#eqms software
Explore tagged Tumblr posts
Text
How QMS Software Can Improve Patient Safety and Product Quality
In the life sciences industry, patient safety and product quality are paramount. Quality Management System (QMS) software plays a crucial role in ensuring both by streamlining processes, enhancing compliance, and mitigating risks. Here's how QMS software contributes to these critical aspects:
1. Enhanced Compliance and Documentation: QMS software ensures that life sciences organizations adhere to stringent regulatory standards such as FDA, EMA, and ISO. By automating documentation processes, it maintains accurate and up-to-date records of quality procedures, changes, and audits. This reduces the risk of non-compliance and ensures that every step in the product lifecycle is thoroughly documented, contributing to improved patient safety.
2. Effective Risk Management: Identifying and managing risks is essential for safeguarding patient health and ensuring high product quality. QMS software provides tools for risk assessment and management, allowing companies to proactively identify potential issues, implement corrective actions, and monitor their effectiveness. This proactive approach minimizes the likelihood of adverse events and enhances overall product reliability.
3. Streamlined Quality Control: By centralizing quality control processes, QMS software facilitates consistent and efficient monitoring of product quality. It automates quality checks, data collection, and analysis, enabling real-time insights into product performance. This ensures that any deviations are promptly addressed, reducing the chance of defective products reaching the market and compromising patient safety.
4. Improved Collaboration and Communication: QMS software fosters better collaboration across teams by providing a unified platform for sharing information, tracking progress, and managing documentation. This improved communication helps ensure that all departments are aligned in their quality management efforts, leading to more cohesive and effective responses to quality issues.
5. Continuous Improvement: A key feature of QMS software is its focus on continuous improvement. Through data analysis and performance metrics, organizations can identify areas for improvement and implement changes that enhance both product quality and patient safety. This iterative process helps in refining processes and maintaining high standards.
In summary, QMS software is a powerful tool for enhancing patient safety and product quality in the life sciences sector. By improving compliance, risk management, quality control, and collaboration, it ensures that products meet rigorous standards and contribute to better health outcomes for patients.
0 notes
Text

What is EQMS Quality Management System or EQMS Software?
EQMS stands for Electronic Quality Management System. It's a powerful tool that helps businesses streamline their quality management processes. From document control to audit management, EQMS Solution ensures compliance with industry standards and regulations. By centralizing data and automating workflows, it enhances efficiency and productivity. Stay ahead of competitors and deliver top-notch products/services with EQMS!
0 notes
Text
Qualityze : Enterprise Quality Management Software | EQMS
Qualityze quality management software solution is completely integrated and brings the required process automation which is needed by customers.��
This software offers Quality Management software such as nonconformance management system, CAPA management software, audit management system, supplier quality management software, document management software to the industries like product management, life sciences, manufacturing, medical devices, electronics, software development, healthcare, pharmaceuticals, logistics and aviation. Qualityze EQMS software helps them comply with applicable regulatory standards by streamlining the workflows.
1 note
·
View note
Text
The Essential Role of Gage R&R Tools in Manufacturing Quality — Omnex Systems

In today’s highly competitive manufacturing landscape, maintaining consistent product quality is more critical than ever. Accuracy in measurements, reliable data, and process consistency are essential components of a successful quality management system. One of the most effective methods to ensure measurement accuracy and system reliability is through Gage Repeatability and Reproducibility (Gage R&R) studies. At Omnex Systems, we understand the importance of using advanced Gage R&R tools to uphold manufacturing excellence.
What Is Gage R&R?
Gage R&R is a subset of Measurement System Analysis (MSA) used to determine the amount of variability in a measurement system caused by the measurement device (repeatability) and the operators using the device (reproducibility). In simpler terms, Gage R&R helps answer a crucial question: Can we trust the data we’re collecting?
Understanding this ensures that the data used for making product and process decisions is accurate, consistent, and not misleading. Without reliable measurement data, any quality control effort may lead to incorrect conclusions, waste, and even product failures.
