#Biosimilars Market Forecast
Text
Biosimilars Unleashed: The Future of Healthcare in the US
Buy Now
What is the Size of US Biosimilar Industry?
US Biosimilar Market is expected to grow at a CAGR of ~ % between 2017-2022 and is expected to reach ~USD Bn by 2028. Biosimilars enhance patient access to essential treatments, especially in therapies with high demand, like oncology, by providing more affordable options. Additionally, Growing evidence of biosimilars' comparable efficacy and safety fosters trust among healthcare professionals, driving adoption.

Click here to Download a sample Report
Biosimilars offer cost savings compared to originator biologics, addressing the need for affordable healthcare solutions in the face of rising medical costs. Favorable regulatory frameworks, like the BPCIA, streamline biosimilar approval processes, encouraging manufacturers to invest in development.
Furthermore, The expiration of patents for numerous reference biologics creates opportunities for biosimilar entry, leading to increased competition and market expansion. Pharmaceutical companies are investing in biosimilar R&D and production, expanding the pipeline and market availability. Supportive healthcare policies and reimbursement models incentivize biosimilar adoption, creating a favorable environment for market growth.
US Biosimilar Market by drug class
The US Biosimilar market is segmented by Monoclonal Antibodies, Recombinant Hormones, Immunomodulators, Anti-inflammatory agents and Others. Based on drug class, Monoclonal Antibodies segment dominates the bio similar market in 2022.
Monoclonal antibodies have diverse applications across various therapeutic areas. From cancer treatment to autoimmune diseases, biosimilar Mabs addressed a wide range of medical needs, leading to a broad and growing market. Biosimilars, with their potential for cost savings while maintaining comparable efficacy and safety, gained significant attention as viable alternatives.
US Biosimilar Market by application
In US Biosimilar market, they are segmented by application into Oncology, Blood disorders, Chronic diseases and autoimmune conditions and Others. On the basis of application, Oncology segment was the dominant in 2022.
The increasing prevalence of cancer and the high cost of traditional biologics used in oncology treatment have created a strong incentive for the adoption of biosimilars. Biosimilars offer the potential to provide similar therapeutic outcomes at a lower cost, making them an attractive option for both healthcare providers and patients.
Additionally, the rigorous clinical trials and regulatory processes that biosimilars undergo to gain approval provide reassurance to healthcare professionals and patients regarding their safety and efficacy. This has led to increased acceptance and adoption of biosimilars in oncology.
US Biosimilar by Region
The US Biosimilar market is segmented by Region into North, East, West and South. In 2022, the dominance region is North region in US Biosimilar market.
The North region benefits from a concentration of healthcare providers and academic institutions that are at the forefront of adopting and integrating biosimilars into their treatment protocols. These institutions are more likely to have the expertise to evaluate and incorporate biosimilars effectively, driving their adoption among healthcare professionals and patients.
Click here to Download a Custom Report
Competition Scenario in US Biosimilar Market
The US biosimilar market has witnessed an evolving competitive landscape, with several key players competing for market share. Prominent pharmaceutical companies such as Amgen, Pfizer, Sandoz (Novartis), and Boehringer Ingelheim have been actively involved in developing and marketing biosimilar products. These established players have utilized their expertise in biologics and significant resources to navigate the regulatory landscape and compete effectively.
The competition in the US biosimilar market is characterized by a balance between established pharmaceutical giants and emerging biotech companies. While the major players possess the advantage of resources and experience, smaller biotech firms are also contributing to the market with innovative approaches and niche biosimilar offerings.
What is the Expected Future Outlook for the Overall US Biosimilar Market?
The US Biosimilar market was valued at USD ~Million in 2022 and is anticipated to reach USD ~ Billion by the end of 2028, witnessing a CAGR of ~% during the forecast period 2022- 2028. The US biosimilar market is likely to experience significant growth in the coming years, driven by several factors. Biosimilars are biologic drugs that are highly similar to already approved reference biologics. They offer potential cost savings, increased competition, and improved patient access to crucial treatments.
Firstly, the regulatory environment is becoming more favorable for biosimilars. The Biologics Price Competition and Innovation Act (BPCIA) established a pathway for biosimilar approval in the US, allowing for a smoother regulatory process. As more biosimilars receive approval, competition in the market is expected to intensify.
Secondly, patents for several blockbuster biologics are expiring or have already expired. This creates opportunities for biosimilar manufacturers to enter the market with more affordable alternatives, offering healthcare systems and patients a choice in treatment options.
Thirdly, as healthcare costs continue to rise, biosimilars present an attractive solution for reducing expenses. Their potential to offer cost savings without compromising therapeutic efficacy could lead to increased adoption by healthcare providers, insurers, and patients alike.
Physician and patient education are crucial, as misconceptions about biosimilars' safety and effectiveness might hinder their adoption. Additionally, legal and market access barriers, including patent litigation and complex distribution systems, could slow down the growth of the biosimilar market.
The biosimilar market witness consolidation as larger pharmaceutical companies acquire or partner with smaller biotech firms to bolster their biosimilar portfolios. This will lead to more resources being devoted to biosimilar development and marketing. Changes in healthcare policies, such as reimbursement models and value-based care initiatives, can influence the biosimilar market's growth. Favourable policies that incentivize biosimilar adoption drives their market growth.
#US Biosimilar Market#US Biosimilar Industry#US Biosimilar Sector#United States Biosimilar Market#US Biosimilar Market forecast#US Biosimilar Market analysis#US Biosimilar Market trends#US Biosimilar Market share#US Biosimilar Market key players#US Biosimilar Market revenue#US Biosimilar Market growth#Monoclonal Antibodies in biosimilar market US#Recombinant Hormones in biosimilar industry US#Oncology in bio similar market US#Blood disorders in biosimilar market US#Research institutes in Biosimilar market US#US similar biotherapeutics products market#Hospitals in Biosimilar market US#Investors in Biosimilar market US#US comparable biologics products industry#US recombinant biosimilars industry#US replicate biologics sector#US analog biologics market#US homologous biologics market#US oncology biosimilar market#US immunology biosimilar sector#US insulin biosimilar industry#US Generics Biologics market challenges#US leading Biosimilar drug providers#US leading Biosimilar drug manufacturers
0 notes
Text
Biosimilars Market
The Biosimilars Market is experiencing rapid growth, offering cost-effective alternatives to biologic drugs. These highly similar versions of approved biologics are gaining popularity for their affordability and accessibility, making healthcare more sustainable. As demand rises, the biosimilars market is poised for continued expansion, benefiting patients and healthcare systems alike.

#Biosimilars Market#Biosimilars#Market Research Reports#Industry Analysis#Market Forecast#Industry Outlook
0 notes
Text
Biosimilars Market Size 2022 Industry Recent Developments and Technology, Size, Trends, Growth, and Forecast Research Report 2030| By R&I

The report is titled as ‘Biosimilars Market: Opportunity Analysis and Future Assessment 2020-2028’. An overview of conceptual frameworks, analytical approaches of the biosimilars market is the main objective of the report, which further consists the market opportunity and insights of the data involved in the making of the respective market. Biosimilars market is expected to grow with a significant rate in the near future.
The global biosimilars market in 2020 is estimated for more than US$ 11,036.2 Mn and expected to reach a value of US$ 1,09,501.8 Mn by 2028 with a significant CAGR of 33.5%.
Request a Sample Copy of this Report @: https://reportsandinsights.com/sample-request/1243
Biosimilars Market Dynamics
Patent expiration in the biological drug industry is the major factor driving the growth of global biosimilars market. Moreover, the introduction of clear regulatory pathways for the introduction of biosimilars to the market by many countries is further encouraging biosimilar development. For instance, recently India and Canada introduced regulations for approval of biosimilar drugs in respective countries. However, unlike generic chemical entities, producing identical copies of innovator cell-based drugs is impossible and takes more time, which is the biggest challenge for the growth of global biosimilars market. Biologics are produced by cells in culture or whole organisms, which are inherently more variable than chemical synthesis methods.
Biosimilars Market Regional Overview
On the basis of region, the global biosimilars market is segmented into six regions namely North America, Latin America, Asia Pacific, Europe, Middle East, and Africa. North America biosimilars market is expected to be the most dominating market throughout the forecast period due to due to the expiry of patents for blockbuster biological drugs. The presence of key players in Europe makes it second-largest market for biosimilars. Asia Pacific biosimilars market is expected to witness delayed growth due to relatively long patent term for biological drugs
MMC Overview
The non-identical approach of Reports and Insights stands with conceptual methods backed up with the data analysis. The novel market understanding approach makes up the standard of the assessment results that give a better opportunity for the customers to put their effort.
