#Aqueous Sodium Carbonate processing
Explore tagged Tumblr posts
Text
Green Semi-Gloss HD CAWN 2321: High-Performance Liquid Photoimageable Solder Resist
Discover Green Semi-Gloss HD CAWN 2321, a thermal hardening liquid solder resist designed for superior PCB protection. RoHS and WEEE compliant, UL listed, and REACH-certified, it meets IPC standards and is trusted in industries including automotive and aerospace. Explore its advanced capabilities today.
#Green semi-gloss solder resist#liquid photoimageable solder resist#CAWN 2321#thermal hardening solder mask#IPC standards solder resist#advanced PCB protection#solder resist for automotive#aerospace solder resist solutions#Aqueous Potassium Carbonate processing#Aqueous Sodium Carbonate processing#solvent-processable solder resist#PCB solder mask solutions#A-Gas Electronic Materials
0 notes
Text
Soda ash dense,
Soda ash dense,
Soda Ash Dense, also known as sodium carbonate (Na₂CO₃), is a white, odorless, and highly soluble inorganic compound widely used across various industries. It is primarily derived from natural sources like trona ore or manufactured synthetically through the Solvay process. The dense form of soda ash is characterized by its high bulk density and low dusting properties, making it preferable for industrial applications requiring superior flow properties and stability.
Physical and Chemical Properties
Chemical Formula: Na₂CO₃
Molecular Weight: 105.99 g/mol
Appearance: White granular powder
Solubility: Highly soluble in water
Density: Higher than light soda ash, making it more suitable for industrial use
pH: Alkaline in nature, typically around 11.6 in aqueous solutions
Key Uses of Soda Ash Dense
Glass Manufacturing:
The primary use of soda ash dense is in glass production, where it acts as a fluxing agent, reducing the melting temperature of silica and enhancing the durability of glass products such as flat glass, containers, and fiberglass.
Detergents and Soaps:
Soda ash dense is a key ingredient in the manufacturing of detergents and soaps. It acts as a water softener, improving cleaning efficiency by reducing the hardness of water.
Chemical Industry:
It is an essential raw material for producing various chemicals, including sodium silicates, sodium bicarbonate, and percarbonates, which are widely used in industrial applications.
Water Treatment:
Used in water purification processes to regulate pH levels and remove acidity, ensuring safe and efficient water treatment for municipal and industrial applications.
Metallurgy:
In non-ferrous metallurgy, soda ash dense is used for ore processing, refining aluminum, and removing phosphates from metal surfaces.
Pulp and Paper Industry:
Acts as a pH regulator and helps in the pulping process by breaking down wood fibers efficiently.
Benefits of Soda Ash Dense
Highly Efficient: Due to its dense nature, it requires lower volumes compared to light soda ash, making it more cost-effective in bulk industrial applications.
Reduced Dusting: Ensures cleaner handling and minimizes product loss during transportation and processing.
Versatility: Its wide range of applications makes it indispensable across multiple industries.
Eco-Friendly: It is a non-toxic, non-hazardous chemical that can be used safely in regulated quantities.
Conclusion
Soda Ash Dense plays a crucial role in various industrial sectors, from glassmaking to water treatment and detergent production. Its superior density, efficiency, and chemical properties make it a preferred choice for manufacturers seeking high-quality and reliable raw materials. With growing industrial demands, soda ash dense remains an essential compound for numerous applications worldwide.
0 notes
Text
Quality Control in Pharmaceutical Industry | QC in Pharma Company

