#Chromatography Consumables
Text
#romatography Consumables#Analytical Reagents#Chromatography Reagents#Chromatography Buffers#Chromatography Reagents Market
0 notes
Text
Biopharmaceutical Processing Equipment and Consumables Market - Global Opportunity Analysis and Industry Forecast (2022-2029)
Meticulous Research®– leading global market research company published a research report titled “Biopharmaceutical Processing Equipment and Consumables Market by Product Type {Filtration, Chromatography [Columns, Equipment], Disposable Bioreactors, Cell Culture Media, Shakers, Services), Application (Vaccine, mAb, R&D), and End User- Forecast to 2029.”
According to this latest publication from Meticulous Research, the global biopharmaceutical processing equipment and consumables market is expected to grow at a CAGR of 10.3% to reach $70.84 billion by 2029. Initiatives supporting the adoption of biopharmaceuticals, capacity expansions of biopharmaceutical manufacturing plants, and growing use of single-use technologies in commercial bioproduction are the factors driving the market growth.
Biopharmaceutical Processing Equipment and Consumables Market: Future Outlook
The global biopharmaceutical processing equipment and consumables market study presents, historical market data in terms of values (2020 and 2021), estimated current data (2022), and forecasts it for 2029 –by Product Type (Filtration Systems, Chromatography Systems {Resins, Columns, & Equipment}, Bioreactors {Reusable Bioreactors, Disposable/Single-use Bioreactors}, Cell Culture Products {Cell Culture Media [Cell Culture Media, by Physical Form–Dry Powder Media, Liquid Media], [Cell Culture Media, by Type–Off-the-Shelf Media, Custom Media], [Cell Culture Media Market, by Source-Chemically Defined Media, Natural Media], Reagents and Supplements, Cell and Cell Lines, Serum}, Mixing Systems, Bioprocessing Containers, Sterilizers, Centrifuges, Incubators, Shakers, Biosafety Cabinets, Other Consumables and Accessories, Services), Application (Commercial Bioproduction {Vaccine Manufacturing, mAb Production, Recombinant Protein Production, Cell and Gene Therapy Production}, Research and Development), End User (Biopharmaceutical/ Biotechnology Companies, Contract Development and Manufacturing Organizations and Contract Research Organizations (CDMOs/CROs), & Academics and Research Institutes), and Geography. The study also evaluates industry competitors and analyzes their market share at global and regional levels.

Download Free Sample PDF Copy: https://www.meticulousresearch.com/download-sample-report/cp_id=4200
Scope of the Report:
Global Biopharmaceutical Processing Equipment and Consumables Market, by Product Type
Resins
Columns
Equipment
Reusable Bioreactors
Disposable/Single-use Bioreactors
Dry Powder Media
Liquid Media
Off-the-Shelf Media
Custom Media
Chemically Defined Media
Natural Media
(Note: Other equipment and consumables include membrane adsorbers, cell disruption reagents, pipettes, syringes, vials, closures, tubing, connectors, and sensors)
Global Biopharmaceutical Processing Equipment and Consumables Market, by Application
Vaccine Manufacturing
mAb Production
Recombinant Protein Production
Cell and Gene Therapy Production
Global Biopharmaceutical Processing Equipment and Consumables Market, by End User
Biopharmaceutical/ Biotechnology Companies
Contract Development and Manufacturing Organizations and Contract Research Organizations (CDMOs/CROs)
Academics and Research Institutes
Biopharmaceutical Processing Equipment and Consumables Market, by Geography
U.S.
Canada
Germany
U.K.
France
Italy
Spain
Switzerland
Rest of Europe (RoE)
China
Japan
India
Rest of APAC (RoAPAC)
Speak to Our Analyst: https://www.meticulousresearch.com/speak-to-analyst/cp_id=4200
Based on product type, the filtration systems segment is estimated to account for the largest share of the market in 2022. The technological developments in filtration technologies and accelerated developments of single-use filtration systems to meet the growing need for single-use bioprocessing systems are contributing to the largest market share.
Based on application, the market is segmented into commercial bioproduction and R&D. The commercial bioproduction segment is estimated to account for the largest share of the market in 2022, owing to the growing number of biopharmaceuticals in the clinical development and nearing patent expiry of biologics.
Based on end user, the biopharmaceutical/biotechnology companies segment is estimated to account for the largest share of the market in 2022. The largest revenue share is attributed to the growing demand for biopharmaceutical equipment for commercial bioproduction in biopharmaceutical/biotechnology companies.
Geographic Review:
This research report analyzes major geographies and provides comprehensive analysis for North America (U.S. and Canada), Europe (Germany, France, U.K., Italy, Spain, Switzerland, and Rest of Europe), Asia-Pacific (China, Japan, India, and RoAPAC), Latin America, and Middle East & Africa.
North America is estimated to command the largest share of the biopharmaceutical processing equipment and consumables market in 2022, followed by Europe and Asia-Pacific. The U.S. is estimated to be the largest shareholding market in North America in 2022. An increase in the biotechnology R&D expenditure, the emergence of infectious diseases, supportive initiatives for increasing adoption of biosimilars, and large number of biopharmaceutical companies are some of the major factors driving the demand for biopharmaceutical processing equipment and consumables in the country.
BUY NOW: https://www.meticulousresearch.com/Checkout/94239620
Key Players
The key players operating in the global biopharmaceutical processing equipment and consumables market are 3M Company (U.S.), Thermo Fisher Scientific, Inc. (U.S.), Bio-Rad Laboratories, Inc. (U.S.), Danaher Corporation (U.S.), Agilent Technologies, Inc (U.S.), Repligen Corporation (U.S.), Sartorius AG (Germany), Merck KGaA (Germany), Eppendorf AG (Germany), and Solaris Biotechnology Srl (Italy).
Key questions answered in the report-
Which are the high-growth market segments in terms of product type, application, end user, and regions/countries?
What was the historical market for biopharmaceutical processing equipment and consumables across the globe?
What are the market forecasts and estimates for the period 2022–2029?
What are the major drivers, restraints, challenges, opportunities, and trends in the global market of biopharmaceutical processing equipment and consumables?
Who are the major players in the global biopharmaceutical processing equipment and consumables market?
How is the competitive landscape, and who are the market leaders in the global biopharmaceutical processing equipment and consumables market?
What are the recent developments in the biopharmaceutical processing equipment and consumables market?
What are the different strategies adopted by the major players in the biopharmaceutical processing equipment and consumables market?
What are the geographical trends and high growth regions/countries?
top 10 companies operating in Biopharmaceutical Processing Equipment And Consumables Market- https://meticulousblog.org/top-10-companies-in-biopharmaceutical-processing-equipment-and-consumables-market/
Contact Us:
Meticulous Research®
Email- [email protected]
Contact Sales- +1-646-781-8004
Connect with us on LinkedIn- https://www.linkedin.com/company/meticulous-research
Connect with us on Twitter- https://twitter.com/MeticulousR123
#Biopharmaceutical Equipment#Biopharmaceutical#Biopharmaceutical Processing#lifescience#Biopharmaceutical Processing Equipment#Biopharmaceutical Processing Equipment and Consumables Market#Biopharmaceutical Processing Equipment and Consumables#healthcare#drug discovery#drug development#medical devices#Filtration#Chromatography#Disposable Bioreactors#Cell Culture Media#Shakers#Vaccine#mAb#health#medical device#medical equipment
0 notes
Note
Ancient Ceramic
What is the ancient ceramic? Is it really a ceramic? Some of the art has the lizards directly interacting and 'using' the surface of the material, what's up with that? Is there an use for salvaged or reclaimed ceramic?
