#US recombinant biosimilars industry
Text
Top 5 players in US Biosimilar Market
Buy Now
STORY OUTLINE
Pfizer: Excelling in the line of Biosimilar drugs with an experience of more than 10 years with presence in over 180 countries.
Amgen: Making pharmaceutical products with an experience of over 40 years and presence in over 100 countries.
Viartis: Presence in over 165 countries, and making Biosimilar drugs in over 75 markets, this pharmaceutical company is another leading contributor of US Biosimilar market.
Coherus Biosciences: Increasing patient access to cost effective medicines with a Biosimilar drugs experience of 13 years.
Biogen: serving humanity through science with a experiences of more than 40 years in the field of biologics.
According to Ken Research, the US Biosimilar market is anticipated to grow at a CAGR of ~40% in the next five years which currently has a market size of ~USD 9.4 Bn.
The US Biosimilar market is rapidly growing and will be witnessing a significant growth in the next five years.
There are various reasons behind the rapid growth of US Biosimilar market. Some of the major reasons behind the growth of US Biosimilar market include the cost effective nature of Biosimilar drugs, rising geriatric population, rising prevalence of chronic diseases, and growing partnerships between companies to develop Biosimilar drugs.
Various companies and players are contributing to their best efforts in the growth of the US Biosimilar market.
This article aims to put light on the contributions done by the major players towards the growth of the US Biosimilar market.
1.Pfizer
Click to read more about Pfizer
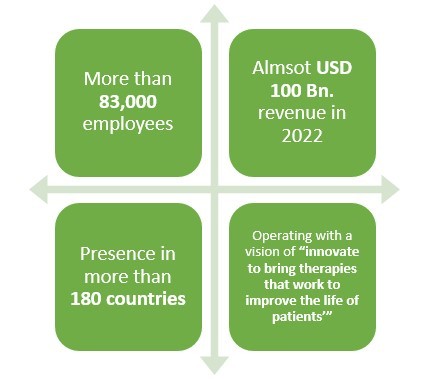
Pfizer is a leading American pharmaceutical company which is operating in the field of generics or original drugs for more than 30 years. But did you know that this pharma not only manufactures biologics but also biosimilar drugs?
Pfizer has been in the business of biosimilar drugs for more than 10 years and have been quite successful as well. With more than 83,000 employees and presence in over 180 countries, this leading pharmaceutical company made almost USD 2 Bn. revenue only from its Biosimilar drugs sale in 2021.
Recently, this pharmaceutical company also collaborated with Samsung in two deals to produce various biosimilar drugs in South Korea. The deal size between these two companies happens to be approximately USD 900 Bn.
The major Biosimilar drugs of this pharmaceutical giant are primarily
ZIRABEV (a Biosimilar of Avastin)
TRAZIMERA (a Biosimilar of Herceptin)
RUXIENCE (a Biosimilar of Rituxan)
RITACRIT (a Biosimilar of Epogen)
NVYEPRIA (a Biosimilar of Neulasta)
NIVESTYM (a Biosimilar of Neupogen)
FILGRASTIM (a Biosimilar of Neupogen).
2.Amgen
Click here to Download a Sample Report
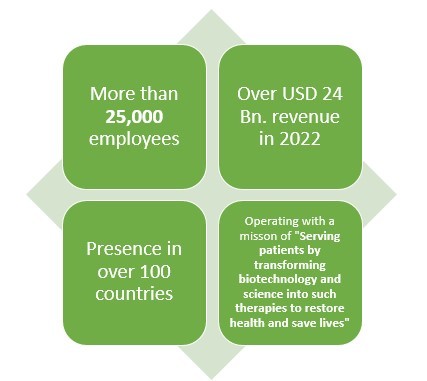
Amgen is another leading American pharmaceutical company which not only makes Biologics or generic drugs but also Biosimilar drugs. This pharmaceutical company has more than 40 years of experience when it comes to pharmaceutical line.
With over 25000 employees and presence in over 100 countries, this pharmaceutical company earned about USD 2 Bn. from their three biosimilar drugs which are reportedly MVASI, KANJITNTI, and AMJEVITA.
This pharma giant has also invested about USD 2 Bn. in the development of Biosimilar drugs.
This pharmaceutical company has made Biosimilar drugs primarily in 4 fields which are General Medicine, Oncology, and Hematology along with, Inflammation.
EPOTEIN ALFA
AMJEVITA
AVSOLA
KANJINTI
MVASI
RIABNI
are the various Biosimilar drugs of Amgen. And, STELARA, EYLEA, SOLIRIS are in their pipeline.
Recently Amgen revealed their Biosimilar report’s 8 version. It revealed a major information which said that the pharmaceutical company saved about USD 10 Bn. through their Biosimilar drugs in the past five years.
3.Viartis

Headquartered in Canonsburg, Pennsylvania, this American pharmaceutical company was founded only in 2020 yet they have achieved massive success in the pharmaceutical products with their revenue being USD 16 ~Bn. in 2022.
With presence in 165 countries and with over 45,000 employees worldwide, this pharmaceutical company makes pharmaceutical products in 10 areas which primarily are Cardiovascular, Dermatology, ophthalmology, Oncology, Gastroenterology, Women’s health, Infectious diseases, Diabetes & Metabolism, Immunology, CNS & Anesthesiology, Respiratory diseases and allergy.
Speaking of their first Biosimilar products, their first ever Biosimilar drug was launched in 2014. They have a variety of Biosimilar drugs which are primarily
TRASTUZUMAB
INSULIN ASPART
PEGFILGRASTIM
INSULIN GLARGINE-YFGN
ADALIMUMAB
BEVACIZUMAB
Their Biosimilar drug Insulin Glargine which is known as SEMGLEE was the first ever interchangeable Biosimilar drug in the United States which was FDA approved.
Their PEGFILGRASTIM also was the first ever FDA approved drug in the United States. They have launched their Biosimilar drugs in over 75 markets worldwide.
4.Coherus Biosciences:
Click here to Ask for a Custom Report
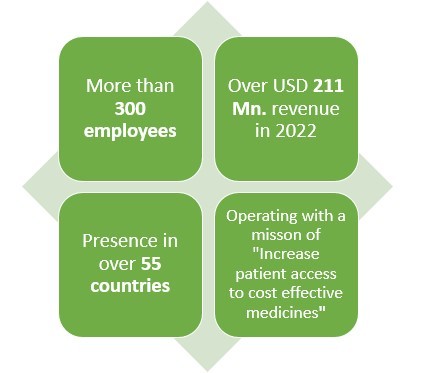
Headquartered in Redwood city, California this American pharmaceutical company earned a revenue of almost USD 211 Mn. In 2022.
With presence in over 55 countries and 300+ employees worldwide, this pharmaceutical company makes products in various areas such as solid tumors, non-small lung cancers, nasopharyngeal carcinoma, small cell lung cancer and hepatocellular carcinoma.
Speaking of their Biosimilar drugs, this pharma has been in the field of creating Biosimilar drugs since 2010 which has given them almost 13 years of experience.
This pharmaceutical company also disclosed that it plans to spend at least USD 1 Tn. on medicines worldwide, out of which at least 40% will be spent on Biosimilar drugs.
Their three major Biosimilar drugs which are also FDA approved include UDENCYA, YUSIMRY, and CIMERLI.
Udencya is a Biosimilar drug of Pegfilgrastim, Yusimry is a Biosimilar drug of Ranibizumab, and Cimerli is a Biosimilar drug of Adalimumab.
5.Biogen
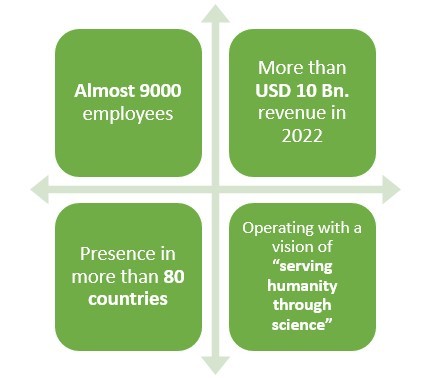
Headquartered in Cambridge, Massachusetts, this American pharmaceutical company earned a revenue of around USD 10 Bn. in 2022.
This company happens to have an experience of more than 40 years when it comes to making pharmaceutical products.
With presence in over 80 countries and more than 9000 employees worldwide, this pharmaceutical company primarily deals in Neurology, Specialized Immunology, Neuropsychiatry, Ophthalmology, and Rare Diseases.
ADUCANUMAB
LECANEMAB
TOFERSEN
ZURANOLONE
LITIFILIMAB
BENAPALI
FLIXABI
IMRALDI
are some of their Biosimilar drugs.
With their Biosimilar drugs, more than 250,000 people have gone on Anti-Tumor Necrosis Factor therapy.
Recently, this pharmaceutical company also made an agreement with Bio-Thera solutions to develop a Biosimilar drug for the treatment of Rheumatoid Arthritis.