The Quality Control department's major and important role is in the Pharmaceutical industry.
The main role of the quality control department in the pharma industry is to check the quality of various products, such as raw materials, in-process samples, and finished products.
Their main agenda is to analyse and control the quality of the products at all stages of the manufacturing of API or Formulations.
QC is done by the Qualitative and Quantitative analysis of specific materials as per Stanard Testing Procedures (STP) or Method of Analysis.
Generally, the QC department is divided into four sections. These are main
Raw Materials
In-process Quality Checks (IPQC)
Finished Products
Stability Studies
Raw materials:
The materials come from outside industries or suppliers and road tankers check the quality of the materials as per in-house specifications or Standard testing procedures.
These are categorised into four parts.
General Raw materials:
These are some chemical analyses, like titrimetry, and chemical analysis methods, such as organic and inorganic acids, bases, salts, etc.
Ex: Hydrochloric acid(HCl), Sulphuric acid(H2SO4), Nitric acid(HNO3), Caustic soda(NaOH), Sodium carbonate(Na2CO3), Methanol, Toluene, Acetone, Dichloromethane etc…
Key Starting Materials (KSM):
These are the building blocks of drug intermediates or used to form the structure of compounds, APIs, or Drug substances.
The sampling method is different from general raw materials.
These are analyzed with both chemical and instrumental analysis.
Ex: Speciality Fine Chemicals, Drug Intermediates etc.
Packing Materials:
PM is used for Products/Compound materials that are stored
Ex: Fibre drums, HDPE, LDPE drums, Polyethene bags, etc…
Hazardous Materials:
HM are harmful or affect body raw materials to handling in careful safety precautions and as per its Material Safety Data Sheets (MSDS) so vendors or suppliers give a certificate of analysis based on these are approved as per customer COAs.
Ex: Sodium Hydride(NaH), Sodium Amide(NaNH2), NaCN etc…
Some catalysts are also approved as per customer COAs
Ex: Raney-Nikel, Palladium/Carbon(Pd/C) used for Hydrogenation reaction.
In-Process samples:
Online chemical and instrumental methods analysis as per in-house specification & STPs carried out samples coming from the manufacturing blocks or production department to time to give results after the process continuously.
Finished Products:
Complete Analysis carried out as per customer or In-house or Pharmacopia specification and Standard Testing Procedures of the final products.
The analysis carried out in the Quality control department is divided into two parts. These are
Chemical Analysis Laboratory (Wet Lab)
Volumetric analysis:
Chemical labs have five types of titrimetric analysis
Acid-Base Titration Ex: Hydrochloric acid (HCl), Sodium Hydroxide (NaOH)
Argentometric titration Ex: Sodium Chloride(NaCl), Aluminum chloride (AlCl3)
Redox Titration Ex: Sodium thiosulphate, Potassium permanganate
Complexometric titration Ex: Calcium chloride (Cacl2), Magnesium (Mg) and Metals
Non-aqueous titration for Drug intermediates and APIs Ex: 2-Amino Pyridine, Isonipotic acid etc..
Gravimetric analysis:
Gravimetric analysis is the mass of an ion in a compound and is determined to find out the mass per cent of the same ion in a known quantity of a compound.
Examples 1) The amount of sulphate as barium sulphate(BaSO4) from sodium sulphate(NaSO4).
2) Content of Nickel in Raney-Nickle catalyst and Palladium in Pd/C catalyst.
Wet laboratory, some important chemical analyses are
Ex: Water content(WC), Loss on drying(LOD), Residue on ignition(ROI), Specific Optical Rotation(SOR), Wt per mL, Thin Layer Chromatography(TLC), Tapped density, Friebilty, Dissolution, Disintegration etc.
Water Analysis:
Softener water: This water is used for boiler purposes to generate steam.
Demineralized or Deionised water: This water is used for chemical analysis and process areas.
Purified water: This water is used for the manufacturing process.
Three samples are collected to be analysed to their specification (WHO) and Standard testing procedures as per scheduled.
Instrumental methods of Chemical analysis
1) Chromatography:
Instrumental analysis to analyse quantitative and qualitative investigates analytes using the help of scientific instruments.
There are main two instrumental analyses carried out for Quality Control in the Pharmaceutical industry.
This technique separates and identifies the mixture of the compounds based on their relative affinity amounts of each compound distributed between a moving mobile phase, and a stationary phase. Mostly used instruments of Quality Control in the Pharmaceutical industry
1) High-Performance Liquid Chromatography (HPLC) 2) Gas Chromatography (GC)
2) Spectrophotometry:
Spectroscopic techniques are to pass a beam of electromagnetic radiation onto an unknown sample and observe to find out the difference between energy levels with reference.
Most commonly used spectrophotometers of Quality Control in the Pharmaceutical industry. There are
1) Ultra-Violet Spectrophotometer (UVS) 2) Fourier-transform infrared spectrometer (FTIR) and NIR 3) Atomic Absorption Spectrometer (AAS) and FAS
These are the main used Research Centres for Structure elucidations and Analytical Method Development.
1) Nuclear Magnetic Resonance Spectrometer (NMR)
2) Mass Spectrometer (MS)
3) Thermo Gravimetric Analysis (TGA)
4) Differential Thermal Analysis (DTA)
Stability Studies:
Stability studies are conducted for a re-test or expiry or a shelf life period for the drug substance or the drug product and recommended storage conditions.
These are analysed as per protocol or stability STP based on the schedule.
1) Hold-time stability studies 2) Long-term, Accelerated, intermediate condition studies
The quality control department follows systematic proper online documentation, Logbooks, Registers, Good Laboratory Practices (GLP) and Good Documentation Practices.
After complete analysis, documented respective analysis signed and checked authorised persons to prepare the certificate of analysis approved by the Head of the department or Designee.
Backup Electronic Data:
All electronic data stored in their servers or external hard disks are Empower network or Lab solution or Open Lab software and its data is backed up and retrieved every week by an IT person.
Conclusion:
The Quality Control department checks each step of the product manufacturing as per specification and standard testing procedures after releasing documented data.
#QC#STP#HPLC#GC#SOR#Analyst#GLP#21CFR11#NABL#LIMS#empower#Labsolution#Pharmacopias#EDQM#TGA#CDSCO#USFDA#ICH#Shimsdzu#Waters#Agilent#Thermoscientific#Remi#Labindia#Perkinelmler#WHO#AR&D#SOP
0 notes
Text
What is the Difference Between PC and PVC in Thermoforming?
Thermoforming is a versatile manufacturing process allowing manufacturers to mold thin and heavy gauge materials.The main principle is to heat and soften the flat hard plastic sheet, then use vacuum adsorption on the surface of the mold, and then form it after cooling. It is widely used in plastic packaging, lighting, advertising, decoration and other industries. Generally divided into heavy gauge thermoforming and thin gauge thermoforming.
Thermoformed products are processed with thermoplastic materials. The product production principle is to heat and soften the flat plastic hard sheet material, then adsorb it to the surface of the mold with vacuum, and then cool it into shape.
Thermoformed products are widely used in the electronics, electrical appliances, and food industries. , hardware tools, cosmetics industry, toy industry, daily necessities industry, medicine, health care products, automobiles, stationery, cultural and sports supplies and other categories of industries.
Plastic thermoforming materials usually use thermoplastic plastics, and common ones include polystyrene (PS), polypropylene (PP), polyethylene (PE), polycarbonate (PC), polyvinyl chloride (PVC), thermoformed acrylic(PMMA), polyethylene terephthalate(PET), polyethylene terephthalate glycol(PET-G), high molecular weight polyethylene etc. Different plastic materials have different properties and application areas.
This article will delve into the complexity and artistry of vacuum forming, pressure forming, focusing on a comparison of two common thermoplastic sheets, PC (polycarbonate) and PVC (polyvinyl chloride).
The Difference between PC and PVC Material
Raw Material
The components of PVC material are mostly polyvinyl chloride, which is a general-purpose plastic.
The components of PC material are mostly carbonate-based, which are engineering plastics.
Property
PC material has the advantages of high strength, good transparency, and good impact resistance; relatively speaking.
PVC material is softer, has average transparency, and has inferior mechanical properties to PC material.
Prices
PVC materials are cheaper, typically only half to a quarter of the price of PC materials.
Application
PC can be used as door and window glass, and PC laminates are widely used in protective windows in banks, embassies, detention centers and public places, as well as in aircraft cabin covers, lighting equipment, industrial safety baffles and bulletproof glass.
PVC materials are mostly used in the construction industry, packaging and other industries. For example, PVC materials can be used to make PVC pipes. PVC pipes have the advantages of easy construction, good corrosion resistance, low fluid resistance, good compressive strength, and long service life. We also It can be subdivided into different types such as drainage pipes, wiring pipes, medical pipes, etc. according to their uses.
Transparency
PC has high transparency, similar to glass, and can be used to make transparent plastic parts such as eyeglass frames and bottles.
In contrast, PVC has poor transparency and is usually translucent or milky white. Many plasticizers can be added to PVC, and after adding them, soft PVC can be added.
Chemical Stability
PVC has stable chemical resistant properties and is highly resistant to oxidants, reducing agents and strong acids. However, it can be corroded by concentrated oxidizing acids such as concentrated sulfuric acid and concentrated nitric acid.
It is also soluble in ethers, ketones, chlorinated aliphatic hydrocarbons and aromatic hydrocarbons. Organic solvents
PC has certain chemical corrosion resistance, resistance to weak acids and neutral oils, and is not resistant to strong acids and alkali. Dilute sodium hydroxide aqueous solution can slowly destroy it, and ammonia, amine or its 10% aqueous solution can cause it to rapidly saponify and degrade.
Mechanical Properties
PC has high mechanical strength, the highest impact strength among plastics, and can even be used as bulletproof material. This clear material forms well and is impact resistant. Its bending and tensile strength is equivalent to that of nylon, and it has high elongation and elastic modulus, but its fatigue resistance is low (less than nylon 66 ), lower compressive strength, better wear resistance (better than ABS), and small creep.
PVC has high mechanical strength, toughness and impact resistance. Its wear resistance at room temperature exceeds vulcanized rubber, and its hardness and rigidity are better than polyethylene.
Heat Resistance
PC Good heat resistance and cold resistance, wide temperature range, can be used for a long time at temperatures of -100℃-140℃, and still has certain toughness at -180℃.
PVC: Thermal stability and light resistance are poor. When it is above 100℃ or exposed to sunlight for a long time, it will decompose to produce hydrogen chloride, which will further auto-catalytically decompose and change color, resulting in a rapid decline in physical and mechanical properties and heat deflection . The use temperature is low (below 60℃)
Characteristics of PC Sheet Thermoforming
It is an amorphous plastic, and the temperatures that need to be controlled are different at different stages.
2) The thermal stability of PC sheet blister is relatively good and can be improved as the relative molecular weight increases.
3) PC sheet blister has good resistance to degeneration and good dimensional stability; but its internal stress is not easy to eliminate.
4) PC sheet blister is easily degraded when exposed to water at high temperatures, and the moisture content is required to be below 0.02% during molding.
5) Cracking may easily occur if care is not taken.
6) During the production process, the apparent viscosity of PC sheets is greatly affected by temperature and less affected by shear rate, and increases with the increase of relative average molecular weight.
7) PC sheet blister has no obvious melting point, the melt viscosity is high, and there are benzene rings in the PC molecular chain, so it is relatively rigid.
Special Processing Method for PC Sheet Thermoforming
Since the blister industry is a relatively new industry, there is currently no textbook-style process solution. We can only summarize relatively suitable processing methods through continuous exploration. In view of the process difficulties of PC material blister, including cracking due to stress, insufficient heating temperature and vacuum strength, and deformation caused by poor cooling, the industry has successively explored some special processing methods.
1) Sheet drying
In view of the fact that PC easily absorbs water, it is generally best to dry PC sheets before heating to absorb the internal moisture.
2) Control mold temperature
We must ensure that the anti-cracking ability and internal stress are balanced so that the cracking phenomenon of PC endurance boards will not occur.
3) Pay attention to molding and cooling time
It is necessary to strictly control the heating forming time not to be too long and ensure sufficient cooling time to prevent the sheet from deforming and curling.
4) Selection of blister machine
For the complex PC blister process, choosing a good blister machine is the first priority.
Characteristics of PVC Thermoforming
PVC sheet has high toughness and is not easy to burn. It will produce chlorine gas when burned, which will have a certain impact on the environment.
PVC is easy to heat seal and can be sealed with a sealing machine and high-frequency edge sealing. It is the main raw material for the production of transparent blister products. PVC sheet is a widely used and popular material.
PVC film can be divided into two types: environmentally friendly and non-environmental. It can be made into various blister packaging products such as transparent, colorful, anti-static, gold-plated, and flocked.
The main features are high transparency, good surface gloss, few crystal points, small water marks, wide use, strong impact resistance, and easy to form.
The products are widely used in toys, food, electronic products, medicine, electrical appliances, gifts, cosmetics, stationery, etc. Product outer packaging.
Conclusion
In conclusion, PC sheets and PVC sheets each have their own advantages and their disadvantages, and which one is better mainly depends on the specific use occasions and customerneeds. If you are more care about plastic part's performance and quality (such as temperature resistance, toughness, transparency), you may want to choose PC sheets. If you pay more attention to no pollution or low-cost needs during production process, then PVC may be a better choice.
0 notes
Text
Sodium Cyanide Market Trends: Insights and Forecasts
Introduction Sodium cyanide is an odorless chemical compound with the formula NaCN. It is a white, water-soluble solid. NaCN has a wide range of industrial and other applications, but it is also notoriously toxic and has sometimes been used for suicide or murder. Let's explore some key facts about this dangerous chemical compound. Chemical Properties and Structure Sodium cyanide has the chemical formula NaCN and a molar mass of 49.01 g/mol. It dissociates in water to give hydroxide (NaOH) and cyanide (CN-) ions. The cyanide ion is linear with carbon and nitrogen separated by a triple bond. It is this CN- ion that is primarily responsible for NaCN 's high toxicity. NaCN is a white solid that melts at 563°C to give a colorless liquid. It is highly soluble in water. Toxicity and Mode of Action Cyanide is an inhibitor of cytochrome c oxidase, an important enzyme in the mitochondrial electron transport chain. By blocking this enzyme, cyanide essentially prevents aerobic respiration from taking place at the cellular level. This leads to a rapid depletion of oxygen to tissues and ultimately causes death due to hypoxia at the tissue and organ level. The lethal dose of NaCN for adult humans is reported to be 200-300 mg. However, as little as 1-5 grams can prove fatal. The primary symptoms of cyanide poisoning include headaches, dizziness, confusion, convulsions and cardiac arrest. Death by cyanide poisoning usually occurs within minutes to an hour. There is no antidote for cyanide poisoning. Treatment focuses on supportive measures in a hospital environment along with use of antidotes like sodium thiosulfate or dicobalt edetate to combat the effects of cyanide. Industrial Uses Despite its obvious toxicity, NaCN has many beneficial industrial applications primarily due to its ability to dissolve minerals containing precious metals like gold and silver. It is widely used for extraction of these metals via cyanidation process in mining operations. In this process, an aqueous solution of NaCN is used to leach gold from minerals into the water to facilitate separation and recovery of gold. NaCN is also used in some cleaning and metal surface treatment applications. Other uses include: Production of nylon - Sodium cyanide serves as an intermediate for adiponitrile, which is further used to make nylon 6,6. Case hardening - It is used in metallurgy for case hardening of steels to increase wear resistance and hardness. Alkylation - In organic chemistry, NaCN acts as an alkylating agent in production of compounds like acrylonitrile. Accidental and Intentional Poisonings There have been many accidental and intentional deaths reported due to sodium cyanide poisoning over the years. Accidental cases may occur due to occupational exposure in industries using cyanide or due to consumption of cyanide-containing products mistaken as food. Intentional poisonings with cyanide have been reported in cases of suicide or murder. Some high-profile murder cases have involved the use of NaCN by the perpetrator. Given the acute toxicity of cyanide ions, even small amounts ingested intentionally can prove rapidly lethal. Proper safety precautions are a must for any activities involving this deadly chemical salt. Regulation and Safe Handling Considering the high human toxicity of NaCN, it is designated as a schedule 2 substance under the Chemical Weapons Convention. Many countries have strict regulations governing its manufacture, transportation, storage and industrial usage. Workers directly handling NaCN must be properly trained in safety procedures like wearing recommended personal protective equipment. Leakage of cyanide solutions into the environment must also be prevented to avoid toxicity to other organisms. Overall, given the risks involved, sodium cyanide requires controlled and regulated use with utmost precautions taken at all stages to prevent accidental poisonings.
0 notes
Text
Tips for Dyeing that Enhance Textile Quality with Direct Dyes

