#deamidization
Text
It's official official today. I have celiacs disease.
Here's my journey to diagnosis in a nut shell. It started off slow and just cascaded starting this past summer:
Moved to a new state ~4.5 years ago and instantly got a weird rash (which has only gotten increasingly miserable). Assumed a local allergy.
Didn't test positive for any local allergens and none of the 4 doctors I saw recognized DH. In particular, the dermatologist literally left me in a puddle of tears. He was so rude about my dermatillomania.
About a year ago, I stumbled upon Dermatitis Herpetiformis (DH) on Google and got suspicious.
Ignorantly decided to go GF to test my theory (don't, for the love of God, do this before you get tested). 80-90% of the rash cleared in about 6-8 months.
While waiting for a new doctor's appointment over the summer, I got like 12+ cavities filled and discovered an enamel pit on one of my front teeth. (I've never had a cavity before and I'm 28.)
General annual blood work shows deficiency in vitamin D + elevated ALT and AST liver enzyme levels.
Finally got an appointment with a new dermatologist, who agreed it was probably DH. First biopsy was inconclusive.
Started my 6 week gluten challenge and obtained a ton of fun new symptoms, like severe head ache, stomach ache, bloating, gas. Plus my DH surged again, with a vengence. Absolutely **fucking miserable** time.
Celiac blood panel shows ttg IgA and Deamidated Gliadin IgA both >250 u/ml (normal range is 0-3 u/ml).
Two new skin biopsies come back positive/consistent with DH.
Additional blood work shows iron deficiency.
DEXA bone density scan (still don't have those results).
Gastroenterologist said my endoscopy results showed extreme damage to the small intestine. Villi are basically completely flat.
So here I am. Systematically going through my kitchen to get rid of gluten-y things. I'm so excited to have an answer and to FINALLY feel better over the next few years!! 🎉



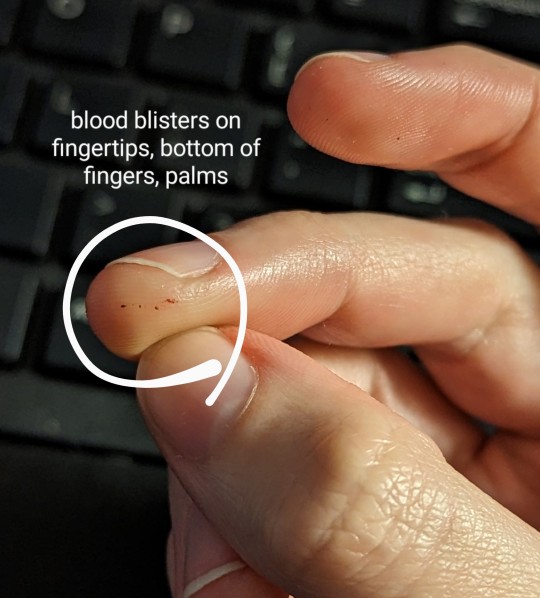
#celiac#celiacs#Dermatitis Herpetiformis#dh#rash#liver#cavities#enamel pit#iron deficient#vitamin d deficiency#endoscopy
4 notes
·
View notes
Text
Introduction
An extensive proteomic analysis was performed on a set of 12 bones of human victims of the eruption that in AD 79 rapidly buried Pompeii and Herculaneum, allowing the detection of molecular signatures imprinted in the surviving protein components. Bone collagen survived the heat of the eruption, bearing a piece of individual biological history encoded in chemical modifications. Here we show that the human bone proteomes from Pompeii are more degraded than those from the inhabitants of Herculaneum, despite the latter were exposed to temperatures much higher than those experienced in Pompeii. The analysis of the specimens from Pompeii shows lower content of non-collagenous proteins, higher deamidation level and higher extent of collagen modification. In Pompeii, the slow decomposition of victims' soft tissues in the natural dry–wet hydrogeological soil cycles damaged their bone proteome more than what was experienced at Herculaneum by the rapid vanishing of body tissues from intense heat, under the environmental condition of a permanent waterlogged burial context. Results herein presented are the first proteomic analyses of bones exposed to eruptive conditions, but also delivered encouraging results for potential biomarkers that might also impact future development of forensic bone proteomics.
Ancient protein analysis provides clues about human and animal life and diseases from the past[ 1]. Bone, a mineralised extracellular matrix rich in connective tissue components, is one of the most abundant sources of ancient proteins and one of the most abundant biomaterials in the archaeological record. The diagenesis, in particular when heat-induced, of organic matter in archaeological bone, initially represented mostly by type I collagen (90%), lipoproteins and mucopolysaccharides[ 2], is a complex phenomenon[ 3],[ 4], linked to several factors in the depositional environment[ 2],[ 5],[ 6]. The analysis of how diagenesis acted to produce or prevent specific modifications led to coin the term "diagenetiforms" to describe the variety of distinct molecular species arising from chemical modifications by environmental conditions[ 7].
Mass spectrometry-based techniques have been widely applied to characterize collagen in ancient proteomes and its chemical modifications occurred pre- and post-mortem[ 8]. Apart from the extensive observation of deamidation of asparagines and glutamines Gln[ 9]–[12] and methionine oxidation[13], a few other diagenetically induced modifications were also detected, such as: (i) aminoadipic acid formation from lysine, (ii) tryptophan oxidation products[13], (iii) advanced glycation end-product (AGEs)[13]–[15], (vi) backbone cleavage[14],[15], while complex oxidative reactions occurring on prolines have been hypothesised but not characterised in details[13],[14]. So far though, no study characterized the modifications occurred post-mortem in the proteome of archaeological bones from individuals who died due to the nearly instantaneous exposure to extreme temperature.
Here we applied a bottom-up proteomic approach to investigate the proteome and the chemical modifications present in the bone proteins of humans from Herculaneum[16] and Pompeii (Italy)[17], who died during the eruption of the Vesuvius in 79 AD. The accurate description of the catastrophic event affecting Herculaneum and Pompeii provided by ancient sources, and the peculiar burial conditions[18]–[20], offer a unique opportunity to analyse lifeways in a relatively large cohort of perfectly coeval individuals who lived and died together[21], whose bone proteome experienced such extreme conditions.
Herculaneum, Pompeii and other Roman settlements up to 20 kms away from the volcano were suddenly hit by successive hot pyroclastic currents and buried by up to tens of meters thick volcanic ash deposits produced by the 79 AD eruption, that killed everyone who had not been evacuated or managed to flee[16]. The skeletal remains were in an excellent state of preservation as a result of the unusual death and burial conditions: instant death caused by hot pyroclastic surges at temperatures between approx. 300 (Pompeii) and 500 °C (Herculaneum)[16],[17],[22].
In Herculaneum, death was followed by vanishing of soft tissues and rapid replacement by volcanic ash[19]. Here, some evidence suggests that the volcanic environment was characterised by a drop in temperature of the first pyroclastic surge during its emplacement. Evidence of rapid cooling of the volcanic ash cloud may account for the preservation of organic tissue residues[22],[23] and organic compounds[24]. At Herculaneum the ash-bed deposit was permanently waterlogged by groundwater[25],[26], as revealed by the early 1980s archaeological investigations of the victims' skeletons on the ancient beach, thus a permanent system of hydraulic pumps was activated[20]. The burial environment of the victims was most likely able to inhibit microbial attack to bone and related diagenetic processes[27].
On the other hand, the temperature of approx. 300 °C experienced by the victims in Pompeii was sufficient as well to instantly kill people, but it was not hot enough to cause rapid soft tissue disappearing as in the case of Herculaneum. Therefore, in Pompeii the victims' corpses were preserved intact inside the ash deposit after its rapid cooling and hardening around them[16]. The cavity formed around the victim's body after the slow disappearance of the flesh would then be filled with plaster of Paris in order to obtain plaster casts, technique adopted for the first time on human victims in 1863 by Giuseppe Fiorelli[28]. Over the last century and up to the present, in the case of bodies of victims found in the ash surge deposit, this technique has been used to replicate the features of the body.
In this paper, our objective was a proteomic profiling of the bones of the eruption victims, using a bottom-up proteomic approach and an unbiased discovery of chemically modified peptides[29], in search for signatures of the high temperatures and environmental conditions the bodies were exposed to. Bone collagen survived even the harshest conditions of temperature imposed by the volcano eruption, bearing encoded in the chemical modifications a piece of individual biological history. For comparison, the bones from a coeval skeletal population from the Campanian region were considered (Baia, Scalandrone locality, II sec. AD, Roman Imperial Age, Puteoli, Naples, Italy). The necropolis site of Baia Scalandrone was chosen as a control because it is coeval with the sites of Herculaneum and Pompeii and, as with these, the burial ground is of volcanic origin. Furthermore, unlike the two Vesuvian sites, the bodies of these individuals were not exposed to heat.
Most interestingly, bones are frequently found in archaeological and forensic contexts, and their characterization for the study of past populations (e.g., age at death or details of funerary practices), or for victim characterization in forensic investigations is of unquestionable relevance[30]–[33]. Actually, burned bones might be the only remains found in forensic scenarios (e.g. from terrorist attacks, explosions or fires) from which identify victims or obtain information, and the present study is expected to contribute to a full molecular characterisation of bones that have been exposed to heat.
Results
Proteome of human bones
A shotgun proteomics approach by LC–MS/MS (Figure S2) was applied to skeletal samples excavated from the archaeological sites of Pompeii (7 samples) and Herculaneum (5 samples)[16],[19]. Samples collected from three individuals from Roman Imperial Age (II sec. AD) cemetery in Baia Scalandrone were also analysed to compare results from the AD 79 eruption victims with this coeval skeletal population. All specimens are illustrated in the Table S1 and Figure S1. Moreover, raw data from proteomic analyses of archaeological bones from a completely independent excavation site, from the Hitotsubashi site (AD 1657–1683) in Tokyo, Japan, were used for comparative evaluation of the results. Samples H-162, H-142 from[34] were selected because proteins have been extracted with almost the same protocol and analysed on the same instrument as herein samples.
Very stringent criteria for protein identification were used: only peptides with score higher than 70 were considered and proteins were considered as identified only when 2 or more peptides have been detected (Table S2).
The number of identified proteins in the samples from Herculaneum, Pompeii and Baia Scalandrone varied among individuals from 2 to 27 and includes collagenous and non-collagenous proteins (NCPs) (Table S3). The expected dominance of collagen in bone tissue is reflected by the result that the two chains of type 1 collagen, namely collagen alpha-1 (I) and collagen alpha-2 (I), were confidently identified in all the samples. Of the 15 samples, 5 contained only type 1 collagen chains (four samples out of seven from Pompeii, 2.8 proteins on average, ± 1.12, and one sample from Baia Scalandrone) while samples from Herculaneum exhibited a higher protein content (13 proteins on average, ± 7). Bone proteome complexity is affected by several factors, including burial age[35]. In this case, since bones from Pompeii and Herculaneum are exactly coeval, the volcanic environmental conditions during death and burial appear to have played a significant role in protein survival in the two different sets of bone samples[36]. Raw data of Control samples were processed with the same constrains as the samples herein analysed, and a large number of proteins, (38 ± 0) were identified, as already reported[34].
Despite a general large variability among the individuals within the different groups (Figure S3), samples can be grouped depending on the site of origin in respect to NCP content. We definitively observed that bone samples from Herculaneum exhibit more NCPs than those from Pompeii. Venn diagram using the ensembles of the proteins identified in each sample group shows that the proteins identified in Pompeii bones are common to all three groups, while several other proteins are shared exclusively by Herculaneum and Baia Scalandrone bones (Figure S4, Table S4).
The non-collagenous proteins identified in this study agree with those expected for archaeological bones[37],[38]. Most of the identified NCP proteins are small leucine-rich proteoglycans (SLRPs) from the extracellular matrix (namely chondroadherin, biglycan, decorin, lumican and osteomodulin), all involved in biomineralisation or interacting with fibrillar collagen, (such as vitronectin and pigment epithelium-derived factor). Moreover, alpha-2-HS-glycoprotein, also known as fetuin-A is a bone matrix protein, known to have a high affinity for apatite. It is worth mentioning that several of the NCPs are related to the coagulation pathway (namely, prothrombin and antithrombin III, and Protein Z-dependent protease inhibitor), and can be functionally connected (Figure S5).
Diagenetically induced modifications in bone proteins
Modifications of amino acids, such as oxidation of methionine, deamidation of asparagine and glutamine, as well as the backbone cleavage, are all degradation phenomena commonly observed and routinely searched for in ancient/aged proteins[13],[39].
Deamidation
To begin with, deamidation of asparagine (N) and glutamine (Q) residues, among the most common and most informative diagenetically derived modifications in proteins[11],[12],[40], was examined. While extensive deamidation increases heterogeneity of the samples, it is a general and relevant glance on the myriad changes that archaeological bone proteins undergo and is influenced by the age and more generally by the preservation state of the bone sample under consideration[11],[40]–[42]. Extensive protein deamidation (N,Q) has been consistently observed in ancient samples, and it has been routinely measured as part of the palaeoproteomics analysis of archaeological and paleontological specimens as a global indicator of sample preservation quality, since rates and levels of deamidation are affected by several chemical and environmental factors[40]. As expected, proteins in our samples are extensively deamidated and asparagine sites are much more deamidated than glutamines[40],[43], (Fig. 1). Moreover, we split the evaluation of the deamidation levels for collagenous (Fig. 1) and non-collagenous proteins (Figure S6) and, in agreement to what already reported by[15], peptides from non-collagenous proteins showed very high to complete deamidation in comparison to peptides derived from collagenous proteins. On average, peptides from Pompeii samples are the most deamidated (see bulk deamidation per archaeological site, Fig. 1).
Graph: Figure 1 Overall percentage of deamidation for asparagine and glutamine residues of collagenous bone proteins from Pompeii, Herculaneum, Baia Scalandrone and control (H-162, H-142 from[34]). Error bars represent standard deviation and numbers above each bar represent the number of deamidation sites the data is based on.
It is worth mentioning that Control samples are significantly more recent (1657–1683 AD)[34] than samples from the Vesuvius area, and they are definitively less deamidated than Pompeii and Herculaneum bone samples. Variations were observed from individual to individual in the three groups from the Vesuvius area (Figure S7–S8). As a general trend, we can confidently assert that the lower the number of surviving proteins the higher the deamidation level, and it is worth observing that the few NCPs identified in samples from Pompeii are almost completely deamidated. Apparently, other factors rather than temperature might have played the biggest role in deamidation. In fact, the skeletal remains from Baia Scalandrone, which were not exposed to hot pyroclastic flows, but had been buried in the volcanic soil of the Campi Flegrei area (Bay of Puteoli, Gulf of Naples, Italy), exhibited a level of deamidation only slightly lower than that measured for bones from Herculaneum and Pompeii.
