#Investors in Biosimilar market US
Text
Biosimilars Unleashed: The Future of Healthcare in the US
Buy Now
What is the Size of US Biosimilar Industry?
US Biosimilar Market is expected to grow at a CAGR of ~ % between 2017-2022 and is expected to reach ~USD Bn by 2028. Biosimilars enhance patient access to essential treatments, especially in therapies with high demand, like oncology, by providing more affordable options. Additionally, Growing evidence of biosimilars' comparable efficacy and safety fosters trust among healthcare professionals, driving adoption.

Click here to Download a sample Report
Biosimilars offer cost savings compared to originator biologics, addressing the need for affordable healthcare solutions in the face of rising medical costs. Favorable regulatory frameworks, like the BPCIA, streamline biosimilar approval processes, encouraging manufacturers to invest in development.
Furthermore, The expiration of patents for numerous reference biologics creates opportunities for biosimilar entry, leading to increased competition and market expansion. Pharmaceutical companies are investing in biosimilar R&D and production, expanding the pipeline and market availability. Supportive healthcare policies and reimbursement models incentivize biosimilar adoption, creating a favorable environment for market growth.
US Biosimilar Market by drug class
The US Biosimilar market is segmented by Monoclonal Antibodies, Recombinant Hormones, Immunomodulators, Anti-inflammatory agents and Others. Based on drug class, Monoclonal Antibodies segment dominates the bio similar market in 2022.
Monoclonal antibodies have diverse applications across various therapeutic areas. From cancer treatment to autoimmune diseases, biosimilar Mabs addressed a wide range of medical needs, leading to a broad and growing market. Biosimilars, with their potential for cost savings while maintaining comparable efficacy and safety, gained significant attention as viable alternatives.
US Biosimilar Market by application
In US Biosimilar market, they are segmented by application into Oncology, Blood disorders, Chronic diseases and autoimmune conditions and Others. On the basis of application, Oncology segment was the dominant in 2022.
The increasing prevalence of cancer and the high cost of traditional biologics used in oncology treatment have created a strong incentive for the adoption of biosimilars. Biosimilars offer the potential to provide similar therapeutic outcomes at a lower cost, making them an attractive option for both healthcare providers and patients.
Additionally, the rigorous clinical trials and regulatory processes that biosimilars undergo to gain approval provide reassurance to healthcare professionals and patients regarding their safety and efficacy. This has led to increased acceptance and adoption of biosimilars in oncology.
US Biosimilar by Region
The US Biosimilar market is segmented by Region into North, East, West and South. In 2022, the dominance region is North region in US Biosimilar market.
The North region benefits from a concentration of healthcare providers and academic institutions that are at the forefront of adopting and integrating biosimilars into their treatment protocols. These institutions are more likely to have the expertise to evaluate and incorporate biosimilars effectively, driving their adoption among healthcare professionals and patients.
Click here to Download a Custom Report
Competition Scenario in US Biosimilar Market
The US biosimilar market has witnessed an evolving competitive landscape, with several key players competing for market share. Prominent pharmaceutical companies such as Amgen, Pfizer, Sandoz (Novartis), and Boehringer Ingelheim have been actively involved in developing and marketing biosimilar products. These established players have utilized their expertise in biologics and significant resources to navigate the regulatory landscape and compete effectively.
The competition in the US biosimilar market is characterized by a balance between established pharmaceutical giants and emerging biotech companies. While the major players possess the advantage of resources and experience, smaller biotech firms are also contributing to the market with innovative approaches and niche biosimilar offerings.
What is the Expected Future Outlook for the Overall US Biosimilar Market?
The US Biosimilar market was valued at USD ~Million in 2022 and is anticipated to reach USD ~ Billion by the end of 2028, witnessing a CAGR of ~% during the forecast period 2022- 2028. The US biosimilar market is likely to experience significant growth in the coming years, driven by several factors. Biosimilars are biologic drugs that are highly similar to already approved reference biologics. They offer potential cost savings, increased competition, and improved patient access to crucial treatments.
Firstly, the regulatory environment is becoming more favorable for biosimilars. The Biologics Price Competition and Innovation Act (BPCIA) established a pathway for biosimilar approval in the US, allowing for a smoother regulatory process. As more biosimilars receive approval, competition in the market is expected to intensify.
Secondly, patents for several blockbuster biologics are expiring or have already expired. This creates opportunities for biosimilar manufacturers to enter the market with more affordable alternatives, offering healthcare systems and patients a choice in treatment options.
Thirdly, as healthcare costs continue to rise, biosimilars present an attractive solution for reducing expenses. Their potential to offer cost savings without compromising therapeutic efficacy could lead to increased adoption by healthcare providers, insurers, and patients alike.
Physician and patient education are crucial, as misconceptions about biosimilars' safety and effectiveness might hinder their adoption. Additionally, legal and market access barriers, including patent litigation and complex distribution systems, could slow down the growth of the biosimilar market.
The biosimilar market witness consolidation as larger pharmaceutical companies acquire or partner with smaller biotech firms to bolster their biosimilar portfolios. This will lead to more resources being devoted to biosimilar development and marketing. Changes in healthcare policies, such as reimbursement models and value-based care initiatives, can influence the biosimilar market's growth. Favourable policies that incentivize biosimilar adoption drives their market growth.
#US Biosimilar Market#US Biosimilar Industry#US Biosimilar Sector#United States Biosimilar Market#US Biosimilar Market forecast#US Biosimilar Market analysis#US Biosimilar Market trends#US Biosimilar Market share#US Biosimilar Market key players#US Biosimilar Market revenue#US Biosimilar Market growth#Monoclonal Antibodies in biosimilar market US#Recombinant Hormones in biosimilar industry US#Oncology in bio similar market US#Blood disorders in biosimilar market US#Research institutes in Biosimilar market US#US similar biotherapeutics products market#Hospitals in Biosimilar market US#Investors in Biosimilar market US#US comparable biologics products industry#US recombinant biosimilars industry#US replicate biologics sector#US analog biologics market#US homologous biologics market#US oncology biosimilar market#US immunology biosimilar sector#US insulin biosimilar industry#US Generics Biologics market challenges#US leading Biosimilar drug providers#US leading Biosimilar drug manufacturers
0 notes
Text
Understanding the Biosimilars Market: Exploring Growth and Market Size Trends

The Biosimilars Market size was valued at USD 29.51 Billion in 2023 and is expected to reach USD 108.8 Billion By 2031 and grow at a CAGR of 17.7% over the forecast period of 2024-2031.The biosimilars market represents a dynamic frontier in healthcare, characterized by its rapid evolution and transformative impact on global access to biologic therapies. As innovator biologics face patent expirations, biosimilars emerge as cost-effective alternatives, driving competition and expanding treatment options across various therapeutic areas. This burgeoning market is propelled by advancements in biotechnology and stringent regulatory frameworks that ensure comparability, safety, and efficacy comparable to reference products. With increasing acceptance from healthcare providers and patients alike, biosimilars not only promise substantial savings but also foster innovation by reallocating resources towards novel therapies. As stakeholders navigate regulatory landscapes and overcome manufacturing complexities, the biosimilars landscape continues to thrive, offering unprecedented opportunities to enhance healthcare sustainability and affordability on a global scale.
Get Sample of This Report @ https://www.snsinsider.com/sample-request/2941
Market Scope & Overview
The market research report can be used by businesses, investors, stakeholders, suppliers, service providers, and distributors to evaluate the Biosimilars Market. To comprehend the situation of the market better, international business and marketing trends are researched. The reliability of the actual numbers was also increased by checking reliable sources to confirm the information. Using information from interviews and the expertise of market research experts, additional forecasts were created. The market research consists of data on the sector, product descriptions, company profiles, financial information, and contact details.
The statistics section of the report includes data on sales volume, price, revenue, gross margin, trends, historical growth, and anticipated future growth. In-depth analyses of each category, market sector, and regional and national market that were studied for the report are included, along with comprehensive statistics for each regional market. For each area, nation, and sub-segment, this Biosimilars Market report also contains historical market analysis and market forecasts.
Market Segmentation Analysis
By Type
Human growth hormone
Erythropoietin
Monoclonal antibodies
Insulin
Granulocyte-Colony Stimulating Factor
By Application
Blood disorders
Oncology diseases
Chronic and autoimmune diseases
Regional Outlook
For each Biosimilars Market region, analysts examined sales, production, and manufacturer data, with a focus on North America, Latin America, Asia Pacific, Europe, and the Middle East and Africa. This section assesses revenue and volume by region throughout the projected period.
Competitive Analysis
By examining the manufacturers' global income, global price, and global output during the predicted period, the reader can ascertain the footprints of the manufacturers. The top rivals in the Biosimilars Market are profiled in this section of the research report. It helps the reader comprehend the alliances and tactics that businesses form in response to market rivalry.
Key Questions Answered in the Biosimilars Market Report
What are the primary possibilities and challenges that the current market climate presents?
According to market research on sales, revenue, and market share, which industry and which category are leading in the target market?
How likely is it that developing market economies will expand in the upcoming years?
What strategies are the leading players doing to gain market share?
Conclusion
In order to present a complete picture of the target market, the research report analyses the worldwide Biosimilars Market and conducts research on consumption, value, year-over-year growth, and future development plans.
Read Full Report @ https://www.snsinsider.com/reports/biosimilars-market-2941
About Us
SNS Insider is a market research and insights firm that has won several awards and earned a solid reputation for service and strategy. We are a strategic partner who can assist you in reframing issues and generating answers to the trickiest business difficulties. For greater consumer insight and client experiences, we leverage the power of experience and people.
When you employ our services, you will collaborate with qualified and experienced staff. We believe it is crucial to collaborate with our clients to ensure that each project is customized to meet their demands. Nobody knows your customers or community better than you do. Therefore, our team needs to ask the correct questions that appeal to your audience in order to collect the best information.
