#pentacarbonyl
Text
Baby moms passed out again so i fuck her in the ass
Handsome hardcore black sex act will make you water
horny girl get hard fuck
BBC Tribute for Slut Julia
Desi girls fucking in friends room and records video
Rubia follando con un viejo
Frivolous fox wakes me up by bouncing boobs
Rica mamada al novio
Ladyboy Topfy Toys and Strokes
Anal and pussy threesome bdsm fuck
#reasonableness#backslidden#placekick#spankee#orgament#condemner#pentacarbonyl#tessellating#barely#pyrographer#indissolubleness#carryings-on#inohymenitic#bootie#wind-swept#puboiliac#skiograph#isophyllous#unobtainable#Cro-Magnon
0 notes
Text
I love whenever people say stuff like this:

So here's a list of chemicals with carbon you should give to these people because clearly carbon isn't bad for you:
Carbon Monoxide, Iron Pentacarbonyl, Hydrogen Cyanide, Sodium Cyanide, Potassium Cyanide, Botulinum Toxin A, Formaldehyde, Ethyl Acrylate, Methacrylic Acid, tert-Butylamine, n-Butylamine, sec-Butylamine, Isobutylamine
1 note
·
View note
Text
Carbonyl Iron Powder | IMP India

The matter of carbonyl iron powder With up to 97.5% 99.5% purity, carbonyl iron powder is a fine particle, high activity iron powder. Purified iron pentacarbonyl is chemically broken down to produce it. Composed of spherical microparticles, it typically has the appearance of a grey powder. This procedure eliminates contaminants like carbon, oxygen, and nitrogen.
For more details read the full blog here: https://imp-india.com/carbonyl-iron-powder-an-insight-on-global-market/
0 notes
Text
Polymerization of 4-Vinyl-1-Cyclohexene Diepoxide by Rhenium Carbonyls Compounds- Juniper Publishers

JUNIPER PUBLISHERS- ACADEMIC JOURNAL OF POLYMER SCIENCE
Abstract
The polymerization of 4-vinyl-1-cyclohexene diepoxide (4-VCHD) by rhenium carbonyl, Re(CO)5X (X = Br, Cl) and dirhenium decacarbonyls Re2(CO)10 has been carried out photochemically at 25oC, and thermally at 25 and 75oC witout cocatalysts. The effects of initiator structure, concentrations, and reaction temperature and time on the polymerization rate is reported here.
Keywords: 4-vinyl-1-cyclohexene diepoxide; Rhenium Carbonyl; Photoinitiated Polymeriation
Introduction
Diepoxy resins have found important commercial applications in UV radiation curing of surface coatings; a dhesives and in the plastic industry [1,2]. Photoinitiated cationic ring-opening polymerizations of cyclohexene oxide (CHO)1 (Figure 1) was conducted using dirhenium decacarbonyl [3] and rhenium carbonyl halides [4] without a cocatalyst. 4-vinyl-1-cyclohexene diepoxide 2 is used as a reactive diluent’s for diepoxides and epoxy resins; this monomer is expected to form crosslinked polymers, if both epoxide groups are polymerized. Cationic photoolymerization of 4-VCHD by diphenyliodonium hexafluorophosphate was carried out and a mixture of crosslinked and benzene soluble polymers were obtained [5], cationic thermal polymerization by BF3. O(C2H5)2 [6], and it has been reported that two epoxy rings can be opened for polymerization selectively by radiation, but not by chemical initiators.
In this paper we report on the polymerization of 4-VCHD (Table 1) by rhenium carbonyl, Re(CO)5X (X = Br, Cl) and dirhenium decacarbonyls Re2(CO)10 photochemically and thermally without cocatalyst Table 2. Insoluble polymer was obtained in photo or thermal polymerization even at low conversion of monomer.
Experimental
Materials
4-vinyl-1-cyclohexene diepoxide (Fluka) was distilled over calcium hydride (CaH2), and the middle fraction was collected. Solvent dichloromethane (Fluka) were dried over calcium hydride and distilled before use. Rhenium carbonyls were obtained from Pressure Chemical Company and used as received.
Instruments
Ultraviolet spectra were obtained on a Cary 2300 spectrophotometer. Infrared spectra were recorded on a Nicolet 50xB FT-IR spectrophotometer.
Polymerization
Photoinitiated polymerization was carried out in a 15mm diameter Pyrex tube using a tight syringe for monomer addition; a homogeneous solution was formed, the reaction tube was then closed with rubber septum, and irradiation was carried out using a merry-go-round photoreactor, Model RPR 100, which rotates continuously by a motor and is surrounded by 16 Hanovia 450 Watt, medium pressure mercury. The light source was equipped with 350 nm wavelength tubes. The samples were placed in the holder and irradiated for the required period.Thermal polymerization was conducted by placing the reaction tube in a water bath at the required temperature for the time indicated in dark. Isolated polymer was washed with dichloroethane, filtered, dried and weighed. The rate of polymerization was calculated gravimetrically as a function of reaction time [7].