Ancient ceramic is definitely a material with properties. It occupies space and possesses mass. It's... probably made out of particles, but lizards aren't actually sure. It's called a ceramic because it's neither organic or metallic, but that's where the similarities to pottery end.
Looking at it with the most powerful microscopes they have, all lizards can see is a single unbroken surface. Chromatography techniques aren't helpful, as nothing in the stuff can be burned or dissolved. Most other material analysis techniques fall flat or give nonsensical results.
Whatever the mechanisms behind it, the material properties of ancient ceramic are that it's superhard, heavy, and conducts flux exceptionally well. When in it's "natural state" as part of an ancient structure, ancient ceramic doesn't just conduct flux, but it consumes it as well. When it's cut off from sources of flux, it slowly "dies," losing it's exceptional strength and hardness. It eventually dissolves away like a sand castle in the sun, leaving fine dust or microscopic, sharp-edged fragments behind.
Salvaged ceramic is rarely used, because it takes constant, concerted effort to keep it alive, and it's too heavy for most applications anyways.
The dust it breaks down into is more useful. It increases the flux conductivity of whatever you mix it into. Lizards often make concrete with it, because the small, fine particles make a great aggregate. As a bonus, it increases the flux conductivity of whatever you're building. Watch out though-- you want to make sure you're building with the dust, and not the carcinogenic, razor-edged fragments that it can also leave behind.
Flux perception is one of lizards' most important senses, and because ancient ceramic is so conductive, laying your hand on it means you can sense a larger region around yourself. Lizards may also be searching for input or output nodes, or perhaps energizing a specific piece of ancient technology connected to the surface they're making contact with.
38 notes
·
View notes
Text
What’s Left on the Neolithic...plate?
ITA version ESP version
Sometimes, not having soap to wash the dishes thoroughly can be useful... especially if we want to uncover our past activities. It is thanks to the residues found in ancient ceramic vessels that a research team, composed of members from the Universitat Autònoma de Barcelona (UAB), the University of Zaragoza, and the University of Strasbourg, has revealed the first direct evidence of the consumption and processing of dairy products since the beginning of the Neolithic period, around 7,500 years ago.
The study utilised the remains of about thirty ceramic vessels obtained from two archaeological sites found in the caves of Chaves and Puyascada, located in the province of Huesca, Spain, to understand the usage and preservation habits of food. The researchers organised and classified the vessels according to various criteria, such as the type of profile, shape, cooking conditions, surface treatment, type of decoration, depth, and volume. Subsequently, lipids were extracted from the ceramic remains using a technique that employs acidified methanol, then analysed through gas chromatography and mass spectrometry.
Thanks to the morphological profiles, the researchers classified the vessels based on their function: preparation with or without heating, serving, and storage. Indeed, a group of ceramic pots was classified as suitable for food preparation, particularly for prolonged boiling thanks to the closed rims that prevent excessive evaporation. Others were suitable for pounding or stirring, as they have thick walls, which would be more resistant to heavy impacts. Regarding organic matter, the residues found in the vessels include ruminant fats, pork, plant products, and dairy, suggesting intentional mixing or subsequent uses.
Another group of small vessels was interpreted as serving containers, used for individual consumption of food and liquids. They could have been easily handled with one hand, so they were likely intended for individual use. The identified ingredients range from animal fats to edible plants and resins. Finally, a last group of vessels, with deep and closed necks, was considered ideal for storing liquids and low-fat foods, such as cereals and legumes.
The analysis of organic residues from the Chaves ceramics indicates that these were mainly used for processing ruminant meat, representing 50% of the residues, and dairy products, which constitute 28%. This is consistent with the mortality profiles of the animals at Chaves, which show that goats, cattle, and pigs were slaughtered young, during the so-called "optimum of meat". At Puyascada, on the other hand, the ceramics were mainly used for dairy products, which account for 54% of the organic residues, while ruminant fats only make up 27%, and pork fat is well represented at 36%. The low percentage of ruminant fats could be due to different preparation and consumption methods compared to Chaves.
The high percentage of dairy fats at Puyascada suggests a priority in the use of ceramics for milk processing. Additionally, the importance of pork fat, despite the low quantitative presence of the species, could indicate specific preparation of pork or its use as a fat reserve. The preparation and consumption of pork fat were widespread practices in Neolithic Iberia, with evidence in many archaeological sites. The data suggest that, while milk was processed and consumed at both sites, ruminant and pork fats were managed differently, reflecting distinct production and consumption strategies.
From the shape of the vessels and the organic residues, scientists have managed to offer a valuable window into the social dynamics and agricultural and livestock practices of Neolithic communities in the Iberian Peninsula.
Other previous studies confirm the production of dairy products in Europe during the Neolithic, but this is the first study that makes a direct comparison between neighbours, between caves located about 100 km apart, describing the diversity of lifestyles.
source: https://link.springer.com/article/10.1007/s12520-024-02001-9#Sec14
examples of ceramic vessels:
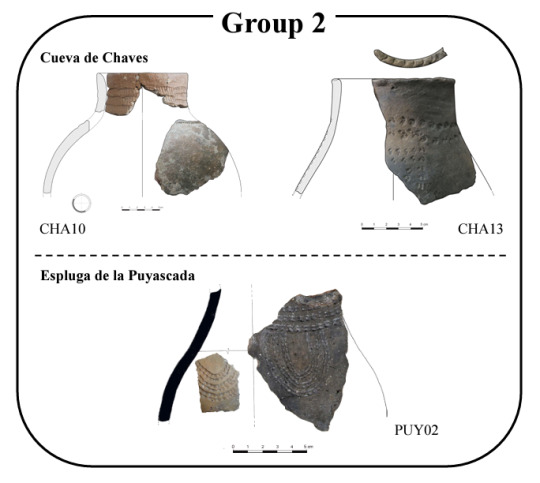
#Archaeology#Neolithic#AncientHistory#PrehistoricCulture#ScientificResearch#Discoveries#AncientPottery#OrganicResidues#PrehistoricFood#Spain#AutonomousUniversityOfBarcelona#UniversityOfZaragoza#UniversityOfStrasbourg
5 notes
·
View notes
Text
I’m an eepy girly today 🥱 but as a chemistry person I do have fun facts anyway.
Did you know decaffeinated coffees still usually have caffeine in them? Gas chromatography studies have confirmed that decaffeinated coffees still contain caffeine, albeit less so than their caffeinated counterparts.