The Two Key Components of Gage R&R
Repeatability: Variation in measurements taken by the same operator using the same instrument under the same conditions.
Reproducibility: Variation in measurements taken by different operators using the same instrument and measuring the same part.
A high-quality measurement system will show minimal variation in both repeatability and reproducibility.
Why Gage R&R Is Critical in Manufacturing
1. Enhances Data Integrity
Accurate data is the backbone of quality assurance. Gage R&R tools verify that the measurements are consistent and valid, reducing the likelihood of errors in decision-making based on faulty data.
2. Supports Lean and Six Sigma Initiatives
Manufacturers pursuing Lean or Six Sigma methodologies rely heavily on statistical data. Gage R&R ensures that the data used in DMAIC (Define, Measure, Analyze, Improve, Control) phases is dependable, leading to more accurate root cause analysis and effective process improvements.
3. Ensures Compliance
Many industries such as automotive, aerospace, and medical devices require strict quality assurance and traceability. Gage R&R studies are often a part of compliance with international standards like ISO 9001, IATF 16949, and AS9100. Omnex Systems' Gage R&R tools ensure seamless compliance and audit readiness.
4. Reduces Waste and Rework
By ensuring your measurement systems are accurate, Gage R&R helps identify true defects versus perceived ones, reducing unnecessary rework and scrap. This directly impacts cost reduction and operational efficiency.
5. Improves Customer Satisfaction
Consistent quality leads to satisfied customers. Gage R&R ensures that every unit produced meets specifications, which builds trust and reduces complaints or returns.
How Omnex Systems Optimizes Gage R&R Studies
At Omnex Systems, we provide a comprehensive suite of tools within our QMS and EQMS platforms that make Gage R&R studies simple, accurate, and efficient. Here’s how:
1. Integrated Measurement System Analysis Tools
Our software includes built-in modules for Gage R&R analysis. These tools automate calculations, generate visual charts, and provide detailed reports that make it easy to interpret the results.
2. User-Friendly Interface
We prioritize usability. Omnex’s Gage R&R tools are designed with intuitive dashboards that allow quality engineers and managers to easily input data, select parameters, and analyze outcomes without requiring advanced statistical knowledge.
3. Real-Time Monitoring and Reporting
With real-time data collection and reporting, manufacturers can make timely decisions based on the most recent and accurate information. Our systems support cloud integration and role-based access, ensuring secure and accessible data sharing.
4. Compliance and Traceability
Omnex Systems ensures that every Gage R&R study is documented and stored with a complete audit trail. This helps companies stay compliant with industry standards and easily respond to audits or customer inquiries.
Real-World Applications of Gage R&R Tools
Automotive Industry
In the automotive sector, precision is critical. A minor variation in part measurements can lead to performance issues or recalls. Gage R&R tools help maintain tight tolerances in machining, assembly, and inspection.
Medical Devices
In medical device manufacturing, where lives are at stake, the reliability of measurement systems must be beyond question. Gage R&R tools help ensure compliance with FDA regulations and ISO 13485.
Electronics Manufacturing
In high-speed production lines with automated inspection systems, Gage R&R ensures that sensors and vision systems are calibrated and accurate, ensuring product reliability.
Best Practices for Conducting a Gage R&R Study
Select Representative Parts: Choose a sample that reflects the entire process variation.
Use Multiple Operators: Include several individuals to account for operator-based variability.
Ensure Consistent Procedures: Standardize the way measurements are taken.
Use Adequate Sample Size: Typically, 10 parts measured by 3 operators, 3 times each, is a common standard.
Review Results Thoroughly: Use control charts, ANOVA tables, and statistical summaries to interpret outcomes.
Interpreting Gage R&R Results
Here are key indicators to watch for:
%GRR < 10%: Excellent – Measurement system is acceptable.
%GRR between 10%-30%: Marginal – May be acceptable depending on application.
