#GC-MS analysis
Explore tagged Tumblr posts
Link
1 note
·
View note
Text
From Separation to Detection: How Gas Chromatography-Mass Spectrometry (GC-MS) Works in Real-World Applications
In today’s world, where science plays a big role in our daily lives, a powerful technique called Gas Chromatography-Mass Spectrometry (GC-MS) has become an essential tool for scientists. Whether it’s checking for harmful chemicals in food, identifying substances in crime investigations, or finding pollutants in the environment, GC-MS helps provide accurate and trusted results. It combines two…
#Applications of GC-MS#Environmental analysis with GC-MS#Gas Chromatography-Mass Spectrometry (GC-MS#GC-MS in food testing#GC-MS in forensic science#GC-MS working principle
0 notes
Text
Also preserved in our archive
By Dr. Sushama R. Chaphalkar, PhD.
In a recent research paper posted to the bioRxiv preprint* server, researchers in the United States investigated the potential effect of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection on cholesterol metabolism, focusing on the role of the viral protein open reading frame 3a (ORF3a).
They found that SARS-CoV-2 causes cholesterol sequestration in lysosomes via the ORF3a protein, which disrupts protein trafficking and reduces the levels of bis(monoacylglycero)phosphate (BMP) in the cell, enhancing viral survival.
Background Coronavirus disease 2019 (COVID-19), caused by SARS-CoV-2, disrupts lipid metabolism, particularly cholesterol homeostasis, which can persist during and after infection. This is linked to disease severity and long-term complications like dyslipidemia and cardiovascular diseases.
Cholesterol is crucial for cellular function and is primarily transported through lysosomes, where proteins like Niemann-Pick C1 and C2 (NPC1 and NPC2) facilitate its release. SARS-CoV-2 may exploit plasma membrane cholesterol to enhance infectivity.
Disruptions in the lysosomal cholesterol pathway can cause cholesterol buildup, impairing cellular functions, and viruses like Ebola are known to hijack this mechanism. Notably, BMP plays a dual role: it aids in cholesterol transport and contributes to viral infection by promoting viral fusion with lysosomal membranes.
In the present study, researchers investigated the potential impact of SARS-CoV-2 infection on cholesterol transport in cells, focusing on the role of the viral protein ORF3a.
About the Study A variety of experimental techniques were employed, including culturing A549, HeLa, and Vero E6 cells, followed by SARS-CoV-2 infection at different multiplicities of infection. SARS-CoV-2 ORF3a-VPS39 interaction was studied using mutations at key residues (notably W193 and Y184, which were identified as critical for this interaction). Immunofluorescence, filipin staining, and confocal microscopy were used to assess cholesterol localization and vesicular dynamics, while high-content imaging quantified cell-specific responses.
Cholesterol levels were measured using gas chromatography-mass spectrometry (GC-MS), and lipid species were analyzed through shotgun lipidomics. For further protein analysis, western blotting was performed to detect secreted NPC2 and cathepsin D, along with cell lysates. Data were analyzed using ImageJ and Prism 9, and statistical significance was determined by t-tests or analysis of variance.
Results and Discussion SARS-CoV-2 infection was found to increase filipin-positive puncta in lysosomes of A549-hACE2 and Vero E6 cells, indicating altered cholesterol distribution, especially in lysosomes, without affecting total cholesterol levels. Among the 28 viral proteins tested, ORF3a showed the strongest increase in filipin puncta, suggesting significant lysosomal cholesterol sequestration.
Notably, SARS-CoV-2 ORF3a localized to lysosomes and caused them to swell, whereas SARS-CoV ORF3a did not induce such effects, highlighting a distinct pathogenic strategy unique to SARS-CoV-2.
ORF3a was found to interact with VPS39, a key component of the HOPS complex involved in cholesterol egress from lysosomes. Key residues W193 and Y184 were shown to form a hydrophobic binding interface critical for this interaction, distinguishing SARS-CoV-2 ORF3a from its SARS-CoV counterpart. Mutations at W193 and Y184 disrupted this interaction, while S171 and H182 had no significant effect.
SARS-CoV-2 ORF3a expression was shown to cause cholesterol accumulation in lysosomes, which was reduced by the W193A mutation. It also led to the mislocalization of NPC2 and increased its secretion, indicating disrupted NPC2 trafficking, likely due to interference with TGN-to-endosome transport. Additionally, BMP levels were significantly reduced in infected cells, which likely exacerbates lysosomal cholesterol sequestration.
In SARS-CoV-2-infected Vero E6 cells, BMP levels were found to decrease at 12 hours post-infection, coinciding with increased cholesterol at 18 hours. In HeLa-Flp-In cells, SARS-CoV-2 ORF3a was found to reduce BMP levels by 20%, with partial rescue in the W193A mutant. Lipidomics confirmed this reduction, correlating BMP loss with cholesterol accumulation and suggesting BMP reduction may contribute to cholesterol sequestration.
SARS-CoV-2 may reduce plasma membrane cholesterol to limit secondary infections, as shown by decreased SARS-CoV-2 infection in NPC1 inhibitor-treated cells. This supports the hypothesis that the virus manipulates cholesterol distribution to optimize replication conditions. Interestingly, the virus also appears to reduce its own infectivity within a single cell, suggesting a self-regulating mechanism to prevent viral overload and ensure broader host-level spread.
Conclusion In conclusion, a novel mechanism by which SARS-CoV-2 disrupts host cell lipid metabolism, specifically through cholesterol sequestration in lysosomes, has been elucidated. By uncovering the specific interaction between the viral protein ORF3a and host protein VPS39, the study highlights a critical role of lysosomal cholesterol trafficking disruption in SARS-CoV-2 pathogenesis.
This discovery opens potential therapeutic avenues to target lipid dysregulation in COVID-19, which could help mitigate both the disease's immediate and long-term metabolic consequences, including dyslipidemia and cardiovascular complications.
Journal reference: Preliminary scientific report. Manipulation of Host Cholesterol by SARS-CoV-2. Aliza Doyle et al., bioRxiv, 2024.11.13.623299 (2024), DOI: 10.1101/2024.11.13.623299,
Study Link: www.biorxiv.org/content/10.1101/2024.11.13.623299v1
#mask up#public health#wear a mask#pandemic#covid#wear a respirator#covid 19#still coviding#coronavirus#sars cov 2
91 notes
·
View notes
Note
We may have a problem with the so-called fiancé, @artofdeductionbysholmes I lost sight of him…
Any update on the lab results? @artofdeductionbysholmes @mollyatthemorgue
The question is: did he disappear on his own terms, or was his disappearance forced?
And yes, I just received @mollyatthemorgue Molly's lab report. See below.



[ID: 3 screenshots of a lab report
Lab Report
Subject: Examination of Paper Sample
Date of Report: June 2, 2024
Lab Technician: Hooper, Molly
Sample ID: Paper-2024-371
Introduction
The purpose of this lab report is to present the findings from the examination of a piece of paper. The analysis aims to identify the physical, chemical, and biological characteristics of the paper, and any potential indicators of its environment and exposure history.
Sample Description
Type: Paper
Condition on Receipt: Intact with minor surface wear, slightly discoloured.
