#burette volume
Text
Measured Volume Burette Set Market Report: Emerging Applications and Future Prospects
The global measured volume burette set market size is expected to reach USD 804.7 million by 2030. The burette sets are intended specifically for the gravity-based administration of precise volumes of infusion fluid. Burette sets with a flexible, clear, and calibrated measured volume container are infusion tools used for precise measurement. Burette chamber is scaled for precise medication measurement for treating chronic illness, newborn and pediatric treatment.
Gain deeper insights on the market and receive your free copy with TOC now @: Measured Volume Burette Set Market Report
The growing global incidence of chronic diseases like cancer and diabetes is driving up demand for measured volumetric burettes. These devices are being adopted by hospitals and other healthcare institutions to provide patients with large volumes of medications and nutrients. Major life sciences and diagnostics companies like Thermo Fisher, B. Braun, Baxter, and others are also expected to support the market's expansion.
During the forecast period, the market is anticipated to be driven by the expanding use of measured volume burettes for paediatric or neonatal treatment. For treating paediatric patients, healthcare centers and other medical establishments commonly use volumetric burette infusion sets. When treating pediatric patients, the burette IV sets, which allow the provider to dispense a precise and controlled quantity of medicine, can be extremely important. Products like Dosifix, a device made available by Braun, are intended for use in treating paediatric and neonatal patients.
#Measured Volume Burette Set Market Size & Share#Global Measured Volume Burette Set Market#Measured Volume Burette Set Market Latest Trends#Measured Volume Burette Set Market Growth Forecast#COVID-19 Impacts On Measured Volume Burette Set Market#Measured Volume Burette Set Market Revenue Value
0 notes
Text
Training Lab Personnel on the Proper Use of Liquid Handling Instruments
Liquid handling instruments, such as pipettes, dispensers, and burettes, are essential tools in laboratories across various scientific disciplines. Proper use of these instruments is crucial for accurate and reliable experimental results. Training lab personnel on the proper handling techniques ensures consistent performance, prevents errors, and protects valuable samples.
Importance of Proper Technique
Accuracy and Precision: Correct use of liquid handling instruments guarantees accurate and precise transfer of liquids, preventing experimental errors and ensuring data reliability.
Sample Integrity: Proper handling techniques minimize contamination risks, preserving the integrity of valuable samples.
Instrument Longevity: Correct use extends the lifespan of liquid handling instruments, reducing the need for frequent repairs or replacements.
Safety: Proper techniques help prevent accidents and injuries, ensuring a safe working environment for laboratory personnel.
Key Training Areas
Instrument Selection:
Match instrument type (micropipettes, micropipettes, dispensers) to the volume range and liquid properties.
Consider factors like accuracy, precision, and ease of use.
Calibration and Maintenance:
Regular pipette calibration ensures accurate dispensing.
Teach proper cleaning and maintenance procedures to prevent contamination and prolong instrument life.
Pipetting Techniques:
Demonstrate correct pipetting techniques, including:
Plunger pressure and speed
Tip immersion depth
Avoiding air bubbles
Proper tip disposal
Emphasize the importance of using appropriate pipette tips for different liquid types.
Dispenser Usage:
Explain the proper operation of dispensers, including:
Setting the desired volume
Filling the reservoir
Dispensing the liquid
Cleaning and maintenance
Address specific considerations for different dispenser types (e.g., serial dispensers, repetitive pipettes).
Burette Handling:
Demonstrate the correct use of E-Burette for titration and other applications.
Teach proper filling, reading, and draining techniques.
Emphasize the importance of avoiding parallax errors when reading the meniscus.
Troubleshooting and Error Correction:
Provide guidance on common issues, such as leaks, air bubbles, and inaccurate dispensing.
Teach troubleshooting techniques to identify and resolve problems effectively.
Training Methods
Hands-on Practice: Encourage trainees to practice using liquid handling instruments under supervision.
Demonstrations: Provide clear demonstrations of proper techniques.
Role-Playing: Simulate laboratory scenarios to reinforce learning.
Quizzes and Assessments: Evaluate trainees' understanding and competency.
Online Resources: Offer access to online tutorials and resources for additional learning.
By providing comprehensive training on the proper use of liquid handling instruments, laboratories can ensure accurate, reliable, and safe experimental procedures. Investing in training not only improves data quality but also contributes to a positive and productive laboratory environment.
#liquid handling instruments#liquid handling#micropipette#pipettes#bottle top dispensers#E-Burette#pipette tips
0 notes
Text
Shop Chemistry Supplies for Students: Essential Tools for Academic Success

Chemistry is a fundamental subject that forms the backbone of scientific understanding and innovation. Whether students are just starting to explore the basics or diving into advanced topics, having the right chemistry supplies is crucial for their success. Shop Chemistry supplies for students At Go Science Crazy, we understand the importance of quality tools and materials for educational achievement. In this article, we will explore the essential chemistry supplies that every student should have and how to choose the best options to enhance learning.
1. Essential Chemistry Lab Equipment
When it comes to chemistry, having the proper lab equipment is vital for conducting experiments safely and effectively. Here are some essential items every student should have:
Beakers and Flasks: These are used for mixing, heating, and storing liquids. Graduated beakers are especially useful for measuring liquids accurately. Erlenmeyer flasks, with their narrow necks, help in swirling solutions without spillage.
Pipettes and Burettes: Precision is key in chemistry. Pipettes are used for transferring small volumes of liquid, while burettes are essential for titrations, allowing for the precise addition of solutions.
Test Tubes: Versatile and durable, test tubes are ideal for conducting small-scale reactions and observing changes. They come in various sizes and are often used in conjunction with a test tube rack.
Bunsen Burners: Used for heating substances, Bunsen burners are a staple in chemistry labs. They provide a controlled flame that can be adjusted for various heating needs.
Safety Equipment: Safety goggles, gloves, and lab coats are non-negotiable in any chemistry lab. They protect students from harmful chemicals and accidental spills, ensuring a safe learning environment.
2. Chemicals and Reagents
Having a well-stocked inventory of chemicals and reagents is crucial for performing a variety of experiments. Some common chemicals and reagents include:
Acids and Bases: Solutions like hydrochloric acid, sulfuric acid, sodium hydroxide, and acetic acid are frequently used in chemical reactions and titrations.
Indicators: Indicators such as phenolphthalein and litmus paper are essential for determining the pH of solutions and understanding the acidic or basic nature of substances.
Salts and Solvents: Sodium chloride, potassium permanganate, and ethanol are examples of salts and solvents used in various experiments.
It’s important for students to handle all chemicals with care and follow safety guidelines to avoid accidents and ensure accurate results.
3. Scientific Notebooks and Calculators
Proper documentation and calculations are essential for any scientific experiment. Here’s why students need these supplies:
Scientific Notebooks: A good scientific notebook helps students record their observations, procedures, and results accurately. This documentation is crucial for analyzing data and drawing conclusions.
Calculators: Chemistry often involves complex calculations, including concentration, molarity, and reaction yields. A scientific calculator with functions for these calculations can be an invaluable tool.
4. Choosing the Right Chemistry Supplies
When shopping for chemistry supplies, consider the following tips to ensure you select the best products:
Quality and Durability: Choose equipment and chemicals from reputable brands known for their quality and reliability. Durable materials will withstand frequent use and ensure consistent performance.
Safety Compliance: Ensure that all safety equipment and chemicals meet industry standards and safety regulations. Properly certified products will provide better protection and reliability.
Budget Considerations: While it’s important to invest in quality supplies, there are also budget-friendly options available. Look for suppliers that offer competitive pricing without compromising on quality.
5. Where to Shop for Chemistry Supplies
Go Science Crazy is your go-to destination for all your chemistry supply needs. Our extensive selection includes everything from basic lab equipment to advanced chemicals and safety gear. With a focus on quality and affordability, we provide students with the tools they need to excel in their chemistry studies. Explore our range of products and find the perfect supplies for your academic journey.
Conclusion
Equipping students with the right chemistry supplies is essential for fostering a productive and safe learning environment. By investing in quality lab equipment, chemicals, and safety gear, students can enhance their understanding of chemistry and achieve academic success. Visit Go Science Crazy today to shop for the best chemistry supplies and set your students up for success in their scientific endeavors.
0 notes
Text
Your Guide to Chemistry Lab Glassware

