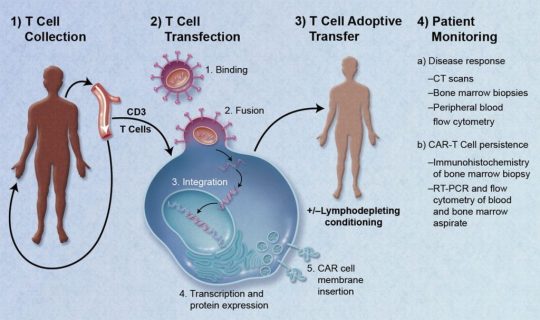
#car t cell therapy
Text
the sample size for this study was only 5 people, so there’s still a long way to go, but the scientists referred to the CAR T-cell treatment as essentially a “reset button” for these participants’ immune systems
#lupus#multiple sclerosis#autoimmune disease#rheumatoid arthritis#rheumatic diseases#car t cell therapy#mac.txt
133 notes
·
View notes
Text
instagram
5 notes
·
View notes
Text
Artemis and the Unintended Odyssey into Oncology
Let me, Artemis, goddess of the hunt, share with you a peculiar twist of fate that led me from the wild forests to the intricacies of modern science, specifically, the battle against cancer with CAR T-cell therapy. This tale starts on a seemingly ordinary day, marked by the mundane task of updating my celestial LinkedIn profile—a task even deities must bow to in the age of digital omnipresence.
It was while scrolling through the Olympian job board that I stumbled upon an advertisement seeking a "Dedicated Hunter of Malignant Foes." Mistaking this for a call to arms against a resurgence of mythic monsters, I promptly applied, envisioning epic battles against beasts of yore. Imagine my surprise when I discovered that the position was in a lab, not on a battlefield, and the foes were not creatures of flesh and blood but cancer cells!
Intrigued by this unexpected turn and ever the adaptable huntress, I decided to don the white coat of a researcher (a garb vastly different from my usual attire). My mission? To harness the mighty T-cells, turning them into specialized hunters known as CAR T-cells. These cells, I learned, were not unlike my hounds, trained to track and vanquish their quarry with precision.
The process, I must confess, involved less running through forests and more navigating the labyrinthine protocols of scientific research. Each step, from engineering the CAR T-cells to observing their relentless pursuit of cancer cells in petri dishes, was a new adventure—an academic hunt, if you will, with its own set of thrills and challenges.
What truly captivated me was the transformation of the T-cells. Much like my own transformation from deity to scientist, these cells were endowed with new powers, crafted through the magic of genetic engineering. They were equipped with a chimeric antigen receptor, a talisman that granted them the ability to see and attack cancer cells—cells that had, until then, hidden like cunning prey in the body’s vast wilderness.
Armed with this newfound knowledge and a burning curiosity, I authored the article "Artemis’s Quiver: Hunting Cancer with CAR T-cell Therapy" to share my unexpected odyssey. It was a humorous twist of fate that led me from celestial hunter to champion of cellular warriors, each of us adapting to our roles in this new age of discovery.
This, dear readers, is how I, Artemis, found myself at the crossroads of mythology and molecular biology, wielding pipettes as skillfully as arrows, in a quest not for glory, but for healing. It’s a wild tale, one that blends ancient myth with modern science, proving that even gods might venture into uncharted territories when the hunt calls for it.
0 notes
Text
The global CAR T-cell therapy market size is expected to be worth around USD 88.52 billion by 2032 and growing at a CAGR of 29.8% from 2023 to 2032. The market size of CAR T-cell therapy was valued at USD 8.44 billion in 2023. The U.S. CAR T-cell therapy market size was valued at USD 1.75 billion in 2023.

1 note
·
View note
Text
Side Effects Of CAR-T Therapy
CAR-T therapy might cause cytokine release syndrome (CRS) that leads to fever, fatigue, low blood pressure, and breathing difficulties. Other general side effects of CAR-T cell therapy include headache, seizures, speech problems, and balance difficulties. The side effects generally start to improve gradually with proper treatment, care, and management.
#car t cell therapy#what is car t therapy#car t cancer treatment#cell therapy overviews#car t therapy for cancer#how does car t cell therapy work#Cell culture#customized primary cells#primary cells#biotech company#stem cells#exosomes#stem cell research center#regenerative medicine#bioengineering#Kosheeka
0 notes
Text
The CAR T Cell Therapy Market Is Driven By Two Main Factors

The global CAR T Cell Therapy Market is estimated to be valued at US$2.26 billion in 2022 and is expected to exhibit a CAGR of 20.9% over the forecast period 2022-2030, as highlighted in a new report published by Coherent Market Insights.