Methods of Analysis
Visual Inspection
Microscopic Examination
Chemical Analysis
X-Ray Fluorescence (XRF)
Gas Chromatography-Mass Spectrometry (GC-MS)
Microbiological Assays
Moisture Content Analysis
Odour Analysis
Results
1. Visual Inspection
Appearance: The paper showed slight discoloration, with faint yellowish-brown stains.
Surface Condition: Minor abrasions were noted. Some dirt and dust particles were visible.
2. Microscopic Examination
Fibre Integrity: The cellulose fibres were mostly intact, with minor signs of surface wear.
Debris: Presence of small soil particles and other unidentified debris.
3. Chemical Analysis
X-Ray Fluorescence (XRF):
Detected Elements: Trace amounts of heavy metals such as lead (Pb), cadmium (Cd), and chromium (Cr).
Surface Contaminants: Minor presence of inorganic substances.
Gas Chromatography-Mass Spectrometry (GC-MS):
Organic Compounds: Detected small amounts of hydrocarbons and other organic pollutants.
Residues: Identified residual chemicals from inks and dyes, with some degradation products.
4. Microbiological Assays
Bacterial Presence: Identified bacterial species including Bacillus and Pseudomonas spp.
Fungal Presence: Traces of fungal spores, likely Aspergillus and Penicillium spp.
5. Moisture Content Analysis
Moisture Level: Moderate moisture content of 12%.
Chemical Composition: The moisture contained slight acidic properties.
6. Odour Analysis
Detected Odours: Mild, musty odour with hints of organic decay.
Discussion
The examination of the paper sample indicates several key findings:
The physical condition and minor wear suggest it was exposed to an environment with abrasive materials and some physical stress.
The presence of heavy metals and organic pollutants detected by XRF and GC-MS indicates exposure to a polluted environment, potentially involving industrial or waste materials.
Microbial assays revealed early stages of microbial colonisation by bacteria and fungi typically associated with organic material decomposition.
The moderate moisture content and slight acidity suggest exposure to a moist environment with some chemical interactions.
Odour analysis confirmed the presence of organic decay-related gases.
The combination of physical wear, chemical contaminants, microbial presence, and environmental indicators suggests that the paper may have been exposed to a mixed waste or polluted environment. The findings are consistent with environments such as waste disposal sites, polluted industrial areas, or other locations with significant organic and inorganic contaminants.
Conclusion
The paper sample shows signs of exposure to a polluted and possibly waste-rich environment. The results indicate physical wear, contamination by heavy metals and organic pollutants, microbial activity, and environmental interactions that are typical of such conditions. Further context about the paper's origin could provide more specific insights.
Lab Technician Signature:
[signature of Molly Hooper]
Reviewed By:
Patrick Miller
/end ID]
22 notes
·
View notes
Note
Hello Ms Pillarsalt, I feel awful at the thought of using you as a pseudo-therapist but I just don't know where else to turn (no one knows I'm gender critical) and you are extremely thoughtful and kind every time you answer asks.
I've become very good internet friends with who I suspected was a trans-identified woman and I just got confirmation of it today. She's sadly long since begun the "" medicalization"" process, but she uniquely has absolutely never mentioned her gender identity at all to me. She doesn't even do pronouns in bio, pronoun introductions, making trans headcanons in her fanart/works, nothing. She even completely ignored my noticing that the indie zine she writes (completely non-trans-related) oneshots for has a website that makes it very very clear they're by and for genderists (which caught me off guard when she sent me the link to it!) like it wasn't even A Thing Central To The Org she's part of.
She has so many common interests with me (which are very much fandoms that are mainly populated by gnc and/or trans-identified women), she's very kind, and we both really enjoy talking to each other for hours and hours. (I really do enjoy our talks, outside of the few times she says something misogynistic of the trans-ideology variety, like tying femaleness to femininity and sexualization "but because I'm *celebrating* it I'm progressive, serve cunt queen ���� but the deep sense of despair I feel when that gets applied to me it must mean I'm not female, there's absolutely nothing inherently wrong with gender roles of course but the feminine one is just not for me", you know the deal.)
So I'm not at all conflicted about being friends with her from an interpersonal standpoint, I've never ever felt like I couldn't befriend trans-identified people because of my beliefs. (Exactly the opposite in fact! I hope that just being Me while being """cis""", and not at all mentioning my gc views ever, can be a positive influence to them!) But this is the first trans-identified friend I've gotten this close to and I was surprised to feel this deep sense of depression and despair about it. Like I feel this way knowing about how widespread misogyny is in general, but when it's affecting someone you know and care about it makes it more real. And less escapable.
It's hard to turn my brain off and not think about how her syntax and fandoms and idolization of male artists and characters to the point of roleplaying and cosplaying are so... female. Her analysis and interest of characters' relationships, her career, her hobbies in general, it's just female all the way down. And yet because it's not *feminine* (it's stereotypical gnc and/or autistic introverted female stuff) she must not be a woman. (I know the thought, I've had it myself.) Her voice is so deep I second-guessed myself about her being a woman at first, and now that I know she is for sure I fear so deeply for her health knowing what she must have done to get it that way. I guess it's possible she used a filter, it was a voice call, but I don't know.
It's just a special kind of despair to see her talk about the things that make her happy and now as she does, she's using that enjoyment as reinforcement that she can't be a woman. It's what all TIFs do (I know, I was one), I think it's partially why they get so invested in their fandoms (aside from likely being autistic, guilty again): indulging in their interests when, according to misogyny, they're "abnormal" for a woman to like, is like they're crafting a big Bat Signal saying "Look everyone, I can't be female, I like [X]!!!" as they do it.
I'm sorry, I'm rambling so much. I just intimately know and empathize with what my friend believes about herself and women as a whole. I want to help her, but because I was (almost) her once I *know* I can't. I can say "I'm being a good influence by being a gnc non-trans-identified woman ^w^" all I want but honestly I don't know if it works. I've never worn makeup or expressed hatred of my body, and yet my sister-in-law still wears a full face every day and got plastic surgery. I think someone has to want to change to be able to. Unfortunately.
I'm rambling again, sorry.
Like I said, on a person-to-person level, my friend and I are so fine. It's just depressing to be reminded of the pervasiveness of misogyny if I want to voice chat with her or see her. Or hear about her passions, knowing they're not just things she enjoys, but also "proof" of her non-female-ness to her. Even if I thought I could say something to change her mind, it'd be completely out of line to say it because she, again, never brings up her gender identity. I just don't know what to do. I love being friends with her, but I hate the misogynistic voyeur inside her that joins all our conversations.

Hi anon, no worries about venting here, I'm always happy to give some input but also don't forget I'm just some guy.
You seem to be a compassionate and intelligent person, and you obviously care a lot about your friend. I've been (and am currently) in similar situations like the one you've described, and I know how distressing it can be to try to balance both your concern for your friend, your moral conflict with their viewpoints, and trying not to offend them lest they push you away or accuse you of phobe crimes. There's no real great solution to your problem, I think you probably already know that.