When it comes to scientific research and experimentation, having the right tools is crucial. Among these tools, chemistry lab glassware holds a special place.
Reliable, durable, and versatile, glassware is fundamental to any well-equipped laboratory. The fate of your experiment hangs in the balance, and the right chemistry lab glassware can tip the scales. From beakers to flasks, every piece of equipment plays a vital role in the scientific pursuit.
The Importance of Chemistry Lab
Scientists can't get much done without reliable lab glassware - it's the foundation upon which experiments are built, observations are made, and breakthroughs are achieved. You can't help but feel the weight of scientific progress when you're surrounded by it - it embodies the dreams, triumphs, and setbacks of chemical pioneers.
One reason glassware dominates the market is its trifecta of benefits: exceptional chemical resistance, remarkable thermal strength, and an affordable price tag that doesn't break the bank. Transparent glassware takes center stage in the lab, allowing learners to track even the subtlest changes in reactions and crystallization processes - and actually see the science unfold.
Types and Uses of Chemistry Lab Glassware
Chemistry lab glassware comes in many shapes and sizes, each designed for specific functions. Here's a look at some of the most commonly used glassware in laboratories:
Beakers: These are simple cylindrical containers used for mixing, stirring, and heating chemicals. Laboratory basics wouldn't be the same without them - they come in a range of sizes and are necessary for everyday lab tasks.
Flasks: Including Erlenmeyer and volumetric flasks, these are used for mixing chemicals and conducting reactions. Erlenmeyer's narrow neck safeguards your mixture, fending off evaporation, whereas volumetric flasks guarantee precision in every measurement.
Pipettes and Burettes: These are used for measuring and transferring precise volumes of liquids. When you're mixing chemicals, precision is everything, and burettes come in handy for accurate measurements during titrations.
Test Tubes: Commonly used for holding small samples, test tubes are essential for qualitative experiments and analysis.
Watch Glasses: These are used as lids for beakers, for evaporating liquids, and as containers for small amounts of solid substances.
Desiccators: These are used for drying substances or keeping them dry. They are essential for maintaining the integrity of hygroscopic chemicals.
Why Choose Chemglass?
Reliable lab glassware is a must-have for any chemist, and Chemglass reliably fills that need. Flawless quality is what you can expect from their products - no imperfections to worry about, just consistent results.
Chemglass glassware is designed to withstand rigorous laboratory conditions, including vacuum applications for multiple hours without leaks, thanks to its thick-walled construction. Whether you need beakers, flasks, pipettes, or desiccators, Chemglass products are crafted with precision to ensure accuracy and reliability in your work.
Their commitment to quality means you can trust Chemglass glassware to deliver consistent and dependable performance, allowing you to focus on your research without worrying about equipment failure.
Elevate Your Laboratory Experience
For scientists, technicians, and researchers, the right chemistry lab glassware is not just a tool but a critical component of their work. The clarity, durability, and versatility of glassware like that from Chemglass enable accurate observations and reliable results. Your research depends on the best tools, and high-quality glassware is no exception – with it, you'll see tangible progress and achievements.
If you're looking for cost-effective options that meet all your experimental needs, consider exploring their lab glassware collection at HiTechTrader. Their range of products ticks all the boxes for durability, precision, and affordability, ensuring you have the best tools at your disposal.
Don't compromise on quality—equip your laboratory with the best chemistry lab glassware and see the difference it makes in your scientific endeavors.
For more information about Gas Chromatography Machine and Analytical Chemistry Equipment please visit:- Hi Tech Trader
0 notes
Text

Labtron Automatic Potentiometric Titrator LPTT-A10, with an integrated burette valve design, comprises a volume titration device, control device,and measurement device. It performs acid-base, redox, non-aqueous, and precipitation titrations, operating between 0 to 100 °C.
0 notes
Text
Unveiling the Essentials: Acid-Base Titration Equipment in the Laboratory
Acid-base titration is a fundamental technique in analytical chemistry, widely employed for determining the concentration of acids or bases in a solution. The precision and accuracy of titration rely heavily on the quality and functionality of the equipment used.

