#US Biosimilar Market growth
Text
Biosimilars Unleashed: The Future of Healthcare in the US
Buy Now
What is the Size of US Biosimilar Industry?
US Biosimilar Market is expected to grow at a CAGR of ~ % between 2017-2022 and is expected to reach ~USD Bn by 2028. Biosimilars enhance patient access to essential treatments, especially in therapies with high demand, like oncology, by providing more affordable options. Additionally, Growing evidence of biosimilars' comparable efficacy and safety fosters trust among healthcare professionals, driving adoption.

Click here to Download a sample Report
Biosimilars offer cost savings compared to originator biologics, addressing the need for affordable healthcare solutions in the face of rising medical costs. Favorable regulatory frameworks, like the BPCIA, streamline biosimilar approval processes, encouraging manufacturers to invest in development.
Furthermore, The expiration of patents for numerous reference biologics creates opportunities for biosimilar entry, leading to increased competition and market expansion. Pharmaceutical companies are investing in biosimilar R&D and production, expanding the pipeline and market availability. Supportive healthcare policies and reimbursement models incentivize biosimilar adoption, creating a favorable environment for market growth.
US Biosimilar Market by drug class
The US Biosimilar market is segmented by Monoclonal Antibodies, Recombinant Hormones, Immunomodulators, Anti-inflammatory agents and Others. Based on drug class, Monoclonal Antibodies segment dominates the bio similar market in 2022.
Monoclonal antibodies have diverse applications across various therapeutic areas. From cancer treatment to autoimmune diseases, biosimilar Mabs addressed a wide range of medical needs, leading to a broad and growing market. Biosimilars, with their potential for cost savings while maintaining comparable efficacy and safety, gained significant attention as viable alternatives.
US Biosimilar Market by application
In US Biosimilar market, they are segmented by application into Oncology, Blood disorders, Chronic diseases and autoimmune conditions and Others. On the basis of application, Oncology segment was the dominant in 2022.
The increasing prevalence of cancer and the high cost of traditional biologics used in oncology treatment have created a strong incentive for the adoption of biosimilars. Biosimilars offer the potential to provide similar therapeutic outcomes at a lower cost, making them an attractive option for both healthcare providers and patients.
Additionally, the rigorous clinical trials and regulatory processes that biosimilars undergo to gain approval provide reassurance to healthcare professionals and patients regarding their safety and efficacy. This has led to increased acceptance and adoption of biosimilars in oncology.
US Biosimilar by Region
The US Biosimilar market is segmented by Region into North, East, West and South. In 2022, the dominance region is North region in US Biosimilar market.
The North region benefits from a concentration of healthcare providers and academic institutions that are at the forefront of adopting and integrating biosimilars into their treatment protocols. These institutions are more likely to have the expertise to evaluate and incorporate biosimilars effectively, driving their adoption among healthcare professionals and patients.
Click here to Download a Custom Report
Competition Scenario in US Biosimilar Market
The US biosimilar market has witnessed an evolving competitive landscape, with several key players competing for market share. Prominent pharmaceutical companies such as Amgen, Pfizer, Sandoz (Novartis), and Boehringer Ingelheim have been actively involved in developing and marketing biosimilar products. These established players have utilized their expertise in biologics and significant resources to navigate the regulatory landscape and compete effectively.
The competition in the US biosimilar market is characterized by a balance between established pharmaceutical giants and emerging biotech companies. While the major players possess the advantage of resources and experience, smaller biotech firms are also contributing to the market with innovative approaches and niche biosimilar offerings.
What is the Expected Future Outlook for the Overall US Biosimilar Market?
The US Biosimilar market was valued at USD ~Million in 2022 and is anticipated to reach USD ~ Billion by the end of 2028, witnessing a CAGR of ~% during the forecast period 2022- 2028. The US biosimilar market is likely to experience significant growth in the coming years, driven by several factors. Biosimilars are biologic drugs that are highly similar to already approved reference biologics. They offer potential cost savings, increased competition, and improved patient access to crucial treatments.
Firstly, the regulatory environment is becoming more favorable for biosimilars. The Biologics Price Competition and Innovation Act (BPCIA) established a pathway for biosimilar approval in the US, allowing for a smoother regulatory process. As more biosimilars receive approval, competition in the market is expected to intensify.
Secondly, patents for several blockbuster biologics are expiring or have already expired. This creates opportunities for biosimilar manufacturers to enter the market with more affordable alternatives, offering healthcare systems and patients a choice in treatment options.
Thirdly, as healthcare costs continue to rise, biosimilars present an attractive solution for reducing expenses. Their potential to offer cost savings without compromising therapeutic efficacy could lead to increased adoption by healthcare providers, insurers, and patients alike.
Physician and patient education are crucial, as misconceptions about biosimilars' safety and effectiveness might hinder their adoption. Additionally, legal and market access barriers, including patent litigation and complex distribution systems, could slow down the growth of the biosimilar market.
The biosimilar market witness consolidation as larger pharmaceutical companies acquire or partner with smaller biotech firms to bolster their biosimilar portfolios. This will lead to more resources being devoted to biosimilar development and marketing. Changes in healthcare policies, such as reimbursement models and value-based care initiatives, can influence the biosimilar market's growth. Favourable policies that incentivize biosimilar adoption drives their market growth.
#US Biosimilar Market#US Biosimilar Industry#US Biosimilar Sector#United States Biosimilar Market#US Biosimilar Market forecast#US Biosimilar Market analysis#US Biosimilar Market trends#US Biosimilar Market share#US Biosimilar Market key players#US Biosimilar Market revenue#US Biosimilar Market growth#Monoclonal Antibodies in biosimilar market US#Recombinant Hormones in biosimilar industry US#Oncology in bio similar market US#Blood disorders in biosimilar market US#Research institutes in Biosimilar market US#US similar biotherapeutics products market#Hospitals in Biosimilar market US#Investors in Biosimilar market US#US comparable biologics products industry#US recombinant biosimilars industry#US replicate biologics sector#US analog biologics market#US homologous biologics market#US oncology biosimilar market#US immunology biosimilar sector#US insulin biosimilar industry#US Generics Biologics market challenges#US leading Biosimilar drug providers#US leading Biosimilar drug manufacturers
0 notes
Text
An Overview of Protein A Resin: Applications and Benefits in Bioprocessing
The global protein A resin market size is expected to reach USD 4.12 Billion in 2032 and register a steady revenue CAGR of 11.4%during the forecast period, according to latest analysis by Emergen Research. Development and launch of highly necessary biosimilar by key biopharmaceutical companies is a key factor driving market revenue growth in. Protein A resins are used for purification and fragmentation of immunoglobulins from biological fluids & cell culture media, immunoprecipitation of proteins, and antigens. For instance, on 13 June 2022, Bio-Rad Laboratories, Inc., a pioneer in life science research as well as clinical diagnostic items introduced CHT-prepacked Foresight Pro Columns. These columns are designed to facilitate downstream process-scale chromatography application areas across various stages of biological drug development and manufacturing.
The recent advancements in the Protein A Resin industry and trends driving the growth of the market. It is an investigative study covering analysis of market drivers, restraints, challenges, threats, and growth prospects in the global Protein A Resin market. The global Protein A Resin market report is a methodical research of the Protein A Resin market done by extensive primary and secondary research. The fundamental purpose of the Protein A Resin market report is to offer an accurate and strategic analysis of the Protein A Resin business sphere.
Get Download Pdf Sample Copy of this Report@ https://www.emergenresearch.com/request-sample/1724
Competitive Terrain:
The global Protein A Resin industry is highly consolidated owing to the presence of renowned companies operating across several international and local segments of the market. These players dominate the industry in terms of their strong geographical reach and a large number of production facilities. The companies are intensely competitive against one another and excel in their individual technological capabilities, as well as product development, innovation, and product pricing strategies.
The leading market contenders listed in the report are:
GE HealthCare, Merck KGaA, Repligen Corporation, Thermo Fisher Scientific Inc., Tosoh Corporation, Purolite, Novasep Holding SAS, Agilent Technologies, Inc., GenScript, and PerkinElmer Inc
Key market aspects studied in the report:
Market Scope: The report explains the scope of various commercial possibilities in the global Protein A Resin market over the upcoming years. The estimated revenue build-up over the forecast years has been included in the report. The report analyzes the key market segments and sub-segments and provides deep insights into the market to assist readers with the formulation of lucrative strategies for business expansion.
Competitive Outlook: The leading companies operating in the Protein A Resin market have been enumerated in this report. This section of the report lays emphasis on the geographical reach and production facilities of these companies. To get ahead of their rivals, the leading players are focusing more on offering products at competitive prices, according to our analysts.
Report Objective: The primary objective of this report is to provide the manufacturers, distributors, suppliers, and buyers engaged in this sector with access to a deeper and improved understanding of the global Protein A Resin market.
