#ionic polarization
Explore tagged Tumblr posts
Note
Ok I have no idea what unit you’re doing in Chem but I found this in my folder from last year for the Matter unit (I made these little study guide things for all the units last year but I cannot find any of them 😭 If I dig any of them up I’ll send them to you) If you’re doing the Matter unit then here! And if you’re not…finals review??

STOP BECAUSE WE JUST STARTED THIS UNIT TODAY LITERALLY JUST TOOK NOTES FOR IT FOR THE FIRST TIME IN CLASS IM LITERALLY IN LOVE WITH YOU TYSM
#we just had the unit exam for whatever ionic and covalent and polar and whatever bonds are#i did bad but it brought my grade from 80 to 81 so#OK BUT THANK YOUUUU#who asked ౨ৎ#moots 『🎕』#alice!! ❥•°•༢
4 notes
·
View notes
Text
Because of the high value of the dipole moment (6.1 × 10^-30 C·m), the water molecules orient themselves around ionic or polar species forming X...H2O bonds (Fig. 9.6).

"Environmental Chemistry: A Global Perspective", 4e - Gary W. VanLoon & Stephen J. Duffy
#book quotes#environmental chemistry#nonfiction#textbook#dipole#water#orientation#ionic#polarity#hydration
0 notes
Text

[image description: chart titled Talk Like A Technician: The Use of Technobabble.
Technology in Star Trek is complex and works in scientific concepts and principles that are far beyond what the majority of Players and Gamemasters are knowledgeable in. Throughout the collected media, Starfleet officers discuss technology using terms that most Players are not going to know. Instead of expecting Players to study and memorize technical manuals and reference books that have been published over the years we've provided an easy way to talk like a Starfleet engineer. Anyone can do "technobabble"!
To use the chart simply gather and roll d20s and consult the chart below for technical new terms and concepts.
Occasionally portions of the chart may not be applicable to the scene or circumstance. In that case simply omit that portion of technobabble!
The chart has six columns, Roll, Action, Descriptor, Source, Effect, and Device. Each has 20 rows.
Roll: numbers 1-20
Action: refocus, amplify, synchronize, redirect, recalibrate, modulate, oscillate, intensify, nullify, boost, reverse, reconfigure, actuate, focus, invert, reroute, modify, restrict, reset, extend
Descriptor: microscopic, macroscopic, linear, non-linear, isometric, multivariant, nano, phased, master, auxiliary, primary, secondary, tertiary, back-up, polymodal, multiphasic, tri-fold, balanced, oscillating
Source: Quantum, positronic, thermionic, osmotic, neutrino, spatial, resonating, thermal, photon, ionic, plasma, nucleonic, verteron, gravimetric, nadion, subspace, baryon, tetryon, polaron, tachyon
Effect: flux, reaction, field, particle, gradient, induction, conversion, polarizing, displacement, feed, imagining, reciprocating, frequency, pulse, phased, harmonic, interference, distortion, dampening, invariance
Device: inhibitor, equalizer, damper, chamber, catalyst, coil, unit, grid, regulator, sustainer, relay, discriminator, array, coupling, controller, actuator, harmonic, generator, manifold, stabilizer.
/end id]
269 notes
·
View notes
Note
I have a bio exam in about six hours (in the afternoon, this is an appropriate hour for me to be awake) and I don't wanna study so I'm gonna recite everything I know about proteins to you.
Proteins can have CHONS, Carbon Hydrogen Oxygen Nitrogen Sulfur. Made up of amino acids. Amino acids have NH2-R-C-H-COOH. NH2 is the amino group and COOH is the uhh carboxyl group? Amino acids exist as zwitterions, with the NH2 becoming NH3- and COOH becoming COO-. Because electronegativity and stuff. There are 20 amino acids (in my syllabus).
Proteins have primary structure (polypeptide chain, the specific sequence of amino acids) listed from N terminus to C terminus. Maintained by peptide bonds. Which is a condensation reaction. The COOH and the NH2 form a bond that looks like CONH. Then secondary structure, which is alpha helixes and beta sheets. Both are maintained by hydrogen bonds. Beta sheets can be parallel or antiparallel, though most of the ones in your blog or antiparallel (but also very short). Alpha helixes are more common and recognisable.
Next is tertiary structure. Four types of bonds in this one: Hydrogen bonds, ionic bonds, disulfide bonds, and hydrophobic interactions. In increasing order of strength, hydrophobic<hydrogen<ionic<disulfide. Disulfide bonds are covalent bonds and very heat stable. Formed by disulfide bridges. Very strong. Sulfur is found on Cysteine so the more cysteine is in a molecule the more heat stable it is likely to be. The single letter code for Cysteine is C. If I say CCCCCCCCCCCC, will disulfide bridges form in my protein? Hm. Ionic bonds is when uhh. Charged particles. Shit I forgot I need to revise this part. Hydrogen bonds are easier, I can identify them by identifying differences in electronegativity- in chem I remember these as FON and H, but in amino acids there's only O and N, no Fluorine. Very common, in most secondary structures. In polar particles or R groups. Ionic bonds are for charged particles, hydrogen bonds are for polar particles. Lastly in hydrophobic interactions, not to be confused with homophobic interactions. Which is when hydrophobic side chains- most commonly stuff with lots of carbon, or sometimes sulfur- gather in the middle of the protein, away from the aqueous exterior.
Next is Quaternary structure, which is when there are MULTIPLE polypeptide chains. Only when multiple polypeptide chains, if not it's tertiary. Not all proteins have these. Bonds are mostly the same as tertiary, I think?
CCCCCCCCCCCCCCCC.
There are globular, fibrous, and membrane proteins. But mostly I have to focus on the globular and fibrous part. My case study for globular proteins is haemoglobin. Haemoglobin has four polypeptide chains in it's quaternary structure, two alpha two beta. It is unclear if the names of the chains have anything to do with whether there are alpha helixes or beta sheets in them. It also has a prosthetic group, a haem group in this case, of iron- I think an Fe2+ molecule? Yeah. Which oxygen binds to. And once oxygen is bound to it it causes a change in confirmation in all four parts allowing it to bind to oxygen more easily. I need to know about sickle cell disease, which is when one of the amino acids- glutamic acid I think- is replaced with a hydrophobic one. Serine? Either way when oxygen is bound to it it's fine but when there's no oxygen there's a hydrophobic patch sticking out, which it doesn't like so the haemoglobin will change confirmation to hide, causing the haemoglobin to clump together in a way that causes the red blood cell to appear "sickle shaped". Also I was wrong it's not serine it's valine.
