#cell therapy
Text

#rap & hip-hop#rap#hip hop#hiphop#music#1990s#90s#goodie mob#cell therapy#soul food#dungeon family#gif
12 notes
·
View notes
Text
Unlocking mRNA’s cancer-fighting potential
New Post has been published on https://thedigitalinsider.com/unlocking-mrnas-cancer-fighting-potential/
Unlocking mRNA’s cancer-fighting potential


What if training your immune system to attack cancer cells was as easy as training it to fight Covid-19? Many people believe the technology behind some Covid-19 vaccines, messenger RNA, holds great promise for stimulating immune responses to cancer.
But using messenger RNA, or mRNA, to get the immune system to mount a prolonged and aggressive attack on cancer cells — while leaving healthy cells alone — has been a major challenge.
The MIT spinout Strand Therapeutics is attempting to solve that problem with an advanced class of mRNA molecules that are designed to sense what type of cells they encounter in the body and to express therapeutic proteins only once they have entered diseased cells.
“It’s about finding ways to deal with the signal-to-noise ratio, the signal being expression in the target tissue and the noise being expression in the nontarget tissue,” Strand CEO Jacob Becraft PhD ’19 explains. “Our technology amplifies the signal to express more proteins for longer while at the same time effectively eliminating the mRNA’s off-target expression.”
Strand is set to begin its first clinical trial in April, which is testing a proprietary, self-replicating mRNA molecule’s ability to express immune signals directly from a tumor, eliciting the immune system to attack and kill the tumor cells directly. It’s also being tested as a possible improvement for existing treatments to a number of solid tumors.
As they work to commercialize its early innovations, Strand’s team is continuing to add capabilities to what it calls its “programmable medicines,” improving mRNA molecules’ ability to sense their environment and generate potent, targeted responses where they’re needed most.
“Self-replicating mRNA was the first thing that we pioneered when we were at MIT and in the first couple years at Strand,” Becraft says. “Now we’ve also moved into approaches like circular mRNAs, which allow each molecule of mRNA to express more of a protein for longer, potentially for weeks at a time. And the bigger our cell-type specific datasets become, the better we are at differentiating cell types, which makes these molecules so targeted we can have a higher level of safety at higher doses and create stronger treatments.”
Making mRNA smarter
Becraft got his first taste of MIT as an undergraduate at the University of Illinois when he secured a summer internship in the lab of MIT Institute Professor Bob Langer.
“That’s where I learned how lab research could be translated into spinout companies,” Becraft recalls.
The experience left enough of an impression on Becraft that he returned to MIT the next fall to earn his PhD, where he worked in the Synthetic Biology Center under professor of bioengineering and electrical engineering and computer science Ron Weiss. During that time, he collaborated with postdoc Tasuku Kitada to create genetic “switches” that could control protein expression in cells.
Becraft and Kitada realized their research could be the foundation of a company around 2017 and started spending time in the Martin Trust Center for MIT Entrepreneurship. They also received support from MIT Sandbox and eventually worked with the Technology Licensing Office to establish Strand’s early intellectual property.
“We started by asking, where is the highest unmet need that also allows us to prove out the thesis of this technology? And where will this approach have therapeutic relevance that is a quantum leap forward from what anyone else is doing?” Becraft says. “The first place we looked was oncology.”
People have been working on cancer immunotherapy, which turns a patient’s immune system against cancer cells, for decades. Scientists in the field have developed drugs that produce some remarkable results in patients with aggressive, late-stage cancers. But most next-generation cancer immunotherapies are based on recombinant (lab-made) proteins that are difficult to deliver to specific targets in the body and don’t remain active for long enough to consistently create a durable response.
More recently, companies like Moderna, whose founders also include MIT alumni, have pioneered the use of mRNAs to create proteins in cells. But to date, those mRNA molecules have not been able to change behavior based on the type of cells they enter, and don’t last for very long in the body.
“If you’re trying to engage the immune system with a tumor cell, the mRNA needs to be expressing from the tumor cell itself, and it needs to be expressing over a long period of time,” Becraft says. “Those challenges are hard to overcome with the first generation of mRNA technologies.”
Strand has developed what it calls the world’s first mRNA programming language that allows the company to specify the tissues its mRNAs express proteins in.
