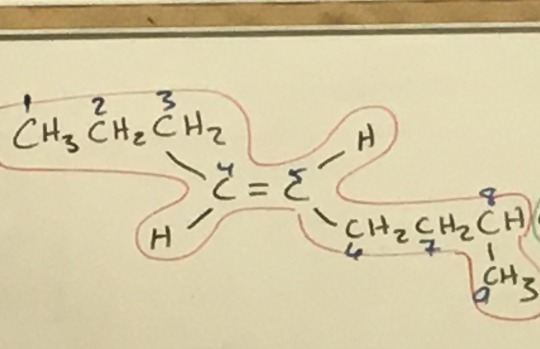
#and trans alkenes
Text
An unintended consequence of partial hydrogenation of the alkene groups in polyunsaturated oils is the isomerisation of a small proportion of the cis alkenes to trans alkenes. These are known as trans-fats and are of concern as they are believed to increase the risk of coronary heart disease. These trans-fats do occur naturally, although only to a small extent. Canola oil naturally has a relatively high level of trans-fats, but most other natural oils have very little trans-fat. Lamb and mutton also naturally contain moderate levels of trans-fats.
"Chemistry" 2e - Blackman, A., Bottle, S., Schmid, S., Mocerino, M., Wille, U.
#book quote#chemistry#nonfiction#textbook#unintended consequences#hydrogenation#alkene#polyunsaturated#oils#isomerization#cis#trans#trans fat#coronary heart disease#risks#canola oil#natural oil#lamb#mutton
2 notes
·
View notes
Text
Look, it’s me!
0 notes
Text
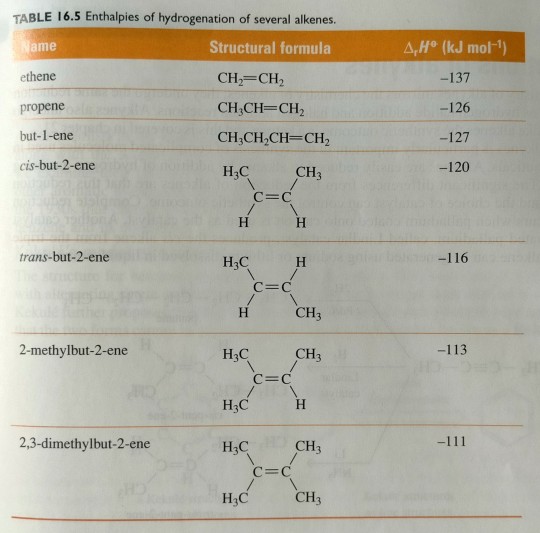
Three important points follow from the information in table 16.5.
The reduction of an alkene to an alkane is an exothermic process. This observation is consistent with the fact that, during hydrogenation, there is net conversion from weaker π bonding to stronger σ bonding; that is, one σ bond (H-H) and one π bond (C=C) are broken, and two new σ bonds (C-H) are formed.
The enthalpies of hydrogenation depend on the degree of substitution of the carbon-carbon double bond: the greater the substitution, the lower the enthalpy of hydrogenation. Compare, for example, the enthalpies of hydrogenation of ethene (no substituents), propene (one substituent), but-1-ene (one substituent) and the cis and trans isomers of but-2-ene (two substituents each).
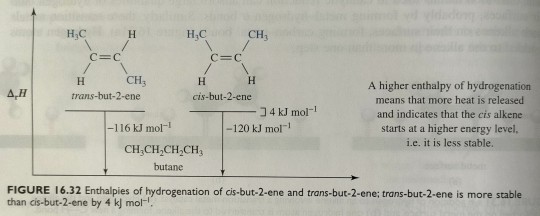
The enthalpy of hydrogenation of a trans alkene is less than that of the isomeric cis alkene. Compare, for example, the enthalpies of hydrogenation of cis-but-2-ene and trans-but-2-ene. Because the reduction of both alkenes gives butane, any difference in their enthalpies of hydrogenation must be due to a difference in relative energy between the two alkenes (figure 16.32). The alkene with the lower (less negative) value of ∆rH° is the more stable alkene. We explain the greater stability of trans alkenes relative to cis alkenes in terms of nonbonded interaction strain. In cis-but-2-ene, the two -CH3 groups are sufficiently close to each other that there is repulsion between their electron clouds. This repulsion is reflected in the larger enthalpy of hydrogenation (decreased stability) of cis-but-2-ene compared with that of trans-but-2-ene.
"Chemistry" 2e - Blackman, A., Bottle, S., Schmid, S., Mocerino, M., Wille, U.