%GRR > 30%: Unacceptable – Measurement system needs improvement.
Omnex Systems’ tools provide clear visualizations and insights into these metrics, enabling fast corrective actions.
Future of Gage R&R with Digital Transformation
With the advent of Industry 4.0 and smart manufacturing, Gage R&R tools are evolving. Omnex Systems is leading this transformation by integrating AI and machine learning to predict measurement system issues before they occur. Our tools now help identify patterns, optimize calibration schedules, and suggest corrective actions—proactively enhancing measurement accuracy and manufacturing quality.
Conclusion
Gage R&R tools play an essential role in safeguarding the quality and consistency of manufactured products. At Omnex Systems, we empower manufacturers with intelligent, user-friendly, and robust tools to conduct effective Gage R&R studies. By ensuring the reliability of your measurement systems, you’re investing in higher quality, better compliance, lower costs, and greater customer satisfaction.
If your organization is looking to strengthen its measurement system analysis and elevate product quality, Omnex Systems has the expertise and technology to help you succeed. Contact us today to learn how our Gage R&R solutions can integrate seamlessly into your quality management framework.
For more info pls visit us Omnex Systems or send mail at [email protected] to get a quote
0 notes
Text
How Enterprise Quality Management Software Powers Continuous Improvement Initiatives
In the complex world of manufacturing and life sciences, continuous improvement is not just a strategic initiative—it is a fundamental practice that determines long-term success. At the heart of this approach lies the ability to capture data, analyze trends, and implement changes efficiently. Enterprise Quality Management Software is crucial in achieving this goal. By consolidating quality processes and enabling real-time insights, enterprise QMS platforms drive continuous improvement at every operational level.
Leveraging Enterprise Quality Management Software for Data-Driven Decisions
The foundation of continuous improvement lies in data. Enterprise quality management software enables organizations to collect, analyze, and act on quality data from diverse sources. This integration of information from production lines, customer feedback, supplier performance, and audit results creates a comprehensive view of quality.
Harnessing Real-Time Data for Proactive Improvements
Unlike traditional systems, enterprise QMS continuously collects data, allowing quality teams to spot trends and address issues before they escalate. This proactive approach minimizes nonconformance and aligns with global quality standards.
Enabling Root Cause Analysis Through Integrated Enterprise QMS
One of the most powerful aspects of an enterprise quality management system is its ability to perform root cause analysis seamlessly. When a defect or nonconformance occurs, the system guides quality professionals through structured problem-solving methods.
Utilizing AI-Driven Insights to Identify Patterns
Modern EQMS platforms integrate artificial intelligence and machine learning to detect patterns that may indicate recurring quality issues. By leveraging this technology, organizations can pinpoint root causes faster and implement corrective actions more effectively.
Enhancing Cross-Functional Collaboration With Enterprise QMS
Continuous improvement requires collaboration between quality, operations, engineering, and supply chain teams. Enterprise quality management software centralizes communication, making it easier to coordinate responses to quality events and implement improvements across departments.
Breaking Down Silos to Accelerate Improvement Cycles
With an enterprise quality management system, cross-functional teams can access the same data and insights, breaking down information silos. This unified approach fosters collaboration and speeds up the resolution of quality challenges.
Driving Process Standardization Across Multiple Sites
Global enterprises face the challenge of maintaining quality consistency across geographically dispersed facilities. Enterprise QMS platforms facilitate standardized processes, ensuring that best practices are replicated regardless of location.
Implementing Global Quality Standards Efficiently
With configurable workflows, templates, and audit protocols, enterprise quality management software helps ensure compliance with ISO, FDA, and other international standards, streamlining quality assurance processes across sites.
Automating CAPA to Reduce Human Error and Improve Consistency
CAPA (Corrective and Preventive Actions) is fundamental to continuous improvement. An enterprise quality management system automates CAPA management, reducing the risk of human error and ensuring consistency in problem resolution.