Emergen Research is Offering Limited Time Discount (Grab a Copy at Discounted Price Now)@ https://www.emergenresearch.com/request-discount/1724
Market Segmentations of the Protein A Resin Market
This market is segmented based on Types, Applications, and Regions. The growth of each segment provides accurate forecasts related to production and sales by Types and Applications, in terms of volume and value for the period between 2022 and 2030. This analysis can help readers looking to expand their business by targeting emerging and niche markets. Market share data is given on both global and regional levels. Regions covered in the report are North America, Europe, Asia Pacific, Latin America, and Middle East & Africa. Research analysts assess the market positions of the leading competitors and provide competitive analysis for each company. For this study, this report segments the global Protein A Resin market on the basis of product, application, and region:
Segments Covered in this report are:
Product Outlook (Revenue, USD Billion; 2019–2032)
Agarose-based Protein A
Glass/Silica-based Protein A
Organic Polymer-based Protein A
Type Outlook (Revenue, USD Billion; 2019–2032)
Recombinant Protein A
Natural Protein A
Application Outlook (Revenue, USD Billion; 2019–2032)
Antibody Purification
Immunoprecipitation
Browse Full Report Description + Research Methodology + Table of Content + Infographics@ https://www.emergenresearch.com/industry-report/protein-a-resin-market
Major Geographies Analyzed in the Report:
North America (U.S., Canada)
Europe (U.K., Italy, Germany, France, Rest of EU)
Asia Pacific (India, Japan, China, South Korea, Australia, Rest of APAC)
Latin America (Chile, Brazil, Argentina, Rest of Latin America)
Middle East & Africa (Saudi Arabia, U.A.E., South Africa, Rest of MEA)
ToC of the report:
Chapter 1: Market overview and scope
Chapter 2: Market outlook
Chapter 3: Impact analysis of COVID-19 pandemic
Chapter 4: Competitive Landscape
Chapter 5: Drivers, Constraints, Opportunities, Limitations
Chapter 6: Key manufacturers of the industry
Chapter 7: Regional analysis
Chapter 8: Market segmentation based on type applications
Chapter 9: Current and Future Trends
Request Customization as per your specific requirement@ https://www.emergenresearch.com/request-for-customization/1724
About Us:
Emergen Research is a market research and consulting company that provides syndicated research reports, customized research reports, and consulting services. Our solutions purely focus on your purpose to locate, target, and analyse consumer behavior shifts across demographics, across industries, and help clients make smarter business decisions. We offer market intelligence studies ensuring relevant and fact-based research across multiple industries, including Healthcare, Touch Points, Chemicals, Types, and Energy. We consistently update our research offerings to ensure our clients are aware of the latest trends existent in the market. Emergen Research has a strong base of experienced analysts from varied areas of expertise. Our industry experience and ability to develop a concrete solution to any research problems provides our clients with the ability to secure an edge over their respective competitors.
Contact Us:
Eric Lee
Corporate Sales Specialist
Emergen Research | Web: www.emergenresearch.com
Direct Line: +1 (604) 757-9756
E-mail: [email protected]
Visit for More Insights: https://www.emergenresearch.com/insights
Explore Our Custom Intelligence services | Growth Consulting Services
Trending Titles: Geocell Market | Pancreatic Cancer Treatment Market
Latest Report: Ceramic Tiles Market | Life Science Analytics Market
0 notes
Text
Exploring the Diagnostic Specialty Antibodies Industry: Trends and Growth Drivers
The diagnostic specialty antibodies market is emerging as a key component in the healthcare and biotechnology sectors. These antibodies are critical for diagnosing a wide range of diseases, from infectious diseases to chronic conditions like cancer and autoimmune disorders. The Diagnostic Specialty Antibodies Market is projected to be valued at USD 28.44 billion in 2024 and is anticipated to reach USD 36.52 billion by 2029, with a compound annual growth rate (CAGR) of 5.13% during the forecast period (2024-2029).
Market Overview
Specialty antibodies are highly specific proteins designed to target and bind to particular antigens, making them invaluable in diagnostic applications. Their precision helps in the early detection and monitoring of diseases, improving patient outcomes and contributing to the efficiency of the healthcare system. The global market for diagnostic specialty antibodies is expanding, driven by the rising prevalence of diseases, advancements in antibody engineering, and growing research activities in biotechnology.
Key Drivers of Market Growth
Rising Prevalence of Chronic Diseases: The increasing number of chronic diseases, such as cancer and autoimmune disorders, is driving the demand for diagnostic antibodies, which play a crucial role in early and accurate detection.
Advances in Biotechnology: Continuous advancements in antibody production and purification techniques are making diagnostic specialty antibodies more efficient, precise, and accessible to a wider range of medical applications.
Increasing Demand for Personalized Medicine: As healthcare shifts towards personalized treatments, specialty antibodies are becoming indispensable for tailored diagnostics, allowing clinicians to make more informed treatment decisions.
Technological Advancements in Diagnostics: Cutting-edge technologies like monoclonal and polyclonal antibodies are revolutionizing diagnostics by providing faster, more reliable test results, aiding in early disease intervention.
Rising Investments in R&D: Significant investments in research and development are enabling the discovery of novel antibodies that can detect specific biomarkers associated with various diseases.
Emerging Trends in the Industry
Adoption of AI in Diagnostics: Artificial Intelligence (AI) is playing a growing role in diagnostic processes, and when combined with antibody-based assays, it enhances the speed and accuracy of disease detection.
Development of Point-of-Care Testing: The push for rapid and decentralized testing solutions is promoting the use of specialty antibodies in point-of-care diagnostics, making them more accessible to a broader patient base.
Increased Use of Biosimilars: With the rise of biosimilars in the pharmaceutical industry, the market for diagnostic antibodies is seeing an influx of cost-effective alternatives to traditional therapeutic antibodies.
Challenges and Opportunities
While the market shows immense potential, challenges such as high production costs and regulatory hurdles can impact growth. However, there are also opportunities for market expansion, especially in developing regions where healthcare infrastructure is evolving, and the need for reliable diagnostic tools is growing.
Conclusion
The diagnostic specialty antibodies industry is on a growth trajectory, fueled by advancements in medical technology, the increasing prevalence of chronic diseases, and the shift towards personalized healthcare. As the demand for precise diagnostics continues to rise, the market is expected to expand, offering new opportunities for innovation and investment.
For a detailed overview and more insights, you can refer to the full market research report by Mordor Intelligence https://www.mordorintelligence.com/industry-reports/diagnostic-specialty-antibodies-market
#diagnostic specialty antibodies market#diagnostic specialty antibodies market size#diagnostic specialty antibodies market share#diagnostic specialty antibodies market trends#diagnostic specialty antibodies market analysis
0 notes
Text
Membrane Technology in Pharmaceuticals: A Comprehensive Market Growth Analysis through 2032
Introduction
Membrane technology has become a transformative force in the pharmaceutical industry, enabling innovation in drug development, purification processes, and biopharmaceutical manufacturing. The Membrane Technology in Pharmaceutical Market is projected to witness significant growth by 2032, driven by the increasing need for efficient separation processes, rising demand for high-quality pharmaceutical products, and advancements in membrane materials and technologies.
This article explores the current landscape, key drivers, and future opportunities for the membrane technology market in pharmaceuticals, providing a comprehensive outlook on trends and growth opportunities through 2032.
Market Overview
Membrane technology involves the use of semi-permeable membranes to separate substances, filter impurities, and purify fluids. It is employed across several pharmaceutical processes, including microfiltration, ultrafiltration, nanofiltration, and reverse osmosis, to enhance product quality and reduce manufacturing costs. The growing application of membrane technology in areas like drug development, sterile filtration, and wastewater treatment has led to an increase in market demand.
Membrane Technology in Pharmaceutical Market Size was estimated at 21.8 (USD Billion) in 2023. The Membrane Technology in Pharmaceutical Market Industry is expected to grow from 23.14 (USD Billion) in 2024 to 37.2 (USD Billion) by 2032. The Membrane Technology In Pharmaceutical Market CAGR (growth rate) is expected to be around 6.11% during the forecast period (2025 - 2032).
The pharmaceutical industry has embraced membrane technology due to its ability to streamline production, ensure purity, and meet stringent regulatory requirements. The increasing complexity of drug formulations, especially biologics, has also accelerated the adoption of membrane technology for precise filtration and separation processes. This technology plays a crucial role in manufacturing high-quality biopharmaceuticals, vaccines, and active pharmaceutical ingredients (APIs), making it indispensable for modern pharmaceutical production.
Key Market Trends
Several trends are driving the growth of the Membrane Technology in Pharmaceutical Market as we look toward 2032, including rising demand for biologics, advancements in nanofiltration, and the increased focus on sustainability in pharmaceutical manufacturing.
Rising Demand for Biopharmaceuticals Biopharmaceuticals, which include biologics and biosimilars, have become an essential part of modern medicine, particularly in the treatment of chronic diseases such as cancer, autoimmune disorders, and diabetes. The production of biologics involves complex processes that require high levels of purity and precision. Membrane technology, particularly ultrafiltration and microfiltration, is used extensively in the purification and filtration of biologics to ensure product quality. The rising demand for biopharmaceuticals is a major driver of growth for the membrane technology market in the pharmaceutical sector.
Advancements in Nanofiltration Technology Nanofiltration has emerged as a key innovation in membrane technology, offering highly selective filtration processes that allow for the separation of smaller particles, ions, and molecules. In pharmaceuticals, nanofiltration is used for removing contaminants, solvents, and unwanted particles during the production process. With ongoing advancements in nanofiltration membranes, such as improved material durability and enhanced permeability, the technology is becoming increasingly effective for complex pharmaceutical processes. These advancements are expected to further drive the adoption of membrane technology in drug development and manufacturing.
Focus on Sustainability and Environmental Concerns The pharmaceutical industry is under increasing pressure to reduce its environmental impact by adopting sustainable manufacturing practices. Membrane technology has emerged as a solution to several environmental challenges faced by the industry. For instance, reverse osmosis and nanofiltration membranes are used for wastewater treatment, reducing the discharge of harmful chemicals into the environment. Additionally, the use of membrane technology can reduce energy consumption and minimize the need for harmful chemical reagents in pharmaceutical processes. As sustainability becomes a growing focus for pharmaceutical companies, the demand for membrane technologies that offer environmentally friendly solutions is expected to increase.
Customization and Process Optimization The ability to customize membrane technology solutions for specific pharmaceutical processes is another factor driving market growth. Pharmaceutical companies require precise filtration systems tailored to their specific manufacturing needs, whether it's for sterilizing drugs, concentrating proteins, or separating complex mixtures. The increasing trend toward customization and optimization of membrane systems allows companies to achieve higher efficiency and lower operational costs. Innovations in membrane materials, such as ceramic and polymer membranes, are enabling better customization and driving the growth of this technology in the pharmaceutical market.