17 notes
·
View notes
Text
Crafting Clear Skin: The Precision of Salicylic Acid Manufacturing
Salicylic acid has long been a cornerstone in skincare and pharmaceutical formulations, celebrated for its remarkable efficacy in treating acne, exfoliating the skin, and managing various dermatological conditions. As a leading ingredient in numerous products, the demand for high-quality salicylic acid is unwavering. Salicylic acid manufacturers play a crucial role in meeting this demand, employing advanced technologies, stringent quality control measures, and innovative processes to produce this essential compound. In this blog, we explore the world of salicylic acid manufacturing, highlighting its significance, processes, benefits, and why it’s a cornerstone of modern skincare and pharmaceutical solutions.

The Importance of Salicylic Acid
Salicylic acid is a beta-hydroxy acid (BHA) derived from natural sources like willow bark and wintergreen leaves or synthesized in laboratories. It is renowned for its ability to penetrate pores, exfoliate dead skin cells, and reduce inflammation, making it a powerful ingredient in acne treatments, chemical peels, and dandruff shampoos. Its keratolytic properties help to soften and shed the outer layer of skin, promoting cell turnover and revealing a smoother, clearer complexion.
For more information salicylic acid manufacturer
Advanced Manufacturing Processes
Manufacturing salicylic acid involves sophisticated chemical processes to ensure purity, potency, and safety. The most common method is the Kolbe-Schmitt reaction, which synthesizes salicylic acid from sodium phenoxide and carbon dioxide under high pressure and temperature. This method yields high-purity salicylic acid, suitable for both pharmaceutical and cosmetic applications. Manufacturers utilize advanced equipment and precise control systems to maintain optimal reaction conditions, ensuring consistent quality and yield.
Quality Control and Assurance
Quality control is paramount in salicylic acid manufacturing. Rigorous testing protocols are implemented at every stage of production, from raw material selection to final product packaging. Analytical techniques such as high-performance liquid chromatography (HPLC), gas chromatography (GC), and mass spectrometry (MS) are used to verify the purity, potency, and stability of salicylic acid. These tests ensure that the final product meets stringent industry standards and regulatory requirements, guaranteeing safety and efficacy for consumers.
Customization and Innovation
Salicylic acid manufacturers often work closely with cosmetic and pharmaceutical companies to develop customized formulations tailored to specific product needs. Whether creating a potent acne treatment, a gentle exfoliating cleanser, or an effective dandruff shampoo, manufacturers provide expertise in optimizing salicylic acid concentrations and formulations for maximum benefit. This collaborative approach fosters innovation, resulting in new and improved products that address evolving consumer demands and dermatological advancements.
Sustainability and Ethical Practices
In response to growing environmental concerns, many salicylic acid manufacturers are adopting sustainable and ethical practices. This includes sourcing raw materials from renewable resources, minimizing waste and emissions, and implementing energy-efficient technologies. Some manufacturers are also exploring greener synthesis methods that reduce environmental impact while maintaining high-quality production standards. These efforts align with the broader industry trend toward sustainability and responsible manufacturing.
Meeting Regulatory Standards
Compliance with regulatory standards is a critical aspect of salicylic acid manufacturing. Regulatory bodies such as the FDA (Food and Drug Administration) and EMA (European Medicines Agency) set stringent guidelines for the production and use of salicylic acid in cosmetic and pharmaceutical products. Manufacturers must adhere to Good Manufacturing Practices (GMP) and ensure their products are free from contaminants, properly labeled, and safe for consumer use. Regular audits and inspections by regulatory authorities help maintain compliance and uphold product integrity.
Future Trends and Innovations
The future of salicylic acid manufacturing is marked by continuous innovation and adaptation to emerging trends. Advances in green chemistry, biotechnology, and nanotechnology are poised to revolutionize production methods, enhancing efficiency and sustainability. Additionally, research into new applications and formulations of salicylic acid promises to expand its role in skincare and healthcare, offering consumers even more effective and versatile solutions.

Conclusion
Salicylic acid manufacturers are at the forefront of producing one of the most versatile and effective ingredients in skincare and pharmaceuticals. Through advanced manufacturing processes, stringent quality control, and a commitment to innovation and sustainability, these manufacturers ensure the consistent supply of high-quality salicylic acid. As consumer demand for effective skincare solutions continues to grow, the role of salicylic acid manufacturers remains vital, driving the development of products that promote healthier, clearer skin and improved well-being. Embrace the power of precision and discover the transformative benefits of expertly crafted salicylic acid.
2 notes
·
View notes
Text
Understanding the Deadly Toxin Found in Certain Mushroom Species
Introduction
Mushrooms are a diverse group of organisms, and while many are safe and edible, some species contain poisonous compounds. β-Amanitin is one such toxin found in certain mushroom species belonging to the genus Amanita. This article aims to explore factual evidence regarding the properties, effects, and potential dangers associated with β-Amanitin.
Understanding β-Amanitin
β-Amanitin is a cyclic peptide toxin produced by various species of mushrooms, including Amanita phalloides (death cap) and Amanita virosa (destroying angel). It is highly stable and resistant to heat, making it a potent toxin even after cooking[¹^]. Once ingested, β-Amanitin targets specific cellular processes, leading to severe liver damage and potentially fatal consequences.
Mechanism of Action
Inhibition of RNA Polymerase II: β-Amanitin specifically inhibits RNA polymerase II, an essential enzyme responsible for transcribing messenger RNA (mRNA) in eukaryotic cells. By binding to RNA polymerase II, β-Amanitin prevents mRNA synthesis, disrupting important cellular processes and ultimately leading to cell death[²^].
Factual Evidence Regarding β-Amanitin
Toxicity and Poisoning: Ingestion of mushrooms containing β-Amanitin can cause acute liver failure, often with delayed symptoms. The initial phase may include gastrointestinal distress, followed by a symptom-free period lasting up to 24 hours. Subsequently, liver damage manifests, characterized by jaundice, hepatic encephalopathy, and potentially progressing to multi-organ failure[³^].
Treatment Challenges: β-Amanitin poisoning is considered a medical emergency, and prompt recognition and appropriate treatment are crucial. Unfortunately, there is no specific antidote for β-Amanitin poisoning. Current management involves supportive care, liver protection measures, and potentially liver transplantation in severe cases[⁴^].
Forensic Toxicology: Due to the potent effects of β-Amanitin and its presence in lethal mushroom species, its detection plays a significant role in forensic toxicology. Analytical techniques such as high-performance liquid chromatography (HPLC) and mass spectrometry (MS) are employed to identify and quantify β-Amanitin in biological samples[⁵^].
Prevention and Awareness
Mushroom Identification: The primary preventive measure is accurate mushroom identification. Proper training and knowledge are crucial for distinguishing edible mushrooms from poisonous species, especially those containing β-Amanitin.
Education and Public Awareness: Raising awareness about the dangers of consuming wild mushrooms without expert guidance is essential. Public education campaigns can help reduce the incidence of β-Amanitin poisoning by promoting safe mushroom foraging practices.
Conclusion
β-Amanitin, a toxic compound found in certain species of mushrooms, poses a significant threat to human health. Its inhibition of RNA polymerase II leads to severe liver damage and potential fatality. Timely recognition of symptoms, along with supportive care and appropriate medical intervention, is vital for managing β-Amanitin poisoning.