There has been a rise in the incidence of cancer globally. According to the World Health Organization (WHO), cancer is the second leading cause of death worldwide, and it is estimated that there will be 29.4 million new cancer cases by 2040. CAR T Cell Therapy offers a highly targeted and personalized approach to cancer treatment, making it a preferred choice for patients and healthcare providers.
Market Key Trends:
One key trend in the CAR T Cell Therapy market is the increasing use of combination therapies. Researchers and clinicians are exploring the potential of combining CAR T Cell Therapy with other treatment modalities, such as immune checkpoint inhibitors, to enhance the anti-tumor effect. This combination approach has shown promising results in clinical trials and is expected to drive market growth in the coming years. For example, a combination of CAR T Cell Therapy and PD-1 inhibitors has demonstrated improved response rates and durable remissions in patients with relapsed or refractory lymphoma.
SWOT Analysis:
Strengths:
1. Personalized and targeted approach to cancer treatment
2. Potential to achieve long-term remissions and cure in certain types of cancer
Weaknesses:
1. High cost and complex manufacturing process
2. Limited availability and accessibility in developing regions
Opportunities:
1. Expansion into new indications, such as solid tumors
2. Collaboration with pharmaceutical companies for clinical development
Threats:
1. Potential side effects, such as cytokine release syndrome and neurotoxicity
2. Competition from alternative therapies, such as immune checkpoint inhibitors
Key Takeaways:
- The global CAR T Cell Therapy market is expected to witness high growth, exhibiting a CAGR of 20.9% over the forecast period, due to increasing investments in research and development and the rising incidence of cancer.
- North America is expected to be the fastest growing and dominating region in the CAR T Cell Therapy market, owing to the presence of key players, favorable reimbursement policies, and well-established healthcare infrastructure.
- Key players operating in the global CAR T cell therapy market include Fate Therapeutics, Mustang Bio, Sorrento Therapeutics, Inc., Bluebird bio, Inc., Pfizer Inc., Gilead Sciences, Inc., Legend Biotech, Aurora Biopharma, CARsgen Therapeutics Co., Ltd, Novartis AG, Johnson & Johnson Services, Inc., and Bristol-Myers Squibb Company. These companies are actively involved in research and development activities and collaborations to drive innovation and expand their market presence.