Growth Opportunities in the Membrane Technology Market
Several growth opportunities exist for the Membrane Technology in Pharmaceutical Market as we approach 2032. From biopharmaceutical manufacturing to sterile filtration and global expansion, the market is poised for significant advancements.
Expansion of Biopharmaceutical Manufacturing The rapid growth of biopharmaceutical manufacturing, driven by the increasing demand for biologics and biosimilars, presents a substantial opportunity for the membrane technology market. Membrane processes like ultrafiltration and microfiltration are essential for purifying biologics, ensuring product consistency, and meeting regulatory standards. As the biopharmaceutical sector continues to expand, the need for advanced membrane technology solutions will increase, providing companies with lucrative market opportunities.
Sterile Filtration and Vaccine Production Membrane technology plays a crucial role in sterile filtration, which is vital for the production of sterile pharmaceuticals and vaccines. As the global focus on public health and immunization programs intensifies, the production of vaccines has become a priority. The COVID-19 pandemic highlighted the importance of vaccines in combating infectious diseases, and the subsequent demand for sterile filtration technologies is expected to drive growth in the membrane technology market for the pharmaceutical sector.
Emerging Markets and Global Expansion Emerging markets, particularly in regions such as Asia-Pacific and Latin America, present significant opportunities for the membrane technology market in the pharmaceutical industry. As these regions invest in healthcare infrastructure and expand their pharmaceutical manufacturing capabilities, the demand for advanced membrane technologies is expected to grow. Companies that invest in global expansion and tailor their offerings to the specific needs of these markets will be well-positioned for success.
Conclusion
The Membrane Technology in Pharmaceutical Market is set for substantial growth by 2032, driven by the rising demand for biologics, advancements in nanofiltration, and the growing emphasis on sustainability. As pharmaceutical companies seek to optimize production processes, ensure product quality, and meet environmental goals, membrane technology will play a pivotal role in shaping the future of the industry. With expanding applications in biopharmaceuticals, vaccine production, and emerging markets, the membrane technology market is poised for continued innovation and growth in the coming decade.
0 notes
Text
Polymer-Based Prefilled Syringe Market: Current Analysis and Forecast (2024-2032)
According to the UnivDatos Market Insights analysis, the increasing incidence of chronic conditions such as diabetes and rheumatoid arthritis, patient preference for self-administration, and reduced risk of contamination & needlestick injuries compared to traditional syringes. will drive the global scenario of the Polymer-Based Prefilled Syringe market. As per their “Polymer-Based Prefilled Syringe Market” report, the global market was valued at USD 2.87 Billion in 2023, growing at a CAGR of about 4.9% during the forecast period from 2024 – 2032.
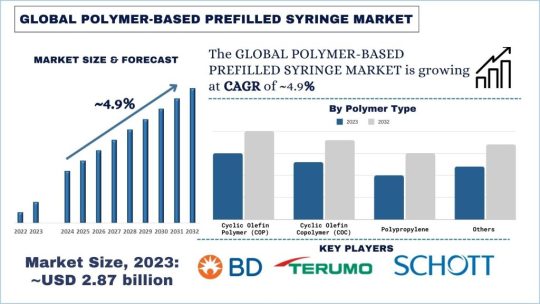
Over the years, there has been a shift of focus in the healthcare sector to develop new and advanced drug delivery systems, and prefilled syringes made of polymers have gained a lot of popularity among pharmaceutical organizations and healthcare centers. Such syringes produced from the highest quality polymers have several advantages over the traditional glass ones: safety, convenience, and a comparatively low price.
Demand
Several factors have led to the high demand for polymer-based prefilled syringes as explained below. Moreover, a trend toward the use of long-lasting drugs, which in turn requires frequent, accurate dosing due to such diseases as diabetes, rheumatoid arthritis, or cancer. Prefilled syringes provide a comfortable and safe way to administer injections to patients who may require multiple injections, hence enhancing the patient’s compliance with treatment and overall positive health impacts.
Applications
Polymer-based prefilled syringes are widely employed across different diseases such as immunology, oncology, and neurology among others. When integrated with the right technologies, they are ideal for dispensing biologic drugs, vaccines, and biosimilar medications, which require accurate dosing and maintaining drug integrity. These syringes are also being employed for administration and this helps the patients to administer their medication at home comfortably.
Access sample report (including graphs, charts, and figures): https://univdatos.com/get-a-free-sample-form-php/?product_id=61521
Factors Influencing the Cost of Syringe
The steps followed in making prefilled syringes from polymer materials include the selection of polymers, injection moulding, and final assembling. Polymeric materials, like cyclic olefin copolymer (COC), cyclic olefin polymer (COP), or their co-polymers, are preferable due to their high chemical and physical stability, and biocompatibility with most drugs. The syringes are then appropriately filled with the right medication and closed in a way that makes them ready for use; they are then sterilized using processes that have been through validation to ensure the product is safe and effective.
Manufacturing
Polymer selection, injection moulding, and subsequent assembly are typical phases of manufacturing pre-filled syringes from polymers. The materials are chosen for their good resistance to chemicals as well as compatibility with most of the drugs; COC or COP are preferred. The syringes are then prepped to contain the right dosage of medicine, closed, and autoclaved in a way that has been certified to be safe for patient use.
Conclusion
Consequently, the prefilled polymer syringes are a breakthrough in the polymer technology of drug delivery systems having the following advantages over glass syringes. Their safety, convenience, and relatively cheaper price make the device an attractive tool for pharma and healthcare providers as they seek better ways of attending to patients and cutting costs. With the increasing trend in technology later in the future, the features of polymer-based prefilled syringes can be developed to enhance the growth and use in the healthcare system.
Contact Us:
UnivDatos Market Insights
Email - [email protected]
Contact Number - +1 9782263411
Website -www.univdatos.com
0 notes
Text
Biological Safety Testing Products and Services Market 2024 | Upcoming Trend in Biological Safety Testing Products and Services Industry by an Expert
The global biological safety testing products and services market is on a robust growth trajectory, valued at $4.42 billion in 2023 and projected to reach $10.51 billion by 2032. This remarkable growth reflects a compound annual growth rate (CAGR) of 10.10% over the forecast period from 2024 to 2032, driven by the increasing demand for safety and efficacy testing across the biopharmaceutical and healthcare sectors.
Biological safety testing is essential for ensuring that medical products, including pharmaceuticals, vaccines, and medical devices, meet stringent safety and efficacy standards before reaching the market. The market encompasses a wide range of testing services, including sterility testing, endotoxin testing, and biocompatibility assessments, all critical for regulatory compliance.
Key Market Drivers
Increasing Biopharmaceutical R&D Activities: The rising investment in biopharmaceutical research and development is a significant factor propelling the market. As the industry focuses on innovative therapies, including monoclonal antibodies, gene therapies, and cell therapies, the need for rigorous biological safety testing becomes paramount. These testing services help ensure that new products are safe for human use, fostering trust in the healthcare system.
Regulatory Compliance and Safety Standards: Stringent regulatory requirements from agencies such as the U.S. Food and Drug Administration (FDA), European Medicines Agency (EMA), and other global health authorities are driving the adoption of biological safety testing services. Compliance with these regulations is critical for companies looking to launch new medical products, creating a steady demand for testing services that ensure product safety and efficacy.
Growing Concerns About Contamination and Quality Assurance: The increasing prevalence of product recalls due to contamination and safety issues has heightened awareness about the importance of biological safety testing. Companies are now more vigilant in their quality assurance processes, recognizing that thorough testing is essential to maintain product integrity and safeguard public health.
Expansion of the Healthcare Sector: The ongoing expansion of the healthcare sector, particularly in emerging markets, is creating new opportunities for biological safety testing services. With the growth of healthcare facilities and the increasing production of biologics and biosimilars, the demand for reliable testing solutions is expected to rise significantly.
Access Free Sample Report: https://www.snsinsider.com/sample-request/4483
Challenges and Opportunities
While the market shows strong potential, it faces challenges such as the high cost of testing services and the need for specialized expertise. However, advancements in technology, including automation and digitalization, are likely to streamline testing processes, reduce costs, and enhance the accuracy of results.
Moreover, the increasing adoption of 3D cell culture systems and organ-on-a-chip technologies offers opportunities for innovative testing solutions. These advancements are expected to improve the efficiency and effectiveness of biological safety testing, providing companies with the tools they need to ensure product safety.
Regional Insights
North America holds the largest share of the biological safety testing market, driven by the presence of leading biopharmaceutical companies, advanced research facilities, and stringent regulatory standards. Europe follows closely, with significant investments in healthcare and biotechnology sectors. The Asia-Pacific region is expected to experience the highest growth rate during the forecast period, supported by the expansion of healthcare infrastructure and increasing research activities in countries such as China, India, and Japan.
Future Outlook
As the demand for innovative biopharmaceutical products continues to rise, the biological safety testing products and services market is set for significant growth. With a projected CAGR of 10.10% from 2024 to 2032, the market is poised to see substantial advancements in testing technologies, helping to meet the increasing demand for safety and efficacy in healthcare products.
In conclusion, the biological safety testing products and services market is entering a dynamic phase of growth, with a valuation expected to rise from $4.42 billion in 2023 to $10.51 billion by 2032. This growth is driven by regulatory compliance, the expansion of biopharmaceutical R&D, and the need for rigorous quality assurance in an increasingly complex healthcare landscape
Other Trending Reports
Dental Suction Systems Market Size
Cosmeceuticals Market Size
Cell Therapy Market Size
Growth Hormone Deficiency Market Size
0 notes
Text
Biosimilars Market: Driving Affordable Healthcare Solutions
The Biosimilars market has become a significant player in modern healthcare, offering cost-effective alternatives to biologics. As the demand for affordable therapeutic options rises, the market for biosimilars is gaining substantial momentum. This article delves into the market trends, segmentation, growth drivers, and leading companies in the biosimilars industry, providing crucial insights for decision-makers.
