#Recombinant Hormones in biosimilar industry US
Text
Biosimilars Unleashed: The Future of Healthcare in the US
Buy Now
What is the Size of US Biosimilar Industry?
US Biosimilar Market is expected to grow at a CAGR of ~ % between 2017-2022 and is expected to reach ~USD Bn by 2028. Biosimilars enhance patient access to essential treatments, especially in therapies with high demand, like oncology, by providing more affordable options. Additionally, Growing evidence of biosimilars' comparable efficacy and safety fosters trust among healthcare professionals, driving adoption.

Click here to Download a sample Report
Biosimilars offer cost savings compared to originator biologics, addressing the need for affordable healthcare solutions in the face of rising medical costs. Favorable regulatory frameworks, like the BPCIA, streamline biosimilar approval processes, encouraging manufacturers to invest in development.
Furthermore, The expiration of patents for numerous reference biologics creates opportunities for biosimilar entry, leading to increased competition and market expansion. Pharmaceutical companies are investing in biosimilar R&D and production, expanding the pipeline and market availability. Supportive healthcare policies and reimbursement models incentivize biosimilar adoption, creating a favorable environment for market growth.
US Biosimilar Market by drug class
The US Biosimilar market is segmented by Monoclonal Antibodies, Recombinant Hormones, Immunomodulators, Anti-inflammatory agents and Others. Based on drug class, Monoclonal Antibodies segment dominates the bio similar market in 2022.
Monoclonal antibodies have diverse applications across various therapeutic areas. From cancer treatment to autoimmune diseases, biosimilar Mabs addressed a wide range of medical needs, leading to a broad and growing market. Biosimilars, with their potential for cost savings while maintaining comparable efficacy and safety, gained significant attention as viable alternatives.
US Biosimilar Market by application
In US Biosimilar market, they are segmented by application into Oncology, Blood disorders, Chronic diseases and autoimmune conditions and Others. On the basis of application, Oncology segment was the dominant in 2022.
The increasing prevalence of cancer and the high cost of traditional biologics used in oncology treatment have created a strong incentive for the adoption of biosimilars. Biosimilars offer the potential to provide similar therapeutic outcomes at a lower cost, making them an attractive option for both healthcare providers and patients.
Additionally, the rigorous clinical trials and regulatory processes that biosimilars undergo to gain approval provide reassurance to healthcare professionals and patients regarding their safety and efficacy. This has led to increased acceptance and adoption of biosimilars in oncology.
US Biosimilar by Region
The US Biosimilar market is segmented by Region into North, East, West and South. In 2022, the dominance region is North region in US Biosimilar market.
The North region benefits from a concentration of healthcare providers and academic institutions that are at the forefront of adopting and integrating biosimilars into their treatment protocols. These institutions are more likely to have the expertise to evaluate and incorporate biosimilars effectively, driving their adoption among healthcare professionals and patients.
Click here to Download a Custom Report
Competition Scenario in US Biosimilar Market
The US biosimilar market has witnessed an evolving competitive landscape, with several key players competing for market share. Prominent pharmaceutical companies such as Amgen, Pfizer, Sandoz (Novartis), and Boehringer Ingelheim have been actively involved in developing and marketing biosimilar products. These established players have utilized their expertise in biologics and significant resources to navigate the regulatory landscape and compete effectively.
The competition in the US biosimilar market is characterized by a balance between established pharmaceutical giants and emerging biotech companies. While the major players possess the advantage of resources and experience, smaller biotech firms are also contributing to the market with innovative approaches and niche biosimilar offerings.
What is the Expected Future Outlook for the Overall US Biosimilar Market?
The US Biosimilar market was valued at USD ~Million in 2022 and is anticipated to reach USD ~ Billion by the end of 2028, witnessing a CAGR of ~% during the forecast period 2022- 2028. The US biosimilar market is likely to experience significant growth in the coming years, driven by several factors. Biosimilars are biologic drugs that are highly similar to already approved reference biologics. They offer potential cost savings, increased competition, and improved patient access to crucial treatments.
Firstly, the regulatory environment is becoming more favorable for biosimilars. The Biologics Price Competition and Innovation Act (BPCIA) established a pathway for biosimilar approval in the US, allowing for a smoother regulatory process. As more biosimilars receive approval, competition in the market is expected to intensify.
Secondly, patents for several blockbuster biologics are expiring or have already expired. This creates opportunities for biosimilar manufacturers to enter the market with more affordable alternatives, offering healthcare systems and patients a choice in treatment options.
Thirdly, as healthcare costs continue to rise, biosimilars present an attractive solution for reducing expenses. Their potential to offer cost savings without compromising therapeutic efficacy could lead to increased adoption by healthcare providers, insurers, and patients alike.
Physician and patient education are crucial, as misconceptions about biosimilars' safety and effectiveness might hinder their adoption. Additionally, legal and market access barriers, including patent litigation and complex distribution systems, could slow down the growth of the biosimilar market.
The biosimilar market witness consolidation as larger pharmaceutical companies acquire or partner with smaller biotech firms to bolster their biosimilar portfolios. This will lead to more resources being devoted to biosimilar development and marketing. Changes in healthcare policies, such as reimbursement models and value-based care initiatives, can influence the biosimilar market's growth. Favourable policies that incentivize biosimilar adoption drives their market growth.
#US Biosimilar Market#US Biosimilar Industry#US Biosimilar Sector#United States Biosimilar Market#US Biosimilar Market forecast#US Biosimilar Market analysis#US Biosimilar Market trends#US Biosimilar Market share#US Biosimilar Market key players#US Biosimilar Market revenue#US Biosimilar Market growth#Monoclonal Antibodies in biosimilar market US#Recombinant Hormones in biosimilar industry US#Oncology in bio similar market US#Blood disorders in biosimilar market US#Research institutes in Biosimilar market US#US similar biotherapeutics products market#Hospitals in Biosimilar market US#Investors in Biosimilar market US#US comparable biologics products industry#US recombinant biosimilars industry#US replicate biologics sector#US analog biologics market#US homologous biologics market#US oncology biosimilar market#US immunology biosimilar sector#US insulin biosimilar industry#US Generics Biologics market challenges#US leading Biosimilar drug providers#US leading Biosimilar drug manufacturers
0 notes
Text
Biosimilars Market Size, Trends, Growth Analysis 2032
Biosimilars Market Overview
An integral component of the Biosimilars Market is the emergence of follow-on biologics. These biologics, which closely resemble existing biologic drugs, offer additional options for patients and healthcare providers. Follow-on biologics undergo rigorous testing to demonstrate similarity to the reference product, ensuring interchangeability and therapeutic equivalence. With their introduction, follow-on biologics stimulate competition in the biopharmaceutical industry, driving down prices and promoting innovation. As the demand for cost-effective biologic therapies continues to grow, the Biosimilars Market stands to benefit from the availability and acceptance of follow-on biologics, expanding treatment options and improving patient outcomes.
According to Market Research Future (MRFR), the biosimilars market insights was valued at USD 29.7 billion in 2023 and is projected to grow from USD 36.79 Billion in 2024 to USD 161.95 billion by 2032, exhibiting a compound annual growth rate (CAGR) of 20.35% during the forecast period (2024 - 2032).
Biosimilars Market: Latest News and Developments
The FDA has approved the first Humira biosimilar. Adalimumab-bwwd (Cyltezo), a Humira biosimilar, received FDA approval in January 2023 for the treatment of rheumatoid arthritis, psoriatic arthritis, ankylosing spondylitis, juvenile idiopathic arthritis, Crohn's disease, ulcerative colitis, plaque psoriasis, and hidradenitis suppurativa.
Rising use of biosimilars in the treatment of cancer. A growing number of biosimilars are being used to treat cancer. The FDA authorised filgrastim-sndz (Zarxio), pegfilgrastim-jmdb (Onpro), and trastuzumab-dkst (Herzuma) as three biosimilars for the treatment of cancer in 2022.
Market Segmentation
Biosimilars industry can be considered with respect to product, applications, and end users.
The products based on which the market has been split into are recombinant glycosylated proteins, recombinant peptides, and recombinant non-glycosylated proteins. Recombinant non-glycosylated proteins are the biggest segment in the global market, thanks to the soaring cases of chronic disorders ranging from growth hormone deficiency to diabetes. As a result, recombinant non-glycosylated proteins are therapeutically used as they are readily available and are cost-effective.
Application-wise, the biosimilars market caters to chronic diseases, oncology, blood disorders, autoimmune diseases, infectious diseases, growth hormone deficiency, and more. Blood disorders have emerged as the top segment, as a result of the rising burden of the condition worldwide and the increased use of biosimilars by virtue of their low cost and the overall reduction in the treatment cost.

Major end users in the global market are hospitals and clinics as well as research institutes. Hospital and clinics are healthcare settings where treatment options and skilled professionals are easily available, and therefore, have emerged as the leading segment in the global industry.
Regional Status
Europe, MEA or Middle East & Africa along with the Americas, APAC or Asia Pacific are the primary markets for biosimilars.
Europe has taken the lead in the global market, as the region houses a vast elderly pool, with close to one fifth of the overall EU population aged more than 65 years. This has given way to several lifestyle-related disorders such as oncology, autoimmune diseases, diabetes, to name a few. Presumably, the scenario has raised the demand for biosimilars and can mean higher market growth over the next few years. In addition to this, numerous blockbuster biologics are on track to lose patent in the coming years, which should present lucrative opportunities to the biosimilar manufacturers.
The North American market displays a bright outlook and can emerge quite lucrative in the coming years, in view of the surging burden of chronic ailments in Canada and the United States. The rising spending on research activities by the healthcare agencies also fuels the market expansion in the region. Favorable reimbursement landscape in the region, especially in the US, encourages healthy competition as it results in lower incentives for the players to compete based on price.
The APAC market is slated to witness considerable growth, with China, South Korea and India offering a host of lucrative opportunities for drug development as well as commercialization. These are generics-driven countries and are known for frequently launching new and advanced manufacturing platforms, which has brought down the costs associated with biosimilar production. In the coming few years, majority of the patent expiries is touted to be in biosimilars, which is deemed as a profitable aspect by leading manufacturers and can translate into substantial market growth.