Direct dyes are celebrated for their vibrant colors and easy application, making them a favorite among textile manufacturers. However, the mastery of using these dyes effectively lies in understanding their properties and integrating best practices that ensure quality and sustainability.
Understanding Direct Dyes
Direct dyes are a class of dyestuffs that are applied directly to the fabric in an aqueous solution, usually with the aid of a substance that enhances the fabric’s affinity for the dye. Predominantly used for cellulosic fibers like cotton, these dyes are valued for their straightforward application process and the vivid colors they produce. Their molecular structures contain substantivity that allows for direct bonding without the need for a mordant, differentiating them from other dye classes, such as vat or reactive dyes. This characteristic makes direct dyes particularly useful for projects requiring consistent, even coverage and a high degree of colorfastness under light exposure.
Pre-Treatment Essentials
Cleaning and Preparing the Fabric: The initial step in the dyeing process involves thoroughly cleaning the fabric. This is crucial as it removes impurities and any residues that might impede the dye uptake. Techniques such as scouring and bleaching are commonly employed to ensure the fabric is pristine, which enhances the uniformity and vibrancy of the dye application.
Applying a Mordant: While direct dyes generally do not require a mordant to bond to the fabric, in some cases, a mild mordant might be used to improve the color’s durability and fastness. This step is particularly useful when dealing with fabrics that will be subjected to rigorous washing conditions or prolonged exposure to environmental elements.
Optimizing Dyeing Conditions
Controlling the Temperature: The temperature at which the dyeing process is conducted plays a pivotal role in the outcome. Most direct dyes require a higher temperature range, typically between 60°C to 100°C. Maintaining this temperature range ensures that the dye molecules are sufficiently energized to bond effectively with the fabric, promoting even distribution and adherence.
Adjusting the pH: The acidity or alkalinity of the dye bath can significantly affect the binding of direct dyes. A slightly alkaline pH is often favored, as it enhances the vibrancy of the dye and aids in the longevity of the color. Adjustments can be made using common agents such as sodium carbonate, which gently shifts the pH without damaging the fabric.
Dye Bath Management: Consistency in the dye bath, in terms of chemical composition and time management, ensures that the dye uptake is uniform across the entire fabric. This includes stirring mechanisms or the use of leveling agents that aid in the even distribution of dye, preventing patchiness and ensuring that every fiber is evenly colored.
After-Treatment for Color Fastness
Soaping: This process involves washing the dyed fabric with a mild soap solution. The primary purpose is to remove any unreacted dye particles that remain on the surface of the fabric. This not only prevents colour bleeding during subsequent washes but also enhances the overall fastness properties of the dye. Soaping is typically done at elevated temperatures to ensure the thorough removal of these excess dyes.
Fixing the Dye: After soaping, a fixing agent is often applied. These agents work by forming a chemical bond between the dye molecules and the fabric fibers, which significantly improves the wash and rub fastness of the dye. Common fixing agents include certain types of polyvalent metal salts, which are chosen based on their compatibility with the fabric and the specific direct dyes used.
Final Rinsing and Drying: The last step in the after-treatment process is a thorough rinsing to eliminate any residual chemicals, followed by drying. Proper drying techniques, whether natural or controlled thermal drying, are crucial as they affect the texture and final appearance of the fabric.
Comparing Direct Dyes vs Reactive Dyes
Color fastness: One of the primary differences between direct dyes and reactive dyes lies in their color fastness. Reactive dyes form covalent bonds with the fabric fibers, making them much more resistant to color fading from washing and sunlight. In contrast, while direct dyes are easier to apply, they tend to have lesser wash fastness, which can be mitigated to some extent through meticulous after-treatment.
Application Techniques: Direct dyes are applied in a neutral or slightly alkaline dye bath and require thorough washing post-application to remove unbound dye. On the other hand, reactive dyes require a slightly more complex process involving an alkaline solution for fixation and acidic conditions for dyeing, which can add to the complexity and cost of the dyeing process.
Environmental Impact: Typically, reactive dyes require more water and produce more waste compared to direct dyes. However, advancements in dye technology and increased awareness of sustainable practices are leading to the development of more eco-friendly variants of both types of dyes, aimed at reducing their environmental footprint.
By understanding the specific characteristics and application methods of direct dyes compared to reactive dyes, manufacturers can make informed decisions that align with their product requirements, sustainability goals, and market demands. Choosing the right dye not only affects the quality and appeal of the final textile product but also impacts the environmental footprint of its production process.
About Meghmani Global
At Meghmani Global, we have honed our expertise in the art and science of dyeing since 1977. Starting with a single product, our portfolio now spans a wide array of dyes, pigments, and chemical products, meeting the needs of various industries globally. Our commitment to quality and sustainability is embedded in our innovative approach, which aims to reduce environmental impact while delivering exceptional value to our customers.
Source URL:
0 notes
Text
Lithium Hydroxide’s Manufacturing Process & Tomorrow's Innovations

The Lithium Hydroxide might sound complicated, but it is in reality an amazing and valuable compound. This white, sometimes sparkling solid, which is an essential element in various industries, surprisingly has some unique applications.
Lithium Hydroxide is a widely used chemical compound, and in this article, we will take a look at its various aspects. We'll be looking into its properties, how it is made (which involves some interesting chemistry, too!), and some of the amazing things it is used for. Lithium Hydroxide, which is used in both air freshening systems on spaceships and in special greases, has a range of unexpected uses. Therefore, please fasten your seatbelt and let us dive into the discussion of this essential chemical!
Introduction
Lithium Hydroxide (LiOH) is a white, solid compound that comes in two forms (hydrated and anhydrous) and is made by reacting lithium carbonate with calcium hydroxide. It is used to create lithium greases, which are valuable lubricants due to their resistance to heat, water, and pressure. In batteries, Lithium Hydroxide is gaining traction as a replacement for lithium carbonate because it allows for bigger batteries with more power, better safety, and longer life. It's already used by Tesla and is likely to be adopted by other electric vehicle makers. Lithium Hydroxide also finds use in alkaline batteries and air scrubbers.
Manufacturing Process
This blog unveils a process for manufacturing Lithium Hydroxide, involving several steps. Initially, lithium sulfate reacts with barium hydroxide in a liquid medium, yielding a Lithium Hydroxide solution through hydroxylation. Subsequently, barium ions in the Lithium Hydroxide solution are eliminated via a barium removal process utilizing a cation exchange resin and/or a chelate resin. Finally, Lithium Hydroxide is precipitated from the treated solution in a crystallization step.
Step-1 Lithium Concentration
The extraction process involves bringing the lithium dissolved solution (aqueous phase) into contact with a solvent (organic phase) and mixing them with agitation using a mixer to transfer lithium ions and similar substances from the dissolved solution to the solvent. Following this, the organic phase and the aqueous phase are separated using a settler based on their differing specific gravities. Depending on factors such as lithium ion concentration, an O/A ratio (organic phase to aqueous phase volume ratio) exceeding 1.5/1.0 may be utilized. To enhance the extraction efficiency of lithium ions, the O/A ratio can be adjusted, and the number of extraction stages can be increased. For the lithium dissolved solution, a phosphonate ester extracting agent, a phosphate ester extracting agent, or a combination of both may be employed as extracting agents. Optionally, the extracting agent could be thinned with a hydrocarbon-based organic solvent, including aromatic, paraffinic, or naphthenic solvents. Ideally, the equilibrium pH range during extraction falls between 7 and 8.
Scrubbing the solvent with the lithium solution effectively eliminates sodium ions that have been extracted into the solvent. By modifying the lithium ion concentration within the lithium solution, lithium ions within the solution can replace the sodium ions present in the solvent, thereby facilitating the efficient removal of sodium ions from the solvent.
The lithium ions present in the scrubbed solvent are subsequently extracted from the solvent through a back-extraction process. This involves stirring and mixing the solvent with a pre-back extraction liquid, typically an acidic aqueous solution, using a mixer or similar equipment. This facilitates the transfer of lithium ions from the solvent to the aqueous phase. The pre-back extraction solution utilized for this process may comprise various inorganic acids such as sulfuric acid, hydrochloric acid, or nitric acid. Among these options, sulfuric acid is favored because it yields a back-extracted liquid containing lithium sulfate, a valuable raw material for Lithium Hydroxide production.
The back-extracted liquid obtained from the back extraction step can undergo additional back extraction cycles, serving as a pre-back extraction liquid. This process further elevates the lithium ion concentration. Moreover, the back-extracted liquid can also be employed in the scrubbing step as a lithium solution. This cyclical approach optimizes the extraction process, enhancing lithium ion concentration and maximizing the utilization of resources.
Step 2 - Hydroxylation Step
In the hydroxylation step, lithium sulfate, obtained from the back-extraction process or similar sources, undergoes a reaction with barium hydroxide in a liquid medium to yield a Lithium Hydroxide solution. This chemical transformation can be represented as follows:
Li2SO4 + Ba(OH)2 → 2LiOH + BaSO4
Consequently, a solution containing dissolved Lithium Hydroxide is generated, while barium sulfate precipitates. The utilization of barium hydroxide proves effective as it facilitates the chemical conversion reaction with lithium sulfate, enabling the production of a Lithium Hydroxide solution.
Step 4 - Barium Removal Step
In the barium removal step following hydroxylation, the Lithium Hydroxide solution undergoes contact with either a cation exchange resin or a chelate resin to eliminate impurities, particularly barium ions. The resin adsorbs these ions from the solution, enhancing the purity of Lithium Hydroxide obtained after subsequent crystallization.
It's crucial to meticulously select the resin and operating conditions during barium removal to ensure efficient extraction of barium ions from the Lithium Hydroxide solution. Resins like weakly acidic cation exchange resins with carboxyl groups or aminophosphoric acid type chelate resins exhibit high selectivity for barium ions while minimizing adsorption of lithium ions.
Moreover, maintaining an alkaline pH (preferably 9 or higher) in the Lithium Hydroxide solution during resin contact optimizes the barium removal efficiency.
Step 5 - Crystallization Step
The crystallization step follows the barium removal process to precipitate Lithium Hydroxide from the solution, yielding pure Lithium Hydroxide. This ensures the removal of impurities, particularly barium ions, resulting in high-purity Lithium Hydroxide suitable for various applications.
Crystallization techniques such as heat concentration or vacuum distillation are employed to precipitate Lithium Hydroxide. Higher temperatures during crystallization expedite the process; however, subsequent drying at temperatures below 60°C prevents the release of water of crystallization, maintaining the product as hydrated Lithium Hydroxide, which is easier to handle. Additional treatments like pulverization may be performed to adjust the physical properties of Lithium Hydroxide as needed.