Monitoring CAPA Effectiveness Through Automated Workflows
By linking CAPA records to quality events, audit results, and risk assessments, the system enables continuous monitoring and validation of corrective actions. This structured approach ensures that improvements are sustainable and measurable.
Integrating Supplier Quality Management for Holistic Improvement
Suppliers play a significant role in product quality. Enterprise quality management software provides tools to assess supplier performance, track Nonconformance, and enforce quality agreements.
Building Collaborative Supplier Quality Networks
With integrated supplier portals, the enterprise QMS fosters transparency and accountability, allowing manufacturers to work proactively with suppliers to resolve quality issues and implement joint improvement initiatives.
Embedding Quality Into Design and Development Phases
Design flaws can significantly impact product quality. By integrating Quality Management early in the product lifecycle, enterprise QMS reduces defects and nonconformance risks during production.
Enabling Design Validation and Risk Assessment
Through design history files (DHF) and risk analysis modules, the enterprise quality management system ensures that potential quality issues are identified and mitigated before manufacturing begins.
Monitoring Performance With Real-Time Quality Dashboards
Tracking progress toward continuous improvement goals requires real-time visibility into key quality metrics. Enterprise quality management software offers customizable dashboards that display nonconformance rates, CAPA completion status, and audit outcomes.
Making Data-Driven Decisions at Every Level
With visualizations and automated reports, decision-makers can quickly identify trends and deviations, enabling timely interventions and strategic planning.
Conclusion: Why ComplianceQuest Is the Future of Continuous Improvement in 2025
To thrive in a dynamic global market, companies must embrace continuous improvement as an ongoing journey rather than a one-time initiative. ComplianceQuest's enterprise quality management software stands at the forefront of this transformation, providing the tools necessary to streamline processes, foster collaboration, and build a culture of quality. In 2025 and beyond, forward-thinking companies will rely on ComplianceQuest to sustain competitive advantage through proactive quality management and data-driven decision-making.
0 notes
Text
This was also an issue in my old workplace, in medical manufacturing.
Our calibration tech was still using software that was abandoned a decade ago, to back up information that otherwise would have had to be manually transferred to a new system by hand.
I personally had to transfer a bunch of shit to a new system by hand when the old eQMS became abandonware, because while we could still use it since we already had it set up, we couldn't count on maintaining permanent access. We couldn't give our IT people the code and say "you maintain this now", because we didn't have access to be able to do that.
This was a software used to maintain and track regulatory compliance. Critical to being able to audit our processes and find errors, or to prove that we had done things correctly. To store and access engineering prints and machine specs and gauge calibrations and operations protocols.
It definitely goes far beyond video games.
abandonware should be public domain. force companies to actively support and provide products if they don't wanna lose the rights to them
128K notes
·
View notes
Text
Unlocking Quality Insights: The Power of APQR Software
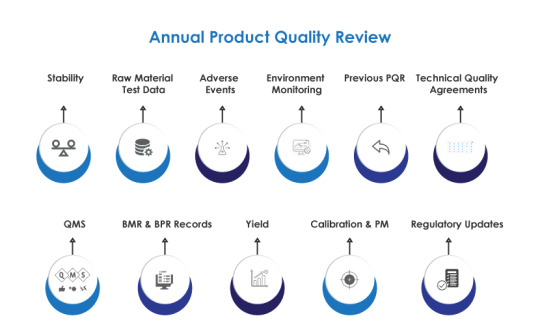
AmpleLogic APQR Software: Elevating Quality Assurance in Pharmaceuticals
In the tightly regulated world of pharmaceuticals, ensuring the quality of every product is not just a commitment but a legal mandate. The Annual Product Quality Review (APQR), often known as the Product Annual Review, serves as the linchpin in upholding current good manufacturing practices (CGMP) regulations, as outlined by the FDA.
AmpleLogic's APQR Software emerges as a powerful solution, streamlining the APQR process and transforming it into a dynamic tool for quality management.