#US Biosimilar Sector#United States Biosimilar Market#US Biosimilar Market forecast#US Biosimilar Market analysis#US Biosimilar Market trends#US Biosimilar Market share#US Biosimilar Market key players#US Biosimilar Market revenue#US Biosimilar Market growth#Monoclonal Antibodies in biosimilar market US#Recombinant Hormones in biosimilar industry US#Oncology in bio similar market US#Blood disorders in biosimilar market US#Research institutes in Biosimilar market US#US similar biotherapeutics products market#Hospitals in Biosimilar market US#Investors in Biosimilar market US#US comparable biologics products industry#US recombinant biosimilars industry#US replicate biologics sector#US analog biologics market
0 notes
Text
Biosimilars Market: Driving Affordable Healthcare Solutions
The Biosimilars market has become a significant player in modern healthcare, offering cost-effective alternatives to biologics. As the demand for affordable therapeutic options rises, the market for biosimilars is gaining substantial momentum. This article delves into the market trends, segmentation, growth drivers, and leading companies in the biosimilars industry, providing crucial insights for decision-makers.
Market Overview
According to SkyQuest's Biosimilars Market report, the global market is currently valued at USD 27.30 billion in 2023, with a projected CAGR of 16.4% over the forecast period. The market's expansion is fueled by patent expirations of key biologics, increasing prevalence of chronic diseases, and a growing emphasis on cost-efficient healthcare solutions.
Request Your Free Sample: - https://www.skyquestt.com/sample-request/biosimilars-market
Market Segmentation
By Product Type:
Recombinant Non-Glycosylated Proteins: Used in the treatment of diseases such as diabetes and cancer.
Recombinant Glycosylated Proteins: Common in oncology and autoimmune disease therapies.
Peptides: An emerging class used for metabolic diseases and cancer treatment.
By Application:
Oncology: High demand for biosimilars due to the rising incidence of cancers and the need for affordable treatments.
Chronic Diseases: Biosimilars for diabetes, arthritis, and cardiovascular diseases are critical for cost-effective long-term care.
Autoimmune Diseases: Increasing prevalence of conditions like rheumatoid arthritis and multiple sclerosis is driving the demand for biosimilars.
Infectious Diseases: Expansion of biosimilars in this sector due to the global burden of infections.
By Manufacturing Type:
In-House Manufacturing: Pharmaceutical companies developing their own biosimilars to maintain control over production.
Contract Manufacturing: Outsourcing production to specialized third-party manufacturers for efficiency and scalability.
By End-User:
Hospitals and Clinics: Major centers for biosimilars administration.
Pharmaceutical Companies: Driving the production and distribution of biosimilars.
Research Institutes: Key players in the innovation and clinical trials of biosimilars.
Key Growth Drivers
Patent Expirations of Biologics: As patents for major biologic drugs expire, the market opens up for biosimilar development.
Rising Healthcare Costs: Increasing demand for cost-effective alternatives to expensive biologic therapies.
Chronic Disease Prevalence: Growing rates of cancer, diabetes, and autoimmune diseases are spurring demand for biosimilars.
Government Initiatives: Regulatory frameworks supporting the approval and adoption of biosimilars.
Read More at: - https://www.skyquestt.com/report/biosimilars-market
Leading Companies in the Market
SkyQuest’s Biosimilars Market report highlights key players driving innovation and expansion, including:
Pfizer Inc.
Novartis AG (Sandoz)
Amgen Inc.
Samsung Bioepis
Biocon
Celltrion Healthcare
Teva Pharmaceuticals
Mylan N.V.
Fresenius Kabi
Hoffmann-La Roche Ltd
Challenges and Opportunities
The biosimilars market faces hurdles such as complex manufacturing processes and regulatory challenges. However, the growing acceptance of biosimilars by healthcare providers, coupled with increasing investments in biosimilar R&D, presents significant opportunities for market players to innovate and expand.
Take Action Now: Secure Your Report Today - https://www.skyquestt.com/buy-now/biosimilars-market
Future Outlook
As healthcare systems worldwide strive to reduce costs without compromising care quality, the biosimilars market is poised for strong growth. Companies that prioritize technological advancements, navigate regulatory frameworks efficiently, and focus on patient access will thrive in this evolving market.
The biosimilars market is shaping the future of affordable healthcare. With increasing demand for cost-effective treatments, decision-makers must keep pace with the trends and opportunities in this dynamic sector. For more comprehensive insights and strategic recommendations, consult SkyQuest’s in-depth Biosimilars Market report.
0 notes
Text
Exploring the Recombinant Chemicals Market: Innovations Shaping the Future
The Recombinant Chemicals Market represents a significant subset of the broader biotechnology and chemical sectors. Recombinant chemicals are produced through genetic engineering techniques, which allow for the precise manipulation of microbial, plant, or animal cells to produce desired chemical compounds. These chemicals have wide applications in pharmaceuticals, agriculture, industrial processes, and research, providing solutions that are more efficient, cost-effective, and sustainable compared to traditional chemical production methods.
The global recombinant chemicals market, valued at US$ 2.9 billion in 2023, is projected to grow at a CAGR of 7.8% from 2024 to 2034. By the end of 2034, the market is expected to reach US$ 6.7 billion, driven by advancements in biotechnology and increasing demand across various industries.
This growth is fueled by the rising need for biopharmaceuticals, green chemicals, and bio-based industrial products. The pharmaceutical sector remains the largest end-user of recombinant chemicals, especially in the production of proteins, enzymes, and other bioactive compounds. Additionally, the agricultural sector is witnessing increased adoption of recombinant chemicals in the development of bio fertilizers and pesticides.
For More Details, Request for a Sample of this Research Report: https://www.transparencymarketresearch.com/recombinant-chemicals-market.html
Market Segmentation
The recombinant chemicals market can be segmented based on various factors:
By Service Type:
Contract Research Services
Manufacturing Services
Custom Synthesis Services
By Sourcing Type:
In-house Production
Outsourced Production
By Application:
Pharmaceuticals
Agriculture
Industrial Enzymes
Cosmetics
Food and Beverages
By Industry Vertical:
Healthcare
Agriculture
Chemical
Biotechnology
Food Processing
By Region:
North America
Europe
Asia-Pacific
Latin America
Middle East & Africa
Regional Analysis
North America: Leading the market due to well-established biotechnology and pharmaceutical industries, as well as heavy investment in R&D for recombinant chemical production.
Europe: The second-largest market, driven by increasing demand for sustainable and bio-based chemicals, as well as stringent regulations on chemical production.
Asia-Pacific: Expected to witness the highest growth during the forecast period due to expanding pharmaceutical and agricultural industries in countries like China and India, along with supportive government initiatives.
Latin America and the Middle East & Africa: These regions are also gaining traction as key markets for recombinant chemicals due to the growing demand for agricultural chemicals and biopharmaceuticals.
Market Drivers and Challenges
Drivers:
Technological Advancements: Continuous innovation in genetic engineering and fermentation technologies is expanding the capabilities of recombinant chemical production.
Sustainability: Recombinant chemicals offer a more environmentally friendly alternative to traditional petrochemical-based products, driving demand in industries focused on sustainability.
Rising Demand for Biopharmaceuticals: The growing need for advanced drugs, including biosimilars, monoclonal antibodies, and vaccines, is fueling demand for recombinant chemicals.
Challenges:
High Production Costs: The initial investment required for setting up recombinant chemical production facilities is high, which may limit market penetration, especially in developing regions.
Regulatory Hurdles: Complex regulatory frameworks, particularly in the pharmaceutical and agricultural sectors, can slow down the commercialization of recombinant chemicals.
Technical Limitations: The scalability of production processes and achieving consistency in yield and purity remain key challenges.
Market Trends
Shift Towards Green Chemicals: With increasing environmental regulations, there is a clear shift toward the development and adoption of bio-based recombinant chemicals.
Expanding Applications: Recombinant chemicals are finding new applications in sectors like food processing, cosmetics, and industrial enzymes, driven by their versatility and efficiency.
Collaborations and Partnerships: Companies are increasingly forming partnerships with research institutions and contract manufacturing organizations (CMOs) to enhance production capabilities and streamline R&D processes.
Future Outlook
The future of the recombinant chemicals market looks promising, with expected breakthroughs in gene editing technologies like CRISPR and the expanding use of synthetic biology. The ongoing shift toward personalized medicine and bio-based solutions in various industries will further drive demand for recombinant chemicals. The market is poised to witness significant investments in R&D and infrastructure development, particularly in the Asia-Pacific and European regions.
Buy this Premium Research Report: https://www.transparencymarketresearch.com/checkout.php?rep_id=86368<ype=S
Key Market Study Points
Analysis of recombinant chemical applications across diverse industries.
Regional market dynamics and their impact on growth projections.
Competitive landscape analysis, focusing on key players and their strategies.
Technological advancements and their role in shaping the market’s future.
Evaluation of the sustainability of recombinant chemical production methods.
Competitive Landscape
The recombinant chemicals market is highly competitive, with key players focusing on innovation, product differentiation, and strategic collaborations. Major players include:
BASF SE
Merck KGaA
Lonza Group
Evonik Industries AG
Genentech, Inc.
These companies are investing in advanced biotechnologies and expanding their recombinant chemical portfolios through mergers, acquisitions, and partnerships with smaller firms or research institutions.
Recent Developments
Mergers and Acquisitions: Several key players have recently acquired smaller biotech firms to strengthen their recombinant chemical capabilities.
Technological Advancements: New methods of gene editing and fermentation are being developed, allowing for more efficient and cost-effective production of recombinant chemicals.
Regulatory Approvals: Recent regulatory approvals for new recombinant-based drugs and chemicals have opened new avenues for market growth, particularly in pharmaceuticals and agriculture.