Applications of Lithium Hydroxide
1. Batteries
Lithium Hydroxide (LiOH) found in batteries, specifically lithium-ion batteries, is a significant contributor to the high electrochemical potential and lightweight characteristic of lithium, which in turn leads to high energy density. Its inherent property of staying stable at the high temperatures in the charging cycles guarantees the batteries' safety. Lithium-ion batteries with LiOH have low discharge rates, and they hold charges over a long time. They are rechargeable, thanks to the lithium ions that are moving back and forth between electrodes that are facilitated by LiOH. This mixture thus leads to lightweight, power-efficient energy storages that are suitable for use in electric vehicles, portable gadgets, and other devices which require high-performance long-lasting power sources.
2. Grease and Lubricants
Lithium Hydroxide finds application in lubricating greases, commonly referred to as lithium grease, enhancing their resistance to water and oxidation. These greases maintain their lubricating efficacy across a broad temperature spectrum, enabling them to endure high-pressure conditions. Their insolubility ensures longevity, making them suitable for humid environments where water exposure is frequent without losing their lubricating properties.
3. Glass & Ceramics
Multiple advantages of Lithium Hydroxide make it applicable in the glass and ceramics industries. Besides the fact that it allows for more accurate dimensional tolerances, it also helps to reduce thermal expansion, which in turn prevents cracks and fractures during production and use. Furthermore, it provides clarity that makes the glass transparent by getting rid of any imperfections that may cause cloudiness. Through the reduction of the melting point of the glass mixtures, Lithium Hydroxide helps energy-efficient manufacturing processes and gives molten glass a better flow. In ceramics, it enhances properties like the strength and thermal shock resistance when it functions as a flux or a filler. Additionally, lithium compounds are capable of coloring glass and ceramics that give them particular colors, which makes them more attractive and appealing.
Market Outlook
The Lithium Hydroxide market is driven by the increasing demand for electric vehicles and consumer electronics. Lithium Hydroxide is a critical component in lithium-ion batteries, which are used in these electric vehicles and consumer electronics. Because of this, the demand for lithium-ion batteries is a major driver of the Lithium Hydroxide market. Additionally, the dominance of lithium-ion batteries in the Lithium Hydroxide market is expected to continue due to ongoing research and development efforts to improve battery performance.
Lithium Hydroxide Major Global Producers
Top companies in the Global Lithium Hydroxide market are Albemarle Corporation, Sociedad Química y Minera de Chile (SQM), Tianqi Lithium Corporation, Ganfeng Lithium Co., Ltd., Livent Corporation, Jiangxi Ganfeng Lithium Co., Ltd., FMC Corporation, Galaxy Resources Limited, Nemaska Lithium Inc., Altura Mining Limited, and Others.
Lithium Hydroxide Market Opportunities
The Lithium Hydroxide market, which is used as the basic material in batteries, ceramics, and other industrial applications, has been growing at a fast rate over the years. Here are some potential opportunities within this market::
Electric Vehicles (EVs) and Energy Storage: The growing demand for electric vehicles and lithium-ion batteries for renewable energy storage necessitates Lithium Hydroxide which has been identified as a crucial component in lithium-ion batteries. Governments across the globe have started enforcing stringent measures on carbon emission and providing incentives for the use of electric vehicles. As a result, the demand for Lithium Hydroxide is highly likely to undergo a sharp increase.
Energy Sector: The transformation of energy sources to include wind turbines and solar panels as the new sources of energy requires energy storage systems that are efficient to avoid intermittent issues. The lithium-ion battery technology, based on Lithium Hydroxide, is the most popular in energy storage solutions for grid stabilization and backup power. As the renewable energy industry is expanding, Lithium Hydroxide, the main component in lithium-ion batteries, is also in high demand.
Consumer Electronics: The development of smartphones, tablets, laptops, and many other portable electronic devices that are driven by lithium-ion batteries has accelerated the demand for this type of batteries. With the ever-growing demand for devices with longer battery life and faster charging capabilities, manufacturers are obliged to use Lithium Hydroxide-based batteries as the majority of consumers are in search of these features.
Conclusion:
In the end, Lithium Hydroxide is the pioneer in the field of innovation and different industries such as battery technology and glass and ceramics production. The diversity and the irreplaceable role of lithium in the production of lithium-ion batteries guarantee its staying power in a techno-evolutionary world which is ever changing. As we are witnessing more and more development of renewable energy and the growth of electric vehicles, the need for Lithium Hydroxide is projected to have a significant increase. No doubt, this technology will play a critical role in the renewable energy storage solutions of the future. Lithium Hydroxide is a chemical that will help us build a greener and more efficient future, and it will be widely used in many fields.
#lithiumhydroxide#lithiumhydroxideprices#lithiumhydroxidemarket#lithiumhydroxidepricetrend#lithiumhydroxidepriceforecast#lithiumhydroxidenews#lithiumhydroxidedemand#lithiumhydroxidesupply#lithiumhydroxidemarketprice#priceoflithiumhydroxide
1 note
·
View note
Text
Green Synthesis of Fe3O4 Nanoparticles from Alternanthera Philoxeroides

Abstract
Iron oxide nanoparticles, notably magnetite (Fe3O4), have become widely used and a key topic of research due to their superparamagnetism and distinctive features. As a result, scientists are diligently looking into new uses for these nanoparticles. The choice and use of synthesis techniques are important variables that might affect the size and characteristics of the nanoparticles (NPs). The use of harmful compounds that are absorbed on the surface of the nanoparticles has been linked to a number of negative impacts of chemical production processes. The Green synthesis of nanoparticles has evolved as an eco-friendly method in response to environmental concerns, giving researchers the chance to internationally investigate the potential of various herbs for nanoparticle synthesis. The aqueous extract of Alternanthera Philoxeroides leaves and the precursors ferric chloride anhydrous (FeCl3 anhydrous) and ferrous chloride tetrahydrate (FeCl2.4H2O) are used in this study to demonstrate a green synthesis approach for manufacturing magnetite nanoparticles. Thermogravimetric analysis (TGA), Fourier transform infrared spectroscopy (FTIR), vibrating-sample magnetometer (VSM), and UV-visible spectroscopy were used to evaluate the produced FeNPs. The presence of functional groups including (-OH), (C-H), and (-NH) was detected in the FTIR findings, showing that organic compounds had been coated on the FeNPs. A maximum absorption peak was detected in the ultraviolet-visible spectra of the aqueous media containing iron nanoparticles at about 330 nm. The magnetic characteristics of the produced FeNPs were verified by VSM testing. Numerous uses for these nanoparticles exist, such as waste water treatment, energy production, and others.

Introduction
Nanoparticles have seen an increase in demand in recent years, due to their use in various fields, including health, catalysis, energy, and drug delivery systems (Nam et al., 2009; Li et al., 2011). The physical, chemical, optical, and electrical properties of these nanomaterials are significantly influenced by the size, shape, and surface morphology of the nanoparticles. Various techniques, including physical vapor deposition, chemical vapor deposition, the solgel method, co-precipitation method, ultrasonic method, electrochemical synthesis, and chemical reduction of metallic ions, are used to create metallic nanoparticles (Horwat et al., 2011; Sobhani et al., 2011; Starowicz et al., 2006). These Syntheses frequently involve toxic, expensive, and unsustainable substances. Due to the simplicity of synthesis, environmental friendliness, and increased stability of nanoparticles, green synthesis approaches based on fungus, microbes, plant and peel extracts are currently being investigated (Balaji et al., 2009; Kumar et al., 2011; Sukirtha et al., 2012).

Because of their high saturation magnetization, Fe3O4 MNPs are simple to magnetically separate in an external magnetic field (Mohamed et al., 2017). Several studies have been done on the synthesis of MNPs using a variety of reducing agents, including hydrazine (Hou et al., 2005). Dimethyl formamide (Jian et al., 2006). Sodium borohydride (Cain et al., 1996). Carbon monoxide (Mondal et al., 2004) and others. The biocompatibility of MNPs is hampered by these highly reactive reducing agents, which also have negative environmental impacts. As a result, there are only a few bio-medical uses for chemically reduced MNPs. MNPs must be strictly biocompatible in order to be used in biomedical applications. Several research using plant extracts to synthesize Fe3O4-NPs have been successful. For instance, Artemisia annua fruit extract (Basavegowda et al., 2014). Perilla frutescens leaf extract (Basavegowda et al., 2014). Tridax procumbens (Senthil et al., 2012). Caricaya papaya extract (Latha et al., 2014). Plantain peel extract (Venkateswarlu et al., 2013). Grape proanthocyanidin seed extract (Narayanan et al., 2012). In the leaf extract, there are a number of polyphenols and acidic compounds available. These Polyphenols from the extract form complexes with metal ions and show both reducing and capping behavior for NPs Senthil et al., 2012. Also, these polyphenolic compounds are biodegradable, nontoxic, and water-soluble at room temperature, which proves that green leaf extract as an effective reducing agent compared to others (Latha et al., 2014). According to the literature analysis, no specific studies have been conducted on the synthesis of Fe3O4-NPs using the alligator weed Alternanthera Philoxeroides, which encourages and pushes us to work on this. We have developed a modified green synthesis method to prepare Fe3O4-NPs using green Alternanthera Philoxeroides extract as a reducing agent, a novel environmentally friendly technique for producing Fe3O4-NPs is suggested in this study.
Source : Green synthesis and characterization of magnetite (Fe3O4) nanoparticles using leaf extract of Alternanthera Philoxeroides for environmental applications
#Magnetite nanoparticles (Fe3O4NPs)#Green synthesis#Alternanthera Philoxeroides#UV- visible Spectroscopy#TGA#DTA#VSM and FTIR
1 note
·
View note
Text
Calcium Carbonate CaCO3
Calcium carbonate is considered to be the “the first and most important thing when building a scientific civilization from scratch” by Senku in Ch 4 where it’s introduced. Their source for CaCO3 comes from smashing shells, as that’s what most seashells are mostly composed of.

The four “deadly important” uses for CaCO3 are:
Agriculture
Mortar
Soap
Gunpowder
Agriculture
The active ingredient in agricultural lime is calcium carbonate, and it works to increase the pH of acidic soil (the higher the pH, the less acidic). The explanation states that “lime gets rid of hydrogen ions” as a higher concentration of hydrogen ions is what makes something acidic. Calcium carbonate reacts with acids, yielding calcium and carbonic acid, which further disintegrates into carbon dioxide and water.
CaCO3(s) + 2 H+(aq) → Ca2+(aq) + CO2(g) + H2O(l)
The agricultural use of calcium carbonate shows up again here in Ch 91.

Acidic soil impacts the production of wheat, so by using CaCO3 to neutralize the pH, Taiju “leveled up” the soil to allow for his wheat to grow.
Mortar
The second use of calcium carbonate is to make mortar, specifically lime mortar, which is made by mixing calcium carbonate with sand and water. This was used by various civilizations in the past, such as in the pyramids build by the Ancient Egyptians and in Indian traditional structures, as well as in ancient Rome and Greece. The sand is used as an aggregate, which is some kind of coarse- to medium-grained particulate material that serves to reinforce things like concrete and other composite materials.
Lime mortar is the “predecessor to cement”, referring to Portland cement which is the most common type of cement used today. The process to make Portland cement seems to be more complicated than making lime mortar, and it can cause chemical burns and other nasty health issues. The Wikipedia page I’m pulling this information from has some other neat things such as how to mix and use lime mortar, and some properties of lime mortar compared to Portland cement.
Soap
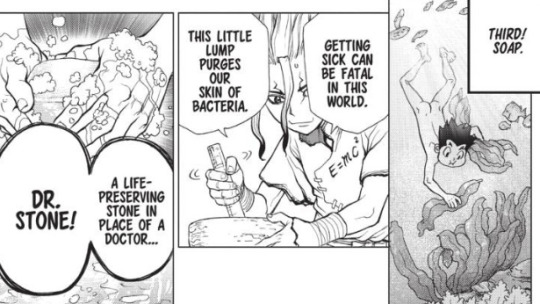
The third use of calcium carbonate is soap. In the manga, it appears that they made soap using seaweed and crushed shells. I can’t find too much about specifically using seaweed and calcium carbonate to make soap, but it seems that seaweed oil can be used for making soap, among other things. Seaweed can also be processed to yield sodium carbonate (Na2CO3, aka soda ash). Mixing solutions of soda ash and lime (calcium oxide here) makes lye, which is used to make "hot process” soap by adding lye to water, cooling it for a few minutes, and then adding that to oils and butters before cooking it for a few hours and placing it into a mold. I’m not sure if that’s the process that Senku used to make soap, though, since we only get a few panels.
Gunpowder
And the fourth use of calcium carbonate is to make gunpowder. The ingredients for gun powder are listed in Ch 8: sulfur, charcoal, and potassium nitrate. Potassium nitrate (KNO3 aka saltpeter) can be extracted from human and animal manure, once bacteria oxidizes the nitrogen-containing organic material into various nitrate salts. It seems potassium nitrate can also be obtained by immersing guano in water for a day, filtering it, and harvesting the crystals, but that doesn’t seem to use calcium carbonate in the process. What does use calcium carbonate is probably the process of treating the calcium carbonate with nitric acid and neutralizing it with ammonia to produce calcium nitrate
CaCO3 + 2 HNO3 → Ca(NO3)2 + CO2 + H2O
And then mixing the aqueous calcium nitrate with potassium carbonate (aka potash) to produce potassium nitrate.
Ca(NO3)2(aq) + K2CO3(aq) → CaCO3(s) + 2 KNO3(aq)
The charcoal is the thing that burns in the combustion reaction for the gunpowder explosion, but the need for oxygen limits the effectiveness of using charcoal on its own as the inner layers would need to wait for the outer layers to burn away in order to access the oxygen needed for the reaction. One way to mitigate that issue is by grinding up the charcoal into powder in order to increase the surface area, and the second way is to mix the charcoal with an oxidizing agent, in this case potassium nitrate, so that the carbon has easier access to oxygen without needing fresh air. The reaction with potassium nitrate and charcoal also uses sulfur in the following unbalanced equation:
KNO3(s) + C(s) + S(s) → N2(g) + CO2(g) + K2S(s)
Here is the reminder not to make gunpowder on your own, kids, because it’s dangerous.