To prevent β-Amanitin poisoning, it is crucial to exercise caution when consuming wild mushrooms and rely on expert identification. Public awareness campaigns can play an important role in educating the general population about the risks associated with consuming unknown mushrooms. Please visit MedChemExpress
(Note: This article is for informational purposes only and should not replace professional medical advice. If there is a suspicion of mushroom poisoning, seek immediate medical attention or contact a poison control center.)
2 notes
·
View notes
Text
The oyster mushroom (Pleurotus ostreatus) is a staple of many kinds of cuisine, prized for its mild flavors and a scent vaguely hinting at anise. These cream-colored mushrooms are also one of several types of carnivorous fungi that prey on nematodes (roundworms) in particular. The mushrooms have evolved a novel mechanism for paralyzing and killing its nematode prey: a toxin contained within lollipop-like structures called toxocysts that, when emitted, cause widespread cell death in roundworms within minutes. Scientists have now identified the specific volatile organic compound responsible for this effect, according to a new paper published in the journal Science Advances.
Carnivorous fungi like the oyster mushroom feed on nematodes because these little creatures are plentiful in soil and provide a handy protein source. Different species have evolved various mechanisms for hunting and consuming their prey. For instance, oomycetes are fungus-like organisms that send out "hunter cells" to search for nematodes. Once they find them, they form cysts near the mouth or anus of the roundworms and then inject themselves into the worms to attack the internal organs. Another group of oomycetes uses cells that behave like prey-seeking harpoons, injecting the fungal spores into the worm to seal its fate.
Other fungi produce spores with irritating shapes like stickles or stilettos. The nematodes swallow the spores, which get caught in the esophagus and germinate by puncturing the worm's gut. There are sticky branch-like structures that act like superglue; death collars that detach when nematodes swim through them, injecting themselves into the worms; and a dozen or so fungal species employ snares that constrict in under a second, squeezing the nematodes to death.
The oyster mushroom eschews these physical traps in favor of a chemical mechanism. P. ostreatus is what's known as a "wood rotter" that targets dead trees, but wood is relatively poor in protein. Its long branching filaments (called hyphae) are the part of the 'shroom that grows into the rotting wood. Those hyphae are home to the toxocysts. When nematodes encounter the toxocysts, the cysts burst, and the nematodes typically become paralyzed and die within minutes. Once the prey is dead, the hyphae grow into the nematode bodies, dissolving the contents and absorbing the slurry for the nutrients.
Read the rest
3 notes
·
View notes
Text
Drug Screening Market: Competitive Insights and Precise Outlook | 2024-2031
Drug Screening Market Overview: A lot of factors, such as geographic growth, segmentation, and market size by value and volume, are taken into account in the SkyQuest Technology Group research to provide a full and accurate analysis of the global Drug Screening market. This outstanding research study was created specifically to provide the most latest data on significant aspects of the global Drug Screening Industry. Numerous market estimates are provided in the analysis, including those for market size, output, revenue, consumption, CAGR, gross margin, price, and other critical factors. The best primary and secondary research methods and tools on the Drug Screening market were used to build it. Numerous research studies are included in it, including ones on pricing analysis, production and consumption analysis, company profile, and manufacturing cost analysis.

Global Drug Screening Market size was valued at USD 8.9 billion in 2022 and is poised to grow from USD 10.37 billion in 2023 to USD 35.18 billion by 2031, growing at a CAGR of 16.5% during the forecast period (2024-2031).
The competitive environment is a crucial element that every key factor needs to be aware of. The study explains the market's competitive landscape so that readers may gauge the degree of both domestic and global rivalry. Additionally, market researchers have provided summaries of each significant firm in the global Drug Screening industry, taking into consideration crucial elements including operational areas, production, and product portfolio. When analyzing the organizations in the study, significant factors including business size, market share, market growth, revenue, production volume, and profitability are also taken into account. The study report uses both qualitative and quantitative data to offer a thorough view of the market. It examines and forecasts the global market in a number of critical industries. The research provides a thorough overview of the industry by segmenting the Drug Screening market into groups based on application, end-user, and location. A thorough research of each market segment was conducted, taking into consideration current and upcoming market trends.
Chance to get a free sample @ https://www.skyquestt.com/sample-request/drug-screening-market
Detailed Segmentation and Classification of the report (Market Size and Forecast - 2031, Y-o-Y growth rate, and CAGR):
The Drug Screening Market can be segmented based on several factors, including product type, application, end-user, and distribution channel. Understanding these segments is crucial for companies looking to target specific markets and tailor their offerings to meet consumer needs.
Product & Service
Drug Screening Services [Laboratory Testing Services, On-site Testing Services], Drug Screening Products [Analytical Instruments (By Type {Breathalyzers “Fuel-cell Breathalyzers, Semiconductor Breathalyzers, Other Breathalyzers”, Immunoassay analyzers, Chromatography instruments}, By Modality {Hand-held drug screening products, Benchtop drug screening products}, Rapid Testing Devices (Urine testing devices {Drug testing cups, Dip cards, Drug testing cassettes}, Oral fluid testing devices), Consumables {Assay kit, Sample collection devices, Calibrators and controls, Other consumables}]
Sample Type
Urine Samples, Breath Samples, Oral Fluid Samples, Hair Samples, Other Samples
Drug Type
Cannabis, Alcohol, Cocaine, Opioids, Amphetamine and Methamphetamine, Other Drugs
End User
Drug Testing Laboratories, Workplaces, Criminal Justice Systems and Law Enforcement Agencies, Hospitals, Drug Treatment Centers, Individual Users, Pain Management Centers, Schools and Colleges, Other End Users
Get your Customized report @ https://www.skyquestt.com/speak-with-analyst/drug-screening-market
Following are the players analyzed in the report:
Quest Diagnostics (US)
Abbott (US)
OraSure Technologies Inc. (US)
Alfa Scientific Designs Inc. (US)
Thermo Fisher Scientific, Inc. (US)
Drägerwerk AG & Co. KGaA (Germany)
Lifeloc Technologies, Inc. (US)
MPD Inc. (US)
Omega Laboratories, Inc. (US)
Premier Biotech, Inc. (US)
Psychemedics Corporation (US)
F. Hoffmann-La Roche Ltd. (Switzerland)
Shimadzu Corporation (Japan)
Siemens Healthineers AG (Germany)
American Bio Medica Corporation (US)
ACM Global Laboratories (US)
CareHealth America Corp. (US)
Clinical Reference Laboratory, Inc. (US)
Intoximeters (US)
Sciteck, Inc. (US)
AccuSourceHR, Inc. (US)
Cordant Health Solutions (US)
Intoxalock (US)
Millennium Health (US)
AdvaCare Pharma (US)
Motives for purchasing this report-
- A full understanding of customer experiences, upcoming trends, and growth drivers may be obtained by market category analysis.
-Drug Screening Market participants will be able to quickly decide on their course of action in order to achieve a competitive advantage thanks to the essential information provided in this area.
The factors affecting the sales prospect are carefully examined by SkyQuest Technology Group across several important categories.
- Analysing market categories can provide detailed insights into consumer experiences, upcoming trends, and growth-promoting factors. A thorough analysis of market manufacturing trends is a crucial component of the study.