Related Reports
Forensic Technology Industry Trends
Functional Service Providers Industry Trends
Genome Editing Industry Trends
Genomics Services Industry Trends
Genotyping Industry Trends
0 notes
Text
Unveiling the Power of Data: Global Digital Pathology Market
The global digital pathology market is on track to witness exponential growth, with a projected valuation of USD 7,035.8 million in 2023. According to the latest market analysis, this dynamic market is expected to surge to an impressive USD 24,961.8 million by 2033, reflecting a remarkable Compound Annual Growth Rate (CAGR) of 13.5% during the forecast period from 2023 to 2033.
Digital pathology, also known as virtual microscopy, is revolutionizing the field of pathology by enabling the digitization of tissue samples for analysis, diagnosis, and research purposes. This transformative technology offers numerous advantages over traditional microscopy, including enhanced accuracy, efficiency, and remote accessibility of pathology data.
The market for digital pathology is expanding due to two main factors: the growing use of advanced analytics in pharmaceutical and medical tests. The industry is expanding as a result of the application of analytics for fraud detection and claim management by government agencies and healthcare financing companies.
Request Your Detailed Report Sample With Your Work Email:
https://www.futuremarketinsights.com/reports/sample/rep-gb-124
Surge in R&D in Digital Pathology to Augment Sales in Forthcoming Years
In recent years, the digital pathology industry has increased investments in biosimilar research and development initiatives, primarily for cancer treatment and Covid-19 treatment, which have substantially fuelled the market expansion.
Contract Research Organizations (CROs), in-vitro diagnostics (IVD) laboratories, clinical laboratories, and regulatory consultants are being assigned biosimilar development responsibilities by pharmaceutical corporations. These enterprises are developing in order to provide more cost-efficient solutions to their customers, requiring the use of digital pathology instruments to diagnose, research, and design effective healthcare solutions.
Cancer diagnostic Opens New Avenues for Digital Pathology Companies
Due to the sheer increasing occurrence of cancer, there is an increase in spending on digital pathology equipment. Complications arising from late cancer diagnosis, particularly in the case of blood cancer and lung cancer, necessitate the use of significant capital resources during therapy and diagnosis. The success of digital pathology in research is driving demand for these systems in the cancer research industry. This is also encouraging government investors to invest in digital pathology systems for clinical diagnosis. A significant amount of money is spent on cancer diagnosis and therapy monitoring. This explains why digital pathology solutions are in high demand.
Competitive Landscape
Danaher Corporation
F. Hoffmann-La Roche AG
Huron Technologies International Inc.
Koninklijke Philips N.V.
Olympus Corporation
Hamamatsu Photonics K.K.
Carl Zeiss AG
Nikon Corporation
3DHISTECH Ltd.
Hologic Inc.
PerkinElmer, Inc.
Visiopharm
OptraSCAN, Inc.
Inspirata, Inc.
Sectra AB
Some of the recent developments in digital pathology market are as follows:
In May 2021, Leica Biosystems joined the European Society for Digital and Integrative Pathology (ESDIP) to assist pathology labs in developing laboratories in accordance with modern digital pathology instruments.
In March 2021, Roche inked a comprehensive merger agreement with GenMark Diagnostics to broaden its product and distribution channel for European consumers.
Key Segments:
Digital Pathology by Product Type:
Digital Pathology Equipment
Whole Slide Scanners
Brightfield Slide Scanners
Fluorescence Slide Scanners
Combination Slide Scanners
Clinical Microscope
Tissue Microarrays
Digital Pathology Software
Image Viewing and Analysis Software
On-premise
Cloud-based
Digital Pathology Information Systems
On-premise
Cloud-based
Digital Pathology Services
Installation and Integration Services
Consulting Services
Maintenance and Validation Services
Digital Pathology by Application:
Clinical Pathology
Molecular Diagnostics
Basic & Applied Research
Drug Development
Others
Digital Pathology by End User:
Hospitals
Diagnostic Laboratories
Pharmaceutical & Biotechnology Companies
Forensic Laboratories
Research Institutes
Contract Research Organizations (CROs)
Clinics
0 notes
Text
Asia Pacific Active Pharmaceutical Ingredients Market Share, Growth, Industry Segmentation, Analysis and Forecast 2027
The reports also help in understanding the Asia Pacific Active Pharmaceutical Ingredients Market Value dynamic, and structure by analyzing the market segments and projecting the Asia Pacific Active Pharmaceutical Ingredients Market Value. Clear representation of competitive analysis of key players by Design, price, financial position, product portfolio, growth strategies, and regional presence in the Asia Pacific Active Pharmaceutical Ingredients Market Value makes the report investor's guide.
Asia Pacific Active Pharmaceutical Ingredients Market Overview:
This Asia Pacific Active Pharmaceutical Ingredients Market industry research provided a comprehensive analysis of the worldwide Asia Pacific Active Pharmaceutical Ingredients Market, taking into account all critical variables such as growth factors, limitations, market advancements, top investment pockets, future prospects, and trends. The research begins by emphasizing the important trends and possibilities that may develop in the near future and have a favorable influence on overall industry growth.
The Asia Pacific Active Pharmaceutical Ingredients Market size is expected to reach US$ 76.00 Bn. by 2029, at a CAGR of 9.35% during the forecast period.
Request a Free PDF Sample Report @
https://www.maximizemarketresearch.com/request-sample/2267
Market Scope:
Asia Pacific Active Pharmaceutical Ingredients Market Research Report analyzed the current state of the definitions, classifications, applications, and industry chain structure. The analysis provides unbiased professional commentary on the present market scenario, prior market performance, production and consumption rates, demand and supply ratios, and income generation forecasts for the projected period. The Asia Pacific Active Pharmaceutical Ingredients Market study also gives information on the leading businesses functioning in the Asia Pacific Active Pharmaceutical Ingredients Market industry's strategic ambitions and company growth strategies. Mergers and acquisitions, government and corporate transactions, partnerships and collaborations, joint ventures, brand promotions, and product launches are among the methods evaluated in the research. To summarise what has been said thus far,
The Asia Pacific Active Pharmaceutical Ingredients Market report presents insights into each of the leading Asia Pacific Active Pharmaceutical Ingredients Market end users along with annual forecasts to 2027. The report provides revenue forecasts with sales and growth rate of the global Asia Pacific Active Pharmaceutical Ingredients Market. Forecasts are also provided for the market's product, application, and geographic segments. Forecasts are produced to help people understand the industry's future outlook and potential.
Segmentation:
Drug type, manufacturer type, synthesis type, therapeutic area, and geography are the several sections based on, which the active pharmaceutical ingredient market for the Asia Pacific is separated. Biotech and synthetic are the various division for the synthesis type segment of the API market. Biotech is one of the fastest-growing segments due to the shift in focus of traditional manufacturers towards biological drugs and high R&D cost for novel biosimilar drugs.
Purchase Inquiry:
Key Players:
The research includes the most recent news and industry developments regarding Asia Pacific Active Pharmaceutical Ingredients Market expansions, acquisitions, growth strategies, joint ventures and collaborations, product launches, market expansions, and so on. Among the main companies in the Asia Pacific Active Pharmaceutical Ingredients Market, the sector is
• Sun Pharmaceuticals
• Dr. Reddy’s Laboratories
• Aurobindo Pharma
• Syn-Tech Chem & Pharm Co., Ltd.
• Everlight Chemical Industrial Corporation
• Yung Shin Pharma Ind.Co., Ltd
• Meiji
• API Corporation
• SK Bioland
• Anzchem
• Auspep
Regional Analysis:
The primary goal of this study is to assist the user in understanding the market in terms of definition, segmentation, market potential, significant trends, and the problems that the industry is experiencing across ten key regions.
COVID-19 Impact Analysis on Asia Pacific Active Pharmaceutical Ingredients Market:
The research details the overall impact of COVID-19 on the Health Insurance Market by providing a micro- and macroeconomic analysis. The precise study focuses on market share and size, which clearly depicts the impact that the pandemic has had and is anticipated to have on the global Health Insurance Market in the future years.
Want your Report customized?
Key Questions answered in the Asia Pacific Active Pharmaceutical Ingredients Market Report are:
What is the function of the Asia Pacific Active Pharmaceutical Ingredients Market?
What is the predicted revenue generation of the Asia Pacific Active Pharmaceutical Ingredients Market?
At what growth rate is the Asia Pacific Active Pharmaceutical Ingredients Market evolving?
Who are the major market giants operating in the Asia Pacific Active Pharmaceutical Ingredients Market?
About Us:
Maximize Market Research provides B2B and B2C research on 12000 high-growth emerging opportunities & technologies as well as threats to the companies across the Healthcare, Pharmaceuticals, Electronics & Communications, Internet of Things, Food and Beverages, Aerospace and Defense, and other manufacturing sectors .
Contact Us:
MAXIMIZE MARKET RESEARCH PVT. LTD
3rd Floor, Navale IT Park Phase 2,
Pune Banglore Highway,
Narhe, Pune, Maharashtra 411041, India.
Asia Pacific Active Pharmaceutical Ingredients Market Size, Share, Analysis, Growth, Trends, Drivers, Opportunity
0 notes
Text
Global Biosimilars Market is estimated to be worth close to $18 billion in 2020 and is projected to expand at a Double-Digit Rate

A biosimilar is a biological product that is similar to a reference biologic in terms of potency, purity, and safety, with no clinically significant changes.
Global Biosimilars Market is estimated close to $18 Bn (2020) growing at a double-digit rate. The market growth is driven by increasing number of biosimilar approvals and growing number of biologics going off-patent.
Loss of patent protection for originator brands to drive the biosimilar growth
The number of loss of protection (LoP) events for originator brands will be a major factor in the expansion of the biosimilar market during the next 5-10 years. In the US alone, 66 biologic U.S. patents are expiring between 2020 and 2025, owing to which the biosimilar market is on the cusp of a significant boost. Oncology is one of the key therapeutic areas in biosimilars market and by 2023, the patents on nearly 20 oncology biologics will expire, which might drive the entry of more biosimilars into the oncology space and propelling oncology biosimilars market.