In conclusion, CAR T Cell Therapy holds great promise in revolutionizing cancer treatment. With increasing investments in research and development and the rising incidence of cancer, the market is expected to witness significant growth in the coming years. However, challenges such as high costs and potential side effects need to be addressed to ensure widespread adoption and accessibility of this innovative therapy.
0 notes
Text
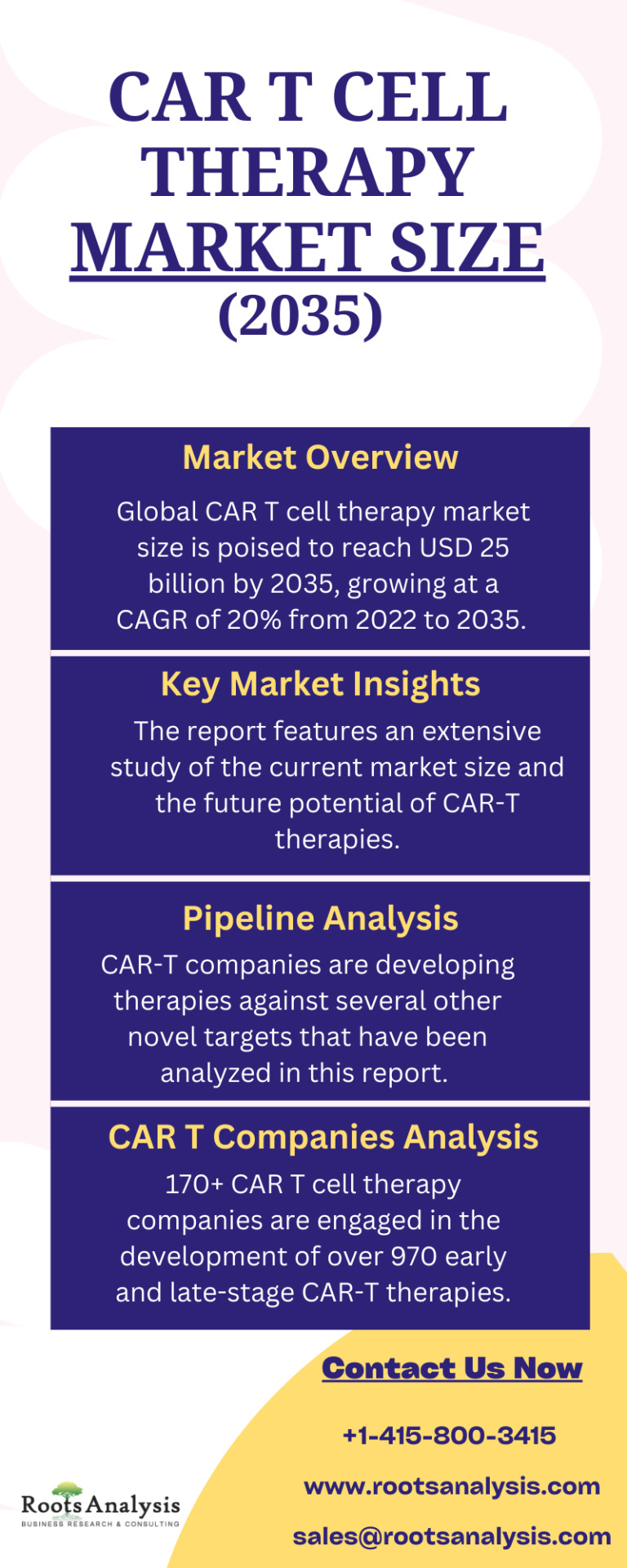
CAR T Cell Therapy Market Size, Share, Trends and Forecast (2035)
Global CAR T cell therapy market size is poised to reach USD 25 billion by 2035, growing at a CAGR of 20% from 2022 to 2035. The report features an extensive study of the current market size and the future potential of CAR-T therapies.
0 notes
Link
#market research future#car t cell therapy market#car t cell therapy market size#car t cell therapy industry#car t cell therapy
0 notes
Photo

The treatment is highly aggressive and is associated with potentially severe adverse events (AEs). Till date, no specific treatment for CAR T-cell therapy has been approved for the treatment of these two disorders. While most AEs are mild, some of them can be life-threatening.
Read More:
http://thriveblogs.weebly.com/article/car-t-cell-therapy-uses-immune-cells-for-fighting-cancer-and-has-shown-promising-results-in-treating-few-types-of-blood-cancers
Click here for CAR T Cell Therapy Market Press Release:
https://www.coherentmarketinsights.com/press-release/global-car-t-cell-therapy-market-to-reach-us-77-billion-by-2028-18
0 notes
Text
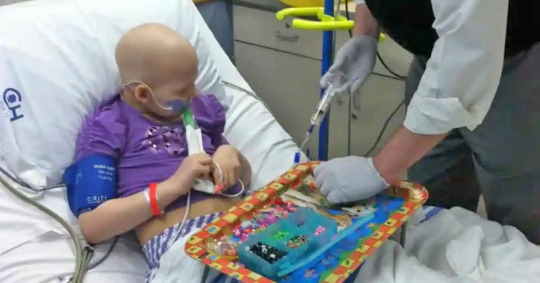
Here's the miraculous story of a teen cured of cancer after receiving CAR T-Cell therapy
Here’s the miraculous story of a teen cured of cancer after receiving CAR T-Cell therapy
In 2010, then 5-year-old Emily Whitehead had just gone to her annual checkup and was declared healthy.
But a week later, Emily’s mom, Kari, noticed that her daughter had bruises on odd parts of her body, including her back and stomach. Her gums started bleeding, and she was waking up in the middle of the night due to unbearable pain.
When Kari, 46, Googled the symptoms, she learned they were the…
View On WordPress
2 notes
·
View notes
Text
The global CAR T-Cell Therapy Market is projected to reach more than USD 22.2 billion by 2032 from USD 2.1 billion in 2023, growing at a CAGR of 30% from 2024-2032.
Key Players in Global CAR T-Cell Therapy Market
Novartis AG
Bluebird Bio, Inc.
Cellectis
Bristol-Myers Squibb
Merck & Co., Inc.
Juno Therapeutics, Inc.
Celyad Oncology
Celgene Corporation
Sorrento Therapeutics, Inc.