Market Overview
According to SkyQuest's Biosimilars Market report, the global market is currently valued at USD 27.30 billion in 2023, with a projected CAGR of 16.4% over the forecast period. The market's expansion is fueled by patent expirations of key biologics, increasing prevalence of chronic diseases, and a growing emphasis on cost-efficient healthcare solutions.
Request Your Free Sample: - https://www.skyquestt.com/sample-request/biosimilars-market
Market Segmentation
By Product Type:
Recombinant Non-Glycosylated Proteins: Used in the treatment of diseases such as diabetes and cancer.
Recombinant Glycosylated Proteins: Common in oncology and autoimmune disease therapies.
Peptides: An emerging class used for metabolic diseases and cancer treatment.
By Application:
Oncology: High demand for biosimilars due to the rising incidence of cancers and the need for affordable treatments.
Chronic Diseases: Biosimilars for diabetes, arthritis, and cardiovascular diseases are critical for cost-effective long-term care.
Autoimmune Diseases: Increasing prevalence of conditions like rheumatoid arthritis and multiple sclerosis is driving the demand for biosimilars.
Infectious Diseases: Expansion of biosimilars in this sector due to the global burden of infections.
By Manufacturing Type:
In-House Manufacturing: Pharmaceutical companies developing their own biosimilars to maintain control over production.
Contract Manufacturing: Outsourcing production to specialized third-party manufacturers for efficiency and scalability.
By End-User:
Hospitals and Clinics: Major centers for biosimilars administration.
Pharmaceutical Companies: Driving the production and distribution of biosimilars.
Research Institutes: Key players in the innovation and clinical trials of biosimilars.
Key Growth Drivers
Patent Expirations of Biologics: As patents for major biologic drugs expire, the market opens up for biosimilar development.
Rising Healthcare Costs: Increasing demand for cost-effective alternatives to expensive biologic therapies.
Chronic Disease Prevalence: Growing rates of cancer, diabetes, and autoimmune diseases are spurring demand for biosimilars.
Government Initiatives: Regulatory frameworks supporting the approval and adoption of biosimilars.
Read More at: - https://www.skyquestt.com/report/biosimilars-market
Leading Companies in the Market
SkyQuest’s Biosimilars Market report highlights key players driving innovation and expansion, including:
Pfizer Inc.
Novartis AG (Sandoz)
Amgen Inc.
Samsung Bioepis
Biocon
Celltrion Healthcare
Teva Pharmaceuticals
Mylan N.V.
Fresenius Kabi
Hoffmann-La Roche Ltd
Challenges and Opportunities
The biosimilars market faces hurdles such as complex manufacturing processes and regulatory challenges. However, the growing acceptance of biosimilars by healthcare providers, coupled with increasing investments in biosimilar R&D, presents significant opportunities for market players to innovate and expand.
Take Action Now: Secure Your Report Today - https://www.skyquestt.com/buy-now/biosimilars-market
Future Outlook
As healthcare systems worldwide strive to reduce costs without compromising care quality, the biosimilars market is poised for strong growth. Companies that prioritize technological advancements, navigate regulatory frameworks efficiently, and focus on patient access will thrive in this evolving market.
The biosimilars market is shaping the future of affordable healthcare. With increasing demand for cost-effective treatments, decision-makers must keep pace with the trends and opportunities in this dynamic sector. For more comprehensive insights and strategic recommendations, consult SkyQuest’s in-depth Biosimilars Market report.
0 notes
Text
The global oncology biosimilars market has grown to $4.7 Billion in 2023 and is set to reach $30.3 Billion by 2032, with a robust CAGR of 22.3%. This marks a major advancement in affordable cancer treatments.
0 notes
Text
Tumor Necrosis Factor Inhibitor Drugs Market — Forecast(2024–2030)
Tumor Necrosis Factor (TNF) Inhibitor Drugs Market size was valued at USD 248.8 Billion in 2023 and is projected to reach USD 13019.65 Billion by 2030, growing at a CAGR of 0.64% during the forecast period 2024–2031.

Market Drivers
Rising Prevalence of Autoimmune Diseases: The demand for TNF inhibitor drugs is growing due to the increasing incidence of autoimmune conditions such as psoriasis, Crohn’s disease, ankylosing spondylitis, and rheumatoid arthritis. These drugs help manage symptoms, reduce inflammation, and slow disease progression in affected individuals.
Request Sample
Aging Population: The global aging population is more susceptible to autoimmune and chronic inflammatory diseases, boosting the need for TNF inhibitor therapies. As the population continues to age, the prevalence of these conditions is expected to rise, driving greater demand for TNF inhibitors.
Broadened Indications and Treatment Options: TNF inhibitors are being prescribed for an expanding range of inflammatory and immune-mediated disorders beyond rheumatoid arthritis and inflammatory bowel disease. This includes conditions like ulcerative colitis, juvenile idiopathic arthritis, and psoriatic arthritis, which contributes to increased adoption and market growth.
Effective Disease Management: TNF inhibitors have proven effective in alleviating symptoms, improving quality of life, and preventing joint damage in autoimmune disease patients. Their ability to manage chronic conditions and reduce inflammation addresses significant unmet medical needs.
Inquiry Before Buying
Advancements in Biotechnology and Drug Development: Innovations in immunology, molecular biology, and biotechnology are leading to the development of new TNF inhibitors with better tolerance, safety, and efficacy profiles. Additionally, the introduction of biosimilar TNF inhibitors provides more affordable treatment options, enhancing patient access.
Patient Preference for Biologic Therapies: Many patients favor biologic therapies, including TNF inhibitors, over traditional disease-modifying antirheumatic drugs (DMARDs) due to their targeted action, longer dosing intervals, and potential for improved outcomes. This preference fuels the market demand.
Increased Healthcare Expenditure: Rising healthcare spending, particularly in developed countries, facilitates access to TNF inhibitor medications. Government healthcare programs, private insurance, and reimbursement policies help make these treatments more affordable and accessible, supporting market growth.
Support from Clinical Guidelines and Evidence: Strong clinical data and treatment guidelines reinforce the use of TNF inhibitors, boosting physician confidence and patient acceptance. Evidence-based recommendations guide the adoption and prescribing practices of these therapies.
Advances in Personalized Medicine: Developments in pharmacogenomics and personalized medicine allow for customized treatment plans based on individual patient needs, condition severity, and therapy response. This personalization enhances treatment efficacy and patient satisfaction, driving market demand.
Market Restraints
High Treatment Costs: TNF inhibitors are often expensive, which can be a barrier to access for patients, healthcare systems, and payers, especially in regions with limited healthcare resources or inadequate insurance coverage. The high cost may lead to underutilization of these therapies.
Buy Now
Safety Concerns and Side Effects: Despite their effectiveness, TNF inhibitors can be associated with risks such as infections, cancer, infusion reactions, and other side effects. Safety concerns may deter both patients and healthcare providers from initiating or continuing treatment, particularly in high-risk groups.
Immunogenicity and Loss of Response: Over time, patients may develop antibodies against TNF inhibitors, leading to treatment failure and reduced efficacy. This immunogenicity can necessitate dose adjustments, alternative therapies, or combination treatments to maintain disease control.
Limited Efficacy in Certain Populations: TNF inhibitors may be less effective in patients with severe or treatment-resistant diseases, those with concurrent health conditions, or individuals with specific genetic profiles. Limited response in these populations can impact the perceived value of these therapies.
Availability of Alternative Therapies: The presence of alternative treatments, including non-pharmacological options, tailored synthetic DMARDs, and other biologic drugs, can pose competition to TNF inhibitors. This competition may affect their market share and acceptance.
Regulatory and Reimbursement Challenges: Various regulatory hurdles, market access barriers, and reimbursement constraints can restrict patient access to TNF inhibitors. Delays in payer policies, formulary restrictions, and regulatory approvals may hinder market penetration.
Impact of Patent Expirations and Biosimilar Competition: As patents for branded TNF inhibitors expire, biosimilars enter the market, increasing competition and driving down prices. This competition can affect the pricing, profitability, and market share of original TNF inhibitor products.
Chronic Nature of Autoimmune Disorders: Autoimmune disorders treated with TNF inhibitors require long-term management, which can be challenging for patients. Continuous treatment adherence, regular monitoring, and follow-up are necessary, which can impact patient compliance and healthcare resource utilization.
Challenges with Drug Administration: TNF inhibitors are often administered intravenously or via subcutaneous injection, which can be uncomfortable and logistically challenging for patients. Issues like injection site reactions, complex dosing schedules, and the need for medical resources can affect treatment adherence and patient satisfaction.
Market Segmentation
The Global TNF Inhibitor Drugs Market is segmented based on:
Drug Type: Includes monoclonal antibodies, recombinant proteins, and biosimilars.
Application: Encompasses rheumatoid arthritis, psoriasis, inflammatory bowel disease, ankylosing spondylitis, juvenile idiopathic arthritis, and other inflammatory and immune-mediated conditions.
Distribution Channel: Comprises hospital pharmacies, retail pharmacies, online pharmacies, and specialty pharmacies.
Geography: Covers regions such as North America, Europe, Asia-Pacific, Latin America, and the Middle East and Africa.
#reseach marketing#marketing#digital marketing#Tumor#brain tumor#three tumors#health & fitness#healthcare#healthylifestyle
0 notes
Text
Exploring the Recombinant Chemicals Market: Innovations Shaping the Future
The Recombinant Chemicals Market represents a significant subset of the broader biotechnology and chemical sectors. Recombinant chemicals are produced through genetic engineering techniques, which allow for the precise manipulation of microbial, plant, or animal cells to produce desired chemical compounds. These chemicals have wide applications in pharmaceuticals, agriculture, industrial processes, and research, providing solutions that are more efficient, cost-effective, and sustainable compared to traditional chemical production methods.