Consistent growth in biosimilar approvals in the US market
The US biosimilars market is growing across multiple therapeutic areas. From 1 approval in 2015, the number of FDA approvals have increased to 10 in 2019 (only 3 approvals in 2020 impacted by COVID as shown in figure below). The growth of the US biosimilars market is being driven by the constant and consistent rise in the number of biosimilar approvals and market accessibility in the US. In 2015, only 1 biosimilar product was available for use in market and this number reached to 19 by end of 2020.
“There is a huge potential for biosimilar market growth as they deliver significant benefits to the healthcare community. Biosimilars help augment overall treatment options, positively impact patients suffering from life-threatening conditions, whilst contributing to the quality and financial sustainability of healthcare systems.” – CEO, Leading Biosimilars Company
Explore Premium Report on Biosimilars Market @ https://meditechinsights.com/global-biosimilars-market/
Geographic Snapshot: Biosimilars Market
Due to the adoption of more liberal policies that have increased market access, Europe is the largest market for biosimilars. However, the US has taken a more protective approach toward originator brands as a result of lobbying from pharma companies. Because of this, the adoption of biosimilars is somewhat delayed in the US. However, the US biosimilar landscape is now developing quickly. There were 29 biosimilars approved in US compared to 11 in EU in the initial six years. Also, in April 2021, the US signed two bipartisan bills to reduce prescription drug prices by supporting generics/biosimilars. Such advancements will fuel the US market growth.
Competitive Landscape: Biosimilars Market
Some of the key players in global biosimilars market include Amgen, Roche, Sandoz, Dr. Reddy’s, Teva, Pfizer, Eli Lilly, Samsung, Biocon, Boehringer, and Mylan.
For More Detailed Insights, Contact Us @ https://meditechinsights.com/contact-us/
About Medi-Tech Insights
Medi-Tech Insights is a healthcare-focused business research & insights firm. Our clients include Fortune 500 companies, blue-chip investors & hyper-growth start-ups. We have completed 100+ projects in Digital Health, Healthcare IT, Medical Technology, Medical Devices & Pharma Services.
Contact:
Ruta Halde
Associate, Medi-Tech Insights
+32 498 86 80 79
0 notes
Text
Global Oncology Biosimilar Market
Global Oncology Biosimilar Treatment Market – Past, present, and way forward
Biosimilar or biogenerics are biotherapeutic products with large and complex structures that are similar to their innovator product in all aspects including their safety, efficacy, and quality.

There is a palpable sense of urgency among researchers and pharmaceutical industries to develop biosimilar for treatment, with its popularity expected to rise in the forthcoming years especially in developing low-cost biosimilar that is easily accessible and affordable for patients.
Market trends show that North America continues to dominate the global oncology biosimilar market followed by Europe, Asia Pacific, Latin America, Middle East, and Africa. The market is purported to reach approximately USD 45 Billion in the year 2026 from the current market of approximately USD 6 Billion.
Understanding the local and global market demand is necessary for companies, research institutes, and investors to study the changing dynamics in the biosimilar market. Segmentation of biosimilar by product, type of cancer, as well as targeted end-user such as hospital/online/retail pharmacies is particularly useful in empowering industries to make informed business decisions. Product segmentation also helps gauge the project’s feasibility so that the pharmaceutical industry can focus on developing safe and commercially viable products. Comprehensive analyses, robust legal and regulatory frameworks, continuing medical education for health care professionals and increased awareness among the public will undoubtedly increase the uptake of biosimilar and help the biosimilar market move ahead competitively.
#Oncology CRO in India#Oncology CRO#Biosimilar Studies in India#Oncology Biosimilar#Biopharmaceutics Services India
3 notes
·
View notes
Text
Primary and Secondary Research For #Bioprocess #Containers Market
The primaries interviewed for this study included experts from the industry, such as CEOs, VPs, directors, sales heads, and marketing managers of tier 1 and tier 2 companies engaged in offering single-use bioprocess containers across the globe, and administrators and purchase managers of biopharmaceutical companies and research institutions.
Extensive primary research was conducted after acquiring knowledge about the global market scenario through secondary research. Primary interviews were conducted from both the demand (biopharmaceutical manufacturing units, CROs & CMOs, research institutes, etc.).
The secondary sources referred to for this research study include publications from government sources; corporate & regulatory filings (such as annual reports, SEC filings, investor presentations, and financial statements); business magazines & research journals; press releases.
Growth in the bioprocess containers market is mainly driven by factors such as the growing biologics market, affordability and sustainability of single-use bioprocess technologies, and rising biopharmaceutical R&D.
For More Info, Download PDF Brochure @ https://www.marketsandmarkets.com/pdfdownloadNew.asp?id=107645832
In recent years, more biologics having higher potency that require smaller production volumes, advancements in biosimilars targeting smaller markets, and ongoing improvements in production yields and efficiencies that create production operations at much smaller scales have increased the use of single-use technologies such as single-use bioprocess containers.
As many patented products are going off-patent in the future, companies are falling short of funds to invest in their R&D pipelines. According to data published in the Generics and Biosimilars Initiative Journal, around twelve biological products with global sales of more than USD 67 billion will be exposed to biosimilar competition by 2020.
The patent expiry of blockbuster drugs has resulted in increased R&D spending by pharmaceutical and biotechnology companies. Patent expiry of biologics leads to increasing R&D of biologics and shifts biologics from the pipeline to commercial production.
Compared to traditional bio-manufacturing technologies, single-use systems (SUS) have many advantages, such as reduced requirements for process validation and higher manufacturing flexibility, which ultimately results in higher operating efficiency and reduced manufacturing costs.
The solid waste generated from single-use assemblies such as bioprocess containers creates several challenges for biomanufacturing companies, such as the disposal of solid waste and an increase in waste management costs.
Read Full Press Release @ https://www.prnewswire.com/news-releases/bioprocess-containers-market-worth-9-6-billion-by-2026--exclusive-report-by-marketsandmarkets-301386429.html
0 notes
Text
Global Oncology Biosimilar Treatment Market – Past, present, and way forward

Biosimilar or bio generics are bio therapeutic products with large and complex structures that are similar to their innovator product in all aspects including their safety, efficacy, and quality.
There is a palpable sense of urge among researchers and pharmaceutical industries to develop biosimilar for treatment, with its popularity expected to rise in the forthcoming years especially in developing low-cost biosimilar that are easily accessible and affordable for patients.
Market trends show that North America continues to dominate the global oncology biosimilar market followed by Europe, Asia Pacific, Latin America, Middle East, and Africa. The market is purported to reach approximately USD 45 Billion in the year 2026 from the current market of approximately USD 6 Billion.
Understanding the local and global market demand is necessary for companies, research institutes, and investors to study the changing dynamics in the biosimilar market. Segmentation of biosimilar by product, type of cancer, as well as targeted end-user such as hospital/online/retail pharmacies is particularly useful in empowering industries to make informed business decisions. Product segmentation also helps gauge the project’s feasibility so that the pharmaceutical industry can focus on developing safe and commercially viable products. Comprehensive analyses, robust legal and regulatory frameworks, continuing medical education for health care professionals and increased awareness among the public will undoubtedly increase the uptake of biosimilar and help the biosimilar market move ahead competitively.
0 notes
Text
Global Pediatric Vaccines Market Research Report with Size, Share, Value, CAGR, Outlook, Analysis, 2027
Global Pediatric Vaccines Market: is expected to reach US$ 54.5 Bn. at a CAGR of 10.7 during the forecast period 2027.
Global Pediatric Vaccines Market Overview:
The global pediatric vaccines market is expected to increase at a CAGR during the forecast period, owing to the growing engagement of various government healthcare as well as private organizations. Rising immunization alertness and destroying the pediatric infectious disease are also boosting the growth of the pediatric vaccines market. Rise in incidence of diseases like chicken pox, typhoid, cholera, measles, and hepatitis, in kids are propelling the growth of the market. However, low availability to remote areas, growing accessibility of biosimilars at low rates, and increasing cost of vaccines are hampering the growth of the market.
Global Pediatric Vaccines Market Report 2020-2027 (Forecast Period) includes an analysis of market growth factors, future analysis, country-level, and regional-level analysis, market distribution, and competitive landscape analysis of major key competitors. Within the analysis report, each phase of the world global market is completely studied. The regional study of the global market will help you in gaining an understanding of the developments of the various geographic markets in recent years and moving ahead. The analysis also includes a wide-ranging synopsis of the key factors of the global market, like market influence and market result factors, drivers, threats, constraints, trends, restraints, and prospects. alternative methods of analysis, like qualitative and quantitative, are also used within the analysis study. Maximize Market Research team analysis provides a unique and in-depth report that helps you to perform detailed research on the global Global Pediatric Vaccines market and make decisions on the future growth factors of the market. The market report provides an overview of the development of the Global Pediatric Vaccines market throughout the forecasted period.
Request for free sample: https://www.maximizemarketresearch.c...t-sample/24882
The Global Pediatric Vaccines market report thoroughly covers insights of key competitors in terms of market, applications, and geographies that will help you in recognizing the competition both domestically as well as globally. The research mentions available micro-market investment options for investors and a full synopsis of the competitive landscape and significant products offered by industries.
The report also includes statistical data that consists of tables and charts which will further help you in business representation. Maximize Market Research also studies the current as well as new trends with the estimates of the market size throughout the forecasted period. Many competitors are also identified in various regions. The report provides PESTEL and Portal analysis too, which helps the clients to calculate every factor of the market.
Global Pediatric Vaccines Market Segmentation:
The Asia Pacific is expected to showcase the highest growth rate in pediatric vaccines market during the forecast period, owing to an increasing larger population. The growing healthcare sector in leading regions such as China and India is boosting the growth of the Asia Pacific. European pediatric vaccines market is the rise, due to the superior government spending such as (NHS) nation healthcare service for the development of the pharmaceutical as well as medical industries. The Middle East & Africa region is expected to grow at a steady pace, owing to the rising research and development activities in the healthcare sector as well as extensive development of healthcare infrastructure.
Global Pediatric Vaccines Market Key Players:
• Merck Sharp & Dohme Corp
• SANOFI
• Indian Immunologicals Limited
• Mitsubishi Tanabe Pharma Corporation
• Sinovac Biotech Ltd.