-These observations offer crucial information on the ways in which market participants are reacting to the most recent developments that are oversaturating the market.
-An in-depth analysis of the numerous organic
Buy your full Market Report now: https://www.skyquestt.com/buy-now/drug-screening-market
FAQs:
1. What are the main vendors' points of strength and weakness?
2. What are the primary business plans of the leading important players for the near future?
3. What will the market size and growth rate be for Drug Screening in the upcoming year?
4. Which prevailing global trends are affecting the Drug Screening market shares of the leading regions? What effect does Covid19 have on the Industry right now?
0 notes
Text
#romatography Consumables#Analytical Reagents#Chromatography Reagents#Chromatography Buffers#Chromatography Reagents Market
0 notes
Text
How UAE Laboratories Ensure Compliance with International Drinking Water Standards | +971 554747210
In the UAE, where access to clean drinking water is essential due to the arid climate and heavy reliance on desalinated water, ensuring the safety of drinking water is a top priority. Regular Drinking Water Testing is crucial for maintaining public health and complying with stringent water quality standards. These standards are often modeled after international guidelines set by organizations such as the World Health Organization (WHO) and the U.S. Environmental Protection Agency (EPA).
UAE laboratories play a key role in ensuring that the country’s drinking water meets both national and international safety standards. By employing advanced technologies and adhering to strict regulatory frameworks, these labs help maintain high water quality standards across the country.
Why Drinking Water Testing is Vital in the UAE
The UAE's drinking water supply is predominantly sourced through desalination plants that convert seawater into freshwater. While this process ensures a reliable water supply in a region with limited natural freshwater resources, it also introduces potential contaminants, including chemical residues from desalination and microbial contamination from water storage and distribution systems.
Regular drinking water testing is therefore essential for identifying and addressing contaminants that could pose health risks. Whether it’s for residential water supplies, commercial establishments, or bottled water production, UAE laboratories are tasked with ensuring that the water consumed is safe and meets international standards.
Key International Drinking Water Standards
The UAE aligns its water quality standards with several globally recognized guidelines. Key organizations that set these standards include:
World Health Organization (WHO): The WHO provides comprehensive guidelines on drinking water quality, covering a wide range of chemical, physical, microbial, and radiological parameters. These guidelines form the basis for many national water quality regulations around the world, including in the UAE.
U.S. Environmental Protection Agency (EPA): The EPA regulates drinking water quality through the Safe Drinking Water Act (SDWA), which sets maximum contaminant levels for various pollutants in public water systems. The UAE often uses these standards as a reference point when developing its own regulations.
European Union (EU) Drinking Water Directive: The EU sets stringent water quality standards for member countries, addressing various chemical and microbial contaminants. These guidelines are also referenced by UAE authorities in formulating national water standards.
How UAE Laboratories Adhere to International Standards
To ensure compliance with these international guidelines, UAE laboratories must meet rigorous standards in their testing protocols. The following steps outline how these labs achieve and maintain compliance with international drinking water standards:
Advanced Testing Technologies:
UAE laboratories use cutting-edge technologies to test drinking water for contaminants. These advanced methods enable accurate and comprehensive detection of both chemical and microbial pollutants.
Mass Spectrometry (MS) and Gas Chromatography (GC-MS): These techniques are used to identify and quantify chemical pollutants, such as pesticides, pharmaceuticals, and industrial chemicals, which may be present in trace amounts.
Inductively Coupled Plasma Mass Spectrometry (ICP-MS): ICP-MS is widely used to detect heavy metals like lead, mercury, and arsenic. The sensitivity of this technology allows for the detection of even minuscule amounts of contaminants, ensuring that water meets stringent international standards.
High-Performance Liquid Chromatography (HPLC): This method is effective in identifying organic contaminants, such as herbicides, solvents, and organic pollutants.
Microbial Testing (PCR): Polymerase Chain Reaction (PCR) technology is employed to detect harmful pathogens like E. coli, Legionella, and Cryptosporidium. These bacteria and viruses can lead to serious waterborne diseases if not properly controlled.
Regular Monitoring and Reporting:
Compliance with international standards requires regular monitoring of drinking water systems. UAE laboratories work with both government agencies and private enterprises to conduct periodic testing and ensure that water quality is consistently maintained.
Real-Time Water Monitoring: Many UAE labs employ real-time water quality sensors in distribution networks to monitor changes in water quality. These sensors can detect fluctuations in pH, turbidity, chlorine levels, and other critical parameters, allowing for immediate intervention if a contamination event occurs.
Scheduled Water Testing: In addition to real-time monitoring, UAE laboratories conduct regular scheduled testing of water samples from various sources, including desalination plants, storage tanks, and water pipelines. These tests help identify long-term trends and ensure compliance with international safety thresholds.
Compliance with National and Regional Regulations:
UAE laboratories are required to adhere to national standards set by the Emirates Authority for Standardization and Metrology (ESMA), which are closely aligned with international guidelines. ESMA regularly updates its regulations to reflect changes in global water quality standards, ensuring that the UAE remains in line with international best practices.
Certification and Accreditation: UAE laboratories must be certified by government bodies such as the Dubai Central Laboratory (DCL) and the Abu Dhabi Quality and Conformity Council (ADQCC). These certifications guarantee that labs are equipped to perform accurate and reliable water testing, and they operate in accordance with international quality management standards, such as ISO 17025 for laboratory competence.
Detection of Emerging Contaminants:
International drinking water standards are continually evolving to account for new and emerging contaminants. UAE laboratories stay ahead of these changes by employing advanced testing methods to detect substances that were previously not considered significant risks.
Pharmaceutical Residues: Increased consumption of medications means that pharmaceutical residues are becoming more prevalent in water supplies. UAE labs now test for these substances to ensure that they do not exceed safe levels.
Endocrine Disrupting Chemicals (EDCs): These chemicals, which can interfere with hormone function, are a growing concern in water quality testing. UAE laboratories use advanced techniques to detect and monitor the presence of EDCs in drinking water.
Ensuring Bottled Water Safety:
In addition to testing public water supplies, UAE laboratories also ensure that bottled water produced and sold in the country meets international standards. Bottled water must undergo rigorous testing for both microbial and chemical contaminants before it is allowed on the market.
Certification and Labeling: All bottled water produced in the UAE must be certified by ESMA, which ensures that it complies with WHO and EPA drinking water guidelines. Bottled water producers are required to submit regular samples for testing, and their products must be clearly labeled with information on water quality and safety.
Public Health Protection and Disease Prevention:
UAE laboratories play a crucial role in protecting public health by identifying potential waterborne disease risks. By regularly testing for pathogens and other harmful microorganisms, these labs help prevent the spread of diseases such as cholera, dysentery, and typhoid.
Legionella Testing: Legionella, a bacterium that thrives in warm water environments, is a common risk in the UAE’s climate. Regular testing for Legionella in water systems helps to prevent outbreaks of Legionnaires' disease, a severe form of pneumonia.
International Collaboration and Knowledge Sharing
To remain at the forefront of water quality testing, UAE laboratories frequently collaborate with international organizations, research institutions, and water quality experts. This collaboration ensures that UAE labs are up-to-date on the latest developments in water testing technologies and methodologies.