A research report on the biosimilars market by Reports and Insights is an in-depth and extensive study of the market based on the necessary data crunching and statistical analysis. It provides a brief view of the dynamics flowing through the market, which includes the factors that support market and the factors that are acting as impedance for the growth of the market. Furthermore, the report includes the various trends and opportunities in the respective market in different regions for a better understanding of readers that helps to analyze the potential of the market.
Wish to Know More About the Study? Click here to get a Report Description: https://reportsandinsights.com/report/global-biosimilars-market
Biosimilars Market Key Players
Some of the key participating players in global Biosimilars market are:
 Pfizer
 Sandoz International
 Teva Pharmaceuticals
 Amgen
 Biocon
 Dr. Reddy’s Laboratories
 Celltrion
 Samsung Biologics
 Synthon Pharmaceuticals, Inc.
 LG Life Sciences
 Biocon
 Hospira
 Merck Serono (Merck Group)
 Biogen Idec Inc.
 Genentech (Roche Group)
Biosimilars Market Segmentation
The global Biosimilars market is segmented on the basis of product type, manufacturing, diseases, and region.
By Product Type
 Recombinant Non-Glycosylated Proteins
 Recombinant Human Growth Hormone (RHGH)
 Granulocyte Colony-Stimulating Factor (Filgrastim)
 Insulin
 Interferons
 Interferon-Beta
 Interferon-Alpha
 Recombinant Glycosylated Proteins
 Erythropoietin (EPO)
 Monoclonal Antibodies (MABS)
 Infliximab
 Rituximab
 Adalimumab
 Other Monoclonal Antibodies
 Follitropin
 Recombinant Peptides
 Glucagon
 Calcitonin
By Type of Manufacturing
 In-House Manufacturing
 Contract Manufacturing
By Disease
 Oncology
 Chronic Diseases
 Autoimmune Diseases
 Blood Disorders
 Growth Hormone Deficiency
 Infectious Diseases
 Other Diseases
By Region
 North America
 Latin America
 Europe
 Asia Pacific
 Middle East
 Africa
To view Top Players, Segmentation and other Statistics of Biosimilars Industry, Get Sample Report @: https://reportsandinsights.com/sample-request/1243
About Reports and Insights:
Reports and Insights is one of the leading market research companies which offers syndicate and consulting research around the globe. At Reports and Insights, we adhere to the client needs and regularly ponder to bring out more valuable and real outcomes for our customers. We are equipped with strategically enhanced group of researchers and analysts that redefines and stabilizes the business polarity in different categorical dimensions of the market.
Contact Us:
Neil Jonathan
1820 Avenue M, Brooklyn
NY 11230, United States
+1-(718) 312-8686
Find Us on LinkedIn: www.linkedin.com/company/report-and-insights/
View Latest Market Updates At: https://marketsresearchanalytics.com
#Biosimilars Market Research#Biosimilars Market Report#Biosimilars Market Share 2021#Biosimilars Market Size 2022#Biosimilars Market Trends#Biosimilars Market Key Players#Global Biosimilars Market Analysis#Biosimilars Industry News#Biosimilars Industry Analysis#Biosimilars Market Forecast#Biosimilars Market CAGR#USA Biosimilars Market#Japan Biosimilars Market#Biosimilars Market Demand#Argentina Biosimilars Market#Australia Biosimilars Market#Belgium Biosimilars Market#Brazil Biosimilars Market#Canada Biosimilars Market#Chile Biosimilars Market#China Biosimilars Market#Columbia Biosimilars Market#Egypt Biosimilars Market#France Biosimilars Market#Germany Biosimilars Market#Global Biosimilars Market#India Biosimilars Market#Indonesia Biosimilars Market#Biosimilars Applications#Biosimilars Industry
0 notes
Text
Polymer-Based Prefilled Syringe Market: Current Analysis and Forecast (2024-2032)
According to the UnivDatos Market Insights analysis, the increasing incidence of chronic conditions such as diabetes and rheumatoid arthritis, patient preference for self-administration, and reduced risk of contamination & needlestick injuries compared to traditional syringes. will drive the global scenario of the Polymer-Based Prefilled Syringe market. As per their “Polymer-Based Prefilled Syringe Market” report, the global market was valued at USD 2.87 Billion in 2023, growing at a CAGR of about 4.9% during the forecast period from 2024 – 2032.
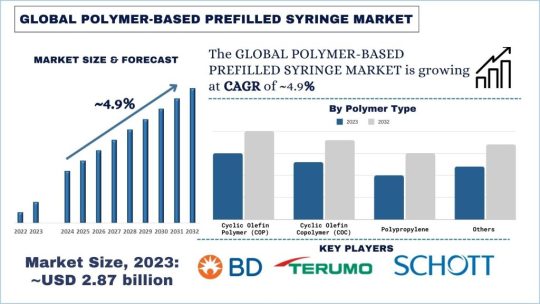
Over the years, there has been a shift of focus in the healthcare sector to develop new and advanced drug delivery systems, and prefilled syringes made of polymers have gained a lot of popularity among pharmaceutical organizations and healthcare centers. Such syringes produced from the highest quality polymers have several advantages over the traditional glass ones: safety, convenience, and a comparatively low price.
Demand
Several factors have led to the high demand for polymer-based prefilled syringes as explained below. Moreover, a trend toward the use of long-lasting drugs, which in turn requires frequent, accurate dosing due to such diseases as diabetes, rheumatoid arthritis, or cancer. Prefilled syringes provide a comfortable and safe way to administer injections to patients who may require multiple injections, hence enhancing the patient’s compliance with treatment and overall positive health impacts.
Applications
Polymer-based prefilled syringes are widely employed across different diseases such as immunology, oncology, and neurology among others. When integrated with the right technologies, they are ideal for dispensing biologic drugs, vaccines, and biosimilar medications, which require accurate dosing and maintaining drug integrity. These syringes are also being employed for administration and this helps the patients to administer their medication at home comfortably.
Access sample report (including graphs, charts, and figures): https://univdatos.com/get-a-free-sample-form-php/?product_id=61521
Factors Influencing the Cost of Syringe
The steps followed in making prefilled syringes from polymer materials include the selection of polymers, injection moulding, and final assembling. Polymeric materials, like cyclic olefin copolymer (COC), cyclic olefin polymer (COP), or their co-polymers, are preferable due to their high chemical and physical stability, and biocompatibility with most drugs. The syringes are then appropriately filled with the right medication and closed in a way that makes them ready for use; they are then sterilized using processes that have been through validation to ensure the product is safe and effective.
Manufacturing
Polymer selection, injection moulding, and subsequent assembly are typical phases of manufacturing pre-filled syringes from polymers. The materials are chosen for their good resistance to chemicals as well as compatibility with most of the drugs; COC or COP are preferred. The syringes are then prepped to contain the right dosage of medicine, closed, and autoclaved in a way that has been certified to be safe for patient use.
Conclusion
Consequently, the prefilled polymer syringes are a breakthrough in the polymer technology of drug delivery systems having the following advantages over glass syringes. Their safety, convenience, and relatively cheaper price make the device an attractive tool for pharma and healthcare providers as they seek better ways of attending to patients and cutting costs. With the increasing trend in technology later in the future, the features of polymer-based prefilled syringes can be developed to enhance the growth and use in the healthcare system.
Contact Us:
UnivDatos Market Insights
Email - [email protected]
Contact Number - +1 9782263411
Website -www.univdatos.com
0 notes
Text
Biological Safety Testing Products and Services Market 2024 | Upcoming Trend in Biological Safety Testing Products and Services Industry by an Expert
The global biological safety testing products and services market is on a robust growth trajectory, valued at $4.42 billion in 2023 and projected to reach $10.51 billion by 2032. This remarkable growth reflects a compound annual growth rate (CAGR) of 10.10% over the forecast period from 2024 to 2032, driven by the increasing demand for safety and efficacy testing across the biopharmaceutical and healthcare sectors.
Biological safety testing is essential for ensuring that medical products, including pharmaceuticals, vaccines, and medical devices, meet stringent safety and efficacy standards before reaching the market. The market encompasses a wide range of testing services, including sterility testing, endotoxin testing, and biocompatibility assessments, all critical for regulatory compliance.