“We built a database that says, ‘Here are all of the different cells that the mRNA could be delivered to, and here are all of their microRNA signatures,’ and then we use computational tools and machine learning to differentiate the cells,” Becraft explains. “For instance, I need to make sure that the messenger RNA turns off when it’s in the liver cell, and I need to make sure that it turns on when it’s in a tumor cell or a T-cell.”
Strand also uses techniques like mRNA self-replication to create more durable protein expression and immune responses.
“The first versions of mRNA therapeutics, like the Covid-19 vaccines, just recapitulate how our body’s natural mRNAs work,” Becraft explains. “Natural mRNAs last for a few days, maybe less, and they express a single protein. They have no context-dependent actions. That means wherever the mRNA is delivered, it’s only going to express a molecule for a short period of time. That’s perfect for a vaccine, but it’s much more limiting when you want to create a protein that’s actually engaging in a biological process, like activating an immune response against a tumor that could take many days or weeks.”
Technology with broad potential
Strand’s first clinical trial is targeting solid tumors like melanoma and triple-negative breast cancer. The company is also actively developing mRNA therapies that could be used to treat blood cancers.
“We’ll be expanding into new areas as we continue to de-risk the translation of the science and create new technologies,” Becraft says.
Strand plans to partner with large pharmaceutical companies as well as investors to continue developing drugs. Further down the line, the founders believe future versions of its mRNA therapies could be used to treat a broad range of diseases.
“Our thesis is: amplified expression in specific, programmed target cells for long periods of time,” Becraft says. “That approach can be utilized for [immunotherapies like] CAR T-cell therapy, both in oncology and autoimmune conditions. There are also many diseases that require cell-type specific delivery and expression of proteins in treatment, everything from kidney disease to types of liver disease. We can envision our technology being used for all of that.”
#Alumni/ae#approach#Behavior#bioengineering#Bioengineering and biotechnology#Biological engineering#Biology#blood#breast cancer#Cancer#cancer cells#cell#cell therapy#cell types#Cells#CEO#challenge#change#Companies#computer#Computer Science#covid#Database#datasets#deal#Disease#Diseases#drug development#drugs#easy
7 notes
·
View notes
Text
FDA Approves Omisirge, a Cell Therapy for Blood Cancer Patients Undergoing Stem Cell Transplantation
The FDA has recently approved a cell therapy called Omisirge (omidubicel-onlv) for patients with blood cancers who are undergoing stem cell transplantation. This allogeneic cord blood-based cell therapy can help speed up the recovery of neutrophils in the body, a type of white blood cell, and reduce the risk of infection. Omisirge is intended for use in adults and pediatric patients 12 years and…

View On WordPress
#allogeneic cord blood-based cell therapy#allogeneic stem cells#bacterial infections#blood cancers#breakthrough therapy#cell therapy#engraftment syndrome#FDA#fungal infections#graft failure#graft-versus-host disease#infection#infusion reactions#innovative therapies#myeloablative conditioning regimen#neutrophils#nicotinamide#Omisirge#randomized multicenter study#rare genetic diseases#secondary malignancies#side effects#stem cell transplantation#transmission of serious infections#umbilical cord blood#white blood cell
5 notes
·
View notes
Text
Europe Single-use Bioreactors Market by Product [Systems, Media Bags (2D, 3D), Filtration Assemblies], Type [Stirred Tank, Wave Induced], Cell [Mammalian, Bacterial, Yeast], Application [Commercial (Mab, Vaccine), Research], End User - Forecast to 2030
#Europe Single-use Bioreactors Market#Single-use Bioreactors Market#Single-use Bioreactors#cell culture#cell therapy#gene therapy#bioprocess#bioprocessing#bioproduction#biotechnology
0 notes
Text