Key Features for Seamless Quality Assurance
AmpleLogic's APQR Software boasts features that significantly enhance the efficiency of the Product Quality Review (PQR) process. It automates the generation of APQR documents based on predefined templates, ensuring consistency and accuracy. Teams can generate PQR reports at any time, and the system includes auto-alerts for delays, ensuring timely reviews.
The software manages master data, offering statistical analysis and trends on critical parameters such as Assay, Water Content, PH, Specific Impurities, and Total Impurities. It goes beyond standard reporting by providing batch-wise trends, enabling tracking of granulation yield, compression yield, coating, and packing yield.
Comprehensive Data Capture for Informed Decision-Making
AmpleLogic's Product Quality Review Software is not just a compliance tool; it is a repository of comprehensive information. It captures batch details, process yields, raw material information, deviations, stability trends, and more. Categorized month-wise and condition-wise, the software offers a holistic overview of a product's journey from production to market.
Beyond the pharmaceutical sector, the software extends its benefits to diverse industries, including Biologics, Medical Devices, Chemical Industry, Contract Manufacturing Organizations, Generics, and Food & Beverages.
Insights through Process Capability Analysis
One of the standout features of AmpleLogic's APR Software is its ability to perform Process Capability Analysis. Leveraging data from Laboratory Information Management Systems (LIMS), Electronic Quality Management Systems (EQMS), and Batch Manufacturing Records, the software employs statistical methods defined in the R Language to calculate key indices—Cp, Cpk, Pp, and Ppk. These indices provide insights into process variations, ensuring manufacturing processes remain robust.
Immediate alerts for Process Deviations add another layer of responsiveness, allowing teams to address issues promptly. The software's continuous process verification capability ensures ongoing scrutiny of manufacturing processes, aligning with Six Sigma standards for consistent quality.
Global Compliance and Adaptability
The APQR Software by AmpleLogic doesn't operate in isolation. It aligns with major regulatory standards, including 21 CFR PART 11, MHRA, and EU Annex 11. This global compliance ensures that the software can seamlessly integrate into the operations of pharmaceutical companies worldwide.
Goodness-of-Fitness Test: Strengthening Analytical Capabilities
Incorporating a Goodness-of-Fitness Test, the software introduces a robust statistical method to validate observed values against model predictions. This test, crucial in decision-making, ensures that the data used for analysis adheres to predefined norms.
The software's adaptive approach, including various transformations and consideration of different distributions, underscores its commitment to data integrity.
Conclusion: Transforming Quality Assurance
In conclusion, AmpleLogic's APQR Software transcends traditional quality assurance practices. It is not merely a tool for compliance but a catalyst for continuous improvement and excellence.
With its global compliance, adaptability across industries, and powerful analytical features, the software positions itself as a transformative force in the pursuit of manufacturing excellence.
In a world where precision and quality are non-negotiable, AmpleLogic's APQR Software emerges as a beacon, ensuring that every product reaching the market adheres to the highest standards.
To gain a more in-depth understanding of our APQR Software, request a demonstration at: Click here to Request Demo
0 notes
Text
Navigating Compliance in Bioscience with eQMS Software: Download Our White Paper!
Uncover the power of eQMS software in ensuring seamless compliance within the dynamic Bioscience industries. 🌱🔒
📌 Staying compliant is the cornerstone of success in Bioscience. Our comprehensive white paper explores how an advanced Electronic Quality Management System (eQMS) can be your ultimate tool for meeting regulatory standards and exceeding quality expectations.
🔍 Key Insights:
- Dive into the challenges Bioscience industries face in adhering to complex regulations.
- Learn how eQMS simplifies documentation, process control, and audits.
- Discover real-world examples of successful compliance integration.
- Elevate your approach to quality management, risk assessment, and continuous improvement.
🚀 Unlock the potential of streamlined compliance processes. Download our white paper now to witness first-hand how eQMS software empowers Bioscience companies to thrive in a regulated landscape.
