#poly4
Explore tagged Tumblr posts
Note
i’d love to see your ocs as a lycanroc. whichever form is up to you :3
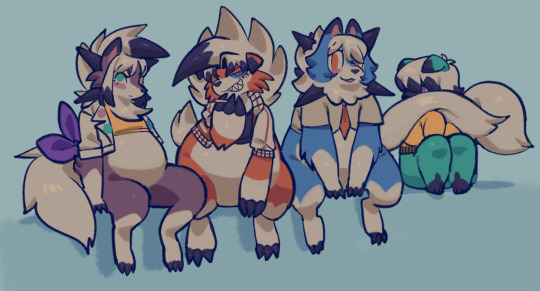
wolf pack :3
#i couldnt decide so i did them all#seraph draws#oc: viv#oc: apollo#oc: josh#oc: charlie#pokemon#lycanroc#rockruff#art#ask tag#poly4
722 notes
·
View notes
Text
Sunset: Geez Flash, you can be SO annoying sometimes! 😑
Flash: But….we’re dating???
Sunset:…😳
Sunset: Doesn’t make you less annoying…😒
Pinkie Pie: Don’t worry Sunbunny & Flashy! Your both annoying and I love you both the same! 😚
Sci Twi: *On the side, watching her girlfriends and boyfriend being the embarrassing adorkable dorks that they’re are*
🙈
“OMG YOU GUYS ARE ALL EQUALLY CUTE AND EMBARRASSING, PLEASE STOP!”
#😣😣😣#😳😳😳#😮💨😮💨😮💨#🫣🫣🫣#sciflashsetpie#sciflash#sunpie#scipie#flashshimmer#flashset#sciset#sunlight#flashpie#polyamory#poly4#poly shipping#poly ship#equestria girls#eqg#mlp#my little pony#mlp eqg#my little pony friendship is magic#mlp fim#sunset shimmer#sci twilight#sci twi#flash sentry#pinkie pie#icorrect quotes
38 notes
·
View notes
Text
I honestly never understood ship wars like deadass.
"oh but this ship is better than that ship" like NO
Every ship is good (as long as said ship isn't toxic or problematic then y'all can hate) but if the ship is good why the hate?
That's why I absolutely ADORE the small Super 4 fandom. No ship wars everyone ships what they want and we all are like "Omg yeah that's SO COOL"
Especially the ships amongst the main 4. They work so well with each other no matter how you pair them they just work.
And if someone can't settle on a ship fuck it Poly4 it is.
I fucking love the Super 4 fandom
22 notes
·
View notes
Text
Also guys I haven't forgot about ship requests you sent me! I'm just currently writing stuff for k2 week and decided not to distract myself before finishing the piece I'm currently working on :) Hope you won't be mad if I do the rest a little late. There's 3 more
Also...I made a tag #lego requests (i know I'm very creative) to navigate my account! Since this is probably not the last time I'm doing requests, they're fun. Also using this tag you can check 3 requests I've already made, there's Stylennyman (poly4), Steek and Kenrietta !! :D
2 notes
·
View notes
Text
Im gonna fucking cry wdym eiden did a poly4 first danc ejwjasjsne
1 note
·
View note
Photo

Wearing my Geologists and Gemmologists hats tonight at the opening of the Woodsmith Mine exhibition @whitbymuseum with the lovely Beth Vetch and Peter Smith one half of the Wood-Smith team. #fromwoodsmithtotheworld #sirius #siriusminerals #polyhalite #poly4 #whitby #whitbyjetresearch
0 notes
Link
Check out this listing I just added to my Poshmark closet: ✨end of summer sale✨ Boutique White Zipper Dress 🤍.
0 notes
Text

getting in the christmas spirit
#sillies putting up xmas decor or smth#seraph draws#oc: charlie#oc: josh#oc: apollo#oc: viv#art#sfw tf#furry#oc#poly4
416 notes
·
View notes
Text
SciFlashSetPie OTP4 Headcanons
Flash introduced his parents to his girlfriends just like this. JK
First I do imagine SunPie getting together first. Then SciFlash after Sci Twi and Timber break up.
SciSet is what brought SciFlashSetPie together.
When the 4 campout on the couch, they mostly sit in the exact same way. From left to right: Sci, Flash, and Sunset. Pinkie lays across on their laps 😊
Pinkie’s favorite games to play with her boyfriend and girlfriends are Spin the bottle and Turth or Dare.
I imagine when they grow older, SciFlash and SunPie get married, but all 4 of them stay close.