A micro-fragmented collagen gel as a stem cell-assembling platform for critical limb ischemia repair
Critical limb ischemia is a condition in which the main blood vessels supplying blood to the legs are blocked, causing blood flow to gradually decrease as atherosclerosis progresses in the peripheral arteries. It is a severe form of peripheral artery disease that causes progressive closure of arteries in the lower extremity, leading to the necrosis of the leg tissue and eventual…
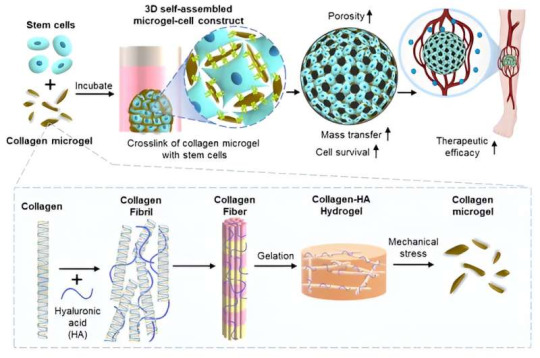
View On WordPress
0 notes
Text
Mira's Lymphoma Warrior Journey: Hope and CAR-T Therapy
Estimated reading time: 7 minutes
The bold headlines proclaimed the promise of CAR T therapy and its efficacy in combating lymphoma’s relentless grip. Mira – a woman who had weathered the storm of cancer diagnosis, treatment, and remission. She paused to reflect on hope with her life-changing lymphoma warrior journey. As the city’s symphony of car horns echoed outside her window, she stepped…

View On WordPress
0 notes
Video
youtube
Danny Brown - Jenn's Terrific Vacation (Official Video)
youtube
Goodie Mob - Cell Therapy (Official HD Video)
youtube
Kassa Overall: Tiny Desk Concert
0 notes
Text
TIL Cell Therapy Market CAGR | Analysis Size & Forecast (2035)
The TIL therapy market, a part of the broader cell therapy market, is experiencing significant growth and is projected to grow at a compounded annual growth rate (CAGR) of 40% during the forecast period. The report also provides sales forecasts for TIL therapies market CAGR that are currently in the mid-to-late stages of development. Get a detailed insights report now!
0 notes
Text
Accelerating CAR T cell therapy: Lipid nanoparticles speed up manufacturing - Technology Org
New Post has been published on https://thedigitalinsider.com/accelerating-car-t-cell-therapy-lipid-nanoparticles-speed-up-manufacturing-technology-org/
Accelerating CAR T cell therapy: Lipid nanoparticles speed up manufacturing - Technology Org
For patients with certain types of cancer, CAR T cell therapy has been nothing short of life-changing. Developed in part by Carl June, Richard W. Vague Professor at Penn Medicine, and approved by the Food and Drug Administration (FDA) in 2017, CAR T cell therapy mobilizes patients’ own immune systems to fight lymphoma and leukemia, among other cancers.
Using activating lipid nanoparticles (aLNPs) to create CAR T cells requires fewer steps and less time.
However, the process for manufacturing CAR T cells itself is time-consuming and costly, requiring multiple steps over days. The state-of-the-art involves extracting patients’ T cells, then activating them with tiny magnetic beads, before giving the T cells genetic instructions to make chimeric antigen receptors (CARs), the specialized receptors that help T cells eliminate cancer cells.
Now, Penn Engineers have developed a novel method for manufacturing CAR T cells, one that takes just 24 hours and requires only one step. This method uses lipid nanoparticles (LNPs), the potent delivery vehicles that played a critical role in the Moderna and Pfizer-BioNTech COVID-19 vaccines.
In a new paper in Advanced Materials, Michael J. Mitchell, Associate Professor in Bioengineering, describes the creation of “activating lipid nanoparticles” (aLNPs), which can activate T cells and deliver the genetic instructions for CARs in a single step, greatly simplifying the CAR T cell manufacturing process. “We wanted to combine these two extremely promising areas of research,” says Ann Metzloff, a doctoral student and NSF Graduate Research Fellow in the Mitchell lab and the paper’s lead author. “How could we apply lipid nanoparticles to CAR T cell therapy?”
In some ways, T cells function like a military reserve unit: in times of health, they remain inactive, but when they detect pathogens, they mobilize, rapidly expanding their numbers before turning to face the threat. Cancer poses a unique challenge to this defense strategy. Since cancer cells are the body’s own, T cells don’t automatically treat cancer as dangerous, hence the need to first “activate” T cells and deliver cancer-detecting CARs in CAR T cell therapy.