Eminent Vendors
Top global key players listed by biosimilars market outlook report include Stada Arzneimittel AG (Germany), Teva Pharmaceuticals (Israel), Biocon (India), Pfizer (US), Sandoz International (Germany), Eli Lily & Company (US), Actavis, Inc. (US), Dr. Reddy’s Laboratories (India), Cipla Ltd (India), Amgen, Inc. (US), Samsung Biologics (South Korea), Hospira Inc.(US), Mylan, Inc.(US), Celltrion (South Korea), to mention a few.
More Related Trending Topics
Medical Foods market
Insomnia market
Blockchain Technology in Healthcare market
Drug Device Combination market
Prefilled Syringes market
0 notes
Text
Electrocompetent Cells Market is Estimated to Witness High Growth Owing to Rising Adoption of Recombinant DNA Technology

Electrocompetent cells are bacterial cells that have been treated to become permeable to plasmid DNA, and can therefore take up DNA from their environment through the process of electroporation. They are widely employed in the transformation of cloning vectors and the isolation of recombinant plasmids. These cells find extensive usage in various molecular biology procedures such as cloning, site-directed mutagenesis, library construction, expression vector construction for the production of therapeutic proteins, recombinant protein expression, and sequencing. The global Electrocompetent Cells market is estimated to be valued at US$ 2.07 Bn in 2023 and is expected to exhibit a CAGR of 10% over the forecast period 2023 to 2030, as highlighted in a new report published by Coherent Market Insights.
Market Opportunity:
The rising adoption of recombinant DNA technology is estimated to present lucrative growth opportunities for the electrocompetent cells market over the forecast period. Recombinant DNA technology finds widespread applications in the production of therapeutics and biologics as well as diagnostics development. It helps in modifying the genome of an organism to produce substances like insulin, human growth hormones, monoclonal antibodies, and various vaccines. Therefore, the increasing investments in biologics and biosimilars development and rising adoption of advanced molecular techniques are estimated to propel the demand for electrocompetent cells globally.
Porter's Analysis
Threat of new entrants: The threat of new entrants is low due to high R&D costs and strong intellectual property protection by existing players in the market.
Bargaining power of buyers: The bargaining power of buyers is high as there are many established players and the product is a commodity with no major differentiation.
Bargaining power of suppliers: The bargaining power of suppliers is moderate as few global suppliers cater to the key raw materials needs of the industry.
Threat of new substitutes: The threat of new substitutes is low as there are no cost-effective alternatives available for competent cells in molecular cloning applications.
Competitive rivalry: The competitive rivalry in the market is high due to many global and local players offering similar products.
SWOT Analysis
Strength: Electrocompetent cells offer advantages like high transformation efficiency, easy production, and cost-effectiveness for molecular cloning. They allow for the uptake and integration of exogenous DNA into bacterial cells.
Weakness: Limitations exist in the transformation efficiency based on the origin of DNA and bacterial strain used. In addition, variations can arise in their performance across different laboratory settings.
Opportunity: Growing R&D investments and rising adoption in cell-based research, biologics production, directed evolution studies provide significant growth opportunities. Also, advancements to improve transformation efficiency and customization as per end-user needs present opportunities.
Threats: Stringent regulations pertaining to the design, production and quality assurance of cell-based products act as a major threat. Further, unfavorable changes in government funding and policies can also negatively impact the market.
Key Takeaways
The global Electrocompetent Cells market is expected to witness high growth over the forecast period of 2023 to 2030. Rising investments in pharmaceutical and biotechnology R&D are driving the uptake of competent cells in molecular cloning applications. The market size for 2024 is projected to reach US$ 2.07 Bn, indicating strong growth.
Key players - Key players operating in the Electrocompetent Cells market include Merck KGaA, Thermo Fisher Scientific, Promega Corporation, Takara Bio, Bio-Rad Laboratories, Lucigen Corporation, New England Biolabs, QIAGEN N.V., Origene Technologies, and Illumina, Inc. These established players cater to the global demand through their widespread production and distribution networks.
0 notes
Text
Global Recombinant Erythropoietin Market Is Estimated To Witness High Growth Owing To Increasing Prevalence of Anemia

The global Recombinant Erythropoietin Market is estimated to be valued at US$ 7.01 billion in 2023 and is expected to exhibit a CAGR of 2% over the forecast period of 2023-2030, according to a new report published by Coherent Market Insights.
Market Overview:
Recombinant erythropoietin (rEPO) is a synthetic form of the hormone erythropoietin, which stimulates the production of red blood cells in the body. It is mainly used to treat anemia associated with chronic kidney disease, cancer chemotherapy, and other conditions that limit the production of red blood cells. The market for recombinant erythropoietin is driven by the increasing prevalence of anemia worldwide. Anemia affects millions of people globally and can lead to fatigue, weakness, and other health complications. Recombinant erythropoietin helps in improving the symptoms of anemia and enhancing quality of life for patients.
Market Key Trends:
One key trend in the recombinant erythropoietin market is the development of biosimilars. Biosimilars are biological products that are highly similar to an already approved reference product in terms of quality, safety, and efficacy. They offer cost-effective alternatives to the original brand-name products, making them more accessible to patients. The increasing demand for affordable treatment options and the expiry of patents for reference products are driving the development and adoption of biosimilars in the recombinant erythropoietin market. For example, companies like Teva Pharmaceutical Industries Ltd., Sandoz International GmbH, and Celltrion Inc. are actively involved in the development of biosimilar versions of recombinant erythropoietin.
PEST Analysis:
Political: The regulatory environment and government policies play a crucial role in the development and approval of recombinant erythropoietin products. Stringent regulations for biosimilar approvals and pricing policies in different regions can impact market growth.
Economic: The increasing healthcare expenditure, particularly in emerging economies, is driving market growth. The rising affordability and accessibility of healthcare services, along with the availability of insurance coverage for treatments, contribute to the demand for recombinant erythropoietin products.
Social: The growing aging population and the rising prevalence of chronic kidney disease and cancer are key social factors driving the demand for recombinant erythropoietin. Additionally, increased awareness and diagnosis of anemia and its consequences are also contributing to market growth.
Technological: Advancements in biotechnology and drug delivery systems are enhancing the efficacy and safety of recombinant erythropoietin products. The development of long-acting formulations and novel delivery methods is improving patient adherence and convenience.
Key Takeaways:
The global Recombinant Erythropoietin Market Growth is expected to witness high growth, exhibiting a CAGR of 2% over the forecast period, due to increasing prevalence of anemia.
North America is the fastest-growing region in the market, owing to the high prevalence of anemia and well-established healthcare infrastructure.
Key players operating in the global recombinant erythropoietin market include Amgen Inc., Johnson & Johnson (Janssen Pharmaceuticals), Pfizer Inc., Roche Holding AG, Novartis AG, Biocon Limited, Teva Pharmaceutical Industries Ltd., LG Chem Ltd., Sandoz International GmbH (a subsidiary of Novartis AG), Intas Pharmaceuticals Ltd., Dr. Reddy's Laboratories Ltd., Celltrion Inc., 3SBio Inc., CJ CheilJedang Corporation, and BioSidus SA. These players focus on strategic collaborations, research and development activities, and product launches to maintain their market position and cater to the growing demand for recombinant erythropoietin.
#Pharmaceutical#Healthcare Industry#Healthcare#Recombinant Erythropoietin#Recombinant Erythropoietin Market
0 notes
Text
Biosimilars Market Size 2022 Industry Recent Developments and Technology, Size, Trends, Growth, and Forecast Research Report 2030| By R&I

The report is titled as ‘Biosimilars Market: Opportunity Analysis and Future Assessment 2020-2028’. An overview of conceptual frameworks, analytical approaches of the biosimilars market is the main objective of the report, which further consists the market opportunity and insights of the data involved in the making of the respective market. Biosimilars market is expected to grow with a significant rate in the near future.
The global biosimilars market in 2020 is estimated for more than US$ 11,036.2 Mn and expected to reach a value of US$ 1,09,501.8 Mn by 2028 with a significant CAGR of 33.5%.
Request a Sample Copy of this Report @: https://reportsandinsights.com/sample-request/1243
Biosimilars Market Dynamics
Patent expiration in the biological drug industry is the major factor driving the growth of global biosimilars market. Moreover, the introduction of clear regulatory pathways for the introduction of biosimilars to the market by many countries is further encouraging biosimilar development. For instance, recently India and Canada introduced regulations for approval of biosimilar drugs in respective countries. However, unlike generic chemical entities, producing identical copies of innovator cell-based drugs is impossible and takes more time, which is the biggest challenge for the growth of global biosimilars market. Biologics are produced by cells in culture or whole organisms, which are inherently more variable than chemical synthesis methods.
Biosimilars Market Regional Overview
On the basis of region, the global biosimilars market is segmented into six regions namely North America, Latin America, Asia Pacific, Europe, Middle East, and Africa. North America biosimilars market is expected to be the most dominating market throughout the forecast period due to due to the expiry of patents for blockbuster biological drugs. The presence of key players in Europe makes it second-largest market for biosimilars. Asia Pacific biosimilars market is expected to witness delayed growth due to relatively long patent term for biological drugs
MMC Overview
The non-identical approach of Reports and Insights stands with conceptual methods backed up with the data analysis. The novel market understanding approach makes up the standard of the assessment results that give a better opportunity for the customers to put their effort.
A research report on the biosimilars market by Reports and Insights is an in-depth and extensive study of the market based on the necessary data crunching and statistical analysis. It provides a brief view of the dynamics flowing through the market, which includes the factors that support market and the factors that are acting as impedance for the growth of the market. Furthermore, the report includes the various trends and opportunities in the respective market in different regions for a better understanding of readers that helps to analyze the potential of the market.