#book quotes#chemistry#nonfiction#textbook#alkene#chemical reactions#ethene#propene#butene#methylbutene#dimethylbutene#enthalpy#cis#trans#stability#hydrogenation#hydrocarbons
1 note
·
View note
Text
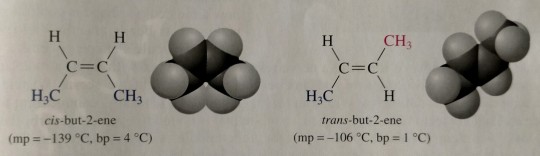
Because of restricted rotation around a carbon-carbon double bond, an alkene in which each carbon of the double bond has two different groups bonded to it shows cis-trans isomerism.
"Chemistry" 2e - Blackman, A., Bottle, S., Schmid, S., Mocerino, M., Wille, U.
#book quote#chemistry#nonfiction#textbook#restriction#rotation#chemical bonding#carbon#hydrogen#hydrocarbons#alkene#cis#trans#isomer#butene
0 notes
Text
In a huge L for cis people Twitter is banning the word "cis", as Elon, a man so transphobic his trans daughter pulled a full name change, thinks it's a slur. Here are some fun alternatives for cisgender people on Twitter who don't want their identity erased by a rich guy:
zis. Cisgender was originally coined as "Zissexuell" by German sexologist Volkmar Sigusch. Go back to your roots cis people, embrace the zis!
C#. For our musically inclined cis pals, consider adopting this note as part of your label!
seppies. For the Star Wars fans! Members of the Confederacy of Independent systems, short C.I.S., are commonly called "seppies" or "seps" by the Clone Troopers. If you're a cis Star Wars fan you can embrace your gender and make a stand against the corrupt Republic in one fell swoop!
Z. As many annoyed chemistry students know cis/trans isomerism nomenclature can only take you so far. Some alkenes have too many different substituents to be effectively called cis or trans. Enter: Cahn-Ingold-Prelog rules! With these you can effectively name alkenes as either Z (equivalent to cis) or E (equivalent to trans). Wanna boast your science skills at your nerdy friends, cis people? Call yourself a Z person and talk about organic chemistry! (May alienate your friends, OC sucks)
28 notes
·
View notes
Text
okay so the wifi i'm on right now is shitting itself despite this being a popular study spot on campus but REGARDLESS i'm studying for my third organic chemistry midterm right now (my class grade is a 90.2% so i'm barely hovering around an A and i'd REALLY like to keep it that way as unrealistic as that may be) and as much as i am absolutely loving this class i'm struggling a little with remembering all of the mechanisms (final exam review sheet posted by professor indicates 40 different reactions we need to know).
granted, a lot of them share the same patterns, but i don't know what format i'll need to compare-contrast all of them in in order to have them down pat for may 2. so right now i'm just focusing on this exam, where we've gotta know
radical substitution of alkanes and allylic substitution, the funky alkyl halide ones with the fishhook arrows. the simplified mechanism looks like halogenation except these are done with heat/light. these go through three stages: initiation from nonradical to radical, propagation from radical to radical, and termination from radical to nonradical. you get your radical intermediate via homolytic cleavage, you react it with a hydrogen coming off of the most substituted carbon, you get your next radical intermediate once the hydrogen is gone, you react all your radicals together to tie things up nicely. if your radical is on a carbon that neighbors a double bond, you have a nice stable allylic radical (thank you resonance), but if your radical is on a double bond, that's a vinyl radical and we don't like those... you also gotta pay attention to where your alkene is because that'll affect your stability too. if you're happening to do your reaction with peroxides, you'll be reacting your intermediate with the less substituted area. a halogen? on MY terminal end?
elimination and substitution reactions Sn1/Sn2/E1/E2. these are silly and complicated and i need to make a chart to understand them better, but the substitution reactions will substitute a nucleophile in for the leaving group, and the elimination reactions will make an alkene out of an alkane using a base, and the 2-reactions are concerted with steric barriers while the 1- reactions have a carbocation intermediate with stability as the barrier.
alkyne reactions, including deprotonation (basically acid-base shit), alkyne formation (using acetylene, which makes good internal alkenes and looks like Sn2, or dibromides, which looks like two E2 reactions and can be done with bromides on the same carbon or on adjacent carbons), halogenation (nonregioselective concerted anti-addition of halogens using Br2/Cl2), hydrohalogenation (markovnikov addition of Br, can happen twice if you have two equivalents of HBr, carbocation intermediate), hydration and hydroboration-oxidation (markov and anti-markov reactions, respectively; adding a hydrogen and an OH, keto-enol tautomerism where you end up with the double bond on the oxygen), and three reduction reactions (Pd/C yields alkane, Lindlar/H2 yields a cis(z)alkene, Na/NH3 yields a trans(E) alkene).
anyway i have practice problems to do and the sn1/sn2/e1/e2 reaction comparison chart to make but i'm drinking my first monster energy (ultra paradise. it's good) and i'm getting dinner w at least one of my friends in 25 minutes... calc homework still due tonight but i think i'll be able to get it... exam in 3 hours... feeling good!