In this blog, we’ll explore the essential acid-base titration equipment that every laboratory should have.
1. Burettes: The Precision Dispensers Burettes are indispensable in titration, allowing for precise measurement and dispensing of titrant. Made of glass with graduated markings, burettes ensure accurate volume readings down to the fraction of a milliliter.
2. Pipettes: Delivering Accuracy Drop by Drop For transferring precise volumes of liquids, pipettes come into play. Volumetric and graduated pipettes enable the careful addition of reactants, a critical aspect in achieving accurate titration results.
3. Titration Flasks: The Reaction Vessels Titration flasks, often conical in shape, are designed for the controlled mixing of the sample and titrant. The narrow neck minimizes solution splashing during the titration process.
4. pH Meter: Gauging the Acidic or Basic Realm A pH meter is crucial for monitoring the pH level of the solution during titration. This digital instrument provides real-time data, ensuring that the endpoint is precisely determined.
5. Indicator Solutions: The Colorful Clues Indicators, such as phenolphthalein or methyl orange, are essential for visually signaling the endpoint of the titration. Their color changes signify the completion of the reaction.
6. Analytical Balance: Weighing with Utmost Precision Accurate measurements start with precise weighing. An analytical balance is used for obtaining the exact quantities of reactants, a critical factor in titration calculations.
7. Stirring Equipment: Ensuring Homogeneous Mixtures Homogeneity in the reaction mixture is vital. Magnetic stirrers or stirring rods assist in achieving uniform mixing during titration.
8. Safety Equipment: Protecting the Analyst Safety goggles, gloves, and a fume hood are essential to ensure the well-being of the analyst. Some titrants and reagents used in acid-base titration can be hazardous, emphasizing the need for a safe working environment.
Conclusion: Mastering Titration with the Right Tools In the world of analytical chemistry, precision and accuracy are paramount. The proper selection and use of acid-base titration equipment contribute significantly to the reliability of results. Laboratories equipped with quality instruments not only ensure accurate analyses but also create a foundation for advancements in scientific research and industrial quality control. The journey through titration becomes smoother and more insightful when guided by the right set of tools.
0 notes
Text
Ensuring Accuracy: Pipette Calibration, Burette Calibration, and Beaker Calibration in Dubai, UAE
Maintaining the integrity of your laboratory measurements is crucial for reliable research and quality control. This is where pipette calibration, burette calibration, and beaker calibration become essential practices. Dubai, UAE, boasts a range of calibration service providers to ensure your volumetric instruments meet the highest standards.
The Importance of Calibration
Volumetric instruments like pipettes, burettes, and beakers are the workhorses of many laboratories. Over time, due to wear and tear or even minor manufacturing inconsistencies, their accuracy can drift. Regular calibration helps identify and rectify these deviations, guaranteeing the validity of your measurements.
Pipette Calibration: Pipettes are used for transferring precise volumes of liquids. Inaccurate pipetting can significantly impact your results. Calibration ensures each pipette delivers the intended volume within acceptable tolerances.
Burette Calibration: Burettes are used for dispensing variable volumes of liquids during titrations. Precise burette calibration safeguards the accuracy of your titrations, leading to reliable data.
Beaker Calibration: While not as critical for some applications, calibrating beakers ensures they accurately reflect the volume they contain. This is particularly important for preparing precise solutions.
Benefits of Regular Calibration
Enhanced Data Integrity: Accurate measurements are the foundation of reliable research and quality control. Regular calibration minimizes errors and ensures data integrity.
Compliance with Regulations: Many industries have regulations mandating regular calibration of laboratory instruments. Calibration certificates serve as proof of compliance.
Cost Savings: Inaccurate measurements can lead to wasted materials, failed experiments, and even product recalls. Calibration helps prevent these costly issues.
Finding Calibration Services in Dubai, UAE
Several reputable laboratories in Dubai, UAE, offer pipette calibration, burette calibration, and beaker calibration services. When choosing a provider, consider factors like:
Accreditation: Look for laboratories accredited by a recognized body like ENAS (Emirates National Accreditation System).
Calibration Capabilities: Ensure the provider can calibrate your specific instruments and volume ranges.
Turnaround Time: Consider the time it takes for calibration and the return of your instruments.
Cost: Calibration costs can vary depending on the complexity of the instruments and the volume ranges involved.
By partnering with a reliable calibration service provider in Dubai, UAE, you can ensure your pipettes, burettes, and beakers deliver the precise measurements your research and quality control processes demand.
0 notes
Text
Theory of Qualitative Analysis
Qualitative analysis is the determinization of the quantity of individual elements or compounds present in a substance.
Two types: Volumetric and Gravimetric.
Volumetric Analysis
General Terms
Titration: aka titrimetry, is a quantitative technique that can be used to calc. the conc. of a given analyte in a mixture
Titrant: The solution of known conc. used in titration (primarily in burette)
Analyte: The solution of unknown conc. which is being analyzed (primarily in the conical flask)
Standard Solution: A solution with known conc. of a substance/element. A known weight of solute is dissolved to make a specific volume of standard solution.
A primary standard solution can be prepared directly and has the following features: highly pure, cheaply available, very soluble in water, neither deliquescent nor hygroscopic, and very stable. Ex: Oxalic acid
Secondary standards are substances whose standard solutions can not be prepared directly. Ex: potassium permanganate.
Indicator: A readily soluble substance that can change color at different pH levels. It should not effect the actual chemical reaction occurring and only 1-2 drops of indicatory should be sufficient to produce the necessary color change.
Endpoint: The point during a titration when an indicator shows that the amount of reactant necessary for a complete reaction has been added to a solution. The end point is approximate to the equivalence point.
Titration Error: The difference between the end point and the equivalence point, can be either positive or negative.
Volumetric analysis measures volume of a reagent needed to react with an analyte.
Volumetric analysis can be classified into acid-base titration, redox titration, precipitation titration, and complexometric titration.
Acid-Base/Neutralization Titration
In this titration, an acid reacts with a base to neutralize completely and form a salt. This can happen when the number of equivalents of an acid/base of unknown conc. is equal to the number of equivalents of the base/acid of known conc.
Acidimetry: the given base is estimated using standard acid solution.
Alkalimetry: the given acid is estimated using standard base solution.
Common indicators used:
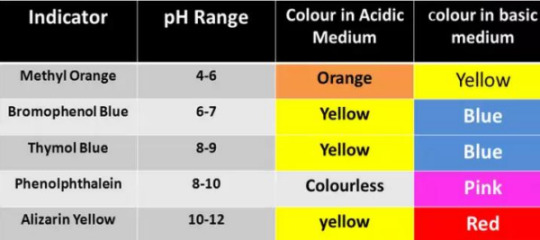
An acid-base indicator is a substance which is one color in acid and another in base. There are two theories which explain how they work: Ostwald and Quinonoid.
Ostwald Theory: An indicator is either a weak organic acid/base. The unionized compound has one color and the ion produced has another color. This is how it ionizes:
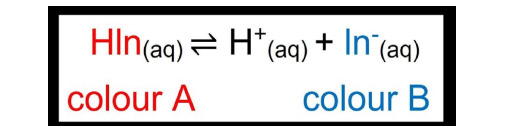
Phenolphthalein: a colorless organic compound which is a weak organic acid. It dissolves in water and dissociates slightly to form H+ ions (colorless) and Ph- ions (pink).
HPh ⇌ H+ + Ph-
i) In Acidic Medium: If the solution is made acidic, then the increase in H+ ion conc. suppresses the dissociation of phenolphthalein due to common ion effect, then the equilibrium shifts towards L.H.S. of the equation and the solution remains clear. Ex: HCl + Phenolphthalein
ii) In Basic Medium: If the solution is made basic, then the OH- ions reacts with the H+ ions to form unionized water molecules, the decrease in the H+ ion conc. shifts the equilibrium towards the R.H.S, therefore more of the indicator ionizes and the solution becomes pink. Ex: NaOH + Phenolphthalein
Methyl Orange: An organic compound which is a weak organic base. It is soluble in water and dissociates to a very small extent.
MeOH ⇌ Me+
i) In Acidic Medium: If the solution is made acidic, then the increase in H+ ions react with OH- ions to form unionized water molecules. Decrease in OH- ion conc. shifts the equilibrium towards the R.H.S., therefore the solution acquires an orange color due to Me+ ions. Ex: HCl + Methyl Orange
ii) In Basic Medium: If the solution is made basic, then the OH- ion conc. suppresses the dissociation of MeOH- due to common ion effect. The equilibrium shifts towards L.H.S and the solution remains pale yellow. Ex: NaOH + Methyl Orange.
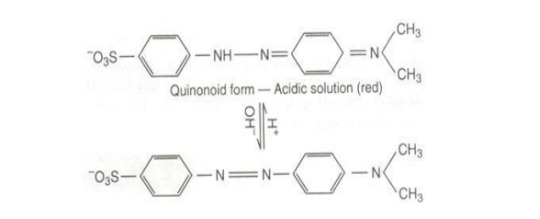
Quinonoid Theory: The color change of an acid-base indicator arises as a result of structural change. It is supposed that an indicator exists as a equilibrium mixture of two tautomeric forms, benzenoid and quinonoid forms.
One form exists in acid and the other in base.
The two forms possess two different colors and as the pH of the solution containing the indicator is changed, the solution shows a change of color.
Phenolphthalein: stable in both forms, Benzenoid - Quinonoid