Results and Discussion
Polymerization of CHO monomer 1 proceeds through the opening of the epoxide ring to give soluble polymer poly (cyclohexene oxide), the product is long sequences of cyclohexane rings interlinked by oxygen atoms, Figure 3, however; the polymerization of the diepoxide monomer 2 was found to proceeds through the opening of both epoxide ring to give crosslinked polymer of poly (4-VCHD), as shown in Figure 4. Evidence for the structure of poly 4VCHD was obtained by studying the FTIR spectrum of the polymer obtained under different conditions. Typical epoxide bands characteristic of the monomer at 890, 850 and 913cm-1 are missing from the polymer spectra, and a new very powerful band at 1087 and 1157cm-1 associated with the ether linkage is present. The new band at 108 cm-1 is the strongest, and its position varies slightly with chemical structure of the polymer. The important characteristic in the polymerization reaction of 4-VCHD by rhenium carbonyls is the start of the polymerization of both of the epoxy rings at the early stages of polymerization, a crosslinked polymer was obtained at 2% conversion is an idication of reaction of both epoxides at the same time (Figure 4). Thermal polymerization of 4VCHD by dirhenium decacarbonyl and the rhenium pentacarbonyl halides is shown in (Table 2).
Re(CO)5Cl > Re(CO)5Br = Re2(CO)10 >> Re(CO)5I
Thermal polymerization of monomer 2 by the rhenium carbonyl halides proceed very slowly at 25oC, and the gel time is 72 hours; and for polymerization at 75oC the gel time fall in the following sequence: Re(CO)5I > Re2(CO)10 > Re(CO)5Br > Re(CO)5Cl (Table 2). This activity is in acrodance with the bond strength between the halide and the rhenium atom. Polymer obtained as a white powder, insoluble in all solvents.
A comparison beteen the bulk photopolymerization of CHO 1 and 4VCHD 2 under the same conditions using Re(CO)5Cl (2.50x10-3M), shows the required time for complete polymerization (100% conversion) of CHO 1 is 10 minutes and for 4VCHD 2 is 5 minutes, this indicates that the reactivity of monomer 2 almost is twice as that of monomr 1, and both epoxide rings react and opened at the same time.
Effect of initiator concentration
The effect of Re(CO)5Cl concentration on 4VCHD photopolymerization in the range (1.80 x10-3 to 3.69 x10-3M) for fxied time of 5 minutes and without solvent is shown in Figure 5, this indicates an increase in the rate of polymerization as the initiator concentration increases, polymer obtained as crystalline solid which is insoluble in aromatic and halogenated hydrocarbon solvents.
Poly4-VCHD characterization
The polymer structure was identified by FTIR spectroscopy. The FT-IR spectrum of poly (4-VCHD) prepared photochemically and thermally by Re(CO)5Cl and Re2(CO)10. Poly (4-VCHD) prepared photochemically by by Re2(CO)10 shows the OH group at 3400, aliphatic (CH2, CH) at 2960, 2850, and 1920, 1720 (CO) and 1655, 1470 ( six member ring in polymer), 1440, 1050, 1087 and 913cm-1 for the C-O-C stretching frequency [8,9]. These assignment suggest that the catalyst is attached to the poly (4-VCHD).
Proposed polymerization mechanism
The photodisproportionation of the complex (Re (CO)5Cl is shown in (Figure 6), L represent a coordinating monomer. The dimerization of the photoexited Re(CO)5X is bridged through the halogen (X), the bridges is broken by the monomer (4-VCHD), further addition of the monomer to the complex induce the initial propagation reaction. For photoinitiation of the polymerization of 4-VCHD by Re2(CO)10 compounds, we suggested the same mechanism as reported for photopolymerization of cyclohexene oxide [8], the growth of the two epoxide functional groups will leads to crosslinked polymer(Figure 6).
Conclusion
Rhenium carbonyls are effective photoinitiator for the polymerization of (4-VCHD) without cocatalyst, in absence of UV light long reaction time is required. Polymerization by Rhenium carbonyls shows that both epoxide ring were opened. The rate of polymerization depends on the structure of the rhenium carbonyl. Insoluble polymer was obtained in photo or thermal polymerization even at low conversion of monomer.
Acknowledgement
Support to this work from university of sharjah is greatfully acknowledged.
For more Open Access Journals in Juniper Publishers please click on: https://juniperpublishers.com
For more articles in Academic Journal of Polymer Science please click on:https://juniperpublishers.com/ajop/index.php
For more Open Access Journals please click on: https://juniperpublishers.com
0 notes
Text
Les métaux de transition
Les métaux de transition sont les éléments du bloc d du tableau de classification périodique de Mendeleïev. Les éléments de cette famille sont ceux dont la bande de valence présente une couche d incomplètement remplie. Cette famille inclut les éléments dont la bande de valence a la configuration suivante :
4s2, 3dx
5s2, 4dx
6s2, 4f14, 5dx
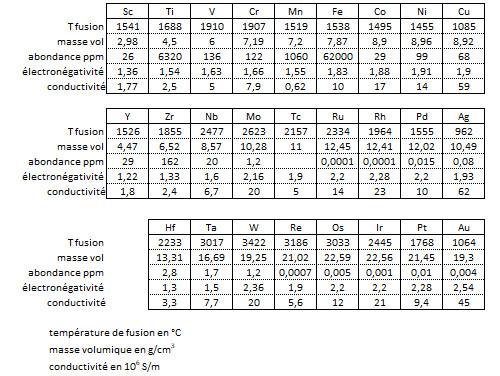
x étant inférieur ou égal à 10. Les éléments de la rangée 7 sont exclus de la famille (ils sont classés dans celle des transactinides). Le lanthane La, premier élément de la rangée 6, est attribué quant à lui à la famille des lanthanides. Le zinc Zn, le cadmium Cd et le mercure Hg, qui font partie du bloc d, ne sont pas des métaux de transition puisque leur sous-couche d est complète. Ils ont d’ailleurs des caractéristiques différentes. Leur température de fusion en particulier est beaucoup plus basse que celle des autres éléments du bloc d : le mercure est liquide à température ambiante ! On les classe dans la famille des métaux pauvres. La famille des métaux de transition comporte 26 éléments. On notera que, parmi ces éléments, le technétium Tc est radioactif (Le 99Tc a une demi-vie de 4,2 millions d’année).