We analysed the distribution of deamidation level along the sequence of collagen type I chains, to explore the possibility of hot spots for deamidation rather than an average distribution. Figure S9 illustrates the deamidation values at single deamidation sites along collagen alpha-1 (I) and alpha-2 (I) chains. The label size indicates the relative intensity of each position in each sample. The values for Control are always well below those calculated for samples from the volcanic areas, in agreement with the global deamidation level calculated in Fig. 1. This difference is even more evident in glutamines, conceivably because glutamine deamidates more slowly. There is a trend in the deamidation; there are some zones where deamidation is more pronounced than others. This trend is almost reproducible in the samples of Pompeii, Herculaneum and Baia Scalandrone suggesting that the deamidation profile is quite robust for samples similar as concerns age and burial soil and also that three-dimensional arrangement might affect the local deamidation level.
Oxidation of methionines
With the same approach the oxidation of methionines (M) was evaluated. Figures S10–S11 illustrate the global oxidation levels of collagenous and non-collagenous proteins in all the samples. Apart from the zero values of control samples, all the proteins in all groups are almost totally oxidized (100%), demonstrating that methionine oxidation follows another pattern than deamidation. Furthermore, we investigate the oxidation values at single oxidation sites along collagen alpha-1 (I) and alpha-2 (I) chains. Almost all oxidation values are either 1 or 0, meaning that methionines are either fully oxidized or not oxidized at all (Figure S12). However, it is worth saying that several methionines were not detected, despite the generally good protein sequence coverage.
Backbone cleavage
Backbone cleavage of the polypeptide chain is also expected as a degradation feature in ancient proteins[15],[44],[45], and can be evaluated since, upon trypsin hydrolysis, semi-tryptic peptides will be generated. Search for semi-tryptic peptides was carried out only on collagen type I chains for comparative purposes, since they are the only polypeptide chains shared among all the samples. The frequency of semitryptic peptides was evaluated as percentage of semitryptic peptides over the total number of identified peptides for each chain, on the basis of spectrum matches (PSMs).
Figure 2 shows the relative abundance of peptide-spectrum matches (PSMs) of semitryptic peptides over the total number of peptides of collagen alpha-1 (I) and alpha-2 (I) chains as a bulk per archaeological site (Fig. 2A) and in the single samples (Fig. 2B). The frequency of backbone cleavages is generally high. However, no clear-cut difference was observed among the samples from the volcanic areas or with the control sample.
Graph: Figure 2 Backbone cleavages in collagen alpha 1(I) and collagen alpha 2 (I) in bone samples from Pompeii, Herculaneum, Baia Scalandrone and control (H-162, H-142[34]). Overall occurrence per samples groups (A) and in the single samples (B), evaluated as percentage of peptide-spectrum matches (PSMs) of semitryptic peptides over the total number of peptide-spectrum matches (tryptic plus semitryptic peptides).
The peptides showed a clear pattern derived from extended terminal hydrolysis occurring in regions of the collagen chains rather than in specific peptide bonds (Figure S13). A manual alignment of all the semitryptic peptides in the four different groups (Pompeii, Herculaneum, Baia Scalandrone, Control) to COL1A1 and COL1A2 sequences, however, reveals that while in case of controls the number cleavages are spread along the sequences, in the samples of Pompeii, Herculaneum and Baia Scalandrone they are localized in some regions of the protein sequences. These hot spots are between 266–286, 481–511, 772–796 and 1051–1118 sites of COL1A1 with a window of ± 2 amino acids, and in COL1A2 between 154–167, 232–250, 320–340, 425–438, 499–517, 681–708, 965–1006 and 1042–1066 with a window of ± 2 amino acids (Figure S14).
The cleavage frequency was then re-evaluated considering the regions rather than the single peptide bonds, by calculating the number of PSMs with semitryptic cleavages identified in a region divided by the total PSMs in the same region, including both tryptic and semitryptic matches. As shown in figure S14, the regions listed above are more hydrolysed in the samples from Pompeii, Herculaneum and Baia Scalandrone. The higher frequency of observed backbone cleavage seems to suggest a different state of preservation of bones embedded in volcanic deposits from those from agricultural soil.
Other diagenetically induced chemical modifications
Data-depended peptide algorithm of MaxQuant[29],[46] was used for an blind search of chemical modifications (CMs) in the samples. The CMs were ranked by their occurrence within the dataset. The modifications were chosen after filtering with localization probabilities of ≥ 80% for modified peptides and occurrence of detection of DP Cluster Mass ≥ 5times for each sample (see Fig. 3). As expected, hydroxylation of prolines is fairly abundant, actually overwhelming most of the other modifications (and therefore omitted from the figure), as well as deamidation at asparagines and glutamines.
Graph: Figure 3 Peptide-spectrum matches (PSMs) of "dependent peptides" with mass shifts in the type I collagen chains in the sample groups of Pompeii, Herculaneum, Baia Scalandrone and control samples (H-142, H-162[34]). Mass shifts were selected after filtering with localization probabilities of ≥ 80% for modified peptides and occurrence of detection of DP Cluster Mass ≥ 5 times for each sample. Reported data only include mass shifts corresponding to known oxidative modifications with matching amino acid targets (Unimod, http://www.unimod.org/).
As a second step, the selected CMs were inserted as variable modifications in standard MaxQuant searches, by setting the modifications as variable in separate runs, for each group separately, as detailed in Table S2. To confirm peptide assignment, we manually inspected MS/MS spectra (and some examples are reported in the supplementary information, Figures S23–S28) thus allowing to confidently assess the site localization of the chemical modifications.
The frequency of modified residues in respect to the amino acid detection is reported in the tables S5 (A–E). Each position was considered only once in this calculation, even when the position was present in overlapping peptides. Furthermore, the frequency of chemical modifications at a specific primary structure position was semiquantitatively evaluated using the MaxQuant calculation of mod/base ratio as reported in[47] (Figures S19–S22).
Interestingly, a high occurrence of mass shifts on lysine (K) and arginine (R) (Figs. 4 and 5) was observed, all, as expected, in correspondence of trypsin missed cleavages, that were interpreted as glycation products, with a high incidence in the samples group of Pompeii. Protein glycation involves the binding of reducing sugar carbonyl groups to protein amino groups, or the reaction of α-dicarbonyls such as glyoxal or methylglyoxal, that are continuously formed during oxidative degradation of sugars, with lysine and arginine residues, leading to a series of molecular reactions collectively called Maillard reaction that generate a variety of complex compounds called advanced glycation end products (AGEs)[48]–[51]. Among lysine-derived AGEs, Nε-(carboxymethyl)lysine (CML) and Nε-(carboxyethyl)lysine (CEL) are the most studied representatives and were significatively observed in the samples from the eruptive area (Fig. 4). Formylation at lysine side chains, oxidative deamination of lysine to aminoadipic acid, another marker of protein carbonyl oxidation[52] that can be associated to decomposition after death[13], and carbamylation, that has been reported as a hallmark of protein aging[53], were all also observed in collagen from samples from Herculaneum and Pompeii. Among arginine-related AGEs we detected the hydroimidazolones MG-H1 and G-H1 formed by reaction of arginine side chain with the oxoaldehydes methylglyoxal and glyoxal[54], respectively, and a substantial formation of ornithine (Figure S15 and fragmentation spectra at Figure S27)[55], that was also recently identified in ancient dental enamel proteins[56].
Graph: Figure 4 Extent of modified lysine residues, reported as percentage of modified over detected (modified plus unmodified) ones.
Graph: Figure 5 Extent of modified arginine residues, reported as percentage of modified over detected (modified plus unmodified) ones.
These modifications are less frequent in the control sample and, within samples from the volcanic areas, such modifications are significantly higher in bone collagen from Pompeii (Figs. 4 and 5).
Histidine is one of the targets of oxidative modifications[39], generating 2-oxohistidine and dioxohistidine that can evolve further to break down to aspartic acid. An extensive oxidation of histidine residues in collagen chains from the bones from the eruptive area was observed (Fig. 6). In fact, more than 65% of collagenous histidine residues in Pompeii and Herculaneum bone samples have been found modified (Table S5D). Interestingly, extensive evolution to aspartic acid has been observed in all the samples coming from the volcanic sites, comprising those from Baia Scalandrone, but not in the control samples, suggesting an influence of the alkalinity of volcanic soil in the final degradation product[57].
Graph: Figure 6 Extent of modified histidine residues, reported as percentage of modified over detected (modified plus unmodified) ones.
Mass shifts that are consistent with the Cα-Cβ bond cleavage of the side chains of serine and threonine, which result in the formation of glycine (G) (− 30.011 Da and − 44.026 Da, respectively) were observed (Figs. 7, S16, S28). This modification resembles what recently reported on histidine residues[39] and generally postulated as a result of radical transfer to backbone following oxidation reactions[58]–[60], although it has never been reported so far for serine and threonine residues. However, this modification is not a prerogative of the bone samples here analysed, from volcanic sites, since it has been consistently observed also in the ancient bone control samples.
Graph: Figure 7 Extent of Cα-Cβ bond cleavage at serine and threonine reported as percentage of modified over detected (modified plus unmodified) residues.
Proline is a rather complex and often neglected target of chemical modification. The abundance of this residue in collagen, exceeding 20% of the total amino acids in human type I collagen, however, increases the rate of detection of modifications on this peculiar residue, although the abundant and variable incidence of hydroxylation makes detection of any other modification quite challenging (see Figure S17 for the occupancy of hydroxylation of proline along the sequences of COL1A1 and COL1A2). It has already been suggested that an increased level of hydroxylated prolines might result from a non-enzymatic oxidation[61]. The peculiar cyclic structure of proline results in an oxidative fate different from that of other aliphatic side chain[62]. Unfortunately, some oxidation products, such as glutamic semialdehyde are isobaric with hydroxylation[62],[63], impairing their unequivocal identification. Nevertheless, consistent formation of pyroglutamic acid from proline (ΔM + 13.980 Da) and di- and tri-oxidation products (ΔM + 31.989 Da and + 47.983 Da respectively), with di-oxidation that also matches formation of glutamic acid (Figure S18), are eventually suggestive of oxidative diagenetic modification (double hydroxylation is not reported as a physiological post-translational modification) (Figures S23–S26). Most interestingly, a mass shift of ΔM − 2.001 Da, consistent with the loss of 2 hydrogens, was repeatedly detected and only in the samples of Pompeii and Herculaneum (Fig. 8). We suggest (Figures S18 and S26) that this mass shift is attributed to 3,4 dehydro-proline, which is the only stable form of the five possible isomers of olefinic proline[64], and could arise from dehydration of 4-hydroxyproline or 3-hydroxyproline. From now it will be called Dhp, with a mass shift of − 18.001 Da from hydroxyproline and ΔM − 2.001 Da from proline.
Graph: Figure 8 Extent of modified proline residues, reported as percentage of modified over detected (modified plus unmodified) ones.
We also explored the occupancy of the non-enzymatic identified modifications along the sequence of COL1A1 and COL1A2. In general, the distribution of modifications is uneven, with residues with high modification occupancy and sites with low occupancy (Figures S19–S22). However, as far as the glycation products, that are the most striking peculiarity of Pompeii samples, rather interestingly, the G-H1 and MG-H1 modifications seem to be localized in some specific arginine positions, namely positions 564, 574, 1014, 1026 and 1034 of COL1A1, and positions 448, 474, 673 and 691 in COL1A2 (Figure S19). Conversely, in agreement with the observation of a higher average modification of lysines (according to Table S5A), glycation products on lysines seems more spread along the polypeptide chain in Pompeii samples (Figure S20). It is worth mentioning that almost all the lysine and arginine were actually covered.
More than 65% of the detected histidines in the samples from Pompeii and Herculaneum have been found modified (Table S5D). Figure S21 reports the occupancy of the identified modifications along the sequence of COL1A1 and COL1A2 in all the sample groups. Pompeii and Herculaneum samples behave quite similarly, and histidine 267 in COL1A1 seems to be a rather hot spot for oxidation.
Proline oxidation products are quite spread along the sequences (Figure S22) and follow the general trend of samples from Pompeii which are more modified than the Herculaneum ones. This is in agreement also with the observation that collectively 8% of prolines have been found to be modified (differently from hydroxylation) in samples from Pompeii and Herculaneum (Table S5C), which are in turn more modified than those from Baia Scalandrone and Control samples. Position 592 seems to be a hot spot in all the cases.
Discussion
Bones can be considered time capsules, and individual history can be imprinted on their organic content[30]. Lack of intracellular proteins, extensive deamidation, backbone cleavage, oxidative chemical modifications, are all taphonomic marks of the diagenesis of organic matter. All these signs characterize the proteins extracted from the bones of human victims from Pompeii and Herculaneum, as a molecular imprint of the effects of the 79 AD eruption.
A striking feature is the almost complete absence of NCPs in the bones from Pompeii compared with those from Herculaneum, thus suggesting an incomplete consumption of the organic matter for the bones from Herculaneum. The latter hypothesis is in agreement with the evidence of preservation of organic tissue residues[22],[23] and organic compounds[24].
Bodies in Pompeii experienced a different fate than those in Herculaneum, and the differences are also imprinted molecularly. The body flesh of Pompeii victims slowly disappeared, thus resulting in cavities between the skeleton and the volcanic ash[19]. In Herculaneum, instead, soft tissues underwent a rapid thermally-induced vanishing resulting in the complete body skeletonization and bones exceptionally well preserved[16]. The different proteomic content observed in Herculaneum bones in comparison with those from Pompeii is the result of the different environmental conditions due to exposure to different pyroclastic flows: the Pompeii victims were affected by the third and fourth pyroclastic surges, while at Herculaneum people were hit and buried by the first surge, which did not reach Pompeii[16]. Local environmental conditions during the eruption such as the peak of maximum temperature of the ash cloud and the time needed for the ash deposit to cool would have produced unique effects on the victims' corpses and their bones.
The pathway of chemical reactions that break down the proteins within the bioapatite cage appears still fairly mysterious, with proteins normally degrading principally via a combination of two parallel as well as interplaying mechanisms: digestion by microbes and chemical modification/degradation[38], with time, temperature and burial environment all contributing to influence the kinetics of protein decay. For instance, the presence of many copper minerals such as sulfates, oxides, carbonates, and phosphates in the volcanic soil may increase the solubility of hydroxyapatite thus leading possibly to partial bone loss[65]. Proteome complexity is generally considered a hallmark of bone degradation, inversely proportional to age, with most of the samples older than 20,000 years containing predominantly and almost exclusively collagen that benefits of the interaction with the bioapatite cage that protects them from degradation[35]. We can observe that in five of the seven Pompeii bones samples, collagen chains were the only proteins to be detected, and in the other two samples, beside collagen, only chondroadherin and biglycan were identified. Moreover, the lower NCPs content, the higher deamidation level and, in general, the higher extent of modification of collagen in the bones from Pompeii in respect to the bone samples from Herculaneum, demonstrate a more degraded state possibly as a result of the slower decomposition of soft tissue.