Methyl Orange: stable in both forms, Quinonoid - Benzenoid
Correct detection of end point will affect the titration calc. and is very important. So it is very important to pick the correct indicator. Acid-base reactions are of 4 types:
Strong acid vs Strong base
Weak acid vs Strong base
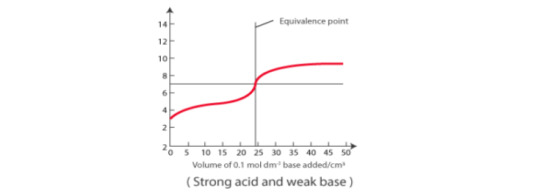
Strong acid vs Weak base
Weak acid vs Weak base
To chose the best indicator, one must understand the pH changes in the above 4. The pH change in the vicinity of the equivalence point is the most important.
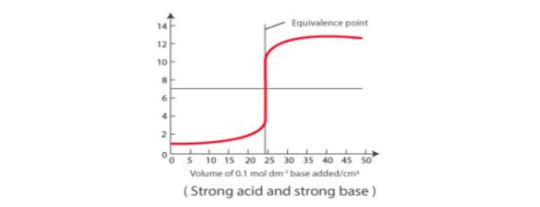
Titration Curve: the curve obtained by plotting pH as y-axis against the volume of alkali added as x-axis.
In each case below, 25ml of acid has been titrated against a standard solution of a base. Each titration curve becomes almost vertical and then bends away again.
The region of abrupt change in pH indicates the equivalence point.
The indicator should be picked so that it changes its color within vertical distance of the curve.
Strong acid vs strong base: pH curve of strong acid (HCl) and strong base (NaOH) is vertical around the pH range 4-10. So phenolphthalein, methyl red, and methyl orange, are suitable.
Weak acid vs strong base: pH curve of weak acid (CH3COOH) and strong base is vertical around the pH range 7-11. so phenolphthalein is okay
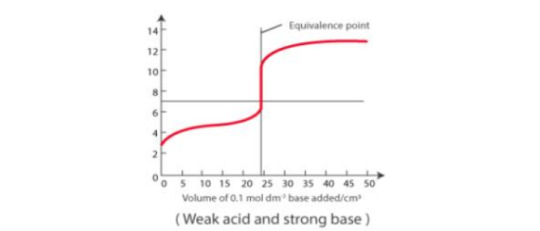
Strong acid vs weak base: pH curve of strong acid with weak base (NH4OH) is vertical around the pH range 4-7, so methyl red and methyl orange are both okay.
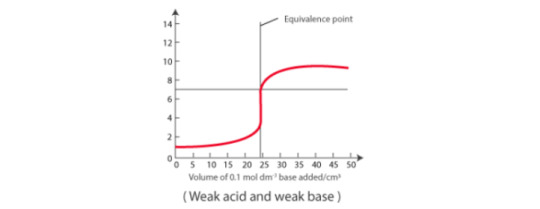
Weak acid vs weak base: pH curve of weak acid and weak base indicates that there is no vertical part, therefore no indicator can be used.
Redox Titration
Redox Titration: reactions that involve the transfer of electrons from one species to another. The species that loses electrons is oxidized while the species that gains electrons is reduced.
Oxidation: addition of O, removal of H+, loss of electrons, increase in the oxidation state of the substance.
Reduction: removal of O, addition of H+, gain of electrons, decrease in the oxidation state of the substance.
Oxidizing agent: the substance which undergoes reduction to induce oxidization. (Ex: KMnO4)
Reducing agent: the substance which undergoes oxidization to induce reduction (Ex: Mohr's salt)
Oxidimetry: Determination of strength of reducing agent with standard solution of oxidizing agent.
Reducimetry: Determination of strength of oxidizing agent with standard solution of reducing agent.
Types of Redox Titrations:
Dichrometry: Estimation which involves the use of potassium dichromate (K2Cr2O7) - primary standard
Iodometry: Estimation involves liberated iodine (I2) - CU(II)+Hypo
Permanganometry: Estimation which involves the use of potassium permanganate (KMnO4) - self indicator
Bromometry: Uses a bromine titrant (Br2)
Cerimetry: Employs cerium salts (IV)
Redox Indicators: these indicators are oxidants and or reductants. Oxidized form has one color while reduced form has another. When slight excess of oxidant is present, the indicatory changes in color and is shows as the end point of the titration. There are 3 types: self, external, and redox.
Self Indicators: The oxidized or reduced form of the titrant is self indicating (Ex: KMnO4)
External Indicator: The indicator is externally added to the solution. It does not take part in the reaction. (Ex: Potassium Ferro cyanide in the ferrous sulphate + K2Cr2O7 experiment)
Redox Indicator: A compound that changes color at specific potential differences. It must have a reduced and oxidized form with different colors and the redox process must be reversible (Ex: Diphenyl amine indicator has blue/violet color when oxidized and is colorless when reduced.
Precipitation Titration
A type of titration were a precipitate is formed during the course of the reaction.
The titrant reacts with the analyte to form an insoluble material and the titration continues till the very last amount of analyte is consumed.
Principle: the quantity of the added precipitating reagent or precipitant is equivalent to the substance being precipitated.
There are three main types based on end point detection: Mohr's, Volhard's and Fajan's Method.
Mohr's Method: This method uses chromate as an indicator. Chromate forms a precipitate with Ag+ but this ppt has a greater solubility than that of AgCl in NaCl vs AgNO3 titrations
Therefore, AgCl is formed first and after all Cl- is consumed, the first drop of excess Ag+ will react with the chromate indicator to give a red ppt.
Volhard's Method: Standard potassium tricyanate is titrated against Ag+ solution containing Fe3+ (Ferric alum indicator)
The excess Ag+ is then titrated with standard SCN- solution till a red color is obtained.
This method is widely used because it can detect the end point very well.
Fajan's Method: Fluorescein and its derivatives are adsorbed to the surface of colloidal AgCl (Reddish tinge on white ppt)
After all chloride is used, the first drop of Ag+ will react with fluorescein (FI-) to form a red color.
Complexometric Titration
Principle: Based on complex formation between the metal ion and ligand.
Particularly useful for determination of a mixture of different metal ions in solution.
An indicator with a marked color change is usually used
EDTA: Ethylene diamine tetra acetic acid, has 4 carboxyl groups and two amine groups that act as electron pair donors.
The ability of EDTA to potentially donate six lone pairs of electrons for the formation of coordinate covalent bonds to metal cations makes EDTA a hexadentate ligand.
However in practice, EDTA is usually only partly ionized and thus forms fewer that six coordinate covalent bonds with metal cations.
EDTA forms stable complex with various metal ions.
The complexation occurs in a single step: there is a sharp change in the metal ion conc. at the equivalence point, the M-EDTA complex is water soluble, and the stoichiometry for all metal ions is the same 1:1 irrespective of charge.
Metal Ion Indicators: Compounds that change color when bonded to metal.
Requirements:
1. Color must be intense enough
2. Color contrast between the indicator and metal-indicator complex must be distinct
3. M-indicator complex should be stable enough to ensure color change but less stable than EDTA.
4. The change in equilibrium bust me rapid and sharp.
5. The color reaction should be selective
6. The indicator must be very sensitive to metal ions.
7. The indicator must be stable in the titration medium.
8. The indicator must me stable in storage too.
9. All of the above must be fulfilled in the pH range of the reaction
10. It should be commercially available and pure.
Examples of metal ion indicators are:
Eriochrome Black T/EBT
Murexide
Xylenol orange
Pyridylazonaphthol/PAN
Types of EDTA Titrations:
Direct
Back
Substitution
Alkalimetric
Indirect
Gravimetric Analysis
A method of analytical chemistry to determine the quantity of analyte based on the mass of a solid.
Principle: The mass of an ion in a pure compound can be determined and used to find the mass percent of that same ion in a known quantity of an impure compound.
Certain conditions must be met: the ion being analyzed must be completely precipitated, the precipitate must be a pure compound, and the precipitate must be easy to filter.
The 7 steps of gravimetry are:
1. Prep. of sample solution
2. Precipitation
3. Digestion
4. Filtration
5. Washing
6. Dying and Ignition
8. Weighing and Calculation
Preparation of Sample Solution
When sample solution is prepared, dilute solution is generally preferred.
Volume is adjusted to suit the amount of precipitating agent.
pH of the solution is adjusted for precipitation to occur.
The desired property of the solution is maintained for precipitation to occur.
Precipitation
The analyte is converted into a soluble precipitate.
Nucleation: Individual particles group together to form a larger colloidal particle called a nuclei. As nucleation increases, a larger number of nuclei appear, leading to more adsorption of impurity.
The initial nucleus grows by the deposition of their precipitate particles to form a crystal or geometric shape.
The greater the super saturation, the more rapid crustal growth rate and colloidal precipitate form.
Increase in the growth rate increases chance of imperfection in the crystal and surface area of precipitate increases, which leads to easy trapping of impurities.
Precipitate Contamination is of two types:
1. Co-Precipitation: Precipitation where soluble compounds in a solution are removed during the course of precipitation. It is of 2 subtypes: surface adsorption and occlusion.
Surface adsorption: Trapping of impurities on the surface of the crystal
Occlusion: Trapping of impurities within the crystal.
2. Post-Precipitation: Where the precipitation of undesirable compound occurs after the formation of the precipitate of the desirable compound, secondary ppt after primary ppt is already formed.
Ex: Ca oxalate ppt in presence of Mg ion. After some time, Mg oxalate forms deposit on Ca oxalate surface.
Digestion
The ppt is left hot, just below boiling point, for up to an hour to digest the particles.
Digestion involves dissolution of small particles and re-precipitation on larger ones when slowly cooled, this results in larger particles.
This process is called Oswald Ripening, it is very useful for colloidal precipitation.
Filtration
Ppt is physically separated from mother liquid.
The filtration method depends on the nature of ppt, the cost of media, and heating temp required for drying.
Examples of filtration mediums are:
Filter paper
Filter pump
Filter mats
Crucible fitted with porus plate.
Crucible to be used at high temps.
Washing
Co-precipitate impurities may be washed off the surface of the ppt even after filtering.
Excess mother liquid on the surface of the ppt can be washed off too.
Many ppts cannot be washed with pure water because peptization would occur.
Ex: NHO3 can be used to wash off AgCl ppt.
Drying and Ignition
The ppt must be pure, stable, and of known composition.
Drying and ignition allow the ppt to be in suitable condition for weighing.
It removes water efficiently.
Appropriate chemical changes occur during heating
Ex: AgCl is dried at 100°-130°C to remove physically bound water.
A higher temp is necessary if the water is chemically bonded, trapped in the crystals, or to ensure appropriate chemical change.
Weighing and Calculation
After ignition, ppt is taken out into a clean crucible and is weighed accuracy on an analytical balance.
Calculations are done.
0 notes
Text
Precision Meets Innovation: Microlit's Digital Burettes
Microlit is where cutting-edge technology and precision engineering converge to redefine volumetric analysis. Our digital burettes are designed to elevate your laboratory experience, offering unmatched accuracy, reliability, and convenience. Explore our range of innovative digital burettes, meticulously crafted to meet the evolving demands of scientific research and analysis.