The sugar mentioned above is also used for combustion. It probably produces more energy compared to burning just charcoal, leading to a bigger explosion.

If you have any questions on or corrections to this post, or have any requests for future posts, feel free to send an ask!
Sources
https://en.wikipedia.org/wiki/Calcium_carbonate
Agriculture
https://en.wikipedia.org/wiki/Agricultural_lime
https://www.khanacademy.org/science/biology/water-acids-and-bases/acids-bases-and-ph/a/acids-bases-ph-and-bufffers
https://www.agprofessional.com/article/liming-acid-soils-optimum-wheat-production
Mortar
https://en.wikipedia.org/wiki/Lime_mortar
https://en.wikipedia.org/wiki/Construction_aggregate
https://en.wikipedia.org/wiki/Portland_cement
Soap
https://en.wikipedia.org/wiki/Soap
https://en.wikipedia.org/wiki/Lye
https://en.wikipedia.org/wiki/Edible_seaweed
https://en.wikipedia.org/wiki/Sodium_carbonate
https://cavemanchemistry.com/oldcave/projects/lime/index.html
Gunpowder
https://en.wikipedia.org/wiki/Potassium_nitrate
https://en.wikipedia.org/wiki/Calcium_nitrate
https://en.wikipedia.org/wiki/Potassium_carbonate
https://cavemanchemistry.com/oldcave/projects/gunpowder/
10 notes
·
View notes
Text
TAFAKKUR: Part 148
Like Dissolves Like
Can you imagine a cup of coffee, sugarless at the top but intensely sweet at the bottom? Likewise, imagine a bowl of soup without salt at the top but over-salted at the bottom. Would these be enjoyable? We owe the joy of uniformly sweetened coffee or perfectly seasoned soup, as well as several vital life processes, to “dissolution.”
The process of dissolution occurs when a solute (solid, liquid, or gas) is placed in a solvent (also solid, liquid, or gas) and dissolves to form a solution which is a homogeneous mixture. A homogeneous mixture is uniform in composition and properties throughout. Pure substances, whether they are elements or compounds, are rarely found in nature. Most materials we encounter are mixtures of two or more substances. A mixture of sand and salt is not homogeneous, while a mixture of salt and water forms a homogeneous mixture (or solution) when enough water is added to dissolve all of the salt present. Likewise, gases dissolve in liquids to form solutions. Fish can survive in water by using their gills to extract the dissolved oxygen. Much of the world around us is made up of aqueous solutions. The oceans and our blood are only two of many examples.
Aqueous solutions are the solutions in which water is the solvent. Water is called the "universal solvent" because it dissolves more substances than any other liquid. The kidneys and water's solvent properties play an essential role in keeping us alive and healthy. The kidneys are responsible for filtering out substances that enter our bodies from the foods and drinks we consume. However, the kidneys have to get rid of these substances when they accumulate. This is where water helps out: being such a great solvent, water washing through the kidneys dissolves these substances and sends them on their way out of our bodies.
Polarity is the property whose job is to ensure water to be such an excellent solvent. Water molecules have a polar arrangement of oxygen and hydrogen atoms—one side (hydrogen) has a positive electrical charge and the other side (oxygen) has a negative charge. This allows the water molecule to become attracted to many polar molecules as well as less-polar molecules. Water can become strongly attracted to a polar compound, such as salt (NaCl); this allows it to disrupt the attractive forces that hold the sodium and chloride ions in the salt compound together, and, thus, dissolves it.
Similarly, glucose, which is a carbohydrate and the most important simple sugar, dissolves in water because polar water molecules attach to the glucose molecules. The six O-H (hydroxyl-) groups in glucose are the polar centers of a glucose molecule. The oxygen in each -O-H has a slight negative charge, and the hydrogen end of the -O-H has a slight positive charge. They are attracted to the water molecules by dipole-dipole forces. When the attractive forces of the water molecules for the glucose exceeds the attractive forces between the glucose and its neighboring glucose molecules, the water can pull the sugar molecule out of the crystal. It is said that water has "dissolved" the sugar molecule. This process continues until either the sugar is completely dissolved or the unattached water molecules are exhausted; in other words, when “the solution is saturated.”
"Like dissolves like" is an expression used by chemists to state that polar solvents dissolve polar solutes; non-polar solvents dissolve non-polar solutes.For example: water is polar, oil is non-polar. Water will not dissolve oil. Water is polar, and table salt (NaCl) is ionic, which is extremely polar. According to the rule of thumb, like dissolves like, and polar dissolves polar; thus, water dissolves table salt.
Polar water molecules are attracted to ions (atoms or groups of atoms with a charge) where "cations" are ions with a positive charge and "anions" have a negative charge. Most ionic compounds have high solubility in water, which means that large concentrations of those compounds can dissolve before the capacity for water molecules to isolate the ions is exceeded. The point at which Na+ and Cl-, for example, would begin to precipitate a salt in seawater is termed "saturation." For NaCl (the mineral "halite"), this only occurs when seawater evaporates and is reduced to about 10% of its original volume. Besides Na+ and Cl- ions, which account for over 85% of the total dissolved solids (salts), seawater contains other important ions such as Mg2+, Ca2+, K+, Sr2+, B+3, SO42-, HCO3-, Br-, and F-, where the positive and negative charges are balanced. As a sign of mercy in its creation, seawater is electrically neutral; otherwise, the flow of current from the sea would be shocking!
All other dissolved substances in seawater are at very low concentrations (parts per million or billion, ppm or ppb: 10-6 to 10-9). This includes important nutrients such as phosphate and nitrate that are cycled by organisms (ions called "bio-limiting") and essential for life. Metals are also found in trace concentrations. There are about 9 million tons of gold dissolved in seawater, which is about equal to all the gold mined on earth throughout history.
The evaporation of about 81-96% of the mass of seawater produces a predictable sequence of mineral salts (as minerals become saturated at a certain point) such as CaCO3 (calcite), CaSO4 (gypsum), NaCl (halite), and the K+ and Mg2+ salts (w/ SO42- and Cl-). There is enough salt in the ocean to cover the earth’s land with a layer 170 meters thick. Such natural deposits from ancient oceans are called "evaporites."
Even though most ionic compounds are highly soluble in water, there are some that are insoluble (or very slightly soluble). Soluble substances can form a solution of at least 0.1M (0.1 moles per liter) at 25 C while insoluble substances cannot.For an ionic substance to dissolve in water, there are two competing factors that determine the enthalpy of the solution ΔHsol, which is the enthalpy (energy) change when a solute is dissolved in a solvent (which is water, in this case):
The lattice energy LE, or the energy of the formation of the crystal between infinitely separated ions. LE is proportional to the charges of its ions. This value is always positive, as energy is required to separate the ions.
The hydration energy of gaseous ions, which is the enthalpy change when gaseous ions dissolve in sufficient water to give an infinitely dilute solution. These values are always negative, as energy is released upon the hydration of ions.
As an example, the LE of calcium carbonate (CaCO3, or calcite), which is insoluble in water, is so large, a great amount of free energy would be required to break the strongly attracted ions apart, and this energy has to come from the enthalpy of hydration. However, the enthalpy of hydration is not large enough to overcome the large lattice energy, hence it does not dissolve in water and exists as a solid (a precipitate form).
It would be very hard to predict whether a precipitate is formed in an aqueous reaction if there were not a number of patterns in the data obtained from measuring the solubility of various salts.These patterns form the basis for the solubility rules7 which can guide predictions of whether a salt will dissolve in water. This sense of order and harmony in creation is an exceptional gift to human beings, for it makes our lives much easier
#allah#god#prophet#sunnah#hadith#quran#islam#muslim#muslimah#hijab#revert#convert#religion#reminder#help#dua#salah#pray#prayer#welcome to islam#how to convert to islam#new muslim#new convert#new revert#revert help#convert help#islam help#muslim help#muhammad#ayah
1 note
·
View note
Text
An Expose of Physical Regenesis & Three-fold Plane of Bodily, Chemical and Spiritual Operation. - DR. GEORGE W. CAREY (Zodiac signs 1-6)
...Our diagram indicates at a glance the chemical formulae that appertain respectively to the zodiacal divisions, but to give a clearer conception as regards their specific qualities and physiological action in relation to the various signs, reference may be had to the following compend :
ARIES : From the teachings of the Chemistry of Life we find that the basis of brain or nerve fluid is a certain mineral salt known as potassium phosphate, or Kali Phos. The Phosphate of Potash. Synonyms : Potassium Phosphate, Kali Phosphoricum, Potassii Phosphas. Formula : K2HPO4 . It may be prepared by mixing aqueous phosphoric acid with a sufficient quantity of potash, hydrate or carbonate, until the reaction is slightly alkaline and evaporating. Triturate to 3d or 6th X. This salt is the great builder of the positive brain cells. Kali phos. unijtes with albumen and by some subtle alchemy transmutes it and forms gray brain matter. When the chemical possibilities of this brain builder are fully understood insane asylums will go out of fashion. Man has been deficient in understanding because his brain receiver did not vibrate to certain subtle influences ; the dynamic cells in the gray matter of nerve were not finely attuned and did not respond hence sin, or falling short of understanding.
TAURUS: Sulphate of Soda. Synonyms: Natrum Sulphate, Sodium Sulphuricum, Sodae or Sodii Sulphas, Glauber's Salts. Formula Na2 (SO4 10H2O). May be obtained by the action of Sulphuric acid on sodium chloride (common salt). This cell-salt is found in the intercellular fluids, liver and pancreas. Its principal work is to regulate the supply of water in the human organism. The blood becomes overcharged with water, either from the oxidation of organic matter or from inhaling air that contains more aqueous vapor (water) than is required to produce normal blood. This condition of air is liable to prevail whenever the temperature is above 70 degrees. One molecule of nat. sulph. has the power (chemical intelligence) to take up and carry away two molecules, or twice its bulk of water. The blood does not become overcharged with water from water taken into the stomach, but from the water lifted by expansion caused by heat above 70 degrees and held in the air and thus breathed into the arteries through the lungs. By the above we see that there is more work for this salt in hot weather than during cold weather. So-called malaria, Latin for bad air, is due to a lack of this tissue salt. Water lifted from swamps or clear streams or lakes by the action of the sun's heat is the same ; for heat does not evaporate and lift poisonous disintegrating organic matter from a swamp or marsh, but the water only.
GEMINI : The Chloride of Potash, or Potassium. Synonyms: Potassium chloride, Kali Muriaticum, Kali Chloratum, Kali Chloridum, Potassi Chloridum. Formula: K Cl. This salt must not be confused with the chlorate of potash, a poison, chemical formula K Clo3 . Chloride of potash may be obtained by neutralizing pure aqueous hydrochloric acid with pure potassium carbonate or hydrate. The cell-salt kali muriaticum (Potassium chloride) is the mineral worker of blood that forms fibrin and properly diffuses it through the tissues of the body. Kali mur molecules are principal agents used in the chemistry of life to build fibrin into the human organism. The skin that covers the face contains the lines and angles that give expression and thus differentiate one person from another. In venous blood fibrin amounts to three in one thousand parts; when the molecules of Kali mur fall below the standard in the blood fibrin thickens, causing what is known as pleurisy, pneumonia, catarrh, diphtheria, etc. When the circulation fails to throw out the thickened fibrin via the glands or mucous membrane, it may stop the action of the heart. Embolus is a Latin word meaning little lump, or balls ; therefore to die of embolus, or "heart failure" generally means that the heart's action was stopped by little lumps of fibrin clogging the auricles and ventricles of the heart. When the blood contains the proper amount of kali mur, fibrin is functional and the symptoms referred to above do not manifest.