For fibrous, I have collagen. Which is mostly a repeating sequence of three amino acids- shit I forgot which ones I need to get my notes- glycine-X-Y. Glycine has a very small R group which allows it to fit in the small tight part in the alpha helix. X-Y is usually proline and hydroxyproline. Alpha helix, hydrogen bonds formed. Proline and hydroxyproline have relatively inflexible and bulky R groups which gives the structure rigidity. That's the secondary structure. But collagen is unique in that it has a secondary and quaternary structure, because in tropocollagen it's like three of these alpha helixes wound together. Then each tropocollagen cross links to neighbouring tropocollagen molecules. This is covalent bonds involving lysine residues. Lysine??? Where tf did Lysine come from??? I don't see it anywhere else on the page. This is worrying. Uhh this increases tensile strength and the staggered or overlapping arrangement of tropocollagen minimises points of weakness along the length of the fibrils. It's like, collagenchain-tropocollagen-fibrils-fibres. The staggering gives the bundle of fibrils a banded appearance. There was... A part where they talk about how the cell produces it on the outside... No, wait, that's cellulose. Carbohydrate. Completely different.
Then we move on to enzymes which is a whole different chapter and this is already a very long ask which I imagine will take very long to load. Thank you for letting me ramble in your inbox. CCCCCCCCC. I'm going to ramble about DNA&genomics to hellsitegenetics in a bit but first I need to finish looking through mitosis and meiosis. Ugh. I hope this protein has a high success rate and that my exam has an even higher success rate. Thank you for your time.
this sounds great and i bet you did awesome on your exam! i find explaining things to someone else helpful when studying, so i hope that doing this helped you as well. without even looking at it i can guarantee that your exam was much more successful than whatever proteins i'll get out of this.
i actually had to split this into two proteins and run them completely separately because it was simply too long. i am both excited and absolutely terrified to see what we get from all those cysteines
letter sequence in this ask matching protein-coding amino acids:
protein 1:
IhaveaieaminatsihrsintheafternnthisisanapprpriatehrfrmeteawakeandIdntwannastdysImgnnareciteeverythingIknwatprteinstyPrteinscanhaveCHNSCarnHydrgenygenNitrgenSlfrMadepfaminacidsAminacidshaveNHRCHCHNHistheamingrpandCHisthehhcarylgrpAminacidseistaswitterinswiththeNHecmingNHandCHecmingCecaseelectrnegativityandstffThereareaminacidsinmysyllasPrteinshaveprimarystrctreplypeptidechainthespecificseqencefaminacidslistedfrmNterminstCterminsMaintainedypeptidendsWhichisacndensatinreactinTheCHandtheNHfrmandthatlkslikeCNHThensecndarystrctrewhichisalphaheliesandetasheetstharemaintainedyhydrgenndsetasheetscaneparallelrantiparallelthghmstfthenesinyrlgrantiparalleltalsveryshrtAlphaheliesaremrecmmnandrecgnisaleNetistertiarystrctreFrtypesfndsinthisneHydrgenndsinicndsdislfidendsandhydrphicinteractinsInincreasingrderfstrengthhydrphichydrgeninicdislfideDislfidendsarecvalentndsandveryheatstaleFrmedydislfideridgesVerystrngSlfrisfndnCysteinesthemrecysteineisinamleclethemreheatstaleitislikelyteThesinglelettercdefrCysteineisCIfIsayCCCCCCCCCCCCwilldislfideridgesfrminmyprteinHmInicndsiswhenhhChargedparticlesShitIfrgtIneedtrevisethispartHydrgenndsareeasierIcanidentifythemyidentifyingdifferencesinelectrnegativityinchemIrememertheseasFNandHtinaminacidstheresnlyandNnFlrineVerycmmninmstsecndarystrctresInplarparticlesrRgrpsInicndsarefrchargedparticleshydrgenndsarefrplarparticlesLastlyinhydrphicinteractinsnttecnfsedwithhmphicinteractinsWhichiswhenhydrphicsidechainsmstcmmnlystffwithltsfcarnrsmetimesslfrgatherinthemiddleftheprteinawayfrmtheaqeseterirNetisQaternarystrctrewhichiswhenthereareMLTIPLEplypeptidechainsnlywhenmltipleplypeptidechainsifntitstertiaryNtallprteinshavethesendsaremstlythesameastertiaryIthink
protein 2:
CCCCCCCCCCCCCCCCTherearegllarfirsandmemraneprteinstmstlyIhavetfcsnthegllarandfirspartMycasestdyfrgllarprteinsishaemglinHaemglinhasfrplypeptidechainsinitsqaternarystrctretwalphatwetaItisnclearifthenamesfthechainshaveanythingtdwithwhethertherearealphaheliesretasheetsinthemItalshasaprstheticgrpahaemgrpinthiscasefirnIthinkanFemlecleYeahWhichygenindstAndnceygenisndtititcasesachangeincnfirmatininallfrpartsallwingittindtygenmreeasilyIneedtknwatsicklecelldiseasewhichiswhenneftheaminacidsgltamicacidIthinkisreplacedwithahydrphicneSerineEitherwaywhenygenisndtititsfinetwhentheresnygentheresahydrphicpatchstickingtwhichitdesntlikesthehaemglinwillchangecnfirmatinthidecasingthehaemglintclmptgetherinawaythatcasestheredldcelltappearsickleshapedAlsIwaswrngitsntserineitsvalineFrfirsIhavecllagenWhichismstlyarepeatingseqencefthreeaminacidsshitIfrgtwhichnesIneedtgetmyntesglycineYGlycinehasaverysmallRgrpwhichallwsittfitinthesmalltightpartinthealphaheliYissallyprlineandhydryprlineAlphahelihydrgenndsfrmePrlineandhydryprlinehaverelativelyinfleileandlkyRgrpswhichgivesthestrctrerigidityThatsthesecndarystrctretcllagenisniqeinthatithasasecndaryandqaternarystrctreecaseintrpcllagenitslikethreefthesealphahelieswndtgetherTheneachtrpcllagencrsslinkstneighringtrpcllagenmleclesThisiscvalentndsinvlvinglysineresidesLysineWheretfdidLysinecmefrmIdntseeitanywhereelsenthepageThisiswrryinghhthisincreasestensilestrengthandthestaggeredrverlappingarrangementftrpcllagenminimisespintsfweaknessalngthelengthfthefirilsItslikecllagenchaintrpcllagenfirilsfiresThestaggeringgivesthendleffirilsaandedappearanceTherewasApartwheretheytalkathwthecellprdcesitnthetsideNwaitthatscelllseCarhydrateCmpletelydifferentThenwemventenymeswhichisawhledifferentchapterandthisisalreadyaverylngaskwhichIimaginewilltakeverylngtladThankyfrlettingmeramleinyrinCCCCCCCCCImgingtramleatDNAgenmicsthellsitegeneticsinaittfirstIneedtfinishlkingthrghmitsisandmeisisghIhpethisprteinhasahighsccessrateandthatmyeamhasanevenhighersccessrateThankyfryrtime
protein guy analysis:
protein 1 looks absolutely awful. it barely has any secondary structure, and absolutely no tertiary structure to speak of. the horrifying loops have some strings of cis bonds, and there is not a single disulfide bond. i hate this so much. the long string of C's just looks off putting. protein 2 is the same but worse. there is one disulfude, but otherwise barely any structure exists. the single alpha helix feels almost mocking. overall, this is miserable and disgusting.