Remarque : certains métaux de transition peuvent avoir une configuration du type ns1, (n-1)dx.
Liaison métallique
L’une des spécificités de ces éléments tient à leur propension à former des cristaux au sein desquels ils sont reliés par une liaison appelée liaison métallique. La liaison métallique est assurée par un nuage électronique délocalisé qui s’étend à tout le cristal (on parle de fluide d’électrons). La géométrie des orbitales de la couche d et le fait qu’elle ne soit pas remplie permet ce type de liaison entre cations métalliques, liaison qui se propage du fait des symétries du cristal. Le principe d’exclusion de Pauli implique cependant une différenciation entre les niveaux d’énergie des électrons de ce nuage délocalisé. Du fait du nombre considérable d’électrons de valence au sein d’un seul cristal, cela conduit à une multitude de niveaux espacés d’un écart infinitésimal. On se retrouve au final avec une bande d’énergie qu’on peut assimiler à un continuum.
Une autre caractéristique de ces éléments est que la bande de valence et la bande de conduction (bande regroupant les niveaux d’énergie théoriquement non occupés) se chevauchent, voire se confondent. Le niveau de Fermi (énergie du plus haut état quantique occupé par un électron à 0 K) est situé au cœur de cette bande commune et la simple agitation thermique permet à des électrons de devenir conducteur. Ceci fait des métaux de transition de bons, voire d’excellents conducteurs électriques (et thermiques, le même raisonnement s’appliquant aux phonons).
Remarque : la répartition des électrons, qui sont des fermions, entre les différents niveaux d’énergie, est régie par la statistique de Fermi-Dirac.
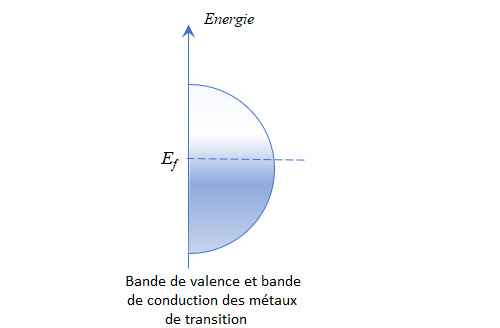
La liaison métallique ne s’applique pas qu’aux cristaux monométalliques. Elle permet aussi de constituer des alliages dans la mesure où les orbitales des couches d de tous les métaux de transition présentent les mêmes symétries.
Propriétés des métaux de transition
La liaison métallique explique un bon nombre des propriétés des métaux de transition et de leurs alliages, parmi lesquelles leur point de fusion et de vaporisation élevés et leur forte densité. La température de fusion de la plupart des métaux de transition est supérieure à 1400°C (elle peut même excéder 3000°C) à l’exception du cuivre, de l’argent et de l’or. Ce sont les métaux des colonnes du milieu du bloc d qui ont la température de fusion la plus élevée (nombre d’électrons non appariés le plus élevé). La température de vaporisation peut monter jusqu’à 6000°C pour le tungstène W !
La liaison métallique explique également les propriétés mécaniques des métaux de transition et de leurs alliages. Ils présentent en général une bonne résistance à la traction et une bonne dureté tout en restant ductiles : ils ne sont pas cassants et ne sont donc pas des matériaux fragiles.
Les propriétés électriques des métaux de transition sont à l’origine de leur caractère opaque, de leur coloration et de leur aspect réfléchissant. Le fluide d’électrons libres empêche en effet toute pénétration des ondes électromagnétiques, ce qui entraîne une réflexion spéculaire qui peut être légèrement colorée (or, cuivre) mais qui est le plus souvent argentée.
Les propriétés magnétiques dépendent quant à elles de la répartition des électrons dans les différentes orbitales (règle de Hund). Très logiquement, ce sont les matériaux qui ont un maximum d’électrons non appariés et pas de doublets qui présentent le plus fort paramagnétisme. C’est le cas du manganèse Mn (spin 5/2).
Un groupe à part : les platinoïdes
Le groupe de platinoïdes (PGM : platinum group metals) occupe une place à part. Ce groupe est composé du platine Pt, du palladium Pd, de l’iridium Ir, du ruthénium Ru, du rhodium Rh, du rhénium Re et de l’osmium Os. Ils font tous partie des lignes 5 et 6 du tableau de classification périodique des éléments. Ce sont des matériaux très denses (densité supérieure à 20g/cm3 pour certains), peu corrosifs et surtout assez peu ductiles.