Key Market Drivers
Increasing Biopharmaceutical R&D Activities: The rising investment in biopharmaceutical research and development is a significant factor propelling the market. As the industry focuses on innovative therapies, including monoclonal antibodies, gene therapies, and cell therapies, the need for rigorous biological safety testing becomes paramount. These testing services help ensure that new products are safe for human use, fostering trust in the healthcare system.
Regulatory Compliance and Safety Standards: Stringent regulatory requirements from agencies such as the U.S. Food and Drug Administration (FDA), European Medicines Agency (EMA), and other global health authorities are driving the adoption of biological safety testing services. Compliance with these regulations is critical for companies looking to launch new medical products, creating a steady demand for testing services that ensure product safety and efficacy.
Growing Concerns About Contamination and Quality Assurance: The increasing prevalence of product recalls due to contamination and safety issues has heightened awareness about the importance of biological safety testing. Companies are now more vigilant in their quality assurance processes, recognizing that thorough testing is essential to maintain product integrity and safeguard public health.
Expansion of the Healthcare Sector: The ongoing expansion of the healthcare sector, particularly in emerging markets, is creating new opportunities for biological safety testing services. With the growth of healthcare facilities and the increasing production of biologics and biosimilars, the demand for reliable testing solutions is expected to rise significantly.
Access Free Sample Report: https://www.snsinsider.com/sample-request/4483
Challenges and Opportunities
While the market shows strong potential, it faces challenges such as the high cost of testing services and the need for specialized expertise. However, advancements in technology, including automation and digitalization, are likely to streamline testing processes, reduce costs, and enhance the accuracy of results.
Moreover, the increasing adoption of 3D cell culture systems and organ-on-a-chip technologies offers opportunities for innovative testing solutions. These advancements are expected to improve the efficiency and effectiveness of biological safety testing, providing companies with the tools they need to ensure product safety.
Regional Insights
North America holds the largest share of the biological safety testing market, driven by the presence of leading biopharmaceutical companies, advanced research facilities, and stringent regulatory standards. Europe follows closely, with significant investments in healthcare and biotechnology sectors. The Asia-Pacific region is expected to experience the highest growth rate during the forecast period, supported by the expansion of healthcare infrastructure and increasing research activities in countries such as China, India, and Japan.
Future Outlook
As the demand for innovative biopharmaceutical products continues to rise, the biological safety testing products and services market is set for significant growth. With a projected CAGR of 10.10% from 2024 to 2032, the market is poised to see substantial advancements in testing technologies, helping to meet the increasing demand for safety and efficacy in healthcare products.
In conclusion, the biological safety testing products and services market is entering a dynamic phase of growth, with a valuation expected to rise from $4.42 billion in 2023 to $10.51 billion by 2032. This growth is driven by regulatory compliance, the expansion of biopharmaceutical R&D, and the need for rigorous quality assurance in an increasingly complex healthcare landscape
Other Trending Reports
Dental Suction Systems Market Size
Cosmeceuticals Market Size
Cell Therapy Market Size
Growth Hormone Deficiency Market Size
0 notes
Text
The Synthetic Biology Market: Innovations, Trends, and Future Outlook
The synthetic biology market is estimated to reach USD 79.39 billion in 2024 and is projected to grow to USD 145.49 billion by 2029, with a compound annual growth rate (CAGR) of 12.88% during this forecast period. This rapidly emerging industry is transforming multiple sectors, including healthcare, agriculture, and environmental sustainability. In this blog, we will explore the key trends, opportunities, and challenges in the synthetic biology market, shedding light on its potential impact across various industries.
Market Overview
Synthetic biology combines biology and engineering to design and construct new biological parts, devices, and systems. This interdisciplinary field is reshaping our understanding of living organisms and enabling innovations in areas such as gene editing, biomanufacturing, and biofuels. With increasing investments from both private and public sectors, the synthetic biology market is poised for substantial growth.
Key Trends
Advancements in Gene Editing Technologies: Technologies like CRISPR-Cas9 are revolutionizing the field of synthetic biology, allowing for precise modifications of DNA. These advancements are accelerating research and development in therapeutics, agriculture, and bioengineering.
Synthetic Biology in Healthcare: The potential of synthetic biology in drug development and personalized medicine is enormous. By engineering microbes to produce complex drugs or vaccines, companies can streamline the production process, making treatments more accessible and cost-effective.
Sustainable Agriculture: Synthetic biology is paving the way for the development of crops with enhanced traits, such as drought resistance or increased yield. This innovation can help address food security issues in a changing climate.
Environmental Applications: The industry is making strides in creating biofuels and biodegradable plastics, reducing reliance on fossil fuels and minimizing environmental impact. Synthetic biology solutions are being developed to tackle pollution and promote sustainability.
Opportunities
Growing Investment: The influx of venture capital and government funding is propelling research and development in synthetic biology. This investment landscape is fostering innovation and the commercialization of new technologies.
Rising Demand for Biopharmaceuticals: As the demand for biologics and biosimilars increases, synthetic biology offers a pathway for efficient production, meeting the needs of the biopharmaceutical industry.
Global Health Challenges: The COVID-19 pandemic has highlighted the importance of rapid response capabilities in healthcare. Synthetic biology can facilitate the rapid development of vaccines and therapeutics, addressing global health challenges more effectively.
Challenges
Regulatory Framework: Navigating the regulatory landscape can be complex, as synthetic biology encompasses a range of applications that fall under different regulatory bodies. This can create uncertainties for companies seeking to bring new products to market.
Ethical Considerations: The manipulation of genetic material raises ethical questions that need to be addressed. Public perception and acceptance of synthetic biology technologies can influence their adoption and commercialization.
Technical Limitations: While the field is advancing rapidly, there are still technical challenges related to the reliability and scalability of synthetic biology processes that need to be overcome.
Future Outlook
The synthetic biology market is expected to continue its rapid growth, driven by technological advancements and increasing applications across various industries. As research progresses, we can anticipate innovative solutions that will address pressing global challenges, from healthcare to environmental sustainability.
Investments in research and development, coupled with collaboration between academia, industry, and regulatory bodies, will be crucial in shaping the future of synthetic biology. Companies that embrace innovation and prioritize ethical considerations will likely lead the way in this dynamic field.
Conclusion
The synthetic biology market stands at the forefront of scientific innovation, with the potential to revolutionize industries and address some of the world’s most pressing challenges. As the industry evolves, stakeholders must navigate the complexities of regulation, ethics, and technology to fully realize the benefits of synthetic biology. The future is bright, and the possibilities are endless as we continue to unlock the potential of living systems through synthetic biology.
For a detailed overview and more insights, you can refer to the full market research report by Mordor Intelligence https://www.mordorintelligence.com/industry-reports/synthetic-biology-market
#marketing#Synthetic Biology Market#Synthetic Biology Market Size#Synthetic Biology Market Share#Synthetic Biology Market Trends#Synthetic Biology Market Growth#Synthetic Biology Market Report
0 notes
Text
Biosimilars Market: Driving Affordable Healthcare Solutions
The Biosimilars market has become a significant player in modern healthcare, offering cost-effective alternatives to biologics. As the demand for affordable therapeutic options rises, the market for biosimilars is gaining substantial momentum. This article delves into the market trends, segmentation, growth drivers, and leading companies in the biosimilars industry, providing crucial insights for decision-makers.
Market Overview
According to SkyQuest's Biosimilars Market report, the global market is currently valued at USD 27.30 billion in 2023, with a projected CAGR of 16.4% over the forecast period. The market's expansion is fueled by patent expirations of key biologics, increasing prevalence of chronic diseases, and a growing emphasis on cost-efficient healthcare solutions.
Request Your Free Sample: - https://www.skyquestt.com/sample-request/biosimilars-market
Market Segmentation
By Product Type:
Recombinant Non-Glycosylated Proteins: Used in the treatment of diseases such as diabetes and cancer.
Recombinant Glycosylated Proteins: Common in oncology and autoimmune disease therapies.
Peptides: An emerging class used for metabolic diseases and cancer treatment.
By Application:
Oncology: High demand for biosimilars due to the rising incidence of cancers and the need for affordable treatments.
Chronic Diseases: Biosimilars for diabetes, arthritis, and cardiovascular diseases are critical for cost-effective long-term care.
Autoimmune Diseases: Increasing prevalence of conditions like rheumatoid arthritis and multiple sclerosis is driving the demand for biosimilars.
Infectious Diseases: Expansion of biosimilars in this sector due to the global burden of infections.