Unparalleled Accuracy: Experience precision like never before with Microlit's digital burettes. Leveraging advanced digital technology and precision engineering, these instruments deliver precise and reproducible measurements with exceptional accuracy, ensuring reliable results for your experiments.
Effortless Operation: Streamline your laboratory workflow with Microlit's digital burettes. Featuring intuitive interfaces and user-friendly controls, these instruments make volumetric analysis simple and efficient. Set your desired volume with ease and let our digital burettes handle the rest, allowing you to focus on your research.
Versatility in Performance: From routine titrations to complex analytical tasks, Microlit's digital burettes offer versatility to suit a wide range of laboratory applications. Choose from our diverse range of models, each designed to meet specific application requirements and experimental protocols with precision and reliability.
Dependable Quality: At Microlit, quality is our commitment. Each digital burette undergoes rigorous testing and quality assurance to ensure superior performance and durability. Trust in Microlit's digital burettes for consistent and reliable results, empowering you to advance your research with confidence.
Upgrade Your Laboratory: Elevate your laboratory capabilities with Microlit's innovative digital burettes. Experience the perfect blend of precision and innovation, and unlock new possibilities in your scientific pursuits. Explore our range of digital burettes today and discover why Microlit is the preferred choice for scientists and researchers worldwide.
0 notes
Text

Lab Glassware:
Lab glassware is a fundamental component of any scientific laboratory. From beakers and flasks to pipettes and test tubes, these specialized vessels play a vital role in countless experiments and analyses. They are used to mix, measure, heat, cool, and store chemicals and solutions, enabling scientists to conduct a wide range of research and development activities.
Essential Properties of Lab Glassware
Chemical resistance: Lab glassware must be able to withstand the corrosive effects of various chemicals used in experiments. Borosilicate glass, a type of glass with low thermal expansion and high chemical resistance, is the most common material used for lab glassware.
Heat resistance: Many laboratory procedures involve heating or cooling substances. Lab glassware needs to be able to withstand high temperatures without cracking or shattering. Borosilicate glass is again the go-to material due to its excellent heat resistance.
Transparency: Scientists need to be able to see what is happening inside their glassware. Lab glassware is typically made of transparent materials like glass, allowing for clear observation of reactions and solutions.
Accuracy: Many laboratory procedures require precise measurements of volumes and concentrations. Lab glassware is often marked with graduations or scales to ensure accurate measurements. Common Types of Lab Glassware
Beaker: A cylindrical glass vessel used for mixing, storing, and heating liquids.
Flask: A flask is a bulbous glass container with a narrow neck, used for various purposes such as boiling liquids, holding reactions, and storing solutions. Different types of flasks include Erlenmeyer flasks, round-bottom flasks, and volumetric flasks.
Pipette: A long, thin glass tube with a calibrated scale used for accurately transferring small volumes of liquids.
Test tube: A small, cylindrical glass tube used for holding and mixing small volumes of liquids.
Burette: A long, graduated glass tube with a stopcock at the bottom, used for dispensing precise volumes of liquids.
Condenser: A tube with a surrounding jacket used to cool vapors and convert them back into liquids.
Choosing the Right Lab Glassware
The type of lab glassware you need will depend on the specific experiment you are conducting. Consider the following factors when choosing lab glassware:
The chemicals you will be using: Make sure the glassware is compatible with the chemicals you will be working with. The temperature range you will be working in: Choose glassware that can withstand the required temperatures.
The volume of liquid you need to handle: Select glassware with the appropriate capacity. The accuracy of your measurements: Use graduated glassware for precise measurements. Safety and Care of Lab Glassware
Lab glassware is delicate and can break easily. It is important to handle it with care to avoid accidents and injuries. Always wear safety glasses and gloves when working with lab glassware. Wash and clean glassware thoroughly after each use. Store glassware properly to prevent it from getting damaged.
0 notes
Text