#a rare brain dump from puzzlehat but making notecards was not being an efficient way to study lawl#perhaps i should make a studyblr...
5 notes
·
View notes
Note
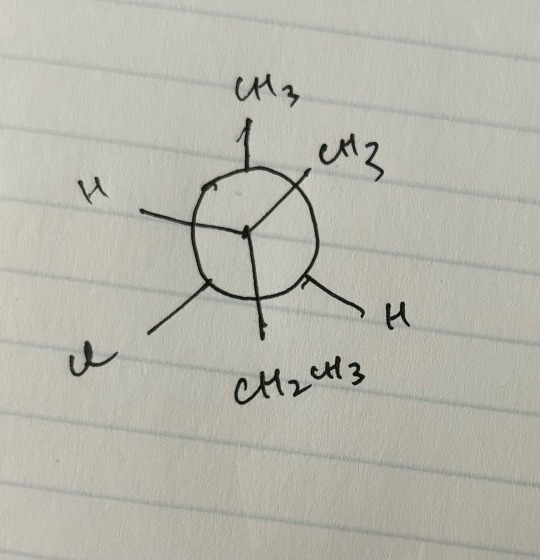
but wait bestie I’m sure that B is trans, since the methyl groups are opposite each other directly and that C is cis because the methyl groups on the alkene carbons are both in the “same” direction (“upwards”?) Why do you say B is cis and C is trans? or is there smth im not seeing hehehh
according to me, B) is cis because the CH2CH3 is given priority over the methyl group while comparing if right and left are on the same side.
a mutual helped me figure it out. so for E2 mechanism, the H needs to be anti-periplanar but in this case it’s not (see image below). so you rotate the bonds to make it anti-periplanar. we wouldn’t have to do so if the Cl was drawn with a wedge instead of a dash, and in that case, the answer would be C).
thank you for tryna help bestie, i appreciate it <3
2 notes
·
View notes
Text
its two in the morning and i just finished a lecture on . idfk. alkene conformation and cis-trans isomers and haskell very gaily explained that the origin of these terms was roman and referred to something either across the river or on the same side of the river as the speaker. and i forgot because this is a two week lab i dont have a postlab report due before class and i dont have to write a procedure. i am so blessed.
3 notes
·
View notes
Text
everything is woke these days smh they didn't even spare chemistry🙄 what do you mean "cis" alkene and "trans" alkene
2 notes
·
View notes
Text
doing cis-trans isomer conversions of alkenes in class and confusing my profs by putting the letters 'HRT' onto every conversion arrow
0 notes
Text
Irradiation at 300 nm converts the trans cinnamide side chain of structure 2 to the cis isomer of structure 3, while the reverse process occurs at 254 nm.
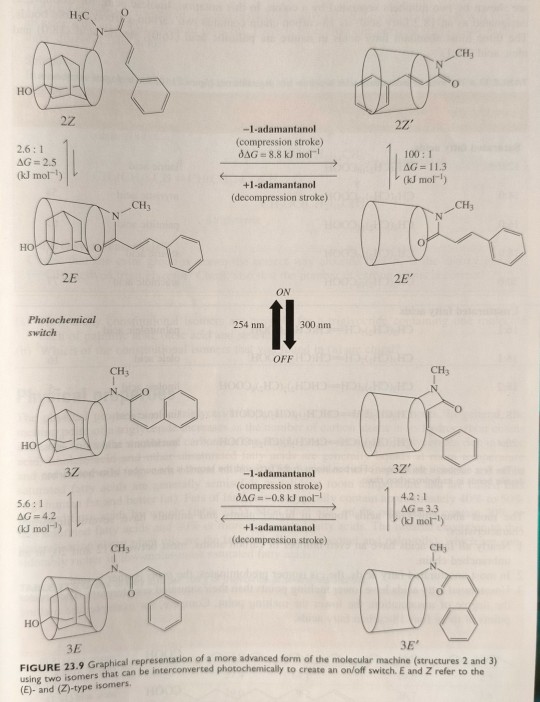
In this case, however, the orientation of the cis double bond prevents the side chain inserting into the cavity and so in that mode the machine is turned off. By contrast, the trans alkene moiety does allow insertion, and so in this mode the machine is turned on. (...) The photoisomerisation of the trans cinnamide side chain of structure 2 and the cis isomer of structure 3 provides the machine with an on/off switch.