Abondance dans la croûte terrestre
Le fer est, de très loin, le plus abondant des métaux de transition dans la croûte terrestre, suivi par le titane et le manganèse. Ce sont les seuls éléments de la liste qui dépasse le taux de 1/1000 en masse. Au-dessus de 1/10000 on trouve le zirconium, le vanadium, le chrome et le nickel. Le cuivre ne vient qu’en 8ème position et, compte tenu de son utilisation intensive dans l’industrie, c’est un matériau dont le coût ne cesse de croître. Le cobalt, l’yttrium, le scandium et le niobium sont dans la gamme des 25 ppm. Hafnium, tantale, tungstène et molybdène sont entre 3 et 1,2 ppm. Tous les autres sont des matériaux rares (0,01 ppm pour le platine, 0,004 ppm pour l’or) voire extrêmement rares (0,0001 ppm pour le ruthénium et le rhodium). Si tous les éléments dont le numéro atomique est inférieur à celui du fer ont été produits par la nucléosynthèse stellaire, tous les autres sont issus de l’explosion de supernovæ. Le fer est en effet l’élément dont le noyau a la plus grande stabilité (voir le post à ce sujet).
Oxydation
A la surface de la Terre, les métaux de transition existent la plupart du temps sous forme oxydée. La sous-couche d se prête bien à la formation d’oxydes. De manière très logique, le nombre de degrés d’oxydation que peut adopter un métal de transition augmente avec le nombre d’électrons non appariés de sa bande de valence (de gauche à droite dans le tableau de classification) puis diminue à partir du moment où les électrons commencent à s’apparier.
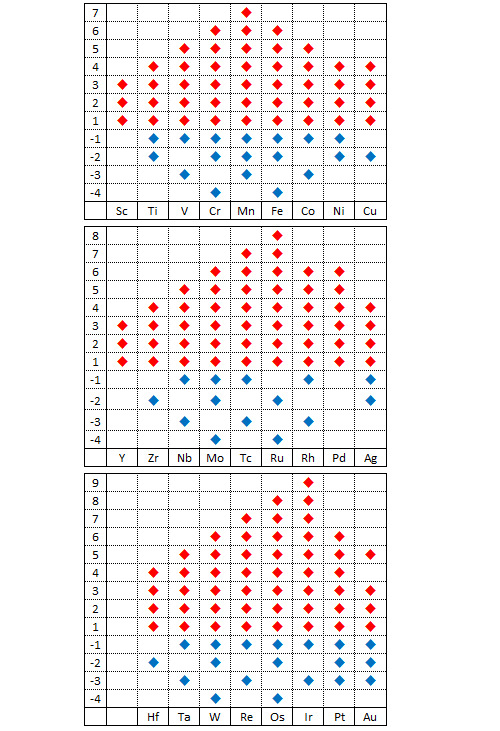
Le tableau qui précède récapitule les différents degrés d’oxydation possibles pour les métaux de transition. Le champion toutes catégories est l’iridium Ir : un tetraoxyde d’iridium IrO4+ a été synthétisé récemment dans lequel il présente un degré d’oxydation +9. Les degrés d’oxydation observés dans la nature sont moins nombreux et l’ion permanganate MnO4- (+7) est l’un des composés métalliques courants avec le degré d’oxydation le plus fort. Le permanganate de potassium est un décapant et un décolorant. Il a été utilisé pendant la guerre comme carburant.
Il existe de nombreux composés métalliques avec un degré d’oxydation zéro (en particulier les composés métal carbonyle comme le pentacarbonyle de fer Fe(CO)5 ou le tetracarbonyle de nickel Ni(CO)4). Les carbonyles de métal sont très utilisés comme catalyseurs ou comme précurseurs en chimie organique. Les degrés d’oxydation négatifs se rencontrent principalement dans les composés organométalliques. C’est le cas par exemple dans l’ion hexacarbonyle de vanadium [V(CO)6]-.
Parmi les métaux de transition, on classe à part les métaux dits nobles compte tenu de leur résistance à la corrosion. Cette famille regroupe l’or Au, l’argent Ag ainsi que tous les métaux platinoïdes à l’exception du rhénium Re. Ces métaux peuvent exister sous forme oxydée mais leur potentiel d’oxydo-réduction positif et relativement élevé rend cette oxydation rare. L’éclat pratiquement inaltérable de l’or en a fait un matériau très prisé dans pratiquement toutes les civilisations.
Composés complexes
Les métaux de transition sont particulièrement intéressants du fait de leur capacité à se coordonner avec des ligands pour former des composés complexes. La chimie des composés complexes est particulièrement riche. Une série de posts lui est consacrée. Les complexes organométalliques jouent un grand rôle en chimie organique : de façon naturelle (dans la chlorophylle, l’hémoglobine et certaines vitamines par exemple) ou artificielle (comme médicaments ou au titre de catalyseurs dans la chimie de synthèse).