By Manufacturing Type:
In-House Manufacturing: Pharmaceutical companies developing their own biosimilars to maintain control over production.
Contract Manufacturing: Outsourcing production to specialized third-party manufacturers for efficiency and scalability.
By End-User:
Hospitals and Clinics: Major centers for biosimilars administration.
Pharmaceutical Companies: Driving the production and distribution of biosimilars.
Research Institutes: Key players in the innovation and clinical trials of biosimilars.
Key Growth Drivers
Patent Expirations of Biologics: As patents for major biologic drugs expire, the market opens up for biosimilar development.
Rising Healthcare Costs: Increasing demand for cost-effective alternatives to expensive biologic therapies.
Chronic Disease Prevalence: Growing rates of cancer, diabetes, and autoimmune diseases are spurring demand for biosimilars.
Government Initiatives: Regulatory frameworks supporting the approval and adoption of biosimilars.
Read More at: - https://www.skyquestt.com/report/biosimilars-market
Leading Companies in the Market
SkyQuest’s Biosimilars Market report highlights key players driving innovation and expansion, including:
Pfizer Inc.
Novartis AG (Sandoz)
Amgen Inc.
Samsung Bioepis
Biocon
Celltrion Healthcare
Teva Pharmaceuticals
Mylan N.V.
Fresenius Kabi
Hoffmann-La Roche Ltd
Challenges and Opportunities
The biosimilars market faces hurdles such as complex manufacturing processes and regulatory challenges. However, the growing acceptance of biosimilars by healthcare providers, coupled with increasing investments in biosimilar R&D, presents significant opportunities for market players to innovate and expand.
Take Action Now: Secure Your Report Today - https://www.skyquestt.com/buy-now/biosimilars-market
Future Outlook
As healthcare systems worldwide strive to reduce costs without compromising care quality, the biosimilars market is poised for strong growth. Companies that prioritize technological advancements, navigate regulatory frameworks efficiently, and focus on patient access will thrive in this evolving market.
The biosimilars market is shaping the future of affordable healthcare. With increasing demand for cost-effective treatments, decision-makers must keep pace with the trends and opportunities in this dynamic sector. For more comprehensive insights and strategic recommendations, consult SkyQuest’s in-depth Biosimilars Market report.
0 notes
Text
Pharmacovigilance Market Size, Share, Growth, Analysis Forecast to 2030
Pharmacovigilance Industry Overview
The global pharmacovigilance market size was estimated at USD 7.32 billion in 2023 and is anticipated to grow at a CAGR of 6.8% from 2024 to 2030.
The rising incidence of Adverse Drug Reactions (ADRs) owing to drug abuse and the prevalence of diseases that require a combination of drugs are the major growth drivers for the market. In addition, an upward shift in the production of novel drugs and the presence of stringent government regulatory frameworks for drug safety are significantly boosting the market growth. For instance, the U.S. FDA and the EU’s European Medical Agency (EMA) formulate regulatory guidelines for all phases of clinical trials. Moreover, advancements in the development of ADR databases and information systems have enabled accurate reporting of information, which can be further utilized by research professionals for prospective clinical studies, thereby fueling overall growth.
Gather more insights about the market drivers, restrains and growth of the Pharmacovigilance Market
A rise in the incidence of chronic diseases, such as cancers, diabetes, and cardiovascular & respiratory disorders, has led to an increase in drug consumption worldwide. According to a WHO report on pharmaceutical consumption, medicines to treat chronic diseases accounted for a larger proportion of the total volume of drug consumption in nonhospital setups. Increasing drug development activities in areas such as personalized medicines, biosimilars, orphan drugs, and companion diagnostics, along with adaptive trial designs, is projected to boost the demand for pharmacovigilance services in the coming years.
Furthermore, the increasing incidence of ADR and drug toxicity is fueling the market growth. According to the National Center for Biotechnology Information (NCBI), approximately 5% of total hospitalizations in a year are due to ADR in Europe. Furthermore, a February 2022 article published in the Journal of Current Medicine Research and Practice titled "Characterization of Seriousness and Outcome of Adverse Drug Reactions in Patients Receiving Cancer Chemotherapy Drugs - A Prospective Observational Study" revealed that serious Adverse Drug Reactions (ADRs) in the U.S. result in over 100,000 deaths annually and have been a major health concern since the past decade.
Browse through Grand View Research's Healthcare IT Industry Research Reports.
The global personalized medicine market was valued at USD 529.28 billion in 2023 and is projected to grow at a CAGR of 8.20% from 2024 to 2030.
The global medical writing market size was valued at USD 3.8 billion in 2022 and is expected to expand at a CAGR of 10.46% from 2023 to 2030.
Key Pharmacovigilance Company Insights
The market is characterized by a few notable players, including Accenture, IQVIA, Cognizant, Aris Global, and IBM Corporation. These manufacturers are actively utilizing strategic initiatives such as mergers and acquisitions to strengthen their market positions. For instance, in October 2023, IQVIA strategically collaborated with argenx to advance treatment to patients with rare autoimmune diseases through innovative and integrated technology-enabled pharmacovigilance (PV) safety services and solutions.
"We look forward to collaborating closely with IQVIA on this important business need. We aim to innovate in all that we do and IQVIA’s technology-enabled PV services and solutions will allow for efficient data integration as we work to bring new treatment options to autoimmune patients”.
- Tim Van Hauwermeiren, CEO, argenx.
In November 2022, Linical Americas (a U.S. subsidiary of The Linical Group) and Science 37 Holdings, Inc. announced a partnership to enable the deployment of hybrid and fully decentralized trials. This partnership will provide enhanced access to Linical’s offerings.
“By partnering with Linical, we have an important new ally in our mission to accelerate clinical research and enable universal access for patients,” “Our technology-enabled Metasite will empower and enhance Linical’s solutions, helping patient’s access new life-changing treatments quicker, in the largest and most prevalent therapeutic areas.”
- ”David Coman, Chief Executive Officer of Science 37
Recent Developments
In March 2023, ICON plc and LEO Pharma announced partnerships to impel execution of clinical trials in medical dermatology space.
“We’ve been exploring several outsourcing models but found a hybrid sourcing model to be the most efficient. Partnering with ICON supports our 2030 strategy as it will help us to bring innovative treatments to patients faster while also supporting a more sustainable business through scalability and flexibility. “ICON’s wealth of services and leading position in clinical development will support LEO Pharma’s R&D strategy building on driving innovation through partnerships and support staying competitive.”
- Jörg Möller, Executive Vice President and head of Global R&D at LEO Pharma
In February 2023, Parexel International Corporation announced the launch of Expert Series-New Medicines, Novel Insights. The series features latest insights from company’s cross-functional experts postanalysis of trends that impact drug development and evidence-based guidance for the biopharmaceutical industry.
“Cutting-edge medicines are becoming more personalized and precise across the therapeutic landscape, while the process to develop those therapies is reaching new heights of complexity. “Parexel’s New Medicines, Novel Insights research series offers expert-led guidance to deliver on the promise of patient-focused drug development and bring impactful treatments to patients more rapidly.”
- Amy McKee, MD, Chief Medical Officer and Head of Oncology Center of Excellence
Key Pharmacovigilance Companies
The following are the leading companies in the pharmacovigilance market These companies collectively hold the largest market share and dictate industry trends
Accenture
IQVIA Inc.
Cognizant
Clinquest Group B.V. (Linical Americas)
IBM
Laboratory Corporation of America Holdings
ArisGlobal
Capgemini
ITClinical
ICON plc.
TAKE Solutions Limited
Parexel International (MA) Corporation
Wipro
United BioSource LLC
BioClinica Inc. (Clario)
ClinChoice (formerly FMD K&L
Order a free sample PDF of the Pharmacovigilance Market Intelligence Study, published by Grand View Research.
0 notes
Text
The global oncology biosimilars market has grown to $4.7 Billion in 2023 and is set to reach $30.3 Billion by 2032, with a robust CAGR of 22.3%. This marks a major advancement in affordable cancer treatments.
0 notes
Text
Shaping the Future: Growth Prospects in Australia’s Pharmaceuticals Market
The Australia pharmaceuticals market is projected to be valued at USD 13.21 billion in 2024 and is anticipated to reach USD 17.89 billion by 2029, registering a compound annual growth rate (CAGR) of 6.25% during the forecast period from 2024 to 2029. The market is set for continued growth, driven by advancements in healthcare, an aging population, and a robust regulatory environment. Based on insights from Mordor Intelligence, here’s an overview of the key factors shaping the future of the industry:
Market Overview: Key Growth Drivers
Aging Population and Chronic Disease Management: Australia's aging population is a significant factor contributing to the growth of the pharmaceuticals market. With increasing cases of chronic diseases such as diabetes, cardiovascular disorders, and cancer, the demand for prescription drugs and specialty treatments is on the rise.