Lab Glassware:
Lab glassware is a fundamental component of any scientific laboratory. From beakers and flasks to pipettes and test tubes, these specialized vessels play a vital role in countless experiments and analyses. They are used to mix, measure, heat, cool, and store chemicals and solutions, enabling scientists to conduct a wide range of research and development activities.
Essential Properties of Lab Glassware
Chemical resistance: Lab glassware must be able to withstand the corrosive effects of various chemicals used in experiments. Borosilicate glass, a type of glass with low thermal expansion and high chemical resistance, is the most common material used for lab glassware.
Heat resistance: Many laboratory procedures involve heating or cooling substances. Lab glassware needs to be able to withstand high temperatures without cracking or shattering. Borosilicate glass is again the go-to material due to its excellent heat resistance.
Transparency: Scientists need to be able to see what is happening inside their glassware. Lab glassware is typically made of transparent materials like glass, allowing for clear observation of reactions and solutions.
Accuracy: Many laboratory procedures require precise measurements of volumes and concentrations. Lab glassware is often marked with graduations or scales to ensure accurate measurements. Common Types of Lab Glassware
Beaker: A cylindrical glass vessel used for mixing, storing, and heating liquids.
Flask: A flask is a bulbous glass container with a narrow neck, used for various purposes such as boiling liquids, holding reactions, and storing solutions. Different types of flasks include Erlenmeyer flasks, round-bottom flasks, and volumetric flasks.
Pipette: A long, thin glass tube with a calibrated scale used for accurately transferring small volumes of liquids.
Test tube: A small, cylindrical glass tube used for holding and mixing small volumes of liquids.
Burette: A long, graduated glass tube with a stopcock at the bottom, used for dispensing precise volumes of liquids.
Condenser: A tube with a surrounding jacket used to cool vapors and convert them back into liquids.
Choosing the Right Lab Glassware
The type of lab glassware you need will depend on the specific experiment you are conducting. Consider the following factors when choosing lab glassware:
The chemicals you will be using: Make sure the glassware is compatible with the chemicals you will be working with. The temperature range you will be working in: Choose glassware that can withstand the required temperatures.
The volume of liquid you need to handle: Select glassware with the appropriate capacity. The accuracy of your measurements: Use graduated glassware for precise measurements. Safety and Care of Lab Glassware
Lab glassware is delicate and can break easily. It is important to handle it with care to avoid accidents and injuries. Always wear safety glasses and gloves when working with lab glassware. Wash and clean glassware thoroughly after each use. Store glassware properly to prevent it from getting damaged.
0 notes
Text
Types of Laboratory Apparatus

Laboratory Apparatus, In the dynamic world of scientific exploration, laboratories serve as the crucibles of innovation and discovery. Central to these hubs of knowledge are various laboratory apparatus, each designed for specific tasks to unlock the secrets of the universe. In this exploration, we will delve into the diverse types of laboratory apparatus, shedding light on their roles and significance in the pursuit of scientific understanding.
Beakers and Flasks: The Workhorses of Experimentation

Beakers and flasks are the stalwarts of any laboratory apparatus, serving as vessels for mixing, measuring, and holding liquids. Beakers, with their cylindrical shape and wide mouth, are ideal for stirring and pouring liquids. On the other hand, flasks, often with a narrower neck, are used for reactions that involve the release of gases, ensuring controlled containment.
Test Tubes and Culture Tubes: Homes of Microcosmic Reactions

Another laboratory apparatus is test tubes, characterized by their slender and elongated shape, are iconic symbols of laboratories. They are employed for small-scale reactions, heating substances, and observing chemical changes. Culture tubes, with a similar design, are tailored for microbial and cellular cultures, facilitating the growth and study of microorganisms.
Pipettes: Precision in Volume

Precision in measuring and transferring small volumes of liquids is the forte of pipettes. Available in various types, including micropipettes and volumetric pipettes, these instruments ensure accuracy in experiments where even a slight deviation can lead to significant errors. Pipettes are indispensable in fields like biology, chemistry, and medical research.
Burettes: The Graduated Flow of Precision

When precise measurement and control of liquids are paramount, burettes step into the limelight. These long, graduated tubes with a stopcock at the bottom allow for meticulous titrations, where the volume of a solution is precisely determined while reacting with another solution. Burettes are vital tools in analytical chemistry, ensuring accuracy in concentration calculations.
Microscopes: Unveiling the Microcosm
Microscopes open windows to the unseen world, enabling scientists to explore the microscopic realms. Light microscopes use visible light to magnify specimens, while electron microscopes employ electron beams for even higher magnification. Microscopes are foundational in fields like biology, medicine, and materials science, revolutionizing our understanding of cellular structures and tiny organisms.
Centrifuges: Separating Forces in a Spin

Centrifuges harness centrifugal force to separate components within a liquid sample based on their density. Commonly used for separating blood components in medical laboratories, centrifuges find applications in various scientific disciplines, including biochemistry and molecular biology. Different types of centrifuges, such as ultracentrifuges and microcentrifuges, cater to specific needs of researchers.
Incubators: Nurturing Growth in a Controlled Environment
Incubators recreate specific environmental conditions to foster the growth of cultures, cells, or microorganisms. They provide a controlled setting for experiments requiring constant temperature, humidity, and CO2 levels. Incubators play a crucial role in biological and medical research, ensuring optimal conditions for cell cultures and experiments.
Autoclaves: Sterilizing for Safety
Sterilization is a non-negotiable aspect of laboratory work, particularly in microbiology and medical research. Autoclaves, using high-pressure steam, eliminate bacteria, viruses, and other contaminants from laboratory equipment and materials. This ensures a sterile environment for experiments and prevents cross-contamination.
Spectrophotometers: Quantifying the Spectrum

Spectrophotometers measure the intensity of light absorbed or transmitted by a sample at different wavelengths. Widely used in chemistry and biochemistry, these instruments provide valuable data about the composition and concentration of substances. Spectrophotometers play a pivotal role in quantifying DNA, proteins, and various chemical compounds.
Ovens and Furnaces: Heat for Transformation
For experiments requiring precise temperature control and heat application, ovens and furnaces step in. These apparatus are crucial for processes like drying, sterilization, and material synthesis. Furnaces, capable of reaching higher temperatures, find applications in metallurgy, ceramics, and material science.
PH Meters: Gauging Acidity and Alkalinity
The acidity or alkalinity of a solution is a fundamental parameter in many scientific investigations. pH meters provide an accurate measure of the hydrogen ion concentration in a solution, allowing researchers to monitor and control the acidity or alkalinity of their experimental environments. These instruments are indispensable in fields like chemistry, biology, and environmental science.
Chromatography Systems: Separating Mixtures with Precision