About Transparency Market Research
Transparency Market Research, a global market research company registered at Wilmington, Delaware, United States, provides custom research and consulting services. Our exclusive blend of quantitative forecasting and trends analysis provides forward-looking insights for thousands of decision makers. Our experienced team of Analysts, Researchers, and Consultants use proprietary data sources and various tools & techniques to gather and analyses information.
Our data repository is continuously updated and revised by a team of research experts, so that it always reflects the latest trends and information. With a broad research and analysis capability, Transparency Market Research employs rigorous primary and secondary research techniques in developing distinctive data sets and research material for business reports.
Contact:
Transparency Market Research Inc.
CORPORATE HEADQUARTER DOWNTOWN,
1000 N. West Street,
Suite 1200, Wilmington, Delaware 19801 USA
Tel: +1-518-618-1030
USA – Canada Toll Free: 866-552-3453
Website: https://www.transparencymarketresearch.com
0 notes
Text
Columnas de cromatografía preempaquetadas para productos farmacéuticos, previsión del tamaño del mercado mundial, clasificación y cuota de mercado de las 11 principa
Según el nuevo informe de investigación de mercado “Informe del Mercado Global del Columnas de cromatografía preempaquetadas para productos farmacéuticos 2024-2030”, publicado por QYResearch, se prevé que el tamaño del mercado mundial del Columnas de cromatografía preempaquetadas para productos farmacéuticos alcance 2.01 mil millones de USD en 2030, con una tasa de crecimiento anual constante del 7.9% durante el período de previsión.
Figure 1. Tamaño del mercado de Columnas de cromatografía preempaquetadas para productos farmacéuticos global (US$ Millión), 2019-2030

Según QYResearch, los principales fabricantes mundiales de Columnas de cromatografía preempaquetadas para productos farmacéuticos incluyen Agilent Technologies, Waters Corporation, Thermo Fisher Scientific, Danaher, Merck, Bio-Rad, Sartorius, Repligen Corporation, Tosoh Bioscience, Astrea Bioseparations, etc. En 2023, las cinco principales entidades mundiales tenían una cuota de aproximadamente 62.0% en términos de ingresos.
Figure 2. Clasificación y cuota de mercado de las 11 principales entidades globales de Columnas de cromatografía preempaquetadas para productos farmacéuticos (la clasificación se basa en los ingresos de 2023, actualizados continuamente)

Here are the key market drivers for the Pharmaceutical Prepacked Chromatography Columns market:
1. Increasing Demand for Biologics and Biosimilars: The growing prevalence of chronic diseases and the rising demand for biopharmaceuticals, such as monoclonal antibodies and recombinant proteins, are driving the need for efficient purification techniques like chromatography.
2. Technological Advancements and Innovation: Continuous improvements in chromatography media, resin chemistries, and column designs are enhancing the performance, efficiency, and versatility of prepacked chromatography columns.
3. Regulatory Requirements and Quality Standards: Stringent regulatory guidelines and good manufacturing practices (GMP) in the pharmaceutical industry mandate the use of reliable and reproducible purification techniques, such as prepacked chromatography columns.
4. Increasing Outsourcing of Pharmaceutical Manufacturing: The pharmaceutical industry's trend towards outsourcing drug manufacturing and purification processes to contract manufacturing organizations (CMOs) and contract research organizations (CROs) is contributing to the demand for prepacked chromatography columns.
5. Expansion of the Biopharmaceutical Industry: The rapid growth of the biopharmaceutical industry, driven by the increasing prevalence of chronic diseases and the development of innovative biological therapies, is a key factor driving the demand for prepacked chromatography columns.
6. Cost-Effectiveness and Operational Efficiency: Prepacked chromatography columns offer cost-effective and time-saving advantages compared to traditional, labor-intensive manual column packing processes.
These market drivers, coupled with the ongoing technological advancements, the increasing demand for biologics and biosimilars, and the emphasis on regulatory compliance and quality standards, are expected to continue driving the growth of the Pharmaceutical Prepacked Chromatography Columns market in the coming years.
Sobre QYResearch
QYResearch se fundó en California (EE.UU.) en 2007 y es una empresa líder mundial en consultoría e investigación de mercados. Con más de 17 años de experiencia y un equipo de investigación profesional en varias ciudades del mundo, QY Research se centra en la consultoría de gestión, los servicios de bases de datos y seminarios, la consultoría de OPI, la investigación de la cadena industrial y la investigación personalizada para ayudar a nuestros clientes a proporcionar un modelo de ingresos no lineal y hacer que tengan éxito. Gozamos de reconocimiento mundial por nuestra amplia cartera de servicios, nuestra buena ciudadanía corporativa y nuestro firme compromiso con la sostenibilidad. Hasta ahora, hemos colaborado con más de 60.000 clientes en los cinco continentes. Trabajemos estrechamente con usted y construyamos un futuro audaz y mejor.
QYResearch es una empresa de consultoría a gran escala de renombre mundial. La industria cubre varios segmentos de mercado de la cadena de la industria de alta tecnología, que abarca la cadena de la industria de semiconductores (equipos y piezas de semiconductores, materiales semiconductores, circuitos integrados, fundición, embalaje y pruebas, dispositivos discretos, sensores, dispositivos optoelectrónicos), cadena de la industria fotovoltaica (equipos, células, módulos, soportes de materiales auxiliares, inversores, terminales de centrales eléctricas), nueva cadena de la industria del automóvil de energía (baterías y materiales, piezas de automóviles, baterías, motores, control electrónico, semiconductores de automoción, etc.. ), cadena de la industria de la comunicación (equipos de sistemas de comunicación, equipos terminales, componentes electrónicos, front-end de RF, módulos ópticos, 4G/5G/6G, banda ancha, IoT, economía digital, IA), cadena de la industria de materiales avanzados (materiales metálicos, materiales poliméricos, materiales cerámicos, nanomateriales, etc.), cadena de la industria de fabricación de maquinaria (máquinas herramienta CNC, maquinaria de construcción, maquinaria eléctrica, automatización 3C, robots industriales, láser, control industrial, drones), alimentación, bebidas y productos farmacéuticos, equipos médicos, agricultura, etc.
#Columnas de cromatografía preempaquetadas para productos farmacéuticos#Pharmaceutical Prepacked Chromatography Columns
0 notes
Text
Biosimilars Market Size, Trends, Growth Analysis 2032
Biosimilars Market Overview
An integral component of the Biosimilars Market is the emergence of follow-on biologics. These biologics, which closely resemble existing biologic drugs, offer additional options for patients and healthcare providers. Follow-on biologics undergo rigorous testing to demonstrate similarity to the reference product, ensuring interchangeability and therapeutic equivalence. With their introduction, follow-on biologics stimulate competition in the biopharmaceutical industry, driving down prices and promoting innovation. As the demand for cost-effective biologic therapies continues to grow, the Biosimilars Market stands to benefit from the availability and acceptance of follow-on biologics, expanding treatment options and improving patient outcomes.
According to Market Research Future (MRFR), the biosimilars market insights was valued at USD 29.7 billion in 2023 and is projected to grow from USD 36.79 Billion in 2024 to USD 161.95 billion by 2032, exhibiting a compound annual growth rate (CAGR) of 20.35% during the forecast period (2024 - 2032).
Biosimilars Market: Latest News and Developments
The FDA has approved the first Humira biosimilar. Adalimumab-bwwd (Cyltezo), a Humira biosimilar, received FDA approval in January 2023 for the treatment of rheumatoid arthritis, psoriatic arthritis, ankylosing spondylitis, juvenile idiopathic arthritis, Crohn's disease, ulcerative colitis, plaque psoriasis, and hidradenitis suppurativa.
Rising use of biosimilars in the treatment of cancer. A growing number of biosimilars are being used to treat cancer. The FDA authorised filgrastim-sndz (Zarxio), pegfilgrastim-jmdb (Onpro), and trastuzumab-dkst (Herzuma) as three biosimilars for the treatment of cancer in 2022.
Market Segmentation
Biosimilars industry can be considered with respect to product, applications, and end users.
The products based on which the market has been split into are recombinant glycosylated proteins, recombinant peptides, and recombinant non-glycosylated proteins. Recombinant non-glycosylated proteins are the biggest segment in the global market, thanks to the soaring cases of chronic disorders ranging from growth hormone deficiency to diabetes. As a result, recombinant non-glycosylated proteins are therapeutically used as they are readily available and are cost-effective.
Application-wise, the biosimilars market caters to chronic diseases, oncology, blood disorders, autoimmune diseases, infectious diseases, growth hormone deficiency, and more. Blood disorders have emerged as the top segment, as a result of the rising burden of the condition worldwide and the increased use of biosimilars by virtue of their low cost and the overall reduction in the treatment cost.

Major end users in the global market are hospitals and clinics as well as research institutes. Hospital and clinics are healthcare settings where treatment options and skilled professionals are easily available, and therefore, have emerged as the leading segment in the global industry.
Regional Status
Europe, MEA or Middle East & Africa along with the Americas, APAC or Asia Pacific are the primary markets for biosimilars.
Europe has taken the lead in the global market, as the region houses a vast elderly pool, with close to one fifth of the overall EU population aged more than 65 years. This has given way to several lifestyle-related disorders such as oncology, autoimmune diseases, diabetes, to name a few. Presumably, the scenario has raised the demand for biosimilars and can mean higher market growth over the next few years. In addition to this, numerous blockbuster biologics are on track to lose patent in the coming years, which should present lucrative opportunities to the biosimilar manufacturers.