Wish to Know More About the Study? Click here to get a Report Description: https://reportsandinsights.com/report/global-biosimilars-market
Biosimilars Market Key Players
Some of the key participating players in global Biosimilars market are:
 Pfizer
 Sandoz International
 Teva Pharmaceuticals
 Amgen
 Biocon
 Dr. Reddy’s Laboratories
 Celltrion
 Samsung Biologics
 Synthon Pharmaceuticals, Inc.
 LG Life Sciences
 Biocon
 Hospira
 Merck Serono (Merck Group)
 Biogen Idec Inc.
 Genentech (Roche Group)
Biosimilars Market Segmentation
The global Biosimilars market is segmented on the basis of product type, manufacturing, diseases, and region.
By Product Type
 Recombinant Non-Glycosylated Proteins
 Recombinant Human Growth Hormone (RHGH)
 Granulocyte Colony-Stimulating Factor (Filgrastim)
 Insulin
 Interferons
 Interferon-Beta
 Interferon-Alpha
 Recombinant Glycosylated Proteins
 Erythropoietin (EPO)
 Monoclonal Antibodies (MABS)
 Infliximab
 Rituximab
 Adalimumab
 Other Monoclonal Antibodies
 Follitropin
 Recombinant Peptides
 Glucagon
 Calcitonin
By Type of Manufacturing
 In-House Manufacturing
 Contract Manufacturing
By Disease
 Oncology
 Chronic Diseases
 Autoimmune Diseases
 Blood Disorders
 Growth Hormone Deficiency
 Infectious Diseases
 Other Diseases
By Region
 North America
 Latin America
 Europe
 Asia Pacific
 Middle East
 Africa
To view Top Players, Segmentation and other Statistics of Biosimilars Industry, Get Sample Report @: https://reportsandinsights.com/sample-request/1243
About Reports and Insights:
Reports and Insights is one of the leading market research companies which offers syndicate and consulting research around the globe. At Reports and Insights, we adhere to the client needs and regularly ponder to bring out more valuable and real outcomes for our customers. We are equipped with strategically enhanced group of researchers and analysts that redefines and stabilizes the business polarity in different categorical dimensions of the market.
Contact Us:
Neil Jonathan
1820 Avenue M, Brooklyn
NY 11230, United States
+1-(718) 312-8686
Find Us on LinkedIn: www.linkedin.com/company/report-and-insights/
View Latest Market Updates At: https://marketsresearchanalytics.com
#Biosimilars Market Research#Biosimilars Market Report#Biosimilars Market Share 2021#Biosimilars Market Size 2022#Biosimilars Market Trends#Biosimilars Market Key Players#Global Biosimilars Market Analysis#Biosimilars Industry News#Biosimilars Industry Analysis#Biosimilars Market Forecast#Biosimilars Market CAGR#USA Biosimilars Market#Japan Biosimilars Market#Biosimilars Market Demand#Argentina Biosimilars Market#Australia Biosimilars Market#Belgium Biosimilars Market#Brazil Biosimilars Market#Canada Biosimilars Market#Chile Biosimilars Market#China Biosimilars Market#Columbia Biosimilars Market#Egypt Biosimilars Market#France Biosimilars Market#Germany Biosimilars Market#Global Biosimilars Market#India Biosimilars Market#Indonesia Biosimilars Market#Biosimilars Applications#Biosimilars Industry
0 notes
Text
Biosimilars Market Size Worth US $ 16.97 Billion By 2025 | CAGR: 26%
Overview
A Biosimilar is described as a biological, medical product which is an identical copy of the original medical product. Market Research Future (MRFR) has published and released a research report about the global biosimilars market that predicts massive enlargement for this market with 26% CAGR during the forecast period between 2016 and 2023. In terms of cash, the market has been anticipated to rise to the US $ 16.97 bn.
Get Request Free Sample @ https://www.marketresearchfuture.com/sample_request/1329
Analyzing the market structure, this report estimates the future growth potential of the market. It observes the strategies of the key players in the market and follows the competitive developments like joint ventures, new product developments, mergers and acquisitions, research and developments (R & D) in the market. This report also covers insights on the major countries/regions in which this industry is flourishing, along with the list and description of untapped regions which could be the potential markets in the future.
The key factors aiding the growth of the global biosimilars market include clinical trial activities for biosimilars, increasing demand for the cheap medical products, increasing incident of different diseases, and strategic collaborations resulting in enhanced productivity. However, lack of awareness and physician skepticism are the major restraining factor regarding market growth.
The segmentation global biosimilars market is on the basis of application, manufacturing, product, and region. The application based segmentation segments the market into blood-related disorders, immunity-related diseases, oncology, and other. Based on manufacturing, the market has contract manufacturing and in-house manufacturing. As per segmentation based on product, the market has been segmented into recombinant glycosylated proteins, recombinant non-glycosylated proteins, and others. Recombinant glycosylated proteins have been further segmented into Monoclonal antibody (mAb) and EPO. Recombinant non-glycosylated proteins have been further segmented into growth hormones, insulin, and other.
The regional segmentation of the global biosimilars market segments the market into continent-based regional markets namely Asia Pacific, North America, Europe, and rest of the world (RoW). According to the report, Europe is the biggest regional market for the biosimilars due to the advanced medical research, increasing the prevalence of diseases and rising geriatric population. Due to technological advancement, Western Europe is a bigger market than Eastern Europe. The key country-specific markets in Western Europe include France, Italy, Spain, Germany, and the United Kingdom (UK), followed by the rest of Western Europe.
North America is the second largest market, being ahead of Europe in terms of technological advancement and being equal to Europe in terms of medical research. North America‘s approach towards biosimilars is not as welcome as Europe‘s is. Major country-specific markets in North America are Canada and the United States of America (USA). The Asia Pacific has been expected to emerge as the fast-growing market during the forecast period. The country-specific markets generating maximum revenue in this region are Australia, India, Japan, South Korea, and China, followed by the rest of the Asia Pacific region.
Key Players
The key players in the global biosimilars market include Accord Healthcare (UK), Amgen Inc. (USA), Astra Zeneca (UK), Biocon Ltd. (India), Celltrion, Inc. (South Korea), Dr. Reddy’s Laboratories Ltd. (India), Eli Lilly (USA), F. Hoffmann-La Roche Ltd. (Switzerland), Novartis (Switzerland), Pfizer Inc. (USA), Samsung Bioepis (South Korea), Sandoz International GmbH (Germany), and Teva Pharmaceuticals Industries Ltd. (Israel).
Latest Industry News
Mundipharma, a network of independent pharma companies, has acquired Spanish biosimilars development company Cinfa Biotech. The deal gives Mundipharma, access to a biosimilar to Amgen’s Neulasta (pegfilgrastim). 10 OCT 2018
The new white paper issued by the Association of European Cancer Leagues, a nonprofit, pan-European organization of national and regional cancer societies, has called for the greater use of biosimilars as a means to increase patient access to cancer treatment, and reduce costs. 10 OCT 2018
Table of Contents
1. REPORT PROLOGUE
2. MARKET INTRODUCTION
2.1. Definition
2.2. Scope of the Study
2.2.1. Research Objective
2.2.2. Assumptions
2.2.3. Limitations
3. RESEARCH METHODOLOGY
3.1. Overview
3.2. Primary Research
3.3. Secondary Research
3.4. Market Size Estimation
4. MARKET DYNAMICS
4.1. Overview
4.2. Drivers
4.3. Restraints
4.4. Opportunities
5. MARKET FACTOR ANALYSIS
5.1. Porter’s Five Forces Analysis
5.1.1. Bargaining Power of Suppliers
5.1.2. Bargaining Power of Buyers
5.1.3. Threat of New Entrants
5.1.4. Threat of Substitutes
5.1.5. Intensity of Rivalry
5.2. Value Chain Analysis
Browse Complete Report @ https://www.marketresearchfuture.com/reports/biosimilars-market-1329
About Market Research Future:
At Market Research Future (MRFR), we enable our customers to unravel the complexity of various industries through our Cooked Research Report (CRR), Half- Cooked Research Reports (HCRR), Raw Research Reports (3R), Continuous-Feed Research (CFR), and Market Research & Consulting Services. MRFR team have supreme objective to provide the optimum quality market research and intelligence services to our clients. Our market research studies by Components, Application, Logistics and market players for global, regional, and country level market segments, enable our clients to see more, know more, and do more, which help to answer all their most important questions. In order to stay updated with technology and work process of the industry, MRFR often plans & conducts meet with the industry experts and industrial visits for its research analyst members.
Contact:
Market Research Future
Office No. 528, Amanora Chambers
Magarpatta Road, Hadapsar,
Pune – 411028
Maharashtra, India
+1 646 845 9312
Email: [email protected]
#Biosimilar market 2021#Biosimilar market report#Biosimilar market Growth Factors#Global Biosimilar market#Biosimilar market share#Bios
1 note
·
View note
Text
Global Biosimilars Market 2020: Historical Analysis, Business Opportunities, Latest Innovations, Top Players and Forecast
Biosimilars Market Synopsis
Market Research Future (MRFR) postulates that the global biosimilars market share is predicted to garner USD 3.35 billion, grabbing a CAGR of 26% throughout the forecast period (2017-2023). The augmenting incidences of several chronic diseases are estimated to favor the Biosimilars Market Growth. Biosimilars are referred to as a biological medical product which are identical copies of the original medical product. They are generally derived from a plant, bacteria, yeast, and other several processes. Biosimilars are neither generics nor treated like generic drugs. Also acknowledged as bio-pharmaceuticals, biosimilars differ from generics in the manufacturing process. They are produced by two main processes, recombinant DNA technology and controlled gene expression.
Biosimilars Market Potential and Pitfalls
With the augmenting incidences of several chronic diseases, growing demand for cheap medical products, and strategic collaborations leading to clinical trials and enhanced productivity, the global biosimilars market is estimated to flourish throughout the appraisal period. Biosimilars are extensively used in the treatment and prevention of chronic diseases like cancer, diabetes, autoimmune disease, cardiovascular diseases (CVDs), kidney failure, rheumatoid arthritis, hematological disease, hormone growth deficiency, and infectious disease. This is likely to create momentum to the market growth during the estimated period. Also, with the growth in the geriatric population, the demand for biosimilars is expected to trigger.