People with deeply held beliefs are not going to be open to changing their minds if they aren't ready to do so yet. It does really suck not to be able to say what you're thinking, but in my opinion, the best thing to do is stay the course - keep doing what you're doing. Keep talking to her like you always have, show her she can trust you. There may be a reason why she never brings up trans-related topics with you; maybe she's already questioning the rhetoric behind the ideology? I know from my own experience and what I've heard from others, when you start questioning gender ideology, the first instinct is BAD THOUGHTS SHUT IT DOWN and to lean into it even harder. That's by design. But you can show her that you are a safe person to talk to about the Bad Thoughts. I know you said that you don't know if "being a good influence" works, and I'm sorry to hear about your sister-in-law. I really believe that modelling feminist behaviour does work to influence your female peers, but it might take her escaping bad influences first for her to realize the misogyny behind her thought processes. It might take years too, that's normal. I think pushing back on her sexist flawed logic without getting too overtly "terfy" could help too. Or you could throw in a "It's nice to see that so many woman are interested in [subject she thinks isn't for women] nowadays!" Anything that encourages self-reflection.
You said "I don't want it to have to be my responsibility, women's responsibility, to combat misogyny alone. But the unfortunate reality is that we're the only ones who care so we're the only ones who can make a difference," and it seems to me like you're taking it on yourself to fix this woman's misogyny problem on your own. Sometimes this just isn't possible. Ultimately, even though she's your friend, you aren't responsible for her actions. She's hurting herself, but only she can choose to stop, so you cannot blame yourself for not digging her out on your own. You're just one woman! Let go of your sense of obligation; you're doing what you can. And if her internalized misogyny gets to be too much for you, take a break and take care of yourself, it doesn't make you a bad feminist. Patience and understanding towards other women and towards yourself are the feminist practices to employ in this situation.
Be well and take care 💜
8 notes
·
View notes
Text


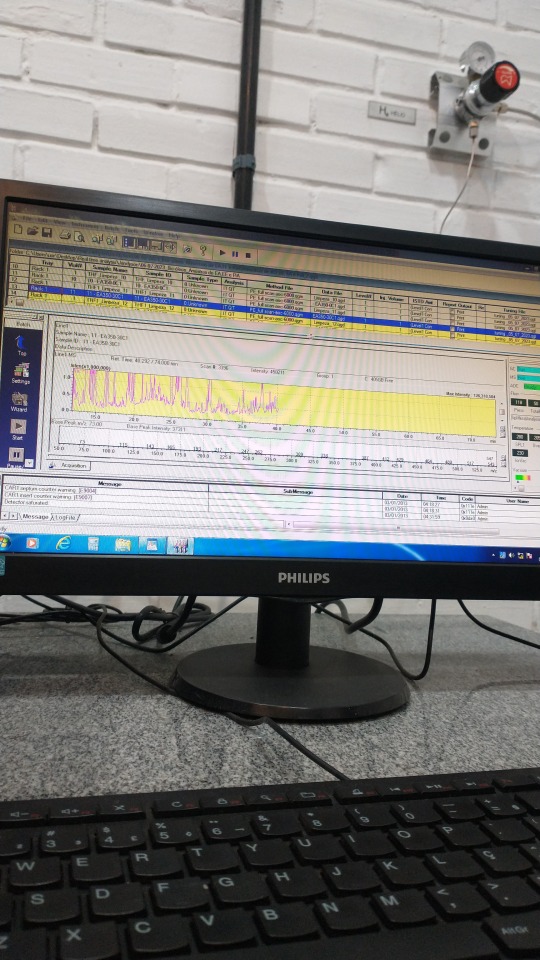
07.07.2023 || Bio-oil analysis by GC/MS at UFRPE - Part II
#chemistry#doutorado#química#study motivation#studying#ufrpe#study#mass spectrometry#gas chromatography#bio oil#htl
4 notes
·
View notes
Text
#health & fitness#GC and GC-MS market#GC and GC-MS Size#GC and GC-MS forcast#GC and GC-MS Price#GC and GC-MS report
2 notes
·
View notes
Text
(Copying this over from my masto: https://tenforward.social/@aspensmonster/114040840755531536 ) Several months later, and apparently there's some contention on the suitability of Py-GC-MS specifically for reliably detecting micro and nano-plastics within high-fat tissues like the brain (or at least polyethylene specifically):
https://cosmicretreat.tumblr.com/post/775774049112571904 (yes, I was originally cued into this development from Tumblr; yes, I will use this incident as an excuse to continue to stay active on Tumblr)
Which leads to two folks on Twitter, both of whom appear to have experience in working with MNPs:
Dr Materic (https://x.com/DusanMateric/status/1886730304859062773)
With three contentions:
Figure S7 [which Dr Materic erroneously labels as Kidney rather than Liver, though his point would stand when comparing either kidney or liver to the brain] versus Figure S10 of Brain, both under light microscopy, showing many more readily apparent microplastics in equivalent areas for the liver/kidney than the brain, contradicting the authors' Py-GC-MS findings.
The flake-like nano-particles of the brain from Figure 2.d are too regular and unlike other nano-particles.
The high measurements of PE are indicative of false-positives due to fatty nature of brain tissue.
and Dr. Bergmann (https://bsky.app/profile/melaniebergma18.bsky.social/post/3lhg664zt6k27 ; could not find original twitter link, though this bluesky link mirrors the content; no, i will not (re)create an X account; yes, scientists need to get the fuck off of X), who also mentions possible unsuitability of Py-GC-MS findings for polyethylene in fatty tissue specifically:
Super important methodological implications for studies on #microplastics in humans, which currently tend to overestimate microplastic levels if Pyrolysis–Gas Chromatography–Mass Spectrometry is used, especcially for fatty tissues.
by linking to the following paper:
which claims that Py-GC-MS is not suited for measuring polyethylene in biological matrices:
It was concluded that Py-GC-MS is currently not a suitable analysis method for PE and PVC in biological matrices due to the presence of interferences and nonspecific pyrolysis products.
===================
The authors of the original paper changed the abstract from the preprint (https://pmc.ncbi.nlm.nih.gov/articles/PMC11100893/) to the published version (https://pubmed.ncbi.nlm.nih.gov/39901044/) in order to imply that they also employed other methods (SEM-EDS) to double-check their work and rule out such false positives:
3c3 [this is supposed to be a diff that tumblr just mangled by treating part of it as a quote -__-]
< Applying pyrolysis gas chromatography-mass spectrometry (Py-GC/MS) methods to isolate and quantify MNPs from human samples, we compared MNP accumulation in kidneys, livers, and brains.
Complementary methods for the robust detection of tissue MNPs, including pyrolysis gas chromatography-mass spectrometry, attenuated total reflectance-Fourier transform infrared spectroscopy and electron microscopy with energy-dispersive spectroscopy, confirm the presence of MNPs in human kidney, liver and brain.
Specifically, Extended figures 6 and 7 show SEM-EDS data on brain samples that, from my limited understanding, purport to demonstrate that the particulates identified, at least in the specimens that underwent SEM-EDS, are not such false positives.