📥 Download Now - eQMS Software | Paperless QMS for Bio Science Industry | Complianceg
0 notes
Text
AI-powered EQMS Software by Qualityze leverages artificial intelligence to transform traditional quality management into a more intelligent, proactive, and efficient system. Built on the secure Salesforce platform, Qualityze integrates AI capabilities to enhance decision-making, automate repetitive tasks, and identify patterns in quality data that may otherwise go unnoticed. This AI-driven approach enables organizations to predict quality risks, recommend corrective actions, and continuously improve product and process quality with greater accuracy and speed. Read more - https://www.qualityze.com/
0 notes
Text

Quality Management Software Systems | EQMS Software
Quality management system is inextricably linked with ISO quality standards and guidelines directed by the FDA and other similar regulatory agencies. Qualityze EQMS software is one of the most comprehensive enterprise quality management software. It helps the organizations to achieve and maintain ISO 9001 certification through automated, highly interactive quality processes that is tailored to an organization specific product, operations and also the business practices. Follow this link to get additional details on Qualityze EQMS software solution - https://www.qualityze.com/
1 note
·
View note
Text
How QMS Software for Food Manufacturing Enhances Quality and Compliance

In the fast-paced world of food manufacturing, even a minor oversight can escalate into a significant issue. Picture a bakery dealing with frequent customer complaints about the texture and taste of its bread, or a dairy processing unit grappling with a product recall because of contamination.
In both scenarios, the absence of a structured, efficient quality management system (QMS) can cause serious disruptions — harming customer trust, product quality, and ultimately, brand reputation.
This is where adopting QMS for food industry becomes essential. It provides food manufacturers with a structured, proactive way to manage product quality, track compliance, and handle risks effectively.
In an industry where consumer safety and regulatory standards are non-negotiable, modern food quality management software ensures that businesses can maintain high-quality standards while streamlining their operations.
Why a Food QMS Software Matters
Food manufacturers today face increasing regulatory pressures, changing consumer expectations, and the constant challenge of maintaining product consistency. Food QMS software not only helps address these concerns but also boosts operational efficiency by automating routine processes like quality checks, compliance monitoring, documentation, and reporting. This reduces the scope for human error, speeds up production cycles, and ensures timely, accurate decision-making.
Imagine if the bakery owner in our earlier example had access to QMS software for food manufacturing industry. By systematically recording customer complaints and tracking product issues, they could quickly pinpoint inconsistencies in the baking process and resolve them efficiently. Similarly, if the dairy plant had robust food quality software, it could have traced the source of contamination, taken corrective action, and prevented costly product recalls.
Features That Make a Food QMS Indispensable
Modern QMS software for food industry solutions like QualityPro eQMS software are designed to cover every critical aspect of quality management in food production. Let’s take a closer look at some of the core capabilities that make this software so valuable:
Change Management: Manages modifications such as ingredient substitutions or process adjustments by documenting and assessing their impact. This ensures smooth communication across teams, preventing workflow disruptions.
Compliance Management: Automates the tracking and documentation of regulatory requirements, making it easier to stay audit-ready and avoid non-compliance penalties.
Complaint Management: Enables food manufacturers to systematically capture, investigate, and resolve customer complaints — improving product quality, consumer trust, and response times.
Non-Conformance (NC) Management: Identifies issues like spoilage, contamination, or mislabeling early in the process, ensuring only safe, high-quality products reach consumers while reducing waste.
Corrective and Preventive Actions (CAPA): Addresses the root causes of recurring issues by implementing long-term preventive strategies, enhancing both product and process reliability.
Inspection Management: Simplifies quality inspections, manages inspection plans, and digitizes records to improve accuracy and accessibility.
Document Management: Centralizes critical documents such as recipes, batch records, compliance certificates, and SOPs, ensuring all team members access the latest, approved information.