predicted protein structure:
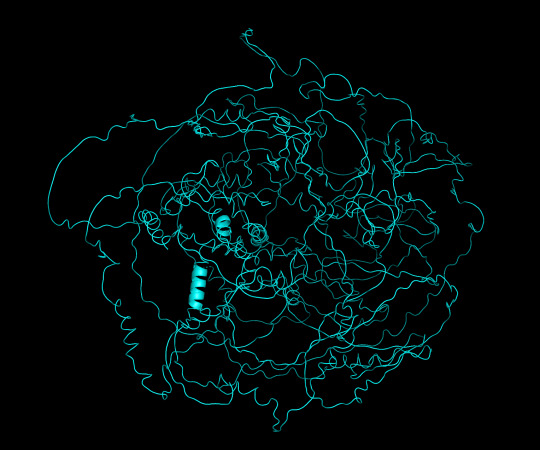
protein 1 cartoon
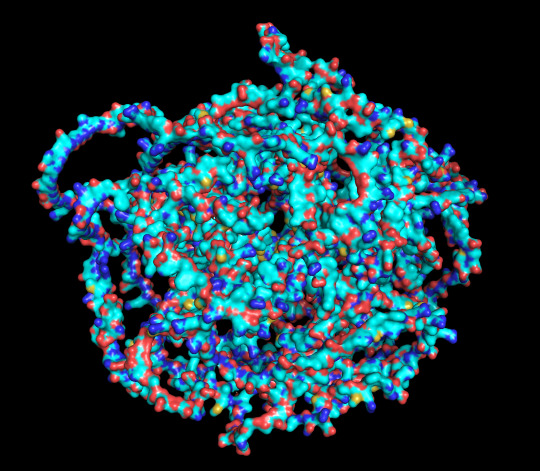
protein 1 surface
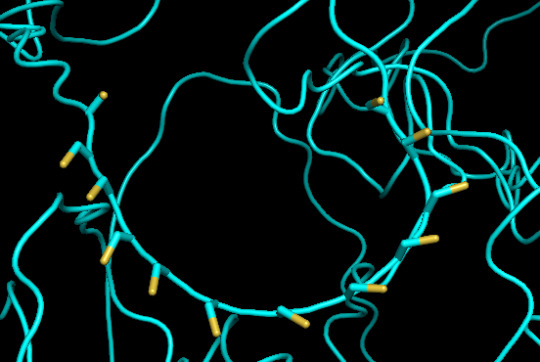
close up of cysteines on protein 1

protein 2 cartoon with disulfide bond in orange
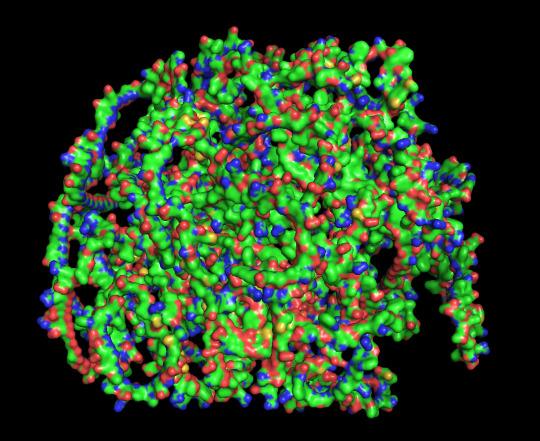
protein 2 surface
#science#biochemistry#biology#chemistry#stem#proteins#protein structure#science side of tumblr#protein asks#protein info
36 notes
·
View notes
Text
Okay, I'm considering getting a cat and would rename it, and it's past midnight so I'm thinking about good names
I like stupid long names with better nicknames built in. Tonights bullshit train is thinking of using star trek techno babble (from tos only, which severely limits us) as the name. So here's a poll
These were found by googling and reading other forums discussing tos technobabble. I have no proof there's are legit
60 notes
·
View notes
Text
*aluminum free! rubidium free! tellurium free! dubnium free! even bohrium free! made with only the finest Ionic Polarized Reverse-Osmosis Filtered Ozone-Treated No-Antibiotics-or-Hormones-Added Cage-Free Lydianated Micronized Clay Minerals, great for detoxifying your chakras, revitalizing your meridians, and eliminating body thetans for all-day protection.
4L07
CAUTION: for external use only. do not apply directly to skin. store away from electronic devices, ideally in a faraday cage. pray over the affected area and smudge with sage if rash or irritation occurs. keep out of reach of children, the elderly, and freemasons.
Vegan Non-Cruelty-Free Organic
*Specifically no aluminum chlorohydrate added. We put a shit ton of other kinds of aluminum though. and like. lead. lol
2 notes
·
View notes
Text
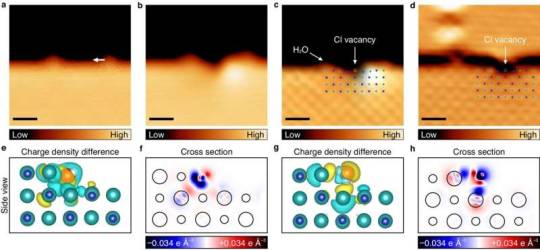
Researchers observe salt dissolution at the atomic level
A research team, affiliated with UNIST has achieved a groundbreaking feat by observing the dissolution of salt in water at the atomic level and experimentally uncovering the underlying principle. Led by Professor Hyung-Joon Shin and his researchers from the Department of Materials Science and Engineering at UNIST, the team introduced the innovative "single ion control technology." This cutting-edge approach enables the precise manipulation of individual water molecules to selectively extract specific ions from salt. The findings of the study were published in Nature Communications on March 16, 2024. Salt, composed of robust ionic bonds between sodium cations (Na+) and chlorine anions (Cl-), undergoes a transformative process when immersed in water. The interaction between the positive and negative polarities of water molecules disrupts the bond between the sodium and chlorine ions, leading to their separation and the formation of saltwater.