The global recombinant chemicals market, valued at US$ 2.9 billion in 2023, is projected to grow at a CAGR of 7.8% from 2024 to 2034. By the end of 2034, the market is expected to reach US$ 6.7 billion, driven by advancements in biotechnology and increasing demand across various industries.
This growth is fueled by the rising need for biopharmaceuticals, green chemicals, and bio-based industrial products. The pharmaceutical sector remains the largest end-user of recombinant chemicals, especially in the production of proteins, enzymes, and other bioactive compounds. Additionally, the agricultural sector is witnessing increased adoption of recombinant chemicals in the development of bio fertilizers and pesticides.
For More Details, Request for a Sample of this Research Report: https://www.transparencymarketresearch.com/recombinant-chemicals-market.html
Market Segmentation
The recombinant chemicals market can be segmented based on various factors:
By Service Type:
Contract Research Services
Manufacturing Services
Custom Synthesis Services
By Sourcing Type:
In-house Production
Outsourced Production
By Application:
Pharmaceuticals
Agriculture
Industrial Enzymes
Cosmetics
Food and Beverages
By Industry Vertical:
Healthcare
Agriculture
Chemical
Biotechnology
Food Processing
By Region:
North America
Europe
Asia-Pacific
Latin America
Middle East & Africa
Regional Analysis
North America: Leading the market due to well-established biotechnology and pharmaceutical industries, as well as heavy investment in R&D for recombinant chemical production.
Europe: The second-largest market, driven by increasing demand for sustainable and bio-based chemicals, as well as stringent regulations on chemical production.
Asia-Pacific: Expected to witness the highest growth during the forecast period due to expanding pharmaceutical and agricultural industries in countries like China and India, along with supportive government initiatives.
Latin America and the Middle East & Africa: These regions are also gaining traction as key markets for recombinant chemicals due to the growing demand for agricultural chemicals and biopharmaceuticals.
Market Drivers and Challenges
Drivers:
Technological Advancements: Continuous innovation in genetic engineering and fermentation technologies is expanding the capabilities of recombinant chemical production.
Sustainability: Recombinant chemicals offer a more environmentally friendly alternative to traditional petrochemical-based products, driving demand in industries focused on sustainability.
Rising Demand for Biopharmaceuticals: The growing need for advanced drugs, including biosimilars, monoclonal antibodies, and vaccines, is fueling demand for recombinant chemicals.
Challenges:
High Production Costs: The initial investment required for setting up recombinant chemical production facilities is high, which may limit market penetration, especially in developing regions.
Regulatory Hurdles: Complex regulatory frameworks, particularly in the pharmaceutical and agricultural sectors, can slow down the commercialization of recombinant chemicals.
Technical Limitations: The scalability of production processes and achieving consistency in yield and purity remain key challenges.
Market Trends
Shift Towards Green Chemicals: With increasing environmental regulations, there is a clear shift toward the development and adoption of bio-based recombinant chemicals.
Expanding Applications: Recombinant chemicals are finding new applications in sectors like food processing, cosmetics, and industrial enzymes, driven by their versatility and efficiency.
Collaborations and Partnerships: Companies are increasingly forming partnerships with research institutions and contract manufacturing organizations (CMOs) to enhance production capabilities and streamline R&D processes.
Future Outlook
The future of the recombinant chemicals market looks promising, with expected breakthroughs in gene editing technologies like CRISPR and the expanding use of synthetic biology. The ongoing shift toward personalized medicine and bio-based solutions in various industries will further drive demand for recombinant chemicals. The market is poised to witness significant investments in R&D and infrastructure development, particularly in the Asia-Pacific and European regions.
Buy this Premium Research Report: https://www.transparencymarketresearch.com/checkout.php?rep_id=86368<ype=S
Key Market Study Points
Analysis of recombinant chemical applications across diverse industries.
Regional market dynamics and their impact on growth projections.
Competitive landscape analysis, focusing on key players and their strategies.
Technological advancements and their role in shaping the market’s future.
Evaluation of the sustainability of recombinant chemical production methods.
Competitive Landscape
The recombinant chemicals market is highly competitive, with key players focusing on innovation, product differentiation, and strategic collaborations. Major players include:
BASF SE
Merck KGaA
Lonza Group
Evonik Industries AG
Genentech, Inc.
These companies are investing in advanced biotechnologies and expanding their recombinant chemical portfolios through mergers, acquisitions, and partnerships with smaller firms or research institutions.
Recent Developments
Mergers and Acquisitions: Several key players have recently acquired smaller biotech firms to strengthen their recombinant chemical capabilities.
Technological Advancements: New methods of gene editing and fermentation are being developed, allowing for more efficient and cost-effective production of recombinant chemicals.
Regulatory Approvals: Recent regulatory approvals for new recombinant-based drugs and chemicals have opened new avenues for market growth, particularly in pharmaceuticals and agriculture.
About Transparency Market Research
Transparency Market Research, a global market research company registered at Wilmington, Delaware, United States, provides custom research and consulting services. Our exclusive blend of quantitative forecasting and trends analysis provides forward-looking insights for thousands of decision makers. Our experienced team of Analysts, Researchers, and Consultants use proprietary data sources and various tools & techniques to gather and analyses information.
Our data repository is continuously updated and revised by a team of research experts, so that it always reflects the latest trends and information. With a broad research and analysis capability, Transparency Market Research employs rigorous primary and secondary research techniques in developing distinctive data sets and research material for business reports.
Contact:
Transparency Market Research Inc.
CORPORATE HEADQUARTER DOWNTOWN,
1000 N. West Street,
Suite 1200, Wilmington, Delaware 19801 USA
Tel: +1-518-618-1030
USA – Canada Toll Free: 866-552-3453
Website: https://www.transparencymarketresearch.com
0 notes
Text
The Erythropoietin (EPO) drug Market is poised for substantial growth, with its market size projected to expand from USD 11,728.5 million in 2024 to USD 24,205.87 million by 2032, reflecting a compound annual growth rate (CAGR) of 9.48%.The global erythropoietin (EPO) drugs market has experienced significant growth in recent years, driven by an increasing prevalence of anemia, particularly among patients with chronic kidney disease (CKD), cancer, and HIV. Erythropoietin, a glycoprotein hormone produced by the kidneys, plays a crucial role in the production of red blood cells (erythropoiesis). The synthetic forms of erythropoietin, known as erythropoiesis-stimulating agents (ESAs), are commonly used to treat anemia by stimulating the bone marrow to produce more red blood cells.
Browse the full report at https://www.credenceresearch.com/report/erythropoietin-drugs-market
Market Dynamics
The erythropoietin drugs market is primarily driven by the rising incidence of chronic diseases such as CKD and cancer. Anemia is a common complication in these diseases, leading to a growing demand for EPO drugs. According to the World Health Organization (WHO), anemia affects approximately 1.62 billion people globally, with iron deficiency anemia being the most prevalent type. This high prevalence, coupled with the increasing number of patients undergoing dialysis, chemotherapy, and antiretroviral therapy, is fueling the demand for EPO drugs.
The market is further bolstered by the growing geriatric population, which is more susceptible to chronic diseases and anemia. Additionally, advancements in biotechnology have led to the development of newer, more effective EPO formulations, enhancing treatment outcomes and expanding the market.
Regional Analysis
The erythropoietin drugs market is geographically segmented into North America, Europe, Asia-Pacific, Latin America, and the Middle East & Africa.
- North America: This region dominates the global market due to the high prevalence of CKD, well-established healthcare infrastructure, and the presence of major pharmaceutical companies. The U.S. is the largest market within this region, driven by a high rate of dialysis procedures and an aging population.
- Europe: The market in Europe is also significant, with countries like Germany, the UK, and France leading due to their advanced healthcare systems and the widespread adoption of biosimilars.
- Asia-Pacific: This region is expected to witness the highest growth rate during the forecast period, attributed to a large patient population, increasing healthcare spending, and growing awareness about anemia management.
- Latin America and the Middle East & Africa: These regions are gradually emerging as potential markets due to improving healthcare infrastructure and increasing access to medical treatments.
Challenges and Opportunities
Despite the positive growth outlook, the erythropoietin drugs market faces several challenges. The high cost of biologics, side effects associated with EPO drugs, and stringent regulatory requirements are some of the key barriers to market growth. Additionally, the emergence of biosimilars poses competition to established biologics, potentially leading to price wars and reduced profit margins for manufacturers.
However, the market also presents significant opportunities. The development of next-generation EPO drugs with improved efficacy and safety profiles, coupled with the expanding applications of these drugs beyond anemia, could drive future growth. Moreover, the increasing focus on personalized medicine and targeted therapies is expected to open new avenues in the erythropoietin drugs market.
Key Player Analysis:
Amgen Inc.
Johnson & Johnson
F. Hoffmann-La Roche Ltd.
Pfizer Inc.
Novartis AG
Biocon Limited
Teva Pharmaceutical Industries Ltd.
Dr. Reddy’s Laboratories Ltd.
LG Life Sciences Ltd.
Wockhardt Ltd.
Segmentation:
by Drug Type
Biologics
Biosimilars
by Product Type
Epoetin-alfa
Epoetin-beta
Darbepoetin-alfa
Others
by Application
Haematology
Kidney Disorder
Cancer
Others
by End User
Hospitals
Homecare
Specialty Clinics
Others
Browse the full report at https://www.credenceresearch.com/report/erythropoietin-drugs-market
About Us:
Credence Research is committed to employee well-being and productivity. Following the COVID-19 pandemic, we have implemented a permanent work-from-home policy for all employees.
Contact:
Credence Research
Please contact us at +91 6232 49 3207
Email: [email protected]
Website: www.credenceresearch.com
0 notes
Text
Top 5 players in US Biosimilar Market
Buy Now
STORY OUTLINE
Pfizer: Excelling in the line of Biosimilar drugs with an experience of more than 10 years with presence in over 180 countries.