Chromatography is a versatile technique employed to separate and analyze complex mixtures. Gas chromatography, liquid chromatography, and thin-layer chromatography are among the various chromatography systems used in laboratories. These systems are vital for applications ranging from pharmaceutical analysis to environmental monitoring.
In Conclusion of type of laboratory apparatus :
The laboratory apparatus discussed here represents the diverse tools that scientists and researchers wield in their quest for knowledge and discovery. Each instrument plays a specific role, contributing to the intricate tapestry of scientific exploration. As technology continues to advance, new and innovative laboratory apparatus will undoubtedly emerge, further enriching the capabilities of laboratories worldwide. In the grand symphony of scientific inquiry, these apparatus stand as the instruments that harmonize to unveil the secrets of the natural world.
0 notes
Text
Liquid Handling Solutions for ELISA and Other Immunoassays
Enzyme-linked immunosorbent assays (ELISA) and other immunoassays are essential tools in laboratories for detecting and quantifying biological molecules such as proteins, antibodies, and hormones. These assays are widely used in diagnostics, research, and pharmaceutical industries due to their sensitivity and specificity. However, for these tests to deliver accurate and reproducible results, precise liquid handling is crucial.
In this blog, we’ll explore how liquid handling instruments play a key role in ELISA and other immunoassays, and what tools can enhance the efficiency and accuracy of these assays.
Understanding the Role of Liquid Handling in ELISA and Immunoassays
ELISA and immunoassays typically involve multiple steps, such as adding reagents, washing, and detecting signals from the sample. Each of these steps requires precise dispensing of liquids to ensure proper binding and reaction between molecules. Even small inconsistencies in pipetting can lead to errors in results, compromising the reliability of the data.
Here are some steps where precise liquid handling is vital:
Sample Dispensing: Accurate measurement of the sample to ensure consistent concentration across wells.
Reagent Addition: Reagents, such as antibodies or substrates, must be added uniformly for reproducibility.
Washing: Thorough washing of wells to remove unbound material and minimize background noise.
Substrate Addition: Ensuring equal amounts of substrate to each well for uniform colorimetric or fluorescent signals.
Liquid Handling Solutions for ELISA and Immunoassays
Let’s take a look at some advanced liquid handling solutions that help labs achieve high-quality ELISA and immunoassay results.
1. Micropipettes
Micropipettes are essential tools for handling small volumes of liquid in immunoassays. Both manual and automated micropipettes are widely used for dispensing reagents and samples in ELISA. Key factors when selecting micropipettes for immunoassays include accuracy, precision, and ergonomic design.
Microlit NERO Micropipettes: Known for its ergonomic design and superior accuracy, the NERO range includes both single-channel and multi-channel micropipettes. Multi-channel options are particularly useful in ELISA, allowing multiple wells to be filled simultaneously, speeding up the process and reducing variability.
2. Electronic Dispensers
Electronic dispensers simplify repetitive pipetting tasks and reduce the chances of human error. They are useful for adding reagents in fixed or variable volumes during different stages of ELISA and immunoassays.
Microlit E-BURETTE: This E-Burette is designed to dispense precise amounts of reagents effortlessly. It eliminates user fatigue and enhances consistency, which is critical when handling large numbers of samples in immunoassays.
3. Bottle Top Dispensers
For laboratories dealing with multiple reagents in large volumes, bottle top dispensers provide an efficient solution. These dispensers allow easy, accurate, and contamination-free transfer of reagents directly from the bottle.
Microlit BEATUS: A bottle top dispenser with excellent chemical compatibility and precision, BEATUS ensures that reagents are safely and accurately dispensed, reducing the risk of cross-contamination in ELISA and immunoassays.
4. Automated Liquid Handling Systems
Automation is transforming laboratories by increasing throughput and minimizing manual intervention. Automated liquid handling systems can perform complex pipetting tasks, including sample preparation, washing, and reagent addition, with precision and speed.
While high-throughput labs benefit greatly from automation, smaller labs can still achieve consistency by using electronic pipettes and semi-automated dispensers for immunoassays.
Best Practices for Liquid Handling in ELISA and Immunoassays
To make the most of liquid handling solutions and improve the accuracy of immunoassays, labs can follow these best practices:
Regular Calibration: Ensure that pipettes and dispensers are regularly calibrated to maintain accuracy.
Use Multi-Channel Pipettes: Multi-channel pipettes can significantly reduce variability between wells, particularly when adding samples or reagents in ELISA plates.
Minimize Air Bubbles: When aspirating and dispensing, be mindful of air bubbles as they can cause inconsistencies in volume and affect results.
Avoid Touching the Sidewalls: During pipetting, avoid touching the sides of wells as this can lead to inaccurate volumes and cross-contamination.
Conclusion
Liquid handling plays a fundamental role in ensuring the success of ELISA and other immunoassays. By using precise and reliable tools like micropipettes, electronic dispensers, and bottle top dispensers, labs can improve the accuracy and reproducibility of their assays.
Microlit’s range of liquid handling instruments is designed to meet the high demands of immunoassays, ensuring that labs can carry out their research and diagnostics with confidence. Whether you’re running routine tests or handling critical samples, investing in high-quality liquid handling solutions will enhance the reliability of your results and streamline your workflows.
#liquid handling instruments#pipetting#liquid handling solutions#E-Burette#bottle top dispensers#electronic pipettes
0 notes
Text
Exploring the World of Lab Glassware: A Comprehensive Guide to Shopping Online

In the ever-evolving landscape of scientific research and experimentation, the need for high-quality lab glassware is paramount. Laboratories across the globe rely on precision and durability when it comes to their equipment. With the convenience of online shopping, acquiring lab glassware has become easier than ever. This article delves into the world of Shopping For Lab Glassware Online, exploring the benefits, considerations, and the diverse range of products available.
1: The Convenience of Online:
Shopping for Lab Glassware In the digital age, the convenience of online shopping has revolutionized the way laboratories source their equipment. Shopping online for lab glassware offers a myriad of advantages, including accessibility, a vast selection, and time efficiency. Laboratories can browse through a diverse range of products without geographical constraints, saving valuable time that can be redirected towards scientific pursuits.
2: Understanding the Types of Lab Glassware:
Before delving into online shopping, it's crucial to understand the various types of lab glassware and their specific applications. From beakers and flasks to test tubes and pipettes, each piece of glassware serves a unique purpose in the laboratory setting. Online platforms provide detailed descriptions and specifications, empowering buyers to make informed decisions based on their experimental requirements.
3: Quality Assurance in Online Lab Glassware Shopping:
Ensuring the quality of lab glassware is of utmost importance in maintaining the integrity of scientific experiments. Reputable online platforms often collaborate with trusted manufacturers, ensuring that their products meet industry standards. Buyers can review product specifications, materials, and customer reviews to gauge the quality of the lab glassware before making a purchase.
Also Read: The Burette: A Vital Tool in the Chemist’s Arsenal
4: Customization Options for Specialized Needs:
Laboratories often have specific requirements that demand customized lab glassware solutions. Online platforms cater to these needs by offering customization options. From volume adjustments to specialized coatings, buyers can work closely with manufacturers to create lab glassware tailored to their unique experiments.
5: Cost Considerations and Budgeting:
Budget constraints are a common concern for laboratories, but online shopping allows for effective cost management. Platforms often provide a range of options with varying price points, enabling laboratories to find quality lab glassware that fits their budget. Additionally, online platforms frequently offer discounts, bulk pricing, and promotions, further maximizing cost-effectiveness.
6: Ensuring Safety in Online Lab Glassware Purchases:
Safety is a top priority in laboratory settings, and purchasing lab glassware online requires careful consideration of safety standards. Reputable online platforms provide detailed safety information, certifications, and adherence to regulations. Buyers should prioritize platforms that prioritize the safety of their products to ensure the well-being of laboratory personnel.
7: Reviews and Ratings:
A Buyer's Guide Before making a purchase, laboratories can benefit from the experiences of others through online reviews and ratings. Genuine feedback from fellow researchers provides valuable insights into the performance, durability, and overall satisfaction with specific lab glassware products. Paying attention to reviews helps laboratories make informed decisions and select products that align with their needs.
8: The Evolution of Lab Glassware Materials:
Advancements in materials science have led to the development of innovative lab glassware materials, offering enhanced properties such as chemical resistance, durability, and transparency. Online platforms showcase the latest materials, allowing laboratories to stay abreast of technological advancements and choose glassware that aligns with the requirements of modern research.
9: Sustainable Practices in Lab Glassware Manufacturing:
As environmental consciousness grows, laboratories are increasingly seeking sustainable lab glassware options. Online platforms are responsive to this demand, featuring glassware manufacturers committed to eco-friendly practices. Buyers can explore options such as recycled glass, reduced packaging waste, and energy-efficient production methods, contributing to a greener laboratory environment.
10: Navigating Shipping and Handling Processes:
Efficient shipping and careful handling are crucial aspects of online lab glassware shopping. Reputable platforms prioritize secure packaging to prevent breakage during transit. Buyers should familiarize themselves with the shipping policies of the online store, including shipping times, tracking options, and return procedures, to ensure a smooth purchasing experience.
Conclusion:
Shopping for lab glassware online opens up a world of possibilities for laboratories, offering convenience, accessibility, and a diverse range of products. From understanding the types of lab glassware to prioritizing quality, safety, and sustainability, this comprehensive guide equips laboratories with the knowledge needed to make informed online purchases. As technology continues to advance, the online marketplace for lab glassware is poised to evolve, providing even more innovative solutions for the scientific community.
Source URL: https://bit.ly/3Oh65XU
0 notes
Text
Measured Volume set for photosensitive and oncology