Pour en savoir plus :
post sur le tableau de classification des éléments
post sur la nucléosynthèse
post sur le noyau des atomes
post sur l’oxydoréduction
post sur le degré d’oxydation
post sur les liaisons chimiques
post sur les complexes
post sur les complexes (approche covalente)
post sur les propriétés magnétiques
post sur le fer
post sur le haut-fourneau
index
#chimie#métal#métal de transition#métaux#fer#cuivre#chrome#platine#palladium#rhodium#catalyse#catalyseur#oxydoréduction#complexe#organométallique#liaison métallique#valence#coordination#iridium#vanadium#métal carbonyle#manganèse#métal noble#conductivité
0 notes
Text
Antiknock Agents Market Major Revenue Boost during the Period between 2020
Antiknock agent is a gasoline additive that works to reduce the engine knocking tendency while trying to accelerate the octane rating of the fuel. Mixture of gas and air in a conventional car engine has a problem with igniting too early and when this happens, it creates a knocking noise. Commonly used antiknock agents are tetraethyl lead, ferrocene, toluene, iron pentacarbonyl, isooctane and methylcyclopentadienyl manganese tricarbonyl. Lead compounds have been used as an antiknock agent for many years. The most commonly used is tetraethyl lead, a transparent and highly toxic dense liquid. It easily dissolves in ethyl, acetone, gasoline and in some other solvents. It boils at around 250°Ð¡. Another commonly used lead antiknock agent is the tetramethyl lead. It is a liquid with pungent smell and boils at around 120°Ð¡. Due to the relatively low boiling temperature, this substance spreads more evenly in gasoline fractions. Tetramethyl lead is more stable than tetraethyl lead at around 700°Ð¡. This ensures higher and better efficiency of the tetramethyl lead as compared to tetraethyl lead in high pressure ratio internal combustion engine vehicles.Commonly found drawback of both the compounds is the high toxicity of the agents, with high impact on the environment and negative influence on the exhaust gas after treatment devices. Hence, for these reasons the use of tetramethyl lead and tetraethyl lead is decreasing and intensive research is carried out for more efficient antiknock agents is in the pipeline.
Read Report Overview @
https://www.transparencymarketresearch.com/antiknock-agents-market.html
Some of the antiknock agents which have already been tested and used at various times are cyclopentadienyl manganese tricarbonyl (CMT), methylcyclopentadienyl manganese tricarbonyl (MMT) and dicyclopentadienyl iron. In terms of higher and better efficiency, manganese compounds are analogous and iron compounds are inferior to lead. CMT is a highly volatile crystalline compound of yellow color. It is stable in air and is easily soluble in organic solvents and is completely insoluble in water. MMT is a low viscosity liquid of light amber color with a grassy smell and has a boiling point of 250°Ð¡. Ferrocene is a solid crystalline substance and has a melting temperature of 180°Ð¡. Iron pentacarbonyl is a straw color liquid with boiling temperature of 105°Ð¡ and freezing temperature of -2°Ð¡. Ferrocenyl dimethyl carbinol is a crystal powder with melting temperature of 70°Ð¡. Organometallic agents create sedimentation of metals on the walls of combustion chamber. Therefore, organometallic anti-knock additives are typically used in combination with materials which convert churly metal oxides into volatile compounds. Due to high toxicity of lead type anti-knock agents, significant disadvantages and high cost associated with it, the research for a special material, which does not comprise any toxic substance, is in the pipeline. Such anti-knock agents are organic amines containing, xylidine methylaniline and extralyne. Stringent environmental norms and increasing investments are some of the key drivers of the antiknock agents market. However, high cost in the manufacturing of antiknock agents can hamper the growth of the market. Advancements in new technology bring huge opportunities for the anti knocks agents market.
Request Report Brochure @
https://www.transparencymarketresearch.com/sample/sample.php?flag=B&rep_id=3450
Some of the key companies in the business of antiknock agents are Shandong Dongchang Fine Chemical Technology Co. Ltd., Wuxi Weite New Engery Co. Ltd., Yingkou Tanyun Chemical Research Institute Corporation and SIMAGCHEM Corporation among others.
0 notes
Link
0 notes
Text
Antiknock Agents Market Analysis, Current and Future Trends 2020
Antiknock agent is a gasoline additive that works to reduce the engine knocking tendency while trying to accelerate the octane rating of the fuel. Mixture of gas and air in a conventional car engine has a problem with igniting too early and when this happens, it creates a knocking noise.
Request Report Brochure @ https://www.transparencymarketresearch.com/sample/sample.php?flag=B&rep_id=3450
Commonly used antiknock agents are tetraethyl lead, ferrocene, toluene, iron pentacarbonyl, isooctane and methylcyclopentadienyl manganese tricarbonyl. Lead compounds have been used as an antiknock agent for many years. The most commonly used is tetraethyl lead, a transparent and highly toxic dense liquid. It easily dissolves in ethyl, acetone, gasoline and in some other solvents. It boils at around 250°Ð¡.
Another commonly used lead antiknock agent is the tetramethyl lead. It is a liquid with pungent smell and boils at around 120°Ð¡. Due to the relatively low boiling temperature, this substance spreads more evenly in gasoline fractions. Tetramethyl lead is more stable than tetraethyl lead at around 700°Ð¡. This ensures higher and better efficiency of the tetramethyl lead as compared to tetraethyl lead in high pressure ratio internal combustion engine vehicles.