Get a FREE Sample with Complete TOC By Considering the COVID-19 impact on Global Market @ https://www.marketresearchfuture.com/sample_request/1329
Biosimilars are constantly gaining prominence over other conventional biologics due to lesser cost as compared to the parent biological drugs. Moreover, different private and government bodies are promoting the use of biosimilars over synthetic drugs and conventional biologics, which is further propelling the growth of the market across the globe. Also, with the rapid growth in the pharmaceutical sector combined with the high cost of existing biological drugs, the market is anticipated to boom.
On the contrary, physician skepticism coupled with the lack of awareness regarding biosimilars is some of the top barriers likely to vitiate the market growth in the coming years. Additionally, complexity and high manufacturing cost can act as roadblocks to the mainstream production of biosimilars. Despite such hiccups, discounted prices of biosimilars will impact the overall biosimilar sales, as patients are the key beneficiaries.
Global Biosimilars Market: Segmental Analysis
The global biosimilars market has been segmented on the basis of product, application, and manufacturing.
By mode of product, the global biosimilars market has been segmented into recombinant non-glycosylated proteins, recombinant glycosylated proteins, and others. Among these, the recombinant glycosylated proteins segment is anticipated to occupy the largest share and is likely to retain its dominance. The growth is credited to the wide therapeutic area of such proteins coupled with the presence biosimilar version of monoclonal antibodies at much lower rates.
By mode of application, the global biosimilars market has been segmented into oncology, immune diseases, blood-related disorders, and others.
By mode of manufacturing, the global biosimilars market has been segmented into contract manufacturing and in-house manufacturing. Among these, the in-house manufacturing is presumed to occupy the largest share owing to the rising demand for cost-effective biosimilar products due to the augmenting incidences of several chronic diseases.
Biosimilars Market Regional Insights
Geographically, the biosimilars market span across regions namely, Europe, Asia Pacific, North America, and the Middle East & Africa.
Among all the regions, the European region is likely to dominate the global biosimilars market and is estimated to retain its dominance in the coming years. The growth is credited to the rising prevalence of diseases coupled with the growth in the geriatric population in this region. Moreover, advanced medical research and technological advancements are further triggering the demand for biosimilars in this region. The growth in the regional market is likely to be stimulated by patent expiry of biologic products combined with the launch of new biosimilars and the advent of new market participants.
The North American region is predicted to occupy the second largest share in the global market owing to technological advancements and extensive medical research. The market is mainly driven by the presence of large research labs such as Amgen, Sandoz, Teva Pharmaceutical, and others.
The Asia Pacific region is anticipated to emerge as the fastest-growing region owing to the immense economic development coupled with the booming biotechnology companies in this region. The country-specific markets in this region generating maximum revenue are India, Australia, South Korea, Japan, and China.
Biosimilars Industry Updates
February 19, 2019: Sandoz, a developer of biosimilar has recently announced the launch of their biosimilar adalimumab, Hyrimoz, in Spain. This biosimilar, referencing Humira, was authorized in the European Union for sale in July 2018. It has been approved for indications of reference adalimumab and is available in 40 mg doses for subcutaneous injection in pre-filled pen or a syringe form.
Obtain Premium Research Report Details @ https://www.marketresearchfuture.com/reports/biosimilars-market-1329
Biosimilars Market Competitive Dashboard
The prominent players operating the global biosimilars market are Biocon Ltd. (India), Eli Lilly (U.S.), Novartis (Switzerland), Celltrion Inc. (South Korea), Dr. Reddy’s Laboratories Ltd. (India), Pfizer Inc. (U.S.), Teva Pharmaceuticals Industries Ltd. (Israel), Sandoz International GmbH (Germany), Astra Zeneca (U.K.), Amgen Inc. (U.S.), Samsung Bioepis (South Korea), F. Hoffmann-La Roche Ltd. (Switzerland), and Accord Healthcare (U.K.).
1 note
·
View note
Text
Erythropoietin Drug Market Size Emerging Growth, Competitor Analysis And Industry Drivers 2027

Market Overview
Erythropoietin, also known as hemotopoietin, is a hormone produced by the kidneys for the continuous production of red blood cells. They are used in the treatment of diseases such as anemia, AIDS, cancer, and others. The global erythropoietin market report by Market Research Future (MRFR) has been compiled after undertaking painstaking primary and secondary research. Predictions and projections outlined in the report for the forecast period are based on current trends, historical events, and technological breakthroughs.
Market Scope:
The global erythropoietin market Size is set to accumulate high revenues at 9.5% CAGR during the forecast period. Large number of patients suffering from anemia, HIV, and end stage renal disease (ESRD) is the primary driver of the market. The hormone is produced in a synthetic form via recombinant technology into darbepoietin alfa, epoetin alfa, epoetin omega, and others. According to the National Institute of Diabetes Digestive and Kidney Diseases (NIDDK), close to 661,000 people were diagnosed with chronic kidney disease. This can fuel the demand for EPO drugs in the market.
The large cases of ESRD has caused a surge of anemia among patients and portended to fuel the demand for erythropoietin drugs. Approval of Epogen and commercialization of the drug as early as 2016 has facilitated market growth. Rise of chemotherapy as a part of cancer treatment can bode well for the market. High incidence of various cancers will be a favorable catalyst for the overall market. The utilization of the drug for treatment of hematology and neurology can affect the industry over the forecast period.
Request Free Sample Copy at: https://www.marketresearchfuture.com/sample_request/1360
Segmental Overview
The global erythropoietin market is segmented by type of product, application, and end user.
By type, it is segmented into biosimilars, first generation formulation, and second generation formulation. The biosimilar segment is expected to generate massive revenue for the global erythropoietin market owing to the expiration of patented drugs by major pharmaceutical companies. Low cost of therapeutics in developing economies of APAC and MEA will facilitate segment growth.
By application, it is segmented into HIV, oncology, renal diseases, and others.
On the basis of end user, it is divided into hospital and pharmacy. The pharmacy can be the biggest end user in the global erythropoietin market owing to having a large distribution network and being able to provide drugs to anemic patients at ease.
Global Erythropoietin Drug Market: Regional Segmentation
On the basis of region, the market has been segmented into North America, South America, Asia Pacific (APAC), Europe, and the Middle East & Africa (MEA). North America represents the largest market for Erythropoietin. This growth can be partly attributed to rising cancer prevalence and increased incidence of renal diseases in the U.S. and Canada. Other factors that favour the market growth in the region include widespread accessibility of cutting-edge medical services and strong government support. North America is followed by Europe and APAC respectively. The market is likely to witness towering gains in APAC in particular. In addition, the APAC erythropoietin drug market is projected to exhibit the fastest growth during the forecast period. Increased penetration of healthcare services and expanding patient population in countries such as China and India. Moreover, increased government effort to improve healthcare in these countries is creating further opportunities. LatAm and the MEA regions hold relatively lower share of the global erythropoietin drug market.
Competitive Landscape
Amgen (US), Teva Pharmaceutical Industries Ltd. (Israel), Emcure Pharmaceuticals (India), Hospira (US), LG Life Sciences Ltd. (Korea), Kyowa Hakko Kirin, Boehringer Ingelheim (US), Intas Pharmaceuticals (India), Hoffmann-La Roche (Switzerland), Johnson & Johnson (US), Celltrion, Inc. (South Korea), 3SBio, Biocon (India), BIOSIDUS (Argentina), and Dahua Pharmaceutical (China).
Browse Detailed TOC with COVID-19 Impact Analysis at:
https://www.marketresearchfuture.com/reports/erythropoietin-drug-market-1360
About Market Research Future:
At Market Research Future (MRFR), we enable our customers to unravel the complexity of various industries through our Cooked Research Report (CRR), Half-Cooked Research Reports (HCRR), & Consulting Services. MRFR team have supreme objective to provide the optimum quality market research and intelligence services to our clients.
Contact us:
Market Research Future (part of Wantstats Research and Media Private Limited),
99 Hudson Street, 5Th Floor,
New York, New York 10013
United States of America
+1 628 258 0071
Email: [email protected]
0 notes
Text
Biosimilars Market Retail Channel Analysis, Profit Margin Study and Competitive Rivalry, 2025
The scope of the global Biosimilars Market was appreciated by US$ 4.36 billion in 2016. It is projected to touch US$ 61.47 billion by the completion of 2025. It is likely to increase at a CAGR of 34.2% during the period of forecast. The most important biological medicines are upcoming the patent precipice. This is the greatest noteworthy motivating feature for the market. For example, Roche’s Mab Thera/Rituxan (rituximab), a monoclonal antibody biologic was accepted by the U.S. Food &Drug Administration (FDA) during November 1997, and its patent was taken in the U.S.A, terminated in September 2016. Several companies for example Pfizer, Amgen, and Boehringer Ingelheim are concentrating on the development of biosimilars medication of rituximab.
Biosimilars are extremely matching to accepted biological medicines. They have alike medicinal possessions using the effectiveness, safety, and potency of original biologic goods. Greater occurrence of long-lasting illnesses for example anemia, cancer, lack of development hormone, and diabetes, is likely to additionally power the growth of the market during the upcoming period.
Classification:
The global biosimilars market can be classified by Application, Product, and Region. By Application, it can be classified as Growth Hormonal Deficit, Oncology, Chronic and Autoimmune Complaints, Blood Disorders, and others. By Product, it can be classified as Recombinant Glycosylated Proteins, Recombinant Non-Glycosylated Proteins.
Companies:
Some of the important companies for the biosimilars market are Mylan N.V., Samsung Bioepis, Teva Pharmaceutical Industries Ltd., Sandoz International GmbH, Amgen Inc., Biocon, Pfizer Inc., Dr. Reddy’s Laboratories Ltd., and F. Hoffmann-La Roche Ltd.
Request free sample to get a complete analysis of the market players @ https://www.millioninsights.com/industry-reports/biosimilars-market/request-sample
Drivers:
The price efficiency of biosimilars medicines and the greater occurrence of long-lasting complaints all over the world are round about the most important reasons for funding to the development of the market.