Until now, the most efficient means of activating T cells has been to extract them from a patient’s bloodstream and then mix those cells with magnetic beads attached to specific antibodies — molecules that provoke an immune response. “The beads are expensive,” says Metzloff. “They also need to be removed with a magnet before you can clinically administer the T cells. However, in doing so, you actually lose a lot of the T cells, too.”
Made primarily of lipids, the same water-repellent molecules that constitute household cooking fats like butter and olive oil, lipid nanoparticles have proven tremendously effective at delivering delicate molecular payloads. Their capsule-like shape can enclose and protect mRNA, which provides instructions for cells to manufacture proteins. Due to the widespread use of the COVID-19 vaccines, says Metzloff, “The safety and efficacy of lipid nanoparticles has been shown in billions of people around the world.”
To incorporate LNPs into the production of CAR T cells, Metzloff and Mitchell wondered if it might be possible to attach the activating antibodies used on the magnetic beads directly to the surface of the LNPs. Employing LNPs this way, they thought, might make it possible to eliminate the need for activating beads in the production process altogether. “This is novel,” says Metzloff, “because we’re using lipid nanoparticles not just to deliver mRNA encoding CARs, but also to initiate an advantageous activation state.”
Over the course of two years, Metzloff carefully optimized the design of the aLNPs. One of the primary challenges was to find the right ratio of one antibody to another. “There were a lot of choices to make,” Metzloff recalls, “since this hadn’t been done before.”
By attaching the antibodies directly to LNPs, the researchers were able to reduce the number of steps involved in the process of manufacturing CAR T cells from three to one, and to halve the time required, from 48 hours to just 24 hours. “This will hopefully have a transformative effect on the process for manufacturing CAR T cells,” says Mitchell. “It currently takes so much time to make them, and thus they are not accessible to many patients around the world who need them.”
CAR T cells manufactured using aLNPs have yet to be tested in humans, but in mouse models, CAR T cells created using the process described in the paper had a significant effect on leukemia, reducing the size of tumors, thereby demonstrating the feasibility of the technology.
Metzloff also sees additional potential for aLNPs. “I think aLNPs could be explored more broadly as a platform to deliver other cargoes to T cells,” she says. “We demonstrated in this paper one specific clinical application, but lipid nanoparticles can be used to encapsulate lots of different things: proteins, different types of mRNA. The aLNPs have broad potential utility for T cell cancer therapy as a whole, beyond this one mRNA CAR T cell application that we’ve shown here.”
Source: University of Pennsylvania
You can offer your link to a page which is relevant to the topic of this post.
#Administration#advanced materials#antibodies#antigen#Art#bioengineering#Biotechnology news#bloodstream#Cancer#cancer cells#Cancer Therapy#CAR T-cell therapy#Cars#cell#cell therapy#Cells#challenge#Chemistry & materials science news#cooking#course#covid#defense#Design#drug#engineers#FDA#Fight#Food#genetic#Giving
0 notes
Text
Cell & Gene Therapy Clinical Trials Market by Phase (Phase I, Phase II), Indication (Oncology, Cardiology) – Global Outlook & Forecast 2023-2031
According to the deep-dive market assessment study by Growth Plus Reports, the global cell & gene therapy clinical trials market was valued at US$ 9.59 billion in 2022 and is expected to register a revenue CAGR of 13.5% to reach US$ 29.69 billion by 2031.
Cell & Gene Therapy Clinical Market Fundamentals
Cell and gene therapy clinical trials refer to research studies conducted to evaluate the safety, efficacy, and potential applications of cell and gene therapies in human patients. These trials aim to assess the therapeutic benefits and risks associated with these innovative treatment approaches. Clinical trials for cell and gene therapies are typically conducted in multiple phases. In early-phase trials (Phase I and Phase II), the primary focus is on evaluating the safety and tolerability of the therapy, determining the optimal dosage and administration route, and gathering preliminary efficacy data. These trials often involve a small number of participants and closely monitor their responses.