Despite the higher temperature that the bodies experienced at Herculaneum than at Pompeii, a good number of NCPS were identified in most of the bones. Only proteins stabilized by the binding to collagen or to the inorganic component of bones survived in Herculaneum, while all other proteins probably decayed rapidly due to the intense heat of the pyroclastic surge. The most common NCPs detected in the Herculaneum bones include Alpha-2-HS-glycoprotein, biglycan, chondroadherin, pigment epithelium derived factor (PEDF), lumican, and prothrombin, all proteins that are known to bind collagen or calcium ions. This evidence is in good agreement with proteins mostly identified in ancient bones[38],[66]. Moreover, it was recently observed that fetuin-A (herein reported as Alpha-2-HS-glycoprotein), a serum glycoprotein, is relatively stable after death[36]. Here we observe that this protein, that prevents mineral precipitation during mineralization process by stabilizing supersaturated mineral solutions by forming soluble colloidal nanospheres[66], is among the NCP survivors to the volcanic environmental conditions at Herculaneum.
Interestingly, in our samples, also Vitronectin survived quite well (it was identified in six of the seven samples from Herculaneum, as frequently as biglycan). This is an abundant multifunctional glycoprotein found in serum, extracellular matrix, and bone, involved in various physiological processes such as cell attachment, spreading, and migration, which interacts also with collagen type I[67].
It is worth mentioning that none of the NCPs recently detected by immunological methods in calcined bone tissue[31] has been identified herein by proteomic approach, while the set of proteins identified is in agreement with those recently identified by similar proteomic approach in rat model bones[68].
NCPs were absent in samples from Pompeii. It might be hypothesized that, in the case of bones from Pompeii, where the body soft tissue survived much longer than in Herculaneum, proteins underwent a massive degradation process, possibly speeded up by the hot burial environment, thus resulting in skeletal remains with the fewest and most modified proteins.
Oxidative modifications in the 79 AD bone samples are extensive, very close to what expected to occur in a cooking process, which is still a debated question on a molecular basis[58]. Diagenetic increase of AGEs correlates with oxidative conditions[69],[70] and extensive glycation products were observed in the samples from Herculaneum and Pompei, always more pronounced in those from Pompeii, likely originating from reactions with the sugars originally present in the extracellular matrix. Histidine was herein confirmed as oxidative target among the amino acids[58] and formation of radicals at C-α backbone can also eventually lead to backbone fragmentation[58], thus suggesting an oxidative origin at the basis of the extensive backbone cleavage observed rather than hydrolysis in an environment where water evaporation is expected.
Several oxidative processes have been postulated to occur on prolines and hydroxyprolines upon heating, according to chemical pathway depicted by Hellwig[58], who predicts hydroperoxides formation from addition of oxygen to radical at the aliphatic side-chain of prolines, as stable intermediates in protein oxidation.
The high incidence of prolines in collagen allowed to highlight the occurrence of oxidative modifications on this peculiar side chain, some of which possibly explained as modifications originating from hydroxyproline (such as that corresponding to a ΔM − 2.001 Da when considering proline as the unmodified amino acid).
It is interesting to observe that modifications (although identified throughout the sequence), appear to be more pronounced in specific regions. In Fig. 9, diagenetic modifications are collectively showed along the collagen sequences, highlighting a different behaviour of the samples from Herculaneum and Pompeii in respect to those of Baia Scalandrone and control samples, that appear clearly less modified, with modifications spread along the sequence. This suggests a strong three-dimensional effect in directing chemical modifications events, an aspect that will deserve further future investigation.
Graph: Figure 9 Comparative analysis of the global "damage signatures" in COL1A1 and COL1A2 from human bones of the different archaeological sites. The figure represents the sum of the average modified/unmodified values of K, R, S, T and P diagenetic modifications (except hydroxyproline and deamidation) at the specific primary structure positions of COL1A1 and COL1A2.
These data do not claim to be conclusive of differences that we have highlighted in the diagenetic processes when comparing skeletal remains from Herculaneum and Pompeii, but rather demonstrate that molecular differences exist and can be seen as a perspective on the chemist's approach to read through the processes that alter proteins in bone during burial. The history written in the molecules, a kind of "chemical life history tracer". Despite the intra-samples' variability observed, paleoproteomic analyses revealed that diagenetic processes generated by different environmental conditions are significantly reflected in the protein survival and modification. Why proteins survived better in the bones of the Herculaneum victims, whose body flesh rapidly disappeared, and why modifications were more evident in Pompeii bones are the main questions to be answered.
In this regard, it is important to highlight that bones from soils subjected to natural dry–wet hydrogeological cycles, as the case of Pompeii, show a low level of organic matter and high porosity[71]. The oxygen-rich environment during dry periods leads to a rapid degradation of the bone's organic matter, and favours as well microbial activity[72]. Water level fluctuation induces leaching out and degradation of collagen due to increased solubility, leading to rapid destruction of the skeleton[73]. In contrast, permanently waterlogged sediments, as is the case with Herculaneum[74], being anoxic, are able to inhibit microbial attack and related diagenetic processes[ 5],[27]. The main characteristic of bone buried in a reducing and waterlogged stable environment is a high level of preservation of the organic and mineral matter, with a consequent low level of porosity/breakdown of the osseous structure[71]. Some microbes were also reported to have a preservative effect over time on bones[73],[75].
Soil chemistry also has an influence on bone preservation. Acidic soils are found to induce bone mineral loss, since an acidic environment is a main condition for bioapatite dissolution[76]. This diagenetic process can be increased by events as repeated wetting/drying soil cycles[77], which in turn may accelerate the degradation of collagen[78]. In an acidic/corrosive soil, rapid bone mineral destruction and chemical alteration by microbial attack will occur. Vice versa, under alkaline to neutral conditions the organic and mineral bone components will be better preserved[76],[78]. This seems to fit the case of the 79 AD eruption, where the chemical composition of volcanic deposits is primarily basic (alkaline-potassium sediment)[79]. A correlation between high fluorine (F) concentrations and alkaline soils has been also highlighted[80]. At Herculaneum, the waterlogged ash bed deposit is characterized by a fluoride-rich environment[25]. Fluorine enrichment of the bone transform bioapatite into a more thermodynamically stable phase[81], thus giving the bone greater hardness, as also detected at Herculaneum[20],[26].
Overall, the volcanic soils from the Campanian region are characterized by a high alkalinity (alkaline-potassium magmatism)[82], with values even more marked for the Phlegraean Fields than those detectable for the Vesuvius area[83],[84]. In addition, the alkalinity of groundwater from the Campanian volcanic areas, which originates from the leaching of alkaline-potassium pyroclastic deposits[85], further supports the evidence of good preservation of organic and mineral matter in the bone[76],[78]. Therefore, contrary to the assumptions of some authors[86], the long-term good preservation of organic matter (i.e., collagen and other proteins) in the Herculaneum bones emerges as the result of the chemical-physical burial environment (ash bed deposit) rather than the effect of a not-so-high ash surge temperature. More in general, the extent of preservation of organic molecules in bones from Herculaneum and Pompeii, on the one hand, and Baia Scalandrone, on the other, regardless of whether or not they were exposed to heat, above all reflects the peculiarity of the interactions between the chemical-physical composition and the hydrogeological regime of the volcanic soils in which the bones were buried, characteristics that being different for each of the sites, produced different effects on the organic bone preservation (Fig. 10).
Graph: Figure 10 Sequence of biological and taphonomic events concerning the 79 AD human victims from Herculaneum and Pompeii, in comparison with the skeletons from the Baia Scalandrone graveyard.
Finally, it is also important to stress that, in an anoxic environment, the extent of bone preservation depends on the mechanism by which the body is buried in the soil over time[78]. In this regard, the sequence of biological and taphonomic events that affected the victims' corpses in Pompeii and Herculaneum, and the way the flesh of the body buried in the ash bed disappeared, appears to play a major role (Fig. 10). In Pompeii, the body tissues of the victims, killed by heat at 250–300 °C[17] and then buried intact, underwent slow decay. The slow decomposition of soft tissues in a cycle of periodic wetting/drying of the soil appears to be the cause of poor preservation of organic matter. At Herculaneum, instead, after the rapid vanishing of soft tissue by ≥ 500 °C exposure[22], the permanently waterlogged ash bed in which the skeleton was buried must have inhibited the microbial chemical modifications, allowing the long-term survival of organic matter. Such a type of environmental context seems to explain the reason of the highlighted good preservation of proteins, as well as the survival of collagen and DNA[24],[87].
Archaeological as well as forensic sciences will possibly benefit from the results herein obtained, since burned human skeletal remains are a common object of study for biological anthropologists, but they also represent a frequent type of evidence in the forensic scenario. Forensic proteomics is still in the early stages of development[88], and the characterization of bone exposed to heat could be useful as an auxiliary strategy[31]. So far, a few proteomic analyses of bones in forensic context have been explored to estimate biological age (age-at-death)[30],[36],[89] and post-mortem interval (PMI)[30],[32],[36] of skeletal tissue[88], or to distinguish individuals[90], looking mainly at residual proteome complexity or to protein deamidation. Our results suggest that additional information can be found by expanding the set of modifications of proteins to look for, unveiling more details about taphonomic agents that may affect bone death processes, leading to find potential biomarkers for medicolegal investigations that can provide information about environmental parameters at the time of death.
Methods
The skeletal elements of 15 individuals from the archaeological sites of Pompeii ( 7), Herculaneum ( 5) and Baia Scalandrone ( 3) were analysed. Table S1 describes each specimen and its related information and Figure S1 shows pictures of the samples and of the EDTA solubilised fraction. All necessary permits were obtained for the study of the human specimen from the Ethics Committee for Biomedical Activities, AOU Federico II, Naples, Italy, Protocol N. 101/17.
Protein extraction, digestion and analyses
Bone samples were prepared as described in[34] with slight modifications. Figure S2 represents the whole procedure, and a detailed description of the protocol is provided in the supplementary materials. Samples were processed as reported in[91], and detailed in the supplementaries. Samples were separated on a 15 cm column (75 μm inner diameter) in-house laser pulled and packed with 1.9 μm C18 beads (Dr. Maisch, Germany) on an EASY-Nlc 1000 (Proxeon, Odense, Denmark) connected to a Q-Exactive HF (Thermo Scientific, Bremen, Germany).
Data analysis
The resulting raw files (EvoG_sample name, in total 15 files) were searched and analysed using the MaxQuant (MQ) software[92] against a UniProt database (759,512 sequences, 37,179,137 residues) with Homo sapiens as taxonomic restriction (20,199 sequences, 928,813 residues). Details of the different runs for standard proteins identification and searches for diagenetically induced modifications are provided in supplementary information and schematised in Table S2.
Author contributions
L.B., G.N., designed research; G.N., I.R.P. and L.B. performed research; P.P. collected samples and cured the anthropological aspects of the conceptualization; G.N., I.R.P. and L.B. analysed data; F.S., F.D.P., G.M., E.C. and L.B. supervised data analysis; G.N., P.P, G.M., E.C. and L.B. wrote the paper.
Funding
G.N., E.C. and L.B were supported by the European Union's Horizon 2020 Research and Innovation Program under the Marie Sklodowska-Curie Grant Agreement No. 722606, TEMPERA (Teaching Emerging Methods in Palaeoproteomics for the European Research Area).
Data availability
LC–MS/MS data have been deposited to ProteomeXchange platform (http://proteomecentral.proteomexchange.org) with the dataset identifier PXD020462.
Competing interests
The authors declare no competing interests.
Supplementary Information
Graph: Supplementary Information.
Supplementary Information
The online version contains supplementary material available at https://doi.org/10.1038/s41598-022-12042-6.
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
1 Warren M. Ancient proteins tell their tales. Nature. 2019; 570: 433-436. 1:CAS:528:DC%2BC1MXht1yktbjK. 31243383. 10.1038/d41586-019-01986-x. 2019Natur.570.433W
2 Kendall C, Eriksen AMH, Kontopoulos I, Collins MJ, Turner-Walker G. Diagenesis of archaeological bone and tooth. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2018; 491: 21-37. 10.1016/j.palaeo.2017.11.041
3 Mamede AP, Gonçalves D, Marques MPM, Batista de Carvalho LAE. Burned bones tell their own stories: a review of methodological approaches to assess heat-induced diagenesis. Appl. Spectrosc. Rev. 2018; 53: 603-635. 10.1080/05704928.2017.1400442. 2018ApSRv.53.603M
4 Marques MPM. Profiling of human burned bones: oxidising versus reducing conditions. Sci. Rep. 2021; 11: 1-13. 10.1038/s41598-020-79139-8. 2021NatSR.11.5M. 1:CAS:528:DC%2BB3MXnsFemsw%3D%3D
5 Hedges REM. Bone diagenesis: an overview of processes. Archaeometry. 2002; 44: 319-328. 1:CAS:528:DC%2BD38XotlOiu7g%3D. 10.1111/1475-4754.00064
6 Collins MJ. The survival of organic matter in bone: a review. Archaeometry. 2002; 3: 383-394. 10.1111/1475-4754.t01-1-00071
7 Cleland TP, Schroeter ER, Colleary C. Diagenetiforms: a new term to explain protein changes as a result of diagenesis in paleoproteomics. J. Proteomics. 2021; 230. 1:CAS:528:DC%2BB3cXitFahs7%2FF. 32992016. 10.1016/j.jprot.2020.103992
8 Van Huizen NA, Ijzermans JNM, Burgers PC, Luider TM. Collagen analysis with mass spectrometry. Mass Spectrom. Rev. 2019; 1: 1-27
9 Perez Hurtado P, O'Connor PB. Deamidation of collagen. Anal. Chem. 2012; 84: 3017-3025. 1:CAS:528:DC%2BC38XhsVSqt7c%3D. 10.1021/ac202980z
Orlando L. Recalibrating equus evolution using the genome sequence of an early Middle Pleistocene horse. Nature. 2013; 499: 74-78. 1:CAS:528:DC%2BC3sXhtVWgt7zI. 23803765. 10.1038/nature12323. 2013Natur.499.74O
Van Doorn NL, Wilson J, Hollund H, Soressi M, Collins MJ. Site-specific deamidation of glutamine: a new marker of bone collagen deterioration. Rapid Commun. Mass Spectrom. 2012; 26: 2319-2327. 22956324. 10.1002/rcm.6351. 2012RCMS.26.2319V. 1:CAS:528:DC%2BC38XhtlWqt7bK
Wilson J, Van Doorn NL, Collins MJ. Assessing the extent of bone degradation using glutamine deamidation in collagen. Anal. Chem. 2012; 84: 9041-9048. 1:CAS:528:DC%2BC38XhsVGisbbF. 23030643. 10.1021/ac301333t
Cappellini E. Proteomic analysis of a pleistocene mammoth femur reveals more than one hundred ancient bone proteins. J. Proteome Res. 2012; 11: 917-926. 1:CAS:528:DC%2BC3MXhsV2nu77M. 22103443. 10.1021/pr200721u
Cleland TP, Schroeter ER, Schweitzer MH. Biologically and diagenetically derived peptide modifications in moa collagens. Proc. R. Soc. B Biol. Sci. 2015; 282: 1-6. 1:CAS:528:DC%2BC2MXhs1eqtbrO
Hill RC. Preserved proteins from extinct Bison latifrons identified by tandem mass spectrometry; hydroxylysine glycosides are a common feature of ancient collagen. Mol. Cell. Proteomics. 2015; 14: 1946-1958. 1:CAS:528:DC%2BC2MXhtFWntrzP. 25948757. 4587324. 10.1074/mcp.M114.047787
Mastrolorenzo G. Archaeology: herculaneum victims of Vesuvius in AD 79. Nature. 2001; 410: 769-770. 1:CAS:528:DC%2BD3MXjtVemt74%3D. 11298433. 10.1038/35071167. 2001Natur.410.769M
Mastrolorenzo G, Petrone P, Pappalardo L, Guarino FM. Lethal thermal impact at periphery of pyroclastic surges: evidences at pompeii. PLoS ONE. 2010; 5. 20559555. 2886100. 10.1371/journal.pone.0011127. 2010PLoSO.511127M. 1:CAS:528:DC%2BC3cXotVyqsb8%3D
Petrone P. Human corpses as time capsules: new perspectives in the study of past mass disasters. J. Anthropol. Sci. 2011; 89: 3-6. 21730365
Petrone P. A hypothesis of sudden body fluid vaporization in the 79 AD victims of Vesuvius. PLoS ONE. 2018; 13. 30256793. 6157861. 10.1371/journal.pone.0203210. 1:CAS:528:DC%2BC1cXitlCisL%2FL
Petrone P. The herculaneum victims of the 79 AD vesuvius eruption: A review. J. Anthropol. Sci. 2019; 97: 1-22
Soncin S. High-resolution dietary reconstruction of victims of the 79 CE Vesuvius eruption at Herculaneum by compound-specific isotope analysis. Sci. Adv. 2021; 7: eabg5791. 1:CAS:528:DC%2BB3MXitVKku7rO. 34433561. 8386925. 10.1126/sciadv.abg5791. 2021SciA.7.5791S
Petrone P. Heat-induced brain vitrification from the Vesuvius eruption in c.e. 79. N. Engl. J. Med. 2020; 382: 383-384. 31971686. 10.1056/NEJMc1909867
Petrone P. Preservation of neurons in an AD 79 vitrified human brain. PLoS ONE. 2020; 15. 1:CAS:528:DC%2BB3cXitVOlurnP. 33022024. 7537897. 10.1371/journal.pone.0240017
Martyn REV. Capturing Roman dietary variability in the catastrophic death assemblage at Herculaneum. J. Archaeol. Sci. Rep. 2018; 19: 1023-1029
Petrone P, Giordano M, Giustino S, Guarino FM. Enduring fluoride health hazard for the vesuvius area population: the case of AD 79 herculaneum. PLoS ONE. 2011; 6. 1:CAS:528:DC%2BC3MXotVWisL4%3D. 21698155. 3116870. 10.1371/journal.pone.0021085. 2011PLoSO.621085P
Petrone P, Guarino FM, Giustino S, Gombos F. Ancient and recent evidence of endemic fluorosis in the Naples area. J. Geochem. Explor. 2013; 131: 14-27. 1:CAS:528:DC%2BC38XhvVyrurrN. 10.1016/j.gexplo.2012.11.012
Tressaud, A. Fluorine and the environment. Fluorine and the environment, agrochemicals, archaeology, green chemistry and water vol. 2 (Elsevier, 2006).