Training Management: Helps businesses keep their teams well-trained in best practices for food safety, hygiene, and quality standards — a crucial factor in minimizing human error and improving production outcomes.
Also Read: How QMS Software is Revolutionizing Quality Management in Chemical Industries
Why Invest in QMS Software for Food Manufacturing?
Choosing a dedicated food QMS like QualityPro is a strategic investment for food manufacturers aiming to stay competitive and compliant. It goes beyond just meeting regulatory requirements; it helps businesses optimize resource utilization, accelerate production timelines, reduce operational risks, and deliver consistent product quality.
Additional features such as smart tagging, cockpit dashboards, complaint tracking, and advanced reporting make QMS software stand out in the market. By integrating a comprehensive quality management system in food industry, manufacturers can protect their brand’s reputation, improve customer satisfaction, and secure long-term operational success.
In Conclusion
In an industry where quality and safety are paramount, relying on spreadsheets or outdated manual processes is a risk no food manufacturer can afford. Investing in QMS software ensures businesses can proactively manage quality, reduce inefficiencies, and deliver products that consistently meet high standards.
Whether you run a bakery, dairy plant, or packaged food unit, embracing reliable food quality management software like QualityPro is a smart move towards safeguarding your operations and elevating your brand.
0 notes
Text
EQMS supports risk management by providing tools to identify, assess, and mitigate risks related to quality and regulatory compliance. It helps organizations evaluate and prioritize risks, implement risk mitigation strategies, and track risk-related activities.
0 notes
Photo

Qualityze EQMS Suite comprises of 8 software for managing quality and process of system, developed on cloud based platform i.e. salesforce.com
0 notes
Text
How Regulations Impact Supplier Quality in Medical Devices
In the highly regulated medical device industry, ensuring supplier quality management is critical to maintaining product safety, efficacy, and compliance. Supplier Quality Management in Medical Devices involves intricate processes that align with stringent regulatory frameworks. This blog explores how regulations shape supplier quality management in the medical device industry and the essential role of quality control and quality assurance in achieving compliance.
Understanding the regulatory landscape for supplier quality management in medical devices
Regulatory agencies worldwide enforce strict standards to ensure that medical devices meet safety and quality requirements. Supplier quality management in medical devices must adapt to these regulations to avoid compliance risks and ensure product reliability.
Key regulatory bodies and standards
The medical device industry must comply with standards set by organizations such as the FDA, ISO, and the European Medicines Agency (EMA). ISO 13485, in particular, governs the quality management systems for medical devices, emphasizing supplier quality and traceability.
Adapting to regional regulatory requirements
Different regions impose unique regulatory requirements. For instance, the FDA’s Quality System Regulation (QSR) and the EU’s Medical Device Regulation (MDR) mandate specific supplier quality management practices. Companies must ensure that their quality assurance frameworks address these regional variations.
The importance of quality control and quality assurance in achieving regulatory compliance
Quality control and quality assurance are the cornerstones of supplier quality management in medical devices. These processes ensure that all components sourced from suppliers meet predefined quality criteria and comply with regulatory standards.
Establishing rigorous quality control measures
Implementing rigorous quality control measures ensures that defects are identified and rectified early in the supply chain. This reduces the risk of non-compliance and enhances the overall quality of medical devices.
Building robust quality assurance frameworks
Quality assurance focuses on creating a proactive approach to Supplier Quality Management by developing processes that prevent defects and ensure compliance. A robust quality assurance system fosters continuous improvement and regulatory adherence.
Implementing traceability and transparency in supplier quality management
Traceability is a regulatory requirement for medical devices and plays a critical role in supplier quality management. Transparent supply chain processes enable organizations to track components from sourcing to final assembly.
Leveraging technology for traceability
Digital tools such as quality management software and electronic quality management systems (eQMS) enhance traceability. These tools provide real-time tracking, ensuring compliance with regulatory mandates and improving supplier accountability.