CANCER : Fluoride of Lime. Synonyms : Calcaria Flurica, Calcium Fluoride. Chemical Formula : CaF2 . This salt is formed by the union of lime and fluorine. The inorganic salts are the workers, controlled and directed by infinite intelligence, that performs the ceaseless miracle of creation or formation. Biologists and physiologists have searched long and patiently for a solution to the mystery of the differentiation of material forms. No ordinary test can detect any difference in the ovum of fish, reptile, bird, beast or man. Chemical analysis reveals the same mineral salts, carbon, oil, fibrine, albumen, sugar, etc., in the life cell, or ovum in the blood, tissue, hair, or bone of the multiple and varied expression of life in material forms. The chemistry of life answers the "Riddle of the Sphinx," and writes above the temple door of investigation: "Let there be light." There is no such thing as dead or inert. All is life. A crystal is an aggregation of living organism. The base of all material manifestation is mineral. "Out of the dust (ashes or mineral) of earth physical man is made." The twelve mineral salts of lime, iron, potash, sodium, silica and magnesium are the foundation stones of every visible form of animal or vegetable. No two forms of the different species of animals have the same combination of this "rock foundation," but all have some of the same minerals. It is quite as important for a student of Biochemistry to understand the process by which certain cellsalts operate to supply a deficiency as it is to know what a particular symptom calls for. Elastic fibre, the chief organic substance in rubber, is formed by a chemical union of the fluoride of lime with albumen, oil, etc. Therefore, we find this salt dominant in the elastic fibre of the body, in the enamel of teeth and connective tissue. A lack of salt in proper amount causes relaxed condition of muscular tissue, falling of the womb and varicose veins. Sometimes there is a non-functional combination of this salt with oil and albumen which forms a solid deposit, causing swelling of stony hardness ; it is a sort of incomplete fibre with other lime salts and vitiated fluids of the body.
LEO : Phosphate of Magnesia. Synonyms : Magnesium Phosphorica. Formula : MgHOPOJ (H2O) . This cell-salt may be made by mixing Phosphate of Soda with Sulphate of Magnesia. This salt is found chiefly in the white fibres of nerves and muscles. The tissues of nerves and muscles are composed of many very fine threads or strands of different colors, each acting as a special telegraph wire, each one having a certain conductile power or quality, special chemical affinity for, certain organic substances, oil or albumen, through and by which the organism is materialized and the process or operations of life are carried on. The imagination might easily conceive the idea that these delicate infinitesimal fibres are strings of the Human Harp, and that molecular minerals are the fingers of infinite Energy striking notes of some Divine Anthem. The white fibres of nerves and muscles need the dynamic action of Magnesia Phosphate especially to keep them in proper tune, or function, for by its chemical action on albumen the special fluid for white nerve or muscle fibre is formed. When the supply of this salt falls below the standard, cramps, sharp shooting pains or some spasmodic condition prevails. Such symptoms are simply calls of nature for more magnesia. The impulsive traits of Leo people are symboled In the pulse, which is a reflex of heart throbs. The phosphate of magnesia, in biochemic therapeutics, is the remedy for all spasmodic impulsive symptoms. This salt supplies the deficient worker or builder in such cases and thus restores normal conditions. A lack of muscular force, or nerve vigor, indicates a disturbance in the operation of the heart cell-salt, magnesia phosphate, which gives the "Lion's spring," or impulse.
VIRGO: Sulphate of Potash. Synonyms : Potassium Sulphate, Kali Sulphos, Potassae Sulphos, Kali Sulphate. Formula : K2SO4 . The miscroscope reveals the fact that, when the tody is in health, little jets of steam are constantly .escaping from the seven million pores of the skin.The human body is a furnace and steam engine. The stomach and bowels burn food by chemical operation as truly as the furnace of a locomotive consumes by combustion. In the case of the locomotive the burning of coal furnishes force which vibrates water and causes an expansion (rate of motion) that we name steam. The average area of skin is estimated to be about 2,000 square inches. The atmospheric pressure being fourteen pounds to the square inch, a person of medium size is subject to a pressure of 40,000 pounds. Each square inch of skin contains 3500 sweat tubes or perspiratory pores (each of which may be likened to a little drain tile) one-fourth of an inch in length, making an aggregate length of the entire surface of the body 201,166 feet, or a tile draining for the body nearly forty miles in length. All tangible elements are the effects of certain rates of motion on the intangible and unseen elements. Nitrogen gas is mineral in solution, or ultimate potency. Oil is made by the union of the sulphate of potassium (potash) with albuminoids and aerial elements. The first element that is disturbed in the organism of those born in the celestial sign Virgo is oil; this break in the function of oils shows a deficiency in potassium sulphate, known in pharmacy as kali sulph. Virgo is represented in the human body by the stomach and bowels, the laboratory in which food is consumed as fuel to set free the minerals, in order that they may enter the blood through the mucous membrane absorbents. The letter X in Hebrew is Samech or Stomach. X or cross, means crucifixion, or charge, transmutation.
42 notes
·
View notes
Text
Carbonate sodium,
Carbonate sodium,
Sodium carbonate (Na₂CO₃), commonly known as soda ash or washing soda, is an inorganic compound with a wide range of industrial, household, and commercial applications. It is a white, odorless, water-soluble powder that exhibits strong alkaline properties. Naturally occurring sodium carbonate is obtained from trona ores, while synthetic production is carried out using the Solvay process.
Chemical and Physical Properties
Chemical Formula: Na₂CO₃
Molar Mass: 105.99 g/mol
Appearance: White crystalline powder
Solubility: Highly soluble in water, forming an alkaline solution
pH Level: Around 11.5 in aqueous solution
Decomposition: Decomposes at high temperatures to form sodium oxide and carbon dioxide
Types of Sodium Carbonate
Light Soda Ash: Fine particles with low bulk density, commonly used in detergent production.
Dense Soda Ash: Coarse particles with higher bulk density, used in glass manufacturing and water treatment.
Washing Soda: Hydrated form (Na₂CO₃·10H₂O) used in household cleaning products.
Uses and Applications of Sodium Carbonate
1. Glass Manufacturing
Sodium carbonate is a key ingredient in glass production. It lowers the melting point of silica, making the manufacturing process more energy-efficient. It is used in making flat glass, container glass, and fiberglass.
2. Detergent and Soap Industry
Sodium carbonate is an essential component in detergents and soaps, helping to remove grease, dirt, and stains by softening water.
3. Water Treatment
It is used to regulate pH levels in water treatment facilities, preventing pipe corrosion and neutralizing acidic water.
4. Chemical Manufacturing
Sodium carbonate is used in the production of various chemicals, including sodium bicarbonate (baking soda), sodium silicate, and sodium hydroxide.
5. Textile and Paper Industry
It acts as a pH regulator and softening agent in textile processing and paper manufacturing.
6. Food Industry
In food processing, it serves as an acidity regulator and stabilizer, commonly found in baking soda and ramen noodles.
7. Metallurgy
Sodium carbonate is used in metal refining processes, including aluminum extraction and ore purification.
Safety and Handling Precautions
Although sodium carbonate is generally safe to use, handling precautions should be followed:
Avoid direct contact with eyes and skin to prevent irritation.
Use gloves and protective eyewear when handling large quantities.
Store in a dry place to prevent moisture absorption.
Conclusion
Sodium carbonate is an essential compound with diverse applications across multiple industries, including glass manufacturing, detergents, water treatment, and chemical production. Its affordability, availability, and versatility make it a crucial industrial chemical. Whether used in large-scale manufacturing or household cleaning, sodium carbonate remains indispensable in everyday life.
0 notes
Text
Caustic Potash Market Size, Growth | Regional Analysis and Forecast to 2032 | ChemAnalyst