Stefani Grete. Uno alla volta. I calchi. (Mondadori Electa S.p.A, 2010).
Savitski MM, Nielsen ML, Zubarev RA. ModifiComb, a new proteomic tool for mapping substoichiometric post-translational modifications, finding novel types of modifications, and fingerprinting complex protein mixtures. Mol. Cell. Proteomics. 2006; 5: 935-948. 1:CAS:528:DC%2BD28XkslCls7o%3D. 16439352. 10.1074/mcp.T500034-MCP200
Mickleburgh HL. Human bone proteomes before and after decomposition: investigating the effects of biological variation and taphonomic alteration on bone protein profiles and the implications for forensic proteomics. J. Proteome Res. 2021; 20: 2533-2546. 1:CAS:528:DC%2BB3MXlslGmt70%3D. 33683123. 8155572. 10.1021/acs.jproteome.0c00992
Díaz-Martín RD, Ambrosio JR, Flores RM, Gonzáles-Pozos S, Valencia-Caballero L. Cytoskeletal and extracellular matrix proteins resist the burning of bones. Forensic Sci. Int. 2019; 305. 31704515. 10.1016/j.forsciint.2019.110027. 1:CAS:528:DC%2BC1MXitFemsbvP
Prieto-Bonete G, Pérez-Cárceles MD, Maurandi-López A, Pérez-Martínez C, Luna A. Association between protein profile and postmortem interval in human bone remains. J. Proteomics. 2019; 192: 54-63. 1:CAS:528:DC%2BC1cXhs1Cgs7jE. 30145274. 10.1016/j.jprot.2018.08.008
Solari A. Cooked bones? method and practice for identifying bones treated at low temperature. Int. J. Osteoarchaeol. 2015; 25: 426-440. 10.1002/oa.2311
Sawafuji R. Proteomic profiling of archaeological human bone. R. Soc. open Sci. 2017; 4. 28680659. 5493901. 10.1098/rsos.161004. 2017RSOS.461004S. 1:CAS:528:DC%2BC1cXisV2jt7bI
Wadsworth C, Buckley M. Proteome degradation in fossils: Investigating the longevity of protein survival in ancient bone. Rapid Commun. Mass Spectrom. 2014; 28: 605-615. 1:CAS:528:DC%2BC2cXisFSjsbk%3D. 24519823. 4282581. 10.1002/rcm.6821. 2014RCMS.28.605W
Procopio N, Williams A, Chamberlain AT, Buckley M. Forensic proteomics for the evaluation of the post-mortem decay in bones. J. Proteomics. 2018; 177: 21-30. 1:CAS:528:DC%2BC1cXivVSmurs%3D. 29407476. 10.1016/j.jprot.2018.01.016
Jiang X. Method development of efficient protein extraction in bone tissue for proteome analysis. J. Proteome Res. 2007; 6: 2287-2294. 1:CAS:528:DC%2BD2sXkvFKlsb4%3D. 17488005. 10.1021/pr070056t
Buckley M, Wadsworth C. Proteome degradation in ancient bone: Diagenesis and phylogenetic potential. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2014; 416: 69-79. 10.1016/j.palaeo.2014.06.026
Mackie M. Palaeoproteomic profiling of conservation layers on a 14th century italian wall painting. Angew. Chemie Int. Ed. 2018; 57: 7369-7374. 1:CAS:528:DC%2BC1cXosFSqtL0%3D. 10.1002/anie.201713020
Schroeter ER, Cleland TP. Glutamine deamidation: an indicator of antiquity, or preservational quality?. Rapid Commun. Mass Spectrom. 2016; 30: 251-255. 1:CAS:528:DC%2BC2MXitVygtbfI. 26689157. 10.1002/rcm.7445. 2016RCMS.30.251S
Pal Chowdhury M. Collagen deamidation in archaeological bone as an assessment for relative decay rates. Archaeometry. 2019; 61: 1382-1398. 1:CAS:528:DC%2BC1MXitVOhtrnF. 10.1111/arcm.12492
Welker F. Variations in glutamine deamidation for a Châtelperronian bone assemblage as measured by peptide mass fingerprinting of collagen. Sci. Technol. Archaeol. Res. 2017; 3: 15-27
Presslee S. Palaeoproteomics resolves sloth relationships. Nat. Ecol. Evol. 2019; 3: 1121-1130. 31171860. 10.1038/s41559-019-0909-z
Orsini S, Yadav A, Dilillo M, McDonnell LA, Bonaduce I. Characterization of degraded proteins in paintings using bottom-up proteomic approaches: new strategies for protein digestion and analysis of data. Anal. Chem. 2018; 90: 6403-6408. 1:CAS:528:DC%2BC1cXptVCitbk%3D. 29733588. 10.1021/acs.analchem.8b00281
Vinciguerra R, De Chiaro A, Pucci P, Marino G, Birolo L. Proteomic strategies for cultural heritage: form bones to paintings. Microchem. J. 2016; 126: 341-348. 1:CAS:528:DC%2BC28XislShsQ%3D%3D. 10.1016/j.microc.2015.12.024
Tyanova S, Temu T, Cox J. The MaxQuant computational platform for mass spectrometry-based shotgun proteomics. Nat. Protoc. 2016; 11: 2301-2319. 1:CAS:528:DC%2BC28XhslynsL7O. 27809316. 10.1038/nprot.2016.136
Martin DR, Dutta P, Mahajan S, Varma S, Stevens SM. Structural and activity characterization of human PHPT1 after oxidative modification. Sci. Rep. 2016; 6: 1-12. 10.1038/s41598-016-0001-8. 1:CAS:528:DC%2BC2sXhslGmur3N
Perrone A, Giovino A, Benny J, Martinelli F. Advanced glycation end products (AGEs): biochemistry, signaling, analytical methods, and epigenetic effects. Oxid. Med. Cell. Longev. 2020; 2020: 3818196. 32256950. 7104326. 10.1155/2020/3818196. 1:CAS:528:DC%2BB3cXit1Glsb7I
Rabbani N, Thornalley PJ. Glycation research in amino acids: a place to call home. Amino Acids. 2012; 42: 1087-1096. 1:CAS:528:DC%2BC38XjsVWhs7w%3D. 20981459. 10.1007/s00726-010-0782-1
Soboleva A, Schmidt R, Vikhnina M, Grishina T, Frolov A. Maillard proteomics: opening new pages. Int. J. Mol. Sci. 2017; 18: 2677. 5751279. 10.3390/ijms18122677. 1:CAS:528:DC%2BC1cXitFCqtbrI
Bachi A, Dalle-Donne I, Scaloni A. Redox proteomics: Chemical principles, methodological approaches and biological/biomedical promises. Chem. Rev. 2013; 113: 596-698. 1:CAS:528:DC%2BC38XhslalurzN. 23181411. 10.1021/cr300073p
Sell DR, Strauch CM, Shen W, Monnier VM. 2-Aminoadipic acid is a marker of protein carbonyl oxidation in the aging human skin: effects of diabetes, renal failure and sepsis. Biochem. J. 2007; 404: 269-277. 1:CAS:528:DC%2BD2sXlt1ejs7w%3D. 17313367. 1868793. 10.1042/BJ20061645
Gorisse L. Protein carbamylation is a hallmark of aging. Proc. Natl. Acad. Sci. U. S. A. 2016; 113: 1191-1196. 1:CAS:528:DC%2BC2MXitV2isL%2FJ. 26712018. 10.1073/pnas.1517096113. 2016PNAS.113.1191G
Chetyrkin S. Glucose autoxidation induces functional damage to proteins via modification of critical arginine residues. Biochemistry. 2011; 50: 6102-6112. 1:CAS:528:DC%2BC3MXns1Knu7w%3D. 21661747. 10.1021/bi200757d
Sell DR, Monnier VM. Conversion of arginine into ornithine by advanced glycation in senescent human collagen and lens crystallins. J. Biol. Chem. 2004; 279: 54173-54184. 1:CAS:528:DC%2BD2cXhtVyltb7E. 15489230. 10.1074/jbc.M408946200
Cappellini E. Early Pleistocene enamel proteome from Dmanisi resolves Stephanorhinus phylogeny. Nature. 2019; 574: 103-107. 1:CAS:528:DC%2BC1MXhslKhsr3O. 31511700. 6894936. 10.1038/s41586-019-1555-y. 2019Natur.574.103C
Hawkins CL, Davies MJ. Detection, identification, and quantification of oxidative protein modifications. J. Biol. Chem. 2019; 294: 19683-19708. 1:CAS:528:DC%2BB3cXjt1ans7Y%3D. 31672919. 6926449. 10.1074/jbc.REV119.006217
Hellwig M. The chemistry of protein oxidation in food. Angew. Chemie Int. Ed. 2019; 58: 16742-16763. 1:CAS:528:DC%2BC1MXhsFSrtLjJ. 10.1002/anie.201814144
Headlam HA, Mortimer A, Easton CJ, Davies MJ. β-Scission of C-3 (β-Carbon) alkoxyl radicals on peptides and proteins: a novel pathway which results in the formation of α-carbon radicals and the loss of amino acid side chains. Chem. Res. Toxicol. 2000; 13: 1087-1095. 1:CAS:528:DC%2BD3cXntFWjtbs%3D. 11087430. 10.1021/tx0001171
Headlam HA, Davies MJ. Markers of protein oxidation: Different oxidants give rise to variable yields of bound and released carbonyl products. Free Radic. Biol. Med. 2004; 36: 1175-1184. 1:CAS:528:DC%2BD2cXjtVemtL4%3D. 15082071. 10.1016/j.freeradbiomed.2004.02.017
Mikšík I, Sedláková P, Pataridis S, Bortolotti F, Gottardo R. Proteins and their modifications in a medieval mummy. Protein Sci. 2016; 25: 2037-2044. 27543755. 5079257. 10.1002/pro.3024. 1:CAS:528:DC%2BC28XhtlOmur3J
Xu G, Chance MR. Hydroxyl radical-mediated modification of proteins as probes for structural proteomics. Chem. Rev. 2007; 107: 3514-3543. 1:CAS:528:DC%2BD2sXos1egs7s%3D. 17683160. 10.1021/cr0682047
Amici A, Levine RL, Tsai L, Stadtman ER. Conversion of amino acid residues in proteins and amino acid homopolymers to carbonyl derivatives by metal-catalyzed oxidation reactions. J. Biol. Chem. 1989; 264: 3341-3346. 1:CAS:528:DyaL1MXhvVygsbk%3D. 2563380. 10.1016/S0021-9258(18)94071-8
Mauger AB, Witkop B. Analogs and homologs of proline and hydroxyproline. Chem. Rev. 1966; 66: 47-86. 1:CAS:528:DyaF28XksFaisw%3D%3D. 10.1021/cr60239a003
Memoli V. Total and fraction content of elements in volcanic soil: Natural or anthropogenic derivation. Sci. Total Environ. 2018; 625: 16-26. 1:CAS:528:DC%2BC2sXitVCktrrJ. 29287209. 10.1016/j.scitotenv.2017.12.223. 2018ScTEn.625.16M
Jahnen-Dechent W, Heiss A, Schäfer C, Ketteler M. Fetuin-A regulation of calcified matrix metabolism. Circ. Res. 2011; 108: 1494-1509. 1:CAS:528:DC%2BC3MXntF2htrk%3D. 21659653. 10.1161/CIRCRESAHA.110.234260
Leavesley DI. Vitronectin—Master controller or micromanager?. IUBMB Life. 2013; 65: 807-818. 1:CAS:528:DC%2BC3sXhsVertbjO. 24030926
Johnston E, Buckley M. Relative protein abundances and biological ageing in whole skeletal elements. J. Proteome Res. 2021; 20: 538-548. 1:CAS:528:DC%2BB3cXitFSltLbF. 33089684. 10.1021/acs.jproteome.0c00555
Wiemann J. Fossilization transforms vertebrate hard tissue proteins into N-heterocyclic polymers. Nat. Commun. 2018; 9: 4741. 30413693. 6226439. 10.1038/s41467-018-07013-3. 2018NatCo.9.4741W. 1:CAS:528:DC%2BC1cXitFKmsLvL
Vistoli G. Advanced glycoxidation and lipoxidation end products (AGEs and ALEs): an overview of their mechanisms of formation. Free Radic. Res. 2013; 47: 3-27. 1:CAS:528:DC%2BC3sXht1Witr%2FP. 23767955. 10.3109/10715762.2013.815348
Reiche I. A multi-analytical study of bone diagenesis: the Neolithic site of Berey (Paris, France). Meas. Sci. Technol. 2003; 14: 1608-1619. 1:CAS:528:DC%2BD3sXnsVKjs7g%3D. 10.1088/0957-0233/14/9/312
Bocherens H. Diagenetic evolution of mammal bones in two French Neolithic sites. Bull. Soc. Géol. France. 1997; 168: 555-564
Kendall C. Diagenesis of archaeological bone and tooth. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2018; 491: 21-37. 10.1016/j.palaeo.2017.11.041
Capasso, L. I fuggiaschi di Ercolano. Paleobiologia delle vittime dell'eruzione vesuviana del 79 d. C. (Roma, 2001).