Ensuring transparency in supplier relationships
Transparency in supplier relationships builds trust and ensures that suppliers adhere to quality and regulatory standards. Regular communication and audits are essential to maintaining transparency.
Addressing challenges in supplier quality management for medical devices
Despite advancements in technology and processes, supplier quality management in medical devices faces several challenges. Organizations must proactively address these challenges to maintain compliance and quality.
Navigating complex supply chains
The medical device industry often relies on global supply chains, which introduce complexities in ensuring consistent quality. A well-implemented supplier quality management system mitigates these challenges.
Managing supplier non-compliance
Non-compliance by suppliers can lead to regulatory penalties and product recalls. Effective Quality Control and Quality Assurance processes help organizations detect and address supplier non-compliance promptly.
Integrating risk management into supplier quality frameworks
Risk management is integral to supplier quality management in medical devices. By identifying and mitigating risks, organizations can ensure the safety and reliability of their products.
Conducting supplier risk assessments
Supplier risk assessments evaluate the potential impact of non-compliance or quality issues on the organization. These assessments inform decision-making and prioritize supplier management efforts.
Aligning risk management with quality assurance
Integrating risk management into Quality Assurance processes ensures a proactive approach to addressing potential issues. This alignment fosters resilience and compliance across the supply chain.
Enhancing supplier performance through audits and evaluations
Supplier audits and evaluations are essential components of supplier quality management in medical devices. These processes ensure that suppliers meet regulatory and organizational standards consistently.
Conducting comprehensive supplier audits
Regular audits provide insights into supplier processes, identifying gaps and areas for improvement. These audits are critical for maintaining compliance with regulations such as ISO 13485 and FDA QSR.
Utilizing performance metrics for evaluations
Tracking performance metrics such as defect rates and delivery timelines helps organizations evaluate supplier reliability. This data-driven approach ensures continuous improvement in supplier quality.
Driving continuous improvement in supplier quality management
Continuous improvement is a regulatory expectation and a business imperative in the medical device industry. Organizations must foster a culture of quality and innovation to meet these expectations.
Leveraging feedback for improvement
Feedback from audits, inspections, and customer interactions informs continuous improvement initiatives. Organizations should use this feedback to refine supplier quality management processes.
Executing corrective and preventive measures
Corrective and preventive actions (CAPA) address identified issues and prevent recurrence. CAPA processes are central to achieving sustained compliance and supplier quality improvement.
Conclusion: Why ComplianceQuest’s Software is essential for business in 2024
In the ever-evolving medical device industry, supplier quality management is critical to ensuring compliance and product excellence. ComplianceQuest’s Software offers a comprehensive solution that integrates quality control and quality assurance processes with advanced analytics and automation. By leveraging ComplianceQuest, organizations can streamline supplier quality management, enhance traceability, and maintain compliance with global regulations. As companies navigate the challenges of 2024, ComplianceQuest stands out as the ultimate partner in achieving quality and regulatory success.
0 notes
Text
Vague wording
Vague wording used in requirement documents
Whether you are freshly minted into the QMS position or you are a founder of a Startup or a product manager with a new project… your strategy requires QMS oversight.
QMS Joe is here to guide you through the plethora of information and support you with handy tips & tricks to streamline your way to compliance.
Use precise requirements as much as possible
If the requirement is not clear enough the employees or the sub-contractor could misunderstand or make incorrect decisions related to the given task that might end in an unplanned surprise, and could easily lead to an unsatisfied customer.
Customer satisfaction can be improved by using precise requirements as much as possible in QMS.

#QMS#eqms#QMS documentation#qmsWrapper#qms planning#standard#software#cloud based software#requirements#quality management system#systems integration#quality#quality manual#quality requirements#ISO#risk#risk management#Iso 9001#iso 13485 documents#ISO 13485#ISO 13485:2016#customer#customer experience#customerreview#customer service#customer satisfaction#planning#resource#tips#tricks
0 notes