manufacturers suffer significant financial losses as a result of recalls, which are frequently caused by contamination, or safety hazards. These losses also stem from potential legal obligations. They may also interfere with supply chains, leading to delays and shortages that impact patients and healthcare providers. All of these factors impede the market growth and impact the general uptake of prefilled syringes.
Opportunity: Increasing demand for biologics and biosimilars
The market for pre-filled syringes has a significant opportunity due to the growing need for biologics and biosimilars. A growing number of people are using biologics, complex drugs made from living things, and biosimilars to treat chronic illnesses. Pre-filled syringes provide a practical and accurate option because these therapies need precise and dependable delivery techniques to guarantee efficacy and patient safety. The market for pre-filled syringes is expected to rise significantly due to their capacity to lower dose errors, increase patient compliance, and improve overall healthcare outcomes. These benefits perfectly complement the growing usage of biologics and biosimilars.
Challenge: Alternative drug delivery methods
In the pre-filled syringe market, alternative drug administration technologies pose a serious threat by providing several options that may be seen as more practical or efficient for particular medical conditions. Various technologies like as autoinjectros, pen injectors, needle-free injectors, transdermal patches, and oral medication formulations offer various benefits like pain reduction, user-friendliness, and increased patient adherence. These substitutes may lessen the need for pre-filled syringes since patients and medical professionals may favor techniques that make injections less uncomfortable or simpler to use. As a result, in order for the pre-filled syringe business to be competitive, it must constantly innovate and highlight its advantages.
North America accounted for the largest market share of the global prefilled syringes industry, by region in the forecast period.
The pre-filled syringe market is anticipated to be dominated by the North American region due to a number of factors, including an advanced healthcare infrastructure, a high prevalence of chronic diseases, and a focus on patient safety and technological innovation. Its dominant position in the market is also a result of the region's well-established pharmaceutical industry and rising demand for practical and precise drug delivery solutions. Furthermore, continued R&D expenditures and favorable regulatory environments in North America contribute to the expansion and use of pre-filled syringes.
The key players in the prefilled syringes market include BD (US), Gerresheimer AG (Germany), SCHOTT (Germany), and West Pharmaceutical Services, Inc. (US), AptarGroup Inc. (US), Nipro (Japan), Baxter (US), Owen Mumford Ltd. (UK), Weigao Meidcal international Co., Ltd. (China), Credence MedSystems, Inc. (US), Novartis AG (Switzerland), Stevanato Group (Italy), Polymedicure (India), MedXL (Canada), Sharps Technology, Inc. (US), Fresenius Kabi (US), DBM S.R.L. (Italy), Taisei Kako Co.,Ltd (Japan), Shandong Province Medicinal Glass Co., Ltd. (China), SHIN YAN SHENO PRECISION INDUSTRIAL CO., LTD (Japan), J.O. PHARMA CO., LTD. (Japan), BMIKOREA (Korea), B. Braun SE (Germany), and Al Shifa Medical Products Co. (Saudi Arabia).
Recent Developments of Prefilled Syringes Industry:
In April 2024, Baxter announced the expansion of its Pharmaceuticals portfolio with the launch of five new injectable products in the US. These launches focus on addressing unmet patient needs in key therapeutic areas, such as anti-infective and anti-hypotensive medications.
In June 2024, SCHOTT Pharma (Germany) constructed a new production facility dedicated to prefillable glass syringes (PFS) at its location in Lukácsháza, Hungary. This strategic step demonstrated the company's proactive approach in offering advanced solutions for the storage of biologics, vaccines, and the newest generation of mRNA-based medications.
In May 2023, Gerresheimer Glass GmbH, a subsidiary of Gerresheimer AG (Germany), signed a purchase agreement to acquire Bormioli Pharma Group. This acquisition strengthens Gerresheimer’s European presence and enhances its position in the pharmaceutical and biotech packaging market.
In May 2023, Baxter(US) signed a final agreement to sell its BioPharma Solutions (BPS) division to Advent International and Warburg Pincus, both renowned investment firms. This deal signifies the divestment of Baxter's contract development and manufacturing arm.
In September 2022, BD (US) launched a next-generation glass prefillable syringe (PFS) that sets a new benchmark for vaccine performance by meeting tighter processability, cosmetics, contamination, and integrity requirements. Leading pharmaceutical companies collaborated to design the innovative BD Effivax Glass Prefillable Syringe to address the growing demand for vaccine manufacturing.