Entire prices of healthcare have augmented owing to the greater costs of patented medicinal preparations, particularly biologics. The administrations of numerous nation-states are stressing the creation of price-operative medication. The U.S.A. is well-known as the nation having the maximum health expenses. It has newly put stress on decreasing the expenses on healthcare. In the same way, price guidelines in Japan and the abridged budget of healthcare in India, have elevated the demands for price repression. This fetches the necessity for developing reasonably priced, better-quality, effective and new-fangled treatments. Hence, hard work to bring down healthcare payments is expected to increase the biosimilar market.
Restraints:
Strict rules by the government for the development and manufacture of biosimilars could hamper the development of the biosimilars industry. Precise strategies are delivered by numerous governing establishments to uphold the care profile and efficiency of planned medications. Dissimilar controlling organizations comprising China Food & Drug Administration, European Medicines Agency, U.S. Food & Drug Administration (FDA), have varied guidelines for endorsements of the medication. The existence of diverse guidelines makes the endorsement procedure of biosimilar medicine extremely time-consuming and tiresome.
Regional Lookout:
By Region, the global biosimilar industry can be classified as North America, Europe, Asia Pacific, and the Rest of the World (RoW). Due to the existence of definite controlling background for biosimilars and the most important biopharmaceutical companies for example GlaxoSmithKline, Merck, AstraZeneca, Johnson & Johnson, Sanofi, Pfizer, and Novartis, Europe has retained the biggest share of the market using an income. Additionally, the finely honed structure of healthcare and an increasing number of product presentations have powered the development of the local market.
Europe was tracked by the Asia Pacific. It was responsible for the most important share of the market during 2016. Increasing demand for less costly healing products and greater occurrence of long-lasting illnesses in the Asia Pacific are backing the development of the local market. Growing concentration on the developments of the product in the nation-states comprising South Korea, India, and China is an additional foremost reason that motivates the market.
North America is estimated to witness a greater CAGR for the duration of the forecast, owing to increasing efforts from the companies to tap development openings in Canada and the U.S.A. The biosimilar controlling path of the U.S.A. was shaped in March 2009, and ever since and there the area has increased substantial momentum, giving fresh openings and challenges. In March 2015, the Food & Drug Administration (FDA) of the U.S.A. permitted the first biosimilar product, Zarxio (filgrastim-sndz).
Browse Related Category Research Reports @ https://industryanalysisandnews.wordpress.com/
0 notes
Text
Top 5 players in US Biosimilar Market
Buy Now
STORY OUTLINE
Pfizer: Excelling in the line of Biosimilar drugs with an experience of more than 10 years with presence in over 180 countries.
Amgen: Making pharmaceutical products with an experience of over 40 years and presence in over 100 countries.
Viartis: Presence in over 165 countries, and making Biosimilar drugs in over 75 markets, this pharmaceutical company is another leading contributor of US Biosimilar market.
Coherus Biosciences: Increasing patient access to cost effective medicines with a Biosimilar drugs experience of 13 years.
Biogen: serving humanity through science with a experiences of more than 40 years in the field of biologics.
According to Ken Research, the US Biosimilar market is anticipated to grow at a CAGR of ~40% in the next five years which currently has a market size of ~USD 9.4 Bn.
The US Biosimilar market is rapidly growing and will be witnessing a significant growth in the next five years.
There are various reasons behind the rapid growth of US Biosimilar market. Some of the major reasons behind the growth of US Biosimilar market include the cost effective nature of Biosimilar drugs, rising geriatric population, rising prevalence of chronic diseases, and growing partnerships between companies to develop Biosimilar drugs.
Various companies and players are contributing to their best efforts in the growth of the US Biosimilar market.
This article aims to put light on the contributions done by the major players towards the growth of the US Biosimilar market.
1.Pfizer
Click to read more about Pfizer
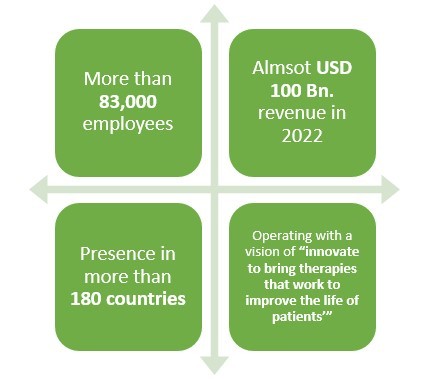
Pfizer is a leading American pharmaceutical company which is operating in the field of generics or original drugs for more than 30 years. But did you know that this pharma not only manufactures biologics but also biosimilar drugs?
Pfizer has been in the business of biosimilar drugs for more than 10 years and have been quite successful as well. With more than 83,000 employees and presence in over 180 countries, this leading pharmaceutical company made almost USD 2 Bn. revenue only from its Biosimilar drugs sale in 2021.
Recently, this pharmaceutical company also collaborated with Samsung in two deals to produce various biosimilar drugs in South Korea. The deal size between these two companies happens to be approximately USD 900 Bn.
The major Biosimilar drugs of this pharmaceutical giant are primarily
ZIRABEV (a Biosimilar of Avastin)
TRAZIMERA (a Biosimilar of Herceptin)
RUXIENCE (a Biosimilar of Rituxan)
RITACRIT (a Biosimilar of Epogen)
NVYEPRIA (a Biosimilar of Neulasta)
NIVESTYM (a Biosimilar of Neupogen)
FILGRASTIM (a Biosimilar of Neupogen).
2.Amgen
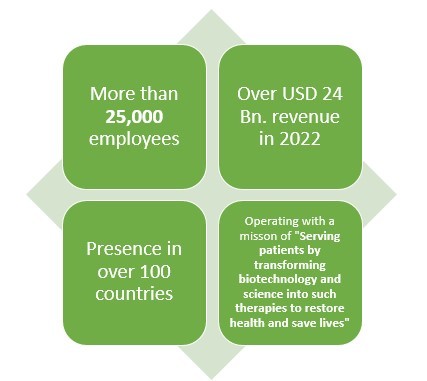
Click here to Download a Sample Report
Amgen is another leading American pharmaceutical company which not only makes Biologics or generic drugs but also Biosimilar drugs. This pharmaceutical company has more than 40 years of experience when it comes to pharmaceutical line.
With over 25000 employees and presence in over 100 countries, this pharmaceutical company earned about USD 2 Bn. from their three biosimilar drugs which are reportedly MVASI, KANJITNTI, and AMJEVITA.
This pharma giant has also invested about USD 2 Bn. in the development of Biosimilar drugs.
This pharmaceutical company has made Biosimilar drugs primarily in 4 fields which are General Medicine, Oncology, and Hematology along with, Inflammation.
EPOTEIN ALFA
AMJEVITA
AVSOLA
KANJINTI
MVASI
RIABNI
are the various Biosimilar drugs of Amgen. And, STELARA, EYLEA, SOLIRIS are in their pipeline.
Recently Amgen revealed their Biosimilar report’s 8 version. It revealed a major information which said that the pharmaceutical company saved about USD 10 Bn. through their Biosimilar drugs in the past five years.
3.Viartis

Headquartered in Canonsburg, Pennsylvania, this American pharmaceutical company was founded only in 2020 yet they have achieved massive success in the pharmaceutical products with their revenue being USD 16 ~Bn. in 2022.
With presence in 165 countries and with over 45,000 employees worldwide, this pharmaceutical company makes pharmaceutical products in 10 areas which primarily are Cardiovascular, Dermatology, ophthalmology, Oncology, Gastroenterology, Women’s health, Infectious diseases, Diabetes & Metabolism, Immunology, CNS & Anesthesiology, Respiratory diseases and allergy.
Speaking of their first Biosimilar products, their first ever Biosimilar drug was launched in 2014. They have a variety of Biosimilar drugs which are primarily
TRASTUZUMAB
INSULIN ASPART
PEGFILGRASTIM
INSULIN GLARGINE-YFGN
ADALIMUMAB
BEVACIZUMAB
Their Biosimilar drug Insulin Glargine which is known as SEMGLEE was the first ever interchangeable Biosimilar drug in the United States which was FDA approved.
Their PEGFILGRASTIM also was the first ever FDA approved drug in the United States. They have launched their Biosimilar drugs in over 75 markets worldwide.
4.Coherus Biosciences:
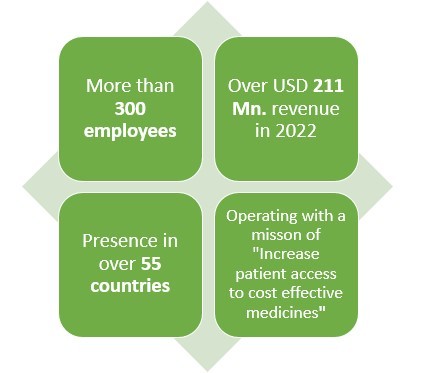
Click here to Ask for a Custom Report
Headquartered in Redwood city, California this American pharmaceutical company earned a revenue of almost USD 211 Mn. In 2022.
With presence in over 55 countries and 300+ employees worldwide, this pharmaceutical company makes products in various areas such as solid tumors, non-small lung cancers, nasopharyngeal carcinoma, small cell lung cancer and hepatocellular carcinoma.
Speaking of their Biosimilar drugs, this pharma has been in the field of creating Biosimilar drugs since 2010 which has given them almost 13 years of experience.
This pharmaceutical company also disclosed that it plans to spend at least USD 1 Tn. on medicines worldwide, out of which at least 40% will be spent on Biosimilar drugs.
Their three major Biosimilar drugs which are also FDA approved include UDENCYA, YUSIMRY, and CIMERLI.
Udencya is a Biosimilar drug of Pegfilgrastim, Yusimry is a Biosimilar drug of Ranibizumab, and Cimerli is a Biosimilar drug of Adalimumab.