The North American market displays a bright outlook and can emerge quite lucrative in the coming years, in view of the surging burden of chronic ailments in Canada and the United States. The rising spending on research activities by the healthcare agencies also fuels the market expansion in the region. Favorable reimbursement landscape in the region, especially in the US, encourages healthy competition as it results in lower incentives for the players to compete based on price.
The APAC market is slated to witness considerable growth, with China, South Korea and India offering a host of lucrative opportunities for drug development as well as commercialization. These are generics-driven countries and are known for frequently launching new and advanced manufacturing platforms, which has brought down the costs associated with biosimilar production. In the coming few years, majority of the patent expiries is touted to be in biosimilars, which is deemed as a profitable aspect by leading manufacturers and can translate into substantial market growth.
Eminent Vendors
Top global key players listed by biosimilars market outlook report include Stada Arzneimittel AG (Germany), Teva Pharmaceuticals (Israel), Biocon (India), Pfizer (US), Sandoz International (Germany), Eli Lily & Company (US), Actavis, Inc. (US), Dr. Reddy’s Laboratories (India), Cipla Ltd (India), Amgen, Inc. (US), Samsung Biologics (South Korea), Hospira Inc.(US), Mylan, Inc.(US), Celltrion (South Korea), to mention a few.
More Related Trending Topics
Medical Foods market
Insomnia market
Blockchain Technology in Healthcare market
Drug Device Combination market
Prefilled Syringes market
0 notes
Text
Epidermal Growth Factors (EGF) Market will grow at highest pace owing to rising use in wound healing applications

Epidermal growth factors (EGFs) are signaling proteins that bind to epidermal growth factor receptors (EGFRs) on the surface of cell within the body. EGFs play an important role in regulating cell growth, proliferation, and cell specialization. These factors promote healing in skin and other epithelial tissues by increasing migration and division of epithelial cells. EGFs are used in cosmetic products and pharmaceutical preparations for wound healing applications. Increased use of EGFs in treatment of chronic and acute wounds is expected to drive the market growth over the forecast period.
The Global Epidermal Growth Factors (EGF) Market is estimated to be valued at US$ 1,349.6 Mn in 2024 and is expected to exhibit a CAGR of 5.9% over the forecast period 2023 to 2030.
Key Takeaways
Key players operating in the Epidermal growth factors (EGF) market are Radiant Inc., Pavay, BIO-FD&C Co. Ltd., BIOEFFECT, LipoTrue, S.L., and Ytkangdaer.
The growing demand for EGFs from wound care industry is the major factor propelling the market growth. EGFs are widely used in treatment of diabetic ulcers, pressure ulcers, venous ulcers and other chronic wounds.
Advancements in recombinant DNA technology has enabled mass production of genetically engineered EGFs. Commercially available EGFs are produced through microbial fermentation or natural extraction processes.
Market Trends
Increased production through biosimilars - Several new players are focused on developing biosimilar version of EGFs through microbial fermentation which is more cost-effective compared to extraction from natural sources. This is expected to increase the product availability and drive down prices.
Rise in aesthetic procedures - With growing aesthetic consciousness, use of EGF in anti-aging cosmetic creams and serums is gaining popularity. EGF helps reduce wrinkles, fine lines and treats skin laxity.
Market Opportunities
EGFs hold huge potential in accelerating wound healing in burn injuries. Ongoing clinical research is exploring efficacy of EGFs in treatment of severe burn wounds.
With increasing life expectancy, age-related medical conditions like pressure ulcers are on rise. EGF based formulations can enable better wound management for elderly population.
0 notes
Text
Overview of Microbial Fermentation Techniques

The Microbial Fermentation Technology Market size was estimated at USD 30,964.20 million in 2022 and is expected to reach USD 48980.988 million by 2030 with a growing CAGR of 5.9% during the forecast period of 2023-2030.The global Microbial Fermentation Technology Market study includes a full analysis of key driving drivers, as well as profiles of prominent firms, essential product features, sales rates, and contact information. A thorough assessment of the most important market trends is also included in the research. Information is gathered through focus groups, surveys, interviews, a geographical and national study, and a comprehensive all-dimensional examination.
Major market factors such as drivers, constraints, opportunities, and threats are examined, as well as how they influence the market. External threats and opportunities have underlying drivers and limits in the global market. Strategic alliances, new product launches, initiatives, transactions, joint activities, and information on top market rivals are all covered by Microbial Fermentation Technology Market research, as well as development factors, limits, and opportunities.
Get Sample Report @ https://www.snsinsider.com/sample-request/3814
Market Segmentation
By Application
Antibiotics
Probiotics Supplements
Monoclonal Antibodies
Recombinant Proteins
Biosimilars
Vaccine
Enzymes
Small Molecules
Others
By End User
Bio-Pharmaceutical Companies
Contract Research Organizations (CROs)
CMOs & CDMOs
Academic & Research Institutes
The global Microbial Fermentation Technology Market analysis summary provides an overview of the topic, including definitions, classifications, applications, and the industrial chain structure. Global business research is offered for emerging markets, including competitive landscape studies and development trends.
Russia-Ukraine War Impact on Microbial Fermentation Technology Market
The impact of the Russia-Ukraine conflict on the worldwide market is detailed in the research paper. While tensions between Russia and Ukraine have been increasing for years, the present military action heightens fears of a long-term conflict within Ukraine, as well as market and global economic implications.
Competitive Scenario
The market report also includes a comprehensive library of future market estimates based on historical data. Customers can get quantitative industry expertise by looking at the most recent market data. The study investigates a variety of significant elements that influence firm players, including as suppliers, end-users, dealers, and others, in order to assist them in strategizing investment and pursuing various Microbial Fermentation Technology Market growth chances. All significant competitors, prices, and positioning, as well as a thorough data collecting strategy, must operate in the same territory.
Report Highlights
Give an overview of the present situation of the target industry, including applications and innovations.
A comprehensive analysis of the Microbial Fermentation Technology Market 's situation amid COVID-19 pandemic and Russia-Ukraine War.
A detailed market analysis, including upstream raw materials, downstream output, and recent growth estimates.
Report Conclusion
The most recent Microbial Fermentation Technology Market study looks at the target market's latest influence. The article looks at how the corporate environment is always changing, as well as the short- and long-term implications.
About Us
SNS Insider is a market research and insights firm that has won several awards and earned a solid reputation for service and strategy. We are a strategic partner who can assist you in re framing issues and generating answers to the trickiest business difficulties. For greater consumer insight and client experiences, we leverage the power of experience and people.
When you employ our services, you will collaborate with qualified and experienced staff. We believe it is crucial to collaborate with our clients to ensure that each project is customized to meet their demands. Nobody knows your customers or community better than you do. Therefore, our team needs to ask the correct questions that appeal to your audience in order to collect the best information.
Related Reports
Nasal Drug Delivery Market Growth Drivers
DNA Synthesis Market Growth Drivers
Osteoporosis Treatment Market Growth Drivers
Immunomodulators Market Growth Drivers
Proteomics Market Growth Drivers
0 notes
Text
Bridging the Gap: How the Global Cell Line Development Services Market is Expanding Access to Care
The demand for cell line development services market is expected to reach a value of USD 6,365.2 million in 2023, marking a significant milestone in the biotechnology sector. Over the following decade, the market is forecasted to double in value, growing at an impressive Compound Annual Growth Rate (CAGR) of 7.4%. By the end of 2033, the market is projected to be valued at an estimated USD 12,957.8 million, according to Future Market Insights.
This extraordinary growth trajectory is fueled by continuous research and technological advancement in the biotechnology sector, driving the demand for innovative cell line development services. With a historical CAGR of 6.5% observed from 2018 to 2022, the market is poised to build upon its solid foundation and reach new heights in the coming years.
Because they make it possible to produce cell lines designated for specific uses such the expression of recombinant proteins, the manufacture of antibodies, and the creation of vaccines, cell line development services are essential to biopharmaceutical research and development. These services, which include cell line banking, engineering, optimization, and characterization, simplify and lower the cost of producing high-quality cell lines for medical and diagnostic applications.
Request Your Detailed Report Sample With Your Work Email:
https://www.futuremarketinsights.com/reports/sample/rep-gb-4339
The biopharmaceutical sector relies on cell line development services to produce vaccines and biologics for a range of therapeutic uses. These services include, among other things, cell line engineering, cell banking, and cell line characterization—all crucial procedures for the creation and production of novel medications.
The market for cell line creation services is expected to grow, and several important factors will make this possible. The significance of efficiency and scalability in cell line development solutions has grown due to the swift progress of biotechnology and the growing demand for biopharmaceuticals. Furthermore, the market’s growth is being supported by the increasing demand for biologic pharmaceuticals due to the increased emphasis on tailored care as well as the rise in infectious and chronic illnesses.
The increasing frequency of neurological disorders and cancer, coupled with the dearth of effective treatment options for these conditions, have created a need for more advanced and efficient treatment pathways. Companies and government agencies are investing funds in research and development initiatives and focusing more on the generation of cell lines in order to identify novel biological pathways for the synthesis of cutting-edge drugs. The increased research and development spending on biosimilars made by departing biopharmaceutical corporations would be advantageous to the cell line development industry.
Market Competition:
Lonza, MabPlex Inc., Thermo Fisher Scientific, Inc., Solentim Ltd., Sigma-Aldrich Corporation, Selexis, Corning, Inc., Wuxi App Tec, Inc., and Sartorius AG are a few of the major players in the worldwide cell line development services market.