• GlaxoSmithKline plc.
• Serum Institute of India Pvt. Ltd.
• Eli Lilly Company
• Pfizer, Inc.
• Bio Med Pvt. Ltd
• Sinova
• Grifols
• AstraZeneca
• Cadila Healthcare Ltd
• CSL Limited.
• Zydus Cadila
• Panacea Biotech
If You Have Any Questions About This Report? Please Contact Us On the link mentioned below: https://www.maximizemarketresearch.c...-market/24882/
Global Pediatric Vaccines Market Regional Analysis:
Based on the regions the market is studied across
Asia-Pacific (Vietnam, Malaysia, Korea, China, Philippines, Thailand, Indonesia, and India, Japan, Australia, ). Africa and the Middle East (Egypt and GCC Countries.). North America (Canada, the United States, and Mexico.). South America (Brazil etc). Europe (France, Italy, Germany, Russia, UK, Turkey, etc.).
IMPACT Of COVID-19 On The Global Pediatric Vaccines Market:
COVID-19 has impacted the world with its fast-spreading effects all over the world. It has impacted every industry and business except for the medical and health care industry which is working 24/7 to stop the spread of the COVID-19 virus, they are working hard to save the lives of people affected by this virus. As mentioned many industries are suffering in this COVID-19 times, our market is also one of them. Our team here at Maximize Market Research has worked hard to provide solutions to these issues that will help your business.
About Us:
Maximize Market Research provides B2B and B2C research on more than 12,000 high growth emerging opportunities technologies as well as threats to the companies across the Healthcare, Pharmaceuticals, Electronics Communications, Internet of Things, Food and Beverages, Aerospace and Defense, and other manufacturing sectors.
Contact Us:
MAXIMIZE MARKET RESEARCH PVT. LTD.
3rd Floor, Navale IT Park Phase 2,
Pune Bengaluru Highway,
Narhe, Pune, Maharashtra 411041, India.
Email: [email protected]
Phone No.: +91 20 6630 3320
Website: www.maximizemarketresearch.com
Get More Report:
http://www.marketwatch.com/story/us-...027-2022-01-17
http://www.marketwatch.com/story/fac...026-2022-01-17
0 notes
Text
Top 5 players in US Biosimilar Market
Buy Now
STORY OUTLINE
Pfizer: Excelling in the line of Biosimilar drugs with an experience of more than 10 years with presence in over 180 countries.
Amgen: Making pharmaceutical products with an experience of over 40 years and presence in over 100 countries.
Viartis: Presence in over 165 countries, and making Biosimilar drugs in over 75 markets, this pharmaceutical company is another leading contributor of US Biosimilar market.
Coherus Biosciences: Increasing patient access to cost effective medicines with a Biosimilar drugs experience of 13 years.
Biogen: serving humanity through science with a experiences of more than 40 years in the field of biologics.
According to Ken Research, the US Biosimilar market is anticipated to grow at a CAGR of ~40% in the next five years which currently has a market size of ~USD 9.4 Bn.
The US Biosimilar market is rapidly growing and will be witnessing a significant growth in the next five years.
There are various reasons behind the rapid growth of US Biosimilar market. Some of the major reasons behind the growth of US Biosimilar market include the cost effective nature of Biosimilar drugs, rising geriatric population, rising prevalence of chronic diseases, and growing partnerships between companies to develop Biosimilar drugs.
Various companies and players are contributing to their best efforts in the growth of the US Biosimilar market.
This article aims to put light on the contributions done by the major players towards the growth of the US Biosimilar market.
1.Pfizer
Click to read more about Pfizer
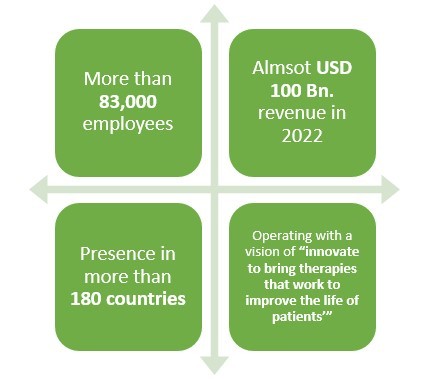
Pfizer is a leading American pharmaceutical company which is operating in the field of generics or original drugs for more than 30 years. But did you know that this pharma not only manufactures biologics but also biosimilar drugs?
Pfizer has been in the business of biosimilar drugs for more than 10 years and have been quite successful as well. With more than 83,000 employees and presence in over 180 countries, this leading pharmaceutical company made almost USD 2 Bn. revenue only from its Biosimilar drugs sale in 2021.
Recently, this pharmaceutical company also collaborated with Samsung in two deals to produce various biosimilar drugs in South Korea. The deal size between these two companies happens to be approximately USD 900 Bn.
The major Biosimilar drugs of this pharmaceutical giant are primarily
ZIRABEV (a Biosimilar of Avastin)
TRAZIMERA (a Biosimilar of Herceptin)
RUXIENCE (a Biosimilar of Rituxan)
RITACRIT (a Biosimilar of Epogen)
NVYEPRIA (a Biosimilar of Neulasta)
NIVESTYM (a Biosimilar of Neupogen)
FILGRASTIM (a Biosimilar of Neupogen).
2.Amgen
Click here to Download a Sample Report
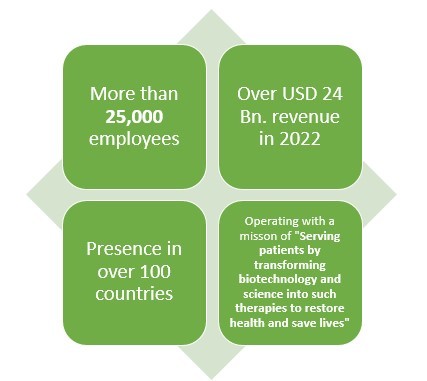
Amgen is another leading American pharmaceutical company which not only makes Biologics or generic drugs but also Biosimilar drugs. This pharmaceutical company has more than 40 years of experience when it comes to pharmaceutical line.
With over 25000 employees and presence in over 100 countries, this pharmaceutical company earned about USD 2 Bn. from their three biosimilar drugs which are reportedly MVASI, KANJITNTI, and AMJEVITA.
This pharma giant has also invested about USD 2 Bn. in the development of Biosimilar drugs.
This pharmaceutical company has made Biosimilar drugs primarily in 4 fields which are General Medicine, Oncology, and Hematology along with, Inflammation.
EPOTEIN ALFA
AMJEVITA
AVSOLA
KANJINTI
MVASI
RIABNI
are the various Biosimilar drugs of Amgen. And, STELARA, EYLEA, SOLIRIS are in their pipeline.
Recently Amgen revealed their Biosimilar report’s 8 version. It revealed a major information which said that the pharmaceutical company saved about USD 10 Bn. through their Biosimilar drugs in the past five years.
3.Viartis

Headquartered in Canonsburg, Pennsylvania, this American pharmaceutical company was founded only in 2020 yet they have achieved massive success in the pharmaceutical products with their revenue being USD 16 ~Bn. in 2022.
With presence in 165 countries and with over 45,000 employees worldwide, this pharmaceutical company makes pharmaceutical products in 10 areas which primarily are Cardiovascular, Dermatology, ophthalmology, Oncology, Gastroenterology, Women’s health, Infectious diseases, Diabetes & Metabolism, Immunology, CNS & Anesthesiology, Respiratory diseases and allergy.
Speaking of their first Biosimilar products, their first ever Biosimilar drug was launched in 2014. They have a variety of Biosimilar drugs which are primarily
TRASTUZUMAB
INSULIN ASPART
PEGFILGRASTIM
INSULIN GLARGINE-YFGN
ADALIMUMAB
BEVACIZUMAB
Their Biosimilar drug Insulin Glargine which is known as SEMGLEE was the first ever interchangeable Biosimilar drug in the United States which was FDA approved.
Their PEGFILGRASTIM also was the first ever FDA approved drug in the United States. They have launched their Biosimilar drugs in over 75 markets worldwide.
4.Coherus Biosciences:
Click here to Ask for a Custom Report
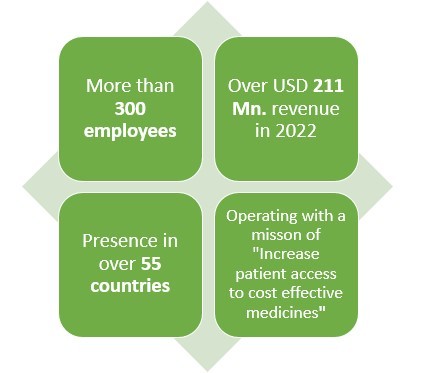
Headquartered in Redwood city, California this American pharmaceutical company earned a revenue of almost USD 211 Mn. In 2022.
With presence in over 55 countries and 300+ employees worldwide, this pharmaceutical company makes products in various areas such as solid tumors, non-small lung cancers, nasopharyngeal carcinoma, small cell lung cancer and hepatocellular carcinoma.
Speaking of their Biosimilar drugs, this pharma has been in the field of creating Biosimilar drugs since 2010 which has given them almost 13 years of experience.
This pharmaceutical company also disclosed that it plans to spend at least USD 1 Tn. on medicines worldwide, out of which at least 40% will be spent on Biosimilar drugs.
Their three major Biosimilar drugs which are also FDA approved include UDENCYA, YUSIMRY, and CIMERLI.
Udencya is a Biosimilar drug of Pegfilgrastim, Yusimry is a Biosimilar drug of Ranibizumab, and Cimerli is a Biosimilar drug of Adalimumab.
5.Biogen
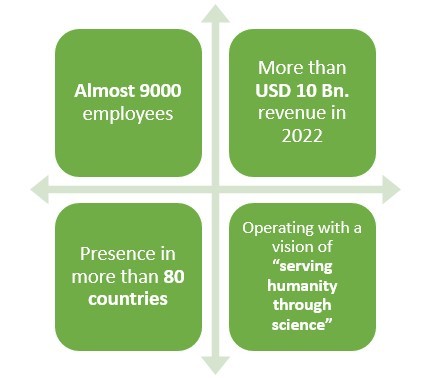
Headquartered in Cambridge, Massachusetts, this American pharmaceutical company earned a revenue of around USD 10 Bn. in 2022.