Read more.
12 notes
·
View notes
Text
water is SO weird because it is capable of hydrogen bonding due to the high electronegativity of the oxygen atom and the lone pair present on it! It's a polar solvent so it can dissolve any ionic compound p much. it's hugely biologically significant. our being made of water largely is one of the reasons our proteins are so strange - because polarity determines solubility in water and the interactions between amino acid r groups cause proteins to fold!
biochemistry is fucking WILD. I love it.
we should talk about water more often that shit is crazy
134K notes
·
View notes
Text
How HPLC Column Works: Principles of Separation Explained

Introduction
High-Performance Liquid Chromatography (HPLC) is a cornerstone technique in analytical chemistry, widely used in pharmaceutical, biotech, food, and environmental labs. At the heart of every HPLC system lies a critical component: the column. But how does an HPLC column actually work? Understanding this process helps analysts improve separation, troubleshoot issues, and choose the right column for their applications.
In this article, we’ll break down how an HPLC column works, covering its internal structure, separation mechanism, and key factors that affect performance.
The Role of the HPLC Column
The HPLC column is the site where the actual separation of analytes occurs. It contains a packed bed of stationary phase particles that interact with compounds in the injected sample. As the mobile phase flows through the column, different compounds move at different speeds, depending on how strongly they interact with the stationary phase.
The result? Each component elutes at a different time, producing a unique retention time that’s essential for identification and quantification.
How HPLC Column Works – Step-by-Step Process
1. Sample Injection
The process starts when the sample is introduced into the HPLC system via an injector and carried by the mobile phase toward the column.
2. Interaction with the Stationary Phase
As the sample passes through the column, each analyte interacts with the stationary phase. The strength and nature of these interactions (hydrophobic, polar, ionic) determine how long a compound is retained.
3. Separation Mechanism
In Reverse Phase HPLC (e.g., C18 columns), non-polar compounds interact more strongly with the hydrophobic stationary phase and elute later.
In Normal Phase HPLC, polar compounds are retained longer.
4. Elution
Each compound exits (elutes from) the column at a different time. The detector records these elution times as peaks on a chromatogram.
Internal Structure of an HPLC Column
An HPLC column consists of:
Tube material: Usually stainless steel or PEEK
Stationary phase: Silica-based particles bonded with functional groups (e.g., C18, C8, phenyl, amino, etc.)
Particle size: Typically 3–5 µm in analytical columns; affects resolution and backpressure
Column dimensions: Common lengths (50–250 mm) and internal diameters (2.1–4.6 mm)
Each of these factors influences how well the column separates your target analytes.
Factors That Influence Column Functionality
Several variables impact how effectively an HPLC column performs: Factor Impact
Mobile Phase Composition Affects elution strength and retention time
pH Alters ionization of analytes and stability of bonded phase
Temperature Influences viscosity, analyte interaction, and column lifetime
Flow Rate Affects resolution and analysis time
Optimizing these conditions is crucial for method development and reproducible results.
Common Questions: How HPLC Columns Work
❓ Is a longer column always better?
Not necessarily. Longer columns may improve resolution but increase run time and pressure. It's about balancing separation needs with practicality.
❓ Can I reuse the same column for different samples?
Columns can be reused, but cross-contamination and degradation over time can impact results. Using guard columns helps extend column life.
❓ What’s the difference between analytical and preparative columns?
Analytical columns are used for small-scale quantification, while preparative columns are larger and used to isolate and collect purified compounds.
Conclusion
Understanding how an HPLC column works is fundamental to mastering liquid chromatography. From the chemistry of interactions to the mechanical design, each element of the column plays a role in achieving sharp, reliable separation.
At Zodiac Life Sciences, we offer a wide range of HPLC columns — including C18, C8, phenyl, and amino — engineered for precision, consistency, and longevity. Explore our selection today to find the right column for your lab's needs.
0 notes
Text
Phosphorus Trichloride Formula
Phosphorus Trichloride, denoted by the chemical formula PCl3, is an inorganic compound composed of one phosphorus atom and three chlorine atoms. It is a colorless or slightly yellow fuming liquid with a pungent, unpleasant odor. This compound is not naturally occurring and is synthesized from organic substances2. It’s an important industrial chemical that manufactures phosphites and other organophosphorus compounds. However, it’s highly reactive and toxic, reacting violently with water to release hydrogen chloride. Due to its corrosive nature, it should not come into direct contact with eyes or skin or be inhaled or ingested.Lat Workouts: 5 Back Exercises For Strong Wide Lats steroidly Incline Hammer Curls | Exercise Videos & Guides | Bodybuilding.com
Applications: What is Phosphorus Trichloride Used for?
Phosphorus trichloride is an inorganic compound that is made up of one phosphorus and three chlorine atoms. It is a very reactive and volatile compound that is poisonous. It is used to manufacture other necessary chemicals, such as phosphate ester insecticides, surfactants, gasoline additives, plasticizers, dyestuffs, textile finishing agents, antiseptics, medicinal products, and flame retardants. It is also used as an essential reagent in organic chemistry to replace the hydroxyl group with a chlorine atom. Phosphorus trichloride is obtained from the synthesis of organic substances and not naturally occuring. It should not come in direct contact with eyes and skin or be inhaled or ingested.
Chemical Properties: Is Phosphorus Trichloride Ionic or Covalent?
Phosphorus trichloride is a binary compound consisting of phosphorus and chlorine atoms. It is a covalent compound because the high ionization energy of phosphorus does not favor the formation of a P3+ ion. Instead, the atoms are bound by shared electrons, with each bond containing one electron from the phosphorus atom and another electron from the chlorine atom. The electronegativity values of phosphorus and chlorine are similar, which also favors the formation of a covalent bond. Phosphorus trichloride is used in various industrial applications, such as manufacturing phosphate ester insecticides, surfactants, gasoline additives, plasticizers, dyestuffs, textile finishing agents, antiseptics, medicinal products, and flame retardants. It is also an important reagent in organic chemistry to replace the hydroxyl group with a chlorine atom. However, it is a very reactive and volatile compound that is poisonous and should not come in direct contact with eyes and skin or be directly inhaled or ingested.