Tjelldén AKE, Kristiansen SM, Birkendal H, Jans MME. The pattern of human bone dissolution-A histological study of Iron Age warriors from a Danish wetland site. Int. J. Osteoarchaeol. 2018; 28: 407-418. 10.1002/oa.2666
Berna F, Matthews A, Weiner S. Solubilities of bone mineral from archaeological sites: the recrystallization window. J. Archaeol. Sci. 2004; 31: 867-882. 10.1016/j.jas.2003.12.003
Pike, A. W. G, Nielsen-Marsh, C. M, & Hedges R. E. M. Bone Dissolution & Hydrology. Proceedings of Archaeological Sciences 1997, British Archaeological Reports (Oxford, 2001).
Nielsen-Marsh CM. Bone diagenesis in the European Holocene II: taphonomic and environmental considerations. J. Archaeol. Sci. 2007; 34: 1523-1531. 10.1016/j.jas.2006.11.012
Civetta L, Galati R, Santacroce R. Magma mixing and convective compositional layering within the Vesuvius magma chamber. Bull. Volcanol. 1991; 53: 287-300. 10.1007/BF00414525. 1991BVol.53.287C
Saxena S, Rani A. Fluoride ion leaching kinetics for alkaline soils of Indian origin. J. Sci. Res. Rep. 2012; 1: 29-40
Straulino L. Approach to the knowledge of preservation of pleistocenic bone. Rev. Mex. de Cienc. Geol. 2019; 36: 170-182. 10.22201/cgeo.20072902e.2019.2.1036
Piochi M, Bruno PP, De Astis G. Relative roles of rifting tectonics and magma ascent processes: Inferences from geophysical, structural, volcanological, and geochemical data for the Neapolitan volcanic region (southern Italy). Geochem. Geophys. Geosyst. 2005; 6: Q07005. 1:CAS:528:DC%2BD2MXpvFOmsbo%3D. 10.1029/2004GC000885. 2005GGG..6.7005P
Pappalardo L. Chemical and isotopical evolution of the Phlegrean magmatic system before the Campanian Ignimbrite (37 ka) and the Neapolitan yellow tuff (12 ka) eruptions. J. Volcanol. Geotherm. Res. 1999; 91: 141-166. 1:CAS:528:DyaK1MXntVaitL8%3D. 10.1016/S0377-0273(99)00033-5. 1999JVGR.91.141P
Isaia, R, Iannuzzi, E, Sbrana, A, Marianelli, P, Donadio, C, Conforti, A, D'Argenio, B. (eds). Note illustrative della Carta Geologica d'Italia alla scala 1:50.000, foglio 446–447, Napoli, ISPRA.
Conticelli, S. et al. Leucite-bearing (kamafugitic/leucititic) and –free (lamproitic) ultrapotassic rocks and associated shoshonites from Italy: constraints on petrogenesis and geodynamics. J. Virt. Explor.36, paper 20, https://doi.org/10.3809/jvirtex.2010.00251 (2010).
Martin R. A re-evaluation of manner of death at Roman Herculaneum following the AD 79 eruption of Vesuvius. Antiquity. 2020. 10.15184/aqy.2019.215
Guarino FM. Recovery and amplification of ancient DNA from Herculaneum victims killed by the 79 AD Vesuvius hot surges. Turk. J. Biol. 2017; 41: 640-648. 1:CAS:528:DC%2BC1cXitV2htr%2FN. 10.3906/biy-1702-48
Duong VA, Park JM, Lim HJ, Lee H. Proteomics in forensic analysis: applications for human samples. Appl. Sci. 2021; 11: 3393. 1:CAS:528:DC%2BB3MXhs1ymsLrM. 10.3390/app11083393
Procopio N, Chamberlain AT, Buckley M. Intra- and interskeletal proteome variations in fresh and buried bones. J. Proteome Res. 2017; 16: 2016-2029. 1:CAS:528:DC%2BC2sXmtlyhu7w%3D. 28436665. 10.1021/acs.jproteome.6b01070
Mason KE, Anex D, Grey T, Hart B, Parker G. Protein-based forensic identification using genetically variant peptides in human bone. Forensic Sci. Int. 2018; 288: 89-96. 1:CAS:528:DC%2BC1cXotlGjsb4%3D. 29738994. 10.1016/j.forsciint.2018.04.016
Lanigan LT. Multi-protease analysis of Pleistocene bone proteomes. J. Proteomics. 2020; 228. 1:CAS:528:DC%2BB3cXhs1GjsbjF. 32652221. 10.1016/j.jprot.2020.103889
Cox J, Mann M. MaxQuant enables high peptide identification rates, individualized p.p.b.-range mass accuracies and proteome-wide protein quantification. Nat. Biotechnol. 2008; 26: 1367-1372. 1:CAS:528:DC%2BD1cXhsVWjtLzJ. 19029910. 10.1038/nbt.1511
0 notes
Text
Mass Spectrometry Analytics For Biologics – Selvita
Biological drugs, also known as biologics, are medications derived from living organisms. Unlike traditional small molecule drugs, which are chemically synthesized, biologics are produced using biotechnology methods, such as recombinant DNA technology or protein engineering. They are used to treat various medical conditions, including autoimmune diseases, cancer, and rare genetic disorders. Biologics are often administered through injection or infusion, and they have become an increasingly important part of modern medicine.
In general, therapeutic proteins exhibit high molecular complexity, making them sensitive to various environmental factors, such as temperature, light, and pH. Even minor changes can cause the biologic to become unstable, leading to decreased efficacy or harmful side effects. Therefore, proper analytical characterization and usage of state-of-the-art techniques allow for mitigating potential risks.
Mass spectrometry (MS) has become an essential tool for characterizing proteins in modern biotechnology and pharmaceutical research. This technology is based on the principles of ionization and mass-to-charge (m/z) separation. It allows researchers to identify and quantify the individual components of a protein sample with a high degree of accuracy and precision. Undoubtedly, MS is a valuable tool for confirming the primary structure of a protein and identifying any post-translational modifications that may affect its function.
Primary structure determination
Primary structure confirmation using mass spectrometry allows for identifying and confirming a protein’s amino acid sequence. It involves breaking down the protein into its constituent peptides and analyzing these peptides to determine their mass-to-charge ratio (m/z). The first step is to cleave the protein into smaller peptides using a protease enzyme, such as trypsin or others. The resulting peptides are then purified and separated using liquid chromatography. The complex peptide mixture can be separated by a nano-LC system where the separation of the analytes takes place into capillary columns to enhance sensitivity. Next, the peptides are ionized using an electrospray ionization source and introduced into the mass spectrometer. The difficulty of obtaining complete coverage of the protein sequence using MS alone is probably the main limitation of this technique, especially for larger proteins. Additionally, some amino acids may be challenging to detect using MS, such as those that are modified or have unusual chemical properties. Therefore utilization of MS-based analysis requires thorough experience.
Modifications of proteins
One of the key applications of mass spectrometry in protein characterization is the identification of post-translational modifications (PTMs). PTMs are chemical modifications that occur after the translation of a protein and can significantly alter its biological activity and function. Almost all proteins, whether produced as recombinant proteins or isolated from natural sources, will carry, to some degree, modified amino acids. Modification may also occur during the sample handling process. Therefore, they need to be monitored to ensure the consistency of production batches. Modifications, like deamidation, oxidation, pyroglutamate formation, etc., may affect protein functionality. Hence, identifying protein modifications and their position is essential in protein characterization.
Among others formation of disulfide bridges significantly influences protein structure and function. Incorrectly paired disulfide bonds result in changing protein properties. For that reason, their mapping is a significant step for confirming proper tertiary structure. Mass spectrometry can be used to identify the specific sites of PTMs, such as phosphorylation or glycosylation, and to determine their abundance. The glycosylation-related heterogeneity of proteins arises from the differences in localization and occupancy of the glycosylation sites and the diversity of the glycan structures expressed on a specific site. To address both levels, glycopeptides analysis and site occupancy evaluation should be utilized.
Aggregation and oligomerization
Oligomers, aggregates, and fragments are biologically active protein proteoforms and common product-related impurities in biopharmaceuticals that impact efficacy, safety, and stability. Identification of low-level impurities may involve labor-intensive chromatographic fraction collection and follow-up experiments. In addition, a significant part of the total protein aggregates can be caused by non-covalent molecular interactions, which are dissociable in denaturing buffers and cannot be characterized by conventional mass spectrometry. Using ammonium acetate solution for SEC allows for preserving non-covalent protein interactions and registers them by MS with high sensitivity and accuracy.
Besides the assessment of biomolecule size variants, native MS allows the characterization of charge heterogeneity through the combination of ion exchange chromatography and native MS. Characterization of variants such as deamidation, which are traditionally unattainable by an intact mass due to their minimal molecular weight differences, can be measured unambiguously by mass and retention time.
Conformational dynamics analysis
Hydrogen–deuterium exchange mass spectrometry (HDx-MS) allows insight into the behavior of the protein in the solution and the correlation of HDx with the structure and dynamics of the molecule. The technique enables the analysis of proteins in terms of interactions with ligands/drugs, other proteins, and lipids or the study of the effect of mutations and post-translational modifications under the same experimental conditions in solution. The main application of HDx-MS is the comparative analysis of different protein conformers. HDx-MS is a complementary method to 3D static structures, allowing for a “dynamic” image of a protein that can explain many biological processes.
In conclusion, mass spectrometry is a powerful tool for the characterization of proteins in modern biotechnology and pharmaceutical research. It allows for identifying post-translational modifications, protein-protein interactions, and quantifying protein abundance, among other applications. With ongoing advances in mass spectrometry technology and sample preparation techniques, mass spectrometry will continue to play a critical role in protein characterization and the development of new therapeutics.
For more information visit our website: https://selvita.com
0 notes
Text
Top-down Proteomics of Myosin Light Chain Isoforms Define Chamber-Specific Expression in the Human Heart
Myosin functions as the "molecular motor" of the sarcomere and generates the contractile force necessary for cardiac muscle contraction. Myosin light chains 1 and 2 (MLC-1 and -2) play important functional roles in regulating the structure of the hexameric myosin molecule. Each of these light chains has an "atrial" and "ventricular" isoform, so called because they are believed to exhibit chamber-restricted expression in the heart. However, recently the chamber-specific expression of MLC isoforms in the human heart has been questioned. Herein, we analyzed the expression of MLC-1 and -2 atrial and ventricular isoforms in each of the four cardiac chambers in adult non-failing donor hearts using top-down mass spectrometry (MS)-based proteomics. Strikingly, we detected an isoform thought to be ventricular, MLC-2v, in the atria and confirmed the protein sequence using tandem MS (MS/MS). For the first time, a putative deamidation post-translation modification (PTM) located on MLC-2v in atrial tissue was localized to amino acid N13. MLC-1v and MLC-2a were the only MLC isoforms exhibiting chamber-restricted expression patterns across all donor hearts. Importantly, our results unambiguously show that MLC-1v, not MLC-2v, is ventricle-specific in adult human hearts. Overall, top-down proteomics allowed us an unbiased analysis of MLC isoform expression throughout the human heart, uncovering previously unexpected isoform expression patterns and PTMs. http://dlvr.it/ShZ9gB
0 notes
Text
What are the factors that affect the stability of peptides?
What are the factors that affect the stability of peptides?
The factors affecting the stability of synthesized peptides include deamidation, oxidation, hydrolysis, disulfide bond , racemization, beta – elimination, aggregation, etc.The results show that the most common degradation products are deamidation products, oxidation products and hydrolysis products.
Among all kinds of amino acids, Asparagine and Glutamine are easy to deamidate (especially under…
View On WordPress
0 notes
Text
methylhexanamine and ustekinumab treatment in psoriatic arthritis: results of a direct comparison
The particular creators examined health #Link# measures, all around health, subconscious hardship and also health behaviours. Results Twenty percent involving participants stayed #Link# steady in the course of the child years, 59% relocated 1 to 2 occasions and 21% relocated at the very least thrice. For some well being steps (other than physical health), there was clearly an increased risk of bad health that will remained elevated for regular moving firm right after realignment pertaining to socio-demographic characteristics and faculty goes (yet was only considerable with regard to illegal drug abuse). Conclusions Risk of illness has been improved within teenage life and adulthood with an increase of residential flexibility when people are young, after changing pertaining to socio-demographic traits and school movements. This became genuine for general health, subconscious hardship and also wellbeing behaviours, however health steps just weren't connected with years as a child range of motion.Release: Coeliac disease testing is frequently determined by discovery associated with IgA anti-tissue transglutaminase (TTGA). IgA deficit (IgAD) is associated with celiac disease and has to end up being discovered to allow utilization of IgG centered assays of these people. The BioPlex (3rd r) 2200 Coeliac IgA and also IgG packages make use of Luminex method use a technique of together calculating TTG and also deamidated gliadin peptide (DGP) antibody quantities by using a completely automated random gain access to analyzer determined by Luminex (R) technological innovation. Separate products are around for IgA (TTGA and DGPA) and also IgG (TTGG along with DGPG) isotypes. Your IgA based kit carries a fresh "IgA Verification Bead" (AVB) to test for IgAD (in smaller than 0.'07 g/L) to ensure that these people are determined and tested using the IgG centered package. Aim: To do a scientific as well as specialized evaluation of the actual BioPlex (Third) Twenty two hundred Celiac IgA and also IgG systems. Techniques: 116 sera from 116 biopsy verified celiac disease people have been screened (Fifty-eight brand-new delivering presentations with a gluten made up of diet regime and 59 known TTGA optimistic individuals over a gluten-free diet program however with alleged inadequate submission). IgAD had been present in A few patients. Capacity to the flag IgAD sera has been evaluated through examination #Link# regarding 29 IgAD along with 190 non-IgAD sera. Uniqueness ended up being computed via 124 unselected consecutive disease manage sera. Final results: Level of responsiveness as well as nature regarding IgAD ended up 100%. Screening process together with TTGA along with including TTGG whenever IgAD was recognized, gave scientific sensitivity regarding 100% regarding celiac disease. Uniqueness ended up being 100% regarding TTGA as well as TTGG, and also 98% and 97% pertaining to DGPA and DGPG respectively. Bottom line: Standby time with the BioPlex (3rd r) 2200 Coeliac IgA along with Coeliac IgG systems in a standard standard protocol gave outstanding level of sensitivity and also nature along with impressive diagnosis associated with IgAD, zero untrue optimistic IgAD flags as well as tiny evidence disturbance via substantial IgA quantities.