One of their favorite activities to do together is cooking. Pinkie and Flash are the cooks of the poly4 while Sunset and Sci Twi usually tries to follow along with their boyfriend and girlfriend. Their meals are decent.
SciFlash is sub and SunPie is dom in their 4 relationship.
If they’re not that comfortable with making their 4 polygamous relationship known, then they’ll just pose as SunPie and SciFlash.
When they’re away from Flash, SciSetPie likes to take group pics, captioning things like “Wish you were here” or “Posing for Babe”.
SciFlashSet loves ramen noodles, so they have this special ramen noodle place that they love to go to eat nice and tasty bowls of ramen together 🍜 Ramen isn’t Pinkie’s FAVORITE food in the world, but she’s open for ANYTHING as long as she she gets to have the experience and enjoys it with her love ones.
Crossover idea: SciFlashSetPie likes to cosplay is Lukadrigaminette (Miraculous Ladybug), Sci Twi as Marinette, Sunset as Kagami, Flash as Luka and Pinkie as Adrien lol
No I don’t plan to make this poly relationship canon in @ask-sonata-dusk, but MAN I kind of wish I did! 🥺
((More could be added later.))
#sciflash#sunpie#sunflash#flashshimmer#FlashPie#pinkieflash#eqg ships#equestria girls#eqg#mlp#my little pony#mlp eqg#my little pony friendship is magic#mlp fim#mlp headcanons#sunset shimmer#flash sentry#sci twilight#pinkie pie#poly4#poly shipping#polymory#polyamorous#polyamory#eqg headcanons#mlp: equestria girls
23 notes
·
View notes
Text
Polymerization of 4-Vinyl-1-Cyclohexene Diepoxide by Rhenium Carbonyls Compounds- Juniper Publishers

JUNIPER PUBLISHERS- ACADEMIC JOURNAL OF POLYMER SCIENCE
Abstract
The polymerization of 4-vinyl-1-cyclohexene diepoxide (4-VCHD) by rhenium carbonyl, Re(CO)5X (X = Br, Cl) and dirhenium decacarbonyls Re2(CO)10 has been carried out photochemically at 25oC, and thermally at 25 and 75oC witout cocatalysts. The effects of initiator structure, concentrations, and reaction temperature and time on the polymerization rate is reported here.
Keywords: 4-vinyl-1-cyclohexene diepoxide; Rhenium Carbonyl; Photoinitiated Polymeriation
Introduction
Diepoxy resins have found important commercial applications in UV radiation curing of surface coatings; a dhesives and in the plastic industry [1,2]. Photoinitiated cationic ring-opening polymerizations of cyclohexene oxide (CHO)1 (Figure 1) was conducted using dirhenium decacarbonyl [3] and rhenium carbonyl halides [4] without a cocatalyst. 4-vinyl-1-cyclohexene diepoxide 2 is used as a reactive diluent’s for diepoxides and epoxy resins; this monomer is expected to form crosslinked polymers, if both epoxide groups are polymerized. Cationic photoolymerization of 4-VCHD by diphenyliodonium hexafluorophosphate was carried out and a mixture of crosslinked and benzene soluble polymers were obtained [5], cationic thermal polymerization by BF3. O(C2H5)2 [6], and it has been reported that two epoxy rings can be opened for polymerization selectively by radiation, but not by chemical initiators.
In this paper we report on the polymerization of 4-VCHD (Table 1) by rhenium carbonyl, Re(CO)5X (X = Br, Cl) and dirhenium decacarbonyls Re2(CO)10 photochemically and thermally without cocatalyst Table 2. Insoluble polymer was obtained in photo or thermal polymerization even at low conversion of monomer.
Experimental
Materials
4-vinyl-1-cyclohexene diepoxide (Fluka) was distilled over calcium hydride (CaH2), and the middle fraction was collected. Solvent dichloromethane (Fluka) were dried over calcium hydride and distilled before use. Rhenium carbonyls were obtained from Pressure Chemical Company and used as received.
Instruments
Ultraviolet spectra were obtained on a Cary 2300 spectrophotometer. Infrared spectra were recorded on a Nicolet 50xB FT-IR spectrophotometer.
Polymerization
Photoinitiated polymerization was carried out in a 15mm diameter Pyrex tube using a tight syringe for monomer addition; a homogeneous solution was formed, the reaction tube was then closed with rubber septum, and irradiation was carried out using a merry-go-round photoreactor, Model RPR 100, which rotates continuously by a motor and is surrounded by 16 Hanovia 450 Watt, medium pressure mercury. The light source was equipped with 350 nm wavelength tubes. The samples were placed in the holder and irradiated for the required period.Thermal polymerization was conducted by placing the reaction tube in a water bath at the required temperature for the time indicated in dark. Isolated polymer was washed with dichloroethane, filtered, dried and weighed. The rate of polymerization was calculated gravimetrically as a function of reaction time [7].