Later in the discussion, the authors explicitly mention possible issues with polyethylene in high-fat tissues within Py-GC-MS -- the (IMO) strongest contention that both of the scientists on Twitter/X/BlueSky make -- and make their case for why their methodology should be less susceptible to those issues; indeed, they argue that their polyethylene counts may well be underestimates (they also make a weak counter-argument to contention 2 by being hand-wavy about "well maybe these nano-fragments are hitherto unknown final byproducts of nanoplastic breakdown, suitable for absorption into brain tissue"):
Lipids have been noted as a potential source of interference in Py-GC/MS analysis of PE[16]. Our method of KOH digestion and physical separation of solids was designed to reduce this concern, rather than augment it with a liquid–liquid extraction in organic solvents that would selectively drive lipid partitioning. Furthermore, the spectra suggest a reduction of longer carbon chains in the pyrolysis chromatogram, which is potentially due to advanced oxidative degradation of the MNPs and excess carbonyl formation that may lead to an underestimation of the concentration, as our standards are created with pristine polymers[17,18]. Finally, given the observed small size of nanoscale particles isolated from the human specimens (typically <200 nm in length), it is likely that ultracentrifugation incompletely collected nanoplastics in the analytical samples, also contributing to potential underestimation. The shape and size of observed nanoparticles in the isolated material from human specimens taxes the limits of modern analytical instrumentation but may reflect an end-stage product of plastic degradation that is uniquely suited for human uptake and accumulation.
And as for contamination, the authors basically argue that they were careful:
Numerous quality control steps ensure that external contaminants are not impacting the results, including Py-GC/MS assessment of KOH and formalin storage control sample ‘blanks’ and measurements of the polymer composition of all plastic tubes and pipette tips that are essential in the digestion and measurement process (Supplementary Figs. 2–4). Decedent specimen collections over the past 30 years were not focused on minimizing external plastic contamination. However, given the consistent nature of handling and processing across all organ samples within objectively clean clinical and forensic settings, the significant accumulation of MNPs in the brain cannot be dismissed as an artifact of contamination. Furthermore, the 2016 samples were stored for 84–96 months compared to only 2–4 months for the 2024 samples, which exhibited greater concentrations of polymer. Thus, contamination from plastic storage vessels should not influence the conclusions.
Certainly sounds like replication could help determine just how careful they really were.
I'm definitely out of my depth here, but I'm curious and following along as best as I can. My gut tells me that, while the authors' SEM-EDS work likely does establish the presence of some MNPs in the brain tissue, the Py-GC-MS measuring technique for quantifying just how much is present might be suspect. Further, the authors' arguments against contamination, as I understand them, still rely on the reliability of Py-GC-MS to accurate quantify MNPs in samples (including their pristines and their blanks). If Py-GC-MS itself is not as suited to the task as thought, then those arguments likely don't hold weight, and so there could also be undetected contamination of the samples.
Yay science!
I wouldn't say that the entire paper is necessarily debunked -- the ACS paper claiming that Py-GC-MS is definitely not suitable for polyethylene *does* mention that it may nevertheless be possible to detect other polymers with the technique (though that's still approaching detection limits of Py-GC-MS) -- but its most catchy headline generator -- "lots of MNPs in your brain" -- likely is.
Ultimately though, this is what science is: nerds nerd-sniping each other over and over until they all (or most of them) come to something approximating a consensus of the truth given all the evidence.
finding enough plastic in human brains to make a spoon is certainly a shocking headline but I just don't have it in me to be shocked anymore. not only can I see the evidence of spoon brain all around me I can literally feel it in myself
42K notes
·
View notes
Text
Beyond the Taboo: How Underwear Odor Research Could Shape the Future of Scent Analysis

If you’ve ever wrinkled your nose at a pile of laundry, you might not realize that the odors lingering on soiled underwear are more than just unpleasant—they’re a goldmine of scientific data. While “underwear sniffing” might sound bizarre or even comical, it’s actually a gateway to advanced scent analysis with real-world applications in health, forensics, and even consumer products.
Why Study Underwear Odors?
Human body odor is a complex cocktail of volatile organic compounds (VOCs) produced by bacteria breaking down sweat and other secretions. Underwear, in particular, acts as a natural collector for these compounds, offering researchers a concentrated sample of an individual’s unique scent profile.
Scientists have long used scent analysis to study everything from disease detection to forensic identification. By analyzing the odors trapped in fabric, researchers can identify patterns linked to health conditions, stress levels, and even genetic differences. This makes underwear—despite its taboo status—an unexpectedly valuable tool in scientific research.
How Scent Analysis Works
Modern scent analysis relies on sophisticated technology:
Gas Chromatography/Mass Spectrometry (GC/MS): This technique separates and identifies the individual chemical components of an odor, allowing scientists to pinpoint specific VOCs.
Electronic Noses: These devices mimic the human sense of smell using arrays of chemical sensors, providing rapid and objective odor assessment.
Human Panels: Trained individuals evaluate odors for intensity and quality, a method still widely used in sensory science and product development.
By combining these tools, researchers can create detailed “scent fingerprints” that may one day be used for personalized health monitoring or biometric identification.
Career Paths in Scent Analysis
While “underwear sniffer” is not a recognized job title, the skills and knowledge required for scent analysis are highly valued in several industries:
Forensic Science: Odor analysis can help identify individuals or track missing persons using scent profiles left on clothing.
Health and Medicine: Researchers are exploring how body odor changes can signal diseases such as diabetes, cancer, or infections.
Textile and Consumer Products: Companies use scent analysis to develop fabrics that resist odor or to test the effectiveness of laundry products.
Personal Care and Cosmetics: Fragrance development relies heavily on understanding how different scents interact with the human body.
To pursue a career in this field, consider studying chemistry, biochemistry, sensory science, or forensic science. Gaining research experience in odor analysis labs and staying current with advances in analytical technology will set you apart in the job market14.
The Bigger Picture: Scent Analysis and Career Development
The world of scent analysis is just one example of how niche scientific fields can offer surprising career opportunities. Career development programs that emphasize interdisciplinary skills, research experience, and adaptability are crucial for students interested in emerging scientific areas1. As industries evolve, the ability to analyze and interpret complex data—including scent data—will become increasingly valuable.
Conclusion
While the idea of “underwear sniffing” might raise eyebrows, it highlights the untapped potential of scent analysis in science and industry. By pushing past taboos and embracing innovative research methods, we open the door to new discoveries and career paths that could shape the future of health, forensics, and consumer technology.
So next time you do the laundry, remember: those lingering odors might just be the key to the next big scientific breakthrough.
https://www.frontiersin.org/articles/10.3389/feduc.2022.999541/full
https://www.allsocialsciencejournal.com/search?q=F-24-14&search=search
https://ijble.com/index.php/journal/article/view/376
https://utppublishing.com/doi/10.3138/jelis.2018-0067
https://www.tandfonline.com/doi/full/10.1080/09585192.2019.1660700
https://www.emerald.com/insight/content/doi/10.1108/CDI-06-2023-0194/full/html
https://www.tandfonline.com/doi/full/10.1080/10611932.2017.1326772
https://journals.sagepub.com/doi/10.1177/08948453231173141
https://www.mdpi.com/2071-1050/14/1/357
https://journals.sagepub.com/doi/10.1177/21582440221078856
0 notes
Video
youtube
GC-MS Analysis Now at Tamilnadu Test House!
We are excited to offer Gas Chromatography-Mass Spectrometry (GC-MS) services for the precise identification and quantification of volatile and semi-volatile compounds.✅ Ideal for: • Pesticide Residue • Essential Oils • Fragrance & Allergen Testing (as per IFRA) • Contaminants in Food, Cosmetics & Pharma • Environmental Pollutants💡 Accurate. Reliable. NABL Accredited. Partner with us for trusted analytical excellence.📞 Contact us today to learn more!