Amgen: Making pharmaceutical products with an experience of over 40 years and presence in over 100 countries.
Viartis: Presence in over 165 countries, and making Biosimilar drugs in over 75 markets, this pharmaceutical company is another leading contributor of US Biosimilar market.
Coherus Biosciences: Increasing patient access to cost effective medicines with a Biosimilar drugs experience of 13 years.
Biogen: serving humanity through science with a experiences of more than 40 years in the field of biologics.
According to Ken Research, the US Biosimilar market is anticipated to grow at a CAGR of ~40% in the next five years which currently has a market size of ~USD 9.4 Bn.
The US Biosimilar market is rapidly growing and will be witnessing a significant growth in the next five years.
There are various reasons behind the rapid growth of US Biosimilar market. Some of the major reasons behind the growth of US Biosimilar market include the cost effective nature of Biosimilar drugs, rising geriatric population, rising prevalence of chronic diseases, and growing partnerships between companies to develop Biosimilar drugs.
Various companies and players are contributing to their best efforts in the growth of the US Biosimilar market.
This article aims to put light on the contributions done by the major players towards the growth of the US Biosimilar market.
1.Pfizer
Click to read more about Pfizer
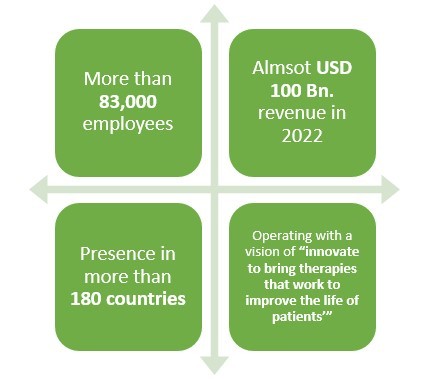
Pfizer is a leading American pharmaceutical company which is operating in the field of generics or original drugs for more than 30 years. But did you know that this pharma not only manufactures biologics but also biosimilar drugs?
Pfizer has been in the business of biosimilar drugs for more than 10 years and have been quite successful as well. With more than 83,000 employees and presence in over 180 countries, this leading pharmaceutical company made almost USD 2 Bn. revenue only from its Biosimilar drugs sale in 2021.
Recently, this pharmaceutical company also collaborated with Samsung in two deals to produce various biosimilar drugs in South Korea. The deal size between these two companies happens to be approximately USD 900 Bn.
The major Biosimilar drugs of this pharmaceutical giant are primarily
ZIRABEV (a Biosimilar of Avastin)
TRAZIMERA (a Biosimilar of Herceptin)
RUXIENCE (a Biosimilar of Rituxan)
RITACRIT (a Biosimilar of Epogen)
NVYEPRIA (a Biosimilar of Neulasta)
NIVESTYM (a Biosimilar of Neupogen)
FILGRASTIM (a Biosimilar of Neupogen).
2.Amgen
Click here to Download a Sample Report
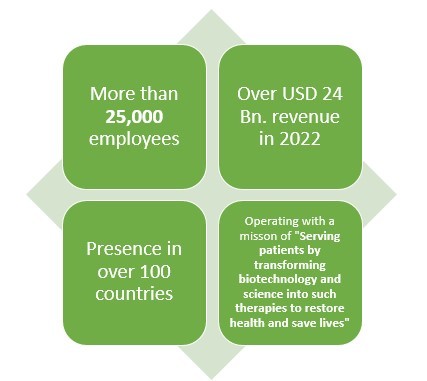
Amgen is another leading American pharmaceutical company which not only makes Biologics or generic drugs but also Biosimilar drugs. This pharmaceutical company has more than 40 years of experience when it comes to pharmaceutical line.
With over 25000 employees and presence in over 100 countries, this pharmaceutical company earned about USD 2 Bn. from their three biosimilar drugs which are reportedly MVASI, KANJITNTI, and AMJEVITA.
This pharma giant has also invested about USD 2 Bn. in the development of Biosimilar drugs.
This pharmaceutical company has made Biosimilar drugs primarily in 4 fields which are General Medicine, Oncology, and Hematology along with, Inflammation.
EPOTEIN ALFA
AMJEVITA
AVSOLA
KANJINTI
MVASI
RIABNI
are the various Biosimilar drugs of Amgen. And, STELARA, EYLEA, SOLIRIS are in their pipeline.
Recently Amgen revealed their Biosimilar report’s 8 version. It revealed a major information which said that the pharmaceutical company saved about USD 10 Bn. through their Biosimilar drugs in the past five years.
3.Viartis

Headquartered in Canonsburg, Pennsylvania, this American pharmaceutical company was founded only in 2020 yet they have achieved massive success in the pharmaceutical products with their revenue being USD 16 ~Bn. in 2022.
With presence in 165 countries and with over 45,000 employees worldwide, this pharmaceutical company makes pharmaceutical products in 10 areas which primarily are Cardiovascular, Dermatology, ophthalmology, Oncology, Gastroenterology, Women’s health, Infectious diseases, Diabetes & Metabolism, Immunology, CNS & Anesthesiology, Respiratory diseases and allergy.
Speaking of their first Biosimilar products, their first ever Biosimilar drug was launched in 2014. They have a variety of Biosimilar drugs which are primarily
TRASTUZUMAB
INSULIN ASPART
PEGFILGRASTIM
INSULIN GLARGINE-YFGN
ADALIMUMAB
BEVACIZUMAB
Their Biosimilar drug Insulin Glargine which is known as SEMGLEE was the first ever interchangeable Biosimilar drug in the United States which was FDA approved.
Their PEGFILGRASTIM also was the first ever FDA approved drug in the United States. They have launched their Biosimilar drugs in over 75 markets worldwide.
4.Coherus Biosciences:
Click here to Ask for a Custom Report
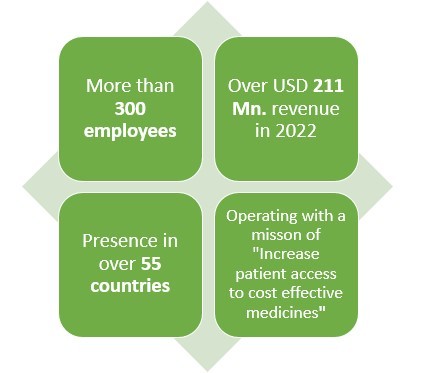
Headquartered in Redwood city, California this American pharmaceutical company earned a revenue of almost USD 211 Mn. In 2022.
With presence in over 55 countries and 300+ employees worldwide, this pharmaceutical company makes products in various areas such as solid tumors, non-small lung cancers, nasopharyngeal carcinoma, small cell lung cancer and hepatocellular carcinoma.
Speaking of their Biosimilar drugs, this pharma has been in the field of creating Biosimilar drugs since 2010 which has given them almost 13 years of experience.
This pharmaceutical company also disclosed that it plans to spend at least USD 1 Tn. on medicines worldwide, out of which at least 40% will be spent on Biosimilar drugs.
Their three major Biosimilar drugs which are also FDA approved include UDENCYA, YUSIMRY, and CIMERLI.
Udencya is a Biosimilar drug of Pegfilgrastim, Yusimry is a Biosimilar drug of Ranibizumab, and Cimerli is a Biosimilar drug of Adalimumab.
5.Biogen
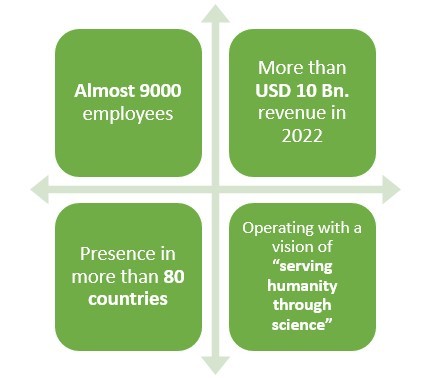
Headquartered in Cambridge, Massachusetts, this American pharmaceutical company earned a revenue of around USD 10 Bn. in 2022.
This company happens to have an experience of more than 40 years when it comes to making pharmaceutical products.
With presence in over 80 countries and more than 9000 employees worldwide, this pharmaceutical company primarily deals in Neurology, Specialized Immunology, Neuropsychiatry, Ophthalmology, and Rare Diseases.
ADUCANUMAB
LECANEMAB
TOFERSEN
ZURANOLONE
LITIFILIMAB
BENAPALI
FLIXABI
IMRALDI
are some of their Biosimilar drugs.
With their Biosimilar drugs, more than 250,000 people have gone on Anti-Tumor Necrosis Factor therapy.
Recently, this pharmaceutical company also made an agreement with Bio-Thera solutions to develop a Biosimilar drug for the treatment of Rheumatoid Arthritis.
#US Biosimilar Sector#United States Biosimilar Market#US Biosimilar Market forecast#US Biosimilar Market analysis#US Biosimilar Market trends#US Biosimilar Market share#US Biosimilar Market key players#US Biosimilar Market revenue#US Biosimilar Market growth#Monoclonal Antibodies in biosimilar market US#Recombinant Hormones in biosimilar industry US#Oncology in bio similar market US#Blood disorders in biosimilar market US#Research institutes in Biosimilar market US#US similar biotherapeutics products market#Hospitals in Biosimilar market US#Investors in Biosimilar market US#US comparable biologics products industry#US recombinant biosimilars industry#US replicate biologics sector#US analog biologics market
0 notes
Text
Prefilled Syringes: A Game-Changer for Drug Delivery

Introduction
The Prefilled Syringes Market is experiencing rapid growth as healthcare providers and patients increasingly prefer prefilled syringes over traditional vial-and-syringe methods. Prefilled syringes offer convenience, accuracy, and safety, reducing the risk of dosing errors and contamination. They are used for a variety of therapeutic applications, including vaccines, biologics, and anticoagulants. The market is expanding globally, driven by increasing chronic disease prevalence, technological advancements in injectable drugs, and the growing demand for self-administration.