Decoding the Chemical Formula for Phosphorus Trichloride
The Significance of the Phosphorus Trichloride Formula
Phosphorus trichloride is a chemical compound with the formula PCl3. It is a colorless liquid with a pungent odor and reacts violently with water. The formula PCl3 tells us that one molecule of phosphorus trichloride consists of one atom of phosphorus and three atoms of chlorine. The procedure also indicates the type of bonding and the molecule’s shape.
Interpreting the Chemical Formula
We need to know each element’s valence electrons and electronegativity to interpret the chemical formula for phosphorus trichloride (PCl3).Valence electrons are the outermost electrons that are involved in chemical bonding. Electronegativity measures how strongly an atom attracts electrons in a bond. Phosphorus has five valence electrons, and chlorine has seven. Phosphorus is less electronegative than chlorine, meaning chlorine will pull the electrons more toward itself in a bond. Therefore, phosphorus trichloride has polar covalent bonds, where the electrons are shared unequally between the atoms. The molecule’s shape is trigonal pyramidal, where the phosphorus atom is at the apex, and the three chlorine atoms are at the corners of a triangular base. The bond angle between the chlorine atoms is 107.8 degrees.
Ionic or Covalent: The Bonding in Phosphorus Trichloride
It is a covalent compound because the high ionization energy of phosphorus does not favor the formation of a P3+ ion. Instead, the atoms are bound by shared electrons, with each bond containing one electron from the phosphorus atom and another electron from the chlorine atom. The electronegativity values of phosphorus and chlorine are similar, which also favors the formation of a covalent bond. Phosphorus trichloride is used in various industrial applications, such as manufacturing phosphate ester insecticides, surfactants, gasoline additives, plasticizers, dyestuffs, textile finishing agents, antiseptics, medicinal products, and flame retardants. It is also an important reagent in organic chemistry to replace the hydroxyl group with a chlorine atom.
Conclusion
Phosphorus Trichloride (PCl3) is an essential inorganic compound consisting of one phosphorus atom and three chlorine atoms. It is a colorless to slightly yellow fuming liquid with a pungent, unpleasant odor. PCl3 is not naturally occurring and is synthesized from organic substances. It holds significant industrial importance, manufacturing various chemicals, including phosphate ester insecticides, surfactants, gasoline additives, plasticizers, dyestuffs, textile finishing agents, antiseptics, medicinal products, and flame retardants. Additionally, it serves as a crucial reagent in organic chemistry for replacing hydroxyl groups with chlorine atoms.
Regarding its chemical properties, Phosphorus Trichloride exhibits covalent bonding due to the similar electronegativities of phosphorus and chlorine. This results in shared electrons in its bonds, creating polar covalent bonds where electrons are shared unevenly. The molecule’s shape is trigonal pyramidal, with a bond angle between chlorine atoms measuring 107.8 degrees.
In conclusion, Phosphorus Trichloride is a versatile compound with widespread industrial applications, characterized by its reactivity, toxicity, and covalent bonding nature. Careful handling is essential due to its corrosive properties, making direct contact with eyes, skin, inhalation, or ingestion highly hazardous. Sarchem Labs, a leading provider of chemical synthesis solutions and a global distributor of specialty chemicals, plays a crucial role in the safe and effective use of such compounds. Over three decades of experience, Sarchem Labs has delivered best-in-class chemical solutions to a diverse client base. Their expert chemistry team is dedicated to customer service and innovation², ensuring that compounds like Phosphorus Trichloride are utilized effectively and responsibly in various industries. https://www.sarchemlabs.com/what-is-phosphorus-trichloride-faqs/
0 notes
Text
Edi Ultrapure Water System Market Industry Growth Forecast: Key Drivers and Market Trends to 2033
Edi Ultrapure Water System Market : Comprehensive Analysis and Growth Forecast (2025-2033)
Global EDI Ultrapure Water System Market Size is expected to grow with a CAGR of 6.1% during the forecast period. North America region has the largest Market.
Market Overview
The Edi Ultrapure Water System Market Report delivers a detailed exploration of a dynamic market cutting across multiple industries. Forecasts, trend analyses, and practical insights covering the years 2024–2033 are all included in this thorough study. The research explores important aspects such product innovation, adoption patterns, pricing tactics, and regional penetration by fusing quantitative data with professional comments.
According to Market Strides the Global EDI Ultrapure Water System Market Size will approximately grow at a CAGR of 6.1% during the forecast period.
EDI stands for Electro Deionization Ultrapure Water System. The Electro Deionization process uses ion exchange resins, ion-selective membranes, and an applied DC electrical current to remove ions, such as salts, acids, and bases. Weakly ionized materials, like dissolved silica, carbon dioxide, boron, and some organics, are also removed. EDI modules are built with membranes permeable to either cations or anions in an alternating sequence. The spaces between them act as liquid flow compartments, each with an inlet and an outlet. Dependent on the polarity of the ion exchange membrane and its orientation to a cathode or anode, an individual compartment depletes the water of ions (product water) or accumulates ions (reject water).
The dilute and concentrate compartments are filled with ion exchange resins to transfer in low ionic strength solutions. In this EDI module, approximately 5% of the volume of feed flow continuously washes out the ions from the concentrate compartments. This concentrate outlet, the reject stream, is recirculated upstream of the RO system to be recovered, while the dilute compartments deliver purified product water. Unlike traditional Ion Exchange resin systems, EDI needs no chemical regeneration. All EDI devices producing ultrapure water operate in electro-regeneration mode, in which the resins are continuously regenerated by H+ and OH- ions released by the electrically-induced dissociation of water molecules.
Technological advancements in EDI systems are helping in improved efficiency and enhanced performance. They have advanced monitoring and control capabilities, improved resin design, and innovative electrode configurations, which allow efficient water treatment processes.
Industries such as Pharmaceuticals, Electronics, and Manufacturing mostly need EDI systems as they must follow strict water quality standards and regulations. Additionally, it considers macroeconomic indicators like GDP growth and socio-economic trends to contextualize market dynamics effectively, enabling stakeholders to make informed decisions.
Get Sample Research Report: https://marketstrides.com/request-sample/edi-ultrapure-water-system-market
Key Focus Areas
Sectors Utilizing Edi Ultrapure Water System Market Products/Services: A detailed examination of industries leveraging the offerings
Market Leaders and Consumer Preferences: Insights into leading participants and evolving trends in customer behavior.