1 note
·
View note
Text
The particular appearance along with localization of V-Temozolomidease and cytokeratin Five throughout postnatal progression of your pig epididymis
mtCAT1 cardiomyocytes had drastically improved mitochondrial membrane layer probable, respiration as well as ATP turnover together with significantly lowered sensitive oxygen species creation along with mobile demise pursuing mitochondrial stress. Summary: These information provide brand-new insights in the function associated with L-arginine transportation within mitochondrial chemistry as well as heart problems. Development of mitochondrial L-arginine supply might be a book therapeutic technique for myocardial ailments involving mitochondrial stress for example center failing and also reperfusion injuries.In the cerebral cortex, pyramidal tissues and interneurons tend to be made inside #Link# far-away germinal zones, so the components that handle his or her specific construction into distinct microcircuits stay the enigma. Take a look at are convinced that cortical interneurons labeled on the clonal amount usually do not deliver aimlessly but alternatively possess a powerful propensity to be able to group within the computer mouse neocortex. This particular actions is usual to different classes involving interneurons, individually of the source. Interneuron groups are normally included within 1 or 2 nearby cortical tiers, are largely produced simply by isochronically made nerves and fill particular levels, since uncovered by simply neutral hierarchical clustering techniques. Our final results advise that distinct progenitor cells bring about interneurons inhabiting #Link# infra- as well as supragranular cortical tiers, which in turn challenges latest opinions regarding cortical neurogenesis. Therefore, specific lineages associated with cortical interneurons are made to be able to mainly reflect the actual laminar structure of the cerebral cortex, as an alternative to the columnar corporation.Whole milk along with cheeses are costly food products, along with their consumption can be distributed one of many inhabitants because of the large nutrients; for this reason #Link# they are usually afflicted by adulterations. Among the widespread unlawful techniques, adding powdered ingredients derivatives looks difficult to find because the adulterant materials have got nearly exactly the same chemical structure regarding liquefied milk. Nonetheless, the prime temperatures (180-200 degrees Chemical) useful for milk natural powder generation could imply the occurrence of a few proteins alterations (elizabeth.gary., glycation, lactosylation, corrosion, deamidation, lack of fluids). The actual revised proteins as well as proteins can then be part of marker pens for that existence of powdered ingredients whole milk. With this function, matrix-assisted laser desorption ionization time-of-flight size spectrometry (MALDI-TOF Microsoft) had been used to examine tryptic absorbs relevant to samples of uncooked fluid (without high temperature remedy), business liquid, and powdered cow's take advantage of. Biological materials had been subjected to two-dimensional teeth whitening gel electrophoresis (2-DE); variations amongst water as well as powdered dairy ended up detected at this time and finally verified simply by MALDI research inside carbamide peroxide gel ingested meats. Several diagnostic peptides associated with powder take advantage of, due to altered whey protein and/or caseins, were discovered.
0 notes
Text
oops I forgot to post yesterday's
100 days of productivity
day 16
CVS/RS
Williams syndrome → supravalvular aortic stenosis
Brugada syndrome → ST-elevation >= 2 mm w/ negative T-waves + incomplete RBBB pattern; pattern becomes clearer with challenge of flecainide
Ebstein's anomaly is assoc w/ Wolff-Parkinson-White
workup of pulmonary HTN: acute vasoreactivity testing/vasodilator testing with prostacyclin/inhaled iloprost → around 5% of sufferers will show >10 mmHg drop in PA pressure, which is considered a positive test result and implies success of CCBs (nifedipine, diltiazem) in treatment; however, 95% of the pop will have a negative test and will require PDE-5 inhibitors or endothelin antagonists for long-term treatment
anti-GBM dz vs GPA: anti-GBM will have a normal ESR/CRP with no sinus or nasal septum involvement
in STEMI 90 minutes after administering tPA repeat ECG must show 50% resolution of ST-elevation; if it doesn't, take patient up for PCI immediately
hyoscine hydrobromide is the preferred agent to reduce respiratory secretions (not to be confused with hyoscine butylbromide)
CNS/Ophthal/ENT
donepezil is assoc w/ insomnia
apraclonidine causes miosis in a normal eye d/t stimulation of alpha-2 receptors; it causes mydriasis in a Horner's pupil due to relative supersensitivity of irideal muscles to alpha-1
hydroxyamphetamine causes mydriasis in Horner's affecting 1st and 2nd order neuron; it engenders no change in 3rd order Horner's
type 1 HSMN (Charcot-Marie-Tooth) is demyelinating; type 2 HSMN is an axonal pathology
GIT/Endocrine
oesophageal stricture in the context of atopy → consider eosinophilic oesophagitis (characteristic rings may not be given in question stem)
pyogenic liver abscess: drain + cipro + metro + amox/(clinda if PCN-allergic)
IgA levels must be measured before interpreting anti-TTG/anti-endomysial antibodies as IgA-deficiencies can create false negatives; if IgA-deficient, measure IgG against deamidated gliadin peptide instead
maximum normal calibre of bowels: 3 cm (small intestine); 6 cm (large intestine); 9 cm (caecum)
splenomegaly is a rare feature of Plummer-Vinson
total parenteral nutrition side effects: blood stream infections, hyperglycaemia, refeeding syndrome when weaning, thrombophlebitis, aminotransferase derangement
hyoscine butylbromide is an agent used in the management of bowel colic (not to be confused with hyoscine hydrobromide)
s/p MI convert all oral drugs to IV insulin infusion (rather than biphasic or basal bolus regimen); evidence not
Onc/Immuno/ID
severe parasitaemia for knowlesi malaria is 1%, as opposed to 2% for other species, d/t short erythrocytic stage
pneumococcus is associated with HSV reactivation (cold sores)
other than MG, thymomas are also assoc w/ pure red cell aplasia, SLE, SIADH and dermatomyositis
giant cell arteritis is invariably characterised by high blood viscosity
Renal/Biochem/Toxo
Fanconi syndrome → type 2 RTA w/ ↓Ca, ↓PO4, glycosuria despite normal blood sugars and amino aciduria
aspirin and sulfasalazine cross-react (as both contain a salicylate moiety)
4 notes
·
View notes
Text

Testing for Celiac Disease
There are a lot of questions regarding the kinds of testing available to diagnose Celiac Disease, who should be tested, and what are the requirements for each.
Knowing your options for testing is crucial to ensure you receive the most accurate diagnosis, as possible.
Learn the following:
• Who should be tested
• Testing children under 3
• The First Step: tTG-IgA Test
• IgA Endomysial antibody (EMA)
• Total serum IgA
• Deamidated gliadin peptide (DGP IgA and IgG)
• Video capsule endoscopy (VCE)
• Intestinal fatty acid binding protein (I-FABP)
• Radiology
• Genetic Testing
• Who should have Celiac HLA testing?
• How do I get tested?
The information has been provided by the Celiac Disease Foundation.
https://www.joeandtheglutenfreelife.com/testing
#gluten free recipes#glutenfree#glutenintolerance#sans gluten#beyond celiac#celiac#coeliac#autoimmune disorders#lupussupport#rheumatoid disease#fibromyalgia#crohn’s disease#hashimoto’s thyroiditis#type 1 diabetes#type1warrior#aids awareness#hiv awareness#lactose intolerance problems
2 notes
·
View notes
Link
Q. How does one test for celiac disease?
.
.
.
A. Order tissue transglutaminase (tTG) IgA, total serum IgA, and maybe IgA deamidated gliadin peptide. Anti-endomysial antibodies are no longer considered useful.
There was a period of time in which anti-gliadin antibodies were thought to be more accurate in kids <2 years of age; now it is thought to be the least accurate serum test. Tissue transglutaminase (tTG) IgA is now thought to be the most accurate test regardless of age, assuming an adequate baseline level of serum IgA.
If the serum tests are positive--or if clinical suspicion remains high despite negative lab tests--proceed to EGD with intestinal biopsy for confirmation of the diagnosis.
Pro-Tip: Test for celiac disease *before* starting a gluten-free diet! Otherwise the sensitivities are too low to be useful.
4 notes
·
View notes
Text
Latex formula for making bladder blood pressure measuring devices ABSTRACT TRIAL PRODUCTION OF RUBBER FOR SPYGMOMANOMETER FREE FROM NITROSAMIN AND PROTEIN ALLERGEN IN FACTORY SCALE. The rubber for sphygmomanometer in this study is: bulb, bladder, and tube which made from irradiated natural rubber latex in factory scale production at PT. Sugih Instrumendo Abadi Padalarang. The irradiated natural latex is prepared by 7 rays' Co radiation vulcanization on natural rubber latex at the doses of 25 kGy, and 3 phr (part hundred ratio of rubber) was added by antioxidant then it was made rubber for sphygmomanometer (bladder, bulb, and tube) by coagulant dipping method. Three important factors: heating temperature (90 ° C and 100 ° C), heating time (4,8,12,116,20,24 hours), and leaching technique (water, solution of ammonia and KOH) has, and the physical and mechanical properties of rubber film (modulus, tensile strength, elongation at break, hardness) has been evaluated. The results show that heating temperature at 90 ° C, and heating time on 8 hour then leached in 0,5% solution of ammonia or KOH are the optimum condition processing. By using this optimum condition the tensile strength of film is 24 MPa, modulus 600% 2.0 MPa, elongation at break 1000%. Hardness is 30 Shore A, with the extractable protein content is around 72-110 nig, and nitrosamine content is not detected (zero). The value of ELISA test method for absorbance of a sensitive human serum again protein allergen is zero (negative), and the response of rubber serum again skin through SPT method is zero (negative), which means that rubber for sphygmomanometer is free from nitrosamine and a protein allergen. Key words: Sphygmomanometer, nitrosamine, protein allergen, irradiated natural rubber latex Preliminary Tensimeter (Sphygmomanometer) is an instrument that can be used to measure arterial blood pressure indirectly (non-invasively) with the help of a stetoscope. This instrument is equipped with a manometer, a packaging container, which is the outside of the tensimeter instrument for placing other parts of the equipment. This packaging container contains, among others: rubber balls, rubber hoses, and bladders wrapped in kai A case that had occurred in America around 1980-1985, namely a nurse who often used a tensimeter, had malignant cancer in her body. After researching it, it turned out that the cause was the nitrosamine from the tensimeter ball which the nurse often used. Eventually the nurse claimed tens of millions of US dollars to tensimeter manufacturers [6]. Another case that has been reported in several publications, is not only nitrosamines but also allergen proteins that can cause allergies in the human body. For example, some workers in hospitals in Jakarta, who use gloves made of natural rubber or factory workers who are in direct contact with natural rubber, show that around 3% of these workers are allergic to natural rubber [7], even if people who are allergic to natural rubber operated on by medics who used rubber finished goods of natural rubber (gloves, condoms and catheters) can cause. With these incidents, since 1987, Europe has limited the content of nitrosamines in rubber finished goods, for example in baby pacifiers, a maximum of 1-10 ppb, and the World Health Organization (WHO) since 1999 has drafted restrictions on extracted protein content ( protein that causes allergies) in rubber products, eg gloves maximum 150 pg / g How is the chemical formula for making latex for medical devices There are actually 3 types of latex that can be used for the production of bladders, balloons, and hoses for tensimeters, namely: concentrated natural latex or concentrated natural latex, chloroprene latex (neoprene), and irradiated natural latex, which by itself the three types of latex have different specifications - different Natural rubber latex consists of rubber particles and a non-rubber material. Natural rubber particles contain polyisoprene, which when irradiated will occur a cross-linking event, meanwhile the non-rubber material in the latex consists of various amino acids containing thiol compounds, as well as aromatic and aliphatic amino acids, carbonyl groups and olifenic groups. As a result of irradiation, it will experience degradation, so there will be various kinds of radicals or can also experience deamination, deamidation, decarboxylation, oxidation of S-H groups, reduction of S-S groups, changes in amino acid side chains and addition / reduction of peptide chains. This event is followed by changes in biological, biochemical, and physicochemical properties, thus forming new products that are easily soluble in water [20-21] When the irradiated rubber film is washed with water, the new compound will come out of the rubber film, consequently the protein value, fat, and the rubber film carbohydrates decreased. Latex Compound Formulation In general, the method of formulating latex compounds for rubber finished goods by means of sulfur vulcanization must be added with vulcanizing agents (sulfur), activating agents (zeng oxides), accelerating agents (carbamate compounds, thiazoles, aldehyde-amines, thiazol sulfoamides, thiophosphates, guanidine, thiourea, or thiocarbonyl sulfenamide) and anti-oxidants (phenyl or amine compounds) into concentrated latex. Because the four types of substances have the potential to produce compounds that are toxic, carcinogenic and allergic [22], it is recommended that they use as little as possible. Table 3 presents the composition of sulfur vulcanized latex compounds and irradiated natural latex used to manufacture bladders, balls and hoses on a factory scale. From this table it shows that only one type of chemical is needed for radiation vulcanization, namely antioxidant phenol compounds with low toxicity and allergy type IV, while for sulfur vulcanization there are 4 types, namely activating agents, accelerators, antioxidants, and sulfur vulcanizing agents. which according to Makuuchi [23] these materials are sufficiently low to high risk of toxicity, type IV allergies, and carcinogens (cancer causing) in the finished rubber products produced. So when viewed from this formulation, irradiated natural latex compounds in addition to more efficient chemicals, it is also a low risk of toxic materials derived from antioxidants. To overcome the toxicity and allergies of type IV from antioxidants, BHT is used as an antioxidant ingredient for irradiated natural latex compounds, because BHT is a non-toxic antioxidant and type IV allergy. In particular, the manufacture of bladders comes from two materials, namely natural and synthetic (neoprene bladder). These two basic ingredients use the same dispersion at different levels. The formula for making natural bladders is as follows: 1. Preparation of natural latex dispersion Yellow package creation Sulfur = 750 gr BHT Yosmox / Vulcanox = 1250 gr Tamol / Vultamol NN 9104 = 50 gr Bentonite / Molding = 13 gr The yellow package material is mixed with water in a 2 liter bucket, White Package Creation ZDBC / LDB (from bayer) = 1250 gr ZDEC / LDA (from Bayer) = 500 gr ZnO (from Bayer) = 750 gr Tamol / Vultamol = 50 gr Bentonite / Molding = 13 gr The white package material is mixed with water in a bucket of 0.5 liter, The two packages are put in the ballmill tank and then grinded for 20 hours 2. 200 liters of concentrated latex that has been brooded for 1 week, given texapon and KOH according to the MST table for then stand by for 2 hours. 3. The rolled yellow package formula is included in 200 liters of concentrated latex. Then on stand by for 1 hour 4. Then put a white package and an additional 200 liters of concentrated latex that has been creased. So that the total concentrated latex is 400 liters 5. Then we get a mixture of natural latex For the manufacture of synthetic latex The difference is in the yellow white package making: Yellow package creation Sulfur = 2464 gr BHT Yosmox / Vulcanox = 4520 gr Tamol / Vultamol NN 9104 = 376 gr Bentonite / Molding = 46 gr The yellow package material is mixed with water in a 2 liter bucket, White Package Creation ZDBC / LDB (from bayer) = 2464 gr Orotan = 752 gr ZnO (from Bayer) = 12300 gr Triton = 400 gr Bentonite / Molding = 100gr 1 synthesis package formula package consists of a mixture of 2 buckets yellow package + 4 buckets white package. To control mixing, enter the formula or water using a 2 liter pitcher.