Market Dynamics
Drivers
Growing Prevalence of Chronic Diseases: The rise in conditions such as diabetes, cardiovascular diseases, and autoimmune disorders has increased the demand for injectable medications, fueling the growth of the prefilled syringes market.
Patient Convenience and Safety: Prefilled syringes offer a more convenient and safer alternative to traditional methods, especially for patients requiring frequent injections.
Challenges
High Production Costs: The manufacturing of prefilled syringes requires stringent quality control and high precision, leading to increased production costs.
Regulatory Compliance: Prefilled syringes must meet rigorous regulatory standards, particularly for biological drugs, creating challenges for manufacturers.
Opportunities
Biologics and Biosimilars: The growing market for biologic drugs presents significant opportunities for the prefilled syringes market, as these drugs often require injectable delivery.
Homecare and Self-Administration: With the increasing demand for home healthcare solutions, prefilled syringes are becoming more popular for self-administration of medications, offering a major growth avenue.
Regional Analysis
North America: The largest market for prefilled syringes, driven by the high prevalence of chronic diseases and advanced healthcare infrastructure.
Europe: Europe is a major market, with countries like Germany and the U.K. leading in the adoption of biologics and injectable therapies.
Asia-Pacific: The region is witnessing rapid growth due to rising healthcare expenditure, increasing chronic disease burden, and expanding access to healthcare in countries like China and India.
Sample pages of Report: https://www.infiniumglobalresearch.com/form/193?name=Sample
Market Segmentation
By Material:
Glass Syringes: Traditionally dominant, but gradually being replaced by plastic due to concerns about breakage and contamination.
Plastic Syringes: Gaining popularity due to their lightweight and durable nature.
By Application:
Diabetes: Prefilled syringes are widely used for insulin administration.
Vaccines: Increasingly used for vaccine delivery due to their convenience and precision.
Competitive Landscape
How much share do large players hold? Companies like BD (Becton, Dickinson and Company), Gerresheimer, and SCHOTT dominate the market, holding a significant share due to their established presence and advanced manufacturing capabilities.
Do big players control the price? Yes, large players with sophisticated manufacturing processes and partnerships with pharmaceutical companies have significant control over pricing in the market.
Do small and mid-size companies challenge the large companies domestically? While smaller companies are entering the market with niche products and regional focus, they face challenges in competing with the scale and pricing power of larger players.
Report Overview : https://www.infiniumglobalresearch.com/market-reports/global-prefilled-syringes-market
Future Outlook
Does new product development really help companies? Yes, innovations in materials, such as plastic prefilled syringes, and the development of safety features like needle shields have helped companies gain market share.
Do sustainable products hold strong customers' minds? Sustainability is becoming increasingly important, with healthcare providers and patients showing a preference for eco-friendly packaging and materials in prefilled syringes.
Conclusion
The prefilled syringes market is poised for substantial growth, driven by increasing chronic disease prevalence, the rise of biologics, and the growing demand for patient convenience. While challenges around cost and regulatory compliance remain, innovations in materials and safety features are expected to drive the market forward.
0 notes
Text
The Bioassay Services Market Is Anticipated To Witness High Growth Owing To Increasing R&D Investment In Pharmaceutical And Biotechnology Industries

The Bioassay Services Market facilitates testing of biomolecule interactions and activities through contracted or outsourced research organizations. Bioassays help evaluate the potency and purity of biotherapeutics, biosimilars, and other biological products in a cost-effective manner. They provide data to support drug development, clinical trials, quality control, and regulatory submissions. As bioassay testing requires specialized expertise and infrastructure, many pharmaceutical and biotech companies prefer outsourcing such services to Contract Research Organizations (CROs).
Bioassays play a vital role in the development and manufacture of biologics such as antibodies, proteins, vaccines, and cell and gene therapies. They aid in determining the biological activity, target specificity, and pharmacological effects of these complex molecules. Through cell-based or receptor-binding assays, bioassays quantify the biological potency of biotherapeutics. This helps ensure their efficacy and safety. They also support characterization, comparability assessment, stability monitoring, and release testing of biologics. As more biologics receive regulatory approvals and enter the market, demand for bioassay services is surging. Advancements in cell-based assays further facilitate high-throughput screening of biotherapeutics.
The Bioassay Services Market is estimated to be valued at US$ 340 Mn in 2024 and is expected to exhibit a CAGR of 30.% over the forecast period 2024-2031.
Key Takeaways
Key players operating in the bioassay services are CCRM,Nexelis,Pacific BioLabs,PPD Laboratories,WuXi Advanced Therapies. They provide a range of bioassay testing and support services to pharmaceutical and biotech clients.
Growing demand for biologics and biosimilars is driving the need for extensive bioassay testing during drug development and manufacturing. As biologics development increases in complexity, requirements from regulatory agencies are also becoming stringent necessitating optimized bioassay data packages.
Advancements in cell-based assays and automation have enabled development of more predictive and high-throughput bioassays systems. 3D cell culture-based assays now better mimic human physiology aiding in selection of safer and more efficacious biotherapeutic candidates.
Market Trends
Increased outsourcing of bioassay testing- Pharma companies prefer outsourcing bioassay activities to specialized CROs to leverage their expertise and infrastructure and focus on core drug development functions. This is expected to boost demand for outsourced bioassay services.
Adoption of non-animal testing methods- Restrictions on animal testing and emphasis on alternative methods by regulators will promote use of human cells and tissue-based bioassay platforms over animal models where applicable. This supports the 3Rs (Replacement, Reduction, and Refinement) principle in drug testing.
Market Opportunities
Emerging biotherapeutic modalities - Cell and gene therapies, RNA/DNA based medicines, biologics for chronic diseases offer significant opportunities as they require extensive biocharacterization and testing during development and lot release.
Partnerships for specialized testing- Demand for niche bioassays for toxins, viruses, vaccines etc. presents opportunities for expert CROs to partner with pharma innovators working in such complex domains.
Impact Of COVID-19 On Bioassay Services Market Growth
The ongoing COVID-19 pandemic has significantly impacted the Bioassay Services Market. During the initial outbreak, the restrictions imposed by governments worldwide led to temporary closures of contract research organization facilities and delays in clinical trial activities. This affected the demand for bioassay services from pharmaceutical and biotechnology companies engaged in drug development. However, as the pandemic progressed, bioassay services emerged as an essential part of vaccine and therapeutic development efforts against COVID-19. Many CROs ramped up their bioassay capabilities to support the surge in COVID-19 related research. This provided some buffer to the market from the adverse impact witnessed initially. Going forward, as vaccine development and therapeutic discovery continues at an accelerated pace, the demand for bioassay services is expected to steadily rise over the forecast period.
The Asia Pacific region currently accounts for the largest share of the Bioassay Services Market in terms of value. Several factors have contributed to the concentration of market in Asia Pacific, primarily the presence of a large pool of clinical trial participants, availability of low-cost and high-quality bioassay services, and growing pharmaceutical outsourcing to countries such as China and India. Within the region, China and India are the top locations for outsourcing of drug discovery and clinical research activities, bolstering service provider revenue. Moreover, investments by governments to promote life sciences R&D have spurred market growth. Going forward, emerging Asian countries with improving regulatory compliance may attract higher outsourcing, sustaining APAC's leading position.
At the same time, the North American region has emerged as the fastest growing market for bioassay services globally. Pre-COVID, growth drivers included rising R&D expenditure of biopharma players based in the US and Canada, well-established pharmaceutical infrastructure, and increasing adoption of outsourcing models. However, the pandemic has significantly accelerated regional market expansion. Heightened research focus on COVID-19 therapeutics and vaccines has boosted service demand. Moreover, the region is home to prominent global CROs with expanded pandemic-relevant testing capabilities. Considering these factors, North America is anticipated to continue outpacing other regions in terms of Bioassay Services Market growth over the forecast period.
Get more insights on this topic: https://www.pressreleasebulletin.com/bioassay-services-market-is-estimated-to-witness-high-growth-owing-to-advancements-in-cell-based-assay-techniques/
Author Bio:
Alice Mutum is a seasoned senior content editor at Coherent Market Insights, leveraging extensive expertise gained from her previous role as a content writer. With seven years in content development, Alice masterfully employs SEO best practices and cutting-edge digital marketing strategies to craft high-ranking, impactful content. As an editor, she meticulously ensures flawless grammar and punctuation, precise data accuracy, and perfect alignment with audience needs in every research report. Alice's dedication to excellence and her strategic approach to content make her an invaluable asset in the world of market insights. (LinkedIn: www.linkedin.com/in/alice-mutum-3b247b137 )
What Are The Key Data Covered In This Bioassay Services Market Report?
:- Market CAGR throughout the predicted period
:- Comprehensive information on the aspects that will drive the Bioassay Services Market's growth between 2024 and 2031.
:- Accurate calculation of the size of the Bioassay Services Market and its contribution to the market, with emphasis on the parent market
:- Realistic forecasts of future trends and changes in consumer behaviour
:- Bioassay Services Market Industry Growth in North America, APAC, Europe, South America, the Middle East, and Africa
:- A complete examination of the market's competitive landscape, as well as extensive information on vendors
:- Detailed examination of the factors that will impede the expansion of Bioassay Services Market vendors
FAQ’s
Q.1 What are the main factors influencing the Bioassay Services Market?
Q.2 Which companies are the major sources in this industry?
Q.3 What are the market’s opportunities, risks, and general structure?
Q.4 Which of the top Bioassay Services Market companies compare in terms of sales, revenue, and prices?
Q.5 Which businesses serve as the Bioassay Services Market’s distributors, traders, and dealers?
Q.6 How are market types and applications and deals, revenue, and value explored?
Q.7 What does a business area’s assessment of agreements, income, and value implicate?