Conclusion
Ensuring compliance with international drinking water testing standards is an ongoing priority for UAE laboratories. Through the use of advanced testing technologies, regular monitoring, and adherence to both national and international guidelines, these labs safeguard the health and well-being of the UAE’s population.
By maintaining stringent standards for drinking water quality, UAE laboratories help prevent waterborne diseases, ensure the safety of bottled water, and protect the environment from harmful pollutants. Their role is crucial in supporting the UAE’s commitment to providing clean, safe drinking water that meets or exceeds international standards.
0 notes
Text
How Textile Testing Labs Help Brands Achieve Regulatory Compliance
In the textile industry, regulatory compliance is critical for brands to ensure product quality, safety, and marketability. With an increasing focus on sustainability, safety, and consumer protection, global regulations governing textile products have become more stringent. Brands that fail to comply with these regulations risk product recalls, hefty fines, and damaged reputations. This is where textile testing laboratories play a crucial role. A textile testing laboratory provides comprehensive testing services that help brands meet global regulatory requirements, ensuring that their products are safe, durable, and fit for purpose.
In this blog, we will discuss how textile testing labs help brands achieve regulatory compliance, the key regulations that impact the textile industry, and the various tests performed by these labs to ensure that textile products meet global standards.
1. Understanding Regulatory Compliance in the Textile Industry
Regulatory compliance in the textile industry involves adhering to a wide range of standards and regulations set by governments, international organizations, and industry bodies. These regulations are designed to ensure product safety, environmental sustainability, and consumer protection. Key regulations affecting the textile industry include:
Consumer Product Safety Improvement Act (CPSIA): In the U.S., CPSIA sets safety requirements for children's products, including textiles, with strict limits on lead content, phthalates, and flammability.
REACH (Registration, Evaluation, Authorisation, and Restriction of Chemicals): This European Union regulation controls the use of chemicals in textile products to minimize health risks to consumers and the environment.
OEKO-TEX® Standard 100: This certification ensures that textiles are free from harmful substances and meet strict safety criteria for consumer use.
ISO (International Organization for Standardization) Standards: ISO provides various standards for textiles, such as ISO 105 for colorfastness and ISO 9001 for quality management systems.
GOTS (Global Organic Textile Standard): GOTS ensures that textiles are made from organic fibers and meet environmental and social criteria throughout the production process.
Textile testing laboratories help brands navigate these complex regulations and ensure that their products comply with the required standards.
2. Chemical Testing for Safety Compliance
One of the major concerns in regulatory compliance for textiles is the presence of harmful chemicals that could pose health risks to consumers. Textile testing laboratories conduct a variety of chemical tests to ensure that textiles are free from toxic substances:
Restricted Substance Testing: Many regulations, such as REACH and OEKO-TEX®, ban or restrict the use of harmful chemicals like azo dyes, formaldehyde, and heavy metals. Textile testing labs use advanced analytical methods like gas chromatography-mass spectrometry (GC-MS) to detect and quantify these substances.
Phthalate and Lead Content Testing: Regulations such as CPSIA restrict the use of phthalates and lead in children’s textiles. Testing labs ensure compliance by performing detailed phthalate and lead content tests on fabrics, trims, and accessories.
Nickel Release Testing: Nickel is a common allergen found in buttons, zippers, and other metal accessories. Textile testing labs perform nickel release testing to ensure compliance with regulations like the EU Nickel Directive.
How It Helps Brands Achieve Compliance:By performing these chemical tests, textile testing labs help brands ensure that their products are safe for consumer use and comply with chemical safety regulations. This reduces the risk of product recalls, legal liabilities, and reputational damage.
3. Physical and Mechanical Testing for Quality and Durability
In addition to chemical safety, regulatory compliance also involves meeting specific quality and durability standards. Textile testing laboratories conduct a range of physical and mechanical tests to assess a fabric's ability to withstand everyday use:
Tensile Strength Testing: This test measures a fabric's resistance to breaking under tension, ensuring it meets performance standards for durability.
Abrasion Resistance Testing: Textile testing labs evaluate a fabric’s ability to resist wear and tear caused by rubbing, which is crucial for high-wear items like upholstery and workwear.
Pilling Resistance Testing: This test assesses the tendency of a fabric to form small balls of fibers on its surface, which can affect its appearance and longevity.
Seam Strength and Slippage Testing: Seam strength and slippage tests measure the strength and durability of seams under stress, ensuring the construction quality of the final product.
How It Helps Brands Achieve Compliance:By conducting these tests, textile testing labs help brands meet quality standards set by regulations such as ISO 9001, ensuring that products are durable, reliable, and meet consumer expectations.
4. Flammability Testing for Safety Standards
Flammability is a critical aspect of regulatory compliance, particularly for children’s clothing, home textiles, and public-use fabrics. Textile testing laboratories perform various flammability tests to ensure that products meet safety standards:
Vertical Flammability Testing: This test measures how quickly a fabric ignites and burns when exposed to a flame. It is crucial for ensuring compliance with flammability regulations, especially for children's sleepwear.
Surface Flash Testing: Surface flash testing assesses the speed at which a fabric's surface ignites and burns, which is particularly important for high-pile fabrics like fleece.
Heat Resistance and Melting Point Testing: Certain standards require that textiles resist ignition or melting when exposed to specific temperatures, ensuring safety in environments where heat exposure is possible.
How It Helps Brands Achieve Compliance:By performing comprehensive flammability testing, textile testing labs help brands meet fire safety regulations, ensuring that products are safe for consumer use and reducing the risk of accidents.
5. Sustainability and Environmental Compliance Testing
Sustainability is becoming increasingly important in the textile industry, with many regulations focusing on eco-friendly practices and materials. Textile testing laboratories perform several tests to ensure environmental compliance:
Eco-Toxicity Testing: This test evaluates the environmental impact of textile chemicals and dyes, ensuring compliance with environmental regulations like REACH and the EPA standards.
Recycled Content Verification: Textile labs verify the percentage of recycled content in fabrics, helping brands comply with certifications like the Global Recycled Standard (GRS).
Biodegradability Testing: Some regulations require textiles to be biodegradable and free from harmful residues. Textile labs perform biodegradability tests to ensure compliance with these standards.
How It Helps Brands Achieve Compliance:By conducting sustainability and environmental compliance testing, textile testing labs help brands meet eco-certification requirements, enhancing their marketability to environmentally conscious consumers.
6. Labeling Verification for Transparency and Consumer Protection
Accurate labeling is essential for regulatory compliance, as it provides consumers with critical information about the product's composition, care instructions, and safety warnings. Textile testing laboratories assist in verifying that labels meet regulatory requirements:
Fiber Content Analysis: Accurate fiber labeling is required under regulations like the Textile Products (Labelling and Fibre Composition) Regulations in the EU. Textile labs perform fiber content analysis to ensure labeling accuracy.
Care Label Testing: Textile testing labs check that care instructions are correct and comply with standards like ISO 3758, preventing consumer dissatisfaction and product damage due to incorrect care.
Safety Label Verification: Certain products, such as children's clothing, require specific safety warnings. Textile labs verify that these labels meet all legal requirements.