The market is extremely competitive as a result of the large number of participants. Regional players are present in key development regions, especially Asia Pacific, but global players like Lonza, MabPlex Inc., Thermo Fisher Scientific, Inc., and Solentim Ltd. still hold a significant share of the market.
In May 2022 – ALSA Ventures, and Lonza, a global development and manufacturing partner to the pharma, biotech and nutrition industries, announced today a framework collaboration agreement to help ALSA’s portfolio of pre-clinical and early clinical biotechs develop and manufacture biologics and small molecule drug candidates.
In March 2021 – Pionyr Immunotherupatics collaborated with Lonza to support their oncology product development. Lonza will help Pionyr with cell line development and offer optimal yield and throughput.
In June 2022 – WuXi Advanced Therapies and Wugen Inc., announced a partnership to produce Wugen’s WU-NK-101, a novel immunotherapy that harnesses the power of memory natural killer (NK) cells to treat cancers. WuXi ATU will provide manufacturing and testing services for WU-NK-101 to enable the delivery of this innovative cell therapy to cancer patients.
Key Companies Profiled:
Lonza
MabPlex Inc.
Thermo Fisher Scientific, Inc.
Solentim Ltd
Sigma-Aldrich Corporation
Selexis
Corning, Inc
Wuxi App Tec Inc.
Sartorius AG
Key Segments Profiled in the Cell Line Development Services Industry Survey
By Product Type:
Media and Reagents
Equipment
Others Cell Line Development Service Products
By Type:
Primary Services
Continuous Services
Hybridomas Services
Recombinant Cell Line Development Services
By Application:
Bioproduction
Diagnostics
Vaccines
Recombinant Protein Therapeutics
Tissue Engineering & Regenerative Medicines
Drug Discovery
Toxicity Testing
Pharmaceutical and Biotechnology Research
By Region:
North America Market
Latin America Market
Europe Market
Asia Pacific Market
Middle East & Africa Market
0 notes
Text
Electrocompetent Cells Market is Estimated to Witness High Growth Owing to Rising Adoption of Recombinant DNA Technology

Electrocompetent cells are bacterial cells that have been treated to become permeable to plasmid DNA, and can therefore take up DNA from their environment through the process of electroporation. They are widely employed in the transformation of cloning vectors and the isolation of recombinant plasmids. These cells find extensive usage in various molecular biology procedures such as cloning, site-directed mutagenesis, library construction, expression vector construction for the production of therapeutic proteins, recombinant protein expression, and sequencing. The global Electrocompetent Cells market is estimated to be valued at US$ 2.07 Bn in 2023 and is expected to exhibit a CAGR of 10% over the forecast period 2023 to 2030, as highlighted in a new report published by Coherent Market Insights.
Market Opportunity:
The rising adoption of recombinant DNA technology is estimated to present lucrative growth opportunities for the electrocompetent cells market over the forecast period. Recombinant DNA technology finds widespread applications in the production of therapeutics and biologics as well as diagnostics development. It helps in modifying the genome of an organism to produce substances like insulin, human growth hormones, monoclonal antibodies, and various vaccines. Therefore, the increasing investments in biologics and biosimilars development and rising adoption of advanced molecular techniques are estimated to propel the demand for electrocompetent cells globally.
Porter's Analysis
Threat of new entrants: The threat of new entrants is low due to high R&D costs and strong intellectual property protection by existing players in the market.
Bargaining power of buyers: The bargaining power of buyers is high as there are many established players and the product is a commodity with no major differentiation.
Bargaining power of suppliers: The bargaining power of suppliers is moderate as few global suppliers cater to the key raw materials needs of the industry.
Threat of new substitutes: The threat of new substitutes is low as there are no cost-effective alternatives available for competent cells in molecular cloning applications.
Competitive rivalry: The competitive rivalry in the market is high due to many global and local players offering similar products.
SWOT Analysis
Strength: Electrocompetent cells offer advantages like high transformation efficiency, easy production, and cost-effectiveness for molecular cloning. They allow for the uptake and integration of exogenous DNA into bacterial cells.
Weakness: Limitations exist in the transformation efficiency based on the origin of DNA and bacterial strain used. In addition, variations can arise in their performance across different laboratory settings.
Opportunity: Growing R&D investments and rising adoption in cell-based research, biologics production, directed evolution studies provide significant growth opportunities. Also, advancements to improve transformation efficiency and customization as per end-user needs present opportunities.
Threats: Stringent regulations pertaining to the design, production and quality assurance of cell-based products act as a major threat. Further, unfavorable changes in government funding and policies can also negatively impact the market.
Key Takeaways
The global Electrocompetent Cells market is expected to witness high growth over the forecast period of 2023 to 2030. Rising investments in pharmaceutical and biotechnology R&D are driving the uptake of competent cells in molecular cloning applications. The market size for 2024 is projected to reach US$ 2.07 Bn, indicating strong growth.
Key players - Key players operating in the Electrocompetent Cells market include Merck KGaA, Thermo Fisher Scientific, Promega Corporation, Takara Bio, Bio-Rad Laboratories, Lucigen Corporation, New England Biolabs, QIAGEN N.V., Origene Technologies, and Illumina, Inc. These established players cater to the global demand through their widespread production and distribution networks.
0 notes
Text
Biosimilars Unleashed: The Future of Healthcare in the US
Buy Now
What is the Size of US Biosimilar Industry?
US Biosimilar Market is expected to grow at a CAGR of ~ % between 2017-2022 and is expected to reach ~USD Bn by 2028. Biosimilars enhance patient access to essential treatments, especially in therapies with high demand, like oncology, by providing more affordable options. Additionally, Growing evidence of biosimilars' comparable efficacy and safety fosters trust among healthcare professionals, driving adoption.

Click here to Download a sample Report
Biosimilars offer cost savings compared to originator biologics, addressing the need for affordable healthcare solutions in the face of rising medical costs. Favorable regulatory frameworks, like the BPCIA, streamline biosimilar approval processes, encouraging manufacturers to invest in development.
Furthermore, The expiration of patents for numerous reference biologics creates opportunities for biosimilar entry, leading to increased competition and market expansion. Pharmaceutical companies are investing in biosimilar R&D and production, expanding the pipeline and market availability. Supportive healthcare policies and reimbursement models incentivize biosimilar adoption, creating a favorable environment for market growth.
US Biosimilar Market by drug class
The US Biosimilar market is segmented by Monoclonal Antibodies, Recombinant Hormones, Immunomodulators, Anti-inflammatory agents and Others. Based on drug class, Monoclonal Antibodies segment dominates the bio similar market in 2022.
Monoclonal antibodies have diverse applications across various therapeutic areas. From cancer treatment to autoimmune diseases, biosimilar Mabs addressed a wide range of medical needs, leading to a broad and growing market. Biosimilars, with their potential for cost savings while maintaining comparable efficacy and safety, gained significant attention as viable alternatives.
US Biosimilar Market by application
In US Biosimilar market, they are segmented by application into Oncology, Blood disorders, Chronic diseases and autoimmune conditions and Others. On the basis of application, Oncology segment was the dominant in 2022.
The increasing prevalence of cancer and the high cost of traditional biologics used in oncology treatment have created a strong incentive for the adoption of biosimilars. Biosimilars offer the potential to provide similar therapeutic outcomes at a lower cost, making them an attractive option for both healthcare providers and patients.
Additionally, the rigorous clinical trials and regulatory processes that biosimilars undergo to gain approval provide reassurance to healthcare professionals and patients regarding their safety and efficacy. This has led to increased acceptance and adoption of biosimilars in oncology.
US Biosimilar by Region
The US Biosimilar market is segmented by Region into North, East, West and South. In 2022, the dominance region is North region in US Biosimilar market.
The North region benefits from a concentration of healthcare providers and academic institutions that are at the forefront of adopting and integrating biosimilars into their treatment protocols. These institutions are more likely to have the expertise to evaluate and incorporate biosimilars effectively, driving their adoption among healthcare professionals and patients.
Click here to Download a Custom Report
Competition Scenario in US Biosimilar Market
The US biosimilar market has witnessed an evolving competitive landscape, with several key players competing for market share. Prominent pharmaceutical companies such as Amgen, Pfizer, Sandoz (Novartis), and Boehringer Ingelheim have been actively involved in developing and marketing biosimilar products. These established players have utilized their expertise in biologics and significant resources to navigate the regulatory landscape and compete effectively.
The competition in the US biosimilar market is characterized by a balance between established pharmaceutical giants and emerging biotech companies. While the major players possess the advantage of resources and experience, smaller biotech firms are also contributing to the market with innovative approaches and niche biosimilar offerings.
What is the Expected Future Outlook for the Overall US Biosimilar Market?
The US Biosimilar market was valued at USD ~Million in 2022 and is anticipated to reach USD ~ Billion by the end of 2028, witnessing a CAGR of ~% during the forecast period 2022- 2028. The US biosimilar market is likely to experience significant growth in the coming years, driven by several factors. Biosimilars are biologic drugs that are highly similar to already approved reference biologics. They offer potential cost savings, increased competition, and improved patient access to crucial treatments.
Firstly, the regulatory environment is becoming more favorable for biosimilars. The Biologics Price Competition and Innovation Act (BPCIA) established a pathway for biosimilar approval in the US, allowing for a smoother regulatory process. As more biosimilars receive approval, competition in the market is expected to intensify.