*Note:
1. Source: Coherent Market Insights, Public sources, Desk research
2. We have leveraged AI tools to mine information and compile it
#Bioassay Services Market Trend#Bioassay Services Market Size#Bioassay Services Market Information#Bioassay Services Market Analysis#Bioassay Services Market Demand
0 notes
Text
Rheumatology Therapeutics Market 2024 : Size, Growth Rate, Business Module, Product Scope, Regional Analysis And Expansions 2033
The Rheumatology Therapeutics Global Market Report 2024 by The Business Research Company provides market overview across 60+ geographies in the seven regions - Asia-Pacific, Western Europe, Eastern Europe, North America, South America, the Middle East, and Africa, encompassing 27 major global industries. The report presents a comprehensive analysis over a ten-year historic period (2010-2021) and extends its insights into a ten-year forecast period (2023-2033).

Learn More On The Rheumatology Therapeutics Market:
https://www.thebusinessresearchcompany.com/report/rheumatology-therapeutics-global-market-report
According to The Business Research Company’s Rheumatology Therapeutics Global Market Report 2024, The rheumatology therapeutics market size is expected to see steady growth in the next few years. It will grow to $51.42 billion in 2028 at a compound annual growth rate (CAGR) of 4.9%. The growth in the forecast period can be attributed to the shift towards personalized medicine approaches, rising adoption of biosimilars in rheumatology, increasing investment in research and development, integration of digital health technologies, and regulatory approvals for novel therapies. Major trends in the forecast period include growth in telemedicine and remote monitoring solutions, expansion of targeted therapies for specific rheumatic conditions, emphasis on patient-centric care models, expansion of biologic treatments beyond monoclonal antibodies, and adoption of value-based pricing models.
The rising prevalence of autoimmune diseases is expected to propel the growth of the rheumatology therapeutics market going forward. An autoimmune disease is a condition where the immune system mistakenly attacks and damages the body's tissues. The prevalence of autoimmune diseases is growing due to a combination of genetic, environmental, and lifestyle factors, including increased awareness and improved diagnostic capabilities. Rheumatology therapeutics are required for autoimmune diseases to manage inflammation, alleviate symptoms, and prevent joint and tissue damage caused by the immune system attacking the body. For instance, in June 2024, according to the Australian Institute of Health and Welfare, an Australia-based government agency, around 514,000 people in Australia, or 2.0% of the population, were estimated to be living with rheumatoid arthritis in 2022. Rheumatoid arthritis accounted for 2.0% of the total disease burden and 16% of the burden for all musculoskeletal conditions in 2023. Therefore, the rising prevalence of autoimmune diseases is driving the growth of the rheumatology therapeutics market.
Get A Free Sample Of The Report (Includes Graphs And Tables):
https://www.thebusinessresearchcompany.com/sample.aspx?id=17246&type=smp
The rheumatology therapeutics market covered in this report is segmented –
1) By Drug Class: Disease Modifying Anti-Rheumatic Drugs, Nonsteroidal Anti-Inflammatory Drugs, Corticosteroids, Uric Acid Drugs, Other Drugs Classes
2) By Distribution Channel: Hospital Pharmacy, Retail Pharmacy, Online Pharmacy
3) By Disease Indication: Rheumatoid Arthritis, Osteoarthritis, Gout, Psoriatic Arthritis, Ankylosing Spondylitis, Other Disease Indications
Major companies operating in the rheumatology therapeutics market are developing innovative solutions, such as intravenous (IV) formulations, to enhance treatment efficacy and patient convenience. An intravenous (IV) formulation refers to a medication or substance that is administered directly into a vein through a needle or catheter. For instance, in October 2023, Novartis AG, a Switzerland-based pharmaceutical company, announced that the US Food and Drug Administration (FDA) had approved Cosentyx, an intravenous (IV) formulation. This form of Cosentyx is uniquely approved to treat adults with psoriatic arthritis (PsA), ankylosing spondylitis (AS), and non-radiographic axial spondyloarthritis (nr-axSpA). It works by specifically targeting and inhibiting interleukin-17A (IL-17A) and is the only non-tumor necrosis factor alpha (TNF-α) IV treatment available for these conditions.
The rheumatology therapeutics market report table of contents includes:
1. Executive Summary
2. Rheumatology Therapeutics Market Characteristics
3. Rheumatology Therapeutics Market Trends And Strategies
4. Rheumatology Therapeutics Market - Macro Economic Scenario
5. Global Rheumatology Therapeutics Market Size and Growth
...........
32. Global Rheumatology Therapeutics Market Competitive Benchmarking
33. Global Rheumatology Therapeutics Market Competitive Dashboard
34. Key Mergers And Acquisitions In The Rheumatology Therapeutics Market
35. Rheumatology Therapeutics Market Future Outlook and Potential Analysis
36. Appendix
Contact Us:
The Business Research Company
Europe: +44 207 1930 708
Asia: +91 88972 63534
Americas: +1 315 623 0293
Email: [email protected]
Follow Us On:
LinkedIn: https://in.linkedin.com/company/the-business-research-company
Twitter: https://twitter.com/tbrc_info
Facebook: https://www.facebook.com/TheBusinessResearchCompany
YouTube: https://www.youtube.com/channel/UC24_fI0rV8cR5DxlCpgmyFQ
Blog: https://blog.tbrc.info/
Healthcare Blog: https://healthcareresearchreports.com/
Global Market Model: https://www.thebusinessresearchcompany.com/global-market-model
0 notes
Text
The Next Decade of the Pharmaceutical Filtration Market: Trends and Predictions

The global pharmaceutical filtration market is on track for impressive growth, with the market size projected to increase from USD 16.4 billion in 2023 to USD 43.9 billion by 2032. This growth, at a compound annual growth rate (CAGR) of 11.56% during the forecast period from 2024 to 2032, is driven by increasing demand for efficient filtration processes in drug manufacturing and rising investments in pharmaceutical R&D.
Pharmaceutical filtration refers to the process of separating contaminants such as particulate matter, microorganisms, and other impurities from drug products, raw materials, and liquids during pharmaceutical manufacturing. It is a critical step to ensure the purity, quality, and safety of pharmaceutical products, which include vaccines, biologics, and small-molecule drugs.
Key Market Drivers
Surge in Biopharmaceutical Production: The rapid expansion of the biopharmaceutical industry, including the development of monoclonal antibodies, gene therapies, and vaccines, is a key driver for the pharmaceutical filtration market. Filtration processes play a crucial role in purifying biologics, ensuring they meet regulatory standards for sterility and safety. As the demand for biopharmaceuticals grows, so does the need for advanced filtration technologies that can handle complex biological products.
Rising Focus on Drug Quality and Safety: Increasing regulatory scrutiny and stringent guidelines from agencies like the U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA) have intensified the focus on drug quality, safety, and purity. Pharmaceutical filtration is essential in meeting these regulatory requirements, minimizing the risk of contamination, and ensuring compliance with Good Manufacturing Practices (GMP). This is particularly crucial for injectable drugs, where any contamination can have severe consequences.
Growth in Vaccine and Biosimilar Development: The global emphasis on vaccine production, driven by the COVID-19 pandemic and other emerging health threats, has heightened the demand for pharmaceutical filtration. Filtration systems are vital in the manufacturing of vaccines, ensuring the removal of unwanted particles and ensuring sterility. Additionally, the growing biosimilar market, which offers more affordable alternatives to biologics, is further boosting the demand for filtration technologies as these products require stringent purification processes.
Technological Advancements in Filtration Systems: Innovations in filtration technologies, such as membrane filtration, depth filtration, and microfiltration, have significantly improved the efficiency and precision of the filtration process. These advanced systems offer higher filtration capacities, faster processing times, and better scalability, making them indispensable in modern pharmaceutical manufacturing. As technology continues to evolve, it will drive the adoption of more efficient and cost-effective filtration solutions across the industry.
Get Free Sample PDF: https://www.snsinsider.com/sample-request/4514
Challenges and Opportunities
While the pharmaceutical filtration market presents significant growth opportunities, it is not without challenges. The high cost of advanced filtration equipment and consumables can be a barrier for smaller pharmaceutical companies, particularly in developing regions. Additionally, the complex nature of biologics requires specialized filtration systems, which can increase production costs.
However, ongoing investments in research and development are expected to address these challenges. Continuous improvements in filtration technology, such as single-use systems and automated filtration processes, offer promising solutions to reduce costs and improve operational efficiency. Moreover, partnerships between pharmaceutical companies and filtration technology providers are likely to drive further innovation in the market.
Regional Insights
North America currently holds the largest share of the pharmaceutical filtration market, driven by a robust pharmaceutical and biopharmaceutical industry, advanced healthcare infrastructure, and strong regulatory frameworks. Europe follows closely, with significant investments in research and stringent regulations driving the need for high-quality filtration solutions.
The Asia-Pacific region is expected to witness the highest growth during the forecast period. The expansion of pharmaceutical manufacturing capabilities in countries such as China, India, and South Korea, combined with rising investments in R&D and a growing focus on healthcare, is propelling the demand for pharmaceutical filtration systems. Additionally, increasing awareness of drug safety and quality standards is boosting the adoption of advanced filtration technologies in these regions.
Future Outlook
As the pharmaceutical industry continues to innovate and expand, the demand for efficient filtration technologies is set to rise. The pharmaceutical filtration market is poised to benefit from the increasing complexity of drug products, particularly biologics and biosimilars, which require highly specialized purification processes. Furthermore, the ongoing advancements in filtration technologies will continue to drive growth, offering new opportunities for market expansion.
In conclusion, the pharmaceutical filtration market is expected to experience robust growth over the next decade, with a projected market value of USD 43.9 billion by 2032. As regulatory requirements become more stringent and the demand for high-quality drug products increases, pharmaceutical filtration technologies will remain a critical component of the drug manufacturing process, ensuring safety, purity, and compliance.
Other Trending Reports
Immunology Market
Medical Imaging Devices Market
Healthcare Mobility Solutions Market
Diabetes Devices Market
0 notes