This company happens to have an experience of more than 40 years when it comes to making pharmaceutical products.
With presence in over 80 countries and more than 9000 employees worldwide, this pharmaceutical company primarily deals in Neurology, Specialized Immunology, Neuropsychiatry, Ophthalmology, and Rare Diseases.
ADUCANUMAB
LECANEMAB
TOFERSEN
ZURANOLONE
LITIFILIMAB
BENAPALI
FLIXABI
IMRALDI
are some of their Biosimilar drugs.
With their Biosimilar drugs, more than 250,000 people have gone on Anti-Tumor Necrosis Factor therapy.
Recently, this pharmaceutical company also made an agreement with Bio-Thera solutions to develop a Biosimilar drug for the treatment of Rheumatoid Arthritis.
#US Biosimilar Sector#United States Biosimilar Market#US Biosimilar Market forecast#US Biosimilar Market analysis#US Biosimilar Market trends#US Biosimilar Market share#US Biosimilar Market key players#US Biosimilar Market revenue#US Biosimilar Market growth#Monoclonal Antibodies in biosimilar market US#Recombinant Hormones in biosimilar industry US#Oncology in bio similar market US#Blood disorders in biosimilar market US#Research institutes in Biosimilar market US#US similar biotherapeutics products market#Hospitals in Biosimilar market US#Investors in Biosimilar market US#US comparable biologics products industry#US recombinant biosimilars industry#US replicate biologics sector#US analog biologics market
0 notes
Text
Oncology Biosimilars Market Overview: Analysis By Product, Portability, Application, Region, Country: Trends, Opportunities and Forecast (2021- 2027)
Oncology Biosimilars Market Overview:
The report on Oncology Biosimilars market covers segments such as By Cancer Type, Product Type, Distribution Channel and Region. The Cancer Type segment includes Lung Cancer, Colorectal cancer, Cervical Cancer, Breast Cancer, Kidney cancer, Stomach cancer, Brain Cancer, and Others. Among the Cancer Type, Breast cancer is anticipated to dominating segment throughout forecast period owing to raising incidence of breast cancer in globe.
Maximize Market Research has published a comprehensive research and analysis of the Oncology Biosimilars Market, which identifies new developments in the Oncology Biosimilars Market and gives business insights to organisations. This research assists manufacturers, suppliers, and investors, as well as CEOs, in identifying opportunities and business optimization techniques for increasing their value in the worldwide Oncology Biosimilars Market. Provides critical information for well-known organisations that are among the best performers. The research covers present and new markets in depth, as well as an assessment of competitiveness in shifting market circumstances.
List of Key players: Following are the number of key players studied to understand the Oncology Biosimilars Market:
• Amgen Inc.
• Allergan, Plc.
• Mylan N.V.
• Samsung Bioepis Co., Ltd.
• Teva Pharmaceutical Industries Ltd.
• Pfizer Inc.
• Celltrion Healthcare
• Novartis International AG
• Biogen Idec, Inc.
• Biocon Limited.
• Celltrion Inc.
• Dr. Reddy’s Laboratories Ltd.
• STADA Arzneimittel AG
• Intas Pharmaceuticals Ltd.
• Sandoz International GmbH
• Apotex Inc.
• BIOCAD.
• Merck & Co., Inc.
• Coherus Biosciences
• F Hoffmann-La Roche
• Momenta Pharmaceuticals Inc.
Enquiry before buying this premium Report:
https://www.maximizemarketresearch.com/request-sample/39767
This study estimates the size of the Oncology Biosimilars Market in terms of both value and volume. It includes a thorough examination of the market's major drivers, restraints, and opportunities. Through the Porter's five forces analysis, the study examines the major elements that contribute to the growth of the Oncology Biosimilars Market. Furthermore, the market participants have been profiled, with a particular emphasis on their product offers.
The research also includes data in the form of statistics, facts, and figures, as well as contact information and sales contact information for the global market's leading players. There is a full picture of the global Oncology Biosimilars Market 's competitive landscape, with all information gathered and deepened with the SWOT analysis. Opportunities for possible industrial expansion have been identified, as have the competitive threats associated.
The report also discusses critical industry niches such as items or services supplied, downstream fields, end-user customers, historical data figures for revenue and sales, market background, and more. To acquire a better understanding of the companies, global Oncology Biosimilars Market-leading main players have been profiled. It offers specific expansions to a variety of high-level industries operating around the globe. The section also includes useful information such as an overview of the firm and its market share, company biographies, and some significant methods used by key companies to build their businesses.
The introduction of new goods and the research involved in the development of innovative products is one of the primary components that is likely to have an impact on the Oncology Biosimilars Market. The study provides information on the sales and market growth of various markets on a regional and national scale. This report is to provide a market analysis in terms of growth patterns, prospects, and market players' contributions to market development. The research also examines market demand growth estimates for products and services.
OBJECTIVES OF THE STUDY
To analyse and forecast the market size of the global Oncology Biosimilars Market, in terms of value and volume
To identify significant trends and factors driving or inhibiting the market growth
To analyse the opportunities in the market for stakeholders by identifying the high-growth segments
To define, describe, and forecast the market by type, application, and region
To forecast the size (in terms of value and volume) of the market and submarkets by five regions, namely, Asia-Pacific, Europe, North America, the Middle East & Africa, and South America
To strategically analyse each submarket with respect to individual growth trend and their contribution to the market
To analyse competitive developments such as expansions, agreements, new product launches, and acquisitions in the market
To strategically profile the key players and comprehensively analyse their growth strategies
Our Research Methodology:
This research study involves the use of extensive secondary sources such as encyclopedia, directories, and databases to identify and collect information useful for this technical, market-oriented, and commercial study of the Oncology Biosimilars Market. Primary sources that include selected experts from related industries and selected suppliers have been interviewed to obtain and verify important information as well as to assess future prospects. As a part of the secondary research process, various sources have been referred to for identifying and collecting information for this study. Secondary sources include annual reports, press releases, and investor presentations of companies; white papers; certified publications, articles from recognized authors; and gold & silver standard websites.
Browse Complete Oncology Biosimilars Market details and List Of Figures Here:
https://www.maximizemarketresearch.com/market-report/oncology-biosimilars-market/39767/
About Us.:
Maximize Market Research has served esteemed clients including Yamaha, Boeing, Sensata, Etnyre, Canada, ALCOR M&A, Microsoft, Harman, and other 200 MNCs worldwide. The Company provides B2B and B2C market research on 5000 high growth emerging technologies & opportunities in Transportation, Chemical, Healthcare, Pharmaceuticals, Electronics & Communications, Internet of Things, Food and Beverages, Aerospace and Defense and other manufacturing sectors.We, at Maximize Market Research, are a strong unified team of industry specialists and analysts across sectors to ensure entire Industry ecosystem is taken in perspective, factoring all recent development, latest trends and futuristic – the technological impact of uniquely specific industries.
Contact Details:
Maximize Market Research Pvt. Ltd.
Phone: US: +1 774 775 2163; APAC: +91-9607365656
Email: [email protected]
Website: www.maximizemarketresearch.com
0 notes
Text
Sales Forecast of Pegfilgrastim Biosimilar Market , Analysis, and Forecast Report till 2031
Fact.MR recently published a market study on the global market for pegfilgrastim biosimilars. The study provides a detailed assessment of key market dynamics, including the drivers, trends, opportunities, and restraints, as well as detailed information about the pegfilgrastim biosimilar market structure. The market study presents exclusive information about how the pegfilgrastim biosimilar market will grow during the forecast period (2020 to 2026).
Key indicators of market growth, which include year-on-year (Y-o-Y) growth of the market and compounded annual growth rate (CAGR) are explained in Fact.MR’s study in a comprehensive manner. This information can help readers understand the quantitative growth prospects of the pegfilgrastim biosimilar market during the forecast period.
Bottom-up approach is always used to obtain Pegfilgrastim Biosimilar insightful data for the specific country/regions.
Click Here To get a Sample Report (Including Full TOC, Table & Figures):-https://www.factmr.com/connectus/sample?flag=S&rep_id=1494
Key Segments of Pegfilgrastim Biosimilar Market
Fact.MR’s study on the pegfilgrastim biosimilar market offers information divided into two important segments-distribution channel and region. This report offers comprehensive data and information about the important market dynamics and growth parameters associated with these categories, for the better understanding of readers.
Distribution Channel
Hospital Pharmacies
Retail Pharmacies
Mail-Order Pharmacies
Region
North America
Latin America
Europe
South Asia
East Asia
Oceania
Middle East & Africa
A comprehensive estimate of the Pegfilgrastim Biosimilar market has been provided through an optimistic scenario as well as a conservative scenario, taking into account the Sales of Pegfilgrastim Biosimilar during the forecast period. Price point comparison by region with the global average price is also considered in the study.
The analysts have used numerous industry-wide prominent business intelligence tools to consolidate facts, figures, and market data into revenue estimations and projections in the Market Insights of Pegfilgrastim Biosimilar.
Key stakeholders in Market including industry players, policymakers, and investors in various countries have been continuously realigning their strategies and approaches to implement them in order to tap into new opportunities.
The Market survey of Pegfilgrastim Biosimilar offers a comprehensive analysis of diverse features, including production capacities analysis of Pegfilgrastim Biosimilar, demand, product developments, revenue generation, and Size of Pegfilgrastim Biosimilar Market across the globe.
Key Takeaways from Pegfilgrastim Biosimilar Market Study
The hospital pharmacies segment under the distribution channel category held half of the global pegfilgrastim biosimilar market share in 2019, owing to increasing cancer treatments such as chemotherapy and others. Retail pharmacies followed due to increasing number of prescriptions.
North America holds almost 3/4 of the global pegfilgrastim biosimilar market, followed by Europe, owing to large number of product launches in these regions.