Get Free Sample PDF including full TOC, Tables and Figures, and Available customizations: https://www.growthplusreports.com/inquiry/request-sample/cell-gene-therapy-clinical-trials-market/9280
Cell & Gene Therapy Clinical Market Dynamics
The rising incidence of genetic disorders, chronic diseases such as cancer and cardiovascular diseases, and other conditions with limited treatment options have created a strong demand for innovative therapies like cell & gene therapy. Clinical trials in this field aim to develop effective treatments for these diseases, driving cell & gene therapy clinical trialsmarket growth. According to the WHO, cardiovascular diseases are the main cause of mortality worldwide, accounting for an estimated 17.9 million lives per year, or 32% of all fatalities worldwide. More than 75% of deaths occur in low and middle-income nations. Rapid advancements in biotechnology, gene editing technologies (such as CRISPR-Cas9), and our understanding of genetics have significantly contributed to developing cell and gene therapies. These advancements have enabled scientists to identify target genes and develop precise therapies, leading to increased clinical trials. Several landmark clinical trials have demonstrated the safety and efficacy of cell and gene therapies in treating various diseases. Positive outcomes in trials for diseases like leukemia, lymphoma, and inherited retinal disorders have generated enthusiasm among researchers, clinicians, and patients, leading to increased participation in clinical trials and further driving the cell & gene therapy clinical trialsmarket demand. The field of cell & gene therapy has attracted significant investments and funding from both public and private sources. Pharmaceutical companies, venture capitalists, and government organizations recognize the potential of these therapies and are investing in research and development, infrastructure, and clinical trials, which are also expected to boost the growth of the cell & gene therapy clinical trialsmarket.
However, developing and conducting cell & gene therapy clinical trials can be extremely expensive due to the complexity of these therapies. Costs are associated with research and development, manufacturing, regulatory compliance, and clinical trial operations. These high costs pose a challenge for small biotech companies and academic institutions with limited resources, restricting the growth of gene therapy clinical trials. While regulatory agencies have made efforts to facilitate the development and approval of cell and gene therapies, navigating the regulatory landscape can still be challenging. Meeting regulatory requirements for safety, efficacy, and quality is essential but can involve complex processes and lengthy approval timelines, which is also hindering the growth of the gene therapy clinical trialsmarket.
Cell & Gene Therapy Clinical Market Ecosystem
The global cell & gene therapy clinical trialsmarket is analyzed from three perspectives: phase, indication, and region.
Cell & Gene Therapy Clinical Market by Phase
Based on the phases, the global cell & gene therapy clinical trialsmarket is segmented into phase I, phase II, phase III, and phase IV.
The phase II segment accounted for the largest revenue share, with a 51% cell & gene therapy clinical trials market share. Phase II trials aim to assess the efficacy of the therapy in a larger patient population. They provide more extensive data on the therapeutic benefits and effectiveness of the treatment. This phase often involves comparing the therapy to existing standard treatments or placebos, allowing for a more comprehensive evaluation of its efficacy. Phase II trials help refine the dosage and administration protocols of the therapy. The initial Phase I trials provide some insight into dosage levels, but Phase II allows for a more systematic exploration of different doses and administration schedules to identify the optimal therapeutic regimen. Phase II trials often involve a larger number of patients, allowing for better selection and inclusion of a diverse patient population. This enables researchers to assess the therapy's efficacy in different subgroups and evaluate its potential benefits across a broader range of patients. Regulatory agencies typically require data from well-designed Phase II trials to support the advancement of therapies to Phase III and subsequent stages. The data collected from Phase II trials are crucial for demonstrating the therapy's efficacy and safety profile, supporting regulatory submissions, and obtaining further approvals for larger-scale trials. Positive results from Phase II trials often generate significant interest from investors, as they indicate the therapy's potential for success. Promising efficacy and safety data from Phase II trials can attract funding and partnerships for further development and commercialization of the therapy, driving market dominance in this phase.
Cell & Gene Therapy Clinical Market by Indication
Based on the indications, the global cell & gene therapy clinical trialsmarket is segmented into oncology, cardiology, CNS, musculoskeletal, infectious diseases, immunology & inflammation, ophthalmology, dermatology, endocrine, metabolic, genetic, hematology, gastroenterology, and others.