Government Healthcare Initiatives: Government support through policies like the Pharmaceutical Benefits Scheme (PBS) helps in subsidizing medicines, making them more accessible to the population. This not only boosts pharmaceutical sales but also encourages pharmaceutical companies to invest in new drug development.
Growth in Biopharmaceuticals: There is a growing focus on biopharmaceuticals, especially in the areas of oncology, immunology, and rare diseases. The development of biologics and biosimilars is expected to further fuel market growth, as these therapies offer new treatment options for complex medical conditions.
Innovations and Trends Shaping the Market
Personalized Medicine: The rise of precision medicine is one of the key trends in Australia’s pharmaceutical landscape. Personalized treatments, which are tailored to individual genetic profiles, are gaining popularity in areas such as oncology and immunology. This trend is driving innovation and investments in genetic research and drug development.
Digital Transformation: The integration of digital health technologies, including telemedicine and electronic health records, is transforming the pharmaceutical industry. These advancements enable better patient outcomes and streamline drug delivery processes, leading to improved healthcare services.
Growth of Generic Pharmaceuticals: As patents on several high-profile drugs expire, the market for generic pharmaceuticals is expanding. Generic drugs provide cost-effective alternatives to branded medicines, contributing to broader market accessibility.
Australia Pharmaceuticals Market Future Outlook
The Australia pharmaceuticals market is expected to see steady growth in the coming years, with increasing demand for advanced therapies and personalized medicine. Innovations in biotechnology and genomics will continue to play a pivotal role in the industry’s expansion, while government support will ensure access to essential medicines.
The market is projected to benefit from continued investments in R&D, particularly in biopharmaceuticals and rare disease treatments. With a growing emphasis on sustainability and digitalization, the pharmaceutical landscape in Australia is set to evolve, offering new growth opportunities for industry players.
Conclusion
The future of Australia’s pharmaceuticals market looks promising, with a strong focus on innovation, patient-centered care, and accessibility. Despite facing challenges such as regulatory barriers and high costs, the market is well-positioned for growth, driven by technological advancements and increasing healthcare demands.
For a detailed overview and more insights, you can refer to the full market research report by Mordor Intelligence: https://www.mordorintelligence.com/industry-reports/australia-pharmaceutical-market
#Australia Pharmaceuticals Market#Australia Pharmaceuticals Market Size#Australia Pharmaceuticals Market Share#Australia Pharmaceuticals Market Growth#Australia Pharmaceuticals#MordorIntelligence#Marketresearchreports#industryresearchreports
0 notes
Text
Tumor Necrosis Factor Inhibitor Drugs Market — Forecast(2024–2030)
Tumor Necrosis Factor (TNF) Inhibitor Drugs Market size was valued at USD 248.8 Billion in 2023 and is projected to reach USD 13019.65 Billion by 2030, growing at a CAGR of 0.64% during the forecast period 2024–2031.

Market Drivers
Rising Prevalence of Autoimmune Diseases: The demand for TNF inhibitor drugs is growing due to the increasing incidence of autoimmune conditions such as psoriasis, Crohn’s disease, ankylosing spondylitis, and rheumatoid arthritis. These drugs help manage symptoms, reduce inflammation, and slow disease progression in affected individuals.
Request Sample
Aging Population: The global aging population is more susceptible to autoimmune and chronic inflammatory diseases, boosting the need for TNF inhibitor therapies. As the population continues to age, the prevalence of these conditions is expected to rise, driving greater demand for TNF inhibitors.
Broadened Indications and Treatment Options: TNF inhibitors are being prescribed for an expanding range of inflammatory and immune-mediated disorders beyond rheumatoid arthritis and inflammatory bowel disease. This includes conditions like ulcerative colitis, juvenile idiopathic arthritis, and psoriatic arthritis, which contributes to increased adoption and market growth.
Effective Disease Management: TNF inhibitors have proven effective in alleviating symptoms, improving quality of life, and preventing joint damage in autoimmune disease patients. Their ability to manage chronic conditions and reduce inflammation addresses significant unmet medical needs.
Inquiry Before Buying
Advancements in Biotechnology and Drug Development: Innovations in immunology, molecular biology, and biotechnology are leading to the development of new TNF inhibitors with better tolerance, safety, and efficacy profiles. Additionally, the introduction of biosimilar TNF inhibitors provides more affordable treatment options, enhancing patient access.
Patient Preference for Biologic Therapies: Many patients favor biologic therapies, including TNF inhibitors, over traditional disease-modifying antirheumatic drugs (DMARDs) due to their targeted action, longer dosing intervals, and potential for improved outcomes. This preference fuels the market demand.
Increased Healthcare Expenditure: Rising healthcare spending, particularly in developed countries, facilitates access to TNF inhibitor medications. Government healthcare programs, private insurance, and reimbursement policies help make these treatments more affordable and accessible, supporting market growth.
Support from Clinical Guidelines and Evidence: Strong clinical data and treatment guidelines reinforce the use of TNF inhibitors, boosting physician confidence and patient acceptance. Evidence-based recommendations guide the adoption and prescribing practices of these therapies.
Advances in Personalized Medicine: Developments in pharmacogenomics and personalized medicine allow for customized treatment plans based on individual patient needs, condition severity, and therapy response. This personalization enhances treatment efficacy and patient satisfaction, driving market demand.
Market Restraints
High Treatment Costs: TNF inhibitors are often expensive, which can be a barrier to access for patients, healthcare systems, and payers, especially in regions with limited healthcare resources or inadequate insurance coverage. The high cost may lead to underutilization of these therapies.
Buy Now
Safety Concerns and Side Effects: Despite their effectiveness, TNF inhibitors can be associated with risks such as infections, cancer, infusion reactions, and other side effects. Safety concerns may deter both patients and healthcare providers from initiating or continuing treatment, particularly in high-risk groups.
Immunogenicity and Loss of Response: Over time, patients may develop antibodies against TNF inhibitors, leading to treatment failure and reduced efficacy. This immunogenicity can necessitate dose adjustments, alternative therapies, or combination treatments to maintain disease control.
Limited Efficacy in Certain Populations: TNF inhibitors may be less effective in patients with severe or treatment-resistant diseases, those with concurrent health conditions, or individuals with specific genetic profiles. Limited response in these populations can impact the perceived value of these therapies.
Availability of Alternative Therapies: The presence of alternative treatments, including non-pharmacological options, tailored synthetic DMARDs, and other biologic drugs, can pose competition to TNF inhibitors. This competition may affect their market share and acceptance.
Regulatory and Reimbursement Challenges: Various regulatory hurdles, market access barriers, and reimbursement constraints can restrict patient access to TNF inhibitors. Delays in payer policies, formulary restrictions, and regulatory approvals may hinder market penetration.
Impact of Patent Expirations and Biosimilar Competition: As patents for branded TNF inhibitors expire, biosimilars enter the market, increasing competition and driving down prices. This competition can affect the pricing, profitability, and market share of original TNF inhibitor products.
Chronic Nature of Autoimmune Disorders: Autoimmune disorders treated with TNF inhibitors require long-term management, which can be challenging for patients. Continuous treatment adherence, regular monitoring, and follow-up are necessary, which can impact patient compliance and healthcare resource utilization.
Challenges with Drug Administration: TNF inhibitors are often administered intravenously or via subcutaneous injection, which can be uncomfortable and logistically challenging for patients. Issues like injection site reactions, complex dosing schedules, and the need for medical resources can affect treatment adherence and patient satisfaction.
Market Segmentation
The Global TNF Inhibitor Drugs Market is segmented based on:
Drug Type: Includes monoclonal antibodies, recombinant proteins, and biosimilars.
Application: Encompasses rheumatoid arthritis, psoriasis, inflammatory bowel disease, ankylosing spondylitis, juvenile idiopathic arthritis, and other inflammatory and immune-mediated conditions.
Distribution Channel: Comprises hospital pharmacies, retail pharmacies, online pharmacies, and specialty pharmacies.
Geography: Covers regions such as North America, Europe, Asia-Pacific, Latin America, and the Middle East and Africa.