The East Asia market year-on-year growth is expected to rapidly surge in the near future. This growth is due to increasing pool of patients and advancements in healthcare with government support, which will propel pegfilgrastim biosimilar market growth in Asian countries.
The COVID-19 pandemic that has swept the world is projected to have only a moderate impact on the progress of the pegfilgrastim biosimilar market.
Technological Innovation in Medical Devices Revolutionizing the Healthcare System
The medical device industry is a heterogeneous, innovative and dynamic sector. From telemedicine to artificial intelligence, robotic surgery and 3D printing, technology is revolutionizing the healthcare industry.
The intersection of healthcare and technology has led to numerous advancements in medical devices. New age medical technology has transformed the way doctors and patients participate and interact with each other. Introduction of advanced medical devices such as drug-device combinations (DDCs), preventive and predictive equipment’s, self-care devices is completely transforming the medical world.
Increasing investments by public and government bodies in healthcare sector is positively impacting the Pegfilgrastim Biosimilar market. Governments across the globe are introducing various initiatives to strength the medical device sector with major emphasis on research and development (R&D).
Pegfilgrastim Biosimilar manufacturing companies and healthcare service providers have started offering personalized patient care and access to complete end-to-end medical device products and services. They are focusing on developing and enhancing product prototyping and minimizing operation cost.
With the onset of COVID-19 pandemic the sales of Pegfilgrastim Biosimilar witnessed a huge upsurge and the trend is likely to continue in the future.
Need More information about Report Methodology? Click here:-https://www.factmr.com/connectus/sample?flag=RM&rep_id=1494
Some Notable Offerings by Fact.MR Report on Pegfilgrastim Biosimilar market:
We will provide you an analysis of the extent to which this Pegfilgrastim Biosimilar market research report acquires commercial characteristics along with examples or instances of information that helps you to understand it better.
We will also help to identify customary/ standard terms and conditions, as offers, worthiness, warranty, and others.
Also, this report will help you to identify any trends to forecast growth rates.
The analyzed report will forecast the general tendency for supply and demand.
Some of the Pegfilgrastim Biosimilar Market insights and estimations that make this study unique in approach and effective in guiding stakeholders in understanding the growth dynamics. The study provides:
Details regarding latest innovations and development in Pegfilgrastim Biosimilar and how it is gaining customer traction during the forecast period.
Analysis about the customer demand of the products and how it is likely to evolve in coming years.
Latest regulations enforced by government bodies and local agencies and their impact on Demand of Pegfilgrastim Biosimilar Market .
Insights about adoption of new technologies and its influence on the Pegfilgrastim Biosimilar market Size.
Overview of the impact of COVID-19 on Pegfilgrastim Biosimilar Market and economic disruptions caused by the pandemic.
Evaluates post-pandemic impact on the Sales of Pegfilgrastim Biosimilar Market during the forecast period.
To get all-in insights on the regional landscape of the Pegfilgrastim Biosimilar Market, Buy Now:-https://www.factmr.com/checkout/1494
After reading the Market insights of Pegfilgrastim Biosimilar Report, readers can:
Understand the drivers, restraints, opportunities and trends affecting the Sales of market.
Analyze key regions holding significant share of total Pegfilgrastim Biosimilar market revenue.
Study the growth outlook of Pegfilgrastim Biosimilar market scenario, including production, consumption, history and forecast.
Learn consumption pattern and impact of each end use & supply side analysis of Pegfilgrastim Biosimilar market.
Investigate the recent R&D projects performed by each market player & competitive analysis of Pegfilgrastim Biosimilar Market Players.
Read More Trending Reports of Fact.MR: – https://www.biospace.com/article/breast-cancer-diagnostics-market-sales-set-to-grow-by-4-7-percent-to-surpass-us-2000-mn-by-2022-end-fact-mr
How Fact.MR Assists in Making Strategic Moves For Pegfilgrastim Biosimilar Market Manufacturer?
The data provided in the Pegfilgrastim Biosimilar market report offers comprehensive analysis of important industry trends. Industry players can use this data to strategize their potential business moves and gain remarkable revenues in the upcoming period.
The report covers the price trend analysis and value chain analysis along with analysis of diverse offering by market players. The main motive of this report is to assist enterprises to make data-driven decisions and strategize their business moves.
More Related Reports by Fact.MR On Healthcare Sector:
Veterinary Endodontics Market :_Forecast, Trend Analysis & Competition Tracking – Global Review 2021 to 2031
Therapeutic Support Surface Market:- Forecast, Trend Analysis & Competition Tracking – Global Review 2021 to 2031
Computer-Assisted Orthopedic Surgery Market :-Forecast, Trend Analysis & Competition Tracking – Global Review 2021 to 2031
About Fact.MR
Market research and consulting agency with a difference! That’s why 80% of Fortune 1,000 companies trust us for making their most critical decisions. We have offices in US and Dublin, whereas our global headquarter is in Dubai. While our experienced consultants employ the latest technologies to extract hard-to-find insights, we believe our USP is the trust clients have on our expertise. Spanning a wide range – from automotive & industry 4.0 to healthcare & retail, our coverage is expansive, but we ensure even the most niche categories are analyzed. Reach out to us with your goals, and we’ll be an able research partner.
Contact:
US Sales Office :
11140 Rockville Pike
Suite 400
Rockville, MD 20852
United States
Tel: +1 (628) 251-1583
E-Mail: [email protected]
Corporate Headquarter:
Unit No: AU-01-H Gold Tower (AU),
Plot No: JLT-PH1-I3A,
Jumeirah Lakes Towers,
Dubai, United Arab Emirates
Visit Our Website: https://www.factmr.com
0 notes
Text
Global Oncology Biosimilar Market
Global Oncology Biosimilar Treatment Market – Past, present, and way forward
Biosimilar or biogenerics are biotherapeutic products with large and complex structures that are similar to their innovator product in all aspects including their safety, efficacy, and quality.
There is a palpable sense of urgency among researchers and pharmaceutical industries to develop biosimilar for treatment, with its popularity expected to rise in the forthcoming years especially in developing low-cost biosimilar that is easily accessible and affordable for patients.
Market trends show that North America continues to dominate the global oncology biosimilar market followed by Europe, Asia Pacific, Latin America, Middle East, and Africa. The market is purported to reach approximately USD 45 Billion in the year 2026 from the current market of approximately USD 6 Billion.
Understanding the local and global market demand is necessary for companies, research institutes, and investors to study the changing dynamics in the biosimilar market. Segmentation of biosimilar by product, type of cancer, as well as targeted end-user such as hospital/online/retail pharmacies is particularly useful in empowering industries to make informed business decisions. Product segmentation also helps gauge the project’s feasibility so that the pharmaceutical industry can focus on developing safe and commercially viable products. Comprehensive analyses, robust legal and regulatory frameworks, continuing medical education for health care professionals and increased awareness among the public will undoubtedly increase the uptake of biosimilar and help the biosimilar market move ahead competitively
0 notes
Text
Why Did Republicans Vote Against Hr3
New Post has been published on https://www.patriotsnet.com/why-did-republicans-vote-against-hr3/
Why Did Republicans Vote Against Hr3
How Would Medicare Negotiate Drug Prices Under Hr 3
Why Do Most White People Vote Republican?
H.R. 3 amends the non-interference clause under current law by adding an exception that allows for the price negotiation process established by the legislation. The negotiation process applies to at least 25 and 50 single-source brand-name drugs lacking generic or biosimilar competitors, selected from among the 125 drugs with the highest net Medicare Part D spending and the 125 drugs with the highest net spending in the U.S., which could include physician-administered drugs covered under Medicare Part B, along with all insulin products. Drugs that are new to market could also be subject to negotiation if their list price is greater than median household income and their projected spending would place them among the list of drugs with the highest spending under Medicare or the U.S. overall.
In determining the maximum fair price, H.R. 3 requires the Secretary to consider research and development costs, market data, production and distribution costs, and existing therapeutic alternatives, including comparative effectiveness data. If a manufacturer offers a price that is no more than the target price, the proposal requires the Secretary to accept this as the maximum fair price for the drug. The agreed-upon negotiated price would be made available to private plan sponsors in Medicare Part D and commercial payers in group and individual markets, and to providers that administer physician-administered drugs.
Prescription Drug Costs Raise Profits For Big Pharma While Lowering Quality Of Life For Millions
Its already been a bad time for pharmaceutical companies. Many have received the brunt end of widespread public dissatisfaction after being criticized in the media and political realms.
Now that healthcare is carrying political narratives, out-of-pocket spending like the kind that would go down for Medicare parts B and D beneficiaries should HR 3 or a similar bill become law is more than just a pundit talking point.
Everyone in America knows someone who is taking an expensive medicine, Robinson said. And they vote, so this is obviously political.
Between patients out-of-pocket costs all the way up to what CMS covers, the United States spent more than $330 billion on prescription drugs in 2017.
That is an enormous draining of dollars from taxpayers out of Medicare and Medicaid, and out of the pockets of Americans who have to pay high premiums, deductibles, and copays, which are driven up significantly by the highest prescription drug costs in the world, Chris Orestis, president of LifeCare Xchange, told Healthline.
Orestis says freeing up even the smallest percentage of this money could raise the standard of living for many people, as well as having a stimulating impact on the economy.
It would also help people who react to the high costs of prescription drugs by rationing their care and dosages.
Republicans Hold Firm Against Socialism
Despite President Trumps frequent use of the bully pulpit to criticize the drug industry, the good news is that only two Republicans joined the Democrats in supporting passage of H.R. 3. House Democrats either did not read or were unmoved by multiple studies suggesting tremendous peril for small and emerging biotechs if the legislation becomes law and our investors run for the hills. More than 130 life sciences investors have publicly confirmed they would have no other choice.
Also Check: Which Republicans Might Vote For Impeachment
If Implemented The Bill Could Save Americans Billions
The Congressional Budget Office estimates that HR 3 would account for about $98 billion in savings over a 10-year period.