Commonly found drawback of both the compounds is the high toxicity of the agents, with high impact on the environment and negative influence on the exhaust gas after treatment devices. Hence, for these reasons the use of tetramethyl lead and tetraethyl lead is decreasing and intensive research is carried out for more efficient antiknock agents is in the pipeline.
Read Report Overview @ https://www.transparencymarketresearch.com/antiknock-agents-market.html
Some of the antiknock agents which have already been tested and used at various times are cyclopentadienyl manganese tricarbonyl (CMT), methylcyclopentadienyl manganese tricarbonyl (MMT) and dicyclopentadienyl iron. In terms of higher and better efficiency, manganese compounds are analogous and iron compounds are inferior to lead. CMT is a highly volatile crystalline compound of yellow color. It is stable in air and is easily soluble in organic solvents and is completely insoluble in water. MMT is a low viscosity liquid of light amber color with a grassy smell and has a boiling point of 250°Ð¡. Ferrocene is a solid crystalline substance and has a melting temperature of 180°Ð¡. Iron pentacarbonyl is a straw color liquid with boiling temperature of 105°Ð¡ and freezing temperature of -2°Ð¡.
About Us
Transparency Market Research (TMR) is a global market intelligence company providing business information reports and services. The company’s exclusive blend of quantitative forecasting and trend analysis provides forward-looking insight for thousands of decision makers. TMR’s experienced team of analysts, researchers, and consultants use proprietary data sources and various tools and techniques to gather and analyze information.
TMR’s data repository is continuously updated and revised by a team of research experts so that it always reflects the latest trends and information. With extensive research and analysis capabilities, Transparency Market Research employs rigorous primary and secondary research techniques to develop distinctive data sets and research material for business reports.
Contact
Transparency Market Research
State Tower,
90 State Street,
Suite 700,
Albany NY – 12207
United States
Tel: +1-518-618-1030
USA – Canada Toll Free: 866-552-3453
Email: [email protected]
Website: http://www.transparencymarketresearch.com
0 notes
Text
Carbonyl Iron Powder and Ultra Fine Iron Powder Industry Report by Application And Size 2022
Global Carbonyl Iron Powder and Ultra Fine Iron Powder Market are segmented on the basis of type as Carbonyl Iron Powder, Atomized Ultra Fine Iron Powder, and Others. Iron powder is powdered iron that is produced with the assistance of various other iron particles. As far as their particle sizes are concerned, they may differ quiet a lot. And the iron characteristics may also vary on the basis of the manufacturing techniques and historical account of a particular iron powder.
Iron powder can be segregated mainly in three categories comprising of reduced iron powder, atomized powder, and electrolyte iron powder. And every kind has a varied application based on their specific properties. However, the studies show that reduced iron powder and atomized iron powder are almost similar in appearance.
A highly pure iron that is made with the help of chemical putrefaction of distilled iron pentacarbonyl is known as carbonyl Iron. As far as the appearance is concerned, it generally looks like a grayish powder made of spherical microparticles. The key impurities that are largely present entails carbon, oxygen, and nitrogen. The most striking factor that is attached with it is its outstanding fineness and spherical morphology that eventually gives rise to exceptional compaction and sintering properties And these properties are further utilized in diamond tool production, metal injection molding, and conventional powder metallurgy. A wide range of applications is attached with the employment of these powders ranging from the manufacturing of magnetic alloys to the production of numerous other steels.
Browse Full Research Report @ https://www.millioninsights.com/industry-reports/carbonyl-iron-powder-and-ultra-fine-iron-powder-market
On the other hand, ultrafine iron powder consists of minute particles. It is said that the particular substance possesses an excellent electrical magnetic optical effect. This not only influences the sintering properties of the material, but also has an impact on the final physical properties of the material. Its major utilization lies in the sector of electromagnetic shielding & powder metallurgy. The key factors that are playing a major role in driving the market growth include technological advancements, rise in the industrialization, augmentation in the applications, and rise in the demand across various industries.
Carbonyl Iron Powder and Ultra Fine Iron Powder Market are likely to witness a robust growth in the next couple of years owing to rise in the scope and applications across various industries. Carbonyl Iron Powder and Ultra Fine Iron Powder Market are segmented on the basis of application as Powder Metallurgy, Electronics Industry, Diamond Tools, Military Industry, Food and Drug Industry, and Others. Carbonyl Iron Powder and Ultra Fine Iron Powder Market are segmented on the basis of geographical location as North America, Europe, Asia-Pacific, South America, and Middle East and Africa.
As far as the geographical location is concerned, North America is the dominant country in the globe and it is simultaneously accounting for the largest share in the market, the reason being technological advancements, rise in the industrialization, and augmented demand across various electronics and metallurgical sectors. On the contrast, Europe and Asia-Pacific are also displaying a robust growth and they will soon emerge as one of the promising regions, owing to emergence of huge market opportunities in the particular regions. The key players operating in the Basf, Jinchuan Group, Gripm, Cnpc Powder, Jiangsu Tianyi, Jilin Jien, Jiangxi Yuean, Sintez-cip, Jfe, Jiangyou Hebao, and Shanxi Xinghua.