#PAULWALKER#HALMA#RIESTER #PAULWALKER#HALMA#RIESTER #PAULWALKER#HALMA#RIESTER #PAULWALKER#HALMA#RIESTER #PAULWALKER#HALMA#RIESTER #PAULWALKER#HALMA#RIESTER #PAULWALKER#HALMA#RIESTER #PAULWALKER#HALMA#RIESTER #PAULWALKER#HALMA#RIESTER #PAULWALKER#HALMA#RIESTER #PAULWALKER#HALMA#RIESTER #PAULWALKER#HALMA#RIESTER #PAULWALKER#HALMA#RIESTER #PAULWALKER#HALMA#RIESTER #PAULWALKER#HALMA#RIESTER #PAULWALKER#HALMA#RIESTER #PAULWALKER#HALMA#RIESTER #PAULWALKER#HALMA#RIESTER #PAULWALKER#HALMA#RIESTER #PAULWALKER#HALMA#RIESTER #PAULWALKER#HALMA#RIESTER #PAULWALKER#HALMA#RIESTER #PAULWALKER#HALMA#RIESTER #PAULWALKER#HALMA#RIESTER
1 note
·
View note
Text

Ancient protein analysis provides clues about human and animal life and diseases from the past1
. Bone, a mineralised extracellular matrix rich in connective tissue components, is one of the most abundant sources of ancient
proteins and one of the most abundant biomaterials in the archaeological record. Te diagenesis, in particular
when heat-induced, of organic matter in archaeological bone, initially represented mostly by type I collagen
(90%), lipoproteins and mucopolysaccharides2
, is a complex phenomenon3,4
, linked to several factors in the
depositional environment2,5,6
. Te analysis of how diagenesis acted to produce or prevent specifc modifcations
led to coin the term “diagenetiforms” to describe the variety of distinct molecular species arising from chemical
modifcations by environmental conditions7
.
Mass spectrometry-based techniques have been widely applied to characterize collagen in ancient proteomes
and its chemical modifcations occurred pre- and post-mortem8
. Apart from the extensive observation of deamidation of asparagines and glutamines Gln9–12 and methionine oxidation13, a few other diagenetically induced
modifcations were also detected, such as: (i) aminoadipic acid formation from lysine, (ii) tryptophan oxidation
products13, (iii) advanced glycation end-product (AGEs)13–15, (vi) backbone cleavage14,15, while complex oxidative reactions occurring on prolines have been hypothesised but not characterised in details13,14. So far though,
no study characterized the modifcations occurred post-mortem in the proteome of archaeological bones from
individuals who died due to the nearly instantaneous exposure to extreme temperature.
Here we applied a bottom-up proteomic approach to investigate the proteome and the chemical modifcations present in the bone proteins of humans from Herculaneum16 and Pompeii (Italy)17, who died during the
eruption of the Vesuvius in 79 AD. Te accurate description of the catastrophic event afecting Herculaneum
OPEN
1
Department of Chemical Sciences, University of Naples Federico II, Naples, Italy. 2
Evolutionary Genomics Section,
Globe Institute, University of Copenhagen, Copenhagen, Denmark. 3
Department of Humanities, University Suor
Orsola Benincasa, Naples, Italy. 4
Department of Medicine, Surgery and Dentistry, University of Salerno, Fisciano,
Salerno, Italy. 5
Archaeological Park of Herculaneum, Ercolano, Naples, Italy. 6
Task Force Di Ateneo “Metodologie
Analitiche per la Salvaguardia dei Beni Culturali”, University of Naples Federico II, Naples, Italy. 7
Department of
Advanced Biomedical Sciences, Departmental Section of Legal Medicine, Anatomy and Histology, University of
Naples Federico II, Naples, Italy. *email: birolo@unina.
0 notes
Text
The Symptoms of Gluten Intolerance You Haven’t Heard About
You just don’t feel good. You’re tired and get frequent headaches, have ongoing skin issues, or struggle with depression—or all of the above. Maybe you’ve wondered if gluten could be the culprit, but because you don’t experience gastrointestinal upset, you’ve since put the thought out of your mind and haven’t mentioned anything to your doctor. Or perhaps you’ve shared your suspicion, but conventional testing ruled out celiac disease (CD) and thus, supposedly, any issues with gluten. In either case, your diet has likely stayed the same … and so have these often-overlooked symptoms of gluten intolerance.
Yes, all of the symptoms mentioned here—and many others you may not have heard about—can be signs that you have a significant degree of gluten intolerance. Even if you don’t run to the bathroom right after enjoying a plate of pasta, and even if standard lab work says otherwise, your body (and brain) may be having serious problems with gluten. Let’s explore these lesser-known symptoms and discuss if going gluten free is right for you.
You’ve heard of the havoc gluten can wreak on your digestive tract, but did you know that gluten intolerance can cause skin problems, depression, and frequent headaches? Check out this article for more symptoms of gluten intolerance you’ve never heard of. #nutrition #chriskresser
Undiagnosed Intolerance Is More Common than You May Think
First off, I want you to know that if you eat gluten and you feel lousy but you don’t have digestive issues—and you have tested negative for CD and been told it’s all in your head—you are not alone. In fact, undiagnosed cases of gluten intolerance are incredibly widespread. Here’s why.
As I’ve written before, wheat contains several different classes of proteins: gliadins (of which there are four different types, including one called alpha-gliadin); glutenins; agglutinins; and prodynorphins. Once wheat is consumed, enzymes in the digestive tract called tissue transglutaminases (or tTGs) help break down the wheat compound. During this process, additional proteins are formed, such as deamidated gliadins and gliadorphins (also called gluteomorphins). Stick with me here—these terms are worth knowing so that you can understand the pitfalls of conventional testing for CD.
CD is a serious form of gluten intolerance, one that can do real damage to the tissues in the small intestine (though its symptoms aren’t merely gut related). CD is characterized by an immune response to one specific gliadin (the aforementioned alpha-gliadin) and one specific type of transglutaminase (tTG-2). But people can—and very much do—react to several other components of wheat and gluten.
Therein lies the problem, because conventional lab testing for CD and gluten intolerance only screens for antibodies to alpha-gliadin and tTG-2.
If your body reacts to any other wheat protein or type of transglutaminase, even severely, you’ll still test negative for CD and intolerance.
Statistics suggest that for every one case of CD that is diagnosed, 6.4 cases remain undiagnosed—the majority of which are atypical forms without gastrointestinal symptoms; even many patients who are eventually diagnosed don’t experience an upset stomach after consuming gluten. (1)
What’s more, the distinct autoimmune response to wheat proteins and transglutaminase enzymes in the gut that defines CD is just one possible expression of gluten intolerance. The many other ways a sensitivity to gluten can affect the body are collectively referred to as non-celiac gluten sensitivity, or NCGS. Cases of gluten intolerance classified as NCGS involve both intestinal and non-digestive reactions to gluten that are not autoimmune or allergic in nature and that resolve when gluten is eliminated from the diet.
There is no definitive diagnostic test for NCGS, making it difficult to put a number on its prevalence. By some estimates, it may occur in as many as one in 20 Americans. (2) And although your doctor and plenty of others out there might still insist that NCGS doesn’t truly exist, several studies have validated it as a distinct clinical condition. (3) As I’ve explained previously, gluten sensitivity is very real. Stories painting NCGS as a collective delusion have gotten it wrong.
Decoding Your (Real) Symptoms
Gluten intolerance can affect nearly every tissue in the body, including the brain, skin, endocrine system, liver, blood vessels, smooth muscles (found in hollow organs such as the intestines), and, yes, stomach.
That’s why it can manifest either in the classic presentation of digestive distress—abdominal pain, bloating, gas, and diarrhea or constipation—or in any of the following, likely surprising, non-digestive symptoms.
Anemia
Although it’s discussed infrequently in popular articles, iron-deficiency anemia is well documented as a symptom of gluten intolerance in scientific studies. (4, 5) In fact, research suggests that it may often be the first noticeable symptom of CD and that up to 75 percent of those with an anemia diagnosis may be gluten intolerant. (6, 7) Gluten intolerance can interfere with the uptake of iron from food, causing malabsorption of this important nutrient. (8) What’s more, because anemia generally saps one’s energy, it can trigger or worsen the next non-digestive sign of gluten intolerance on this list.
Fatigue
Many gluten-intolerant individuals report feeling tired and fatigued, especially right after eating, you guessed it, gluten. (9) Research has linked NCGS to chronic fatigue symptoms in some people. (10) As with chronic fatigue syndrome, symptoms of gluten intolerance can also include muscle fatigue and muscle and joint pain.
Brain Fog
This type of cognitive dysfunction can be a sign of gluten sensitivity. Those affected often describe experiencing “foggy mind” symptoms such as an inability to focus and concentrate; some also describe feeling mentally fatigued. (11, 12)
Headaches
Headache is a frequent finding in NCGS, with one recent study reporting the symptom in more than half of its participants. (13, 14, 15) Migraine in particular is an associated symptom. (16, 17)
Eczema and Other Skin Disorders
As with fatigue, brain fog, and headaches, people with NCGS may notice a worsening of skin symptoms such as eczema, rash, and undefined dermatitis after ingesting gluten-containing foods. The most commonly reported skin lesions include those similar to subacute eczema, as well as the bumps and blisters indicative of dermatitis herpetiformis, or Duhring’s disease—to which CD is closely linked. Those who are gluten intolerant may also experience scaly patches resembling psoriasis. Lesions are typically found on the muscles of the upper limbs. (18, 19, 20)
Depression and Anxiety
One of the main reasons gluten sensitivity often goes unrecognized and untreated, researchers theorize, is because mental health issues can be a hallmark of this condition. Data suggests that up to 22 percent of patients with CD develop such dysfunctions, with anxiety and depression occurring most commonly. One study found that CD patients were more likely than others to feel anxious in the face of threatening situations, while additional research has linked conditions such as panic disorder and social phobia to gluten response. Depression and related mood disorders appear to occur with both NCGS and CD. (21, 22)
Here’s the good news: The majority of studies cited here not only investigated whether or not these symptoms are signs of gluten intolerance, but also whether or not they can be addressed by going gluten free. And it turns out, these problems improved or completely resolved with adherence to a gluten-free diet.
But more on that in a minute.
Beware These Surprising Consequences of Intolerance
While the symptoms mentioned above are what will most likely clue you in to your body’s negative response to gluten, they aren’t the only effects of intolerance to be aware of. In fact, a variety of chronic diseases may develop due to long-term CD or NCGS, including: (23, 24)
Epilepsy
Attention-deficit hyperactivity disorder, or ADHD
Autism spectrum disorders
Schizophrenia
Type 1 diabetes
Osteoporosis
Multiple sclerosis
Hashimoto’s
Peripheral neuropathy
Amyotrophic lateral sclerosis, or ALS
In one study, researchers found a strong link between gluten sensitivity and neurological complications—especially those in which the cause was unknown. (25) Research has even shown that, for some people with gluten sensitivity, the primary symptom they experience is a neurological dysfunction. (26) The data suggests that nearly 60 percent of people with neurological dysfunction of unknown origin test positive for anti-gliadin antibodies. (27)
Challenge Yourself: Do You Feel Better On a Gluten-Free Diet?
If you’re currently experiencing any of the symptoms of gluten intolerance I shared in this article and can’t seem to find relief, or if you have received a diagnosis of any of the above linked diseases or disorders and you and your doctor have not found a probable cause or resolution, gluten could very well be a trigger for you.
Because of the limitations of current testing for CD and the lack of diagnostic options for NCGS, the most reliable test for gluten intolerance is a “gluten challenge.”
This involves removing gluten from your diet completely for a period of at least 30 days—60 days is best—then adding it back in after that time has elapsed. If your symptoms and/or diagnosis improve during the elimination period and return when gluten is reintroduced, let your healthcare provider know. You have NCGS or atypical CD.
Though I consider this to be the gold-standard test for gluten intolerance, Cyrex Laboratories does offer a comprehensive blood panel that screens for all of the wheat and gluten proteins and transglutaminase enzymes discussed earlier. It can be a helpful diagnostic tool, but it shouldn’t replace a gluten challenge. (Note: It must be ordered by your physician or another healthcare provider.)
How Will You Deal with Your Symptoms of Gluten Intolerance?
If you experience improvement on a gluten-free diet and plan to continue eating this way, you can feel confident that there is no risk in terms of nutrient deficiencies to removing gluten from your diet. (28) If anything, my experience has shown me that people who eat gluten-free are more likely to increase their intake of essential nutrients, especially if they replace breads and other flour products with whole foods.
Have you experienced any of these symptoms? Are you planning a gluten challenge to determine once and for all if gluten is the culprit? Let me know below in the comments!
The post The Symptoms of Gluten Intolerance You Haven’t Heard About appeared first on Chris Kresser.
Source: http://chriskresser.com
November 20, 2018 at 09:17PM
5 notes
·
View notes
Text
Gluten Intolerance, Wheat Allergies, and Celiac Disease - It's More Complicated Than You Think
Is “gluten free” a fad? No, it’s going to be a thing for as long as we are producing wheat and bread the way we’re doing it. A lot has changed in the bread industry – it’s not just one thing.
People often comment about how bread didn’t cause problems with our health before GMOs and Roundup were prevalent in our food supply. Our farming practices have changed, and fairly recently, wheat has started being sprayed with Roundup. The newest speculation is that wheat is not the problem – that the problem is glyphosate, the active ingredient in Roundup. People also often suspect that wheat has been genetically modified. And, of course, there are those who believe the whole gluten-elimination thing is ridiculous and that most people are jumping on the gluten-free bandwagon because it’s trendy.