Secondly, patents for several blockbuster biologics are expiring or have already expired. This creates opportunities for biosimilar manufacturers to enter the market with more affordable alternatives, offering healthcare systems and patients a choice in treatment options.
Thirdly, as healthcare costs continue to rise, biosimilars present an attractive solution for reducing expenses. Their potential to offer cost savings without compromising therapeutic efficacy could lead to increased adoption by healthcare providers, insurers, and patients alike.
Physician and patient education are crucial, as misconceptions about biosimilars' safety and effectiveness might hinder their adoption. Additionally, legal and market access barriers, including patent litigation and complex distribution systems, could slow down the growth of the biosimilar market.
The biosimilar market witness consolidation as larger pharmaceutical companies acquire or partner with smaller biotech firms to bolster their biosimilar portfolios. This will lead to more resources being devoted to biosimilar development and marketing. Changes in healthcare policies, such as reimbursement models and value-based care initiatives, can influence the biosimilar market's growth. Favourable policies that incentivize biosimilar adoption drives their market growth.
#US Biosimilar Market#US Biosimilar Industry#US Biosimilar Sector#United States Biosimilar Market#US Biosimilar Market forecast#US Biosimilar Market analysis#US Biosimilar Market trends#US Biosimilar Market share#US Biosimilar Market key players#US Biosimilar Market revenue#US Biosimilar Market growth#Monoclonal Antibodies in biosimilar market US#Recombinant Hormones in biosimilar industry US#Oncology in bio similar market US#Blood disorders in biosimilar market US#Research institutes in Biosimilar market US#US similar biotherapeutics products market#Hospitals in Biosimilar market US#Investors in Biosimilar market US#US comparable biologics products industry#US recombinant biosimilars industry#US replicate biologics sector#US analog biologics market#US homologous biologics market#US oncology biosimilar market#US immunology biosimilar sector#US insulin biosimilar industry#US Generics Biologics market challenges#US leading Biosimilar drug providers#US leading Biosimilar drug manufacturers
0 notes
Text
Biopharmaceuticals Industry Drives High Growth Through Innovation In Cell And Gene Therapies
The biopharmaceuticals industry develops medicines from living cells and organisms to treat a wide range of medical conditions. Biopharmaceuticals, also known as biologics, include vaccines, blood components, allergenics, somatic cells, gene therapies, tissues, and recombinant therapeutic proteins. They can be composed of sugars, proteins, nucleic acids, or complex combinations of these substances, or may be living cells or tissues. The global biopharmaceutical industry is increasingly focusing on new cell and gene therapies to treat cancer and genetic diseases. Cell therapy often uses stem cells to replace or repair damaged tissues and cells, while gene therapy aims to fix defects by manipulating genes inside patient's cells and tissues. Major biopharmaceutical companies are making substantial investments in R&D for cutting-edge cell and gene therapies with the potential to treat previously untreatable medical conditions.
The global biopharmaceuticals Market is estimated to be valued at US$ 397.24 Bn in 2023 and is expected to exhibit a CAGR of 9.4% over the forecast period 2023 to 2030, as highlighted in a new report published by Coherent Market Insights.
Market Dynamics:
The growth of the global biopharmaceuticals market is driven significantly by continued innovation in cell and gene therapies. According to the report by CMI, biopharma companies invested over $100 billion globally in R&D for cell and gene therapies in 2021 alone. Cell therapies have shown promising results in cancer immunotherapy by leveraging the immune system to fight tumors. Additionally, gene therapies aim to treat genetic diseases such as hemophilia at their root cause by fixing the defective genes. The high success rate of recent gene therapy clinical trials is fueling further investments in this area. While cell and gene therapies offer potential cures, their development requires substantial funding and brings high risks. Overall, continued biomedical advances are critical to maximize the healthcare outcomes from the multi-billion dollar biopharma industry.
SWOT Analysis
Strength: The biopharmaceuticals market is dominated by large companies with widespread R&D facilities and huge financial resources. These large players hold a major share of the market and have strong brand recognition. Biopharmaceuticals have minimal side effects compared to traditional drugs. They can be customized for individual patients which improves treatment effectiveness.
Weakness: Biopharmaceuticals have a lengthy and complex development process making them highly capital intensive. The development of biologics requires specialized expertise and infrastructure which increases costs significantly. Some biopharmaceuticals also have short shelf lives and require specialized storage and distribution networks.
Opportunity: The rapidly aging global population is increasing the demand for therapies to treat chronic diseases. Biopharmaceuticals are increasingly being used to treat conditions like cancer, immunological disorders, and cardiovascular diseases. Developing economies in Asia Pacific and Latin America offer huge market potential due to rising healthcare expenditure.
Threats: Price controls and drug reimbursement policies enacted by various governments pose pricing challenges. Stricter regulations for approval and safety monitoring also increase compliance costs. Expiry of patents for blockbuster drugs results in competition from cheaper biosimilars. Outsourcing of manufacturing and lax regulatory oversight in some nations threatens quality assurance.
Key Takeaways
The global biopharmaceuticals market growth is expected to witness high growth over the forecast period supported by rising demand for specialty drugs and biologics globally. The global biopharmaceuticals Market is estimated to be valued at US$ 397.24 Bn in 2023 and is expected to exhibit a CAGR of 9.4% over the forecast period 2023 to 2030.
The North American region currently dominates the market owing to advanced research capabilities and strong presence of leading players. However, Asia Pacific is emerging as the fastest growing regional market due to growing health awareness, increasing healthcare spending, and improving research infrastructure in countries like China, India, and South Korea. The presence of contract manufacturing hubs in India and China coupled with improving research capabilities is attracting significant investments from global pharmaceutical giants. Furthermore, rising affluence, increased awareness, and favorable government policies are driving healthcare spending upwards in major Asian economies. Countries like India, China, South Korea, and Singapore are actively promoting the development of biologics and biosimilars.
Key players
Key players operating in the biopharmaceuticals market are Accenture, Teleperformance SE, Infosys Limited (Infosys BPM), WNS (Holdings) Ltd., HCL Technologies Limited, AMDOCS, CBRE Group Inc., Sodexo, NCR Corporation, TTEC Holdings, Inc., Wipro Limited, and Capgemini. These companies offer integrated services and solutions across the value chain to help pharmaceutical companies optimize operations and reduce costs.
Get more insights on this topic: https://www.newsstatix.com/biopharmaceuticals-market-industry-insights-trends-biopharmaceuticals-market/ Explore more information on this topic, Please visit: https://filmik.in/molecular-cytogenetics-the-future-of-genomic-research/
#Biopharmaceuticals#Biopharmaceuticals Market#Biopharmaceuticals Market size#Biopharmaceuticals Market share#Biopharmaceuticals Market demand#Biopharmaceuticals Market analysis
0 notes
Text
Biotechnology in Hyderabad covers every aspect of your job search, from beginning to end

Biotechnology is a multidisciplinary area that is applied to pharmaceuticals, agriculture, food sciences, and forestry sciences. Its application results in improved medicines, more productive crops, and even more resilient materials, among others. BIO is the world's largest trade association representing biotechnology companies, academic institutions, state biotechnology centers and related organizations across the United States and in more than 30 other nations. It uses living cells or any of their components to develop products with specific aims. In the early years, the main achievement of biotechnology was the ability to produce naturally occurring therapeutic molecules in larger quantities than could be derived from conventional sources such as plasma, animal organs, and human cadavers. Recombinant proteins are also less likely to be contaminated with pathogens or to provoke allergic reactions. Today, biotechnology researchers seek to discover the root molecular causes of disease and to intervene precisely at that level. Sometimes this means producing therapeutic proteins that augment the body’s own supplies or that make up for genetic deficiencies, as in the first generation of biotech medications. Medical Biotechnology helps in preventing human diseases. The medical research studies are conducted by the medical biotechnologists and these biotechnologists work for academic and industrial needs. They are one of the top Biotechnology Recruitment Agency in Hyderabad.
Today, Pharma companies are more and more moving to new areas such as generics, vaccines, research, biosimilars and gene therapies, just to name a few. Some of the big pharma companies are even developing their generics. Pursuing a career in Biotechnology is a lucrative career and you can earn handsome pay. You will be receiving an attractive salary package as you pursue a career in Biotechnology. Moreover, the job prospects are promising and as per the recent studies, the employment in Biotechnology sector is expected to increase. Best Biotechnology Recruitment Agency in Hyderabad provides placement support. Biotechnology can support environmental sustainability through synthetic biosynthesis, which employs live organisms like bacteria, fungi, or plants to manufacture fuels, chemicals, and other materials. Producing adequate food for both humans and animals will remain a challenge while the world's population continues to grow at an uncontrollable rate. The World Economic Forum's Council on Biotechnology claims that, although debatable, genetically modified crops can aid in the solution to this issue.
Biotechnology is a field that uses biological systems to produce technological products and systems. Many types of biotechnology professionals collaborate to research and test new biotech innovations, making it a diverse and interesting field to pursue. Along with the technical part and engineering applications, biotechnology is giving rise to various new fields with humongous job opportunities. Biotechnologists are at the forefront of the continuous search to find new, sustainable food sources. They can work in various work environments like industrial sector, medical, food manufacturing, healthcare and pharmaceuticals. Professionals in this field can specialize in one or more subfields like genomics, bioinformatics, and proteomics. Any students with biology, mathematics, physics and chemistry in their 12 can take up Biotechnology as their specialization in their undergraduate course. Biomedical equipment technicians are responsible for overseeing the operation of biomedical machines and tools. They inspect machines according to technical specifications, record any issues and make repairs to surpass inspection requirements. They install software updates and make hardware adjustments to reprogram machines for new uses. Environmental biotechnology serves to create more sustainable crops, as well as to optimize natural resources. Some faculties of microorganisms, fungi, enzymes, and plants are used to recover contaminated ecosystems.