Request Sample Copy of this Market Research @ https://www.millioninsights.com/industry-reports/carbonyl-iron-powder-and-ultra-fine-iron-powder-market/request-sample
#Carbonyl Iron Powder and Ultra Fine Iron Powder Market#Carbonyl Iron Powder and Ultra Fine Iron Powder Industry#Carbonyl Iron Powder and Ultra Fine Iron Powder Market Size#Carbonyl Iron Powder and Ultra Fine Iron Powder Market Share#Carbonyl Iron Powder and Ultra Fine Iron Powder Market Trend#Carbonyl Iron Powder and Ultra Fine Iron Powder Market Growth
0 notes
Link
Carbonyl iron is a highly pure iron, prepared by chemical decomposition of purified iron pentacarbonyl. It usually has the appearance of grey powder, composed of spherical micro-particles. Most of the impurities are carbon, oxygen, and nitrogen. Scope of the Report: This report focuses on the Carbonyl Iron Powder in North America market, especially in United States, Canada and Mexico. This report categorizes the market based on manufacturers, countries, type and application. Market Segment by Manufacturers, this report covers BASF Sintez-CIP Jiangsu Tianyi Jilin Jien Jiangxi Yuean Shanxi Xinghua Jiangyou Hebao Jinchuan Group CNPC Powder Market Segment by Countries, covering United States Canada Mexico Market Segment by Type, covers Fe?98% Fe: 98-99% Fe?99% Market Segment by Applications, can be divided into Powder Metallurgy Electronics Industry Diamond Tools Military Industry Food and Drug Industry Others There are 15 Chapters to deeply display the North America Carbonyl Iron Powder market. Chapter 1, to describe Carbonyl Iron Powder Introduction, product type and application, market overview, market analysis by countries, market opportunities, market risk, market driving force; Chapter 2, to analyze the manufacturers of Carbonyl Iron Powder, with profile, main business, news, sales, price, revenue and market share in 2016 and 2017; Chapter 3, to display the competitive situation among the top manufacturers, with profile, main business, news, sales, price, revenue and market share in 2016 and 2017; Chapter 4, to show the North America market by countries, covering United States, Canada and Mexico, with sales, revenue and market share of Carbonyl Iron Powder, for each country, from 2012 to 2017; Chapter 5 and 6, to show the market by type and application, with sales, price, revenue, market share and growth rate by type, application, from 2012 to 2017; Chapter 7, 8 and 9, to analyze the segment market in United States, Canada and Mexico, by manufacturers, type and application, with sales, price, revenue and market share by manufacturers, types and applications; Chapter 10, Carbonyl Iron Powder market forecast, by countries, type and application, with sales, price and revenue, from 2017 to 2022; Chapter 11, to analyze the manufacturing cost, key raw materials and manufacturing process etc. Chapter 12, to analyze the industrial chain, sourcing strategy and downstream end users (buyers); Chapter 13, to describe sales channel, distributors, traders, dealers etc. Chapter 14 and 15, to describe Carbonyl Iron Powder Research Findings and Conclusion, Appendix, methodology and data source
#carbonyl iron powder market#carbonyl iron powder market trends#carbonyl iron powder market size#carbonyl iron powder market data#carbonyl iron powder market structure#carbonyl iron powder industry analysis#carbonyl iron powder market research#carbonyl iron powder market report
0 notes
Text
Carbonyl Iron Powder and Ultra Fine Iron Powder Market Segmentation, Opportunities, Trends & Future Scope to 2022
Global Carbonyl Iron Powder and Ultra Fine Iron Powder Market are segmented on the basis of type as Carbonyl Iron Powder, Atomized Ultra Fine Iron Powder, and Others.Iron powder is powdered iron that is produced with the assistance of various other iron particles. As far as their particle sizes are concerned, they may differ quiet a lot. And the iron characteristics may also vary on the basis of the manufacturing techniques and historical account of a particular iron powder.
Browse Full Research Report @ https://www.millioninsights.com/industry-reports/carbonyl-iron-powder-and-ultra-fine-iron-powder-market
Iron powder can be segregated mainly in three categories comprising of reduced iron powder, atomized powder, and electrolyte iron powder. And every kind has a varied application based on their specific properties. However, the studies show that reduced iron powder and atomized iron powder are almost similar in appearance.A highly pure iron that is made with the help of chemical putrefaction of distilled iron pentacarbonyl is known as carbonyl Iron.
As far as the appearance is concerned, it generally looks like a grayish powder made of spherical microparticles. The key impurities that are largely present entails carbon, oxygen, and nitrogen.
The most striking factor that is attached with it is its outstanding fineness and spherical morphology that eventually gives rise to exceptional compaction and sintering properties. And these properties are further utilized in diamond tool production, metal injection molding, and conventional powder metallurgy. A wide range of applications is attached with the employment of these powders ranging from the manufacturing of magnetic alloys to the production of numerous other steels.
On the other hand, ultrafine iron powder consists of minute particles. It is said that the particular substance possesses an excellent electrical magnetic optical effect. This not only influences the sintering properties of the material, but also has an impact on the final physical properties of the material. Its major utilization lies in the sector of electromagnetic shielding & powder metallurgy.
Request Sample Copy of this Market Research @ https://www.millioninsights.com/industry-reports/carbonyl-iron-powder-and-ultra-fine-iron-powder-market/request-sample
The key factors that are playing a major role in driving the market growth include technological advancements, rise in the industrialization, augmentation in the applications, and rise in the demand across various industries.