How It Helps Brands Achieve Compliance:By ensuring labeling accuracy, textile testing labs help brands comply with labeling laws, providing consumers with clear, reliable information and reducing the risk of legal issues.
7. Third-Party Testing and Certification for Credibility
Many global standards and certifications require third-party testing to ensure unbiased results and compliance. Engaging an accredited textile testing laboratory provides credibility and confidence in the product's quality and safety.
Why It’s Important:Third-party testing is often mandatory to obtain certifications such as OEKO-TEX®, GOTS, and Fair Trade Certification. These certifications provide a competitive advantage in global markets.
How It Helps Brands Achieve Compliance:By partnering with third-party textile testing laboratories, brands can demonstrate a commitment to quality, safety, and sustainability, enhancing their reputation and market access.
Conclusion
Textile testing laboratories are invaluable partners in helping brands achieve regulatory compliance. From chemical testing for safety and mechanical testing for durability to flammability, sustainability, and labeling verification, these labs provide comprehensive testing services that ensure products meet global standards.
In an increasingly regulated global market, compliance is not just a legal requirement; it is a critical factor for brand reputation, consumer trust, and market success. By leveraging the expertise of textile testing laboratories, brands can navigate complex regulatory landscapes, reduce risks, and deliver products that meet the highest standards of quality, safety, and sustainability.
0 notes
Text
How To Check Purity Of Shilajit
Several things have been said about Shilajit. The Himalayan region, which includes parts of Afghanistan, Tibet, Asia, India, and northern Chile, is where this herb is primarily found naturally. This plant is well known for its adaptogenic and antioxidant properties. Because of Ayurveda's numerous benefits, may brands have entered the market as interest in it has grown. Selecting a brand to buy is frequently a challenging choice for customers. This article will explain how to check the purity of shilajit.
What is shilajit
Shilajit is a naturally occurring resin with two distinct colors: brown and black. It is created by the centuries-long breakdown of plants, minerals, and animals under intense heat and pressure. This substance contains fatty acids, vitamins, minerals, and humic acid and offers various benefits
How to check purity of shilajit
This substance is available under many different brands, but it is impossible to determine which are genuine. Two methods are available for checking it: one is simple, requires little equipment and ingredients, and can be done at home; the other is large-scale and requires significant investment in machinery and other resources.
Home based test
Below are a few tests which can be done at home with minimum equipment and ingredient
Solubility test procedure
You will need shilajit, which you have, and water at room temperature for this test. It is necessary to thoroughly dissolve shilajit in water; if the mixture is mixed and no residue remains, giving you a golden or dark brown tint, then your shilajit is authentic; otherwise, it is not
2. Burn test procedure
Gas and the flame shilajit you will use are required for this test. Put this product on a fire. It might not be the original shilajit if it emits excessive smoke and leaves a large amount of residue after burning. The original will burn with the most negligible ash and without emitting smoke.
3. Pliability test procedure
The shilajit you were considering utilizing for this experiment will also need to be added to your refrigerator. After putting it in the fridge, mold your shilajit into a different form for a few minutes. The original shilajit becomes sticky and brittle when shaped by hand.
4. Color test procedure
Colour is an excellent way to determine Shilajit's purity. The original Shilajit was dark and brown. A different color could result from contaminants.
5. Acid test procedure
This test requires weak acid and the product itself. Add vinegar or another mild acid to shilajit. A pure shilajit will not react violently; it will dissolve. If it fizzes or reacts strongly, additives or impurities may be present.
Large scale test
Spectroscopic analysis
This analysis identifies the chemical makeup of the shilajit you own. Although the chemical makeup of the impure form will differ, these tests cannot be carried out at home. The right lab and equipment are needed to perform this test.
2. Chromatography technique
Compounds in shilajit are separated and identified using thin-layer chromatography and high-performance liquid chromatography. These techniques help guarantee that products are free of impurities and contain the anticipated bioactive compounds.
3. Heavy metal testing
These tests determine whether mercury, arsenic, or metals are present in shilajit. Not all of these compounds are present in the original shilajit because they are not beneficial to health.
4. Microbiological testing
Using methods like microbial culture or PCR, tests are carried out for bacteria, fungi, and pathogens to make sure that these natural herbs are safe to consume
5. Standardization
This test ensures that the product meets all safety standards and is safe to consume by all age groups.
Conclusion
Shilajit contains a wealth of minerals, vitamins, and fulvic acid. This semi-liquid resin compound offers several advantages, including improving physical endurance, immunity, energy, sexual function, heart health, muscle gain, etc. As previously mentioned, several methods exist to determine if this compound is original. Please take note that consuming shilajit raw can have adverse effects on your health. Additionally, it is crucial to see a doctor before taking any brand of shilajit in any quantity.
0 notes
Text
Cannabis Testing Comprehensive Review
As Long Beach solidifies its role as a leading hub in the cannabis industry, cannabis testing in Long Beach becomes increasingly critical for ensuring product safety, compliance, and quality. This comprehensive review explores why cannabis testing in Long Beach is essential, details the testing process, and outlines the benefits it offers to both consumers and businesses.
Why Cannabis Testing in Long Beach is Essential
Cannabis testing in Long Beach plays a pivotal role in the industry for several reasons:
Ensures Product Safety: Cannabis testing in Long Beach identifies harmful contaminants such as pesticides, heavy metals, and microbial impurities. This testing is crucial for guaranteeing that products are safe for consumer use and adhere to health standards.
Accurately Measures Potency: Cannabis testing in Long Beach determines the concentrations of key cannabinoids like THC (tetrahydrocannabinol) and CBD (cannabidiol). This precise measurement helps consumers understand the strength of their cannabis products, leading to more informed and consistent usage.
Supports Regulatory Compliance: Adherence to local and state regulations through cannabis testing in Long Beach ensures that products meet legal standards. This compliance helps businesses avoid penalties and operate within the bounds of the law.
The Cannabis Testing in Long Beach Process
Understanding the cannabis testing in Long Beach process is essential for maximizing its benefits:
Sample Collection: The process starts with collecting samples from cannabis products. Proper sampling methods are critical to ensure that samples are representative and uncontaminated.
Laboratory Analysis: Samples are analyzed in accredited laboratories specializing in cannabis testing in Long Beach. Key analyses include:
Potency Testing: Measures the levels of cannabinoids, including THC and CBD.
Terpene Analysis: Identifies and quantifies terpenes that contribute to the aroma and flavor of cannabis.
Contaminant Screening: Detects the presence of pesticides, heavy metals, residual solvents, and microbial contaminants.
Interpreting Results: Results from cannabis testing in Long Beach are reviewed to ensure they meet established safety and quality standards. This step confirms whether the cannabinoid levels and contaminant levels align with product claims and regulatory requirements.
Reporting: Detailed reports from cannabis testing in Long Beach provide comprehensive data on cannabinoid concentrations, contaminants, and overall product quality. These reports are crucial for transparency and regulatory adherence.
Benefits of Cannabis Testing in Long Beach
Investing in cannabis testing in Long Beach offers numerous advantages:
Enhanced Consumer Safety: By identifying contaminants and verifying potency, cannabis testing in Long Beach helps ensure that cannabis products are safe for consumers, minimizing potential health risks.