#reseach marketing#marketing#digital marketing#Tumor#brain tumor#three tumors#health & fitness#healthcare#healthylifestyle
0 notes
Text
Top 5 players in US Biosimilar Market
Buy Now
STORY OUTLINE
Pfizer: Excelling in the line of Biosimilar drugs with an experience of more than 10 years with presence in over 180 countries.
Amgen: Making pharmaceutical products with an experience of over 40 years and presence in over 100 countries.
Viartis: Presence in over 165 countries, and making Biosimilar drugs in over 75 markets, this pharmaceutical company is another leading contributor of US Biosimilar market.
Coherus Biosciences: Increasing patient access to cost effective medicines with a Biosimilar drugs experience of 13 years.
Biogen: serving humanity through science with a experiences of more than 40 years in the field of biologics.
According to Ken Research, the US Biosimilar market is anticipated to grow at a CAGR of ~40% in the next five years which currently has a market size of ~USD 9.4 Bn.
The US Biosimilar market is rapidly growing and will be witnessing a significant growth in the next five years.
There are various reasons behind the rapid growth of US Biosimilar market. Some of the major reasons behind the growth of US Biosimilar market include the cost effective nature of Biosimilar drugs, rising geriatric population, rising prevalence of chronic diseases, and growing partnerships between companies to develop Biosimilar drugs.
Various companies and players are contributing to their best efforts in the growth of the US Biosimilar market.
This article aims to put light on the contributions done by the major players towards the growth of the US Biosimilar market.
1.Pfizer
Click to read more about Pfizer
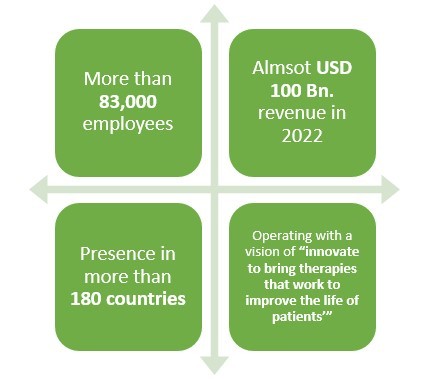
Pfizer is a leading American pharmaceutical company which is operating in the field of generics or original drugs for more than 30 years. But did you know that this pharma not only manufactures biologics but also biosimilar drugs?
Pfizer has been in the business of biosimilar drugs for more than 10 years and have been quite successful as well. With more than 83,000 employees and presence in over 180 countries, this leading pharmaceutical company made almost USD 2 Bn. revenue only from its Biosimilar drugs sale in 2021.
Recently, this pharmaceutical company also collaborated with Samsung in two deals to produce various biosimilar drugs in South Korea. The deal size between these two companies happens to be approximately USD 900 Bn.
The major Biosimilar drugs of this pharmaceutical giant are primarily
ZIRABEV (a Biosimilar of Avastin)
TRAZIMERA (a Biosimilar of Herceptin)
RUXIENCE (a Biosimilar of Rituxan)
RITACRIT (a Biosimilar of Epogen)
NVYEPRIA (a Biosimilar of Neulasta)
NIVESTYM (a Biosimilar of Neupogen)
FILGRASTIM (a Biosimilar of Neupogen).
2.Amgen
Click here to Download a Sample Report
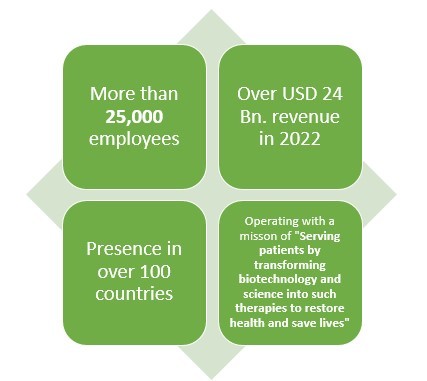
Amgen is another leading American pharmaceutical company which not only makes Biologics or generic drugs but also Biosimilar drugs. This pharmaceutical company has more than 40 years of experience when it comes to pharmaceutical line.
With over 25000 employees and presence in over 100 countries, this pharmaceutical company earned about USD 2 Bn. from their three biosimilar drugs which are reportedly MVASI, KANJITNTI, and AMJEVITA.
This pharma giant has also invested about USD 2 Bn. in the development of Biosimilar drugs.
This pharmaceutical company has made Biosimilar drugs primarily in 4 fields which are General Medicine, Oncology, and Hematology along with, Inflammation.
EPOTEIN ALFA
AMJEVITA
AVSOLA
KANJINTI
MVASI
RIABNI
are the various Biosimilar drugs of Amgen. And, STELARA, EYLEA, SOLIRIS are in their pipeline.
Recently Amgen revealed their Biosimilar report’s 8 version. It revealed a major information which said that the pharmaceutical company saved about USD 10 Bn. through their Biosimilar drugs in the past five years.
3.Viartis

Headquartered in Canonsburg, Pennsylvania, this American pharmaceutical company was founded only in 2020 yet they have achieved massive success in the pharmaceutical products with their revenue being USD 16 ~Bn. in 2022.
With presence in 165 countries and with over 45,000 employees worldwide, this pharmaceutical company makes pharmaceutical products in 10 areas which primarily are Cardiovascular, Dermatology, ophthalmology, Oncology, Gastroenterology, Women’s health, Infectious diseases, Diabetes & Metabolism, Immunology, CNS & Anesthesiology, Respiratory diseases and allergy.
Speaking of their first Biosimilar products, their first ever Biosimilar drug was launched in 2014. They have a variety of Biosimilar drugs which are primarily
TRASTUZUMAB
INSULIN ASPART
PEGFILGRASTIM
INSULIN GLARGINE-YFGN
ADALIMUMAB
BEVACIZUMAB
Their Biosimilar drug Insulin Glargine which is known as SEMGLEE was the first ever interchangeable Biosimilar drug in the United States which was FDA approved.
Their PEGFILGRASTIM also was the first ever FDA approved drug in the United States. They have launched their Biosimilar drugs in over 75 markets worldwide.
4.Coherus Biosciences:
Click here to Ask for a Custom Report
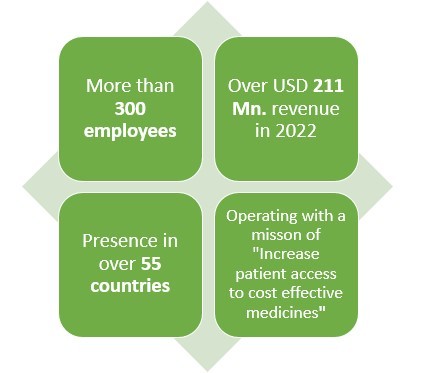
Headquartered in Redwood city, California this American pharmaceutical company earned a revenue of almost USD 211 Mn. In 2022.
With presence in over 55 countries and 300+ employees worldwide, this pharmaceutical company makes products in various areas such as solid tumors, non-small lung cancers, nasopharyngeal carcinoma, small cell lung cancer and hepatocellular carcinoma.
Speaking of their Biosimilar drugs, this pharma has been in the field of creating Biosimilar drugs since 2010 which has given them almost 13 years of experience.
This pharmaceutical company also disclosed that it plans to spend at least USD 1 Tn. on medicines worldwide, out of which at least 40% will be spent on Biosimilar drugs.
Their three major Biosimilar drugs which are also FDA approved include UDENCYA, YUSIMRY, and CIMERLI.
Udencya is a Biosimilar drug of Pegfilgrastim, Yusimry is a Biosimilar drug of Ranibizumab, and Cimerli is a Biosimilar drug of Adalimumab.
5.Biogen
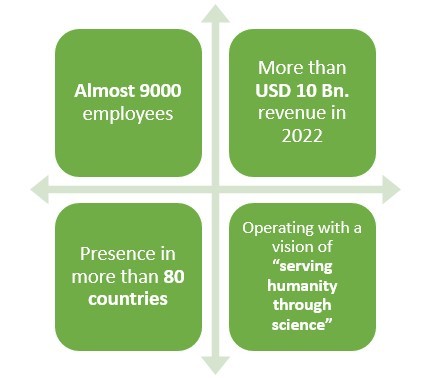
Headquartered in Cambridge, Massachusetts, this American pharmaceutical company earned a revenue of around USD 10 Bn. in 2022.
This company happens to have an experience of more than 40 years when it comes to making pharmaceutical products.
With presence in over 80 countries and more than 9000 employees worldwide, this pharmaceutical company primarily deals in Neurology, Specialized Immunology, Neuropsychiatry, Ophthalmology, and Rare Diseases.