Measured volume set
A measured volume set for photosensitive and oncology purposes would typically refer to a specialized infusion set designed to address the specific needs and requirements of administering light-sensitive medications or chemotherapy drugs used in cancer treatment.
These sets may include additional features to protect the medication from light exposure and ensure safe administration. Here are some potential components and considerations for a measured volume set for photosensitive and oncology use:
Light-Blocking Tubing: The tubing used in the set may be constructed with materials that are opaque or have light-blocking properties. This helps to minimize or prevent exposure of light-sensitive medications or blood products to external light sources.
Non-PVC Construction: Some medications or chemotherapy drugs can interact with PVC materials, potentially leading to degradation or leaching of harmful substances. Non-PVC materials, such as polyurethane or other plastics, may be used to ensure compatibility and safety.
Light-Protective Drip Chamber: The drip chamber included in the set may be designed to shield the medication from light exposure. It may feature additional layers or coatings to block out light and protect the medication from degradation.
Light-Resistant Infusion Bag: For medications that are particularly sensitive to light, the set may include a specialized light-resistant infusion bag. These bags are typically made of opaque or light-blocking materials to minimize light exposure during storage and administration.
Compatibility with Oncology Medications: The set should be specifically designed to be compatible with chemotherapy drugs commonly used in cancer treatment. This ensures the safe and effective administration of these medications.
Accurate Measuring Device: A measuring device, such as a drip chamber with volume markings or a burette, is included to accurately measure the volume of medication being administered during the infusion. This helps healthcare providers control the dosage and ensure precise delivery.
It is important to note that the specific design and features of measured volume sets for photosensitive and oncology use can vary among manufacturers and healthcare facilities. These sets are typically used under the guidance of healthcare professionals experienced in administering light-sensitive medications or chemotherapy treatments to ensure patient safety and efficacy.
#medicalequipment#doctor#medicalproducts#madeinindia#hospital#medical#health#nurse#larsmedicare#medicaldevices
0 notes
Text
Liquid Handling System Market Research Report
Liquid Handling System Market, by Type (Electronic, Automated, Manual), Product (Pipette, Consumables, Workstation, Microplate Dispensers, Burette), Application (Drug Discovery, Genomics, Clinical Diagnostics, Proteomics) and Geography (North America, Europe, Asia-Pacific, Middle East and Africa and South America)
The global Liquid Handling System market size is projected to reach USD 5.2 billion by 2026 at a CAGR of 9% from USD 3.2 billion in 2021 during the forecast period 2021-2028.

Liquid Handling System contains a variety of devices which are used to perform various liquid Handling activities. It is used for the automation of various processes such as dispensing the reagent, addition of desired quantity of fluid to a container etc. These are able to transfer liquid in very small volumes like micro or nano litre level without any error. There are various types of liquid handling system based on the amount of volume to be dispensed. These range from a modernized syringe or pipette.
Due to the recent expansion and growth of global pharmaceutical market along with the increased focus on data precision and the technological advancement in the sector are some of the factors that have supported long-term expansion for Liquid Handling System Market.
The COVID-19 pandemic is causing widespread concern and economic hardship for consumers, businesses, and communities across the globe.
Request For Free Sample Report: https://www.delvens.com/get-free-sample/liquid-handling-system-market-trends-forecast-till-2028
Key Players
Danaher Corporation
Thermo Fisher Scientific Inc.
Eppendorf AG
Tecan Group Ltd.
Gardner Denver Medical
Mettler-Toledo International Inc.
Hamilton Company
PerkinElmer, Inc.
Sartorius AG
Corning Incorporated
Gilson Inc.
Agilent Technologies Inc.
Qiagen N.V.
Lonza Group Ltd
Brooks Automation Inc.
Integra Holding AG
Endress+Hauser AG
Labcyte Inc.
BioTek Instruments Inc.
TTP Labtech Ltd
Metrohm AG
BRAND GMBH + CO KG Tomtec Inc.
Hudson Robotics Inc.
Orochem Technologies Inc.
To Grow Your Business Revenue, Make an Inquiry Before Buying at: https://www.delvens.com/Inquire-before-buying/liquid-handling-system-market-trends-forecast-till-2028
Reasons to Acquire
Increase your understanding of the market for identifying the best and suitable strategies and decisions on the basis of sales or revenue fluctuations in terms of volume and value, distribution chain analysis, market trends and factors
Gain authentic and granular data access for Liquid Handling System market so as to understand the trends and the factors involved behind changing market situations
Qualitative and quantitative data utilization to discover arrays of future growth from the market trends of leaders to market visionaries and then recognize the significant areas to compete in the future
In-depth analysis of the changing trends of the market by visualizing the historic and forecast year growth patterns
Report Scope
Liquid Handling System Market is segmented into type, product, application and geography.
On the basis of Type
Electronic
Automated
Manual
On the basis of Product
Pipettes
Electronic
Manual
Semi-automated
Consumables
Disposable tips
Tubes & plates
Reagent containers
Other consumables
Microplate reagent dispensers
Liquid handling workstations
Burettes
Microplate washers
Software
Other products
On the basis of Application
Drug discovery
HTS
Compound weighing and dissolution
ADME screening
Other drug discovery applications
Genomics
Genotyping
NGS
PCR
DNA/RNA purification
Other genomics applications
Clinical diagnostics
Sample preparation
ELISA
Other clinical diagnostics applications
Proteomics
Other applications
On the basis of Region
Asia Pacific
North America
Europe
South America
Middle East & Africa
Purchase the Report: https://www.delvens.com/checkout/liquid-handling-system-market-trends-forecast-till-2028
About Us:
Delvens is a strategic advisory and consulting company headquartered in New Delhi, India. The company holds expertise in providing syndicated research reports, customized research reports and consulting services. Delvens qualitative and quantitative data is highly utilized by each level from niche to major markets, serving more than 1K prominent companies by assuring to provide the information on country, regional and global business environment. We have a database for more than 45 industries in more than 115+ major countries globally.
Delvens database assists the clients by providing in-depth information in crucial business decisions. Delvens offers significant facts and figures across various industries namely Healthcare, IT & Telecom, Chemicals & Materials, Semiconductor & Electronics, Energy, Pharmaceutical, Consumer Goods & Services, Food & Beverages. Our company provides an exhaustive and comprehensive understanding of the business environment.
Contact Us:
UNIT NO. 2126, TOWER B,
21ST FLOOR ALPHATHUM
SECTOR 90 NOIDA 201305, IN
0 notes