Regulatory Assurance: Compliance with state and local regulations through cannabis testing in Long Beach helps businesses avoid legal issues and maintain adherence to industry standards.
Improved Product Quality: Regular cannabis testing in Long Beach ensures that products consistently meet quality standards, leading to higher customer satisfaction and increased trust in the brand.
Market Differentiation: Products that undergo rigorous cannabis testing in Long Beach can distinguish themselves in a competitive market, enhancing brand reputation and attracting discerning consumers.
Choosing the Right Facility for Cannabis Testing in Long Beach
To maximize the benefits of cannabis testing in Long Beach, selecting the right testing facility is crucial:
Certification and Accreditation: Choose a laboratory accredited for cannabis testing in Long Beach to ensure accuracy and reliability in testing results.
Advanced Technology: Opt for labs that utilize state-of-the-art technology, such as High-Performance Liquid Chromatography (HPLC) and Gas Chromatography (GC), for precise analysis.
Comprehensive Services: Select facilities offering a full range of tests, including potency, terpene analysis, and contaminant screening, to ensure a thorough evaluation of your products.
Expertise and Reputation: Partner with laboratories known for their expertise in cannabis testing in Long Beach and a solid reputation for delivering accurate and reliable results.
Conclusion
Cannabis testing in Long Beach is fundamental to maintaining high standards in the cannabis industry. By investing in detailed cannabis testing in Long Beach, businesses can ensure product safety, meet regulatory requirements, and enhance product quality.
For optimal results, choose a well-accredited laboratory that provides comprehensive testing services and uses advanced technology. This approach will help you fully leverage the benefits of cannabis testing in Long Beach and excel in the competitive cannabis market.
0 notes
Text
Chromatography Systems Market SWOT Analysis, Forecast 2024-2032
Chromatography Systems Market Overview
The Chromatography Systems Market is experiencing steady growth driven by diverse applications across various industries such as pharmaceuticals, biotechnology, food and beverage, environmental analysis, and others. Chromatography, a technique used for separating and analyzing complex mixtures, has become an indispensable tool in laboratories worldwide, fueling the demand for chromatography systems.
One of the primary drivers of this market is the increasing need for drug development and research in the pharmaceutical and biotechnology sectors. Chromatography plays a crucial role in drug discovery, development, and quality control processes, ensuring the safety and efficacy of pharmaceutical products. Moreover, the growing prevalence of chronic diseases and the subsequent demand for innovative therapies have further propelled the adoption of chromatography systems in drug development.
In the food and beverage industry, chromatography systems are extensively utilized for quality testing, ensuring compliance with regulatory standards, and detecting contaminants. With rising consumer awareness about food safety and authenticity, there is a heightened emphasis on implementing robust analytical techniques like chromatography to maintain product integrity and meet regulatory requirements.
Environmental analysis is another significant application area driving the demand for chromatography systems. These systems are employed for monitoring air, water, and soil quality, as well as analyzing pollutants and contaminants. With increasing environmental concerns and stringent regulations aimed at safeguarding ecosystems and public health, the demand for chromatography solutions for environmental testing continues to grow.

Technological advancements and innovations in chromatography systems are also contributing to market expansion. Manufacturers are introducing advanced chromatography instruments with enhanced capabilities such as higher throughput, improved sensitivity, and greater automation, catering to the evolving needs of laboratories and research facilities.
Chromatography Systems Market Analysis
The Chromatography systems Market is expected to reach USD 15.9 billion by 2032 at 6% CAGR during the forecast period 2023-2032. Chromatography simply put, is a laboratory method used for separating mixture and in various ways. It is a vital technique for pharmaceutical industry, biotechnology, agriculture and life science research. Chromatography is used in various applications right from production-scale use in the purification step to analyzing miniscule samples. Its most vital applications in various industries include testing drinking water, monitoring air quality, drug detection in urine as well as other body fluids, in species identification and chemical fingerprinting, in pharmaceutical industries for purifying materials and analyzing chemical compounds to trace contaminants as well as separating chiral compounds. Besides, in the food industry it is used for analysis and separation of proteins, vitamins, preservatives and additives along with detecting contaminants and toxins in food. Some of the key benefits of using chromatography include separating highly complex mixtures, mixture components that are separated through chromatography can be gathered individually, chromatography works on different samples such as tissue extracts, water and air samples, pesticides, plastics, food particles and drugs, it needs minimal sample volumes and precise purification, separation and analyses is possible with the help of chromatography.
There are abundant factors that is propelling the growth of the chromatography systems market. These factors as per the MRFR (Market Research Future) report include increased significance of chromatography in forensic labs, laboratories and pharmaceuticals, advancement in technology, growing number of research activities, government grants and funds for research, need for portable systems, demand for chromatography instruments and systems for protein purification, easy affordability, new product launches and need for automated separation techniques. On the contrary, soaring price of chromatography systems, need for skilled people for handling chromatography systems, less awareness, alternative separation techniques and affordability issues are factors that may restrict the growth of the chromatography systems market.
Key Players
Key players profiled in the chromatography systems market players include WATERS, Thermo Fisher Scientific Inc., Siemens Industry Inc., Shimadzu Corporation, QUADREX CORPORATION, Phenomenex Inc., PerkinElmer Inc., Pall Corporation, PAC L.P., OI Analytical/Xylem Inc., Novasep Holding S.A.S., JASCO Inc., GL Sciences Inc., GE Healthcare, Bio-Rad Laboratories Inc., Apix Analytics, Apex Chromatography Pvt. Ltd., and Agilent Technologies.
Chromatography Systems Market Segmentation
MRFR report offers a broad segmental analysis of the chromatography systems market on the basis of type and end users.
Based on type, it is segmented into gas chromatography, liquid chromatography and others. Liquid chromatography is again segmented into low pressure liquid chromatography (LPLC), ultra-high-pressure liquid chromatography (UHPLC) and high-pressure liquid chromatography. The others segment is further segmented into thin layer chromatography, column chromatography, ion exchange chromatography and affinity chromatography. Of these, gas chromatography will have the largest share in the market over the predicted years owing to upsurge in adoption of chromatography systems in biotechnology and pharmaceutical industry and its enhanced applications.
Based on end users, the chromatography systems market is segmented into agriculture & food industry, hospital & research laboratories, pharmaceutical & biotechnology industry and others.
Chromatography Systems Market Regional Analysis
By region, the chromatography systems market covers growth opportunities and latest trends across North America, Europe, Asia Pacific and Middle East and Africa. Of these, North America will lead the chromatography systems market over the predicted years. This is owing to growing research activities for various government investments, biologics development and medicines and high incidence of soft tissue sarcoma and cancer in the region. The chromatography systems market in Europe will have the second major share followed by Asia Pacific owing to rising incidence of cancer, growing awareness about advanced cancer treatments, increasing healthcare expenditure for cancer and related diseases, investment of pharmaceutical companies here from western regions and also expansion of local organizations.
Related Report’s
Ophthalmic Drugs and Devices market
Healthcare Biometrics market
Fill Finish Manufacturing market
Wearable Medical Device market
Corporate Wellness market
0 notes