The price negotiation provisions would lower spending by about $456 billion, but covering dental, vision, and hearing under the Medicare program would raise spending by approximately $358 billion.
Dr. James C. Robinson, PhD, MPH, the Leonard D. Schaeffer professor of health economics and the director of the Berkeley Center for Health Technology, says HR 3 would help bring the costs of drugs in the United States closer to the International Reference Price, or what other wealthier countries pay.
For reference, the United States pays 60 percent more than Germany, another country with high healthcare costs.
The big issue is the part of HR 3 that allows CMS to negotiate drug prices under Medicare Part D, which Robinson says is predominantly run by private interests.
If youre pharma, this is bad, he told Healthline.
So What Exactly Would This Legislation Do


The centerpiece of the House Democrats’ bill would require the government to negotiate the cost of up to 250 brand-name drugs that don’t have competition and cost the U.S. health care system the most money. Insulin for diabetes would have to be included. The maximum price negotiated would be capped: It couldn’t be more than 120 percent of the average price charged in other industrialized countries that typically pay less than the United States. Drug companies would either play ball or face an excise tax starting at 65 percent and rising to 95 percent. Private insurance plans could also use the negotiated prices.
The nonpartisan Congressional Budget Office estimated this part of the bill would save $456 billion over a 10-year period. Its projected to cost drug companies at least $500 billion in revenue, which CBO predicts would cut research funding and result in about eight fewer new drugs coming to market in the first 10 years the law was in effect, and 30 fewer drugs in the following decade.
The legislation also tries to restrain companies abilities to launch new drugs at astronomical prices by allowing the government to negotiate the cost of any medicines that come to market at list prices higher than the U.S. median household income. The plan would also cap seniors’ annual out-of-pocket spending for outpatient prescription drugs at $2,000.
Recommended Reading: Are There Any Republicans Running Against Donald Trump
Govtrackus Is Taking A New Focus On Civic Education
Help us develop the tools to bring real-time legislative data into the classroom.
If youve visited a bill page on GovTrack.us recently, you may have noticed a new study guide tab located just below the bill title. This is part of a new project to develop better tools for bringing real-time legislative data into the classroom. We hope to enable educators to build lesson plans centered around any bill or vote in Congress, even those as recent as yesterday.
Were looking for feedback from educators about how GovTrack can be used and improved for your classroom. If you teach United States government and would like to speak with us about bringing legislative data into your classroom, please reach out!
House Republicans Vote Against Slashing Costs For Prescription Drugs
A bill to lower the cost of prescription drugs passed the House with mostly Democratic support.
The House of Representatives took the issue of high prescription drug costs head on Thursday, passing a bill that promises to lower the costs of medication associated with cancer, asthma, and many other conditions.
By a 230-192 vote, H.R.3, the Elijah E. Cummings Lower Drug Costs Now Act, passed on a largely party line vote. Every Democrat supported the legislation, joined by only 2 Republicans, with the lone House independent, Rep. Justin Amash of Michigan, voting no. The bill was named after the late Rep. Elijah Cummings , who passed away earlier this year.
According to NPR, the legislation would allow the federal government to negotiate the cost of prescription drugs for Medicare, limit out-of-pocket costs for Medicare participants, and prevent drug price hikes. The Trump administration vowed to veto the legislation if it ever comes to his desk.
��In my district alone, H.R. 3 could lower breast cancer medication by $45,100, and diabetics could save up to 94% on the cost of insulin,” Rep. Lucy McBath said in an email. “I have heard heartbreaking stories from people in my community who are forced to skip doses or ration their insulin,” she added, noting too many Americans “worry about paying for their lifesaving prescription drugs.”
Rep. Ami Bera , a doctor a member of the moderate New Democrat Coalition, joined McBath in supporting the legislation.
TAGS
Read Also: Why Are The Republicans So Evil
Democrats Pass Us Bill To Lower Drug Prices That Trump Threatens To Veto
By Lisa Lambert
3 Min Read
WASHINGTON – – The U.S. House of Representatives on Thursday approved legislation aimed at driving down the prices that seniors pay for prescription drugs, but the bills future is clouded by President Donald Trumps threat of a veto and lack of support in the Senate.
The Democrat-led chamber voted 230 to 192, largely along party lines, to approve the measure that would allow the Medicare insurance program for seniors to negotiate prices for dozens of prescription drugs, including insulin. The lower drug prices would also be available to private insurance companies.
Ive seen grown men cry on the campaign trail because they cannot meet the prescription drug cost, whether they have a spouse that is ill or a child with a pre-existing conditions, Speaker of the House Nancy Pelosi told reporters ahead of the vote. This will make all the difference in the world.
The bill would cap prices for the countrys most expensive drugs using an international index and impose hefty fines for manufacturers that do not negotiate.
The pricing system would save the government $456 billion over 10 years, according to estimates from the non-partisan Congressional Budget Office, much of which would go toward extending Medicare coverage for vision, hearing and dental care.
The bill also would prevent price-gouging on new drugs for those with private health insurance.
Democrats And Hr 3 Taking A Harder Line Against Biomedical Innovation
Lifelong Republicans Explain Why They’re Voting Against Trump | NowThis
Shockingly, some Democrats went on the record saying a trade-off lower prices now for fewer cures in the future is acceptable. Rep. Darren Soto said, I frankly think its worth it. He should tell that to the people whose lives wont be saved by the medicines that wont be made.
House Democrats have unanimously moved to a hard-left position against the biotechnology industry, and we have some real work ahead of us to bring moderates back into the fold. In a single week, Pelosis party unanimously supported socialized price controls on drugs and persuaded the White House to strip important intellectual property protections for biologic medicines out of the U.S.-Canada-Mexico Trade Agreement.
Also Check: Why Did Democrats And Republicans Switch
Combining The Two Bills Sets Up A Political Minefield For Republicans Who Are Torn Between The Two Issues
The House is set to vote Thursday on legislation meant to lower prescription drug prices and strengthen the individual health insurance exchanges, setting up a political minefield for Republicans who are torn between the two issues.
Democratic leaders decision to combine legislation that would make it easier to bring generic drugs to market with bills that would bolster the 2010 health care law does;not damage the prospects of passage for the package of bills. But that does make it certain that most Republicans will vote against the bipartisan drug pricing legislation.
The decision to merge the bills, which Democrats say was made so that savings achieved through the drug pricing measures would pay for spending under the health insurance legislation, could open Republicans up to attacks that they voted against legislation to lower drug prices, an issue that polls show is of great concern to both Republicans and Democrats.
These are very separate issues, said Rep. Greg Walden, R-Ore., the ranking member on the Energy and Commerce Committee. How we deal with bad practices in getting drugs and samples and all that to consumer and competition in the market is different than paying for more navigators and wiping out state-regulated health insurance.
Theyre just waiting to cut the TV ads, he added.
Rep. , R-N.C., the chairman of the House Freedom Caucus, said he wasnt concerned about the show vote, but said it was a missed unique opportunity.
House Is At Work While Senate Stalls
Most people Ive spoken with in Connecticut are astounded to learn that the House of Representatives has passed more than 400 bills over this past year.
Theyre equally struck by the fact that 275 of those bills were bipartisanly supported.
They were then deeply disappointed to learn that the Senate has not taken any of them up over the past year.
The media coverage, including social media, has been focused on impeachment, a divided Congress, and a divided nation. Is it any wonder then why people have little faith in government and are fed up with the process?
Yet, the truth is much has been done by one chamber, the House of Representatives, while the President and the Senate continue to falsely assert the only thing were working on is impeachment.
The summary below indicates what the House of Representatives has passed to help the American people.
Hailing from the state where the activism around the tragedy at Sandy Hook took place, Im proud that the House of Representatives took action and passed three gun violence prevention bills in 2019. Gun violence is an epidemic that is tearing our communities apart, and we need to make responsible changes now.
In addition to voting to lower prescription drugs, Democrats are also working to protect those with pre-existing conditions and strengthen affordable health care for Americans.
These are just a few of the 400-plus bills the House has passed that are awaiting consideration in the Senate.
You May Like: What Republicans Are Voting Against Trump
How Did Pelosi Keep Various Democratic Factions On Board
She carefully crafted legislation that could appeal to diverse wings of her caucus.
Moderate Democrats pushed for a bill that could become law and wasnt just a messaging document. Progressives argued that since no Pelosi bill would get the time of day from McConnell, the party should go bold.
The Pelosi bill attempts to appease both camps by focusing on government price negotiations for the highest-cost, highest-use drugs that enjoy patent-protected monopolies. Unlike previous Democratic attempts at drug price negotiations, it contains hefty sticks to guarantee the government gets prices down for those drugs. But it would allow the status quo to prevail for most medicines, letting the private market continue to dictate their prices.
Progressive Democrats appeared to have the upper hand over moderates as the bill went through its final iterations. By threatening to block the bill from getting a vote if Pelosi didn’t make last-minute changes, progressives won two big last-minute concessions, expanding the scope of the legislation.
Why Are There Pro


H.R. 3 has been protested almost since the day it was introduced. The No Taxpayer Funding for Abortion Act, which does pretty much what it says on the tin imposes a series of draconian restrictions on abortion funding made waves at first because of its forcible rape clause, which aimed to restrict the definition of rape as it applied to rape and incest exemptions, and which garnered a significant amount of coverage and protest this February. Of course, even with that infamous clause removed, H.R. 3 aims to deprive people of Medicaid funding for abortions, meaning that it aims to deprive poor women of abortions, meaning that it targets specifically the most vulnerable people within the population. And good news for those of us in the middle: If you are lucky enough to have insurance, H.R. 3 will also prohibit your insurance company from covering your abortion. So, hurrah! All will be delightfully well-oppressed.
The most upsetting thing about H.R. 3, however, is the fact that it passed the House on May 4. And how it passed: On a 251-to-175 vote. Every single Republican present voted for it. And so did 16 Democrats.
Which is why those pro-life Democrats stick out. In a House that was already going to pass the measure, against a united and determined Republican anti-choice strategy, they added sixteen unnecessary voices of support. The question of why why such a thing as a pro-life Democrat even exists gets more terrifying the more closely you consider it.