Request 10% Customization:
#Global Prefilled Syringes Market#Global Prefilled Syringes Market Analysis#Global Prefilled Syringes Market Forecast
0 notes
Text
Pharmaceutical Analytical Testing Outsourcing Market 2024-2030: Trends and Opportunities
The global pharmaceutical analytical testing outsourcing market size was valued at USD 8.3 billion in 2023 and is projected to grow at a CAGR of 8.4% from 2024 to 2030.
Growth in the market can be attributed due to increasing focus toward safety, & quality; regulation, pricing benefits of outsourcing, and rising number of end use. Besides, increasing R&D investment is one of the critical sustainability strategies adopted by market players. As, not all companies have an infrastructure for analytical testing. Therefore, outsourcing these operations is a suitable option, which helps to save time and cost.
Gather more insights about the market drivers, restrains and growth of the Pharmaceutical Analytical Testing Outsourcing Market
Other factors, such as the changing regulations for in vivo and in vitro tests, are also expected to propel the growth of the market for pharmaceutical analytical testing outsourcing. Besides, high demand for quality generic drugs, analytical testing methods, improved the quality and transparency of the review & approval process, and encourage new drug R&D in line with global development is anticipated to fuel the market growth.
In addition, innovation or new product development is directly proportional to the demand for testing services due to pricing concerns, competitive pressures, and lead-time to market, companies are opting for outsourcing of testing services. Moreover, focus on customized care and technological advancements, which has resulted in rapid development of new products. Likewise, development of biosimilar, combination products, and other innovative medicines has fueled the demand for pharmaceutical analytical testing services.
Browse through Grand View Research's Medical Devices Industry Research Reports.
• The global pharmaceutical regulatory affairs market size was estimated at USD 8.84 billion in 2023 and is projected to grow at a CAGR of 7.16% from 2024 to 2030.
• The global pharmaceutical packaging market size was valued at USD 139.37 billion in 2023 and is expected to grow a compound annual growth rate (CAGR) of 9.7% from 2024 to 2030.
Pharmaceutical Analytical Testing Outsourcing Market Segmentation
Grand View Research has segmented the prebiotic ingredient market based on type, application, and region:
Type Outlook (Revenue, USD Million, 2017 - 2028)
• Inulin
• Oligosaccharide (MOS/GOS/FOS)
• Others
Applications Outlook (Revenue, USD Million, 2017 - 2028)
• Food & Beverage
• Dietary Supplements
• Others
Regional Outlook (Revenue, USD Million, 2017 - 2028)
• North America
• Europe
• Asia Pacific
• Central & South America
• Middle East & Africa
Key Companies & Market Share Insights
Companies' are focusing on innovating new types of prebiotic ingredients to meet the increasing demand. New players are entering the market owing to low market entry barriers and higher market potential. The increasing demand for prebiotic ingredients offers an opportunity for product development in the regions of high demand. Some of the prominent players in the global prebiotic ingredient market include:
• Beneo-Orafti SA
• Tereos Group
• Ingredion Inc.
• Cargill Inc.
• DowDuPont Inc
• Royal FrieslandCampina N.V.
• Kerry Group
• Cosucra-groupe Warcoing SA
• Sensus BV
Recent Developments
• In December 2023, Agno Pharma acquired Lubrizol Particle Sciences Inc. The acquisition encompasses the drug product formulation technology integral to Particle Sciences Inc. including the PA, Bethlehem development & manufacturing site.
• In November 2023, Eurofins acquired Quasfar supporting the pharmaceutical industry in Latin America, enhancing its capabilities in R&D, providing support in the early stages of pharmaceutical product development.
Order a free sample PDF of the Pharmaceutical Analytical Testing Outsourcing Market Intelligence Study, published by Grand View Research.
#Pharmaceutical Analytical Testing Outsourcing Market#Pharmaceutical Analytical Testing Outsourcing Industry#Pharmaceutical Analytical Testing Outsourcing#Pharmaceutical analytical testing outsourcing market size#Global pharmaceutical analytical testing outsourcing market
0 notes
Text
Remicade Biosimilar Market Share, Overview, Competitive Analysis and Forecast 2031
0 notes