0 notes
Link
1 note
·
View note
Text
Bulk Carrier Oils for Cosmetics, Pharma & Food Industries

Carrier oils are the unsung heroes behind creams, capsules, tinctures, and even salad dressings. Bulk purchasing offers economies of scale and ensures product consistency. In this blog, we go beyond generic content to provide insights into innovations, supply strategies, and compliance checkpoints that can set your brand apart.
What Are Carrier Oils?
Definition and Function Carrier oils, also known as base oils, are plant-derived fats used to dilute essential or active oils. Their function varies by industry: in cosmetics, they moisturize and support active ingredient delivery; in pharmaceuticals, they serve as excipients; in food, they contribute to texture and nutrition. Unlike essential oils, carrier oils are non-volatile but may contain beneficial phytochemicals such as vitamin E and fatty acids.
Types of Carrier Oils in Bulk MCT Oil: Extracted from coconut or palm, highly valued in pharmaceuticals and keto-focused foods.
Almond, Grapeseed, Jojoba: Popular in cosmetics for their moisturizing and non-comedogenic properties.
Sunflower, Sesame, Olive: Commonly used in food and nutraceutical products.
Hemp Seed, Apricot Kernel, Avocado: Specialized oils offering fatty acid diversity and product differentiation.
Why Choose Bulk Carrier Oils?
Cost Efficiency
Buying in bulk significantly reduces per-unit costs. Packaging, shipping, and handling expenses decrease with volume. In a tightening supply market for ingredients like argan or meadowfoam oil, bulk buying gives you negotiation leverage and stable pricing.
Supply Chain Advantages
Bulk suppliers provide consistent batches, which is crucial for products with INCI labeling or seasonal formulations. Pre-tested warehouse stock minimizes lead times, avoiding delays due to seasonal harvests or shipping congestion.
Carrier Oils in Key Industries

Carrier oils serve as the base for lotions, balms, serums, and lip glosses. Jojoba mimics sebum, almond is lightweight, and avocado is intensely nourishing. Many oils contain bioactive compounds that allow for added claims like anti-aging or skin barrier support.
Pharmaceuticals MCT oil is essential in both oral and topical pharmaceutical formulations. It facilitates accurate dosing, intestinal absorption, and meets GRAS and GMP standards. Each order typically undergoes rigorous testing, including endotoxin and sterility checks.
Food and Nutraceuticals Cold-pressed sunflower or olive oil enhances products such as flavored vinegars, meal replacements, and omega-enriched beverages. Organic certifications and clean labels appeal to health-conscious consumers.
Standards and Purity in Bulk Carrier Oils
Certificates & Lab Testing
Always request a Certificate of Analysis (COA) including:
GC-MS or GPC profiles
Peroxide value
Free fatty acid content
Microbial count
Heavy metal residues
Food-grade oils require stricter testing thresholds than cosmetic or pharmaceutical grades.
Stability, Shelf Life, and Oxidation Carrier oils degrade over time. Monitor peroxide levels monthly. Add antioxidants (like tocopherols) and store in nitrogen-flushed, opaque containers at temperatures below 25 °C.
Bulk Pure Essential Oils vs. Carrier Oils

Complementary Roles Carrier oils dilute essential oils for safe topical or ingestible use. They enhance spreadability and dermal absorption — olive oil’s vitamin E, for instance, helps lavender oil penetrate deeper.
Handling, Blending, and Dilution Use inert, stainless-steel or HDPE vessels. Blending should follow precise weight-based ratios. Minor seasonal variations (e.g., 0.5% difference in essential oil potency) may require adjusting carrier oil volumes.
Sourcing Bulk Oils: Wholesale Essential Oils Near Me
/media/4a0fe76350fb6156dbbf5358def53141
Local vs. International Suppliers
Local suppliers offer faster delivery, lower freight costs, and easy returns — plus you can often inspect samples or facilities. International suppliers from countries like India, Morocco, or Indonesia offer wider selections and competitive prices, though with longer lead times and regulatory complexity.
Evaluate:
Traceability: Where was the plant cultivated?
Third-Party Testing: Do they provide verified lab reports?
MOQ: What is the minimum order quantity?
Logistics, Tariffs, and Regulations Importing bulk oils requires:
FDA Prior Notice (for food-grade oils in the U.S.)
Harmonized Tariff Schedule compliance
Freight coordination and cold-chain logistics
Consider HS codes, documentation, and customs brokers. FedEx/DHL may offer cold-chain shipping, though at higher costs. Partnering with 3PL specialists for organic/cosmetic ingredients can reduce complexity.
Strategic Packaging & Storage
Containers and Material Compatibility Oils must be stored in HDPE, aluminum, or stainless steel — never PVC or reactive metals. Follow best practices:
Tamper-proof seals
Nitrogen-flushed drums
Use of MSDS for material compatibility
Temperature, Light, and Moisture Control To avoid rancidity:
Store at 15–25°C
Use UV-resistant containers
Dehumidify if necessary
Label batches with “Received On” and “Best Before” dates
Quality Control Approach
Batch Testing and Traceability Each batch should be tested for:
Free fatty acids Peroxide levels Refractive index Microbial and heavy metal contamination
Insist on COA and MSDS for every order. Use barcoding or RFID tagging for traceability.
Documentation & GMP Compliance
For resale — especially in regulated sectors like food and pharma — ensure oils are sourced from GMP-compliant facilities. Compliance should include:
ISO, HACCP, or FDA certification Staff training Proper documentation for audits
Regulations & Compliance FDA (US) and EU Standards In the U.S., the FDA regulates food and pharmaceutical-grade oils. They must be GRAS and processed in registered facilities.
In the EU, REACH registration and compliance with Cosmetic Regulation (EC) №1223/2009 are essential.
INCI labeling and allergen disclosures required
Ensure all documentation is updated and verified
GRAS, Cosmetic, and Pharma Designations Different uses require different certifications:
GRAS for edible use
Cosmetic safety testing and compatibility reports
Pharma: USP, EP, or JP standards for sterility and purity
Sustainability and Ethical Sourcing
Certifications: Organic, Fair‑Trade, RSPO
Valuable certifications include:
USDA/EU Organic — No synthetic chemicals
Fair Trade — Ethical labor practices
RSPO — Sustainable palm oil
These build brand trust and meet requirements for premium retailers.
Environmental Impact and Carbon Footprint Partner with eco-conscious suppliers that:
Use renewable energy
Provide recyclable/biodegradable packaging
Offer carbon offset programs
Consumers are increasingly willing to pay more for green products.
Customization for End‑Products Tailored Blends Custom carrier oil blends offer convenience and consistency:
Facial oils: Jojoba + Camellia + Squalane
Massage oils: Coconut + Sesame
Culinary: Olive + Grapeseed
Blending services often include added preservatives and essential oils.
Private-Label and OEM Options
Suppliers may offer:
White-label packaging under your brand Regulatory-compliant labeling Pre-prepared marketing collateral
Ideal for startups seeking rapid market entry.
How to Choose a Reliable Supplier Due Diligence Checklist Do they provide third-party COAs? Are they ISO/FDA/GMP certified? Can you get samples? Do they trace products to the source? Do they store oils in temperature-controlled facilities?
AI is being used to predict:
Oxidation levels Shelf life Microbial growth Blockchain may soon ensure full traceability — from harvest to warehouse.
Regional Demand and Market Growth
The Asia-Pacific region is booming in both demand and production. North America and Europe continue to prioritize organic and sustainable oils.