ADUCANUMAB
LECANEMAB
TOFERSEN
ZURANOLONE
LITIFILIMAB
BENAPALI
FLIXABI
IMRALDI
are some of their Biosimilar drugs.
With their Biosimilar drugs, more than 250,000 people have gone on Anti-Tumor Necrosis Factor therapy.
Recently, this pharmaceutical company also made an agreement with Bio-Thera solutions to develop a Biosimilar drug for the treatment of Rheumatoid Arthritis.
#US Biosimilar Sector#United States Biosimilar Market#US Biosimilar Market forecast#US Biosimilar Market analysis#US Biosimilar Market trends#US Biosimilar Market share#US Biosimilar Market key players#US Biosimilar Market revenue#US Biosimilar Market growth#Monoclonal Antibodies in biosimilar market US#Recombinant Hormones in biosimilar industry US#Oncology in bio similar market US#Blood disorders in biosimilar market US#Research institutes in Biosimilar market US#US similar biotherapeutics products market#Hospitals in Biosimilar market US#Investors in Biosimilar market US#US comparable biologics products industry#US recombinant biosimilars industry#US replicate biologics sector#US analog biologics market
0 notes
Text
Navigating the Dynamic Landscape of the Biosimilars Market
In recent years, the global pharmaceutical industry has witnessed a transformative shift with the emergence of biosimilars. These innovative biologic medicines, often referred to as "follow-on biologics," are designed to be highly similar to existing reference biologics, offering a more cost-effective alternative without compromising on safety and efficacy. The biosimilars market has gained significant traction, revolutionizing patient access to essential treatments and reshaping the competitive landscape of the healthcare sector.
The Rise of Biosimilars: A Game-Changer in Healthcare
Biosimilars have swiftly gained prominence due to their potential to alleviate the financial burden of expensive biologics while maintaining high therapeutic standards. Unlike generic versions of small-molecule drugs, creating biosimilars involves intricate processes due to the complexity of biologic molecules. Rigorous analytical and clinical studies are conducted to demonstrate similarity in structure, function, and clinical outcomes, assuring both healthcare professionals and patients of their reliability.
Market Trends and Drivers
The biosimilars market is fueled by several key factors. One of the primary drivers is the impending patent expiration of several blockbuster biologics. As these patents lapse, it opens the door for biosimilar manufacturers to introduce affordable alternatives, fostering healthy competition and potentially lowering overall healthcare costs.
Moreover, regulatory agencies around the world are increasingly establishing clear pathways for biosimilar approval. This provides manufacturers with a streamlined route to market, encouraging more players to invest in biosimilar research and development. The resulting increase in product availability benefits patients, as they gain access to life-changing treatments that may have been previously out of reach.
Challenges and Opportunities
While the biosimilars market holds immense promise, it also presents challenges. Building trust among healthcare professionals and patients is paramount, as skepticism regarding biosimilar efficacy and safety still exists. Education and communication efforts are essential to dispel misconceptions and promote informed decision-making.
Additionally, manufacturing biosimilars involves maintaining stringent quality control and consistency, given the complexity of biologic production. Companies must invest in state-of-the-art manufacturing facilities and robust supply chains to ensure their products meet the required standards.
Global Market Outlook
The biosimilars market's growth is not confined to a specific region; it's a global phenomenon. Europe has led the way in biosimilar adoption, thanks to a well-defined regulatory framework. The Asia-Pacific region is also showing remarkable progress, driven by a large patient population and increasing healthcare expenditure. In North America, the biosimilars landscape is evolving, with the United States gradually embracing these alternatives to address rising healthcare costs.
The Road Ahead
As the biosimilars market continues to evolve, collaboration between regulatory authorities, healthcare providers, and manufacturers remains crucial. Establishing clear guidelines and promoting knowledge sharing will further bolster confidence in biosimilars. Ongoing research into novel therapeutic areas and innovative manufacturing techniques will drive product diversification and unlock new opportunities.
In conclusion, the biosimilars market is a disruptive force with the potential to democratize access to biologic therapies. By offering cost-effective alternatives to expensive reference biologics, biosimilars are revolutionizing healthcare systems worldwide. As regulatory processes mature and understanding grows, biosimilars will play an increasingly vital role in providing effective, accessible, and affordable treatments to patients in need.
#biosimilars market#biosimilars#market research reports#market research#industry analysis#market forecast
0 notes
Text
Exploring the Recombinant Chemicals Market: Innovations Shaping the Future
The Recombinant Chemicals Market represents a significant subset of the broader biotechnology and chemical sectors. Recombinant chemicals are produced through genetic engineering techniques, which allow for the precise manipulation of microbial, plant, or animal cells to produce desired chemical compounds. These chemicals have wide applications in pharmaceuticals, agriculture, industrial processes, and research, providing solutions that are more efficient, cost-effective, and sustainable compared to traditional chemical production methods.
The global recombinant chemicals market, valued at US$ 2.9 billion in 2023, is projected to grow at a CAGR of 7.8% from 2024 to 2034. By the end of 2034, the market is expected to reach US$ 6.7 billion, driven by advancements in biotechnology and increasing demand across various industries.
This growth is fueled by the rising need for biopharmaceuticals, green chemicals, and bio-based industrial products. The pharmaceutical sector remains the largest end-user of recombinant chemicals, especially in the production of proteins, enzymes, and other bioactive compounds. Additionally, the agricultural sector is witnessing increased adoption of recombinant chemicals in the development of bio fertilizers and pesticides.
For More Details, Request for a Sample of this Research Report: https://www.transparencymarketresearch.com/recombinant-chemicals-market.html
Market Segmentation
The recombinant chemicals market can be segmented based on various factors:
By Service Type:
Contract Research Services
Manufacturing Services
Custom Synthesis Services
By Sourcing Type:
In-house Production
Outsourced Production
By Application:
Pharmaceuticals
Agriculture
Industrial Enzymes
Cosmetics
Food and Beverages
By Industry Vertical:
Healthcare
Agriculture
Chemical
Biotechnology
Food Processing
By Region:
North America
Europe
Asia-Pacific
Latin America
Middle East & Africa
Regional Analysis
North America: Leading the market due to well-established biotechnology and pharmaceutical industries, as well as heavy investment in R&D for recombinant chemical production.
Europe: The second-largest market, driven by increasing demand for sustainable and bio-based chemicals, as well as stringent regulations on chemical production.
Asia-Pacific: Expected to witness the highest growth during the forecast period due to expanding pharmaceutical and agricultural industries in countries like China and India, along with supportive government initiatives.
Latin America and the Middle East & Africa: These regions are also gaining traction as key markets for recombinant chemicals due to the growing demand for agricultural chemicals and biopharmaceuticals.
Market Drivers and Challenges
Drivers:
Technological Advancements: Continuous innovation in genetic engineering and fermentation technologies is expanding the capabilities of recombinant chemical production.
Sustainability: Recombinant chemicals offer a more environmentally friendly alternative to traditional petrochemical-based products, driving demand in industries focused on sustainability.
Rising Demand for Biopharmaceuticals: The growing need for advanced drugs, including biosimilars, monoclonal antibodies, and vaccines, is fueling demand for recombinant chemicals.
Challenges:
High Production Costs: The initial investment required for setting up recombinant chemical production facilities is high, which may limit market penetration, especially in developing regions.
Regulatory Hurdles: Complex regulatory frameworks, particularly in the pharmaceutical and agricultural sectors, can slow down the commercialization of recombinant chemicals.
Technical Limitations: The scalability of production processes and achieving consistency in yield and purity remain key challenges.
Market Trends
Shift Towards Green Chemicals: With increasing environmental regulations, there is a clear shift toward the development and adoption of bio-based recombinant chemicals.
Expanding Applications: Recombinant chemicals are finding new applications in sectors like food processing, cosmetics, and industrial enzymes, driven by their versatility and efficiency.
Collaborations and Partnerships: Companies are increasingly forming partnerships with research institutions and contract manufacturing organizations (CMOs) to enhance production capabilities and streamline R&D processes.
Future Outlook
The future of the recombinant chemicals market looks promising, with expected breakthroughs in gene editing technologies like CRISPR and the expanding use of synthetic biology. The ongoing shift toward personalized medicine and bio-based solutions in various industries will further drive demand for recombinant chemicals. The market is poised to witness significant investments in R&D and infrastructure development, particularly in the Asia-Pacific and European regions.