Related: How to Eliminate IBS, IBD, Leaky Gut
In my experience, if one suffers from a chronic illness of any kind, they must remove gluten from their diet in order to get well. I have yet to see an exception. So what’s the problem? Is it the glyphosate or the wheat or something else? The truth is it’s not just one thing. Everyone would already know this if most humans weren’t so bad at thinking in terms of systems. We tend to think linearly and look for singular cause and effects, but rarely if ever are complex problems solved by such simplistic thinking. There are multiple reasons one gets sick, with a cold or a chronic disease, just like there are multiple reasons why our planet’s ecosystem is changing. This is why you can’t blame the rise of autism on just glyphosate, or GMOs, or increased vaccinations, or diminishing food quality, or environmental degradation – they all correlate, it’s all of the above.
Related: Best Supplements To Kill Candida and Everything Else You Ever Wanted To Know About Fungal Infections
There is a very complex system that is causing the decline of American health, and it’s not just the bread. And yes, our health is in decline. If you doubt that…here, google it and take your pick. Our lifespan is actually decreasing.
What’s the difference between Gluten Intolerance, Wheat Allergies, and Celiac Disease
Conventional medicine states that celiac disease and non-celiac gluten sensitivity have a lot of symptoms in common but identifies a key difference. Non-celiac gluten sensitivity is not a genetic disease and does not cause an autoimmune reaction, and celiac disease is a genetic autoimmune disease. A wheat allergy is an allergic reaction to any of the hundreds of proteins in wheat. Gluten intolerance used to be a catch-all phrase for any problem with eating gluten, but now it’s being relegated to mean Non-celiac gluten sensitivity.
Non-celiac Gluten Sensitivity
Non-celiac gluten sensitivity is believed to be the most prevalent of the gluten-related disorders, but it’s not as well defined as the other two. It’s not an autoimmune reaction nor is it an allergic reaction. There are no tests or biomarkers to identify this disorder. Other components of gluten-grains may be causing symptoms. In order for non-celiac gluten sensitivity to be diagnosed, a doctor will rule out celiac disease and wheat allergies or other possible causes of the symptoms first.
Common Symptoms for Non-celiac Gluten Sensitivity
Fatigue
Mental fatigue, aka “brain fog”
Headaches
Migraines
Bone or joint pain
Gastrointestinal distress
Gas
Bloating
Cramping
Indigestion
Abdominal pain
Diarrhea
Constipation
It’s said that individuals with gluten sensitivity do not experience damage to the small intestine or develop tissue transglutaminase antibodies like they do with celiac disease. Non-celiac gluten sensitivity has been linked to a variety of health problems including, diabetes, allergies, autism spectrum disorders, and much more.
Related: How to Avoid GMOs in 2018 – And Everything Else You Should Know About Genetic Engineering
Gastroenterologists looking for celiac disease typically test for a few specific antibodies, and if found, they do an intestinal biopsy to determine if tissue damage is present. Chris Kresser addresses the issue with this kind of testing in 3 Reasons Gluten Intolerance May Be More Serious Than Celiac Disease, which I highly recommend reading. He states:
According to some estimates, for every diagnosed case of celiac disease (CD), there are 6.4 undiagnosed cases that remain undiagnosed—the majority of which are atypical or “silent” forms with no damage to the gut. (1) This silent form of CD is far from harmless; it is associated with a nearly fourfold increase in the risk of death. (2)
I believe that patients with NCGS are even more likely than patients with CD to go undiagnosed. Most gastroenterologists today know how to screen for celiac disease. They will typically test for antibodies to antibodies to alpha gliadin, transglutaminase-2, deamidated gliadin, and endomysium, and if positive do a biopsy to determine if tissue damage is present.
However, we now know that people can (and do) react to several other components of wheat above and beyond alpha gliadin, the component that is implicated in CD. These include other epitopes of gliadin (beta, gamma, omega), glutenin, wheat germ agglutinin (WGA), gluteomorphin, and deamidated gliadin. What’s more, people can react to other types of tissue transglutaminase, including type 3—primarily found in the skin—and type 6—primarily found in the brain. (3, 4, 5, 6, 7, 8)
Celiac Disease
Celiac disease is considered a genetic, autoimmune disorder. Ninety-eight percent of people with celiac disease carry one or both of two very specific genes, HLA DQ2 and DQ8. On the other hand, so does up to 25-30% of the general population. Carrying one or both of these genes does not mean you have celiac disease nor does it mean you will develop it. Doctors often use gene testing to rule out celiac disease, but there are some cases where people who do not have either of the genes still tested out to have celiac disease.
Though celiac disease is said to be genetic, genes cause predispositions and our diet and environment adjust our genes. Environment can alter gene activity without changing the DNA sequence. This is called gene expression. I also believe that the environment and diet can actually alter the DNA sequence, but from what I’m seeing, current science doesn’t agree with me on this. Regardless, how your genes affect you is altered by our diet and our environment, and those traits can be passed down to our offspring as well. In other words, a predisposition to celiac disease may be hereditary, but whether or not we have celiac disease could depend on our genetic health, which depends on our overall health, which depends on our lifestyle. And this can all be traced to gut health – you cannot have a healthy gut without a healthy lifestyle, and our gut health is something most of us have complete control over.
Related: Gluten, Candida, Leaky Gut Syndrome, and Autoimmune Diseases
Common Symptoms of Celiac Disease
Fatigue
Mental fatigue, aka “brain fog”
Headaches
Migraines
Bone or joint pain
Gastrointestinal distress
Gas
Bloating
Cramping
Indigestion
Abdominal pain
Diarrhea
Constipation
Arthritis
Dermatitis
Eczema
Osteoporosis
Liver disorders
Depression or anxiety
Peripheral neuropathy
Seizures
Migraines
Irregular menstruation
Miscarriages
Canker sores
Doctors believe that in order to develop the disease, a person needs to have the genetic predisposition while they are consuming gluten and to subsequently have the disease activated. Activation triggers are said to potentially be stress, trauma, and viral infections. I contend that vaccines and antibiotics are the two most common triggers for the disease. Damaging the gut is what leads to problems with wheat, but we’ll get more into that below.
Wheat Allergies
Celiac disease and non-celiac gluten sensitivity have many symptoms in common, but wheat allergies are often much more distinctive. Symptoms include itching, hives, or anaphylaxis which is a life-threatening reaction. A wheat allergy is an immune reaction to any of the hundreds of proteins in wheat. It is possible for a person to be allergic to wheat and to have non-celiac gluten sensitivity or celiac disease at the same time.
What About Roundup?
Monsanto introduced glyphosate under the trade name Roundup in 1974 shortly after DDT was banned. It wasn’t used very much until the late 1990s when Monsanto genetically engineered seeds to withstand high doses of Roundup, and the product took off. Eager to sell more of its flagship herbicide, Monsanto has encouraged farmers to use their glyphosate as a desiccant. Wheat can be harvested quicker and easier if you dry it all out ahead of time with Roundup. It’s also used in this way on wheat, barley, oats, canola, flax, peas, lentils, soybeans, dry beans, and sugar cane.
Studies have concluded that chronically ill people have higher levels of glyphosate in their bodies. Glyphosate has been attributed to an increased prevalence of most of our common chronic conditions including, but not limited to ADHD, Alzheimer’s, birth defects, autism, cancer, kidney disorder, irritable bowel syndrome, Parkinson’s disease, depression, diabetes, heart disease, thyroid disorders, liver disorders, multiple sclerosis, reproductive issues, adrenal failure, obesity, asthma, and of course, celiac disease.
It’s not hard to understand why. Glyphosate is poison and so are the other ingredients in Roundup. People have to wear protective gear to apply the product. It is designed to kill. It kills plants by preventing them from making certain proteins. Just imagine what that does to one’s gut ecology.
How Wheat Has Changed
The wheat we have now is very different from what our ancestors consumed. Modern dwarf wheat is hybridized. That isn’t a GMO, but the genes of our wheat plant have certainly been modified to grow faster, and to be more resilient. We used to eat wheat called einkorn, which was actually one of the very first grains we humans cultivated more than 10,000 years ago. When you read in the Bible about how we should eat bread, this is the wheat it refers to.
There is a lot more gluten in modern wheat than there is in einkorn, and the gluten that einkorn wheat does contain is different. Einkorn also has 15 percent less starch and 30 percent more protein. Modern wheat has a lower nutrient content and a different protein structure. In fact, many with celiac and gluten intolerance report being able to eat einkorn without issue.
Also, that blood sugar spike experienced after eating bread does not happen with einkorn.
So I conducted a simple experiment on myself. On an empty stomach, I ate 4 oz of einkorn bread. On another occasion I ate 4 oz of bread that dietitian, Margaret Pfeiffer, made with whole wheat flour bought at the grocery store. Both flours were finely ground and nothing was added beyond water, yeast, olive oil, and a touch of salt.” – Einkorn and blood sugar
“Ancient wheat diets caused a downregulation of key regulatory genes involved in glucose and fat metabolism, equivalent to a prevention or delay of diabetes development. Spelt and rye induced a low acute glycemic response compared to wheat.” – NCBI
How Bread Making Has Changed
Most commercial bread contains bromides, added starches, refined sugars, added gluten (vital wheat gluten), preservatives, artificial flavorings, leveling agents, and stabilizers. Potassium bromate is an additive used in commercial bread and baked goods that make the products lighter and fluffier. Bromines are part of the halide family, a group of elements that includes fluorine, chlorine, and iodine, which are all endocrine disruptors that cause digestive issues and a host of other health problems.
Related: Sugar Leads to Depression – World’s First Trial Proves Gut and Brain are Linked (Protocol Included)
Baking Soda, baking powder, and cream of tartar are often used in place of yeast or in addition to rapid rise yeast to make the bread rise quickly and more uniformly. Modern bread rises for a couple of hours or less, whereas homemade bread traditionally takes at least 12 hours to rise. I got curious about the difference between baking soda and baking powder, and I thought you might be as well, hence the video below.
youtube
Traditional bread recipes typically utilized a few common ingredients including flour, yeast, salt, water, a sweetener, and some spices or herbs.
Related: Holistic Guide to Healing the Endocrine System and Balancing Our Hormones
Refined flours started to be widely used around 1880 which caused worldwide epidemics of pellagra and beriberi. Refining the flours removes bran and germ which increases shelf life. It also removed the B vitamins. Previous iterations of bread did use bolted or sifted flour which did refine the wheat somewhat, but it didn’t remove all of the bran, germ, and endosperm, and that flour was never bleached.
Bread with Whole Grains that are gently stone ground just before mixing the dough and then allowed to ferment slowly and naturally, in other words — authentic sourdough. That’s how the Egyptians made it 6,000 years ago.”
Bread was fundamentally redesigned. Refined flours, large quantities of commercial yeast, and a combination of additives and intense energy created the modern industrial bread. Fast mixing, fast rise, fast baking. Industrial bread is made far too fast.” – Mario Repetto
How Our Gut Biology Has Changed
We keep eating more and more sugar. In the early 1700s, the average sugar consumption was about 4 pounds a year. By 1800 we were at 18 pounds a year. By 1900 we were up to 60 pounds of sugar a year. Today the average American consumes between 130 and 150 pounds of sugar every year.
Sugar feeds pathogens. Our healthiest gut bacteria like the healthiest foods: vegetables and herbs. Nature wouldn’t work any other way; how could it? You’re probably thinking, “What about fruit?” We don’t eat the fruit we used to eat. Like wheat, our fruit has been radically altered through hybridization. But that’s another article (I’m working on it). For now, just Google “wild banana” or “what watermelon used to look like“.
We get way more sugar than our ancestors got even if we cut out refined foods. This causes an abundance of Candida. I believe Candida is prevalent in every single person with chronic illness. Everyone has yeast but when yeast is left unchecked they turn into pathogenic fungi. Tests for Candida aren’t accurate. Candida, when in it’s in the virulent fungal form, will make the gut more permeable. When this happens food proteins are absorbed into the body before they are digested. This causes allergies. This is one of the main causes of allergies, but there are others at play as well. In my experience, every single person who has cut refined sugar out of their lives and decreased their body’s Candida was able to rid themselves of seasonal, environmental, and food allergies. Every single time!
In addition to that, a study published in The Lancet showed that the candida protein HWP-1 is similar in structure to gluten.
A candida infection in the gut can cause an immune system reaction to HWP-1, which then stimulates an allergic reaction to the gluten in wheat and other grains and may trigger celiac disease in genetically susceptible people.” – Leyla Muedin, RD
Wheat proteins can also cause an immune response against the thyroid.
An obvious explanation is that the initial attack on the thyroid by anti-tTG autoantibodies of celiac leads to thyroid inflammation and presentation of TPO, with a second round of autoantibodies produced to TPO resulting in Hashimoto’s Thyroiditis.” – Dr. Art Ayers
Celiac disease and hypothyroidism beget more chronic autoimmune issues. Allergies lead to autoimmune disease. Allergies lead to chronic health issues. Medical science has established this. Medical science is just starting to understand the fact that a permeable gut causes allergies. Science also has established that an abundance of Candida causes a permeable gut. What they haven’t figured out yet is just how prevalent the permeable gut issue really is. But the bottom line is that our poor diet leads to allergies and almost all that commonly ails us.
Suggestions
If you have a healthy gut, make your own sourdough bread using heirloom wheat and the old-school practices. If you have any chronic illness, then you do not have a healthy gut. Here’s how you fix it. If you’re not well, wait until you get well before consuming any kind of bread. And don’t think of old-fashioned bread as healthy. Vegetables are healthy. Bread is at its best a neutral food with some health benefits and easy calories that can help sustain life like brown rice and millet. Vegetables and herbs heal the body.
Obviously, stay the heck away from poisons! Glyphosate is a cocktail of poisons. Science has firmly established this. And avoid GMOs as well. They weren’t designed with our health in mind, they were designed for profit, and in most cases, to sell more Roundup.
The hard truth is that letting companies cook your food for you leads to poor health. People often ask me, “If you can cure cancer why aren’t you rich?” If I could cure cancer and figure out how to do it while still eating refined, prepackaged, and processed foods that we humans have grown accustomed to, I would be rich. But people would rather die for convenience food than give it up. Obviously. We see this everywhere.
Being well long-term means preparing all your own food yourself the right way, or being rich and hiring someone else to do it. There is no shortcut. Certainly not with bread.
Sources:
Your Ancestors Didn’t Eat The Same Type Of Wheat That You Do (And They Were Healthier) – Off The Grid News
4 Ways Modern Bread is Different From Traditional Bread – Our Heritage of Health
The Real Problem With Bread (It’s Probably Not Gluten) – Mother Jones
Problems Linked to Monsanto’s RoundUp – EcoWatch
15 Health Problems Linked to Monsanto’s Roundup – EcoWatch
Consumption of Sugar – Sugar and Sweetener Guide
Gluten Intolerance, Wheat Allergies, and Celiac Disease – It’s More Complicated Than You Think was originally published on Organic Lifestyle Magazine
3 notes
·
View notes
Text
Deamidated Gliadin Peptide IgA
Deamidated Gliadin Peptide IgA
View On WordPress
0 notes