5.Biogen
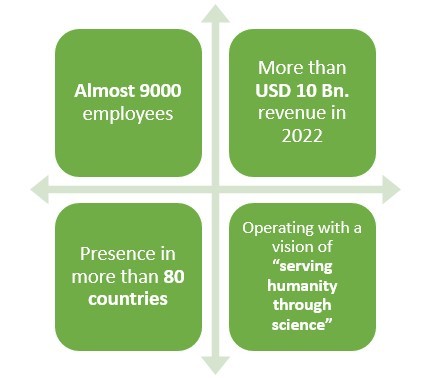
Headquartered in Cambridge, Massachusetts, this American pharmaceutical company earned a revenue of around USD 10 Bn. in 2022.
This company happens to have an experience of more than 40 years when it comes to making pharmaceutical products.
With presence in over 80 countries and more than 9000 employees worldwide, this pharmaceutical company primarily deals in Neurology, Specialized Immunology, Neuropsychiatry, Ophthalmology, and Rare Diseases.
ADUCANUMAB
LECANEMAB
TOFERSEN
ZURANOLONE
LITIFILIMAB
BENAPALI
FLIXABI
IMRALDI
are some of their Biosimilar drugs.
With their Biosimilar drugs, more than 250,000 people have gone on Anti-Tumor Necrosis Factor therapy.
Recently, this pharmaceutical company also made an agreement with Bio-Thera solutions to develop a Biosimilar drug for the treatment of Rheumatoid Arthritis.
#US Biosimilar Sector#United States Biosimilar Market#US Biosimilar Market forecast#US Biosimilar Market analysis#US Biosimilar Market trends#US Biosimilar Market share#US Biosimilar Market key players#US Biosimilar Market revenue#US Biosimilar Market growth#Monoclonal Antibodies in biosimilar market US#Recombinant Hormones in biosimilar industry US#Oncology in bio similar market US#Blood disorders in biosimilar market US#Research institutes in Biosimilar market US#US similar biotherapeutics products market#Hospitals in Biosimilar market US#Investors in Biosimilar market US#US comparable biologics products industry#US recombinant biosimilars industry#US replicate biologics sector#US analog biologics market
0 notes
Text
Biosimilars Market Trends Industry Share, Leading Players Strategy, Development Plans, Emerging Demand, CAGR, Drivers and Opportunity Outlook with Top Growth Companies
A Biosimilars Market Trends is described as a biological, medical product which is an identical copy of the original medical product. Market Research Future (MRFR) has published and released a research report about the global biosimilars market that predicts massive enlargement for this market with 26% CAGR during the forecast period between 2016 and 2023. In terms of cash, the market has been anticipated to rise to the US $ 16.97 bn.Get Request Free Sample @ https://www.marketresearchfuture.com/sample_request/1329Analyzing the market structure, this report estimates the future growth potential of the market. It observes the strategies of the key players in the market and follows the competitive developments like joint ventures, new product developments, mergers and acquisitions, research and developments (R & D) in the market. This report also covers insights on the major countries/regions in which this industry is flourishing, along with the list and description of untapped regions which could be the potential markets in the future.The key factors aiding the growth of the global biosimilars market include clinical trial activities for biosimilars, increasing demand for the cheap medical products, increasing incident of different diseases, and strategic collaborations resulting in enhanced productivity. However, lack of awareness and physician skepticism are the major restraining factor regarding market growth.The segmentation global biosimilars market is on the basis of application, manufacturing, product, and region. The application based segmentation segments the market into blood-related disorders, immunity-related diseases, oncology, and other. Based on manufacturing, the market has contract manufacturing and in-house manufacturing. As per segmentation based on product, the market has been segmented into recombinant glycosylated proteins, recombinant non-glycosylated proteins, and others. Recombinant glycosylated proteins have been further segmented into Monoclonal antibody (mAb) and EPO. Recombinant non-glycosylated proteins have been further segmented into growth hormones, insulin, and other.The regional segmentation of the global biosimilars market segments the market into continent-based regional markets namely Asia Pacific, North America, Europe, and rest of the world (RoW). According to the report, Europe is the biggest regional market for the biosimilars due to the advanced medical research, increasing the prevalence of diseases and rising geriatric population. Due to technological advancement, Western Europe is a bigger market than Eastern Europe. The key country-specific markets in Western Europe include France, Italy, Spain, Germany, and the United Kingdom (UK), followed by the rest of Western Europe.North America is the second largest market, being ahead of Europe in terms of technological advancement and being equal to Europe in terms of medical research. North America‘s approach towards biosimilars is not as welcome as Europe‘s is. Major country-specific markets in North America are Canada and the United States of America (USA). The Asia Pacific has been expected to emerge as the fast-growing market during the forecast period. The country-specific markets generating maximum revenue in this region are Australia, India, Japan, South Korea, and China, followed by the rest of the Asia Pacific region.Key PlayersThe key players in the global biosimilars market include Accord Healthcare (UK), Amgen Inc. (USA), Astra Zeneca (UK), Biocon Ltd. (India), Celltrion, Inc. (South Korea), Dr. Reddy’s Laboratories Ltd. (India), Eli Lilly (USA), F. Hoffmann-La Roche Ltd. (Switzerland), Novartis (Switzerland), Pfizer Inc. (USA), Samsung Bioepis (South Korea), Sandoz International GmbH (Germany), and Teva Pharmaceuticals Industries Ltd. (Israel).Latest Industry NewsMundipharma, a network of independent pharma companies, has acquired Spanish biosimilars development company Cinfa Biotech. The deal gives Mundipharma, access to a biosimilar to Amgen’s Neulasta
(pegfilgrastim). 10 OCT 2018
The new white paper issued by the Association of European Cancer Leagues, a nonprofit, pan-European organization of national and regional cancer societies, has called for the greater use of biosimilars as a means to increase patient access to cancer treatment, and reduce costs. 10 OCT 2018
Table of Contents1. REPORT PROLOGUE2. MARKET INTRODUCTION2.1. Definition2.2. Scope of the Study2.2.1. Research Objective2.2.2. Assumptions2.2.3. Limitations3. RESEARCH METHODOLOGY3.1. Overview3.2. Primary Research3.3. Secondary Research3.4. Market Size Estimation4. MARKET DYNAMICS4.1. Overview4.2. Drivers4.3. Restraints4.4. Opportunities5. MARKET FACTOR ANALYSIS5.1. Porter’s Five Forces Analysis5.1.1. Bargaining Power of Suppliers5.1.2. Bargaining Power of Buyers5.1.3. Threat of New Entrants5.1.4. Threat of Substitutes5.1.5. Intensity of Rivalry5.2. Value Chain AnalysisBrowse Complete Report @ https://www.marketresearchfuture.com/reports/biosimilars-market-1329About Market Research Future:At Market Research Future (MRFR), we enable our customers to unravel the complexity of various industries through our Cooked Research Report (CRR), Half- Cooked Research Reports (HCRR), Raw Research Reports (3R), Continuous-Feed Research (CFR), and Market Research & Consulting Services. MRFR team have supreme objective to provide the optimum quality market research and intelligence services to our clients. Our market research studies by Components, Application, Logistics and market players for global, regional, and country level market segments, enable our clients to see more, know more, and do more, which help to answer all their most important questions. In order to stay updated with technology and work process of the industry, MRFR often plans & conducts meet with the industry experts and industrial visits for its research analyst members.Contact:Market Research FutureOffice No. 528, Amanora ChambersMagarpatta Road, Hadapsar,Pune – 411028Maharashtra, India+1 646 845 9312Email: [email protected] Market Trends
#Biosimilars Market#Biosimilars Market Industry#Global Biosimilars Market#Biosimilars Market Size#Biosimilars Market Share#Biosimilars Market Research#Biosimilars Market Growth#Biosimilars Market Trend#Biosimilars Market Analysis#Biosimilars Market MRFR
0 notes
Text
Global Biosimilars Market Overview, Experiments, Evolution, Key Players And Forecast Until 2027


The global Biosimilars market was valued at US$ xx million in the year 2019. The market is estimated to be valued at US$ xx million in the year 2020, and is expected to reach US$ xx million by the year 2027, with an estimated CAGR of xx% during the forecast period (2021-2027). The research study also includes exhaustive information about market dynamics such as drivers, restraints, opportunities, technological advancements, future trends, supply chain analysis, patent landscape, pricing analysis, regulatory and reimbursement framework for precise market estimations and forecasts.
Additionally, the business intelligence study encompasses the COVID-19 scenario as follows:
· Long-Term, Mid-Term, and Short-Term Impact of COVID-19
· Pre- and Post-COVID-19 Scenario
· Disruptions in the Supply Chain
Get Free PDF Brochure Of This Report @ https://www.fostermarketresearch.com/product/industry/3/224/Pdf%20Brochure/
Competitive Insights
Leading and emerging companies of the global Biosimilarsmarket were identified based on their current offerings. Further, the Biosimilars industry is one of the most competitive industries, with the leading players actively competing against each other to gain a greater share in the industry. Some of the major companies in the Biosimilars market are: Novartis AG, Mylan NV, Pfizer Inc., Sandoz International GmbH, Biocon Ltd., Dr Reddy’s Laboratories Ltd., Amgen Inc., Teva Pharmaceuticals Inc., Celltrion Inc., Samsung Bioepis, Innovent Biologics, Inc., Bioton S.A., Biosidus, Stada Arzneimittel AG, and Fresenius Kabi AG, among others.
The competitive landscape of the Biosimilars industry exhibits an inclination towards emerging strategies including product launches, partnerships, collaborations and contracts, acquisitions, funding, and other developments to achieve the objectives faster.