According to ChemAnalyst report, “Caustic Potash Market Analysis: Plant Capacity, Production, Operating Efficiency, Demand & Supply, End Use, Sales Channel, Region, Competition, Trade, Customer & Price Intelligence Market Analysis, 2015-2035”, Global Caustic Potash demand valued at around 2.8 million tonnes as of 2021, and the market is anticipated to grow at a CAGR of 4% by 2035. Caustic potash demand is propelled by the major end-user such as fertilizer, soap & detergent, chemical industry, etc and the market is expected to escalate in the upcoming period. Caustic Potash is produced in line with the safety requirements on human health and the environment before being released onto the market, which further improves the standard compliance.
Caustic Potash, chemically referred to as Potassium Hydroxide (KOH), is an inorganic compound, that is available in several forms including pellets, flakes, crystals, powder, and aqueous. The KOF aqueous known as Lye or Potash Lye which is used to produce chlorine and hydrogen (formed as co-products). Caustic Potash appears to be white, clear colourless liquid or slightly yellow lumps, possessing strong base (alkali), corrosive to metals and tissue, and found to be non-combustible and get dissolves easily in water with discharge of much heat. Generally, KOH is manufactured by the electrolysis of a potassium chloride (KCl) solution by using membrane electrolytic cells process.
Read Full Report Here: https://www.chemanalyst.com/industry-report/caustic-potash-market-671
Caustic Potash has gained attention in various grades including food grade, automotive, pharmaceutical grade, agricultural grade, technical grade, etc. Potassium Hydroxide possess a wide range of applications such as fertilizer production, soaps and detergents, potassium chemical industries, alkaline battery manufacturing, potassium phosphate chemicals, paints, biodiesel preparation, rubber preparation, etc. KOH is also essential in petroleum refining, runway de-icing and useful in pH balancing. It is similar to and just as effective as caustic soda or sodium hydroxide (NaOH), which is the most commonly used in cleaning agent and fertilizer. KOH is utilized as dehydrating agent for drying gases, lubricant in the extrusion pressing of high melting alloys, methylating agent, alkaline builder in detergent formulations, etc. In agricultural sector, KOH-based fertilizer is extensively used to improve drought tolerance of the crops, crop yield and enhance the productivity. In food industry, Caustic Potash act as a food thickening, food additives and pH control agent. The FDA considers it to be risk-free product due to which it is used in the chocolate, wine preservation and pharmaceutical industry. Potassium Hydroxide is gaining popularity with rising demand of potassium-based chemicals for instance potassium carbonate, potassium phosphates, potassium soaps, potassium acetate, etc. In addition, KOH is a versatile cleaning agent used in the formulation of personal care products such as liquid lotions, soaps, hairsprays, and shampoos, and as a strong base, it reacts with grease, making it a useful ingredient in drain and oven cleaners as well as in non-phosphate detergents. Due to these factors Caustic potash demand is anticipated to escalate in the market globally.
Taking into consideration, KOH is more environmentally friendly than NaOH but high-priced. Due to possessing alkaline properties it may cause serious chemical burns on skin and permanent eye damage. KOH can also be corrosive to stainless steel tanks if used in extensive high concentrations.
Due to global pandemic COVID-19, demand of Caustic Potash got decreased as the chlorine derivative demand has limited the supply of caustic soda, a by-product of chlorine. By imposing countryfies lockdowns and social distancing norms by government authority, which led to the decline of sales and demand. However, the market is expected to be recovered and pick of the space eventually.
Among different regions, APAC region hold around 49% of the global consumption of Caustic Potash and the demand is expected to increase in the forecast period owing to population, APAC region construction sector is largest in the world, and it is increasing at a healthy rate which will increase the demand of Caustic Potash. Countries like India and China are making investment in construction sector to develop the economy. Most chlor-alkali production companies are trying to involve the new mercury-free chlor-potash plant to avoid any possible mercury contamination, and from technological point of view, Europe is the most advanced around the world to produce pure potassium derivative. Healthy growth is anticipated for the KOH market in the forecast year propel by the rising need to increase crop productivity to feed the expanding global population that is urging demand for KOH-based fertilizers.
Sample Request Online: Caustic Potash Market
Caustic Potash Market Analysis: Plant Capacity, Production, Operating Efficiency, Demand & Supply, Application, End Use, Distribution Channel, Region, Competition, Trade, Customer & Price Intelligence Market Analysis, 2015-2035”, some of the major players operating in Caustic Potash market include Gujarat Alkalies and Chemical Limited, The Andhra Sugars Limited., TGV SRAAC LIMITED, Meghmani Finechem Ltd. UNID Company Ltd., Chengdu Huarong Chemical Co., Ltd., Tangshan Sunfar Silicon Industries Co.,Ltd., Inner Mongolia Rida Taifeng Chemical Co., Ltd., Asahi Glass Co., Ltd., Altair Chimica S.p.A, INOVYN, Neolyse Ibbenbüren GmbH, Vynova Group, Ercros S.A, and Others.
“Growing demand of Caustic Potash from the major end-use industries including agricultural, chemical, pharmaceutical, and others to manufacture a wide range of products is expected to accelerate the Caustic Potash market across the globe in the next ten years. Rising awareness regarding hygiene in several countries is propelling the demand of Caustic Potash around the world. In India, Initiatives such as Swachh Bharat Abhyan, is taken into consideration in order to promote health and hygiene, which is likely to boost the Caustic Potash market in the future.” said Mr. Karan Chechi, Research Director with TechSci Research, a research-based management consulting firm promoting ChemAnalyst worldwide.
About Us:
ChemAnalyst is a subsidiary of Techsci Research, which was established in 2008, and has been providing exceptional management consulting to its clients across the globe for over a decade now. For the past four years, ChemAnalyst has been a prominent provider of Chemical commodity prices in more than 15 countries. We are a team of more than 100 Chemical Analysts who are committed to provide in-depth market insights and real-time price movement for 300+ chemical and petrochemical products. ChemAnalyst has reverberated as a preferred pricing supplier among Procurement managers and Strategy professionals worldwide. On our platform, we provide an algorithm-based subscription where users can track and compare years of historical data and prices based on grades and incoterms (CIF, CFR, FOB, & EX-Works) in just one go.
The ChemAnalyst team also assists clients with Market Analysis for over 1200 chemicals including assessing demand & supply gaps, locating verified suppliers, choosing whether to trade or manufacture, developing Procurement Strategies, monitoring imports and exports of Chemicals, and much more. The users will not only be able to analyze historical data for past years but will also get to inspect detailed forecasts for the upcoming years. With access to local field teams, the company provides high-quality, reliable market analysis data for more than 40 countries.
ChemAnalyst is your one-stop solution for all data-related needs. We at ChemAnalyst are dedicated to accommodate all of our world-class clients with their data and insights needs via our comprehensive online platform.
Contact Us:
ChemAnalyst
B-44 Sector-57 Noida,
National Capital Region
Tel: 0120-4523990
Mob: +91-8882805349
Email: [email protected]
Website: https://www.chemanalyst.com/
0 notes
Text
AKD - Inevitable paper sizing
Alkyl ketene dimers (AKDs) are a family of organic compounds based on the 4-membered ring system of oxetan-2-one, which is also the central structural element of propiolactone and diketene. Attached to the oxetane ring of technically relevant alkyl ketene dimers there is a C12 – C16 alkyl group in the 3-position and a C13 – C17 alkylidene group in the 4-position.

The main application of alkylated ketene dimer is in the sizing of paper and cardboard, as well as in the hydrophobization of cellulose fibers. The products thus modified are distinguished by higher mechanical strengths and less penetration of water, inks, or printing inks.[1]
AKD’s feature hydrophobic alkyl groups extending from a beta-propiolactone ring. A specific example is derived from the dimerization of the ketene of stearic acid. This ketene is generated by pyrolysis of stearoyl chloride.[2] AKD’s react with the hydroxyl groups on the cellulose via an esterification reaction. The esterification is competitive with hydrolysis of the AKD. Prior to the development of AKD’s, hydrophobicity was imparted by incorporating rosin into the paper.
Related to AKDs, is alkenyl succinic anhydride which is another substitute used in sizing applications (ASA). As for AKDs, ASA reacts with hydroxy groups of the cellulose to form an ester, anchoring the hydrophobic group to the surface. ASA is prepared by thendne reaction of unsaturated hydrocarbons with maleic anhydride.
A continuous process in which long-chain carboxylic acid chloride and tertiary amine (e. g. dimethyl isopropylamine, dimethylcyclohexylamine or triethylamine) is supplied separately without solvents to a tube reactor, kneader or preferably a twin-screw extruder or planetary roller extruder and reacted at temperatures between 90 and 110 °C, delivers lactone contents of over 90% at short reaction times. Processing is carried out by phase separation or acidic extraction.[19]
Alkylated ketene dimers as paper sizing agents
The problems with the acidic (aluminum sulfate-mediated) mass sizing of paper with alkaline-digested colophony resins introduced since the early 19th century led beside the use of alkaline flocculants (such as chalk or calcium carbonate as the alkali reserve) to the search for alternative materials for sizing in a neutral or alkaline environment. In addition to the significantly more reactive alkenyl succinic anhydrides (which do also hydrolyze rapidly in the presence of water) alkylated ketene dimers were preferred surface and mass sizes in the paper industry from the 1960s onwards.
Industrially applied AKDs are derived from fatty acids with chain lengths between C14 (myristic acid) to C22 (behenic acid); palmityl (C16) diketene and stearyl (C18) ketene and mixtures thereof are preferably used, as well as fatty acid mixtures from the hydrolysis of animal and vegetable fats. Because of the chain length of the original fatty acids, AKD are waxy solids with melting points between 42oC and about 70 °C. Mixtures of alkylated ketene dimers and water are dispersions at temperatures below 40 °C or emulsions at temperatures above 45 °C. Liquid AKDs are widely used, they are based on unsaturated fatty acids like oleic acid or branched fatty acids, like iso-stearic acid.
Aqueous alkyl diketene dispersions generally contain 10-20 wt% of AKD, as well as active protective colloids (particularly polycations such as cationic starch, copolymers of N-vinylpyrrolidone and quaternized N-vinylimidazole, acylated polyethyleneimines or cationic high molecular weight polyacrylamides with an average molar mass up to 7 million g/mol) and other stabilizers (usually anionic surfactants, for example, lignin sulfonates or condensation products of naphthalene-sulfonic acid sodium salt and formaldehyde). Such stabilized AKD dispersions are active and stable at room temperature for up to three months and also tolerate the addition of different fillers for paper or cardboard (e.g. kaolin, chalk, talc, titanium dioxide, calcium sulfate, aluminum oxide, etc.) from 5 to 25%. The amounts of alkyl ketene dimers used for the sizing of paper and paper products are preferably in the range from 0.15 to 0.8 wt%, sometimes from 0.05 to 0.2 wt%, based on the dry paper stock.
Paper sizing with alkylated ketene dimers
For paper sizing with AKD, a three-step process was proposed which, despite controversial discussions in the 1990s, seems to describe the processes that are taking place.
Decisive criteria for the quality of the hydrophobicity of papers are
The retention of the AKD particles on the wet paper mass on the paper screen
The spreading of the AKD particles on the surface and the penetration in the paper mass
The chemical reaction of the hydroxyl groups of the cellulose (esterification) with the alkylated ketene dimers to form beta-ketocarboxylic esters.
The molecular structure (i.e., molar mass and cross-linking degree), the molar charge density of cationic groups, the exact dosage of the cationic polymer as a dispersion stabilizer and retention aid as well as keeping the other process parameters such as temperature, pH and residence times is crucial.
After removal of excess water – also to avoid hydrolysis of the AKD to the beta-keto acid and subsequent decarboxylation to the ketone – follows the cracking of the stabilized AKD particles on the base paper mass, the melting of the solid AKD wax (at approx. 90 °C), the spreading of the liquid AKD wax by surface diffusion on the cellulose fibers, and the formation of closed hydrophobic layers. The thickness of the hydrophobic layers depends on the AKD concentration in the dispersion.
Ad 3. The hydrophobization of cellulose fibers with alkylated ketene dimers takes place most effectively in neutral or preferably weakly alkaline media (pH 7.5-9.0). The reaction temperature is generally 90-110 °C, with approximately 40% of the AKD used reacting with the cellulose. After the reaction contact angles of >100° are measured, indicating the hydrophobic character of the AKD-modified model surfaces. The esterification of hydroxyl groups of cellulose fibers was also demonstrated by comparison reactions with 14C-labeled AKD.
The sizing with AKD is suitable for the permanent hydrophobization of newsprint, printing and writing paper and cardboard used as a container for liquids (including foodstuffs such as milk), as well as for the improvement of shape stability and runnability.
Commercial Production
At Advance Chemicals, undergoing robust testing frequently, the production of AKD is strictly under defined and controlled conditions thus giving out world call AKD for application in various grades of paper making.
0 notes
Text
What is a Home Water Filtration System and What Are the Benefits?
Clean Water vs Pure Water
Last week, Melbourne was crowned Australia’s best city for tap water. The title was judged by theWater Industry Operators Australiaand the prize is based on colour, clarity, odour and taste. By all reports, it tasted delicious! But just because tap water tastesgood, and isclean, does that mean it is pure?
All of Melbourne’s Water undergoes treatment if it is sourced from unprotected catchments. This involves filtration and disinfection. Water is disinfected at the point where it enters the supply system, and again at specified points along the delivery system (Greater Western Water, 2022). Greater Western Water’sDrinking Water Quality Report, 2021-22 states that our city’s water undergoes the following water treatment processes:
Fluoridation; Adding fluorosilicic acid (as per Fluoridation Act 1973)
Chemical Treatment and Disinfection; by Chlorination, and a second chlorine dosing (using liquid sodium hypochlorite) in four locations, including our Werribee catchment.
pH Correction; Little River water supply is dosed with gaseous carbon dioxide
Other filtration activities in certain catchments; Coagulation and flocculation for clarification, sand filtration, drum screen filtration, dual media pressure filters, cartridge filters, reverse osmosis, remineralisation, sludge handling, dissolved air flotation, oxidation, absorption coagulation
Other added substances in certain catchments; polymer alum, lime, ferric sulphate/ sulphuric acid/polydadmac, antiscalant, sodium hydroxide, sodium bisulfite, membrane cleaning, chemicals (caustic, detergent, acid), hydrated lime, carbon dioxide, chlorine gas, aluminium chlorihydrate, polyelectrolyte, sodium hydroxide (caustic soda), potassium permanganate, powdered activated carbon, sodium hypochlorite sodium, flurosilicic acid, sodium hexafluorosilicate, aqueous ammonia sodium, aluminium sulphate and sodium carbonate (soda ash).
Greater Western Water state that the purpose of the above is totake a preventative measures and multiple barrier risk management approach.
We’re Melbourneplumbers, and it is our mission to ensure Melburnians have access to safe drinking water in order to prevent waterborne diseases; plumbing plays a vital role in protecting public health. We are also cognizant of the difference betweencleantreated water, andpurefiltered water. Reading a list of the added substances above (detergent anyone?!) sounds more like what you’d read on the side of a can ofCoca-Cola, rather than straight from the tap. The reason for this is completely understandable, they need to keep their pipes clean in order to deliver water to you disease-free.
For the general population, tap water is perfectly fine to drink, cook, clean and bathe in. But we’ll go into detail below some of the problems that can occur with treated tap water, and how whole home water filtration systems can help in solving these.