Miltenyi Biotech
Intellia Therapeutics
Pfizer, Inc.
Autolus Therapeutics
Gilead Sciences, Inc. (Kite Pharma Inc.)
Cartesian Therapeutics, Inc.
Caribou Biosciences, Inc.
Other Prominent Players
0 notes
Text
The Cancer Treatment In China: Uncover Some Hidden Facts About It
It's true that in the domain of cancer management and research trials, China always shines as the beacon of progress and innovation. With its strong healthcare infrastructure and research abilities, China can offer top benefits for participants in research endeavors and cancer patients. Are you someone who wants to know more about the outstanding cancer treatment in China or cancer trials in China? If Yes. This writing piece is the best place where people can know more about it in the best manner.
Cancer Treatment in China: Know Everything About It
China is known for its state-of-the-art medical or healthcare facilities equipped with top-notch technology for cancer treatment, research, and diagnosis. Patients now have access to ground-breaking interventions that greatly improve outcomes and alter the standard of care, including innovative imaging techniques, precision radiation therapies, and minimally invasive surgical procedures.
Cancer management in China takes a collaborative, multidisciplinary approach that includes professionals in oncology, surgery, imaging, and pathology. This holistic approach guarantees that patients receive personalized, comprehensive care that is suited to their specific needs, resulting in better treatment outcomes and a higher quality of life.

One distinguishing feature of cancer therapy in China is its lower cost when compared to several Western countries. The lower cost of medical services such as chemotherapy, surgery, and radiation therapy makes cancer treatment more accessible to a wider range of patients. The cancer treatment in China is worth the popularity for many reasons. You should uncover top facts about the cancer clinical trials in China if you want the best experience.
It's true that China has established itself as the top global leader in cancer research trials, offering patients a wide range of choices to acquire novel medications and contribute to scientific progress. China's oncology research system is active and growing constantly, with multiple research institutions and biotechnology platforms fully dedicated to cancer research.
#cancer treatment in China#cancer clinical trials in China#Stem cell therapy in China#CAR T Cell therapy in India
0 notes
Text
Trailblazing Triumphs: CAR T-Cell Therapy Market Successes 2033

In 2022, the global Chimeric Antigen Receptor (CAR) T-Cell Therapy Market was valued at US$ 2.54 billion, and is poised for substantial growth. Forecasts predict that the market will expand to US$ 3.5 billion by 2023 and is projected to reach an impressive US$ 6.82 billion by 2033, maintaining a steady CAGR of 6.9% from 2023 onwards.
This remarkable trajectory is primarily attributed to advancements in technology and the escalating demand for personalized treatment options. Notably, from 2018 to 2022, the CAR T-cell therapy market witnessed a robust growth at a CAGR of 7%.A distinguishing feature of CAR T-cell therapy lies in its remarkable efficacy.
Clinical trials have demonstrated response rates of up to 90% in certain leukemia and lymphoma cases, a substantial leap compared to conventional cancer treatments which typically yield response rates averaging 20-30%.
Click Here to Access Your Visuals-Packed Report!https://www.futuremarketinsights.com/reports/sample/rep-gb-16808
0 notes
Text
Accelerating CAR T cell therapy: Lipid nanoparticles speed up manufacturing - Technology Org
New Post has been published on https://thedigitalinsider.com/accelerating-car-t-cell-therapy-lipid-nanoparticles-speed-up-manufacturing-technology-org/
Accelerating CAR T cell therapy: Lipid nanoparticles speed up manufacturing - Technology Org
For patients with certain types of cancer, CAR T cell therapy has been nothing short of life-changing. Developed in part by Carl June, Richard W. Vague Professor at Penn Medicine, and approved by the Food and Drug Administration (FDA) in 2017, CAR T cell therapy mobilizes patients’ own immune systems to fight lymphoma and leukemia, among other cancers.
Using activating lipid nanoparticles (aLNPs) to create CAR T cells requires fewer steps and less time.
However, the process for manufacturing CAR T cells itself is time-consuming and costly, requiring multiple steps over days. The state-of-the-art involves extracting patients’ T cells, then activating them with tiny magnetic beads, before giving the T cells genetic instructions to make chimeric antigen receptors (CARs), the specialized receptors that help T cells eliminate cancer cells.
Now, Penn Engineers have developed a novel method for manufacturing CAR T cells, one that takes just 24 hours and requires only one step. This method uses lipid nanoparticles (LNPs), the potent delivery vehicles that played a critical role in the Moderna and Pfizer-BioNTech COVID-19 vaccines.