Changing demographics and growing demand for food, fuel and agricultural and environmental sustainability are among the key challenges the world faces today. You will investigate the economic basis for current biotechnology structures and areas of future demand, including the global pharmaceutical industry. You will learn how technology can be applied to solve pressing real-world biological problems and gain the skills and expertise needed for future developments in biotechnology. Within healthcare biotech is already benefiting more than 350 million patients around the world through the use of biotech medicine to treat and prevent every day and chronic illnesses. It also offers new, improved and adapted agricultural crops to reduce poverty and can increase food security for a growing global population. No other sector has the same promise of extraordinary rewards for investors as biotech stocks who will benefit from the new drugs and treatments that are developed. Top Biotechnology Recruitment Agency in Hyderabad provides the best service.
#BiotechnologyRecruitmentAgencyinHyderabad#BestBiotechnologyRecruitmentAgencyinHyderabad#TopBiotechnologyRecruitmentAgencyinHyderabad
0 notes
Text
Global Recombinant Erythropoietin Market Is Estimated To Witness High Growth Owing To Increasing Prevalence of Anemia

The global Recombinant Erythropoietin Market is estimated to be valued at US$ 7.01 billion in 2023 and is expected to exhibit a CAGR of 2% over the forecast period of 2023-2030, according to a new report published by Coherent Market Insights.
Market Overview:
Recombinant erythropoietin (rEPO) is a synthetic form of the hormone erythropoietin, which stimulates the production of red blood cells in the body. It is mainly used to treat anemia associated with chronic kidney disease, cancer chemotherapy, and other conditions that limit the production of red blood cells. The market for recombinant erythropoietin is driven by the increasing prevalence of anemia worldwide. Anemia affects millions of people globally and can lead to fatigue, weakness, and other health complications. Recombinant erythropoietin helps in improving the symptoms of anemia and enhancing quality of life for patients.
Market Key Trends:
One key trend in the recombinant erythropoietin market is the development of biosimilars. Biosimilars are biological products that are highly similar to an already approved reference product in terms of quality, safety, and efficacy. They offer cost-effective alternatives to the original brand-name products, making them more accessible to patients. The increasing demand for affordable treatment options and the expiry of patents for reference products are driving the development and adoption of biosimilars in the recombinant erythropoietin market. For example, companies like Teva Pharmaceutical Industries Ltd., Sandoz International GmbH, and Celltrion Inc. are actively involved in the development of biosimilar versions of recombinant erythropoietin.
PEST Analysis:
Political: The regulatory environment and government policies play a crucial role in the development and approval of recombinant erythropoietin products. Stringent regulations for biosimilar approvals and pricing policies in different regions can impact market growth.
Economic: The increasing healthcare expenditure, particularly in emerging economies, is driving market growth. The rising affordability and accessibility of healthcare services, along with the availability of insurance coverage for treatments, contribute to the demand for recombinant erythropoietin products.
Social: The growing aging population and the rising prevalence of chronic kidney disease and cancer are key social factors driving the demand for recombinant erythropoietin. Additionally, increased awareness and diagnosis of anemia and its consequences are also contributing to market growth.
Technological: Advancements in biotechnology and drug delivery systems are enhancing the efficacy and safety of recombinant erythropoietin products. The development of long-acting formulations and novel delivery methods is improving patient adherence and convenience.
Key Takeaways:
The global Recombinant Erythropoietin Market Growth is expected to witness high growth, exhibiting a CAGR of 2% over the forecast period, due to increasing prevalence of anemia.
North America is the fastest-growing region in the market, owing to the high prevalence of anemia and well-established healthcare infrastructure.
Key players operating in the global recombinant erythropoietin market include Amgen Inc., Johnson & Johnson (Janssen Pharmaceuticals), Pfizer Inc., Roche Holding AG, Novartis AG, Biocon Limited, Teva Pharmaceutical Industries Ltd., LG Chem Ltd., Sandoz International GmbH (a subsidiary of Novartis AG), Intas Pharmaceuticals Ltd., Dr. Reddy's Laboratories Ltd., Celltrion Inc., 3SBio Inc., CJ CheilJedang Corporation, and BioSidus SA. These players focus on strategic collaborations, research and development activities, and product launches to maintain their market position and cater to the growing demand for recombinant erythropoietin.
#Pharmaceutical#Healthcare Industry#Healthcare#Recombinant Erythropoietin#Recombinant Erythropoietin Market
0 notes
Text
Biopharmaceutical Contract Manufacturing Market Is Expected To Generate A Revenue Of USD 17.9 Billion By 2030
The latest market report published by Credence Research, Inc. “Global Biopharmaceutical Contract Manufacturing Market: Growth, Future Prospects, and Competitive Analysis, 2016 – 2028. The global biopharmaceutical contract manufacturing market has grown steadily in recent years. It is expected to grow at a CAGR of 9.5% between 2023 and 2030. The market was valued at USD 8.8 Billion in 2022 and is expected to reach USD 17.9 Billion in 2030.
The biopharmaceutical contract manufacturing market is experiencing significant growth, and key trends are shaping its trajectory. One of the main factors driving this expansion is the increasing demand for specialized expertise in drug development and production. Pharmaceutical companies are increasingly outsourcing their manufacturing processes to contract manufacturers who possess advanced technical know-how and state-of-the-art facilities. Another notable trend is the rising number of partnerships between biopharmaceutical companies and contract manufacturers, fostering innovation through collaboration. This allows both parties to combine their strengths, leading to faster product commercialization and improved patient outcomes. Additionally, advancements in technology have revolutionized the biopharmaceutical sector, enabling more efficient manufacturing processes that can handle complex biological products such as cell therapies or gene therapies.
The biopharmaceutical industry has been witnessing substantial growth due to advancements in biotechnology and the rising prevalence of chronic diseases worldwide. The development of complex biologics and personalized medicine has further fueled the demand for efficient contract manufacturing services.
Outsourcing Trend Boosting Market Expansion
The trend of outsourcing biopharmaceutical manufacturing activities has gained traction in recent years, as pharmaceutical companies seek to focus on their core competencies, such as research and development, while relying on specialized contract manufacturing organizations (CMOs) for manufacturing and production. Outsourcing allows companies to reduce capital investment and operational costs while accessing state-of-the-art facilities and expertise of established CMOs.
Key Market Drivers and Opportunities
The Biopharmaceuticals Contract Manufacturing Market is driven by several key factors, including:
1. Rising Demand for Biologics: The increasing demand for biologics, including monoclonal antibodies, recombinant proteins, and vaccines, has stimulated the need for contract manufacturing services. Biologics offer targeted therapies and novel treatment options for various diseases, prompting pharmaceutical companies to outsource manufacturing to meet the rising demand.
2. Growing Complexity of Biopharmaceuticals: The complexity of biopharmaceutical products requires specialized manufacturing capabilities and stringent quality control measures. Contract manufacturing organizations equipped with advanced technology and regulatory expertise are better positioned to produce these complex biologics efficiently.
3. Advancements in Bioprocessing Technology: Continuous innovations in bioprocessing technologies, such as single-use systems, perfusion bioreactors, and cell culture media, have enhanced the productivity and cost-effectiveness of biopharmaceutical manufacturing. CMOs adopting these advanced technologies gain a competitive advantage in the market.
4. Expansion of Biopharmaceutical Pipelines: The robust growth of biopharmaceutical pipelines, driven by extensive research and development activities, has led to an increased need for contract manufacturing services. As pharmaceutical companies seek to expedite the development and commercialization of their products, outsourcing manufacturing processes becomes a strategic option.
5. Growing Biosimilars Market: The emergence of biosimilars as cost-effective alternatives to originator biologics has boosted their adoption worldwide. Contract manufacturing organizations play a crucial role in the production of biosimilars, contributing to the market growth.
Browse 190 pages report Biopharmaceutical Contract Manufacturing Market By Source (Mammalian, Non-mammalian) By Service (Process Development, Downstream., Upstream, Fill & Finish Operations, Analytical & QC studies, Packaging) - Growth, Future Prospects & Competitive Analysis, 2016 – 2030)- https://www.credenceresearch.com/report/biopharmaceutical-contract-manufacturing-market
North America Leads the Market
North America holds the largest share in the Biopharmaceuticals Contract Manufacturing Market and is expected to maintain its dominance during the forecast period. The region's well-established biopharmaceutical industry, favorable regulatory environment, and significant investments in biotechnology research contribute to its market leadership.
Asia Pacific: A Lucrative Market
Asia Pacific is emerging as a lucrative market for biopharmaceutical contract manufacturing services. The region offers cost-effective manufacturing solutions and a large pool of skilled workforce, making it an attractive destination for outsourcing biopharmaceutical production.
Conclusion
The Biopharmaceuticals Contract Manufacturing Market is witnessing significant growth, driven by the increasing trend of outsourcing and the growing demand for biologics. As pharmaceutical companies focus on innovation and pipeline expansion, contract manufacturing organizations play a crucial role in meeting the manufacturing needs of the industry. North America remains a dominant market, while Asia Pacific presents promising growth opportunities.