Markets are expanding to include:
Veterinary applications Sports supplements Hypoallergenic baby care
The bulk oil industry is projected to reach $12 billion by 2030, with carrier oils leading over essential oils due to their safety and flexibility.
Conclusion
Carrier oils are no longer just raw materials — they are strategic ingredients in food, pharma, and cosmetics. Sourcing them in bulk with attention to quality, compliance, and sustainability gives your brand an edge in consistency, profitability, and consumer trust.
FAQs 1. What defines a carrier oil? A plant-based oil used to dilute essential oils or actives. It “carries” them safely to the skin, bloodstream, or food product.
2. Can I use carrier oils in food products? Yes — if they’re food-grade and GRAS-certified. Examples: olive, coconut, sunflower, sesame oils.
3. How long does bulk carrier oil last? Depends on the type and storage. MCT oil can last up to 2 years. Unrefined oils: 6–12 months when stored properly.
4. How do I compare suppliers? Evaluate certifications, lab reports, pricing, logistics, and reputation. Always start with a sample.
5. Are all carrier oils allergen-free? No. Some like almond or peanut oil can trigger allergies. Disclose allergens and use hypoallergenic alternatives for sensitive applications.
#carrier oil#nature oils#naturesnaturalindia#carrier oil supplier#bulk carrier oil#essential oil#natures carrier oil
1 note
·
View note
Text
Herbal Formulations – Food Research Lab
With rising global demand for safe, effective, and clean-label botanical solutions, herbal products have taken center stage. From wellness supplements and functional beverages to skincare and topical formulations, consumers are seeking nature-inspired innovations with scientific backing. At Food Research Lab (FRL), we offer comprehensive herbal product development and innovation services & solutions that blend traditional wisdom with modern formulation expertise. 👉 Discover more: Herbal Formulations – Food Research Lab
Your Partner in Herbal Innovation
Herbal products have gone mainstream across categories including pharmaceuticals, nutraceuticals, cosmeceuticals, and F&B. As experienced herbal product development and innovation consultants, we help brands—from startups to large manufacturers—develop high-performance herbal products with a strong scientific foundation.
Our expertise includes: • Custom herbal blends aligned with target health benefits • Standardization of herbal extracts and bioactives • Clean-label, vegan, and organic formulation design • Phytochemical analysis and botanical sourcing • Regulatory support (FSSAI, AYUSH, EFSA, FDA, and more)
We guide you through every step—from concept development to compliance documentation and shelf-life validation.
End-to-End Herbal Formulation & Scale-Up Services
Translating traditional herbal concepts into commercial-ready products demands technical precision. Our herbal formulation development and scale-up solutions ensure your product is safe, stable, and effective—whether in capsule, powder, liquid, or topical form.
Our capabilities include: • Phytochemical profiling and ingredient compatibility testing • Optimizing dosage and improving bioavailability • Enhancing solubility and choosing the right excipients • Microbial and stability testing under accelerated and real-time conditions • Pilot-scale production, small batch trials, and tech transfer
We deliver lab-proven formulations ready for scale and commercialization.
Herbal Innovation Across Categories
We offer formulation expertise across a wide range of herbal applications, ensuring your products are compliant, stable, and aligned with consumer needs:
• Herbal drinks (detox blends, immunity boosters) • Topical creams, gels, and herbal ointments • Nutraceuticals (capsules, tablets, gummies) • Traditional Ayurvedic products (churnas, arishtas, asavas) • Functional foods enriched with botanicals • Cosmeceuticals with plant-based actives
Our approach fuses botanical heritage with modern formulation technology to deliver distinctive products.
Herbal R&D Services for Outsourcing & Innovation
For companies looking to outsource development, FRL offers specialized herbal product development and innovation contract R&D services with full confidentiality and scalability.
We support you with: • Botanical ingredient sourcing, analysis, and IP consultation • Scientific substantiation of herbal health claims • Custom extraction processes and phytochemical standardization • Advanced delivery formats including nanoformulations and liposomal systems • Preparation of regulatory dossiers for international markets
Our integrated team of botanists, chemists, and regulatory professionals ensures a seamless, end-to-end R&D experience.
Our Scientific Approach to Herbal Formulation
At FRL, we combine ancient herbal knowledge with rigorous scientific methods to create products that meet both traditional and modern expectations.
Our scientific edge includes: • Traceability of herbal raw materials • Advanced analytical tools like HPLC, GC-MS, FTIR, and UV-Vis • Support for clinical studies and documentation of efficacy • Process innovation from decoction to supercritical CO₂ extraction • Global regulatory compliance and documentation readiness
We solve key challenges such as formulation stability, dosage accuracy, and international compliance to help you succeed in the competitive herbal marketplace.
Examples of Herbal Product Success Stories
Here are a few product innovations showcasing our herbal formulation and scale-up expertise: • Stress-relief Ashwagandha gummies • Cognitive boosters with Brahmi extracts • Tulsi and Mulethi-based herbal cough syrup • Anti-acne face cream with Neem and Turmeric • Triphala capsules for digestive wellness • Aloe vera and Hibiscus shampoo with natural preservation
Each formulation is supported by validated botanical science, precise extraction, and regulatory alignment.
Why Partner with Food Research Lab?
FRL is more than just a formulation service—we are herbal product development and innovation consultants dedicated to helping you build impactful, compliant, and market-ready herbal products. Whether launching one SKU or an entire range, we offer:
• Secure and confidential R&D lab services • Scientific validation of herbal ingredients and actives • Category-spanning formulation (topical, ingestible, cosmetic) • Market readiness testing and packaging recommendations • Global scaling solutions with cost-effective sourcing strategies
With our multidisciplinary expertise, your product journey is in trusted hands.
Let’s Build the Future of Herbal Innovation
As botanical products continue to grow in popularity, you need a partner who understands both the roots of tradition and the rigors of modern science. From formulation to scale-up, FRL offers herbal product development and innovation contract R&D services designed to help you succeed.
👉 Ready to bring your herbal vision to life? Explore our capabilities at: Herbal Formulations – Food Research Lab
#Herbal product Development#erbal Formulation Development and Scale-Up Solutions#Herbal product Development and Innovation Contract R&D services & solutions#Herbal product Development and Innovation Consultants services & solutions
0 notes
Text
Mass Spectrometry Market: Key Trends Fueling Growth in Precision Analysis, Healthcare, and Emerging Applications
Market Overview
The global mass spectrometry market is projected to be valued at USD 7.19 billion in 2025 and is anticipated to reach USD 9.74 billion by 2030, growing at a compound annual growth rate (CAGR) of 6.25% during the forecast period from 2025 to 2030. Mass spectrometry is a highly sensitive and accurate analytical technique used to determine the mass-to-charge ratio of ions. Its applications span drug discovery, proteomics, metabolomics, toxicology, clinical diagnostics, and more. With advancements in ionization techniques and hybrid systems, the technology is now more accessible, automated, and user-friendly.
Leading market research firms suggest that ongoing investments in life sciences research, coupled with stricter regulations for food and environmental safety, are fueling sustained demand for mass spectrometry systems globally.
Key Trends Driving Market Growth
Integration with Chromatography Systems Hybrid technologies like LC-MS (liquid chromatography–mass spectrometry) and GC-MS (gas chromatography–mass spectrometry) are becoming standard due to their ability to deliver comprehensive, high-resolution results. These systems are vital in pharmaceutical and clinical settings.