Buy this Premium Research Report: https://www.transparencymarketresearch.com/checkout.php?rep_id=86368<ype=S
Key Market Study Points
Analysis of recombinant chemical applications across diverse industries.
Regional market dynamics and their impact on growth projections.
Competitive landscape analysis, focusing on key players and their strategies.
Technological advancements and their role in shaping the market’s future.
Evaluation of the sustainability of recombinant chemical production methods.
Competitive Landscape
The recombinant chemicals market is highly competitive, with key players focusing on innovation, product differentiation, and strategic collaborations. Major players include:
BASF SE
Merck KGaA
Lonza Group
Evonik Industries AG
Genentech, Inc.
These companies are investing in advanced biotechnologies and expanding their recombinant chemical portfolios through mergers, acquisitions, and partnerships with smaller firms or research institutions.
Recent Developments
Mergers and Acquisitions: Several key players have recently acquired smaller biotech firms to strengthen their recombinant chemical capabilities.
Technological Advancements: New methods of gene editing and fermentation are being developed, allowing for more efficient and cost-effective production of recombinant chemicals.
Regulatory Approvals: Recent regulatory approvals for new recombinant-based drugs and chemicals have opened new avenues for market growth, particularly in pharmaceuticals and agriculture.
About Transparency Market Research
Transparency Market Research, a global market research company registered at Wilmington, Delaware, United States, provides custom research and consulting services. Our exclusive blend of quantitative forecasting and trends analysis provides forward-looking insights for thousands of decision makers. Our experienced team of Analysts, Researchers, and Consultants use proprietary data sources and various tools & techniques to gather and analyses information.
Our data repository is continuously updated and revised by a team of research experts, so that it always reflects the latest trends and information. With a broad research and analysis capability, Transparency Market Research employs rigorous primary and secondary research techniques in developing distinctive data sets and research material for business reports.
Contact:
Transparency Market Research Inc.
CORPORATE HEADQUARTER DOWNTOWN,
1000 N. West Street,
Suite 1200, Wilmington, Delaware 19801 USA
Tel: +1-518-618-1030
USA – Canada Toll Free: 866-552-3453
Website: https://www.transparencymarketresearch.com
0 notes
Text
Human Insulin Market to Reach USD 24.71 Billion by 2031

Steady Growth Projected Amid Rising Diabetes Prevalence
The human insulin market, valued at USD 19.21 billion in 2023, is anticipated to grow to USD 24.71 billion by 2031. This growth represents a Compound Annual Growth Rate (CAGR) of 3.2% over the forecast period from 2024 to 2031. The market expansion is largely driven by the increasing prevalence of diabetes worldwide and the rising demand for effective insulin therapies.
Key Drivers of Market Growth
Rising Diabetes Incidence: The global increase in diabetes cases, particularly Type 1 and Type 2 diabetes, is a significant factor driving the demand for human insulin. As the prevalence of diabetes continues to rise, there is a growing need for advanced insulin therapies.
Technological Advancements: Innovations in insulin delivery systems and the development of more efficient insulin formulations are contributing to market growth. The advent of smart insulin pens and continuous glucose monitoring systems are enhancing patient management and adherence.
Increasing Healthcare Access: Expanding access to healthcare in emerging markets is also contributing to the growth of the human insulin market. As healthcare infrastructure improves and more people gain access to diabetes management resources, the demand for insulin is expected to rise.
Market Trends and Developments
The market is witnessing trends such as the development of biosimilar insulins, which offer more cost-effective treatment options compared to traditional insulin products. Additionally, there is a growing focus on personalized medicine, with the aim of tailoring insulin treatments to individual patient needs.
Regional Insights
North America currently dominates the human insulin market due to advanced healthcare infrastructure and high diabetes prevalence. However, the Asia-Pacific region is projected to experience the fastest growth during the forecast period, driven by rising diabetes cases and improving healthcare systems in countries like India and China.
Future Outlook
With ongoing advancements in insulin technology and increasing global diabetes rates, the human insulin market is set for steady growth. Companies are expected to continue investing in research and development to enhance insulin products and delivery methods, ensuring better management of diabetes and contributing to the market's expansion through 2031.
0 notes
Text
Cancer Biologics Market Future Outlook: Predictions and Analysis
The global cancer biologics market is projected to experience robust growth, with its market size expected to expand from USD 102.2 billion in 2023 to an impressive USD 195.5 billion by 2032. The market is set to grow at a compound annual growth rate (CAGR) of 7.5% during the forecast period from 2024 to 2032, driven by technological advancements in biologic therapies and an increasing global burden of cancer.
Cancer biologics are advanced therapeutic agents derived from living organisms or their products, such as proteins, DNA, and antibodies, designed to target specific cancer cells. Unlike traditional chemotherapy, biologics are often more precise and offer the potential to minimize damage to healthy cells, making them a preferred treatment option for various types of cancer.
Get Free Sample PDF: https://www.snsinsider.com/sample-request/4512
Key Drivers of Market Growth
Rising Cancer Incidence: The global rise in cancer cases is a significant factor propelling the demand for biologics. According to the World Health Organization (WHO), cancer is one of the leading causes of death globally, with millions of new cases diagnosed each year. As the global population ages, the incidence of cancer is expected to increase, driving the demand for effective and innovative treatment options like biologics.
Advances in Biotechnology and Immunotherapy: Recent advancements in biotechnology, particularly in immunotherapy and targeted therapies, are revolutionizing cancer treatment. Cancer biologics, such as monoclonal antibodies, cell-based therapies, and checkpoint inhibitors, have shown great promise in improving patient outcomes. The success of immuno-oncology therapies like CAR-T cell therapies and immune checkpoint inhibitors has expanded treatment options for patients and created a surge in market demand.
Personalized Medicine and Precision Oncology: The trend toward personalized medicine and precision oncology is another critical growth driver for cancer biologics. By tailoring treatments based on individual genetic profiles and tumor characteristics, biologics offer a more targeted approach to cancer treatment. This reduces the likelihood of adverse side effects and enhances treatment efficacy, particularly for patients with rare or aggressive cancers.
Favorable Regulatory Approvals: The regulatory landscape for cancer biologics has also improved in recent years, with several breakthrough therapies receiving fast-track approvals from regulatory bodies like the U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA). These expedited approval processes have encouraged pharmaceutical companies to invest in research and development (R&D) for new biologics, accelerating the market’s growth.
Challenges and Opportunities
While the cancer biologics market holds great promise, several challenges remain. High development costs, complex manufacturing processes, and the need for advanced infrastructure to produce biologics can act as barriers for smaller biotech firms. Additionally, the cost of cancer biologic therapies can be prohibitively expensive, limiting access for patients in low- and middle-income countries.
However, significant opportunities exist, particularly in the areas of biosimilars and next-generation biologics. As patents for several blockbuster biologic drugs expire, the market for biosimilars—cheaper, highly similar alternatives—will expand, offering more affordable treatment options for cancer patients. Moreover, continuous innovation in biopharmaceuticals, including advancements in gene editing and cell-based therapies, will open new pathways for cancer treatment, further driving market growth.
Regional Insights
North America continues to dominate the cancer biologics market, attributed to its strong healthcare infrastructure, high levels of investment in R&D, and a large patient population. The U.S. market, in particular, benefits from government support for cancer research and early adoption of innovative therapies.
Europe follows closely, with significant investments in biotechnology and increasing access to advanced cancer treatments. The Asia-Pacific region is expected to witness the fastest growth during the forecast period due to rising cancer incidence, improving healthcare infrastructure, and increasing government initiatives to promote cancer research. Countries like China and India are becoming key players in the market, with growing R&D activities and expanding access to biologic treatments.
Future Outlook
The future of the cancer biologics market looks promising, with continued advancements in biotechnology, precision medicine, and immunotherapy expected to drive significant growth. With a projected CAGR of 7.5% from 2024 to 2032, the market is on track to nearly double in size, reaching USD 195.5 billion by 2032. Biologics will play a central role in the ongoing battle against cancer, offering new hope for patients and transforming cancer care worldwide.
In conclusion, the cancer biologics market is set for robust growth, driven by rising cancer incidence, advancements in biotechnology, and the increasing adoption of personalized medicine. From USD 102.2 billion in 2023, the market is expected to reach USD 195.5 billion by 2032, significantly impacting the global healthcare landscape.
Other Trending Reports
Smart Fertility Tracker Market
Venous Thromboembolism Treatment Market
Automated Liquid Handling Technologies Market
Digestive Health Supplements Market
0 notes