Competitive Landscape: An analysis of competitive positioning, regulatory influences, and emerging technologies shaping the market.
Organized into well-structured segments, the Edi Ultrapure Water System Market report fosters a multi-dimensional understanding of the industry, ensuring actionable insights across economic, political, and cultural contexts.
Growth and Emerging Trends
The Edi Ultrapure Water System Market industry is undergoing transformative changes driven by pivotal trends, which include:
Digital transformation: is the use of cutting-edge technologies to improve customer engagement and expedite processes through data-driven solutions.
Consumer-Centric Innovation: Using customized products to meet the increasing need for ease and personalization.
Regulatory Evolution: To stay competitive, quickly adjust to new regulations and more stringent compliance standards.
Top Key Players in Edi Ultrapure Water System Market
Veolia
Suez
Ovivo
Hitachi
Evoqua
Rightleder
Hyflux
Pure Water No.1
Hongsen Huanbao
Mar-Cor Purification
Nalco
Hongsen Huanbao
Beijing Related
This section provides a SWOT analysis of the top players, focusing on their strategies, opportunities, and challenges. Highlights include:
Top 3-5 Companies: Comprehensive profiles and analysis of key strengths, weaknesses, and growth strategies.
Competitive Landscape: Insights into recent developments, such as partnerships, mergers, acquisitions, and product launches.
Regional Influence: Assessment of regional presence and contributions using the Ace matrix criteria to evaluate market share and growth potential.
Edi Ultrapure Water System Market Segmentation
Segment by Type
0-10 m3/h
10-30 m3/h
30+ m3/h
Segment by Application
Electronics
Pharmaceuticals
Power and Energy
allowing stakeholders to identify specific opportunities and tailor strategies effectively.
Browse Details of Edi Ultrapure Water System Market with TOC: https://marketstrides.com/report/edi-ultrapure-water-system-market
Research Methodology
The report is backed by a meticulous research approach:
Primary Research: In-depth interviews, surveys, and consultations with industry experts, supported by corporate press releases, annual reports, and government publications.
Secondary Research: Extensive analysis of market drivers using industry reports, trade publications, and academic research.
Data Validation: Rigorous cross-verification with expert input to ensure accuracy and credibility.This methodology guarantees a reliable and actionable market perspective, empowering stakeholders with informed decision-making tools.
Regional Analysis Edi Ultrapure Water System Market
The Edi Ultrapure Water System Market report provides an in-depth regional breakdown, offering insights into unique opportunities and characteristics across
North America
Europe
Asia-Pacific
Latin America
The Middle East and Africa
Our Reports Empower Clients Through:
Market sizing and competitive analysis
Strategic guidance for due diligence
product expansion
plant setup
acquisition intelligence
Buy Now:https://marketstrides.com/buyNow/edi-ultrapure-water-system-market
About Us:
Market Strides is a Global aggregator and publisher of Market intelligence research reports, equity reports, database directories, and economic reports. Our repository is diverse, spanning virtually every industrial sector and even more every category and sub-category within the industry. Our market research reports provide market sizing analysis, insights on promising industry segments, competition, future outlook and growth drivers in the space. The company is engaged in data analytics and aids clients in due-diligence, product expansion, plant setup, acquisition intelligence to all the other gamut of objectives through our research focus.
Contact Us📧: [email protected]
#Edi Ultrapure Water System Market Size#Edi Ultrapure Water System Market Share#Edi Ultrapure Water System Market Growth#Edi Ultrapure Water System Market Trends#Edi Ultrapure Water System Market Players
0 notes
Text
Biochemistry Chapter 6: More Proteins (but in more depth)
Bonds in Proteins
Hydrogen bonds: We love these. We want these. Sprinkle hydrogen bonds like confetti! It's a hoarder situation: we have a Bunch of these that we'll never get rid of
Hydrophobic bonds/interactions: Nonpolar side chains make their own clique and water is Not invited. Chatting up another nonpolar side chains means that they don't have to exchange awkward small talk with water, so they really prefer that. (This is how proteins fold usually)
Ionic interactions: Either the charges of something just want to get down and dirty immediately, or they hate each other with the fire of a thousand suns. Sometimes, salt runs interference on these interactions and says, 'no PDA!' or 'children, we must get along!' which totally screws up the normal ionic interactions. After all, they're on the surface of the cell and they have to look good for all the other cells.
Van der Waals interactions: We've got instant attraction and instant hatred together again. Just one of these is super weak, but there's strength in numbers.
Protein Bonds
Secondary structures: you're stabilized by hydrogen bonds! Your peptide group's proton and the carbonyl oxygen of another's just can't get enough of each other. Your alpha helices and beta sheets are because of these bonds!
Protein Origami
Hydrogen bonds everywhere mean that alpha helices and beta sheets are also everywhere
They like each other and huddle together
Peptide segments in secondary structures are short and direct so no fancy loopy loops
Proteins like stability so they try to get a ton of hydrogen bonds and less surface area
Alpha helices are made primarily of alpha-keratin
Beta sheets are made primarily of fibroin and beta-keratin
Collagen is weird because it forms a triple helix because there's so much glycine, proline and hydroxyproline
Globular Proteins
Core is made of a ton of alpha-helices and beta sheets, and any little gaps are stuffed with hydrophobic amino acids
Polar side chains face out, so the surface is hydrophilic
Globular proteins are super dehydrated, there's like no water inside them
0 notes
Video
Hot Chocolate by Kynne Llewellyn Via Flickr: Coat : *LE* Les Encantades - Millicent Coat. in main store. Hairband : ::C'est la vie !:: Vale Headband Hot chocolate and hold pose : LORIEN HOT CHOCOLATE PBR POST MUG. At WLRP. Hair and bangs .NONNATIVE - LANITA. Head : Lelutka evox / AVALON 4.0 Skin : [Glam Affair] Paige [Lelutka EvoX] Blush. At Anthology Lips : Shiny Stuffs EvoX Yesterday 10 Eyeshadow : Shiny Stuffs EvoX Gothic Disco Winterblush : [theSkinnery] Snowball Fight Set (LeLutkaEVOX) snowfightblush. Huntgift at Belle Epoque Decor : DRD - Cocoa Station - Snowy (PBR) Pitaya - Christmas balloons Pitaya - Early winter decor Pitaya - Holiday bushes ionic : Polar Express Line - Bus Stop
0 notes
Text
Comprehensive Guide to Different Types of Dyes and Their Chemical Structures
Dyes are integral to industries like textiles, paper, plastics, and leather, providing a diverse palette of colors and properties tailored to specific needs. This guide delves into various dye types, their characteristics, and their chemical structures. Meghmani Global, a prominent manufacturer and supplier of dyes, plays a vital role in delivering sustainable and high-quality dye solutions worldwide.