Rise in Proteomics and Biomarker Discovery Mass spectrometry is essential for proteomic analysis, allowing researchers to identify and quantify proteins in complex biological samples. This is vital for disease diagnosis, personalized medicine, and the development of targeted therapies.
Growing Use in Clinical Diagnostics As healthcare systems move toward precision diagnostics, MS is increasingly being adopted in laboratories for testing vitamin D levels, detecting inborn errors of metabolism, and analyzing complex blood samples with high accuracy.
Advancements in High-Resolution Mass Spectrometry (HRMS) Innovations in HRMS technologies such as time-of-flight (TOF) and Orbitrap analyzers have enhanced speed, accuracy, and throughput, making these systems indispensable in high-end research and commercial labs.
Environmental and Food Safety Testing Governments and regulatory bodies worldwide are mandating strict monitoring of pollutants, pesticides, and contaminants. Mass spectrometry offers a gold standard for detecting trace levels of toxic substances, boosting its use in public health monitoring.
Miniaturization and Portability There is a rising trend toward portable and compact MS devices, especially in defense, forensics, and on-site environmental testing. These innovations are making mass spectrometry more versatile and field-deployable.
Competitive Landscape
The market is moderately consolidated, with leading players like Thermo Fisher Scientific Inc., Agilent Technologies Inc., SCIEX (a Danaher Corporation company), Bruker Corporation, Waters Corporation, and PerkinElmer Inc. dominating the industry. These companies focus on innovation, mergers, and strategic collaborations to enhance their technological capabilities and global reach.
Emerging players and academic partnerships are contributing to niche advancements, particularly in affordable MS solutions and AI-driven data analysis.
Regional Insights
North America remains the largest market due to strong investments in pharmaceutical R&D, a well-established healthcare infrastructure, and early adoption of advanced technologies. Europe follows closely, with significant contributions from the UK, Germany, and France.
Asia-Pacific is emerging as a high-growth region, driven by expanding research capabilities, government funding, and increasing demand from the biopharmaceutical and environmental sectors, particularly in China, India, and Japan.
Outlook and Conclusion
As industries demand more accurate, fast, and cost-efficient analytical solutions, the mass spectrometry market is set to grow steadily across all major regions. From enabling cutting-edge research to ensuring public health and safety, the importance of MS in modern science cannot be overstated.
Looking ahead, the market will benefit from further miniaturization, automation, and AI integration, all of which will expand its accessibility and application scope. Mass spectrometry will continue to be a cornerstone technology in the global analytical ecosystem—supporting innovation, ensuring compliance, and enhancing scientific discovery.
Read More
About Mordor Intelligence:
Mordor Intelligence is a trusted partner for businesses seeking comprehensive and actionable market intelligence. Our global reach, expert team, and tailored solutions empower organizations and individuals to make informed decisions, navigate complex markets, and achieve their strategic goals.
With a team of over 550 domain experts and on-ground specialists spanning 150+ countries, Mordor Intelligence possesses a unique understanding of the global business landscape. This expertise translates into comprehensive syndicated and custom research reports covering a wide spectrum of industries, including aerospace & defense, agriculture, animal nutrition and wellness, automation, automotive, chemicals & materials, consumer goods & services, electronics, energy & power, financial services, food & beverages, healthcare, hospitality & tourism, information & communications technology, investment opportunities, and logistics.
For any inquiries or to access the full report, please contact:
[email protected] https://www.mordorintelligence.com/
0 notes
Text
The Role of Cosmetic Testing Labs in Abu Dhabi in Supporting Halal Certification for Cosmetics | +971 554747210
As demand for halal-certified cosmetics continues to rise across the Middle East and beyond, the need for stringent verification processes has never been more important. Consumers today are not only seeking beauty products that are safe and effective, but also compliant with their religious and ethical values. In the United Arab Emirates, particularly in Abu Dhabi, cosmetic testing labs are playing a vital role in supporting the halal certification process for cosmetic and personal care products.
This blog explores how a cosmetic testing lab in Abu Dhabi contributes to halal compliance by verifying ingredient purity, detecting prohibited substances, and aligning with Islamic guidelines—ensuring that products meet both local regulatory and religious standards.
Understanding Halal Certification in Cosmetics
Halal, an Arabic term meaning “permissible,” refers to anything that adheres to Islamic law. In cosmetics, halal certification assures consumers that the products:
Do not contain haram (forbidden) ingredients, such as alcohol or animal derivatives from non-halal sources.
Are manufactured under hygienic conditions that prevent cross-contamination.
Do not involve animal testing, depending on certification authority requirements.
In the UAE, the Emirates Authority for Standardization and Metrology (ESMA) oversees halal certification through its Emirates Halal Accreditation Scheme (EHAS). To receive this certification, companies must provide scientific proof—often from a cosmetic testing lab in Abu Dhabi—that their products are free from non-halal substances and produced according to halal guidelines.
How Cosmetic Testing Labs in Abu Dhabi Support Halal Certification
1. Ingredient Verification and Source Authentication
The first step in halal compliance is confirming that all ingredients used in the cosmetic product are halal-compliant. Cosmetic testing labs conduct:
Source validation – Labs investigate the origin of ingredients such as glycerin, collagen, and stearic acid to ensure they are not derived from pork or non-halal slaughtered animals.
Certificate of analysis (CoA) review – Labs cross-verify supplier documentation with their analytical results to confirm ingredient purity.
This helps cosmetic companies in Abu Dhabi maintain transparency and confidence in their formulations.
2. Detection of Prohibited Substances
Advanced analytical techniques are employed by Abu Dhabi cosmetic testing labs to detect the presence of haram substances such as:
Alcohols – Ethanol and methanol can be tested using Gas Chromatography (GC) to confirm the absence or quantify their concentration.
Pig-derived ingredients – Gelatin, enzymes, and other animal derivatives are tested using methods like Polymerase Chain Reaction (PCR) and Liquid Chromatography-Mass Spectrometry (LC-MS).
Contaminants from non-halal sources – Cross-contamination during production is a major concern; labs test final products to confirm absence of impurities.
These tests provide scientific backing for halal certifiers to confidently approve products.
3. Preservative and Fragrance Analysis
Preservatives and fragrances in cosmetics can sometimes contain hidden animal-derived compounds or alcohol-based solvents. Cosmetic testing labs in Abu Dhabi conduct:
Fragrance composition analysis
Preservative origin tracing
Solvent testing for alcohols
This ensures that every component, even in trace amounts, complies with halal requirements.
Aligning with UAE Halal Standards and Global Guidelines
ESMA and EHAS Compliance
Abu Dhabi’s cosmetic testing labs are well-versed with ESMA’s guidelines for halal cosmetics and provide testing results in accordance with these standards. Their results are accepted by UAE authorities and halal certification bodies as credible evidence of compliance.
International Compatibility
Many cosmetic brands operating in Abu Dhabi also export to Malaysia, Indonesia, and the GCC—countries with strict halal regulations. Cosmetic testing labs in Abu Dhabi ensure compatibility with:
Malaysian JAKIM standards
GSO/GCC Halal standards
Southeast Asian halal cosmetic frameworks
This makes them a strategic partner for companies looking to go global with their halal-certified products.