"Chemistry" 2e - Blackman, A., Bottle, S., Schmid, S., Mocerino, M., Wille, U.
#book quote#chemistry#nonfiction#textbook#irradiation#trans#cinnamide#isomer#on off#double bond#alkene#moiety#insertion#nanotechnology#machine#photochemistry#uv light#ultraviolet light
2 notes
·
View notes
Text
Today I partecipated in a chemistry competion where since my category bundles 5th year as well, it's bound to have topic that i didn't do yet, and ok.
But what the fuck is cis-trans alkenes isomerism???
0 notes
Text
i learned today at school that cis and trans are also like chemistry terms(we're learning about alkenes)
0 notes
Link
0 notes
Text
Sella Turcica Abnormalities, Dental care Grow older and also Dentistry Problems inside Shine Young children.
This research targeted to research the actual possibility associated with populace pharmacokinetic custom modeling rendering for you to assess the rate involving gastric emptying (GE) and also little intestinal tract transit occasion (Web site) as a result of medicines which affect Uniform motility inside fed as well as fasted pet dogs. Paracetamol and also sulfapyridine (sulfasalazine metabolite) pharmacokinetics were chosen while guns pertaining to Kenmore and SITE, respectively. Approaches: In 2 distinct scientific studies, under raised on along with fasted conditions, half a dozen guy beagle dogs acquired the Fifteen minutes intravenous infusion of car, atropine (0.July mg/kg) or erythromycin (A single mg/kg) as well as the intragastric supervision of a mixture of paracetamol (Twenty four mg/kg) and sulfasalazine (Twenty mg/kg). Foods was given ahead of or even with 6 following medication supervision within the fed and fasted examine, respectively. Blood samples had been collected regarding evaluation regarding paracetamol as well as sulfapyridine within plasma televisions. Inhabitants pharmacokinetic examination of paracetamol along with sulfapyridine throughout plasma televisions was utilized to determine the charge associated with Kenmore and SITE. Benefits: The actual quantitative parameter quotations shown expose and also important affect of atropine, erythromycin and food about General electric and SITE Compared to fasted situations intake of food postponed Whirlpool inside pharmacologically dealt with pet dogs and SITE has been reduced soon after therapy with car or truck or erythromycin. Atropine considerably overdue Whirlpool within given as well as fasted problems however the effect on SITE was evident just underneath provided condition. Erythromycin, in comparison#keep##links#, increased Whirlpool only inside fasted situations, and usually overdue Website Discussion: Inhabitants pharmacokinetic modeling regarding paracetamol and sulfapyridine offers a ideal preclinical non-invasive fresh way of quantification of drug- and food-induced alterations in the rate associated with Kenmore and SITE within informed beagle canines for usage safely evaluations to predict modifications in Uniform transit and/or to spell out your pharmacokinetic profile of medication underneath advancement. (H) The new year Elsevier Incorporated. Almost all protection under the law MLN8237 earmarked.Even though steady in CH(A couple of)Craigslist(2), hexane or even THF, inside the presence of#keep##links# MeOH, self-promoted dimerization with the triarylphosphine-alkene A single, any ligand regarding Pd-catalyzed responses, created a rare racemic bis(phosphine) Only two within substantial generate. The response of two with Pd(dba)(Only two), as well as oxidative inclusion of p-IC(Some)They would(Some)NO(A couple of), exhibited a new trans-chelated Pd(The second) aryl iodide sophisticated.Single measure involving diethylcarbamazine (12 ,) found in manage programs works throughout damaging the transmitting regarding filariasis. As a way to investigate effect of ambitious treatment on Wuchereria bancrofti (Wb) microfilariae, DEC was given for you to 28 individuals who were positive for the going around filarial antigen (CFA) assay however did not have clinical manifestations associated with filariasis, at Some mg/kg/day for Twelve nights and also once more six months later on utilizing the same dosing routine. (h) '08 Elsevier Incorporated. Just about all protection under the law reserved.
#PR-171#LBH589#MLN8237#LXH254#CL13900#VX-445#AG-221#PF-02341066#DS-3201#NN2211#CX-5461#IACS-10759#GSK1349572#RP56976#SAG#ICI 46474#MC3#E-616452#NSC 127716#JNJ-42756493#BI 10773#VP-16#PF-6463922#GW-572016#AZD1152-HQPA#SCH727965#VX-661#GDC-0973#INCB028050#GSK1265744
0 notes