Carbonyl Iron Powder and Ultra Fine Iron Powder Market are likely to witness a robust growth in the next couple of years owing to rise in the scope and applications across various industries.
Carbonyl Iron Powder and Ultra Fine Iron Powder Market are segmented on the basis of application as Powder Metallurgy, Electronics Industry, Diamond Tools, Military Industry, Food and Drug Industry, and Others.
Carbonyl Iron Powder and Ultra Fine Iron Powder Market are segmented on the basis of geographical location as North America, Europe, Asia-Pacific, South America, and Middle East and Africa.
As far as the geographical location is concerned, North America is the dominant country in the globe and it is simultaneously accounting for the largest share in the market, the reason being technological advancements, rise in the industrialization, and augmented demand across various electronics and metallurgical sectors. On the contrast, Europe and Asia-Pacific are also displaying a robust growth and they will soon emerge as one of the promising regions, owing to emergence of huge market opportunities in the particular regions. The key players operating in the Basf, Jinchuan Group, Gripm, Cnpc Powder, Jiangsu Tianyi, Jilin Jien, Jiangxi Yuean, Sintez-cip, Jfe, Jiangyou Hebao, and Shanxi Xinghua.
0 notes
Text
Antiknock Agents Market For Near Future; Global Industry Analysis 2020
Antiknock agent is a gasoline additive that works to reduce the engine knocking tendency while trying to accelerate the octane rating of the fuel. Mixture of gas and air in a conventional car engine has a problem with igniting too early and when this happens, it creates a knocking noise. Commonly used antiknock agents are tetraethyl lead, ferrocene, toluene, iron pentacarbonyl, isooctane and methylcyclopentadienyl manganese tricarbonyl. Lead compounds have been used as an antiknock agent for many years. The most commonly used is tetraethyl lead, a transparent and highly toxic dense liquid. It easily dissolves in ethyl, acetone, gasoline and in some other solvents. It boils at around 250°Ð¡.
Read Report Overview @ https://www.transparencymarketresearch.com/antiknock-agents-market.html
Another commonly used lead antiknock agent is the tetramethyl lead. It is a liquid with pungent smell and boils at around 120°Ð¡. Due to the relatively low boiling temperature, this substance spreads more evenly in gasoline fractions. Tetramethyl lead is more stable than tetraethyl lead at around 700°Ð¡. This ensures higher and better efficiency of the tetramethyl lead as compared to tetraethyl lead in high pressure ratio internal combustion engine vehicles. Commonly found drawback of both the compounds is the high toxicity of the agents, with high impact on the environment and negative influence on the exhaust gas after treatment devices.
Hence, for these reasons the use of tetramethyl lead and tetraethyl lead is decreasing and intensive research is carried out for more efficient antiknock agents is in the pipeline. Some of the antiknock agents which have already been tested and used at various times are cyclopentadienyl manganese tricarbonyl (CMT), methylcyclopentadienyl manganese tricarbonyl (MMT) and dicyclopentadienyl iron. In terms of higher and better efficiency, manganese compounds are analogous and iron compounds are inferior to lead. CMT is a highly volatile crystalline compound of yellow color. It is stable in air and is easily soluble in organic solvents and is completely insoluble in water. MMT is a low viscosity liquid of light amber color with a grassy smell and has a boiling point of 250°Ð¡. Ferrocene is a solid crystalline substance and has a melting temperature of 180°Ð¡.
Iron pentacarbonyl is a straw color liquid with boiling temperature of 105°Ð¡ and freezing temperature of -2°Ð¡.
Request Report Brochure @ https://www.transparencymarketresearch.com/sample/sample.php?flag=B&rep_id=3450
Ferrocenyl dimethyl carbinol is a crystal powder with melting temperature of 70°Ð¡. Organometallic agents create sedimentation of metals on the walls of combustion chamber. Therefore, organometallic anti-knock additives are typically used in combination with materials which convert churly metal oxides into volatile compounds. Due to high toxicity of lead type anti-knock agents, significant disadvantages and high cost associated with it, the research for a special material, which does not comprise any toxic substance, is in the pipeline. Such anti-knock agents are organic amines containing, xylidine methylaniline and extralyne.
0 notes
Text
Carbonyl Iron Powder Market,Forecast to 2022
Carbonyl Iron Powder Market,Forecast to 2022
Carbonyl iron is a highly pure iron, prepared by chemical decomposition of purified iron pentacarbonyl. It usually has the appearance of grey powder, composed of spherical micro-particles. Most of the impurities are carbon, oxygen, and nitrogen.
Date Published: 2017/08/21
Region: Global
Delivery Format: PDF
Pages: 116
Category: Chemical
Scope of the Report:
This report focuses on the Carbonyl…
View On WordPress
#Carbonyl Iron Powder Market data#Carbonyl Iron Powder Market discount#Carbonyl Iron Powder Market industrial analysis#Carbonyl Iron Powder Market research reports#Carbonyl Iron Powder Market resourses#Carbonyl Iron Powder Market risk#Carbonyl Iron Powder Market samples#Carbonyl Iron Powder Market share#Carbonyl Iron Powder Market trends#Global Carbonyl Iron Powder Market
0 notes