The oncology segment accounted for the prominent cell & gene therapy clinical trials market share in 2022, with a 45% market share. Oncology represents a significant unmet medical need, with a wide range of cancers having limited treatment options. According to GLOBOCAN 2020 report, approximately 10 million deaths were cases by cancer in 2020. Cell and gene therapies offer potential breakthroughs in cancer treatment by targeting specific genetic alterations or enhancing the immune system's ability to recognize and eliminate cancer cells. The urgent need for effective cancer treatments drives the focus on oncology in clinical trials. Oncology has been a well-established area of research and development for many years. This has led to a deeper understanding of cancer biology, genetic alterations, and immune responses in the context of cancer. The infrastructure and expertise required for conducting clinical trials in oncology are well-established. Oncology research centers, academic institutions, and specialized hospitals often have the necessary infrastructure, multidisciplinary teams, and patient populations available to conduct cell & gene therapy clinical trials. The market potential for oncology treatments is significant, given the high prevalence of cancer and the increasing demand for more effective therapies. Successful cell and gene therapies in oncology have the potential for commercial success, attracting investments from both pharmaceutical companies and venture capitalists. This market potential further drives the growth of the oncology segment in the cell & gene therapy clinical trials market.
Cell & Gene Therapy Clinical Market by Region
Geographically, the global cell & gene therapy clinical trials market has been segmented into North America, Europe, Asia Pacific, Latin America, and the Middle East & Africa.
The North America region has the largest cell & gene therapy clinical trials market size in terms of revenue generation accounting for around 46.2% share of the market. North America boasts a strong biotechnology and pharmaceutical industry, with numerous research institutions, academic centers, and biotech companies at the forefront of cell & gene therapy development. These entities contribute to the discovery, development, and commercialization of innovative therapies, driving the growth of clinical trials in the region. North America has a well-established, advanced healthcare infrastructure, including specialized treatment centers, research institutions, and clinical trial networks. This infrastructure supports the conduct of clinical trials by providing access to patient populations, expert clinicians, and specialized facilities required to deliver cell and gene therapies. North America is a hub for biotech and venture capital investments, attracting significant funding for cell & gene therapy research and development. Academic institutions, government agencies, and private investors provide financial support to advance clinical trials in the region. North America fosters a collaborative environment, with academic institutions, biotech companies, and research organizations working together on cell & gene therapy projects. Collaborations between academia and industry and partnerships between different stakeholders facilitate knowledge sharing, access to resources, and the progression of clinical trials.
Cell & Gene Therapy Clinical Market Competitive Landscape
The prominent players operating in the global cell & gene therapy clinical trials market are:
ICON Plc
IQVIA
Charles River Laboratories International, Inc.
Laboratory Corporation of America Holdings
Syneos Health
PAREXEL International Corp.
Medpace Holdings, Inc.
PPD Inc.
Novotech
Veristat, LLC
Cell & Gene Therapy Clinical Market Strategic Developments
In June 2023, Arrowhead Pharmaceuticals submitted an application seeking approval to initiate a Phase I clinical trial of ARO-SOD1 to treat amyotrophic lateral sclerosis (ALS) harbouring superoxide dismutase 1 (SOD1) mutations. In compliance with the Australian Department of Health and Ageing's Therapeutic Goods Administration's clinical trial notification process, the company filed the application to an ethics committee. The RNAi-based experimental drug ARO-SOD1 is being tested in adults with ALS with SOD1 mutations in a dose-escalation, placebo-controlled, randomized research.
In June 2023, Beacon Therapeutics entered into the gene therapy field with a $120m Series A financing. The new ocular gene therapy firm was founded by integrating Applied Genetic Technologies Corporation's (AGTC) late-stage X-linked retinitis pigmentosa (XLRP) program with two unique preclinical studies. The finance includes AGTC's acquisition and funds to help speed Beacon Therapeutics' candidate development, with participation from Oxford Science Enterprises (OSE). The total amount of funding was £96 million ($120 million).
Visit our report store at https://www.growthplusreports.com/report-store

#gene therapy#clinical trials#cell therapy#gene editing#clinical research#regenerative medicine#Clinical Trial Management#precision medicine
0 notes
Text
Thing People Get Wrong About Benefits of Stem Cell Therapy

You may find yourself in an emotionally charged situation if you engage yourself in a discussion related to stem cell. While the controversy about stem cell therapy will cross the religious and political beliefs, it is controversial topic of discussion for everybody.
Due to personal biasness, much of the contention is the result of misleading and incorrect information. While the clinical research that was necessary to advance this field of medicine, personal biasness has slowed it down.