Results and Discussion
Polymerization of CHO monomer 1 proceeds through the opening of the epoxide ring to give soluble polymer poly (cyclohexene oxide), the product is long sequences of cyclohexane rings interlinked by oxygen atoms, Figure 3, however; the polymerization of the diepoxide monomer 2 was found to proceeds through the opening of both epoxide ring to give crosslinked polymer of poly (4-VCHD), as shown in Figure 4. Evidence for the structure of poly 4VCHD was obtained by studying the FTIR spectrum of the polymer obtained under different conditions. Typical epoxide bands characteristic of the monomer at 890, 850 and 913cm-1 are missing from the polymer spectra, and a new very powerful band at 1087 and 1157cm-1 associated with the ether linkage is present. The new band at 108 cm-1 is the strongest, and its position varies slightly with chemical structure of the polymer. The important characteristic in the polymerization reaction of 4-VCHD by rhenium carbonyls is the start of the polymerization of both of the epoxy rings at the early stages of polymerization, a crosslinked polymer was obtained at 2% conversion is an idication of reaction of both epoxides at the same time (Figure 4). Thermal polymerization of 4VCHD by dirhenium decacarbonyl and the rhenium pentacarbonyl halides is shown in (Table 2).
Re(CO)5Cl > Re(CO)5Br = Re2(CO)10 >> Re(CO)5I
Thermal polymerization of monomer 2 by the rhenium carbonyl halides proceed very slowly at 25oC, and the gel time is 72 hours; and for polymerization at 75oC the gel time fall in the following sequence: Re(CO)5I > Re2(CO)10 > Re(CO)5Br > Re(CO)5Cl (Table 2). This activity is in acrodance with the bond strength between the halide and the rhenium atom. Polymer obtained as a white powder, insoluble in all solvents.
A comparison beteen the bulk photopolymerization of CHO 1 and 4VCHD 2 under the same conditions using Re(CO)5Cl (2.50x10-3M), shows the required time for complete polymerization (100% conversion) of CHO 1 is 10 minutes and for 4VCHD 2 is 5 minutes, this indicates that the reactivity of monomer 2 almost is twice as that of monomr 1, and both epoxide rings react and opened at the same time.
Effect of initiator concentration
The effect of Re(CO)5Cl concentration on 4VCHD photopolymerization in the range (1.80 x10-3 to 3.69 x10-3M) for fxied time of 5 minutes and without solvent is shown in Figure 5, this indicates an increase in the rate of polymerization as the initiator concentration increases, polymer obtained as crystalline solid which is insoluble in aromatic and halogenated hydrocarbon solvents.
Poly4-VCHD characterization
The polymer structure was identified by FTIR spectroscopy. The FT-IR spectrum of poly (4-VCHD) prepared photochemically and thermally by Re(CO)5Cl and Re2(CO)10. Poly (4-VCHD) prepared photochemically by by Re2(CO)10 shows the OH group at 3400, aliphatic (CH2, CH) at 2960, 2850, and 1920, 1720 (CO) and 1655, 1470 ( six member ring in polymer), 1440, 1050, 1087 and 913cm-1 for the C-O-C stretching frequency [8,9]. These assignment suggest that the catalyst is attached to the poly (4-VCHD).
Proposed polymerization mechanism
The photodisproportionation of the complex (Re (CO)5Cl is shown in (Figure 6), L represent a coordinating monomer. The dimerization of the photoexited Re(CO)5X is bridged through the halogen (X), the bridges is broken by the monomer (4-VCHD), further addition of the monomer to the complex induce the initial propagation reaction. For photoinitiation of the polymerization of 4-VCHD by Re2(CO)10 compounds, we suggested the same mechanism as reported for photopolymerization of cyclohexene oxide [8], the growth of the two epoxide functional groups will leads to crosslinked polymer(Figure 6).
Conclusion
Rhenium carbonyls are effective photoinitiator for the polymerization of (4-VCHD) without cocatalyst, in absence of UV light long reaction time is required. Polymerization by Rhenium carbonyls shows that both epoxide ring were opened. The rate of polymerization depends on the structure of the rhenium carbonyl. Insoluble polymer was obtained in photo or thermal polymerization even at low conversion of monomer.
Acknowledgement
Support to this work from university of sharjah is greatfully acknowledged.