Don’t Miss: Who Won The Midterm Elections Democrats Or Republicans
What Is Your Analysis Of This Vote
What trends do you see in this vote?
Members of Congress side together for many reasons beside being in the same political party, especially so for less prominent legislation or legislation specific to a certain region. What might have determined how the roll call came out in this case? Does it look like Members of Congress voted based on party, geography, or some other reason?
One tool that will be helpful in answering this question is the cartogram at the top of the page. A cartogram is a stylized map of the United States that shows each district as an identical hexagon. This view allows you to see the how the representatives from each district voted arranged by their geography and colored by their political party. What trends can you see in the cartogram for this vote?
How did your representative vote?
There is one vote here that should be more important to you than all the others. These are the votes cast by your representative, which is meant to represent you and your community. Do you agree with how your representative voted? Why do you think they voted the way they did?
If you dont already know who your Members of Congress are you can find them by entering your address here.
Each votes study guide is a little different we automatically choose which questions to include based on the information we have available about the vote.Study guides are a new feature to GovTrack. You can help us improve them by filling out this survey or by sending your feedback to .
Theres Two Approaches To Drug Pricing Reform And Both Are Stalled
The House bill H.R.3 has a few mechanisms for reducing prescription drug prices, but most notably, it would allow the US health department to directly negotiate the prices it will pay for up to 250 drugs every year. The Congressional Budget Office has estimated the bill would save Medicare up to $450 billion over 10 years because of those new negotiating powers. CBO has also projected about eight fewer drugs would come to the market in the next decade because of the decrease in revenues for drug makers.
Despite Trumps promises on the 2016 campaign trail that he would support proposals allowing Medicare drug negotiations, the White House threatened to veto the House plan. They called it a plan to institute government price controls, and said it would limit access to medicine, a favored talking point of the pharmaceutical lobby.
Even without this veto threat, H.R.3 is expected to be dead-on-arrival in the Senate. Senate Majority Leader Mitch McConnell has shown no interest in taking up the bill. It did, however, garner some small measure of bipartisan support although Trump has thrown the weight of the White House against the bill, it did receive two House Republican votes in December.
Instead, Trump has aligned himself more with Republican Sen. Chuck Grassley, who has advanced a narrower set of reforms from his perch as the Senate Finance Committee chair.
Will you support Voxs explanatory journalism?
You May Like: Did Republicans Shut Down The Government
0 notes
Text
Global Erythropoietin - Stimulating Agents market Size, Share, growth, Trends and Forecast 2021-2025
Erythropoiesis is the process which produces red blood cells in body. Erythropoiesis-stimulating agents (ESA) are drugs which are used to stimulate the bone marrow to produce red blood cells and are used to treat anemia due to end stage kidney disease, chemotherapy or major surgery. These drugs are used to maintain hemoglobin at the lowest level which minimizes transfusions in human body and best meets a person's needs. First authorized for medical usage in the United States in 1989, Erythropoiesis-stimulating agents (ESA) are present on the World Health Organization's List of Essential Medicines- the most efficient and secure medicines needed in a health system. Currently the erythropoietin agents which are commercially availabe include epoetin alfa and darbepoetin alfa, as well as biosimilars.
Get Sample Reports Here - https://www.kennethresearch.com/sample-request-10064939
Market Dynamics
The growth of the global market of Erythropoiesis stimulating agents is mainly driven by increasing number of patients suffering from anemic condition induced due to cancer, HIV, and kidney failures. These are very chronic diseases and are responsible for large number of deaths worldwide. Increasing awareness of therapies in the treatment of anemia also pushes the market upwards. However, stringent regulatory guidelines and side effects such as hypersensitivity, hypertension, or seizures, associated with erythropoiesis-stimulating agents are restraining the growth of the market.
Market Segmentation
The Erythropoietin Stimulating Agents Market can be segmented on the basis of type and application. On the basis of type, market is categorized into two- biologics and biosimilars. Further the biologic erythropoietin drugs can be classified into epoetin-alfa, epoetin-beta, darbepoetin-alfa and epoetin-theta on the basis of biological properties. The biosimilars erythropoietin drugs is further subdivided into epoetin-zeta, epoetin-omega, epoetin-lambda and epoetin-delta. The segmentation on the basis of application includes renal disorder, cancer chemotherapy, anti-retroviral treatment, anemia, neural diseases and others.
Regional/Geographic Analysis
The market have been geographically segmented into North America, Europe, Asia-Pacific, Middle East and Africa, and South America. The North America have been subdivided into USA, Canada and Mexico. The market in Europe have been subdivided into UK, Germany, France, Italy, Spain, and rest of Europe. The market in Asia-Pacific have been subdivided into Japan, China, India, South Korea, Australia & New Zealand, and rest of Asia-Pacific. The Middle East and Africa have been subdivided into GCC countries, South Africa, and rest of Middle East and Africa. The South America have been segmented into Brazil, Argentina and rest of South America. North America accounted for the largest market value but Asia-Pacific market is expected to grow at a higher CAGR during the forecast period. The emerging economies and new products with better efficacy will be a big opportunity for the market.
Key Players
Some of the major players in the biologics erythropoietin drug market are Amgen Inc., Hoffmann-La Roche and Johnson & Johnson and major players in biosimilar erythropoietin drugs market are Sandoz, Hospira, 3SBio, BioSidus and Teva Pharmaceutical Industries Ltd among others.
Report ContentsRegional AnalysisReport Highlights
Market segments
Market Drivers, Restraints and Opportunities
Market Size & Forecast 2016 to 2022
Supply & Demand Value Chain
Market - Current Trends
Competition & Major Companies
Technology and R&D Status
Porters Five Force Analysis
Strategic and Critical Success Factor Analysis of Key Players
About Kenneth Research
Kenneth Research is a reselling agency providing market research solutions in different verticals such as Automotive and Transportation, Chemicals and Materials, Healthcare, Food & Beverage and Consumer Packaged Goods, Semiconductors, Electronics & ICT, Packaging, and Others. Our portfolio includes set of market research insights such as market sizing and market forecasting, market share analysis and key positioning of the players (manufacturers, deals and distributors, etc), understanding the competitive landscape and their business at a ground level and many more. Our research experts deliver the offerings efficiently and effectively within a stipulated time. The market study provided by Kenneth Research helps the Industry veterans/investors to think and to act wisely in their overall strategy formulation
Contact Us
Name: David
Email: [email protected]
Phone: +1 313 462 0609
1412, Broadway,
21st Floor Suite MA111,
New York, NY 10018
0 notes
Text
Biosimilar of Remicade Market: Global Industry Analysis By Size, Share, Growth, Sourcing Strategy, Scope, Demand And Forecast To 2028

A new report on the Biosimilar of Remicade Market research study, published by Reports and Data, provides an in-depth survey of the dominant participants of the industry –the basis points for which are the financial highlights, company outline, SWOT Analysis, Product Portfolio, as well as major strategies and the expansion plans of the new and potential contenders. This report is also anticipated to reflect consistent growth in the coming years since consumers are now more aware of product quality. This market analysis of an industry is a crucial factor that numerous stakeholders, such as investors, traders, suppliers, and others, will find beneficial.
The research offered by the Biosimilar of Remicade report has been formulated through key analytical tools and extensive primary and secondary research further validated and verified by industry experts, industry professionals and analysts. The report includes SWOT analysis, Porter’s Five Forces analysis, feasibility analysis, and investment return analysis to impart better understanding of the Biosimilar of Remicade market dynamics.
Key companies in the Biosimilar of Remicade market include:
Janssen Biotech Inc., Merck &Co., Pfizer Inc. (AC. Hospira), Celltrion Inc., Alvogen, Napp Pharmaceuticals, and Nippon Kayaku are some major players in the global Remicade (infliximab mAb) market.
Get a sample of the report @ https://www.reportsanddata.com/sample-enquiry-form/4029
For the purpose of this report, Reports and Data has segmented the Disposable Blood Pressure Cuffs market on the basis of Product Type, end use, and region:
Approved Indication Outlook (Revenue, USD Billion; 2018 – 2028)
Ulcerative Colitis
Rheumatoid Arthritis
Psoriatic Arthritis
Plaque Psoriasis
Crohn’s Disease
Ankylosing Spondylitis
Research findings and conclusion are offered through detail graphs, tables, charts, figures, and other pictorial representations. The report also includes a feasibility analysis and investment return analysis and aims to offer beneficial information that might help in formulating new business strategies and expansion plans. The report is a comprehensive overview of the industry, sales channel, distribution network, key strategies, growth prospects, drivers and restraints, and the key technological and product developments in the Biosimilar of Remicade market.
Ask for discount @ https://www.reportsanddata.com/discount-enquiry-form/4029
The report provides insights on the following pointers:
· Market Penetration: Comprehensive information on the product portfolios of the top players in the Biosimilar of Remicade market.
· Product Development/Innovation: Detailed insights on the upcoming technologies, R&D activities, and product launches in the market.
· Competitive Assessment: In-depth assessment of the market strategies, geographic and business segments of the leading players in the market.
· Market Development: Comprehensive information about emerging markets. This report analyzes the market for various segments across geographies.
· Market Diversification: Exhaustive information about new products, untapped geographies, recent developments, and investments in the Biosimilar of Remicade market.
Key Questions Answered in this Research Study:
What is the global production, production value and consumption value?
Who are the global key manufacturers of the market? How are their operating situation?
What are the types and applications of market?
What is the market share value of each type and application?
What are the upstream raw materials and manufacturing equipment?
What is the manufacturing process?
Economic impact on the market and development trends of market.
What will be the market size and the growth rate be in 2028?
What are the key factors driving the market?
What are the key market trends impacting the growth of the market?
What are the challenges to market growth?
What are the market opportunities and threats faced by the vendors in the market?
To know more about the report @ https://www.reportsanddata.com/report-detail/biosimilar-of-remicade-market
Thank you for reading our report. For further inquiry, please get in touch with us. Our team will ensure the report is customized to meet your requirements.
0 notes