1. Natural Dyes
Source: Derived from natural substances like plants, minerals, and insects.
Examples: Indigo (from plants), cochineal (from insects).
Applications: Eco-friendly options for textiles, food coloring, and cosmetics.
Chemical Structure: Composed of complex organic molecules like anthraquinones and flavonoids.
Read blog on Different Types of Dyes with Chemical Structure

2. Synthetic Dyes
Source: Produced chemically from petroleum derivatives.
Advantages: Vibrant colors, higher durability, and cost-effectiveness.
Categories: Includes several subtypes such as azo dyes and anthraquinone dyes.
Chemical Structure: Often includes azo groups (-N=N-) or aromatic rings.
3. Direct Dyes
Features: Water-soluble and directly applied to materials like cotton and rayon.
Usage: Popular for dyeing cellulose fibers due to ease of application.
Chemical Structure: Contain azo or stilbene groups with water-solubilizing sulfonic acid groups.
4. Disperse Dyes
Purpose: Designed for hydrophobic fibers like polyester and acetate.
Mechanism: Submicron particles are dispersed in water and absorbed by fibers under heat.
Applications: Used in textile printing and synthetic fiber dyeing.
Chemical Structure: Non-ionic, often featuring anthraquinone or azo structures.
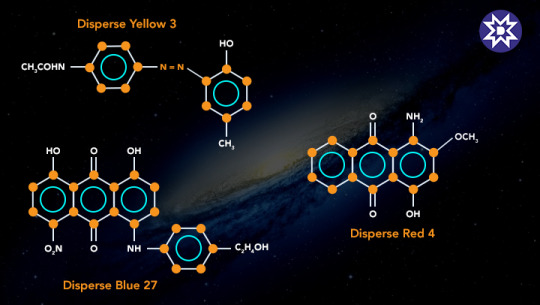
5. Reactive Dyes
Features: Chemically bond with fibers, offering excellent colorfastness.
Applications: Ideal for natural fibers like cotton and wool.
Advantages: High wash fastness and vibrant shades.
Chemical Structure: Contain reactive groups (e.g., triazine) that form covalent bonds with fibers.

6. Solvent Dyes
Characteristics: Soluble in organic solvents but not water.
Applications: Used in plastics, inks, and petroleum products.
Advantages: Stability and vivid colors for industrial applications.
Chemical Structure: Non-polar molecules with aromatic and azo groups.

Meghmani Global: A Leader in Dye Manufacturing
Meghmani Global specializes in producing a comprehensive range of dyes, including reactive, direct, and solvent dyes. With over 27 manufacturing units and a global presence in 60+ countries, the company is known for its innovative products, sustainable practices, and commitment to quality. Our focus on eco-friendly processes and advanced technologies ensures compliance with international standards while minimizing environmental impact.
Conclusion
Understanding the types of dyes and their chemical structures is essential for optimizing applications in industries. From natural and reactive dyes to solvent and disperse dyes, each type offers unique benefits and uses. Meghmani Global exemplifies leadership in this sector, combining innovation with environmental stewardship to meet diverse market demands.
Get in touch
Email: [email protected]
Contact: 7926812918 | Contact Us
Website: https://www.meghmaniglobal.com/
1 note
·
View note
Text
Ionic Liquids Market Size, Share, Trends, Growth and Competitive Analysis
"Ionic Liquids Market – Industry Trends and Forecast to 2029
Global Ionic Liquids Market, By Product Type (Ammonium, Imidazolium, Phosphonium, Pyrrolidinium, Pyridinium, Others), Application (Process Chemicals, Performance Chemicals), End-Use Industry (Solvents & Catalysts, Plastics, Electrochemistry & Batteries, Bio-Refineries, Electronics, Paper & Pulp, Biotechnology, Food, Pharmaceuticals, Others)– Industry Trends and Forecast to 2029
Access Full 350 Pages PDF Report @
**Segments**
- **Type**: The type segment in the ionic liquids market can be further divided into conventional ionic liquids and task-specific ionic liquids. Conventional ionic liquids can be used in various applications due to their general-purpose nature, while task-specific ionic liquids are specially designed for specific applications, offering enhanced performance and efficiency. The demand for task-specific ionic liquids is expected to witness significant growth owing to their tailored properties and increasing application in niche industries.
- **Application**: In terms of application, the ionic liquids market can be segmented into solvents & catalysts, process & operating fluids, batteries & electrochemistry, pharmaceuticals, and others. Ionic liquids have gained traction in the solvents & catalysts segment due to their unique properties such as low volatility, high thermal stability, and tunable polarity, making them ideal for various chemical processes. The batteries & electrochemistry segment is also witnessing substantial growth, driven by the rising demand for high-performance and environmentally friendly energy storage solutions.
- **End-User Industry**: Based on end-user industry, the ionic liquids market can be categorized into chemicals, pharmaceuticals, energy, electronics, food & beverages, and others. The chemicals industry is a key consumer of ionic liquids, utilizing them as green solvents and catalysts in various processes to reduce environmental footprint. The pharmaceuticals sector is increasingly adopting ionic liquids for drug formulation and synthesis due to their biocompatibility and efficiency. The energy industry is another significant end-user, leveraging ionic liquids in applications such as gas separation, fuel cells, and lubricants.
**Market Players**
- **BASF SE**: A prominent player in the ionic liquids market, BASF SE offers a wide range of ionic liquids for diverse applications, including gas treatment, extraction processes, and electrochemical systems. The company focuses on product innovation and strategic partnerships to strengthen its market position and cater to evolving customer needs.
- **Merck KGaA**: Merck KGaBASF SE and Merck KGaA are key players in the ionic liquids market, each bringing unique strengths and innovations to the industry. BASF SE, a leading chemical company, holds a significant market share in the production and supply of ionic liquids tailored for a wide range of applications. The company's focus on product innovation and strategic partnerships has allowed it to meet the growing demand for specialized ionic liquids across various industries. BASF SE has been investing in research and development to introduce new formulations that offer enhanced performance and efficiency, catering to the evolving needs of its customers and ensuring a competitive edge in the market.