For more Open Access Journals in Juniper Publishers please click on: https://juniperpublishers.com
For more articles in Academic Journal of Polymer Science please click on:https://juniperpublishers.com/ajop/index.php
For more Open Access Journals please click on: https://juniperpublishers.com
0 notes
Text
Sirius Minerals, Britain’s first deep mine in decades, is in a hole

SINKING DEEP mine shafts is hard, says Graham Clarke, operations director at Sirius Minerals, a company digging the first properly deep mine—a mile down—in Britain for over 40 years. At a certain depth, earth movement can cause trouble, or an aquifer needs sealing. One of Sirius’s shafts has descended 120 metres beneath the North York Moors on the way to a 260m-year-old, 2.69bn-tonne fertiliser deposit. Mr Clarke is surprised to have got so far. “Everyone said we would never get planning permission, then when we did they said we wouldn’t get our first funding.”
The naysayers may yet win. Last month the FTSE-250-listed firm cancelled a $500m junk bond issue. It would have unlocked a $2.5bn loan from JPMorgan Chase, or enough in all to finish the £3.1bn ($4bn) project. Now Sirius could run out of money. Investors got spooked by market conditions, Brexit and the risks of shaft-sinking. Sirius also let it be known that the government in August refused a request to guarantee $1bn of bonds in 18 months’ time, even though state backing via export credit agencies is common in project finance.
Get our daily newsletter
Upgrade your inbox and get our Daily Dispatch and Editor's Picks.
A challenge for Sirius, as well as the shafts, is getting its product, polyhalite, widely accepted by farmers. It is a speciality potash that is chloride-free (an advantage, since chlorine tolerance varies across crops). It has more nutrients that plants need than the two mainstream products, sulphate of potash and muriate of potash. Produced at scale, polyhalite has potential to disrupt the global fertiliser industry.
That explains why existing powerful players, such as EuroChem, a Russian-owned giant with revenues of $5.6bn, have been talking down polyhalite, presumably adding to bond investors’ worries. Nevertheless, Sirius has struck multi-year sales agreements with customers to supply in total 13.8m tonnes a year at the peak. That will claim most of the mine’s initial production capacity. One is a ten-year deal announced on October 11th to supply polyhalite to Muntajat, a giant Qatari petrochemicals firm.
But Sirius’s current funding—it has raised over £1.5bn—will run out by March. It has slowed operations; eventually it would have to fill in and cap its shafts and close off the mile that it has already dug of a 23-mile-long tunnel to carry the polyhalite to Teesside to be processed and shipped off as “Poly4” to fertiliser firms around the world. Never before has a mine been joined to a long tunnel—the design avoids unsightly railway tracks over the moors.
At full capacity, Sirius’s positive impact on the local economy could be substantial. The company plans to ship £2.5bn-worth of polyhalite a year at full production and send an annual £470m to the exchequer. It employs 1,200, mostly local, workers and will need 2,800 more. In Teesside its tunnel cuts into the earth from an old ICI site near shut-down steelworks; local politicians such as Ben Houchen, Tees Valley’s mayor, are backers. So are around 85,000 retail investors, many from Yorkshire and the north-east, who piled into Sirius shares over the past eight years. Many lost out when Sirius’s bond issue was cancelled.
One reason the government declined to back the financing—in addition to its fears about the risk to the taxpayer—was that politicians reckon there are other funding options, say people close to the firm. Sirius is to come up with alternative plans by the end of the month. One option could be a strategic partner with a hefty balance-sheet. Given the push that the government has put behind boosting the north, it might not let the project fail. Even for believers in Sirius, however, the light at the end of the tunnel is getting hard to make out. ■
This article appeared in the Britain section of the print edition under the headline "In a hole"
https://ift.tt/31qSq58
0 notes
Note
Kiss prompt the poly4 babs: Accidental kisses that turn into a giggling fit



5 notes
·
View notes
Link
via MaxforLive.com New Devices
0 notes
Text
Sirius Minerals share price falls on funding deal. This is what I’d do now
Shares in Sirius Minerals (LSE: SXX) were down by 17% at the time of writing on Tuesday morning. The fall came after the company revealed details of its long-awaited $3.8bn financing deal.
The good news is that this funding should see the mine through to completion, barring further cost overruns. But this cash will come at a fairly high cost, much of which is still unknown.
In this article I’ll explain what today’s news means, and what I’d do about Sirius Minerals shares.
Shareholders face dilution
In March, Sirius abandoned funding talks with its planned lenders to discuss a possible financing deal with US bank JPMorgan. This deal will now go ahead.