The Importance of ISO/IEC 17025 Accreditation
Leading cosmetic testing labs in Abu Dhabi are accredited under ISO/IEC 17025, the international standard for testing and calibration laboratories. This accreditation ensures:
Reliable, reproducible test results
Use of validated and internationally recognized methods
Credibility with regulators and certifiers
For halal certification, using an ISO-accredited lab adds credibility to the testing process and helps accelerate approval timelines.
Benefits for Cosmetic Companies Seeking Halal Certification
Partnering with a certified cosmetic testing lab in Abu Dhabi offers several advantages to manufacturers and importers:
1. Faster Halal Certification Process
With ready access to required tests and experienced analysts, labs streamline the documentation and verification process for halal authorities.
2. Regulatory Compliance
Cosmetic testing labs also help ensure products meet other regulatory requirements for safety, labeling, and product registration in the UAE alongside halal compliance.
3. Market Access and Consumer Trust
Halal-certified products enjoy strong demand in the Middle East, Southeast Asia, and among Muslim consumers globally. Reliable lab testing builds consumer confidence and opens new markets.
4. Reduced Risk of Non-Compliance
Failure to comply with halal standards can lead to recalls, reputational damage, and financial losses. Labs help mitigate these risks with accurate and timely testing.
Common Cosmetic Products Requiring Halal Testing
Lipsticks and lip balms (may contain animal-derived waxes or pigments)
Moisturizers and creams (contain emulsifiers or collagen)
Soaps and cleansers (may include alcohol or glycerin)
Perfumes and deodorants (contain ethanol or synthetic fragrances)
Hair care products (may include keratin or protein complexes)
Each of these products must undergo thorough ingredient and microbial testing to ensure full halal compliance.
Conclusion
As halal cosmetics gain popularity in the UAE and around the world, ensuring authentic halal certification is more important than ever. Cosmetic testing labs in Abu Dhabi are at the heart of this process—providing scientific validation, detecting non-compliant substances, and aligning with both religious and regulatory standards.
By leveraging advanced testing technologies and deep regulatory knowledge, these labs empower manufacturers to produce halal-certified products that are trusted by consumers and approved by certifying bodies. Whether you're a local manufacturer or an international brand entering the Middle Eastern market, working with a reputable cosmetic testing lab in Abu Dhabi is your essential step toward safe, compliant, and successful halal-certified cosmetics.
#cosmetic testing lab#cosmetic testing#cosmetic testing services#testing lab near me#testing lab uae
0 notes
Text
Global Chromatography Instruments Market to Rise With Drug Quality Control Measures by 2030
The global chromatography instruments market is set to witness a growth rate of 5% in the next 5 years. Growing application in pharmaceutical and biotech R&D; growing food safety concerns, environmental testing and pollution control; adoption of hyphenated techniques; growing importance of chromatography tests in the drug approval process; and rise in demand for quality control in manufacturing are some of the key factors driving the chromatography instruments market.
Chromatography instruments are analytical tools used to separate, identify, and quantify components within complex mixtures. These instruments operate based on the principle of differential partitioning between a mobile phase and a stationary phase. Common types include gas chromatography (GC), liquid chromatography (LC), and high-performance liquid chromatography (HPLC), each suited for specific sample types and applications. Chromatography instruments are essential in various fields such as pharmaceuticals, biotechnology, environmental analysis, food safety, and chemical manufacturing. They enable high-precision analysis for quality control, drug development, and regulatory compliance, making them indispensable in both research and industrial laboratories worldwide.
Download a free sample report now 👉 https://meditechinsights.com/chromatography-instruments-market/request-sample/
Growth of proteomics and genomics markets: A key market opportunity
The growing proteomics and genomics markets present a significant opportunity for the chromatography instruments market. These fields require highly sensitive, accurate, and high-throughput analytical tools to separate, identify, and quantify biomolecules such as proteins, peptides, nucleic acids, and metabolites. Chromatography techniques; especially liquid chromatography coupled with mass spectrometry (LC-MS); are vital for analyzing complex biological samples in genomics and proteomics research. As demand for personalized medicine, biomarker discovery, and disease pathway analysis rises, research institutions and biotech firms are increasingly investing in advanced chromatography systems to support these applications, thereby expanding market potential and driving innovation in instrument development.
Chromatography instruments powering the shift to decentralized clinical trials
The growth of remote and decentralized clinical trials is a key trend driving the chromatography instruments market. These trials leverage chromatography instruments to collect continuous, real-time patient data outside traditional clinical settings, reducing the need for in-person visits. This approach enhances patient recruitment, retention, and compliance while lowering operational costs. Wearables, mobile apps, and sensors enable seamless remote monitoring, making trials more inclusive and geographically flexible. As regulatory bodies increasingly recognize digital endpoints, the adoption of decentralized trial models is accelerating, positioning chromatography instruments as essential tools in transforming clinical research into a more patient-centric process.
Competitive Landscape Analysis
The global chromatography instruments market is marked by the presence of established and emerging market players such as Agilent Technologies, Inc., Thermo Fisher Scientific Inc., Shimadzu Corporation, Waters Corporation, PerkinElmer, Merck KGaA, Phenomenex Inc. (a Danaher company), Bio-Rad Laboratories, Inc., GE Healthcare (Cytiva), Hitachi, Ltd.; among others. Some of the key strategies adopted by market players include new product development, strategic partnerships and collaborations, and geographic expansion.
Download a sample report for in-depth competitive insightshttps://meditechinsights.com/chromatography-instruments-market/request-sample/
Global Chromatography Instruments Market Segmentation
This report by Medi-Tech Insights provides the size of the global chromatography instruments market at the regional- and country-level from 2023 to 2030. The report further segments the market based on system, consumables, accessories, end user.
Market Size & Forecast (2023-2030), By Systems, USD Million
Liquid Chromatography
Gas Chromatography
Thin-Layer Chromatography
Supercritical Fluid Chromatography
Others
Market Size & Forecast (2023-2030), By Consumables, USD Million
Columns
Solvents
Syringes
Others
Market Size & Forecast (2023-2030), By Accessories, USD Million
Column Accessories
Auto-Sampler Accessories
Pumps
Others
Market Size & Forecast (2023-2030), By End User, USD Million
Life Sciences Industry
Academic & Research Institutes
Environmental Agencies
Food & Beverage Industry
Oil & Gas Industry
Others
Market Size & Forecast (2023-2030), By Region, USD Million
North America
US
Canada
Europe
UK
Germany
France
Italy
Spain
Rest of Europe
Asia Pacific
China
India
Japan
Rest of Asia Pacific
Latin America
Middle East & Africa
About Medi-Tech Insights
Medi-Tech Insights is a healthcare-focused business research & insights firm. Our clients include Fortune 500 companies, blue-chip investors & hyper-growth start-ups. We have completed 100+ projects in Digital Health, Healthcare IT, Medical Technology, Medical Devices & Pharma Services in the areas of market assessments, due diligence, competitive intelligence, market sizing and forecasting, pricing analysis & go-to-market strategy. Our methodology includes rigorous secondary research combined with deep-dive interviews with industry-leading CXO, VPs, and key demand/supply side decision-makers.
Contact:
Ruta Halde Associate, Medi-Tech Insights +32 498 86 80 79 [email protected]
0 notes