Our reports fill up the gaps and provide you with all the detailed analysis of competitive companies involving:
· Analyzing competitive strategies and techniques
· Competitive positioning of key players
· PORTER’s and SWOT analysis for competitive risk analysis
· Competitive Share Analysis
· Financial analysis of players to determine their withstand capacity
· Analyzing their sales path and product study
For More Information Of This Report @ https://www.fostermarketresearch.com/product/industry/3/224/
Key Segments Covered in the global Biosimilars market report are:
· By Application: (Oncology, Gastroenterology, Immunology, Cardiology, and Others)
· By Drug Class: (Monoclonal Antibodies (Adalimumab, Infliximab, Rituximab, Trastuzumab, Bevacizumab, and Others), Recombinant Hormones (Insulin, Human Growth Hormone, Follicle-Stimulating Hormone, and Others), Recombinant Growth Factor (Granulocyte Colony-Stimulating Factor, Erythropoietin, and Other), Immunomodulators (Interleukins, Cytokines, Immunomodulatory Imide Drugs (IMID), and Others), Anti-Inflammatory Agents (TNF Inhibitors and Other Anti-inflammatory Agents)
· By Distribution Channel: (Hospital Pharmacies, Retail Pharmacies and Online Pharmacies)
Regional Coverage:
The global Biosimilars market segregates into five regions including North America, Europe, Asia-Pacific, Latin America, and Middle East and Africa. North America followed by Europe held the major share of the global market (in terms of value) in 2020. However, Asia-Pacific region exhibit highest CAGR (%) during the forecast period (2021-2027). Our research study further sub-divides regions into countries as follows:
· North America - the U.S. and Canada
· Europe - Germany, the U.K., France, Spain, Italy, Russia, and Rest-of-Europe
· Asia-Pacific - China, India, Japan, South Korea, Australia, ASEAN, and Rest-of-Asia-Pacific
· Latin America - Mexico, Brazil, and Rest-of-Latin America
· Middle East and Africa - Gulf Cooperation Council (GCC) Countries, South Africa, Israel, and Rest-of-Middle East and Africa
Market Dynamics:
Key players are raising their funding to continue the study of biosimilars pipeline, which is anticipated to boost growth of the global biosimilars market in the foreseen period. For instance, in January 2019, Alvotech raised its funding to US$ 300 million to continue the growth of its biosimilars pipeline. Company’s pipeline consists of six biosimilar monoclonal antibodies for treatment of cancer, autoimmune diseases, and inflammatory diseases.
Buy Now This Business Strategic Report To Improve Your Profits @ https://www.fostermarketresearch.com/product/buy/3/224/
About Foster Market Research:
Foster Market Research is a global market intelligence and advisory firm engaged in providing data-driven research extract from rigorous analysis, to the clients to make critical business decisions and execute them successfully. Foster connects over various distribution channels and numerous markets for great understanding of the trends and market to deliver our clients with accurate data.
Our focus is on providing market research that delivers a positive impact on your business. We work continuously to provide our clients with the most accurate analytics data and research reports without any delay so as to improve their business strategies and provide them with rich customer experience.
Contact Us:
1701 Royal Lane,
#1306, Dallas
Tx-75229
Phone: +1 469 4981929
Email: [email protected]
0 notes
Text
Biosimilars Market is Experiencing Significant Growth Due to the Rising US$ 61.47 billion by 2025
Mar 08, 2021: Synopsis:
The scope of the global Biosimilars Market was appreciated by US$ 4.36 billion in 2016. It is projected to touch US$ 61.47 billion by the completion of 2025. It is likely to increase at a CAGR of 34.2% during the period of forecast. Most important biological medicines are upcoming the patent precipice. This is the greatest noteworthy motivating feature for the market. For example, Roche’s Mab Thera/Rituxan (rituximab), a monoclonal antibody biologic was accepted by the U.S. Food &Drug Administration (FDA) during November 1997 and its patent taken in the U.S.A, terminated in September 2016. A number of companies for example Pfizer, Amgen and Boehringer Ingelheim are concentrating on the development of biosimilars medication of rituximab.
Biosimilars are extremely matching to accepted biologic medicines. They have alike medicinal possessions by means of the effectiveness, safety, and the potency to original biologic goods. Greater occurrence of long-lasting illnesses for example anemia, cancer, lack of development hormone and diabetes, is likely to additionally power the growth of the market during the upcoming period.
Download sample Copy of This Report at: https://www.millioninsights.com/industry-reports/biosimilars-market/request-sample
Drivers:
The price efficiency of biosimilars medicines and greater occurrence of long-lasting complaints all over the world, are round about the most important reasons funding to the development of the market.
Entire prices of healthcare have augmented owing to the greater costs of patented medicinal preparations, particularly biologics. The administrations of numerous nation state are stressing on creation of price operative medication. The U.S.A. is well-known as the nation having the maximum health expenses. It has newly put stress on decreasing the expenses on healthcare. In the same way, price guideline in Japan and abridged budget of healthcare in India, have elevated the demands for price repression. This fetches the necessity for developing reasonably priced, better-quality, effective and new-fangled treatments. Hence, hard work to bring down healthcare payments is expected to increase the biosimilar market.
Read Complete Report With TOC @ https://www.millioninsights.com/industry-reports/biosimilars-market
Restraints:
Strict rules by the government for the development and manufacture of biosimilars could hamper the development of the biosimilars industry. Precise strategies are delivered by numerous governing establishments to uphold care profile and efficiency of planned medications. Dissimilar controlling organizations comprising China Food & Drug Administration, European Medicines Agency, U.S. Food & Drug Administration (FDA), have varied guidelines for endorsements of the medication. Existence of diverse guidelines make endorsement procedure of biosimilar medicine extremely time consuming and tiresome.
Classification:
The global biosimilars market can be classified by Application, Product, and Region. By Application, it can be classified as Growth Hormonal Deficit, Oncology, Chronic and Autoimmune Complaints, Blood Disorders, and others. By Product, it can be classified as Recombinant Glycosylated Proteins, Recombinant Non-Glycosylated Proteins.
Regional Lookout:
By Region the global biosimilar industry can be classified as North America, Europe, Asia Pacific, and Rest of the World (RoW). Due to the existence of definite controlling background for biosimilars and the most important biopharmaceutical companies for example GlaxoSmithKline, Merck, AstraZeneca, Johnson & Johnson, Sanofi, Pfizer, and Novartis, Europe has retained the biggest share of the market by means of an income. Additionally, finely honed structure of healthcare and increasing number of product presentations have powered the development of the local market.
Get in touch
At Million Insights, we work with the aim to reach the highest levels of customer satisfaction. Our representatives strive to understand diverse client requirements and cater to the same with the most innovative and functional solutions.
Contact Person:
Ryan Manuel
Research Support Specialist, USA
Email: [email protected]
Global Headquarters
Million Insights
Felton Office Plaza 6265 Highway 9 Felton, California 95018, United States
Phone: 1-408-610-2300
Email: [email protected]
Asia Pacific
Million Insights
Office No. 302, 3rd Floor, Manikchand Galleria, Model Colony, Shivaji Nagar, Pune, MH, 411016 India
Phone: 91-20-65300184
0 notes
Text
Biosimilar Market Research Report
Growth Opportunities in the global biosimilar market look promising over the next six years. An increase in fast food consumption among people and a rise in the inactive lifestyle are the key factors driving the growth of the global biosimilar market.
Introduction of the Biosimilar Market:
Biosimilar refers to the type of biologic medical product, which is similar in reference to biologic. It does not have a clinically meaningful difference with regards to safety, purity, and potency. It is a type of drug which is produced by proteins and pieces of proteins (either natural or artificial). Biosimilars are made from living cells and require expertise and state-of-the-art technology in development and manufacturing. Biosimilar goes through multiple paths for FDA approval. Once approved, it needs special approval, which must be considered before it gets replaced automatically for its brand name drug.
Request for a FREE sample of this report: https://www.gmiresearch.com/report/global-biosimilar-market/sample-request
Biosimilar Market Dynamics (including market size, share, trends, forecast, growth, forecast, and industry analysis)
The global biosimilar market is projected to witness strong growth in the market during the forecast period. This is mainly due to growth in the penetration of chronic diseases worldwide driven by the increase in fast food consumption among people and the rise in inactive lifestyle. According to the biosimilar market forecast, the decreased clinical trials have improved patients' access to the treatment due to the low cost of biosimilars. This is a major factor in boosting the growth of the biosimilars market share in terms of revenue. There has been an upsurge in demand for the market in the upcoming years include an increase in the number of diagnoses of specific pathologies and the growth in the introduction of new biologic products. As per the biosimilar market research, the increasing geriatric population, growing R&D activities across different regions like Asia-Pacific, and rising focus on minimizing the healthcare cost across developing economies are the major factors fuelling the growth of the global biosimilar market size across the world. On the other hand, the presence of several complexities in the manufacturing process and limited definitive standards for approval are the factors restraining the growth of the global biosimilar market.
Based on the region, the Europe region is projected to dominate the market during the forecast period. This is attributed to the strong demand for biosimilars among the population, driven by the growing number of patients with chronic diseases across the region. Additionally, the presence of various key biopharmaceutical companies in the region and an increase in the geriatric population are some other key drivers propelling the growth of the biosimilars market in the region.
Key Players of the Global Biosimilar Market:
· Pfizer Inc.
· Sandoz International GmbH
· Teva Pharmaceutical Industries Ltd.
· Eli Lilly and Company
· Biocon
· Celltrion Healthcare Co., Ltd.
· Samsung Biologics
· Amgen Inc.
· Dr. Reddy’s Laboratories Ltd.
· Viatris Inc.