The first stage is a $400m (£310m) share placing. This will be launched immediately. The new shares are expected to be issued at 15p-18p. My sums suggest this will result in about 1.9bn new shares being issued, adding about 40% to the group’s current share count.
This means that future earnings will have to be spread among a larger number of shares, meaning that earnings per share will be lower. This is known as dilution.
And even more dilution
Unfortunately, today’s placing may just be the start of the dilution. Sirius is also going to issue $644m of new convertible bonds, due in 2027. This will raise $400m of new cash and allow the group to refinance $244m of existing bonds.
Convertible bonds can be converted into shares, hence the name. The conversion price is expected to be somewhere between 18p and 22.5p. So if the Sirius share price heads upwards in future years, bondholders may choose to convert their bonds into shares.
If all the bonds are converted, I estimate that about 2.4bn new shares might be issued, adding a further 35% to Sirius’s share count.
Overall, my sums suggest that the placing and convertible bonds could reduce the value of each existing Sirius share by nearly 50%.
What comes next?
The share placing and convertible bonds should provide $800m of new cash. The next $500m is expected to come from some standard (non-convertible) bonds issued later this year. The interest rate and repayment terms for these bonds have not yet been agreed.
The final $2.5bn needed will initially come from a giant overdraft provided by JPMorgan. As the project develops, the plan is for this short-term financing to be converted into further bond issues.
Good news vs bad news
The good news is that Sirius Minerals has secured the funding it needs to complete the build of the Woodsmith mine in North Yorkshire. Unless costs rise again, this funding should see the firm through until production hits 10m tonnes per annum (mtpa). This is expected in mid-2024.
Another piece of good news is that prospective customers still seem interested. The company now has supply agreements for 10.7mtpa of POLY4 fertiliser, up from 8.2mtpa at the end of 2018.
The bad news is that shareholders will face significant dilution.
Another concern is that most of the firm’s borrowing costs are still unknown. This is a risk for equity investors, as debt costs are likely to be a significant drain on cash in the early years of production.
My view: At under 20p, I think Sirius is a speculative long-term buy. But it’s not without risk and certainly isn’t a short-term trade, in my opinion.
You Really Could Make A Million
Of course, picking the right shares and the strategy to be successful in the stock market isn't easy. But you can get ahead of the herd by reading the Motley Fool's FREE guide, "10 Steps To Making A Million In The Market".
The Motley Fool's experts show how a seven-figure-sum stock portfolio is within the reach of many ordinary investors in this straightforward step-by-step guide. Simply click here for your free copy.
More reading
Investors are taking a gamble on Sirius Minerals and the UKOG share price
Why I believe the Sirius Minerals share price could soon return to 40p
The Sirius Minerals share price is rising: is it time to buy?
The Sirius Minerals share price: What’s next?
Forget the National Lottery. I’m having a punt on the Sirius Minerals share price
Roland Head has no position in any of the shares mentioned. The Motley Fool UK has no position in any of the shares mentioned. Views expressed on the companies mentioned in this article are those of the writer and therefore may differ from the official recommendations we make in our subscription services such as Share Advisor, Hidden Winners and Pro. Here at The Motley Fool we believe that considering a diverse range of insights makes us better investors.
0 notes
Text
McLaughlin & Harvey wins Teesside potash port facility
Yorkshire potash mine developer Sirius Mineral has selected contractor Mclaughlin & Harvey to deliver a major port materials handling facility at Teesside.
Construction of the port and a special handling and storage facility at Wilton will see more than £400m invested in Teesside and will regenerate the former Redcar steelworks port frontage.
Chris Fraser, Managing Director and CEO of Sirius, said: “We are pleased to have entered into another significant construction contract for the ongoing construction of our world-leading polyhalite project.
“In 18 months we have made great progress and are now nearing the completion of the procurement programme to support our stage 2 financing process.
“The construction of our port infrastructure is another example of the level of investment and skilled job creation we are delivering in both Teesside and the UK as we develop a project that has the potential to make the UK a world leader in the fertilizer industry.”
The port handling facility will receive polyhalite fertilizer after it has been transported from the Woodsmith mine site through the 37km underground mineral transport system and processed into the finished product, POLY4, at the Wilton Materials Handling Facility.
At peak production the Company expects to export 20mtonnes per annum of POLY4 to markets all over the world.
Ben Houchen, Mayor of the Tees Valley, said: The successful development of the Sirius project will see over £400m invested in Teesside and will importantly see the regeneration of the former Redcar steelworks port frontage.
“We expect many more regeneration projects will be announced on the South Tees Development Corporation site very soon.”
0 notes