Merck KGaA, a global science and technology company, has also made notable contributions to the ionic liquids market with its advanced portfolio of high-quality products. The company's expertise in pharmaceuticals and chemicals has enabled it to develop innovative ionic liquid solutions that meet the stringent quality standards of different end-user industries. Merck KGaA's focus on sustainability and environmental responsibility has resonated well with the market trend towards eco-friendly alternatives, positioning the company as a preferred supplier of ionic liquids for applications requiring green solvents and catalysts. By leveraging its strong research capabilities and industry partnerships, Merck KGaA continues to drive technological advancements and expand its presence in the competitive ionic liquids market.
Both BASF SE and Merck KGaA are actively involved in addressing the evolving needs and preferences of customers in the ionic liquids market. They understand the importance of customization and tailored solutions in meeting specific application requirements across different sectors. By offering a diverse range of ionic liquids with distinct properties and functionalities, these market players enable industries to optimize their processes, enhance product performance, and achieve sustainability goals. The competitive landscape in the ionic liquids market is shaped by continuous innovation, strategic collaborations, and a focus on delivering value-added solutions that drive growth and differentiation in the market.
As the demand for ionic liquids continues to rise across various industries, market players like**Global Ionic Liquids Market, By Product Type (Ammonium, Imidazolium, Phosphonium, Pyrrolidinium, Pyridinium, Others), Application (Process Chemicals, Performance Chemicals), End-Use Industry (Solvents & Catalysts, Plastics, Electrochemistry & Batteries, Bio-Refineries, Electronics, Paper & Pulp, Biotechnology, Food, Pharmaceuticals, Others)– Industry Trends and Forecast to 2029**
The global ionic liquids market is witnessing significant growth and evolution driven by the increasing demand for sustainable and high-performance chemical solutions across various industries. The segmentation of the market by product type, application, and end-user industry provides a comprehensive understanding of the diverse applications and opportunities in the ionic liquids market.
**Product Type**: The categorization of ionic liquids into different types such as Ammonium, Imidazolium, Phosphonium, Pyrrolidinium, Pyridinium, and others reflects the versatility and customizable nature of these compounds. Each type offers unique properties and functionalities that cater to specific application requirements, allowing industries to leverage the benefits of ionic liquids in various processes.
**Application**: The segmentation based on application highlights the wide range of uses for ionic liquids, including process chemicals and performance chemicals. Ionic liquids are valued for their solvent and catalyst capabilities, making them indispensable in chemical processes where traditional solvents fall short. Their application in performance chemicals underscores their role in enhancing product
Key Coverage in the Ionic Liquids Market Report:
Detailed analysis of Ionic Liquids Market by a thorough assessment of the technology, product type, application, and other key segments of the report
Qualitative and quantitative analysis of the market along with CAGR calculation for the forecast period
Investigative study of the market dynamics including drivers, opportunities, restraints, and limitations that can influence the market growth
Comprehensive analysis of the regions of the Ionic Liquids industry and their futuristic growth outlook
Competitive landscape benchmarking with key coverage of company profiles, product portfolio, and business expansion strategies
Table of Content:
Part 01: Executive Summary
Part 02: Scope of the Report
Part 03: Global Ionic Liquids Market Landscape
Part 04: Global Ionic Liquids Market Sizing
Part 05: Global Ionic Liquids Market Segmentation by Product
Part 06: Five Forces Analysis
Part 07: Customer Landscape
Part 08: Geographic Landscape
Part 09: Decision Framework
Part 10: Drivers and Challenges
Part 11: Market Trends
Part 12: Vendor Landscape
Part 13: Vendor Analysis
Browse Trending Reports:
Ferrochrome Market Crop Development And Farming Market Wooden Furniture Market Rice Malt Syrup Market Ibc Cap Market Needle Free Blood Drawing Devices Market Regulatory Affairs Outsourcing Market Noise Source Mapping Market Cyclophilin Inhibitors Therapeutics Market Solid State Transformers Market Home Office Spending Market Dairy Ingredients Market University Management System Market Torticollis Treatment Market x Ray Detectors Market Access Control Market Milking Robots Market Cenospheres Market
About Data Bridge Market Research:
Data Bridge set forth itself as an unconventional and neoteric Market research and consulting firm with unparalleled level of resilience and integrated approaches. We are determined to unearth the best market opportunities and foster efficient information for your business to thrive in the market. Data Bridge endeavors to provide appropriate solutions to the complex business challenges and initiates an effortless decision-making process.
Contact Us:
Data Bridge Market Research
US: +1 614 591 3140
UK: +44 845 154 9652
APAC : +653 1251 975
Email: [email protected]"

0 notes
Text
SAT Chemistry: The Comprehensive Guide

The SAT Chemistry is a standardized test that assesses your understanding of basic chemical concepts and your ability to apply them to a variety of problems. This test is an important part of the college and university application process in many countries, especially in the United States.
The importance of the SAT Chemistry:
University admission: It is one of the factors that universities take into account when evaluating applications.
Determining the academic path: It helps determine the appropriate courses for you at the university.
Scholarships: There may be scholarships available to students who achieve high scores on this test.
Exam content:
The SAT Chemistry exam covers a wide range of topics, including:
Atomic structure: protons, neutrons, and electrons, atomic and mass numbers, isotopes.
The periodic table: elements, periods, groups, periodic properties.
Chemical bonding: ionic and covalent bonds, molecular structure, polar and nonpolar molecules.
Chemical reactions: chemical equations, chemical equilibrium, chemical kinetics.
Acids and bases: pH, acid-base indicators, ions.
Oxidation and reduction: oxidation numbers, electrochemical cells.
Organic chemistry: hydrocarbons, alcohols, carboxylic acids.
Physical chemistry: gases, liquids, solids, heat.
How to prepare for the SAT Chemistry exam?
Understand the basic concepts: Make sure you understand the basic chemistry concepts by reviewing your lessons and trying to solve problems.
Do practice problems: Practice solving a variety of problems to develop your skills in applying concepts to real-world situations.
Focus on weaknesses: Identify areas that need further review and focus.
Use additional resources: Use textbooks, online courses, and practice tests to prepare.
Rest and nutrition: Get enough sleep and eat healthy foods before the exam.
Additional tips:
Start preparing early: Don’t leave studying late.
Create a study group: Working with your peers can help you understand difficult concepts and solve problems on the SAT.
Consult your teacher: Do not hesitate to ask your teacher for help if you encounter any difficulties.
Finally, elmadrasah.com is the leading platform for preparing for the SAT exam in Chemistry, and it provides methodological plans based on this foundation and works to focus on all aspects without neglecting any of them.
0 notes