In a new paper in Advanced Materials, Michael J. Mitchell, Associate Professor in Bioengineering, describes the creation of “activating lipid nanoparticles” (aLNPs), which can activate T cells and deliver the genetic instructions for CARs in a single step, greatly simplifying the CAR T cell manufacturing process. “We wanted to combine these two extremely promising areas of research,” says Ann Metzloff, a doctoral student and NSF Graduate Research Fellow in the Mitchell lab and the paper’s lead author. “How could we apply lipid nanoparticles to CAR T cell therapy?”
In some ways, T cells function like a military reserve unit: in times of health, they remain inactive, but when they detect pathogens, they mobilize, rapidly expanding their numbers before turning to face the threat. Cancer poses a unique challenge to this defense strategy. Since cancer cells are the body’s own, T cells don’t automatically treat cancer as dangerous, hence the need to first “activate” T cells and deliver cancer-detecting CARs in CAR T cell therapy.
Until now, the most efficient means of activating T cells has been to extract them from a patient’s bloodstream and then mix those cells with magnetic beads attached to specific antibodies — molecules that provoke an immune response. “The beads are expensive,” says Metzloff. “They also need to be removed with a magnet before you can clinically administer the T cells. However, in doing so, you actually lose a lot of the T cells, too.”
Made primarily of lipids, the same water-repellent molecules that constitute household cooking fats like butter and olive oil, lipid nanoparticles have proven tremendously effective at delivering delicate molecular payloads. Their capsule-like shape can enclose and protect mRNA, which provides instructions for cells to manufacture proteins. Due to the widespread use of the COVID-19 vaccines, says Metzloff, “The safety and efficacy of lipid nanoparticles has been shown in billions of people around the world.”
To incorporate LNPs into the production of CAR T cells, Metzloff and Mitchell wondered if it might be possible to attach the activating antibodies used on the magnetic beads directly to the surface of the LNPs. Employing LNPs this way, they thought, might make it possible to eliminate the need for activating beads in the production process altogether. “This is novel,” says Metzloff, “because we’re using lipid nanoparticles not just to deliver mRNA encoding CARs, but also to initiate an advantageous activation state.”
Over the course of two years, Metzloff carefully optimized the design of the aLNPs. One of the primary challenges was to find the right ratio of one antibody to another. “There were a lot of choices to make,” Metzloff recalls, “since this hadn’t been done before.”
By attaching the antibodies directly to LNPs, the researchers were able to reduce the number of steps involved in the process of manufacturing CAR T cells from three to one, and to halve the time required, from 48 hours to just 24 hours. “This will hopefully have a transformative effect on the process for manufacturing CAR T cells,” says Mitchell. “It currently takes so much time to make them, and thus they are not accessible to many patients around the world who need them.”
CAR T cells manufactured using aLNPs have yet to be tested in humans, but in mouse models, CAR T cells created using the process described in the paper had a significant effect on leukemia, reducing the size of tumors, thereby demonstrating the feasibility of the technology.
Metzloff also sees additional potential for aLNPs. “I think aLNPs could be explored more broadly as a platform to deliver other cargoes to T cells,” she says. “We demonstrated in this paper one specific clinical application, but lipid nanoparticles can be used to encapsulate lots of different things: proteins, different types of mRNA. The aLNPs have broad potential utility for T cell cancer therapy as a whole, beyond this one mRNA CAR T cell application that we’ve shown here.”
Source: University of Pennsylvania
You can offer your link to a page which is relevant to the topic of this post.
#Administration#advanced materials#antibodies#antigen#Art#bioengineering#Biotechnology news#bloodstream#Cancer#cancer cells#Cancer Therapy#CAR T-cell therapy#Cars#cell#cell therapy#Cells#challenge#Chemistry & materials science news#cooking#course#covid#defense#Design#drug#engineers#FDA#Fight#Food#genetic#Giving
0 notes
Text
https://cynochat.com/read-blog/185784_car-t-cell-therapy-market-size-overview-share-and-forecast-2031.html
0 notes
Text

CAR T Cell Therapy Market in Clinical Trials | Market Size(2035)
Global CAR T cell therapy market size is poised to reach USD 25 billion by 2035, growing at a CAGR of 20% from 2022 to 2035. The roots analysis report features an extensive study of the current market size and the future potential of CAR-T therapies.
0 notes