When researchers discovered that cells were building blocks of life, cell research started in the mid — 1880s. Doctors had performed 200 allogeneic stem cell transplants in human by 1950s and 60s without any success.
While treating immune deficiency through bone marrow transplant, stems cells in the late 1960s were also used for treatment of leukemia and aplastic anemia.
It is seen that there are some myths and facts related to stem cell, which are discussed in this article. Let us dig it in.
Stem Cells Are Taken from Embryos
True/False
It is said that practices, and clinics do use embryonic stem cells for their procedures. When it comes to medical injection therapy, it is not the case. It is seen that you can classify stem cell into two categorise viz: embryonic and adult cell.
It is seen that doctors can easily replicate the stem cells into other types of cells, which they use through the adult mesenchymal cells. These cells are very undifferentiated with the normal adult cells.
Before injecting to the affected site, these adults stem cells are taken from the patient’s stem cell or adipose tissue. Doctors can use platelet rich plasma injections for curing the condition.
Stem Cell Is Unsafe
False
If you go to an unreliable, and unauthorized clinic, you will know that doctors are providing unsafe stem cell therapy. It is seen that doctors will not provide such improper practices, as you do not have worry about it.
It is the trained team of doctors, who will perform such therapies. These doctors will take an autologous approach so that you do not get a transmitted infectious disease. While doctors try to make it risk free, you will know that the therapy is minimally invasive.
After analyzing the medical history of patients, doctors will go ahead with the injection therapy. Before administering the injection therapy, they will brief you about the process.
Therapy is Prone to Body Rejection
False
There is no chance of body rejecting the injected cells, as stem cells is autologous. While there are chances of elimination of chances of rejection which is a cause of concern in donor transplant, your immune system would not treat them as a foreign invasion.
While assisting the degenerative alignments, and condition, the stem cells will stimulate the body’s natural healing response. It will provide a long — lasting and quick solution.
Therapy Will Cure Several Aliments
True
Doctors can treat several illnesses, and medical conditions, as it has become possible with stem cell. While used for the healing sports related injuries like bruised tendons, torn ligaments and muscle pain, the therapy is very useful for the musculoskeletal injuries.
The therapy is also very effective for the osteoarthritis. It is seen stem cell therapy will promote cartilage regeneration.
Re Adult Stem Cells as Good — Or Better — Than Embryonic Stem Cells?
While helping for future therapies, adult stem cells have great potential and are extremely valuable. While adult stem cells can only follow certain paths, embryonic cells can grow virtually into any cell type in the body.
While the embryonic stem cells are not so flexible in treating all types of diseases, adult stem cells do not grow indefinitely in the labs.
Final Thoughts
There are lots of benefits for stem cell. You should not remain in notion that stem cell is a dangerous therapy for your disease.
0 notes
Text
Restoring Dopamine Production in Parkinson's Disease?
Replacing Lost Neurons in Parkinson's Disease with Bemdaneprocel and ANPD001 cell therapies
Bemdaneprocel dopaminergic neuron cell therapy by BlueRock Therapeutics, A Bayers company
Key points
Focus on Allogeneic therapy – a drug product developed from human embryonic stem cells.
Recently completed Phase I study.
One-year interim data presents
Dopaminergic neurons survived and engrafted into the brain upon surgical implantation
High dose cohort presented improvement in "on" status (2.16 hr)
Low dose cohort showed improvement in "on" status (0.72 hr)
BlueRock Therapeutics is starting Phase II very soon.
ANPD001 Dopaminergic neuron precursor cell therapy by Aspen Neuroscience recently received FDA clearance, to begin with clinical trial
Focus on autologous therapy, i.e., collecting patient skin cells, reprogramming them to iPSCs, and developing dopamine neuronal precursor cells as a drug product.
Phase 1 clinical trial starts soon.
#FDA approved clinical trials#medicine#health#mental health#mentalheathawareness#positive mental attitude#neuroscientist#neuroscience#dopaminergic neuron cell therapy#aspen neuroscience#cell therapy#parkinsons disease#parkinsonsawareness#stem cells#dyskinesia#Parkinson disease treatment#Bayers company#Blue rock therapeutics#fda approved clinical trials
0 notes