Biosimilar Market Segmentation:
Segmentation by Product:
· Insulin
· Monoclonal Antibodies
· Erythropoietin
· Granulocyte Colony-Stimulating Factor
· Etanercept
· Recombinant Human Growth Hormone
· Teriparatide
· Follitropin
· Interferons
· Enoxaparin Sodium
· Calcitonin
· Glucagon
Segmentation by Type of Manufacturing:
· Contract Manufacturing
· In-House Manufacturing
Segmentation by Indication:
· Chronic Diseases
· Oncology
· Blood Disorders
· Autoimmune Diseases
· Infectious Diseases
· Growth Hormone Deficiency
· Other Indications
Segmentation by Region:
· North America
o United States of America
o Canada
· Asia Pacific
o China
o Japan
o India
o Rest of APAC
· Europe
o United Kingdom
o Germany
o France
o Spain
o Rest of Europe
· RoW
o Brazil
o South Africa
o Saudi Arabia
o UAE
o Rest of the world (remaining countries of the LAMEA region)
About GMI Research
GMI Research is a market research and consulting company that offers business sights and market research reports for every enterprise, including small & medium enterprises and large organizations. Our research team helps the clients to understand the impact of market dynamics such as market size, share, drivers, growth opportunities, and other aspects. We have a team of analysts and industry experts who conduct market intelligence studies to ensure relevant and fact-based research across a wide range of sectors such as FMCG, Technology, Energy, Healthcare, and other industries. We collect relevant information about the industry using both internal and external databases. Our main focus is to keep our clients abridged of the emerging opportunities and challenges in a wide range of industries. We provide step-by-step assistance to our client through strategic and consulting services to reach a managerial and actionable decision. Featured in the ‘Top 20 Most Promising Market Research Consultants’ list of Silicon India Magazine in 2018, we at GMI Research are always looking forward to helping businesses stay ahead of the curve.
Media Contact
Company Name: GMI RESEARCH
Contact Person: Sarah Nash
Email: [email protected]
Phone: Europe – +353 1 442 8820; US – +1 860 881 2270
Address: Dublin, Ireland
Website: www.gmiresearch.com
0 notes
Text
Biosimilars Market Size, Share, Demand, Analysis and Forecast Report to 2027
The Biosimilars Market report, at first, has given a brief understanding of the industry through basic overview. This overview includes the market definition, key applications of the product, and the recent manufacturing technology employed for such production. The global Biosimilars market has been analyzed in detail to gain an understanding of the competitive landscape, key regional status, and recent trends noted in the relevant industry.
Request Sample Copy of this Report @ https://qualiketresearch.com/request-sample/Biosimilars-Market/request-sample
Biosimilars are highly identical to approved biological drugs. They have similar properties in terms of safety, potency, and efficacy to original biologic products but they have minor differences in clinically inactive components. Regulations for biosimilars play important role in maintaining the viability and balance between reference product and biosimilars product. Various regulatory authorizes like FDA and EMA actively regulate the development and commercialization of biosimilars.
Market Drivers
The rise in number of biosimilar approvals is the key driving factor which is expected to boost the global biosimilars market growth. For instance, in July 2018, the USFDA (U.S. Food and Drug Administration) had introduced a biosimilars action plan to encourage the development of biosimilars. Furthermore, increases in prevalence of chronic diseases like cancer will positively contribute the market growth. For instance, in 2018, as per the NCI (National Cancer Institute) information approximates 1,735,350 new cases of cancer likely to be diagnosed in United States. Moreover, increase in research and development activities as well as government initiatives will fuel the global biosimilars market growth during this forecast period. Many governments are emphasizing on cost-effective drug synthesis. The United States is the popular country with highest health expenditure.
Market Restraints
However, a complexity in manufacturing is the major restraining factor which is expected to hinder the global biosimilars market growth. Also, stringent rules and regulations for development and manufacturing of biosimilars will affect the market growth.
Market Key Players
Various key players are discussed in this report such as Novartis AG, Pfizer Inc. Celltrion Healthcare Co., Ltd, Teva Pharmaceutical, Biocon Limited, Dr. Reddy's Laboratories, Amgen, Inc.,and Sanofi S.A.
Market Taxonomy
By Drug Class
Recombinant Human Growth Hormone
Granulocyte Colony-Stimulating Factor
Fusion Proteins
Insulin
Erythropoietin
Monoclonal Antibodies
Follitropin
Anticoagulants
Others
By Therapy Type
Immunology
Oncology
Hematology
Hormone Therapy
Metabolic Disorders
Others
By Distribution Channel
Hospital Pharmacies
Retail Pharmacies
Specialty Pharmacies
By Region
North America
Latin America
Europe
Asia Pacific
Middle East & Africa
Get discount on this report @ https://qualiketresearch.com/request-sample/Biosimilars-Market/ask-for-discount
About Us:-
QualiKet Research is a leading Market Research and Competitive Intelligence partner helping leaders across the world to develop robust strategy and stay ahead for evolution by providing actionable insights about ever changing market scenario, competition and customers. QualiKet Research is dedicated to enhancing the ability of faster decision making by providing timely and scalable intelligence. We use different intelligence tools to come up with evidence that showcases the threats and opportunities which helps our clients outperform their competition.
0 notes
Text
Epo Biomarkers Market Size, Share, Outlook, and Opportunity Analysis, 2019– 2027

Epo Biomarkers Market, by Biomarker Type (Recombinant Human Erythropoietin, Erythropoietin Alfa, Erythropoietin Beta, Erythropoietin Zeta, Erythropoietin Theta, and Darbepoietin Alfa), by Application (End-stage Renal Disorder, Cancer, Rheumatoid Arthritis, AIDS, Myelodysplastic Syndrome, Neurology, Hematology, and Others), by End User (Diagnostic Centers, Hospitals, and Ambulatory Care and Surgical Centers), and by Region (North America, Latin America, Europe, Asia Pacific, Middle East, and Africa) - Size, Share, Outlook, and Opportunity Analysis, 2020 - 2027
Erythropoietin (Epo) is a glycoprotein hormone that consists 165 amino acids with four complex carbohydrate chains attached to the peptide at four linkage sites. Epo adjusts red blood cell production to meet the tissue oxygen demand. Epo biomarkers are used for treating cancers and associated anemia, AIDS, and mostly in end-stage renal disorders (ESRD). There are five different type of epo biomarkers. Commercially used epo for humans is known as recombinant human erythropoietin (rHuEPO).
Increasing research and development in epo biomarker technology and high prevalence of ESRD is expected to boost growth of the global epo biomarker market. For instance, in 2015, Molecular Health — a cloud-based healthcare decision support technology that aids in evidence-based treatment decisions and improved outcomes — confirmed the discovery that ephrin-type B receptor 4 (EPHB4) is an alternative epo receptor.
* The sample copy includes: Report Summary, Table of Contents, Segmentation, Competitive Landscape, Report Structure, Methodology.
Request a sample copy of this report: https://www.coherentmarketinsights.com/insight/request-sample/4057
Global Epo Biomarkers Market: Drivers
The epo biomarkers market is expected to witness significant growth over the forecast period, owing to increasing launch and adoption of erythropoietin alfa in cancer patients who are suffering from anemia. For instance, in November 2018, Pfizer launched epoetin alfa biosimilar — Retacrit — at 33% discounted rate than the original drug in the U.S. for treatment of anemia caused by chronic kidney disease and chemotherapy.
Furthermore, increasing prevalence of ESRD, which causes kidney failure and is often fatal is expected to boost the market growth. According to the U.S. Renal Data System Annual Data Report of 2016, over 660,000 people in the U.S. were treated for kidney failure or ESRD. Out of these, 468,000 were dialysis patients and over 193,000 had a functioning kidney transplant.
Global Epo Biomarkers Market: Restraints
A major restraining factor for the epo biomarkers market growth is the over-production of red blood cells due to over-expression of epo biomarkers, which leads to absolute polycythemia and other conditions. Overproduction of epo may be an adaptive response associated with conditions that produce tissue hypoxia such as living at high altitude, chronic obstructive pulmonary disease, cyanotic heart disease, and sleep apnea. According to an article published in Biospace Journal in 2015, in the U.S. between 40% and 90% of cancer patients suffer from chemotherapy-induced anemia. Use of epo in cancer patients that suffer from chemotherapy-induced anemia was restricted by the Food and Drug Administration (FDA) due to increasing adverse events including decreased survival and increased risk of tumor progression and recurrence.
Browse Research Report: https://www.coherentmarketinsights.com/ongoing-insight/epo-biomarkers-market-4057
Global Epo Biomarkers Market: Regional Analysis
North America is expected to hold a dominant position in the epo biomarkers market over the forecast period, owing to robust healthcare infrastructure and increasing prevalence of cancer in the region. According to World Health Organization’s (WHO) 2018 report, around 609,640 deaths were recorded due to cancer and around 1.7 million new cases of cancer were diagnosed in 2018 in the U.S.
Asia Pacific is expected to witness significant growth in the epo biomarkers market, owing to presence of major companies such as 3Sbio Group, Biocon Ltd., Genscript, Intas Pharmaceuticals Ltd., Kyowa Hakko Kirin Co., Ltd., and Shandong Kexing Bioproducts Co. Ltd. Moreover, increasing prevalence of AIDS in India, China, Pakistan, and Bangladesh is also contributing to epo biomarker market growth. For instance, according to the UNAIDS Data 2018 for Asia Pacific region, in India 2.1 million people are living with HIV AIDS at the end of 2017.
Global Epo Biomarkers Market: Competitive Landscape
Key players operating in the global epo biomarkers market include, 3Sbio Group, Agilent Technologies, Inc., Amgen Inc., Bioagilytix Labs, Biocon Ltd., Bio-Rad Laboratories, Inc., F. Hoffmann-La Roche Ltd., Galenica Ag, Genscript, Glaxosmithkline Plc., Intas Pharmaceuticals Ltd., Johnson & Johnson, Inc., Kyowa Hakko Kirin Co., Ltd., Teva Pharmaceutical Industries Ltd., Merck Kgaa, Myriad Rbm, Novartis Ag, Pacific Biomarkers, Pfizer Inc., Shandong Kexing Bioproducts Co. Ltd., Siemens Ag, and Thermo Fisher Scientific.
Buy-Now this research report: https://www.coherentmarketinsights.com/insight/buy-now/4057
About Coherent Market Insights:
Coherent Market Insights is a prominent market research and consulting firm offering action-ready syndicated research reports, custom market analysis, consulting services, and competitive analysis through various recommendations related to emerging market trends, technologies, and potential absolute dollar opportunity.
Contact Us:
mailto:[email protected]
U.S. Office:
Name: Mr. Shah
Coherent Market Insights 1001 4th Ave,
# 3200 Seattle, WA 98154, U.S.
US : +1-206-701-6702
UK : +44-020-8133-4027
JAPAN : +050-5539-1737
0 notes