Why to Buy This Report-
The report provides a qualitative as well as quantitative analysis of the global Biopharmaceutical Contract Manufacturing Market by segments, current trends, drivers, restraints, opportunities, challenges, and market dynamics with the historical period from 2016-2020, the base year- 2021, and the projection period 2022-2028.
The report includes information on the competitive landscape, such as how the market's top competitors operate at the global, regional, and country levels.
Major nations in each region with their import/export statistics
The global Biopharmaceutical Contract Manufacturing Market report also includes the analysis of the market at a global, regional, and country-level along with key market trends, major players analysis, market growth strategies, and key application areas.
Browse Full Report: https://www.credenceresearch.com/report/biopharmaceutical-contract-manufacturing-market
Visit: https://www.credenceresearch.com/
Related Report: https://www.credenceresearch.com/report/temperature-sensitive-packaging-market
Related Report: https://www.credenceresearch.com/report/seaweed-coated-carton-market
Browse Our Blog: https://www.linkedin.com/pulse/biopharmaceutical-contract-manufacturing-market-share-priyanshi-singh
Browse Our Blog: https://tealfeed.com/biopharmaceutical-contract-manufacturing-market-size-worth-5q5ef
About Us -
Credence Research is a viable intelligence and market research platform that provides quantitative B2B research to more than 10,000 clients worldwide and is built on the Give principle. The company is a market research and consulting firm serving governments, non-legislative associations, non-profit organizations, and various organizations worldwide. We help our clients improve their execution in a lasting way and understand their most imperative objectives. For nearly a century, we’ve built a company well-prepared for this task.
Contact Us:
Office No 3 Second Floor, Abhilasha Bhawan, Pinto Park, Gwalior [M.P] 474005 India
0 notes
Text
Chromatography Resin Market In-Depth Analysis with Booming Trends Supporting Growth and Forecast 2023 to 2033
ESOMAR-certified consulting firm Future Market Insights’ report projects the global chromatography resin market to rise at an impressive pace between 2021 and 2031. Growing adoption of affinity chromatography resin technology is expected to increase the demand of chromatography resins across the globe. Alongside this, increase in demand for monoclonal antibody production will improve the sales over the coming years.
Demand for chromatography resins is rising extensively within the biopharmaceutical companies and research institutes. Burgeoning adoption of synthetic chromatography resin for the separation of biosimilars and development of monoclonal antibodies will spur the growth.
As per the FMI’s analysis, affinity chromatography resin sales are set to grow at over 11.3% CAGR, to surpass a valuation of approx. US$ 879.6 Mn by 2031-end. However, stringent regulatory policies, availability of cost-effective alternatives, and high cost of the chromatography technologies are some of the factors limiting the market growth.
Get Latest Sample Copy@ https://www.futuremarketinsights.com/reports/sample/rep-gb-1349
Increasing funding by governments and initiatives undertaken to curb the mortality rate due to non-communicable diseases will spur the adoption of chromatography resins across the globe. Also, increasing expenditure on the research & development of drug delivery among the industry giants will also boost the market demand.
According to the FMI’s analysis, the global chromatography resins market is anticipated to reach a valuation of nearly US$ 2215 Mn, registering a steady growth at 6.90% CAGR between 2021 and 2031.
“Increasing research & development activities aimed at the development of economic monoclonal antibody drugs within the biopharmaceutical companies are expected to provide lucrative opportunities for the market players,” says the FMI analyst.
Competitive Landscape
Agilent Technologies, Bio-Rad Laboratories Inc., GE Healthcare, Waters Corporation, Expedeon Ltd., Thermo Fisher Scientific Inc., Pall Corporation, Merck KGaA, PerkinElmer Inc., Kaneka Corporation, Shimadzu Corporation, Knauer GmbH, Tosoch Bioscience, Avantor Performance Materials Inc., Mitsubishi Chemical Corporation, W.R. Grace & Co., JSR Micro Inc., and Life Technology Corporation among others are some key players operating in the chromatography resin market.
As per the FMI’s analysis, the market is dominated by top 5 players in the global chromatography resin market, accounting for over 70% of market share. Top 5 players include Bio-Rad Laboratories, Inc., GE Healthcare, Tosoh Corporation, Merck KGaA, and Thermo Fisher Scientific Inc.
Market players operating in chromatography resins market are also forming tie-ups and targeted mergers & collaboration with other top healthcare companies for the expansion of their product portfolios.
For instance, in February 2021, Repligen Corporation, a global life sciences company focused on bioprocessing technology leadership, and Navigo Proteins GmbH, a prominent protein engineering company specializing in novel affinity ligand development, announced the commercial launch of NGL COVID-19 Spike Protein Affinity Resin, a novel affinity resin used in the purification of COVID-19 vaccines.
On February 4th, 2021 Avitide, another leading player, finalized the development of an affinity bioprocess resin, AVIPure-COV2S, for the purification of recombinant COVID-19 vaccines.
Ask from Market Research Expert – https://www.futuremarketinsights.com/ask-question/rep-gb-1349
More Insights on the Global Chromatography Resin Market
In its latest report, FMI provides an incisive coverage on the global chromatography resin market, providing historical data for the period of 2016-2020 and forecast statistics for the period of 2021-2031. In order to understand the global market potential, its growth, and scope, the market is segmented on the basis of product type (native and synthetic), technology type (affinity chromatography, anion exchange chromatography, cation exchange chromatography, size exclusion, and hydrophobic interaction), end user type (biopharmaceutical companies, clinical research organizations, and academic institutes), and across major regions (North America, Latin America, Eastern Europe, Asia Pacific excluding Japan, Western Europe, Japan and Middle East & Africa)
Chromatography Resin Market by Category
By Product Type:
Native
Synthetic
By Technology Type:
Affinity Chromatography
Anion Exchange Chromatography
Cation Exchange Chromatography
Size Exclusion
Hydrophobic Interaction
By End User Type:
Biopharmaceutical Companies
Clinical Research Organizations
Academic Institutes
0 notes
Text
Recombinant Human Epidermal Growth Factor (EGF) Market is Estimated to Witness High Growth Owing to Wide Range of Applications

Recombinant human epidermal growth factor (EGF) is a peptide growth factor that regulates cell proliferation, differentiation and survival. EGF is important for processes like wound healing by stimulating cell division and growth. Recombinant EGF produced through recombinant DNA technology mimics human EGF and is used extensively in cell culture applications. EGF promotes the growth, proliferation, and survival of various cell types, including epithelial cells and fibroblasts. It finds applications in cell culture media, wound healing products and tissues engineering due to its abilities to stimulate cell growth and proliferation.
The global recombinant human EGF market is estimated to be valued at US$ 10,251.1 Mn in 2023 and is expected to exhibit a CAGR of 9.3% over the forecast period 2023 to 2030, as highlighted in a new report published by Coherent Market Insights.
Market Opportunity:
The wide range of applications of recombinant EGF offers significant growth opportunities in the market. EGF is extensively used in cell culture applications to promote cell growth. It stimulates the proliferation of fibroblasts, keratinocytes, endothelial cells and hepatocytes and is routinely added to cell culture media. The growing biosimilars industry and cell therapy development is expected to drive the demand for recombinant EGF as it is widely used in cell expansion processes. EGF promotes wound healing by stimulating cell proliferation and growth of fibroblasts and keratinocytes involved in wound repair. The rising prevalence of chronic wounds worldwide represents major opportunity for recombinant EGF products in wound care management over the forecast period.
Porter's Analysis
Threat of new entrants: Low economies of scale and capital requirements limit threat of new entrants in the recombinant human EGF market. However, intellectual property rights can pose a barrier.
Bargaining power of buyers: Large buyers in the pharmaceutical and biotechnology industries can negotiate lower prices. However, specialized nature of products limits switching.
Bargaining power of suppliers: A few global players supply recombinant human EGF, giving them significant influence over prices. However, intellectual property also provides suppliers power over market.
Threat of new substitutes: Substitutes are limited as recombinant human EGF has sought after properties for cell culture and research. However, ongoing innovation could introduce new substitutes.
Competitive rivalry: Key players compete on quality, reliability of supply and price. However, market is characterized by partnerships and licensing between players.
SWOT Analysis
Strengths: Recombinant human EGF finds wide applications in cell culture and research. Established distribution networks of key players.
Key Takeaways
The global recombinant human EGF market is expected to witness high growth over the forecast period supported by ongoing research in pharmacology, biotechnology and stem cell therapy. Key regional trends include the Asia Pacific market expected to grow at a high rate during the forecast period owing to increasing government investments in life sciences research in countries like China and India and presence of contract research organizations. Europe is also a lucrative market for recombinant human EGF driven by initiatives to boost research.
Key players operating in the recombinant human EGF market are Thermo Fisher Scientific, FUJIFILM Irvine Scientific, Inc., ScienCell Research Laboratories, R&D Systems, Abcam PLC, Cell Sciences, Inc., Eurofins DiscoverX, PeproTech, Inc., RayBiotech, Inc., Prospec-Tany TechnoGene Ltd., Miltenyi Biotec, Tonbo Biosciences, BioLegend, Inc., EnQuire BioReagents, STEMCELL Technologies Inc., Cell Guidance Systems Ltd., Creative BioMart, Sino Biological Inc., and BioVision, Inc.
0 notes