Types of Water Problems
ChoiceAustralia has conducted research, in conjunction with Sydney Water, to determine the 5 most common contaminants in Australian tap water:
Stained plumbing/clothes. Likely caused by iron, manganese, copper.
Red/Brown slime in water pipes – Caused by Bacteria feeding on Iron
Discolouration of water – Turbidity (cloudy water) from hydrogen sulphide, iron, manganese, humic and tannic acids
Unusual colour or taste – caused by Hydrogen sulphide, low pH, iron, zinc, copper, lead, total dissolved solids, chloride, bacteria or algae, chlorine, paint soaking into plastic piping
Corrosive Water – likely caused by pH, copper, lead
Greater Western Water, our local water authority, has the task of ensuring water is treated enough to not be a risk to public health, but not treated too much to cause other problems to the end consumer.
Many people who are concerned about the processes of water treatment often opt for filtered water, or drinking water from water bottles. The following highlights what whole house water filtration systems are, and what benefits they can deliver to the end user.
What is a Home Water Filtration System?
There are many options on the market, but we consider those fromComplete Home Filtrationto be the jewel in the crown of the water filtration market. They are Australia’smost awarded water filter, including winning a Telstra Best of Business Awards Winner in 2022.
Essentially, a complete home water filtration is more than a kitchen mixer tap water filter; it filters all water entering your home for showering, drinking, washing etc by removing unwanted particles, chemicals and pollutants out of the water. Systems range in price from a simple reverse osmosis system (≈$1,000) to a whole house system (≈$6,000).
The benefits of removing chemicals, such as chlorine, out of your water once it enters your home are discussed below.
Health
Allergies are more prevalent in modern society, mainly due to pollution, dietary changes and less exposure to microbes. One such allergy iscercarial dermatitis, or chlorine rash. Symptoms of this allergy include an itchy, red rash, scaling of skin, small hives and swollen/tender skin. A frustrating allergy for someone to have as it can flare each time they shower due to the levels of chlorine in a municipal water supply.
Chlorine is a natural element compared to bleach, however, is used to treat and disinfect (and is a main active ingredient in bleach) thus likely to irritate those with skin sensitivities.
Chlorine often causes people to have lung irritations, and in extreme cases those with sensitivity to chlorine can feel unwell just breathing in shower vapour.
I’m getting controversial here, but chlorine has actually been linked to cancer and has been considered a carcinogen. Having a family member recently diagnosed with a Gastrointestinal Carcinoid, I’ve been researching causes. The World Journal of Gastrointestinal Oncology stated in 2016 that“published reports have revealed increased risk of colorectal cancers in people exposed to drinking water or chemical derivatives of chlorination”(World J Gastrointest Oncol. 2016 Apr 15; 8(4): 402–409). I should note this is a peer reviewed journal, not a subreddit of conspiracy theorists (I do love them though).
Skin and Hair
My 40’s are coming for me as fast as theSnowpiercertrain is perpetually lapping the globe at top speed (IYKYK). For the first time in my life I’m beginning to consider buying blood-of-a-virgin anti-wrinkle cream, not just the nivea face + body + pets. I’m finding my skin so dry and irritated when I exit the shower and I’m certain it’s not due to the volcanic water temperature…
Chlorine is a contributing factor in drying out your skin after you shower. It strips away your essential oils and opens these pores up, causing your skin to dry out. It causes itchiness and dryness and can trigger eczema.
Along with my skin ageing, my hair is becoming less of an ochre brown and more of a dusty silver… so I visit Thomas and his team atUNIK Hair Designin Point Cook to give me a paint job every now and again. Chlorine can strip dyed hair of colour, and dry it out, so many home filtration users enjoy its beauty benefits along with its health benefits.
Appliances
Water hardness refers to calcium and magnesium, amongst other minerals, in our water. If water is too ‘hard’ if it has higher than usual amounts of these minerals in the supply.
If not filtered out, these minerals cause deposits on appliances when water is heated (such as calcium on showerheads, in kettles and on coffee machines. Hard water means more soap and detergent is used for washing and cleaning, as its harder to lather.
Environment
Finally, home water filtration systems void the use of bottled water and thus reduce plastic pollution and fossil fuel consumption. If reducing your carbon footprint is important to you, and you’d like pure water without using plastic water bottles, whole house water filtration could be an option for you.
Installing a whole house water filtration system
If you’re interested in discussing the benefits of investing in a home water filtration system (which needs to be installed by a licensed plumber) get in touch with us on9931 0905.
0 notes
Text
SSD Chemical Solution.
Many people have described SSD as Species Sensitivity Distributions (SSD). In a nutshell, SSD is a common tool used for setting safe limits on chemical concentrations in surface waters.
Chemical activation involves carbonization and activation in a single step in which the raw material, impregnated with certain chemical agents, is thermally decomposed. This process is usually applied where the precursor is wood.
SSD Chemical is a type of cleaning chemical that is used by many experts who want to clean black money at home. This black money cleaning solution is a standardized chemical solution generally used to remove excess stains from every type of currency.
Buy SSD CHEMICAL, ACTIVATION POWDER for Bulk cleaning.
However, the handling process of removing the stains in currency using the SSD must be handled by an expert who knows exactly how to go about it.
ACTIVATION POWDER is an aqueous inorganic salt that is used as a hydrogen peroxide carrier. ACTIVATION POWDER is a compound that reacts with hydrogen peroxide in an aqueous solution to form peroxy acids which when added to ordinary SSD SOLUTION, could be used for the BLACK MONEY cleaning process
What is SSD Chemical Formula? The three main power elements of the SSD Solution Chemical Formula are; Sodium chloride NaCl, Sulphated Ash 0.2%, and Sulfuric acid H2SO4. -A compound of Mercury Mercuric Nitric Dioxide liquid (brown/blue in color).
The species sensitivity distribution (SSD) is a statistical approach that is used to estimate either the concentration of a chemical that is hazardous to no more than x% of all species (the HCx) or the proportion of species potentially affected by a given concentration of a chemical.
Authentic US$100 bills are coated with a protective layer of glue and then dipped into a solution of tincture of iodine. The bill, when dried, looks and feels like black sugar paper. The mass of notes is real sugar paper; when the victim picks a "note" for cleaning, it is switched with the iodine-coated note.
Money laundering is illegal because it is a way for criminals to profit from crime and often involves more than one illegal activity. Both the act and origin of money laundering make it illegal.
Will the bank take washed money?
But while a wash cycle may make your money look untainted, it nonetheless ruins the bills; hot water can damage security features, and detergents change the way cash reflects light, which currency-sorting machines detect. Banks shred washed money
What Are Common Ways to Launder Money? The traditional forms of laundering money, include smurfing, using mules, and opening shell corporations. Other methods include buying and selling commodities, investing in various assets like real estate, gambling, and counterfeiting.
BFC Sodium Thiosulphate
Can dirty money be trace?
Profits gained from criminal activity are often referred to as 'dirty money. This is because the money is linked directly to the crime and can be traced. Due to this, criminals need to 'clean' the money so that it appears legal and can be used for investments.
0 notes